Summary
Introduction
Infants with myelomeningocele are at risk for chronic kidney disease caused by neurogenic bladder dysfunction. Urodynamic evaluation plays a key role to risk stratify individuals for renal deterioration.
Objective
To present baseline urodynamic findings from the Urologic Management to Preserve Initial Renal function for young children with spina bifida (UMPIRE) protocol, to present the process that showed inadequacies of our original classification scheme, and to propose a refined definition of bladder hostility and categorization.
Study design
The UMPIRE protocol follows a cohort of newborns with myelomeningocele at nine children’s hospitals in the United States. Infants are started on clean intermittent catheterization shortly after birth. If residual volumes are low and there is no or mild hydronephrosis, catheterization is discontinued. Baseline urodynamics are obtained at or before 3 months of age to determine further management. Based on protocol-specific definitions, urodynamic studies were reviewed by the clinical site in addition to a central review team; and if necessary, by all site urologists to achieve 100% concurrence.
Results
We reviewed 157 newborn urodynamic studies performed between May 2015 and September 2017. Of these 157 infants, 54.8% were boys (86/157). Myelomeningocele closure was performed in-utero in 18.4% (29/157) and postnatally in 81.5% (128/157) of newborns. After primary review, reviewers agreed on overall bladder categorization in 50% (79/157) of studies. Concurrence ultimately reached 100% with further standardization of interpretation. We found that it was not possible to reliably differentiate a bladder contraction due to detrusor overactivity from a volitional voiding contraction in an infant. We revised our categorization system to group the “normal” and “safe” categories together as “low risk”. Additionally, diagnosis of detrusor sphincter dyssynergia (DSD) with surface patch electrodes could not be supported by other elements of the urodynamics study. We excluded DSD from our revised high risk category. The final categorizations were high risk in 15% (23/157); intermediate risk in 61% (96/157); and low risk in 24% (38/157).
Conclusion
We found pitfalls with our original categorization for bladder hostility. Notably, DSD could not be reliably measured with surface patch of electrodes. The effect of this change on future renal outcomes remains to be defined.
Keywords: Urodynamics, Meningomyelocele, Urinary bladder, Neurogenic, Infant
Graphical Abstract
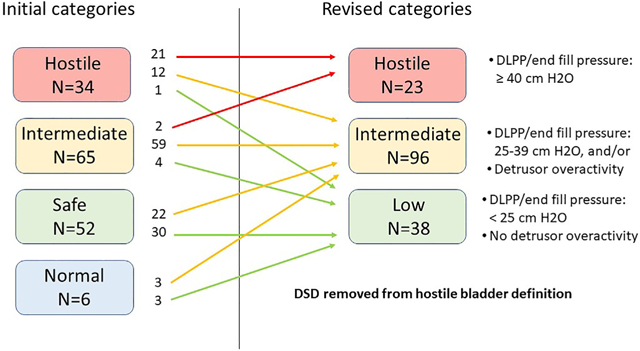
summary figure Change in urodynamic bladder categorization.
Introduction
Children born with myelomeningocele are at risk for chronic kidney disease (CKD) caused by neurogenic bladder dysfunction. Despite improvements in care and attention to kidney preservation, CKD continues to be a common outcome of spina bifida in adulthood [1,2]. Because the severity of bladder dysfunction differs in individuals with spina bifida and changes over time, urodynamic evaluation has played a key role as an objective measure to risk stratify individuals for renal deterioration. More aggressive urologic management is recommended for those individuals found to have a high risk, hostile bladder.
