Supplemental Digital Content is Available in the Text.
Key Words: advanced HIV disease, test and treat, CD4 testing, ART suppression
Abstract
Background:
Earlier antiretroviral therapy (ART) may decrease progression to advanced HIV disease (AHD) with CD4 count of <200 cells per cubic millimeter or clinical sequelae. We assessed factors associated with AHD among people living with HIV before and during the “test and treat” era.
Setting:
The African Cohort Study prospectively enrolls adults with and without HIV from 12 clinics in Uganda, Kenya, Tanzania, and Nigeria.
Methods:
Enrollment evaluations included clinical history, physical examination, and laboratory testing. Generalized estimating equations were used to estimate adjusted odds ratios and 95% confidence intervals for factors associated with CD4 count of <200 cells per cubic millimeter at study visits.
Results:
From 2013 to 2021, 3059 people living with HIV with available CD4 at enrollment were included; median age was 38 years [interquartile range: 30–46 years], and 41.3% were men. From 2013 to 2021, the prevalence of CD4 count of <200 cells per cubic millimeter decreased from 10.5% to 3.1%, whereas the percentage on ART increased from 76.6% to 100% (P <0.001). Factors associated with higher odds of CD4 count of <200 cells per cubic millimeter were male sex (adjusted odds ratio 1.56 [confidence interval: 1.29 to 1.89]), being 30–39 years (1.42 [1.11–1.82]) or older (compared with <30), have World Health Organization stage 2 disease (1.91 [1.48–2.49]) or higher (compared with stage 1), and HIV diagnosis eras 2013–2015 (2.19 [1.42–3.37]) or later (compared with <2006). Compared with ART-naive, unsuppressed participants, being viral load suppressed on ART, regardless of ART duration, was associated with lower odds of CD4 count of <200 cells per cubic millimeter (<6 months on ART: 0.45 [0.34–0.58]).
Conclusion:
With ART scale-up, AHD has declined. Efforts targeting timely initiation of suppressive ART may further reduce AHD risk.
INTRODUCTION
By the end of 2021, 38.4 million people globally were living with HIV and 28.7 million people (73%) were accessing antiretroviral therapy (ART); despite the progress in scale-up of ART, 650,000 people died of AIDS-related illness in 2021.1 Advanced HIV disease (AHD) remains common in many countries, defined as having a CD4 cell count of <200 cells per cubic millimeter or World Health Organization (WHO) clinical stage 3 or 4 disease for adults, adolescents, and children older than 5 years, which increases the risk of morbidity and mortality.2
WHO recommends a package of interventions for people living with HIV (PLHIV) who present with AHD, including screening for, prophylaxis, and/or management of common opportunistic infections, including tuberculosis and cryptococcal disease.2–4 Since the implementation of Universal Test and Treat (UTT), spearheaded by the US President's Emergency Plan for AIDS Relief (PEPFAR),5 there has been a shift in country investments from CD4 testing to viral load (VL) testing for monitoring response to ART.6 Because PLHIV are being initiated on ART sooner, rather than based on CD4 criteria, there has been an expectation that earlier ART may decrease the prevalence of AHD.
Longitudinal cohorts spanning both the before and during the “test and treat” periods have noted a decline in AHD prevalence among countries in Southern Africa, but this response has been modest.7,8 Understanding of temporal changes and other drivers of AHD prevalence in other regions in sub-Saharan Africa is limited. To understand the impact of ART scale-up on AHD, we focused on a cohort where consistent cross-sectional data collections occurred upon enrollment over several years, assessed trends in AHD and ART uptake and described factors associated with AHD among adult PLHIV across 4 countries in sub-Saharan Africa before and during the “test and treat” era. Because noninfectious comorbid diseases (NCDs) contribute to morbidity and mortality among PLHIV in resource-rich countries with higher ART uptake, we also assessed the impact of NCDs on AHD.9,10
METHODS
Study Design and Participants
These longitudinal analyses used data from the African Cohort Study (AFRICOS), a prospective cohort established in 2013 that enrolls persons with and without HIV at 12 PEPFAR-supported facilities in Uganda, Kenya, Tanzania, and Nigeria. Initially, individuals were eligible if they were aged ≥18 years and consented to data and specimen collection. Beginning in January 2020, individuals aged 15–17 years were also eligible for enrollment. Most of the participants living with HIV were invited to the study based on random selection from existing clinic patient lists (stratified by gender and ART status) or new enrollees to the clinic, whereas a minority (<5%) were recruited from other HIV studies performed by the AFRICOS group locally.11 As a result, some participants were already on ART at the point of enrollment into AFRICOS. For our analyses, we excluded persons without HIV and included PLHIV with available CD4 at AFRICOS enrollment.
Data Collection/Measures
AFRICOS participants were administered a medical history and physical examination and completed a demographic and sociobehavioral questionnaire, and underwent phlebotomy upon enrollment, as previously described.11 In addition, every 6 months, follow-up study visits occurred, and participants provided medical history, completed a physical examination, and underwent laboratory assessments. If a participant misses a study visit but later returns, they continue to be included in the cohort, and the missed visit is documented. Participants stop contributing information to follow-up visits if they die or are lost to follow-up, as defined as no contact for 360 days since missed visit.
