Abstract
Background:
Skin toxicities are the most common adverse events related to immunotherapy, such as reactive cutaneous capillary endothelial proliferation (RCCEP) following treatment with the anti-programmed cell death-1 antibody camrelizumab.
Objective:
This study aimed to comprehensively analyze the clinical features and prognostic value of RCCEP in patients with malignancies who received camrelizumab alone (Camre) or in combination with the angiogenesis-targeted agent apatinib (Camre-Apa) or chemotherapy (Camre-Chemo).
Design:
A large-scale pooled analysis.
Methods:
Individual patient-level data were derived from 10 clinical trials of camrelizumab monotherapy, camrelizumab plus apatinib, or camrelizumab plus chemotherapy (n = 1305).
Results:
RCCEP occurred in 77.0% (516/670) of patients with Camre, 23.6% (70/296) with Camre-Apa, and 67.8% (230/339) with Camre-Chemo. Most RCCEP lesions were grade 1 or 2 in severity. The median time to onset was 0.8 months [interquartile range (IQR), 0.6–1.2] with Camre, 5.0 months (IQR, 2.7–8.0) with Camre-Apa, and 1.6 months (IQR, 1.0–4.2) with Camre-Chemo; and the median duration was 4.8 months (IQR, 2.6–8.8), 4.4 months (IQR, 1.7–8.9), and 7.2 months (IQR, 4.1–14.3), respectively. In all the three groups, patients with RCCEP showed significantly better clinical outcomes compared with those without [objective response rate: 23.8% versus 1.9% with Camre, 48.6% versus 21.2% with Camre-Apa, and 78.7% versus 54.1% with Camre-Chemo; median progression-free survival: 3.2 versus 1.7 months (hazard ratio (HR) = 0.36), 10.2 versus 4.5 months (HR = 0.39), and 12.7 versus 7.3 months (HR = 0.38); median overall survival: 13.3 versus 3.8 months (HR = 0.34), 29.2 versus 13.5 months (HR = 0.46), and not reached versus 12.8 months (HR = 0.19); all p < 0.0001].
Conclusion:
Although RCCEP occurred frequently with camrelizumab, most lesions were mild and self-limiting. The occurrence of RCCEP was strongly associated with the antitumor activity and survival of camrelizumab, both as monotherapy and in combination therapy.
Keywords: camrelizumab, clinical efficacy, immune-related adverse event, prognostic marker, reactive cutaneous capillary endothelial proliferation
Introduction
Immune checkpoint inhibitors (ICIs) are a class of immunotherapeutic agents that inhibit the immune escape of tumor cells and activate the antitumor immune response, mainly including monoclonal antibodies against programmed cell death-1 (PD-1), programmed death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated protein 4.1,2 Nowadays, ICIs have been widely used for the treatment of multiple malignancies, but several issues remain to be addressed. The objective response rate (ORR) with ICI monotherapy is low in an unselected population of many tumor types. 3 In addition, ICIs have the risk of causing immune-related adverse events (irAEs) affecting multiple tissues and organ systems including the skin and mucous membranes as well as the respiratory, gastrointestinal, and musculoskeletal systems.4,5 Therefore, reasonable treatment strategies, such as a combination of drugs with different mechanisms of action, should be proactively investigated to improve the efficacy of ICIs. 6 Furthermore, close observation and active prevention and management of irAEs are necessary, and it is essential to identify prognostic biomarkers to screen populations that could benefit from ICIs. In recent years, molecular biomarkers, such as PD-L1 expression, tumor mutational burden, and microsatellite instability-high (MSI-H)/DNA mismatch repair-deficient (dMMR), have been approved for predicting responses to immunotherapy in patients with non-small-cell lung cancer (NSCLC) and melanoma.7–9 However, given that these markers are rare in many tumor types and not sufficiently stable,10,11 new clinical or blood/tissue biomarkers are required for application in clinical practice.
Camrelizumab is a humanized monoclonal antibody that targets PD-1 with high affinity and blocks the binding of PD-1 to its ligands, thereby activating or restoring the body’s immune response and consequently exerting the antitumor activity. 1 PD-1 is a highly glycosylated inhibitory receptor predominantly expressed in T cells. Polymorphism at the N-glycosylation sites of PD-1 affects antibody binding. Previous studies showed that camrelizumab binds to PD-1 via the CDRH2 domain on its heavy chain, and the glycosylation of asparagine 58 (N58) promotes interaction with camrelizumab, while its light chain sterically inhibits PD-1/PD-L1 interaction.12,13 Camrelizumab has been approved by the National Medical Products Administration of China for the treatment of relapsed or refractory classical Hodgkin lymphoma, 14 advanced or metastatic esophageal squamous cell carcinoma,15,16 hepatocellular carcinoma (HCC),13,17 nasopharyngeal carcinoma (NPC), 18 and non-squamous and squamous NSCLC.19,20
Reactive cutaneous capillary endothelial proliferation (RCCEP) is the most common irAE of camrelizumab monotherapy. Previous reports from individual clinical trials mainly presented the incidence of RCCEP following treatment with camrelizumab alone or in combination.6,13–16,18–40 There is a lack of systematic reporting on clinical characteristics of RCCEP, such as time to onset, duration, and remission data, especially in patients treated with a camrelizumab combination. Similarly, the correlation between RCCEP and efficacy was analyzed from individual clinical studies. It has been reported that the occurrence of RCCEP was associated with better clinical outcomes with camrelizumab monotherapy13,23,24,41; however, there are no reports on the correlation following camrelizumab combination therapy. In this context, we pooled data from 10 clinical trials of camrelizumab with a large patient population involving multiple types of cancers to explore the clinical features and prognostic value of RCCEP, not only post-camrelizumab monotherapy but also following camrelizumab combination therapy. The comprehensive nature of pooled analysis could provide important references for clinical practice and subsequent studies of camrelizumab.