The Urologic Management to Preserve Initial Renal Function for young children with spina bifida (UMPIRE) protocol was conceived and initiated by the Centers for Disease Control and Prevention (CDC) in 2015 to study the urologic management of children with myelomeningocele beginning with birth at multiple centers in the US. A central tenet of this protocol was to use urodynamic evaluation shortly after birth to guide bladder management in a strict prospective protocol in hopes of preserving renal function by identifying bladder risk. Although there are not specific guidelines that define bladder risk, the findings of elevated intravesical pressure at time of urethral leakage [3] and/or detrusor sphincter dyssynergia (DSD) [4] are widely accepted to confer high risk. Investigators met to develop a mutually agreed upon schema to perform and interpret the urodynamic studies upon which management was based [5]. We herein present baseline urodynamic findings in this modern cohort. We anticipated that the proportion of infants with high risk bladder might differ from historical cohorts because of the advent of prenatal intervention. We did not anticipate the challenges in reaching consistent performance and interpretation of urodynamic studies and the inadequacy of our initial classification scheme and methodology to identify the high risk bladder.
Because of several authors’ experiences in other multi-center studies [6–8], there were a priori concerns about urodynamic interrater reliability. We hypothesized that our initial classification scheme with strict definitions for reporting urodynamic findings would be sufficient to overcome these concerns. We describe our initial classification scheme and how we had a higher rate of discordance than expected. As a result, we proceeded with enhanced review and led us to refine the standardization of urodynamic interpretation in this population and to redefine the criteria for bladder hostility.
Materials and methods
The UMPIRE iterative quality improvement protocol follows a cohort of newborns with myelomeningocele at nine children’s hospitals in the United States (Table 1) [5]. This work has been approved by the Institutional Review Boards at all nine clinical sites. This cohort includes both individuals with pre- and post-natal myelomeningocele closure. Briefly, infants are started on clean intermittent catheterization shortly after birth. If residual volumes are low and renal/bladder ultrasound shows no or mild hydronephrosis (less than or equal to Society for Fetal Urology grade [9] 2), catheterization is discontinued. If not, catheterization is continued every 4 h while the patient is awake. Baseline urodynamics are obtained at or before 3 months of age. Urodynamics includes fluoroscopy, if available. If fluoroscopy is not available, voiding cysto-urethrography is performed. All but one site used surface patch electrode electromyography (EMG) rather than needle EMG.
Table 1.
Urologic Management to Preserve Initial Renal function for young children with spina bifida (UMPIRE) clinical sites.
| UMPIRE clinical sites |
|---|
| Alabama (Birmingham): University of Alabama–Birmingham |
| California (Los Angeles): Children’s Hospital of Los Angeles |
| Illinois (Chicago): Lurie Children’s Hospital of Chicago |
| North Carolina (Durham): Duke University Medical Center |
| Oregon (Portland): Oregon Health Sciences University |
| Tennessee (Nashville): Monroe Carell Jr. Children’s Hospital at Vanderbilt |
| Texas (Houston): Texas Children’s Hospital |
| Utah (Salt Lake City): Primary Children’s Hospital |
| Washington (Seattle): Seattle Children’s Hospital |
Table 2 lists the urodynamic parameters collected by individual sites and submitted to CDC. Estimated bladder capacity of the infant was calculated by the following weight-based formula: Capacity (mL) = weight (kg) × 7. Because of concerns about urodynamic interpretation, after more than 100 baseline urodynamic studies from 100 infants had been accrued, each of these studies underwent a central review process by participating urologists not affiliated with the initial study. Each site urologist rereviewed the submitted urodynamic studies using the strict definitions which had been further clarified prior to review. Detrusor leak point pressure (DLPP) was defined as detrusor pressure associated with urine leakage in the absence of detrusor contraction. End filling pressure was defined as the pressure measured immediately prior to a voiding or detrusor overactivity (DO) contraction; alternatively, this may be measured at the end of filling if the study is terminated due to discomfort or other cause. End filling pressure was used if there was no DLPP noted. The presence of DO was defined as ≥2 contractions both ≥15 cm H2O over baseline. DSD was diagnosed by EMG, fluoroscopy, or both. The overall bladder status was categorized as normal, safe, intermediate, or hostile. Table 3a provides the initial definitions used for these bladder categories.
Table 2.