All participants had an assessment of serum creatinine, glucose, and cholesterol performed annually. Enrollment year was stratified according to timing of implementation of UTT policy: 2013–2014 for pre-UTT, 2015–2016 for early UTT, and 2017–2021 for broader implementation of UTT. Participants with recorded dates of HIV diagnosis were categorized by WHO guideline era (<2006, 2006–2009, 2010–2012, 2013–2015, ≥2016) to account for the changes in recommendations for ART initiation over time as previously described.12 Demographic and sociobehavioral variables included sex, age, education level, clinical care site, and food security (defined as having enough food to eat). Body mass index (BMI) was calculated using participants' height and weight, then categorized as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), or overweight (≥25 kg/m2). NCDs were defined as previously described.11 Anemia was defined as hemoglobin <13 g/dL for men or <12 g/dL for women. Elevated blood pressure was defined as a systolic blood pressure measurement of >139 mm Hg, a diastolic blood pressure measurement of >89 mm Hg, or receipt of antihypertensive medications. Any abnormal blood pressures were repeated for confirmation during the study visit, and the second blood pressure was recorded. Hypercholesterolemia was defined as a total fasting cholesterol of >199 mg/dL or receipt of lipid-lowering medications. Dysglycemia was defined as a fasting glucose of >99 mg/dL, nonfasting glucose of >199 mg/dL, or receipt of hypoglycemic medications. Renal insufficiency was defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2, calculated using the Modification of Diet in Renal Disease equation based on serum creatinine captured at entry.13 Historical ART start dates and laboratory results were obtained from medical record review. ART medication use (also known as ART uptake) was defined as not on ART or on ART at each visit, and VL suppression (VLS) was defined as <1000 copies per milliliter. PLHIV had CD4 T-lymphocyte count (CD4) in cells per cubic millimeter and HIV RNA (“viral load” or VL) testing performed at enrollment and at 6-month follow-up visits. CD4 count was assessed using BD FACSCount, BD FACSCalibur, BD FACSCanto II, or BD FACSPresto; HIV RNA was assessed using several different platforms over the course of the study, as previously described.14 Serum cryptococcal antigen (CrAg) was measured at enrollment for PLHIV with CD4 of <200 cells per cubic millimeter using IMMY Lateral Flow Assay method. TB epidemiology and diagnostic testing results for AFRICOS have previously been described.15
Statistical Analyses
All data were recorded on paper case report forms and double-entered into the ClinPlus platform (DZS Software Solutions, Bound Brock, NJ). Analyses were performed in SAS 9.3 (SAS, Cary, NC) and Stata 14.0 (StataCorp, College Station, TX). For the longitudinal descriptive analysis, we included all participants ≥15 years of age enrolled from January 21, 2013, to September 1, 2021, with available CD4 data at enrollment; participants were categorized by year of study enrollment, and we compared the percentage of participants with CD4 <200 cells per cubic millimeter and percentage on ART (ART uptake) during the study visits; in addition, we compared the trends in percentage of CD4 count <200 cells per cubic millimeter by UTT eras at study visits. We assessed the frequency of NCDs and CrAg prevalence at enrollment. Generalized estimating equation (GEE) regression modeling was performed for participants with available data to estimate unadjusted and adjusted odds ratio with 95% confidence intervals (CI) for factors potentially associated with CD4 count of <200 cells per cubic millimeter at each study visit. Epidemiologically plausible variables were considered a priori for the initial variable list. Backward selection was used to select the variables included in the final adjusted GEE model.
Ethical Considerations
The study was approved by Institutional Review Boards of the Walter Reed Army Institute of Research, Makerere University School of Public Health, Kenya Medical Research Institute, Tanzania National Institute of Medical Research, and Nigerian Ministry of Defense. All participants provided written informed consent before enrollment. The secondary analysis assessing factors associated with CD4 count of <200 cells per cubic millimeter was approved by the US Centers for Disease Control and Prevention (CDC) and was conducted consistent with applicable federal law and CDC policy. A waiver of consent was obtained as the procedures only involved analyzing previously collected deidentified data, and the project was considered only minimal risk to the participants.
RESULTS
Participant characteristics at enrollment are presented in Table 1. A total of 3097 PLHIV aged ≥15 years were enrolled from 2013 to 2021, and 3059 with available CD4 data (98.8%) at enrollment were included in these analyses. Among those included, the median age was 38 years [interquartile range, 31–46 years], 1262 (41.3%) were men, 575 (18.8%) had a CD4 count of <200 cells per cubic millimeter, 1215 (39.7%) had WHO clinical stage 3 or 4 disease, and 2203 (72.0%) were on ART at enrollment. At the most recent visit, 8.0% of participants had a CD4 count of <200 cells per cubic millimeter; including all visits, 8.7% had a CD4 count of <200 cells per cubic millimeter.
TABLE 1.
Participant Characteristics by Country at AFRICOS Enrollment*
| Uganda | Kenya | Tanzania | Nigeria | Total | P | |
| N | 550 | 1586 | 574 | 349 | 3059 | |
| Enrollment year | <0.001 | |||||
| 2013–2014 | 222 (40.4%) | 630 (39.7%) | 157 (27.4%) | 91 (26.1%) | 1100 (35.9%) | |
| 2015–2016 | 247 (44.9%) | 807 (50.9%) | 218 (38.0%) | 158 (45.3%) | 1430 (46.7%) | |