Methods
Study design and patients
This pooled study comprehensively analyzed the incidence, time to onset, duration, and remission of RCCEP in patients with advanced or metastatic malignancies who received camrelizumab alone or in combination with other agents, as well as the associations between the occurrence of RCCEP and clinical outcomes including objective response and survival benefits. Data were derived from 10 clinical trials of camrelizumab in China (ClinicalTrials.gov Identifier: NCT02721589, 40 NCT02742935, 42 NCT03463876, 36 NCT03092895,30,35,38 NCT03417895, 33 NCT03472365, 39 NCT02989922, 13 NCT03099382, 15 NCT03134872, 19 and NCT03707509 18 ). As of 30 June 2021, a total of 1305 patients received camrelizumab; among whom, 670 were treated with camrelizumab alone (Camre group), 296 with camrelizumab combined with the antiangiogenic agent apatinib (Camre-Apa group), and 339 with camrelizumab combined with cytotoxic chemotherapy [gemcitabine plus cisplatin (n = 134) or pemetrexed plus carboplatin (n = 205); Camre-Chemo group], respectively (Supplemental Table S1). In all 10 trials, tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors (version 1.1), and all patients were followed up for survival. The reporting of this study refers to the STROBE statement (Supplemental Material 1).
RCCEP grade and remission
The grading criteria for RCCEP were defined as follows: grade 1, nodule(s) with a maximum diameter of ⩽10 mm, with or without rupture and bleeding; grade 2, nodule(s) with a maximum diameter of >10 mm, with or without rupture and bleeding; grade 3, generalized nodules throughout the body, which may be complicated by skin infection; grade 4, multiple and generalized nodules, life-threatening condition; and grade 5, death. 24 RCCEP remission was defined as regression of all RCCEP lesions.
Statistical analyses
Descriptive statistics were used to describe the baseline characteristics and the incidence, time to onset, duration, remission rate, and time to remission of RCCEP in the Camre, Camre-Apa, and Camre-chemo groups, respectively. In each group, ORR (defined as the percentage of patients whose best overall response was complete or partial response), progression-free survival (PFS; defined as the time from the first dose of study treatment or randomization to the first documented disease progression or death from any cause, whichever occurred first), and overall survival (OS; defined as the time from the first dose of study treatment or randomization to death from any cause) were tested in patients who experienced RCCEP and those who did not. The two-sided 95% confidence interval (CI) for ORR was calculated using the Clopper–Pearson method; comparisons were done with the Mantel–Haenszel χ2 test. The median PFS and OS were estimated using the Kaplan–Meier method, and the corresponding 95% CIs were calculated using the Brookmeyer–Crowley method; comparisons were done with the log-rank method. The Cox proportional hazards model was used to estimate hazard ratios (HRs) with 95% CIs. All analyses were performed using SAS version 9.4.
Results
Patient characteristics
Patients with a variety of tumor types, including NSCLC, HCC, NPC, esophageal cancer (EC), and small-cell lung cancer (SCLC), were included in this study. The baseline characteristics of patients are shown in Table 1. The median age of patients in the Camre, Camre-Apa, and Camre-chemo groups were 54 years (IQR, 46–62), 54 years (IQR, 46–62), and 57 years (IQR, 49–63), respectively. The median duration of camrelizumab treatment was 3.7 months (IQR, 1.8–8.3), 6.0 months (IQR, 2.8–11.8), and 7.9 months (IQR, 4.9–14.0), respectively.
Table 1.
Patients characteristics.
| Items | Camrelizumab monotherapy (N = 670) |
Camrelizumab plus apatinib (N = 296) |
Camrelizumab plus chemotherapy (N = 339) |
|---|---|---|---|
| Age (years), median (IQR) | 54 (46–62) | 54 (46–62) | 57 (49–63) |
| Sex, n (%) | |||
| Male | 579 (86.4%) | 257 (86.8%) | 259 (76.4%) |
| Female | 91 (13.6%) | 39 (13.2%) | 80 (23.6%) |
| ECOG PS, n (%) | |||
| 0 | 217 (32.4%) | 141 (47.6%) | 96 (28.3%) |
| 1 | 452 (67.5%) | 155 (52.4%) | 243 (71.7%) |
| 2 | 1 (0.1%) | 0 | 0 |
| Tumor type, n (%) | |||
| HCC | 222 (33.1%) | 211 (71.3%) | 0 |
| Extensive SCLC | 0 | 59 (19.9%) | 0 |
| NPC | 97 (14.5%) | 0 | 134 (39.5%) |
| NSCLC | 34 (5.1%) | 0 | 205 (60.5%) |
| EC | 275 (41.0%) | 0 | 0 |
| Others | 42 (6.3%) | 26 (8.8%) | 0 |
| Camrelizumab exposure (months), median (IQR) | 3.7 (1.8–8.3) | 6.0 (2.8–11.8) | 7.9 (4.9–14.0) |
EC, esophageal cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; HCC, hepatocellular carcinoma; IQR, interquartile range; NPC, nasopharyngeal carcinoma; NSCLC, non-small-cell lung cancer; SCLC, small-cell lung cancer.
Clinical features of treatment-related RCCEP
RCCEP were classified as ‘red nevus-like’, ‘pearl-like’, ‘mulberry-like’, ‘patch-like’, and ‘tumor-like’ lesions (Supplemental Figure S1). Overall, the incidence of RCCEP of any grade was the highest in patients treated with Camre (516 out of 670 patients, 77.0%) and lowest with Camre-Apa (70 out of 296, 23.6%). For those who received Camre-Chemo, 230 (67.8%) of the 339 patients developed RCCEP. The majority of RCCEP lesions were grade 1 or 2 in severity, grade 3 RCCEP rarely occurred (0.3% with Camre, 0.7% with Camre-Apa, and 1.2% with Camre-Chemo), and no grade 4 or 5 RCCEP were reported (Table 2).
Table 2.