Urodynamic data collected.
| Bladder capacity | ____mL |
| Detrusor pressure at 50% estimated bladder capacity | ____mL, OR not volume not reached |
| Detrusor leak point pressure, OR End filling pressure | ____cm H2O |
| Post void residual: | ____mL |
| Detrusor overactivity | Yes, or No |
| Detrusor sphincter dyssynergia | Yes, or No |
| Shape of bladder | Smooth and round, or Smooth and oblong, or Trabeculated |
| Bladder neck outlet appearance | Open, or Closed |
| Right kidney vesicoureteral reflux | Grades 0 – 5 |
| Left kidney vesicoureteral reflux | Grades 0 – 5 |
| Bladder category | Normal, or Safe, or Intermediate, or Hostile |
Table 3.
Bladder categorization definitions – initial and revised.
| A. Initial bladder categories | B. Revised bladder categories | ||
|---|---|---|---|
| Normal | The bladder should have normal capacity and compliance and empty to near completion at a relatively low detrusor pressure. Leakage before voiding, detrusor overactivity, and DSD are not seen. | Low | Bladder capacity can be normal, low, or high. Compliance is normal or mildly decreased, but the end filling pressure or DLPP must be < 25 cm H2O. Leakage may be present. Detrusor overactivity should not be |
| Safe | Bladder capacity can be normal, low, or high. | present on CMG. | |
| Compliance is normal or mildly decreased, but the end filling pressure or DLPP must be < 25 cm H2O. If bladder capacity and compliance are normal, emptying is poor or absent. (This factor should not be sole reason to choose abnormal in infants as many do not empty completely.) Leakage may be present. Definitive evidence of DSD is not present on either EMG or fluoroscopy. Detrusor overactivity should not be present on CMG. | |||
| Intermediate | This category is a grey zone for bladders that are neither safe nor hostile (see below). Bladder capacity may be low, normal, or high. Compliance is reduced with end filling pressure (measured prior to DO or voiding contraction if present) or DLPP of 25–39 cm H2O. Detrusor overactivity (≥2 contractions of ≥ 15 cm H2O over baseline) or voiding contractions may be present but are not accompanied by DSD. | Intermediate | This category is a grey zone for bladders that are neither safe nor hostile (see below). Bladder capacity may be low, normal, or high. Compliance is reduced with end filling pressure (measured prior to DO or voiding contraction if present) or DLPP of 25–39 cm H2O. Detrusor overactivity (≥2 contractions of ≥ 15 cm H2O over baseline) or voiding contractions may be present. |
| Hostile | This pattern should be obvious and is noted if one or both of the following criteria are present: a. The bladder has poor compliance with an end filling pressure or DLPP ≥ 40 cm H2O. b. Bladder contractions or detrusor overactivity, if present, are accompanied by DSD. |
High risk | The bladder has poor compliance with an end filling pressure or DLPP ≥ 40 cm H2O. |
CMG: cystometrogram; DLPP: detrusor leak point pressure; DO: detrusor overactivity; DSD: detrusor sphincter dyssynergia.
De-identified pressure-volume cystometrogram tracings and available fluoroscopy images were uploaded to a secure site for central review. A cover sheet summarized the urodynamic findings and categorization submitted by the performing urologist. This included: detrusor pressure at 50% expected bladder capacity, DLPP/end filling pressure, DO, DSD, and overall bladder categorization.
Primary review plan: In addition to the clinical site interpretation, each urodynamic study was reviewed by two of four volunteer reviewing urologists (EY, JW, DT, CA) on the central review team so that each study was reviewed by 3 pediatric urologists from 3 different clinical sites. The reviewing urologists were asked if they agreed or disagreed with the original interpretation. If all reviewing urologists did not agree with bladder categorization, bladder categorization was defined as discordant.