| 2017–2021 | 81 (14.7%) | 150 (9.5%) | 199 (34.7%) | 100 (28.7%) | 530 (17.3%) | |
| Sex | 0.48 | |||||
| Female | 330 (60.0%) | 911 (57.4%) | 343 (59.8%) | 213 (61.0%) | 1797 (58.7%) | |
| Male | 220 (40.0%) | 675 (42.6%) | 231 (40.2%) | 136 (39.0%) | 1262 (41.3%) | |
| Age, yr | <0.001 | |||||
| 15–29 | 150 (27.3%) | 294 (18.5%) | 149 (26.0%) | 89 (25.5%) | 682 (22.3%) | |
| 30–39 | 172 (31.3%) | 535 (33.7%) | 162 (28.2%) | 133 (38.1%) | 1002 (32.7%) | |
| 40–49 | 148 (26.9%) | 454 (28.6%) | 146 (25.4%) | 95 (27.2%) | 843(27.6%) | |
| 50+ | 80 (14.5%) | 303 (19.1%) | 117 (20.4%) | 32 (9.2%) | 532 (17.4%) | |
| Education | <0.001 | |||||
| None or some primary | 339 (61.6%) | 551 (34.7%) | 88 (15.3%) | 18 (5.2%) | 996 (32.5%) | |
| Primary or some secondary | 185 (33.6%) | 609 (38.4%) | 359 (62.5%) | 74 (21.2%) | 1227 (40.1%) | |
| Secondary and above | 26 (4.7%) | 425 (26.8%) | 126 (22.0%) | 257 (73.6%) | 834 (27.3%) | |
| Missing | 0 (0.0%) | 1 (0.1%) | 1 (0.2%) | 0 (0.0%) | 2 (0.1%) | |
| Had enough food to eat | <0.001 | |||||
| No | 122 (22.2%) | 748 (47.1%) | 91 (15.9%) | 88 (25.2%) | 1049 (34.3%) | |
| Yes | 428 (77.8%) | 837 (52.8%) | 482 (84.0%) | 261 (74.8%) | 2008 (65.6%) | |
| Missing | 0 (0.0%) | 1 (0.1%) | 1 (0.2%) | 0 (0.0%) | 2 (0.1%) | |
| Currently employed | <0.001 | |||||
| No | 64 (11.6%) | 1248 (78.7%) | 487 (84.8%) | 82 (23.5%) | 1881 (61.5%) | |
| Yes | 486 (88.4%) | 337 (21.2%) | 86 (15.0%) | 267 (76.5%) | 1176 (38.4%) | |
| Missing | 0 (0.0%) | 1 (0.1%) | 1 (0.2%) | 0 (0.0%) | 2 (0.1%) | |
| CD4 (cells/mm3), median (IQR) | 421 (259–613) | 416 (262–595) | 327 (193–497) | 448 (234–664) | 401 (242–588) | <0.001 |
| CD4, cells/mm3 | <0.001 | |||||
| <100 | 56 (10.2%) | 111 (7.0%) | 60 (10.5%) | 24 (6.9%) | 251 (8.2%) | |
| 100–199 | 46 (8.4%) | 150 (9.5%) | 89 (15.5%) | 39 (11.2%) | 324 (10.6%) | |
| 200–499 | 234 (42.5%) | 729 (45.9%) | 285 (49.7%) | 139 (39.8%) | 1387 (45.3%) | |
| 500+ | 214 (38.9%) | 596 (37.6%) | 140 (24.4%) | 147 (42.1%) | 1097 (35.8%) | |
| WHO clinical stage | <0.001 | |||||
| 1 | 161 (29.3%) | 367 (23.1%) | 176 (30.7%) | 191 (54.7%) | 895 (29.2%) | |
| 2 | 177 (32.2%) | 541 (34.1%) | 140 (24.4%) | 83 (23.8%) | 941 (30.8%) | |
| 3 | 182 (33.1%) | 591 (37.3%) | 194 (33.8%) | 60 (17.2%) | 1027 (33.6%) | |
| 4 | 25 (4.5%) | 86 (5.4%) | 63 (11.0%) | 14 (4.0%) | 188 (6.1%) | |
| Missing | 5 (0.9%) | 1 (0.1%) | 1 (0.2%) | 1 (0.3%) | 8 (0.3%) | |
| HIV diagnosis year | <0.001 | |||||
| <2006 | 47 (8.5%) | 123 (7.8%) | 56 (9.8%) | 25 (7.2%) | 251 (8.2%) | |
| 2006–2009 | 136 (24.7%) | 431 (27.2%) | 130 (22.6%) | 79 (22.6%) | 776 (25.4%) | |
| 2010–2012 | 63 (11.5%) | 364 (23.0%) | 56 (9.8%) | 67 (19.2%) | 550 (18.0%) | |
| 2013–2015 | 198 (36.0%) | 475 (29.9%) | 161 (28.0%) | 145 (41.5%) | 979 (32.0%) | |
| 2016+ | 102 (18.5%) | 166 (10.5%) | 167 (29.1%) | 29 (8.3%) | 464 (15.2%) | |
| Missing | 4 (0.7%) | 27 (1.7%) | 4 (0.7%) | 4 (1.1%) | 39 (1.3%) | |
| On ART | <0.001 | |||||
| ART naive | 302 (54.9%) | 297 (18.7%) | 155 (27.0%) | 100 (28.7%) | 854 (27.9%) | |
| On ART | 248 (45.1%) | 1289 (81.3%) | 418 (72.8%) | 248 (71.1%) | 2203 (72.0%) | |
| Missing | 0 (0.0%) | 0 (0.0%) | 1 (0.2%) | 1 (0.3%) | 2 (0.1%) | |
| ART duration†, viral suppression status‡ | <0.001 | |||||
| ART naive, not suppressed | 287 (52.2%) | 236 (14.9%) | 140 (24.4%) | 90 (25.8%) | 753 (24.6%) | |
| <6 months, not suppressed | 14 (2.5%) | 51 (3.2%) | 52 (9.1%) | 16 (4.6%) | 133 (4.3%) | |
| 6 months–<2 yrs, not suppressed | 3 (0.5%) | 21 (1.3%) | 7 (1.2%) | 2 (0.6%) | 33 (1.1%) | |
| 2+ yrs, not suppressed | 9 (1.6%) | 95 (6.0%) | 42 (7.3%) | 22 (6.3%) | 168 (5.5%) | |
| ART naive, suppressed | 15 (2.7%) | 59 (3.7%) | 12 (2.1%) | 9 (2.6%) | 95 (3.1%) | |
| <6 months, suppressed | 21 (3.8%) | 179 (11.3%) | 72 (12.5%) | 31 (8.9%) | 303 (9.9%) | |
| 6 months–<2 yrs, suppressed | 56 (10.2%) | 241 (15.2%) | 56 (9.8%) | 32 (9.2%) | 385 (12.6%) | |
| 2+ yrs, suppressed | 138 (25.1%) | 654 (41.2%) | 170 (29.6%) | 140 (40.1%) | 1102 (36.0%) | |
| Missing | 7 (1.3%) | 50 (3.2%) | 23 (4.0%) | 7 (2.0%) | 87 (2.8%) | |
| Dysglycemia‖ | <0.001 | |||||
| No | 521 (94.7%) | 1390 (87.6%) | 537 (93.6%) | 282 (80.8%) | 2730 (89.2%) | |
| Yes | 17 (3.1%) | 172 (10.8%) | 34 (5.9%) | 65 (18.6%) | 288 (9.4%) | |
| Missing | 12 (2.2%) | 24 (1.5%) | 3 (0.5%) | 2 (0.6%) | 41 (1.3%) | |
| Elevated blood pressure¶ | <0.001 | |||||
| No | 519 (94.4%) | 1399 (88.2%) | 471 (82.1%) | 295 (84.5%) | 2684 (87.7%) | |
| Yes | 31 (5.6%) | 186 (11.7%) | 100 (17.4%) | 54 (15.5%) | 371 (12.1%) | |
| Missing | 0 (0.0%) | 1 (0.1%) | 3 (0.5%) | 0 (0.0%) | 4 (0.1%) | |
| Hypercholesterolemia# | <0.001 | |||||
| No | 488 (88.7%) | 1272 (80.2%) | 451 (78.6%) | 261 (74.8%) | 2472 (80.8%) | |
| Yes | 54 (9.8%) | 289 (18.2%) | 119 (20.7%) | 86 (24.6%) | 548 (17.9%) | |
| Missing | 8 (1.5%) | 25 (1.6%) | 4 (0.7%) | 2 (0.6%) | 39 (1.3%) | |
| Renal insufficiency** | 0.13 | |||||
| No | 534 (97.1%) | 1561 (98.4%) | 565 (98.4%) | 337 (96.6%) | 2997 (98.0%) | |
| Yes | 7 (1.3%) | 16 (1.0%) | 7 (1.2%) | 9 (2.6%) | 39 (1.3%) | |
| Missing | 9 (1.6%) | 9 (0.6%) | 2 (0.3%) | 3 (0.9%) | 23 (0.8%) | |
| Anemia†† | <0.001 | |||||
| No | 349 (63.5%) | 1155 (72.8%) | 440 (76.7%) | 169 (48.4%) | 2113 (69.1%) | |
| Yes | 201 (36.5%) | 431 (27.2%) | 134 (23.3%) | 180 (51.6%) | 946 (30.9%) | |
| BMI‡‡, kg/m2 | <0.001 | |||||
| Underweight (<18.5 kg/m2) | 82 (14.9%) | 203 (12.8%) | 43 (7.5%) | 25 (7.2%) | 353 (11.5%) | |
| Normal (18.5–24.99 kg/m2) | 373 (67.8%) | 1039 (65.5%) | 348 (60.6%) | 186 (53.3%) | 1946 (63.6%) | |
| Overweight (25+ kg/m2) | 95 (17.3%) | 344 (21.7%) | 183 (31.9%) | 138 (39.5%) | 760 (24.8%) |
*This analysis includes data for 3059 participants with available CD4 data at enrollment.