Clinical features of RCCEP.
| Items | Camrelizumab monotherapy (N = 670) | Camrelizumab plus apatinib (N = 296) | Camrelizumab plus chemotherapy (N = 339) |
|---|---|---|---|
| Grade of RCCEP, n/N (%) | |||
| All grade | 516/670 (77.0%) | 70/296 (23.6%) | 230/339 (67.8%) |
| Grade 1 | 434/670 (64.8%) | 57/296 (19.3%) | 185/339 (54.6%) |
| Grade 2 | 80/670 (11.9%) | 11/296 (3.7%) | 41/339 (12.1%) |
| Grade 3 | 2/670 (0.3%) | 2/296 (0.7%) | 4/339 (1.2%) |
| Time to onset (months), median (IQR) | 0.8 (0.6–1.2) | 5.0 (2.7–8.0) | 1.6 (1.0–4.2) |
| Duration of RCCEP lesions (months), median (IQR) | 4.8 (2.6–8.8) | 4.4 (1.7–8.9) | 7.2 (4.1–14.3) |
| RCCEP remission rate, n/N1 (%) | 332/516 (64.3%) | 46/70 (65.7%) | 110/230 (47.8%) |
| Time to remission of RCCEP (months), median (IQR) | 4.2 (2.5–7.6) | 3.0 (1.2–5.6) | 6.2 (3.6–9.7) |
N is the number of patients in the group; N1 is the number of patients with RCCEP in the group.
IQR, interquartile range; RCCEP, reactive cutaneous capillary endothelial proliferation.
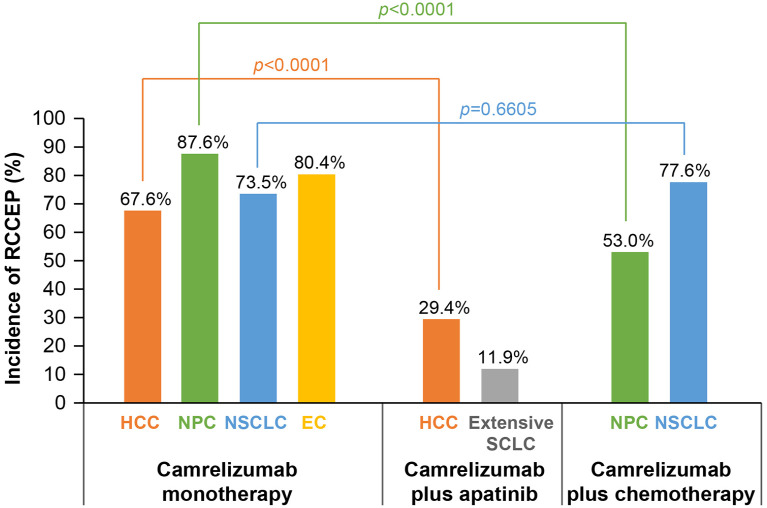
For Camre monotherapy, the incidence of RCCEP was similar among different tumor types, ranging from 67.6% to 87.6% (Figure 1). In patients with HCC, the incidence of RCCEP after Camre-Apa treatment was significantly lower than that after treatment with Camre alone (29.4% versus 67.6%; p < 0.0001). Notably, in patients with NSCLC, the incidence of RCCEP after Camre-Chemo was similar to that after treatment with Camre alone (77.6% versus 73.5%; p = 0.6605). However, in patients with NPC, the incidence was significantly lower with Camre-Chemo than that with Camre alone (53.0% versus 87.6%; p < 0.0001).
Figure 1.
Incidence of RCCEP by tumor type.
Fisher’s exact test was used to calculate p value.
EC, esophageal cancer; HCC, hepatocellular carcinoma; NPC, nasopharyngeal carcinoma; NSCLC, non-small-cell lung cancer; RCCEP, reactive cutaneous capillary endothelial proliferation; SCLC, small-cell lung cancer.
In the Camre group, the median time to RCCEP onset was 0.8 months (IQR, 0.6–1.2), and the median duration of RCCEP lesions was 4.8 months (IQR, 2.6–8.8; Table 2). In the Camre-Chemo group, the median time to RCCEP onset was 1.6 months (IQR, 1.0–4.2) with a median duration of RCCEP lesions of 7.2 months (IQR, 4.1–14.3), which were longer compared with the Camre group. In the Camre-Apa group, the median time to RCCEP onset was 5.0 months (IQR, 2.7–8.0), the longest among the three groups; and the median duration of RCCEP was 4.4 months (IQR, 1.7–8.9).
The remission rate of RCCEP lesions was similar between the Camre and Camre-Apa groups (64.3% versus 65.7%), while the Camre-Chemo group showed the lowest remission rate (47.8%; Table 2). The median time to remission was 4.2 months (IQR, 2.5–7.6) with Camre alone, 6.2 months (IQR, 3.6–9.7) with Camre-Chemo, and only 3.0 months (IQR, 1.2–5.6) with Camre-Apa (Table 2), indicating more rapid RCCEP remission when combined with an antiangiogenic agent.
One (0.1%) patient in the Camre group and two (0.6%) patients in the Camre-Chemo group discontinued Camre due to RCCEP, while no patients in the Camre-Apa discontinued Camre due to RCCEP.
Correlations between the development of RCCEP and clinical outcomes
The ORR was higher in those who experienced RCCEP compared with those who did not in the Camre group [23.8% (95% CI, 20.2–27.8%) versus 1.9% (95% CI, 0.4–5.6%), p < 0.0001]. A similar pattern was observed in the Camre-Apa group [48.6% (95% CI, 36.4–60.8%) versus 21.2% (95% CI, 16.1–27.2%), p < 0.0001] and the Camre-Chemo group [78.7% (95% CI, 72.8–83.8%) versus 54.1% (95% CI, 44.3–63.7%), p < 0.0001]. The significant association between RCCEP occurrence and higher ORR was consistently observed across all the tumor types analyzed, regardless of the treatment group (Table 3).
Table 3.