Additional secondary review plan: We initially anticipated that with standardized definitions for interpretation, all 3 pediatric urologists would agree on bladder categorization for a large proportion of the studies. When we found a higher rate of discordance than anticipated, we developed an additional secondary review plan. For urodynamic studies in which all 3 pediatric urologists did not agree on bladder characterization, the full central review team (EY, JW, DT, CA, EC, JR, ST) further discussed the study at an in-person meeting in September 2019. Feedback from the central review team was then returned to the clinical site urologist. If discordance in bladder categorization persisted between the original site and the central review team, the study was presented in February 2020 at an in-person meeting of all urologists from the nine sites for consensus opinion. Concurrence regarding final bladder categorization for each study was measured after each of these reviews.
Results
We reviewed 157 newborn baseline urodynamic studies performed between May 2015 and September 2017. Of these 157 infants, 54.8% were boys (86/157). Myelomeningocele closure was performed in-utero in 18.4% (29/157) and postnatally in 81.5% (128/157). After primary review, all three reviewers initially agreed on overall bladder categorization in 50% (79/157) of tests. Because of this low agreement, we proceeded to additional secondary review of the urodynamics studies with bladder categorization discordance.
At the in-person central review of urodynamic studies in September 2019, variation in study technique across institutions became apparent, particularly filling rate, when to stop filling, when to obtain fluoroscopy images, and when to perform a second fill. After the central review meeting, points of disagreement were clarified, and concurrence regarding bladder categorization increased to 68% (106/157). Agreement increased as interpretation definitions were further clarified using multiple examples of actual urodynamic studies. When this clarifying information was provided to the original site performing urologist, concurrence between the performing urologist and the central review team increased to 94% (147/157).
The outstanding 6% (10/157) of studies were reviewed in-person by UMPIRE study urologists from all sites in February 2020, with concurrence established in 100%. This team included STT, EBY, JCR, DT, CA, JSW, EV, DBJ, and 3 additional pediatric urologists (see acknowledgments). During this process, details of standardization of technique and interpretation of urodynamics were discussed and further specified to be used for the UMPIRE protocol moving forward. Yerkes et al. [10]. Provides lessons learned from this review process and provides detail on standardization and interpretation of pediatric urodynamics for multi-institutional research and clinical care in spina bifida.
Fig. 1 summarizes the outcomes of re-interpretation of bladder categorization from the initial interpretation to our final baseline urodynamic categorization for this cohort. The six bladders initially categorized as normal were deemed not normal and were upgraded to low or intermediate risk. During the in-person review, it was determined that it was not possible to reliably differentiate a bladder contraction due to DO from a volitional voiding contraction in an infant. We revised our categorization system to eliminate the “normal” and “safe” categories and define a new group as “low risk”. Initially, 34 bladders were categorized as hostile; 13 studies were downgraded to lower categories. Downgraded bladders were initially categorized as hostile because of DSD as determined from increased sphincter activity utilizing surface patch EMG electrodes. Upon careful review, the diagnosis of DSD could not be supported by the rest of the urodynamics study including the fluoroscopic images and concordant sphincter and detrusor contractions. In order to confirm diagnosis of DSD, there had to be increased activity present during the entire detrusor contraction and for every contraction. Fluoroscopic confirmation included evidence of narrowing of the external sphincter and dilation of the urethra proximal to the sphincter with contrast flowing through the urethra.
Fig. 1.