ART duration was ascertained before CD4 measurement at enrollment. Participants enrolled were either ART naive or already on ART.
VL suppression defined as VL <1000 copies per milliliter.
§ART naive and VL suppressed included ARV-naive participants with VL <50 copies per milliliter or VL load ≥50 and <1000 copies per milliliter, for 2 consecutive visits.
Dysglycemia was defined as a fasting glucose >99 mg/dL, nonfasting glucose >199 mg/dL, or receipt of hypoglycemic medications.
Elevated blood pressure was defined as a systolic blood pressure measurement of >139 mm Hg, a diastolic blood pressure measurement of >89 mm Hg, or receipt of antihypertensive medications, with abnormal blood pressures repeated for confirmation during the study visit.
Hypercholesterolemia was defined as a total fasting cholesterol of >199 mg/dL or receipt of lipid-lowering medications.
Renal insufficiency was defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2.
Anemia was defined as hemoglobin of <13 g/dL for men or <12 g/dL for women.
BMI was categorized as underweight (<18.5), normal (18.5–24.9), or overweight (≥25).
Bold entries correspond to factors significantly associated witth CD4 <200.
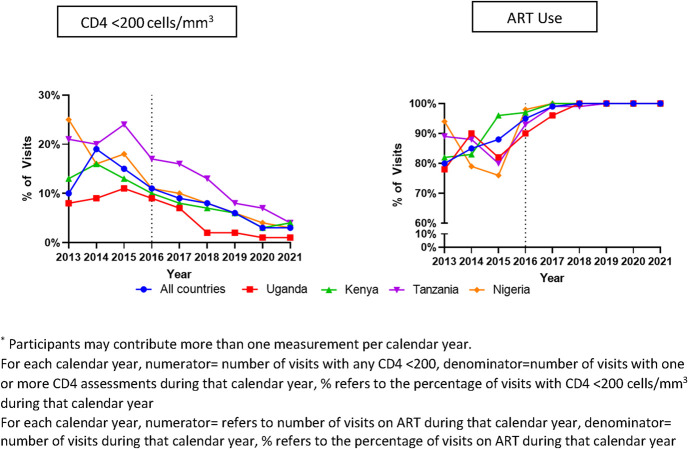
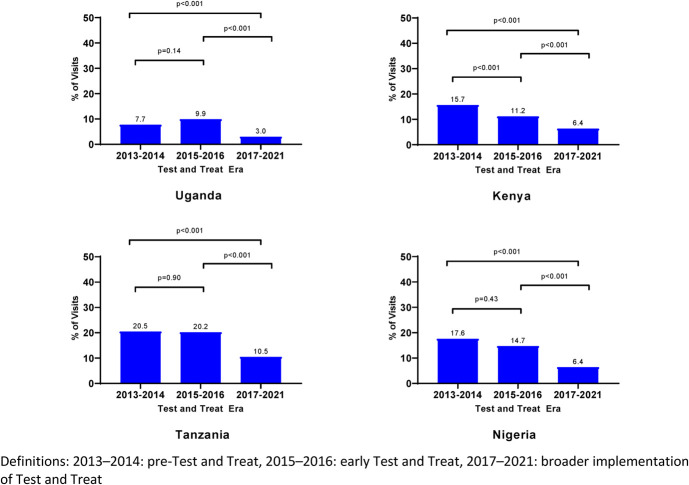
Across all countries at study visits, the prevalence of CD4 count of <200 cells per cubic millimeter declined from 2013 (10.5%) to 2021 (3.1%) (P < 0.001) and peaked in 2014 (19.1%) (Fig. 1, Table 1, Supplemental Digital Content, http://links.lww.com/QAI/C233). Overall, by UTT era, the prevalence of CD4 count of <200 cells per cubic millimeter declined from a peak in 2013–2014 (14.1%) to 2015–2016 (12.5%) and nadired in 2017–2021 (6.5%). Across all 4 countries, the overall AHD prevalence for the 2017–2021 era differed significantly from the earlier 2 eras (P < 0.001) (Fig. 2). Across all countries, the prevalence of CD4 count of <200 cells per cubic millimeter declined across all 3 eras (P = 0.001); country-specific differences are shown in Figure 3. The prevalence was lowest in Uganda across all eras (Fig. 3). Across all countries at study visits, ART use increased from its lowest in 2013 (80.2%) to 2021 (100%) (P < 0.001, Fig. 1); however, few new participants were enrolled in 2020 (n = 21) because of the COVID-19 pandemic.
FIGURE 1.
Trends in percentage of visits in participants with CD4 <200 cells per cubic millimeter and ART use by country and year at each study visit.
FIGURE 2.
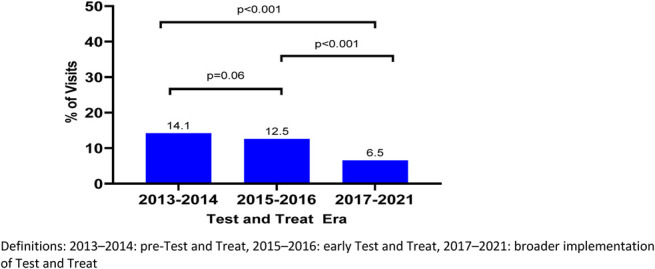
Prevalence of AHD at AFRICOS Study visits by test and treat era, all countries.