Correlation between RCCEP and tumor response.
| Items | Number of patients | ORR | Difference in ORR | ||||
|---|---|---|---|---|---|---|---|
| Total, N | With RCCEP, n (%) | Without RCCEP, n (%) | With RCCEP, % (95% CI) |
Without RCCEP, % (95% CI) |
With RCCEP − without RCCEP, % (95% CI) | p Value | |
| Camrelizumab monotherapy | |||||||
| HCC | 217 | 145 (66.8%) | 72 (33.2%) | 18.6% (12.6–25.9) | 4.2% (0.9–11.7) | 14.4% (6.6–22.3) | 0.0038 |
| EC | 228 | 182 (79.8%) | 46 (20.2%) | 25.3% (19.1–32.2) | 0.0% (0.0–6.3) | 25.3% (19.0–31.6) | 0.0001 |
| All | 670 | 516 (77.0%) | 154 (23.0%) | 23.8% (20.2–27.8) | 1.9% (0.4–5.6) | 21.9% (17.6–26.2) | <0.0001 |
| Camrelizumab plus apatinib | |||||||
| PLC | 28 | 6 (21.4%) | 22 (78.6%) | 33.3% (4.3–77.7) | 4.5% (0.1–22.8) | 28.8% (−9.9 to 67.5) | 0.0472 |
| Extensive SCLC | 59 | 7 (11.9%) | 52 (88.1%) | 57.1% (18.4–90.1) | 30.8% (18.7–45.1) | 26.4% (−12.4 to 65.1) | 0.1700 |
| GC/GEJC | 19 | 0 (0.0%) | 19 (100.0%) | / | 10.5% (1.3–33.1) | / | / |
| HCC | 190 | 57 (30.0%) | 133 (70.0%) | 49.1% (35.6–62.7) | 21.8% (15.1–29.8) | 27.3% (12.6–42.1) | 0.0002 |
| All | 296 | 70 (23.6%) | 226 (76.4%) | 48.6% (36.4–60.8) | 21.2% (16.1–27.2) | 27.3% (14.5–40.2) | <0.0001 |
| Camrelizumab plus chemotherapy | |||||||
| NSCLC | 205 | 159 (77.6%) | 46 (22.4%) | 69.8% (62.0–76.8) | 28.3% (16.0–43.5) | 41.6% (26.7–56.4) | <0.0001 |
| NPC | 134 | 71 (53.0%) | 63 (47.0%) | 98.6% (92.4–100.0) | 73.0% (60.3–83.4) | 25.6% (14.3–36.9) | <0.0001 |
| All | 339 | 230 (67.8%) | 109 (32.2%) | 78.7% (72.8–83.8) | 54.1% (44.3–63.7) | 24.6% (13.8–35.3) | <0.0001 |
Mantel–Haenszel χ2 test was used to calculate the p value.
EC, esophageal cancer; GC/GEJC, gastric or gastroesophageal junction cancer; HCC, hepatocellular carcinoma; NPC, nasopharyngeal carcinoma; NSCLC, non-small-cell lung cancer; ORR, objective response rate; PLC, primary liver cancer; RCCEP, reactive cutaneous capillary endothelial proliferation; SCLC, small-cell lung cancer.
In addition, the ORR was higher in the Camre-Apa or Camre-Chemo group than that in the Camre group, both in the populations with and without RCCEP (Table 3). Although the incidence of RCCEP was significantly lower with the Camre-Apa than with Camre, the antitumor activity was not compromised due to the synergistic effects of camrelizumab and the antiangiogenic drug.
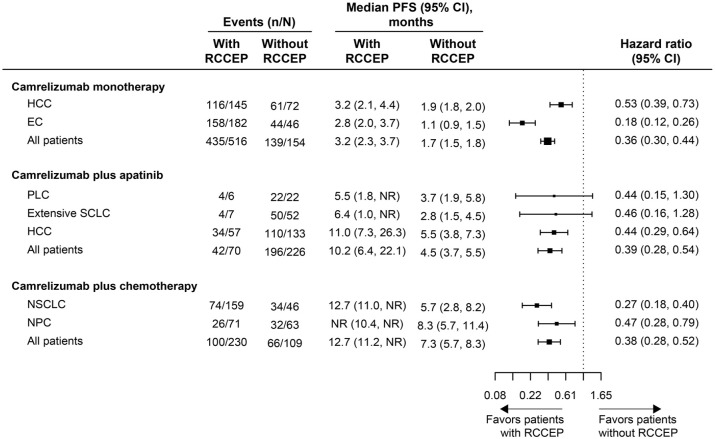
PFS was significantly prolonged in patients who developed RCCEP compared with those who did not in the Camre, Camre-Apa, and Camre-Chemo groups [HR, 0.36 (95% CI, 0.30–0.44), 0.39 (95% CI, 0.28–0.54), and 0.38 (95% CI, 0.28–0.52), respectively; all log-rank p < 0.0001; Figure 2]. Among patients with a specific tumor type, consistent results were found in the Camre group [HR, 0.18 (95% CI, 0.12–0.26) for EC and 0.53 (95% CI, 0.39–0.73) for HCC; log-rank p < 0.0001] and the Camre-Chemo group [0.27 (95% CI, 0.18–0.40) for NSCLC and 0.47 (95% CI, 0.28–0.79) for NPC; log-rank p < 0.0001 and p = 0.0035]. In the Camre-Apa group, the median PFS was also significantly longer in HCC patients with RCCEP compared with those without [HR, 0.44 (95% CI, 0.29–0.64); log-rank p < 0.0001], but the differences were not significant in patients with primary liver cancer (PLC) and extensive SCLC (log-rank p > 0.05), which might be attributed to the relatively small sample size of PLC and SCLC patients (Figure 2).
Figure 2.
Forest plot for PFS by tumor type in patients with versus without RCCEP.
EC, esophageal cancer; HCC, hepatocellular carcinoma; NPC, nasopharyngeal carcinoma; NR, not reached; NSCLC, non-small-cell lung cancer; PFS, progression-free survival; PLC, primary liver cancer; RCCEP, reactive cutaneous capillary endothelial proliferation; SCLC, small-cell lung cancer.