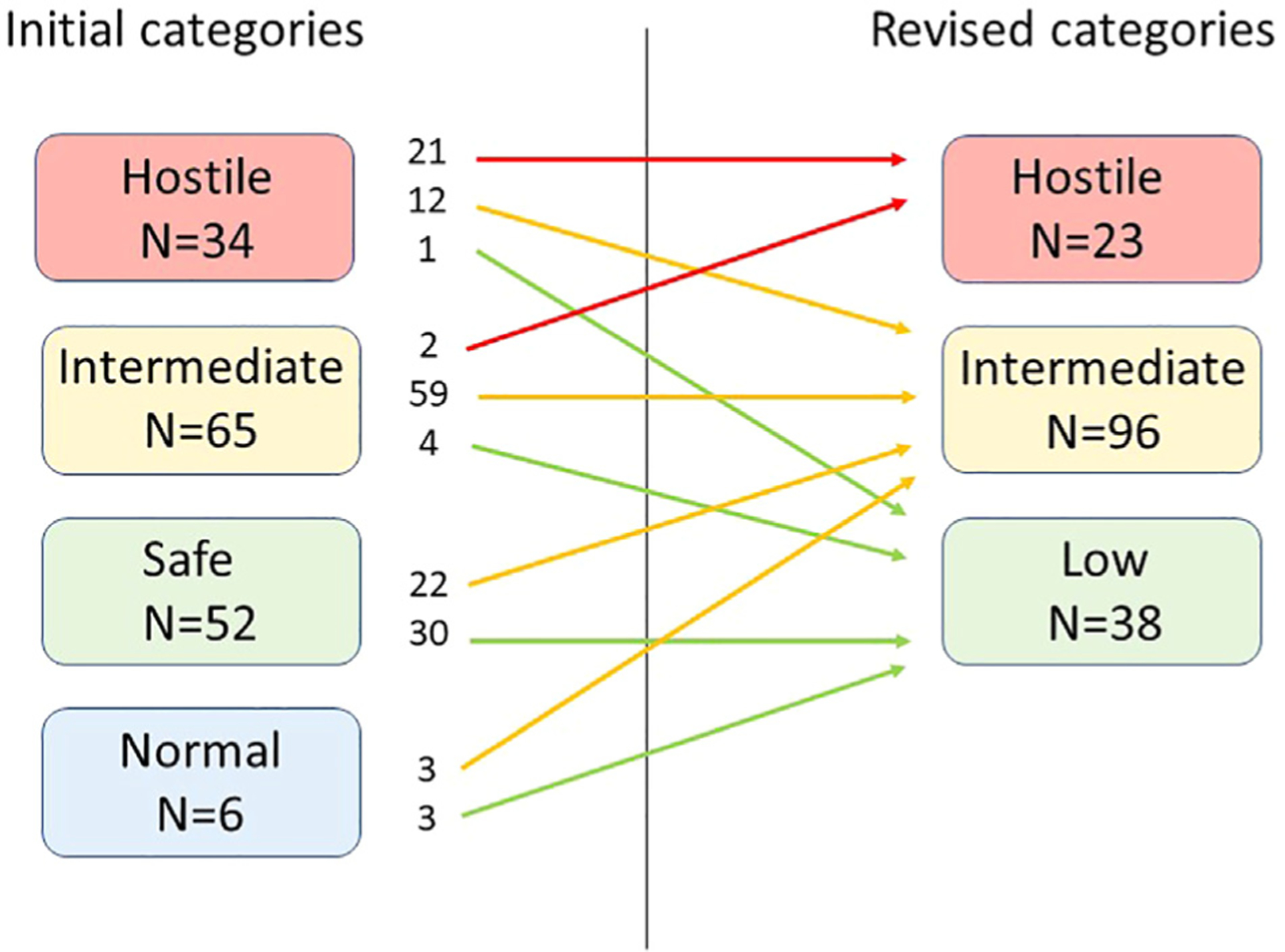
Change in bladder urodynamic categorization.
Table 3b shows our revised categorization of bladder hostility based on this review. Final consensus of baseline urodynamic tests indicated a high risk bladder in 15% (23/157); intermediate risk for 61% (96/157); and low risk for 24% (38/157).
Discussion
This initial report of baseline urodynamic findings has taken longer than we initially anticipated. Our intent was to publish these data as soon as they were available. However, during our multi-institutional data review we encountered multiple barriers to standardized interpretation of urodynamics. During this process, we discovered that even experienced practitioners of urodynamic studies in infants had difficulty in consistent performance and interpretation of studies despite detailed prospective guidelines. We recognized that our initial categorization scheme based on existing guidelines was not sufficient. Accordingly, we redefined and reorganized our urodynamic categorization scheme.