FIGURE 3.
Prevalence of AHD at AFRICOS Study visits by test and treat era, by country.
Comorbidities
Among all participants at enrollment (n = 3059), 946 (30.9%) had anemia, 548 (17.9%) had hypercholesteremia, and 371 (12.1%) had elevated blood pressure. Elevated blood pressure, hypercholesterolemia, anemia, and being underweight or normal weight were significantly more common among those with a CD4 count of <200 cells per cubic millimeter compared with those with a CD4 ≥200 cells per cubic millimeter (P < 0.01). Among the 575 participants with CD4 count of <200 cells per cubic millimeter at enrollment, 551 (95.8%) received serum CrAg testing at enrollment and 16 (2.9%) were CrAg positive—12 (75%) with a CD4count of ≤100 cells per cubic millimeter and 4 (25%) with a CD4 count of 100–199 cells per cubic millimeter.
Factors Associated With CD4 count of <200 cells per cubic millimeter
Factors associated with CD4 count of <200 cells per cubic millimeter at study visits are presented in Table 2. In the adjusted model, factors associated with higher odds of CD4 count <200 cells per cubic millimeter at study visits were male sex, enrollment in Kenya, Tanzania, and Nigeria (compared with Uganda), having at least a primary education or higher (compared with none or some primary education), HIV diagnosis eras 2013–2015 or after 2016 (compared with before 2006), dysglycemia, anemia, and WHO stage 2 disease or higher (compared with stage 1). As compared with participants aged <30 years, odds of CD4 <200 cells per cubic millimeter were higher for those aged 30–39, 40–49, and 50+ years. As compared with being ART naive and not VLS, odds of CD4 count of <200 cells per cubic millimeter were lower for those on ART for 2+ years and not VLS and those on ART (regardless of the duration) and VLS; among ART duration/suppression combinations, the lowest odds of CD4 count of <200 cells per cubic millimeter was associated with being on ART for 2+ years and VLS. Being normal weight or overweight (compared with being underweight) also reduced the odds of CD4 count of <200 cells per cubic millimeter.
TABLE 2.
Factors Associated With CD4 <200 Cells per cubic millimeter at AFRICOS Study Visits
| Unadjusted OR | 95% CI | Adjusted OR* | 95% CI | |
| Sex | ||||
| Female | Ref | — | — | — |
| Male | 2.08 | 1.75–2.47 | 1.56 | 1.29–1.89 |
| Age, yr | ||||
| 18–29 | Ref | — | — | — |
| 30–39 | 0.99 | 0.81–1.21 | 1.42 | 1.11–1.82 |
| 40–49 | 0.74 | 0.60–0.92 | 1.50 | 1.15–1.97 |
| 50+ | 0.63 | 0.49–0.81 | 1.57 | 1.16–2.12 |
| Country | ||||
| Uganda | — | — | — | — |
| Kenya | 1.32 | 1.03–1.68 | 1.51 | 1.16–1.96 |
| Tanzania | 2.14 | 1.62–2.82 | 1.82 | 1.33–2.51 |
| Nigeria | 1.57 | 1.13–2.20 | 1.52 | 1.02–2.25 |
| Education† | ||||
| None/some primary | Ref | — | — | — |
| Primary or some secondary | 1.36 | 1.15–1.60 | 1.45 | 1.18–1.79 |
| Secondary and above | 1.23 | 1.00–1.52 | 1.32 | 1.02–1.71 |
| WHO clinical stage | ||||
| 1 | Ref | – | – | – |
| 2 | 1.38 | 1.08–1.76 | 1.91 | 1.48–2.49 |
| 3 | 1.69 | 1.34–2.13 | 2.76 | 2.12–3.60 |
| 4 | 1.72 | 1.19–2.48 | 2.63 | 1.74–4.00 |
| HIV diagnosis year | ||||
| <2006 | — | — | — | — |
| 2006–2009 | 1.13 | 0.69–1.86 | 1.08 | 0.69–1.69 |
| 2010–2012 | 1.60 | 0.97–2.65 | 1.57 | 0.98–2.50 |
| 2013–2015 | 4.13 | 2.61–6.54 | 2.19 | 1.42–3.37 |
| 2016+ | 3.70 | 2.28–6.00 | 2.27 | 1.41–3.63 |
| ART duration, viral suppression status† | ||||
| ART naive, not suppressed | Ref | — | — | — |
| <6 mo, not suppressed | 1.18 | 0.86–1.60 | 0.96 | 0.65–1.41 |
| 6 mo–<2 yrs, not suppressed | 0.81 | 0.60–1.08 | 0.78 | 0.55–1.21 |
| 2+ yrs, not suppressed | 0.65 | 0.53–0.81 | 0.72 | 0.56–0.94 |
| ART naive, suppressed‡ | 0.39 | 0.25–0.62 | 0.40 | 0.23–0.70 |
| <6 mo, suppressed | 0.50 | 0.42–0.60 | 0.45 | 0.34–0.58 |
| 6 mo–<2 yrs, suppressed | 0.32 | 0.28–0.37 | 0.27 | 0.23–0.33 |
| 2+ yrs, suppressed | 0.13 | 0.11–0.15 | 0.11 | 0.09–0.14 |
| Dysglycemia§ | ||||
| No | Ref | — | — | — |
| Yes | 1.18 | 1.02–1.38 | 1.31 | 1.10–1.56 |
| Anemia‖ | ||||
| No | Ref | — | — | — |
| Yes | 1.62 | 1.46–1.80 | 1.41 | 1.23–1.63 |
| BMI¶, kg/m2 | ||||
| Underweight (<18.5) | Ref | — | — | — |
| Normal (18.5–24.99) | 0.70 | 0.60–0.80 | 0.77 | 0.63–0.94 |
| Overweight (≥25) | 0.34 | 0.28–0.41 | 0.48 | 0.37–0.63 |
Backward selection was used to select the variables included in the final-adjusted GEE model. Variables included in the adjusted model were sex, age, education, WHO clinical stage, time since HIV diagnosis, interaction terms: duration on ART and VL suppression status, dysglycemia, anemia, and BMI.
Viral suppression was defined as VL <1000 copies per milliliter.
ART naive and VL suppressed included ARV-naive participants with VL <50 copies per milliliter or VL load ≥50 and <1000 copies per milliliter, for 2 consecutive visits.