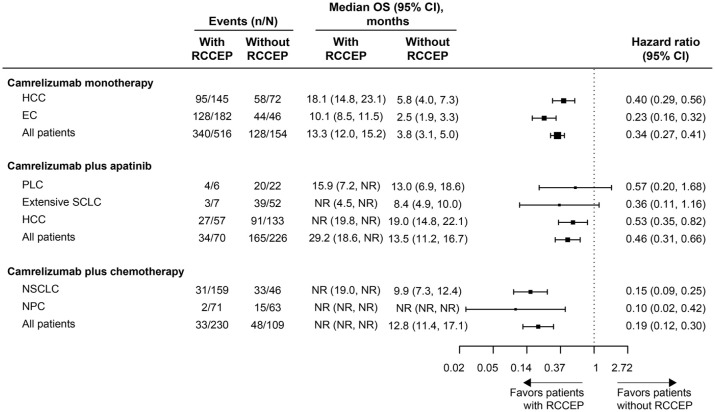
OS was significantly prolonged in patients who developed RCCEP compared with those who did not in the Camre, Camre-Apa, and Camre-Chemo groups [HR, 0.34 (95% CI, 0.27–0.41), 0.46 (95% CI, 0.31–0.66), and 0.19 (95% CI, 0.12–0.30), respectively; log-rank p < 0.0001; Figure 3]. Among patients with a specific tumor type, consistent results were found in the Camre group [HR, 0.23 (95% CI, 0.16–0.32) for EC and 0.40 (95% CI, 0.29–0.56) for HCC; log-rank p < 0.0001] and the Camre-Chemo group [0.10 (95% CI, 0.02–0.42) for NPC and 0.15 (95% CI, 0.09–0.25) for NSCLC; log-rank p = 0.0001 and p < 0.0001, respectively]. In the Camre-Apa group, the median OS was also significantly longer in HCC patients with RCCEP compared with those without [HR, 0.53 (95% CI, 0.35–0.82); log-rank p = 0.0037], whereas similar to the findings of PFS, the differences in OS between patients with and without RCCEP were not significant in patients with PLC and extensive SCLC (log-rank p > 0.05; Figure 3).
Figure 3.
Forest plot for OS by tumor type in patients with versus without RCCEP.
EC, esophageal cancer; HCC, hepatocellular carcinoma; NPC, nasopharyngeal carcinoma; NSCLC, non-small-cell lung cancer; OS, overall survival; PLC, primary liver cancer; RCCEP, reactive cutaneous capillary endothelial proliferation; SCLC, small-cell lung cancer.
Discussion
Camrelizumab demonstrated potent clinical efficacy in multiple unresectable or metastatic malignancies, with limited toxicities.13–16,18,19,42 Preclinical studies have demonstrated a single-digit nanomolar binding affinity to human PD-1, and potent PD-1/PD-L1 blocking activity as well as the T cell activation in vitro. The distinct binding sites and the high and persistent receptor occupancy of camrelizumab may contribute to its broad spectrum and high antitumor activity. 42
Skin toxicities are the most frequently observed AEs of ICIs, with an incidence of 30–40%, mainly including maculopapular rash (eczema-like), dermatitis, and pruritus.43–45 RCCEP is the most common irAE of camrelizumab. The lesions mainly occurred shortly after the initiation of treatment (cycles 1–2). Most RCCEP lesions were in grade 1 or 2. The incidence of grade 3 lesions was very low (0.3% with camrelizumab monotherapy, 0.7% with camrelizumab plus apatinib, and 1.0% with camrelizumab plus chemotherapy), and there were no reported cases of life-threatening grade 4 or fatal grade 5 RCCEP. In addition, there was no bleeding report from the gastrointestinal mucosa, bronchial mucosa, or abdominal organs.13,15,24,26 The majority of lesions occurred on the skin surface of the head, neck, face, and trunk, whereas a very small proportion of patients had the lesions in other sites, such as gingivae, oral mucosa, nasal mucosa, and palpebral conjunctiva.20,24,38 Morphologically, the most common types were ‘red nevus-like’ and ‘pearl-like’ lesions. 24 The histopathological signature of RCCEP is characterized primarily by capillary endothelial hyperplasia and capillary hyperplasia in the dermis.24,41 As for ‘red nevus-like’, the lesions were located in the reticular layer of the dermis, with the proliferative capillaries sparsely arranged; the lining endothelial cells were enlarged but exhibited no atypia and were all uniformly mono-layered; and mostly, single red blood cells could be seen within the lumen of these capillaries. As for ‘pearl-like’, the lesions were located in the reticular layer of the dermis, composed of proliferative capillaries; the capillaries had multiple layers of endothelial cells, with multiple red blood cells visible within the lumen; the capillaries were arranged in a lobulated or nodular pattern, with fibrous connective tissue between the lobules; larger nutrient vessels within or between the lobules or interstitial fibrosis were found in some cases. Most RCCEP lesions were managed through close monitoring, with no special treatment administered. The symptomatic therapies reported for RCCEP included minor resection, laser therapy, local hemostatic therapy, cryotherapy, and local hormone treatment.
The present study showed that camrelizumab monotherapy resulted in the highest incidence of RCCEP (77.0%), which was consistent with those reported previously.13,15,25,27,34,37 The mechanism by which camrelizumab causes RCCEP is not fully understood. When combined with the antiangiogenic agent apatinib, the incidence was substantially lower (23.6%) compared with camrelizumab alone, in line with the findings in other studies of camrelizumab plus apatinib22,28,29 or another antiangiogenic agent famitinib.31,32 Our previous immunohistochemical analysis found strong capillary endothelial cell staining (CD31) in the dermis, as well as markedly high expressions of endothelial cell proliferation markers (Ki-67) and vascular endothelial growth factor-A (VEGF-A) in the lesion tissues, indicating activation of VEGFR2 signaling. 24 Immunofluorescence co-staining revealed a massive increase in CD4+ T cells that were distributed around the capillaries of the lesion tissues, accompanied by high expression of the Th2 cytokine IL-4, which stimulated differentiation of CD163+ M2 macrophages and accumulation of CD163+ M2 macrophages adjacent to blood vessels and possibly promoted vascular proliferation through VEGF-A release. 24 Therefore, combination with apatinib might prohibit the development of RCCEP by targeting VEGFR2 to block angiogenesis. In addition, a delayed onset, shortened duration, and increased remission rate were also found following camrelizumab plus apatinib therapy. However, inconsistent results were observed when combined with chemotherapeutics. Compared with camrelizumab monotherapy, combination with gemcitabine and cisplatin resulted in a significantly lower RCCEP incidence in NPC patients (53.0% versus 87.6%, p < 0.0001), while combination with pemetrexed and carboplatin in NSCLC did not (77.6% versus 73.5%, p = 0.6605). It has been reported that gemcitabine showed the capacity to inhibit VEGF expression. 46 Thus, a combination of different cytotoxic agents might predominantly explain the inconsistent results. Whether tumor type is also an impact factor remains to be further investigated.