Our new categorization scheme combines the previously “normal” and “safe” categories into a single “low risk” category. It was also felt that “high risk” was a better predictive descriptor than “hostile” bladder. Theoretically, a normal bladder fills at low pressure up to expected bladder capacity, and the bladder empties at a low pressure with an efficient detrusor contraction coordinated appropriately with the relaxation of the external sphincter. However, many healthy infants will have high voiding pressures and detrusor-sphincter dyscoordination on urodynamics [11,12]. Performing urodynamic studies on infants and toddlers can be particularly challenging because the patient may be constantly crying or moving which affects both bladder pressure and sphincter EMG activity. We do not expect this consolidation will have a clinical impact.
Of our 157 infants, 14.6% were recategorized as having a high risk bladder. This number of newborns with high risk bladder is low compared to historical cohorts. Of 148 newborns from 1979 to 1990 at a single institution, 45% had the hostile bladder characteristic of DSD [13]. Bladder function in a modern newborn cohort has many reasons to differ from historical cohorts. More centers are using a proactive treatment approach from birth as recommended by the European Association of Urology (EAU)/European Society for Paediatric Urology (ESPU) guidelines [14]. Additionally, the number of infants who have undergone in utero closure continues to increase, and it is possible that this may result in lower risk bladders upon initial evaluation. Our number of patients with a high risk bladder is also lower when compared to a recent cohort from Brazil. In this cohort of 100 infants who had undergone in utero repair, 41.1% had DSD, and 52.6% were considered high risk because of DLPP > 40 cm H2O or filling pressures > 40 cm H2O [15]. These differences may be due to actual physiologic changes, variations in high risk categorization, or a combination of both.
One likely reason for our lower percentage of high risk bladders is that we have essentially removed DSD as a criterion that we initially used to categorize bladders as hostile. While DSD has been a known risk factor for renal deterioration in infants with myelomeningocele, it has also been shown to have low interrater reliability [8]. When first described [4], concentric needle electrodes were used to measure bioelectric activity. In contrast, 8 sites in our cohort used surface-patch electrodes and only one site used dual wire electrodes, to document striated urethral sphincter activity. In non-neurogenic populations, concentric needle electrodes have showed better reliability than surface electrodes [16,17]. At this time, equipment to support concentric needle EMG is not available at any of our sites, and dual-wire electrodes are no longer commercially available. Utilizing DSD to define a high risk bladder is problematic using surface-patch EMG. Surface-patch EMG can mimic the appearance of DSD, but artifact with any patient movement and grounding out from urinary leakage makes interpretation problematic. In addition, the diagnosis was not supported by the rest of the study including fluoroscopic images and exact temporal concurrence between detrusor and sphincter contractions. In our study 13 bladders initially categorized as hostile because of DSD were downgraded to a lower risk category. We continue to note DSD in our data collection if supported by fluoroscopic images during voiding.
We continue to use urodynamic findings of elevated DLPP [3] to categorize a bladder as high risk. If no leakage was noted or if leakage only occurred during a detrusor contraction or patient movement, end filling pressure was used for categorization. A future goal of our efforts is to determine if this bladder risk categorization holds up over multiple sequential studies in individuals over time using ultrasound to define upper tract deterioration, as opposed to the findings on intravenous pyelograms and VCUGs in the initial McGuire et al. study [3].
We do not yet know the implications of this narrowed definition of bladder hostility. In the UMPIRE protocol, only those infants with high risk bladder or grade 5 vesicoureteral reflux routinely are restarted on intermittent catheterization and antimuscarinic medication after baseline urodynamic study. In the expanded intermediate category, there will likely be a subset of individuals who are at higher risk for renal deterioration. With continued data accrual of renal outcomes as well as longitudinal urodynamic follow up in this protocol, we may be able to elucidate other urodynamic risk factors for bladder and upper tract deterioration that can be reliably measured at multiple sites with widely available equipment.