Dysglycemia was defined as a fasting glucose of >99 mg/dL, nonfasting glucose of >199 mg/dL, or receipt of hypoglycemic medications.
Anemia was defined as hemoglobin of <13 g/dL for men or <12 g/dL for women.
BMI was categorized as underweight (<18.5), normal (18.5–24.9), or overweight (≥25).
OR, odds ratio; VLNS, VL non suppressed.
Bold entries correspond to factors significantly associated witth CD4 <200.
DISCUSSIONS
Aligning with the significant scale-up of ART among PLHIV in the AFRICOS cohort study in Uganda, Kenya, Tanzania, and Nigeria, AHD prevalence persistently declined across UTT eras. Although prior studies comparing the pre-UTT and early UTT eras have noted a decline in the prevalence of CD4 count of <200 cells per cubic millimeter, most studies in the early UTT and early period of broader implementation have noted a persistence of AHD up to 2018, with an approximate prevalence of 20%–30% despite an increase in CD4 over time.7,8,16–18 In contrast to persistence noted in more recent studies, our findings from this large longitudinal cohort adds to the literature by highlighting a significant decline in the prevalence of AHD, particularly over the past 5 years of our study (2017–2021) not only because of the inclusion of recent years but also because of the high ART uptake in the AFRICOS cohort.
Our study found that male sex, age ≥30 years, and having at least a WHO clinical stage 2 condition were associated with an increased risk of CD4 <200 cells per cubic millimeter. Before UTT and early UTT eras, male sex, older age, and advanced clinical stage disease have been reported as risk factors for CD4 count of <200 cells per cubic millimeter like our study.8,19–21 A study from South Africa noted that individuals aged 30–50 years were 2.6 times more likely to present late for HIV care (with CD4 ≤200 cells per cubic millimeter and or stage 3/4 disease) compared with those aged 12–20 years.22 Men and PLHIV aged ≥30 years may be at greater risk for AHD as shown because of delays in HIV diagnosis leading to delayed ART initiation or may experience interruptions in ART.23 In addition, perceived social norms and structural/social barriers may also impact men's access to HIV care, leading to higher viral loads.24 These findings suggest a need for strategies that engage young adults and older PLHIV as well as men earlier into care and ensure continuity of treatment to reduce risk for AHD. Country-specific differences in the CD4 count of <200 cells per cubic millimeter decline likely reflects differences in baseline prevalence of CD4 count of <200 cells per cubic millimeter and additional country-specific factors or policies that may have influenced “test and treat” scale-up and HIV care continuum.
Interestingly, having at least completed a primary education compared with none or some primary education was associated with a CD4 count of <200 cells per cubic millimeter in our study, which differs from previous studies. A prior AFRICOS study noted that participants with none or some primary education had the longest median time to ART initiation across WHO guideline eras from 2006 to 2016, although education was not significantly associated with time to ART initiation.12 Several studies have noted a lack of formal education or lower levels of education (ie, not having a primary or secondary education) as predictors of late HIV diagnosis, AHD, or late presentation to care (defined as CD4 count of <350 cells per cubic millimeter or AIDS defining condition including WHO stage 3 or 4 disease), although those studies had varying definitions of low level of education.21,25 There may be additional factors that impact an association between education attained and risk for AHD that were not accounted for in our model such as challenges in clinic attendance amidst busy work schedules for persons with higher levels of education. This highlights the need for differentiated service delivery (DSD) treatment models that adapt HIV serves to meet the needs of PLHIV.26
Our study noted that HIV diagnosis era, specifically being diagnosed with HIV during 2013–2015 or after 2016 was associated with a higher risk of CD4 count of <200 cells per cubic millimeter. The association between the later HIV diagnosis eras and lower CD4 counts may reflect changes in AFRICOS enrollment over time as enrollment in the later years targeted more newly diagnosed persons who may not have had a chance to experience the benefits of ART on CD4 count recovery. The interval between HIV diagnosis and ART initiation has decreased over time in the AFRICOS cohort, and most PLHIV are now initiating ART within 2 weeks.12 In addition, it also highlights that the universe of AHD also includes persons who may have interrupted prior ART and self-reported as ART naive. Interestingly, the Botswana Combination Prevention Project implemented from October 2013 to March 2018 found that AHD as defined by CD4 count of ≤200 cells per cubic millimeter became more common after UTT was adopted compared with beforehand (24.7% versus 15.5%; P < 0.001); however, half of those with AHD self-reported prior disengagement.18 Prior studies have noted that the increasing contribution of ART interruption and gaps in retention on AHD and HIV mortality.27–29
We found that regardless of ART duration, being VLS reduced the risk of having CD4 count of <200 cells per cubic millimeter. Balachandra et al17 also noted that in the 2015–2016 Zimbabwe population HIV impact assessment (ZIMPHIA), PLHIV who were not VLS (defined as < 1000 copies/mL) were 7 times more likely to have a CD4 count of <200 cells per cubic millimeter than those with VLS. In addition, we found that the protective effect of VLS was proportional to the duration on ART. Similarly, concurrent AHD (CD4 <200 cells per cubic millimeter) and VLS in ZIMPHIA was associated with a shorter ART duration of 6 months to 2 years, compared with more than 2 years. These findings highlight the importance of continuity of treatment for durable VL suppression, which prevents the depletion of CD4 cells and/or allow sufficient time for immune reconstitution. Our findings of a lower risk of AHD among PLHIV who were ART naive with VLS likely reflects some component of misclassification of ART status and HIV elite controllers or long-term nonprogressors enrolled in our study, known to have stable levels of CD4 and a strong immune response and VL under 1000 copies per milliliter.30,31
Our study assessed cryptococcal antigenemia among those with AHD. Cryptococcal disease is a common opportunistic infection among those with AHD, and cryptococcal antigenemia prevalence varies across studies and regions.32 Our study noted a CrAg prevalence of 2.9% among those with a CD4 count of <200 cells per cubic millimeter; a 2018 systematic review reported a pooled CrAg prevalence of 6.5% (95% CI: 5.7% to 7.3%; 54 studies) among those with CD4 count of ≤100 cells per cubic millimeter and 2.0% (95% CI: 1.2% to 2.7%; 21 studies) among those with a CD4 count of 101–200 cells per cubic millimeter.32 The CrAg prevalence in our study cannot be attributed to the lack of CD4 testing and results availability but may have been attenuated by the high ART uptake in our study. TB is still the leading cause of death of among PLHIV and has previously been reported as the most common comorbidity among hospitalized AHD patients.27,33 In the AFRICOS cohort, in addition to having a CD4 count of <200 cells per cubic millimeter, a shorter time to HIV diagnosis and being on ART for < 6 months have previously been described as risk factors for incident TB in a prior publication.15