Combinations of ICIs with other agents such as tumor angiogenesis inhibitors and chemotherapeutics have synergistic potential.47–50 The present study showed improved ORR with camrelizumab plus apatinib or chemotherapeutics over camrelizumab alone, regardless of tumor type. Antiangiogenic drugs can normalize the tortuous tumor vasculature, alleviate hypoxia in the tumor microenvironment, suppress T regulatory cells, relieve immunosuppression, and promote tumor infiltration by effector immune cells, consequently enhancing the antitumor immunity by ICIs as monotherapy.47,49,50 It is generally believed that cytotoxic drugs can enhance the antitumor immune effect of ICI monotherapy by stimulating T-cell function and driving activation of immune checkpoints in cancer cells. 48
Nevertheless, only a subset of the population benefits from either treatment with ICIs alone or in combination with other drugs, with limited survival.7,9,10,51,52 Thus, there is a warranted need for further investigation of reliable biomarkers that can predict efficacy, disease progression, and survival. Several studies have proven that patients with MSI-H/dMMR benefited from treatment with PD-1 inhibitors. In the Keynote-158 and Keynote-164 trials, the ORR with pembrolizumab was 34.3% in patients with non-colorectal cancer and 33.0% in those with metastatic MSI-H/dMMR colorectal cancer, respectively.51,52 In the CheckMate-142 trial, the ORR with nivolumab was 31.3% in patients with metastatic MSI-H/dMMR colorectal cancer. 9 However, the overall incidence of MSI-H/dMMR in tumors is very low (approximately 15% in early-stage metastatic colorectal cancer and less than 5% at advanced stage).7,10 Some studies used AEs of drugs to predict clinical efficacy and survival benefits, such as hand–foot skin reaction following treatment with regorafenib, hypertension, and proteinuria following bevacizumab, and skin rash following cetuximab, the occurrence of which was associated with higher ORR and/or longer median PFS and OS.53–55 irAEs of ICIs have also been found to serve as markers of clinical outcomes.56,57 The present study showed that the ORR in patients who developed RCCEP was higher than that in patients who did not, both following treatment with camrelizumab alone and combination therapies. Similar trends were observed for PFS and OS. The development of RCCEP significantly reduced the risk of disease progression or death and the risk of death in patients treated with camrelizumab alone or in combination with other therapies. Thus, RCCEP represented a potential marker for predicting the objective response and survival benefits of camrelizumab in clinical practice.
The present study is the largest pooled analysis to date and comprehensively investigated the characteristics of RCCEP and its correlation with clinical outcomes, across a wide range of malignancies and different treatment strategies. Nevertheless, the study has some limitations. First, this was a pooled study, which might weaken the evidence level. Second, the patients in the 10 included trials were all from China, making it difficult to contextualize the findings to other countries and regions around the world. Third, visible differences in the histopathological features of RCCEP, depending on the therapy type (monotherapy or combination involving camrelizumab), are crucial for a comprehensive understanding of this AE, which needs further investigation. Fourth, the characteristics of each morphological type of RCCEP (such as incidence, site, size, color, grade, and treatment) merit attention in future clinical studies. Last, the quality of life and cost-effectiveness of camrelizumab as monotherapy or in combination warrant investigations in future studies.
Conclusion
In summary, this large-scale pooled analysis of 10 clinical trials for the treatment of malignancies found that although RCCEP was a common irAE of camrelizumab, the lesions were generally mild and self-limiting. Furthermore, combination with antiangiogenic agents markedly reduced the occurrence of RCCEP. RCCEP represented a potential clinical indicator for predicting ORR, PFS, and OS following camrelizumab, both as monotherapy and combination therapy, and warrants wide use.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359241242607 for Reactive cutaneous capillary endothelial proliferation following camrelizumab monotherapy or combination therapy for multi-cancers: a large-scale pooled analysis of 10 studies in China by Wenshu Qu, Feng Wang, Shukui Qin, Yuqi Sun and Chuanpei Huang in Therapeutic Advances in Medical Oncology
Acknowledgments
All the trials were sponsored by Jiangsu Hengrui Pharmaceuticals Co., Ltd. We thank the patients and their families as well as the study teams of the included clinical trials (including but not limited to Binghe Xu, Jianming Xu, Jie Wang, Lin Shen, Jing Huang, Caicun Zhou, and Li Zhang). We would also like to acknowledge Hui Dong, PhD (Medical Writer at Hengrui) for the editorial support.
Footnotes
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Wenshu Qu, Department of Medical Oncology, Affiliated Jinling Hospital, Nanjing University of Chinese Medicine, Nanjing, China.
Feng Wang, Department of Medical Oncology, Affiliated Jinling Hospital, Nanjing University of Chinese Medicine, Nanjing, China.
Shukui Qin, Department of Medical Oncology, Affiliated Jinling Hospital, Nanjing University of Chinese Medicine, No. 34, 34 Biao, Yanggongjing Street, Nanjing 210002, China.
Yuqi Sun, Jiangsu Hengrui Pharmaceuticals Co., Ltd, Shanghai, China.
Chuanpei Huang, Jiangsu Hengrui Pharmaceuticals Co., Ltd, Shanghai, China.