The International Children’s Continence Society (ICCS) recommends that urodynamic testing be performed in the first 2–3 months of life [18]. Early, proactive management of the hostile bladder is believed to decrease the risk of CKD [19], which remains prevalent in individuals with myelomeningocele [20]. Despite using existing guidelines from ICCS [21], we discovered differences in urodynamics technique and interpretation among experienced pediatric urologists across our sites that required further standardization. Overall recommendations for urodynamics standardization of technique and interpretation in the myelomeningocele population will be addressed separately [10]. Until the pediatric urologic community has a well-defined standard for technique and interpretation, it is critical that individual authors include in their methods how urodynamics are performed, how urodynamic parameters such as DSD were measured, and how bladder hostility is defined.
Despite the efforts of our multi-institutional collaboration, our study has several limitations. Of the nine sites, only one had the interpreting urologist in the room during the entire study; however, most sites had the interpreting urologist supervising the study to determine if repeat filling cycles were required. We are only reporting our baseline urodynamic data at this time, and our findings are not correlated to renal outcomes. The oldest children in our cohort are approaching six years of age. By following this cohort over the next 5–10 years, we hope to be able to correlate baseline urodynamic data with renal function deterioration to determine which infants are truly at risk. As we proceed with this longitudinal protocol, we will continue to rereview submitted urodynamics so that we reliably correlate these findings to radiographic findings and clinical outcomes. Our study does not present the details of the full urodynamic study, such as bladder capacity or specific DLPP values. In the newborn, these measurements can be quite difficult, and we worked to standardize these parameters as well. In the end, these specific values did not change our categorization. Finally, although not specifically a limitation of this study, urodynamic studies previously interpreted using the original categorization schema will be reassessed before they can be used in future data analysis. Additionally, when evaluating renal outcomes in the future, some individuals in the intermediate category will have received treatment for high risk bladder (intermittent catheterization and antimuscarinic medication) because of their original bladder categorization.
Conclusions
On critical review of our baseline urodynamics in infants with myelomeningocele, we found pitfalls with our original categorization system for bladder hostility. Notably, DSD could not be reliably measured with the surface patch electrodes that are the only currently commercially available tools to our sites. We present a new categorization system. The effect of this change on future renal outcomes in the UMPIRE protocol remains to be determined. Future work in this cohort will assess renal outcomes to improve newborn risk stratification.
Acknowledgments
This work is supported by collaborative agreements with the Centers for Disease Control and Prevention (1U01 DD001234, 1U01DD001236, 1U01DD001263, 1U01DD001271, 1U01DD001273, 1U01DD001276, 1U01DD001282, 1U01DD00 1284, 1U01DD001071) The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
We thank the many individuals who have been part of the UMPIRE team since its inception. In particular, for this manuscript, we would like to acknowledge the pediatric urologists at the February 2020 meeting where we discussed the last details of the future standardization of UMPIRE urodynamics: Joan S. Ko (JSK), David I. Chu (DIC), and Elizabeth B. Roth (EBR).
Abbreviations
- CDC
Centers for Disease Control and Prevention
- CKD
chronic kidney disease
- CMG
cystometrogram
- DLPP
detrusor leak point pressure
- DO
detrusor overactivity
- DSD
detrusor sphincter dyssynergia
- EAU
European Association of Urology
- EMG
electromyography
- ESPU
European Society for Paediatric Urology
- ICCS
International Children’s Continence Society
- UMPIRE
Urologic Management to Preserve Initial Renal function for young children with spina bifida
- VCUG
voiding cysto-urethrography
Footnotes
Conflicts of interest
None.