The prevalence of NCDs in our study is similar to a prior AFRICOS study, which also demonstrated that ART use was associated with hypercholesterolemia and dysglycemia.11 We found that dysglycemia, anemia, and being underweight were associated with AHD. There have been inconsistent results from studies exploring the associations between CD4 cell count and dysglycemia among PLHIV.34,35 Therefore, the relationship between dysglycemia and AHD in our study may be impacted by multiple factors including the high ART uptake in our study or the impact of specific ART regimen. Anemia has also been previously reported to be associated with AHD but is not specific to patients with AHD.36 Anemia in HIV is relatively common as PLHIV are at high risk of anemia due to inadequate iron intake, HIV-related opportunistic infections, including TB, and inflammation.37,38 Our findings highlight the importance of integrating NCD care within a DSD model for PLHIV, even those with AHD.39,40
Our study is subject to several limitations. We did not systematically capture treatment interruption among persons on ART, which may impact AHD risk; studies have noted that an increasing proportion of people with AHD are ART experienced and interrupt treatment.19,27,29 Furthermore, we saw a higher prevalence of AHD before 2017 during the same period that accounted for most of the participant enrollment, so we cannot determine the extent to which the lower enrollment from 2017 and onward affected AHD prevalence. In addition, our results may not be generalizable to other settings with lower ART uptake. Furthermore, the uptake of CD4 testing in our study was high (nearly 100%) and likely not reflective of other PEPFAR supported countries where support for CD4 has declined over time.7 By nature of our study, the AFRICOS cohort has robust systems for clinical services, laboratory diagnostics, and patient follow-up, which may differ from real-world clinical settings, which could lead to bias in outcomes and limits generalizability. The AFRICOS cohort is well-established prospective cohort with high ART uptake; a prior publication from this cohort noted that complete ART adherence, with no missed doses in the past 30 days, was reported by 87.8% of PLHIV, whereas 92.4% had viral suppression of <1000 copies per milliliter across all available visits and that only a small fraction (2.8%) had been on ART for <6 months at the participants most recent follow-up visit.41 Nevertheless, our findings highlight the persistent challenge of AHD in the early phase of ART scale-up and the ensuing decline with increasing ART uptake.
PEPFAR, which supports HIV programs in AFRICOS countries, recommends implementation of the AHD package of care, although coverage is variable across countries.26 Continued efforts toward early HIV diagnosis, timely ART initiation, and promoting VLS are needed to reduce the risk for AHD. In addition, additional interventions are needed to engage men earlier into HIV testing and treatment and ensure continuity of treatment for VLS. DSD models that address barriers to continuity of treatment for target populations warrant further scale-up. Furthermore, there is a strong need to ensure availability of AHD diagnostics, including rapid CD4 tests, for timely identification of AHD and diagnostics for opportunistic infections.
Supplementary Material
ACKNOWLEDGMENTS
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the funding agencies. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70–25.
Footnotes
Supported by the Military Infectious Disease Research Program and also conducted in collaboration with a President's Emergency Plan for AIDS Relief–supported basic program evaluation through the U.S. Department of Defense (DoD) and funded through a cooperative agreement (W81XWH-11-2-0174, W81XWH-18-2-0040) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the US DoD's contributions were supported by the National Institute of Mental Health (K24MH098759) and the Global Brain Health Institute.
Meetings at which parts of the data were presented at IDWeek 2020; October 22, 2020; Virtual presentation.
The authors have no funding or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
Contributor Information
Allahna L. Esber, Email: allahnaesber@gmail.com.
Nicole Dear, Email: NDear@hivresearch.org.
Heather N. Paulin, Email: ydi2@cdc.gov.
Michael Iroezindu, Email: miroezindu@wrp-n.org.
Emmanuel Bahemana, Email: ebahemana@wrp.or.tz.
Hannah Kibuuka, Email: hkibuuka@muwrp.org.
John Owuoth, Email: John.Owuoth@usamru-k.org.
Jonah Maswai, Email: Jonah.Maswai@usamru-k.org.
Neha Shah, Email: NShah@hivresearch.org.
Trevor A. Crowell, Email: tcrowell@hivresearch.org.
Julie A. Ake, Email: jake@hivresearch.org.
Christina S. Polyak, Email: christinaspolyak@gmail.com.
REFERENCES
- 1.UNAIDS. Global HIV & AIDS Statistics—2022 Fact Sheet. Available at: https://www.unaids.org/en/resources/fact-sheet. Accessed September 10, 2022. [Google Scholar]
- 2.WHO . Guidelines For Managing Advanced HIV Disease And Rapid Initiation Of Antiretroviral Therapy. Geneva: World Health Organization; 2017. [PubMed] [Google Scholar]
- 3.WHO . Guidelines for the Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children: Supplement to the 2016 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Geneva: World Health Organization; 2018. [PubMed] [Google Scholar]
- 4.WHO . Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public Health approach. Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach. Geneva: World Health Organization; 2021. [PubMed] [Google Scholar]
- 5.The United States President's Emergency Plan for AIDS Relief. Available at: https://www.state.gov/pepfar/. Accessed September 24, 2020. [Google Scholar]
- 6.PEPFAR Country/Regional Operational Plan (COP/ROP) Guidance 2017. Available at: https://mz.usembassy.gov/wp-content/uploads/sites/182/2017/04/FINAL_COP17_guidance-1.pdf. Accessed September 1, 2022. [Google Scholar]
- 7.Zaniewski E, Dao Ostinelli CH, Chammartin F, et al. Trends in CD4 and viral load testing 2005 to 2018: multi-cohort study of people living with HIV in Southern Africa. J Int AIDS Soc. 2020;23:e25546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmona S, Bor J, Nattey C, et al. Persistent high burden of advanced HIV disease among patients seeking care in South Africa's National HIV Program: data from a nationwide laboratory cohort. Clin Infect Dis. 2018;66:S111–s117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasse B, Ledergerber B, Furrer H, et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011;53:1130–1139. [DOI] [PubMed] [Google Scholar]