Declarations
Ethics approval and consent to participate: All analyses were based on previous studies; thus, no ethical approval or patient consent was needed.
Consent for publication: Not applicable.
Author contributions: Wenshu Qu: Data curation; Investigation; Resources; Validation; Visualization; Writing – original draft.
Feng Wang: Data curation; Investigation; Methodology; Resources; Validation; Writing – review & editing.
Shukui Qin: Conceptualization; Data curation; Investigation; Methodology; Resources; Supervision; Validation; Writing – review & editing.
Yuqi Sun: Formal analysis; Investigation; Resources; Software; Validation; Visualization; Writing – review & editing.
Chuanpei Huang: Investigation; Resources; Validation; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The studies included in this pooled analysis were funded by Jiangsu Hengrui Pharmaceuticals Co., Ltd.
Yuqi Sun and Chuanpei Huang are employees of Jiangsu Hengrui Pharmaceuticals Co., Ltd. All other authors declare no competing interests.
Availability of data and materials: All data generated or analyzed during this study are included in this published article. Further inquiries can be directed to the corresponding author.
References
- 1. Bardhan K, Anagnostou T, Boussiotis VA. The PD1:PD-L1/2 pathway from discovery to clinical implementation. Front Immunol 2016; 7: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gong J, Chehrazi-Raffle A, Reddi S, et al. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J Immunother Cancer 2018; 6: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hirsch L, Zitvogel L, Eggermont A, et al. PD-Loma: A cancer entity with a shared sensitivity to the PD-1/PD-L1 pathway blockade. Br J Cancer 2019; 120: 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dougan M, Blidner AG, Choi J, et al. Multinational Association of Supportive Care in Cancer (MASCC) 2020 clinical practice recommendations for the management of severe gastrointestinal and hepatic toxicities from checkpoint inhibitors. Support Care Cancer 2020; 28: 6129–6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kostine M, Finckh A, Bingham CO, et al. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Ann Rheum Dis 2021; 80: 36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang J, Su S, Li J, et al. Efficacy and safety of camrelizumab monotherapy and combination therapy for cancers: A systematic review and meta-analysis. Front Oncol 2021; 11: 695512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015; 372: 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chan TA, Yarchoan M, Jaffee E, et al. Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann Oncol 2019; 30: 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol 2017; 18: 1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goldstein J, Tran B, Ensor J, et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H). Ann Oncol 2014; 25: 1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sclafani F. PD-1 inhibition in metastatic dMMR/MSI-H colorectal cancer. Lancet Oncol 2017; 18: 1141–1142. [DOI] [PubMed] [Google Scholar]
- 12. Liu K, Tan S, Jin W, et al. N-glycosylation of PD-1 promotes binding of camrelizumab. EMBO Rep 2020; 21: e51444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: A multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol 2020; 21: 571–580. [DOI] [PubMed] [Google Scholar]
- 14. Song Y, Wu J, Chen X, et al. A single-arm, multicenter, phase II study of camrelizumab in relapsed or refractory classical hodgkin lymphoma. Clin Cancer Res 2019; 25: 7363–7369. [DOI] [PubMed] [Google Scholar]
- 15. Huang J, Xu J, Chen Y, et al. Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): A multicentre, randomised, open-label, phase 3 study. Lancet Oncol 2020; 21: 832–842. [DOI] [PubMed] [Google Scholar]
- 16. Luo H, Lu J, Bai Y, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: The ESCORT-1st randomized clinical trial. JAMA 2021; 326: 916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qin S, Chan SL, Gu S, et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): A randomised, open-label, international phase 3 study. Lancet 2023; 402: 1133–1146. [DOI] [PubMed] [Google Scholar]
- 18. Yang Y, Qu S, Li J, et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2021; 22: 1162–1174. [DOI] [PubMed] [Google Scholar]
- 19. Zhou C, Chen G, Huang Y, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): A randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med 2021; 9: 305–314. [DOI] [PubMed] [Google Scholar]
- 20. Ren S, Chen J, Xu X, et al. Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC (CameL-Sq): A phase 3 trial. J Thorac Oncol 2022; 17: 544–557. [DOI] [PubMed] [Google Scholar]
- 21. Hui-Mei P, Guang-Ming H, Xiao-Ling Q, et al. Reactive cutaneous capillary endothelial proliferation caused by camrelizumab: Sixteen case reports. Indian J Dermatol 2023; 68: 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang K, Li B, Li M, et al. The safety and efficacy of camrelizumab and its combination with apatinib in various solid cancers. Front Pharmacol 2020; 11: 568477. [Google Scholar]
- 23. Ding Q, Liu Y, Ju H, et al. Reactive cutaneous capillary endothelial proliferation predicted the efficacy of camrelizumab in patients with recurrent/metastatic head and neck squamous cell carcinoma. Med Oral Patol Oral Cir Bucal 2023; 28: e525–e529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang F, Qin S, Sun X, et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: Data derived from a multicenter phase 2 trial. J Hematol Oncol 2020; 13: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ren Z, Qin S, Meng Z, et al. A phase 2 study of camrelizumab for advanced hepatocellular carcinoma: Two-year outcomes and continued treatment beyond first RECIST-defined progression. Liver Cancer 2021; 10: 500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lickliter JD, Gan HK, Voskoboynik M, et al. A first-in-human dose finding study of camrelizumab in patients with advanced or metastatic cancer in Australia. Drug Des Devel Ther 2020; 14: 1177–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang Y, Zhou T, Chen X, et al. Efficacy, safety, and biomarker analysis of camrelizumab in previously treated recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN study). J Immunother Cancer 2021; 9: e003790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu J, Liu Q, Li Y, et al. Efficacy and safety of camrelizumab combined with apatinib in advanced triple-negative breast cancer: An open-label phase II trial. J Immunother Cancer 2020; 8: e000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou C, Wang Y, Zhao J, et al. Efficacy and biomarker analysis of camrelizumab in combination with apatinib in patients with advanced nonsquamous NSCLC previously treated with chemotherapy. Clin Cancer Res 2021; 27: 1296–1304. [DOI] [PubMed] [Google Scholar]