- 10.Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53:1120–1126. [DOI] [PubMed] [Google Scholar]
- 11.Ake JA, Polyak CS, Crowell TA, et al. Noninfectious comorbidity in the African cohort study. Clin Infect Dis. 2019;69:639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esber AL, Coakley P, Ake JA, et al. Decreasing time to antiretroviral therapy initiation after HIV diagnosis in a clinic-based observational cohort study in four African countries. J Int AIDS Soc. 2020;23:e25446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 14.Kibuuka H, Musingye E, Mwesigwa B, et al. Predictors of all-cause mortality among people with human immunodeficiency virus (HIV) in a prospective cohort study in East Africa and Nigeria. Clin Infect Dis. 2022;75:657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganesan K, Mwesigwa R, Dear N, et al. Epidemiology of tuberculosis among people living with HIV in the African cohort study from 2013 to 2021. J Acquir Immune Defic Syndr. 2023;92:359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auld AF, Shiraishi RW, Oboho I, et al. Trends in prevalence of advanced HIV disease at antiretroviral therapy enrollment - 10 countries, 2004-2015. MMWR Morb Mortal Wkly Rep. 2017;66:558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balachandra S, Rogers JH, Ruangtragool L, et al. Concurrent advanced HIV disease and viral load suppression in a high-burden setting: findings from the 2015-6 ZIMPHIA survey. PLoS One. 2020;15:e0230205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vinikoor MJ, Hachaambwa L. Advanced HIV disease during the ‘treat all’ era in Botswana. AIDA. 2020;34:2321–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chihana ML, Huerga H, Van Cutsem G, et al. Distribution of advanced HIV disease from three high HIV prevalence settings in Sub-Saharan Africa: a secondary analysis data from three population-based cross-sectional surveys in Eshowe (South Africa), Ndhiwa (Kenya) and Chiradzulu (Malawi). Glob Health Action. 2019;12:1679472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benzekri NA, Sambou JF, Ndong S, et al. Prevalence, predictors, and management of advanced HIV disease among individuals initiating ART in Senegal, West Africa. BMC Infect Dis. 2019;19:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sogbanmu OO, Goon DT, Obi LC, et al. Socio-demographic and clinical determinants of late presentation among patients newly diagnosed with HIV in the Eastern Cape, South Africa. Medicine (Baltimore). 2019;98:e14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fomundam HN, Tesfay AR, Mushipe SA, et al. Prevalence and predictors of late presentation for HIV care in South Africa. S Afr Med J. 2017;107:1058–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giles ML, Achhra AC, Abraham AG, et al. Sex-based differences in antiretroviral therapy initiation, switching and treatment interruptions: global overview from the International Epidemiologic Databases to Evaluate AIDS (IeDEA). J Int AIDS Soc. 2018;21:e25149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nardell MF, Adeoti O, Peters C, et al. Men missing from the HIV care continuum in sub-Saharan Africa: a meta-analysis and meta-synthesis. J Int AIDS Soc. 2022;25:e25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu X, Liang B, Zhou C, et al. HIV late presentation and advanced HIV disease among patients with newly diagnosed HIV/AIDS in Southwestern China: a large-scale cross-sectional study. AIDS Res Ther. 2019;16:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.PEPFAR Country/Regional Operational Guidance 2022. Available at: https://www.state.gov/wp-content/uploads/2022/02/COP22-Guidance-Final_508-Compliant-3.pdf. Accessed September 1, 2023. [Google Scholar]
- 27.Ousley J, Niyibizi AA, Wanjala S, et al. High proportions of patients with advanced HIV are antiretroviral therapy experienced: hospitalization outcomes from 2 sub-Saharan African sites. Clin Infect Dis. 2018;66:S126–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haachambwa L, Kandiwo N, Zulu PM, et al. Care continuum and postdischarge outcomes among HIV-infected adults admitted to the hospital in Zambia. Open Forum Infect Dis. 2019;6:ofz336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osler M, Hilderbrand K, Goemaere E, et al. The continuing burden of advanced HIV disease over 10 Years of increasing antiretroviral therapy coverage in South Africa. Clin Infect Dis. 2018;66:S118–s125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez-Galindez C, Pernas M, Casado C, et al. Elite controllers and lessons learned for HIV-1 cure. Curr Opin Virol. 2019;38:31–36. [DOI] [PubMed] [Google Scholar]
- 31.Li JZ, Blankson JN. How elite controllers and posttreatment controllers inform our search for an HIV-1 cure. J Clin Invest. 2021;131:e149414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ford N, Shubber Z, Jarvis JN, et al. CD4 cell count threshold for cryptococcal antigen screening of HIV-infected individuals: a systematic review and meta-analysis. Clin Infect Dis. 2018;66:S152–s159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.UNAIDS. Global HIV & AIDS Statistics — 2021 Fact Sheet. Available at: https://www.unaids.org/en/resources/fact-sheet. Accessed September 1, 2023. [Google Scholar]
- 34.Boufassa F, Goujard C, Viard JP, et al. Immune deficiency could be an early risk factor for altered insulin sensitivity in antiretroviral-naive HIV-1-infected patients: the ANRS COPANA cohort. Antivir Ther. 2012;17:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Sadr WM, Mullin CM, Carr A, et al. Effects of HIV disease on lipid, glucose and insulin levels: results from a large antiretroviral-naive cohort. HIV Med. 2005;6:114–121. [DOI] [PubMed] [Google Scholar]
- 36.Abioye AI, Andersen CT, Sudfeld CR, et al. Anemia, iron status, and HIV: a systematic review of the evidence. Adv Nutr. 2020;11:1334–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Brien ME, Kupka R, Msamanga GI, et al. Anemia is an independent predictor of mortality and immunologic progression of disease among women with HIV in Tanzania. J Acquir Immune Defic Syndr. 2005;40:219–225. [DOI] [PubMed] [Google Scholar]
- 38.Nandlal V, Moodley D, Grobler A, et al. Anaemia in pregnancy is associated with advanced HIV disease. PLoS One. 2014;9:e106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCombe G, Lim J, Hout MCV, et al. Integrating care for diabetes and hypertension with HIV care in sub-Saharan Africa: a scoping review. Int J Integr Care. 2022;22:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ehrenkranz P, Grimsrud A, Holmes CB, et al. Expanding the vision for differentiated service delivery: a call for more inclusive and truly patient-centered care for people living with HIV. J Acquir Immune Defic Syndr. 2021;86:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mbah P, Iroezindu M, Esber AL, et al. Assessing the impact of HIV support groups on antiretroviral therapy adherence and viral suppression in the African cohort study. BMC Infect Dis. 2021;21:694. [DOI] [PMC free article] [PubMed] [Google Scholar]