- 30. Chen X, Qin S, Gu S, et al. Camrelizumab plus oxaliplatin-based chemotherapy as first-line therapy for advanced biliary tract cancer: A multicenter, phase 2 trial. Int J Cancer 2021; 149: 1944–1954. [DOI] [PubMed] [Google Scholar]
- 31. Qu YY, Zhang HL, Guo H, et al. Camrelizumab plus famitinib in patients with advanced or metastatic renal cell carcinoma: Data from an open-label, multicenter phase II basket study. Clin Cancer Res 2021; 27: 5838–5846. [DOI] [PubMed] [Google Scholar]
- 32. Qu YY, Sun Z, Han W, et al. Camrelizumab plus famitinib for advanced or metastatic urothelial carcinoma after platinum-based therapy: Data from a multicohort phase 2 study. J Immunother Cancer 2022; 10: e004427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fan Y, Zhao J, Wang Q, et al. Camrelizumab plus apatinib in extensive-stage SCLC (PASSION): A multicenter, two-stage, phase 2 trial. J Thorac Oncol 2021; 16: 299–309. [DOI] [PubMed] [Google Scholar]
- 34. Yang JJ, Huang C, Fan Y, et al. Camrelizumab in different PD-L1 expression cohorts of pre-treated advanced or metastatic non-small cell lung cancer: A phase II study. Cancer Immunol Immunother 2022; 71: 1393–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mei K, Qin S, Chen Z, et al. Camrelizumab in combination with apatinib in second-line or above therapy for advanced primary liver cancer: Cohort A report in a multicenter phase Ib/II trial. J Immunother Cancer 2021; 9: e002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu J, Shen J, Gu S, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): A nonrandomized, open-label, phase II trial. Clin Cancer Res 2021; 27: 1003–1011. [DOI] [PubMed] [Google Scholar]
- 37. Wu J, Song Y, Chen X, et al. Camrelizumab for relapsed or refractory classical Hodgkin lymphoma: Extended follow-up of the multicenter, single-arm, phase 2 study. Int J Cancer 2022; 150: 984–992. [DOI] [PubMed] [Google Scholar]
- 38. Li H, Qin S, Liu Y, et al. Camrelizumab combined with FOLFOX4 regimen as first-line therapy for advanced hepatocellular carcinomas: A sub-cohort of a multicenter phase Ib/II study. Drug Des Devel Ther 2021; 15: 1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peng Z, Wei J, Wang F, et al. Camrelizumab combined with chemotherapy followed by camrelizumab plus apatinib as first-line therapy for advanced gastric or gastroesophageal junction adenocarcinoma. Clin Cancer Res 2021; 27: 3069–3078. [DOI] [PubMed] [Google Scholar]
- 40. Fang W, Yang Y, Ma Y, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: Results from two single-arm, phase 1 trials. Lancet Oncol 2018; 19: 1338–1350. [DOI] [PubMed] [Google Scholar]
- 41. Chen X, Ma L, Wang X, et al. Reactive capillary hemangiomas: A novel dermatologic toxicity following anti-PD-1 treatment with SHR-1210. Cancer Biol Med 2019; 16: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mo H, Huang J, Xu J, et al. Safety, anti-tumour activity, and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody in advanced solid tumours: A dose-escalation, phase 1 study. Br J Cancer 2018; 119: 538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer 2016; 60: 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017; 28: iv119–iv142. [DOI] [PubMed] [Google Scholar]
- 45. Coleman E, Ko C, Dai F, et al. Inflammatory eruptions associated with immune checkpoint inhibitor therapy: A single-institution retrospective analysis with stratification of reactions by toxicity and implications for management. J Am Acad Dermatol 2019; 80: 990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kuwahara K, Sasaki T, Kobayashi K, et al. Gemcitabine suppresses malignant ascites of human pancreatic cancer: Correlation with VEGF expression in ascites. Oncol Rep 2004; 11: 73–80. [PubMed] [Google Scholar]
- 47. Lanitis E, Irving M, Coukos G. Targeting the tumor vasculature to enhance T cell activity. Curr Opin Immunol 2015; 33: 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Galluzzi L, Humeau J, Buque A, et al. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol 2020; 17: 725–741. [DOI] [PubMed] [Google Scholar]
- 49. Khan KA, Kerbel RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol 2018; 15: 310–324. [DOI] [PubMed] [Google Scholar]
- 50. Fukumura D, Kloepper J, Amoozgar Z, et al. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat Rev Clin Oncol 2018; 15: 325–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol 2020; 38: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Le DT, Kim TW, Van Cutsem E, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol 2020; 38: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Abdel-Rahman O, Fouad M. Correlation of cetuximab-induced skin rash and outcomes of solid tumor patients treated with cetuximab: A systematic review and meta-analysis. Crit Rev Oncol Hematol 2015; 93: 127–135. [DOI] [PubMed] [Google Scholar]
- 54. Tanaka H, Takahashi K, Yamaguchi K, et al. Hypertension and proteinuria as predictive factors of effects of bevacizumab on advanced breast cancer in Japan. Biol Pharm Bull 2018; 41: 644–648. [DOI] [PubMed] [Google Scholar]
- 55. Yoo C, Byeon S, Bang Y, et al. Regorafenib in previously treated advanced hepatocellular carcinoma: Impact of prior immunotherapy and adverse events. Liver Int 2020; 40: 2263–2271. [DOI] [PubMed] [Google Scholar]
- 56. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur J Cancer 2016; 54: 139–148. [DOI] [PubMed] [Google Scholar]
- 57. Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 2018; 4: 374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359241242607 for Reactive cutaneous capillary endothelial proliferation following camrelizumab monotherapy or combination therapy for multi-cancers: a large-scale pooled analysis of 10 studies in China by Wenshu Qu, Feng Wang, Shukui Qin, Yuqi Sun and Chuanpei Huang in Therapeutic Advances in Medical Oncology