References
- [1].Santiago-Lastra Y, Cameron AP, Lai J, et al. Urological surveillance and medical complications in the United States adult spina bifida population. Urology 2019;123:287–92. [DOI] [PubMed] [Google Scholar]
- [2].Wang HH, Lloyd JC, Wiener JS, et al. Nationwide trends and variations in urological surgical interventions and renal outcome in patients with spina bifida. J Urol 2016;195: 1189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].McGuire EJ, Woodside JR, Borden TA, et al. Prognostic value of urodynamic testing in myelodysplastic patients. J Urol 1981;126:205–9. [DOI] [PubMed] [Google Scholar]
- [4].Bauer SB, Hallett M, Khoshbin S, et al. Predictive value of urodynamic evaluation in newborns with myelodysplasia. J Am Med Assoc 1984;252:650–2. [PubMed] [Google Scholar]
- [5].Routh JC, Cheng EY, Austin JC, et al. Design and methodological considerations of the centers for disease Control and prevention urologic and renal protocol for the newborn and young child with spina bifida. J Urol 2016;196:1728–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Joseph DB, Borer JG, De Filippo RE, et al. Autologous cell seeded biodegradable scaffold for augmentation cystoplasty: phase II study in children and adolescents with spina bifida. J Urol 2014;191:1389–95. [DOI] [PubMed] [Google Scholar]
- [7].Brock JW 3rd, Carr MC, Adzick NS, et al. Bladder function after fetal surgery for myelomeningocele. Pediatrics 2015;136: e906–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dudley AG, Adams MC, Brock JW 3rd, et al. Interrater reliability in interpretation of neuropathic pediatric urodynamic tracings: an expanded multicenter study. J Urol 2018;199: 1337–43. [DOI] [PubMed] [Google Scholar]
- [9].Fernbach SK, Maizels M, Conway JJ. Ultrasound grading of hydronephrosis: introduction to the system used by the Society for Fetal Urology. Pediatr Radiol 1993;23:478–80. [DOI] [PubMed] [Google Scholar]
- [10].Yerkes EB, Cheng EY, Wiener JS, et al. Translating pediatric urodynamics from clinic into collaborative research: lessons and recommendations from the UMPIRE study group. J Pediatr Urol 1993;17:718–27. [DOI] [PubMed] [Google Scholar]
- [11].Yeung CK, Godley ML, Dhillon HK, et al. Urodynamic patterns in infants with normal lower urinary tracts or primary vesicoureteric reflux. Br J Urol 1998;81:461–7. [DOI] [PubMed] [Google Scholar]
- [12].Bachelard M, Sillen U, Hansson S, et al. Urodynamic pattern in asymptomatic infants: siblings of children with vesicoureteral reflux. J Urol 1999;162:1733–7. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- [13].Lais A, Kasabian NG, Dyro FM, et al. The neurosurgical implications of continuous neurourological surveillance of children with myelodysplasia. J Urol 1993;150:1879–83. [DOI] [PubMed] [Google Scholar]
- [14].Stein R, Bogaert G, Dogan HS, et al. EAU/ESPU guidelines on the management of neurogenic bladder in children and adolescent part I diagnostics and conservative treatment. Neurourol Urodyn 2020;39:45–57. [DOI] [PubMed] [Google Scholar]
- [15].Macedo A Jr, Ottoni SL, Garrone G, et al. In utero myelomeningocele repair and urological outcomes: the first 100 cases of a prospective analysis. Is there an improvement in bladder function? BJU Int 2019;123:676–81. [DOI] [PubMed] [Google Scholar]
- [16].Brostrom S, Jennum P, Lose G. Motor evoked potentials from the striated urethral sphincter: a comparison of concentric needle and surface electrodes. Neurourol Urodyn 2003;22: 123–9. [DOI] [PubMed] [Google Scholar]
- [17].Mahajan ST, Fitzgerald MP, Kenton K, et al. Concentric needle electrodes are superior to perineal surface-patch electrodes for electromyographic documentation of urethral sphincter relaxation during voiding. BJU Int 2006;97:117–20. [DOI] [PubMed] [Google Scholar]
- [18].Bauer SB, Austin PF, Rawashdeh YF, et al. International Children’s Continence Society’s recommendations for initial diagnostic evaluation and follow-up in congenital neuropathic bladder and bowel dysfunction in children. Neurourol Urodyn 2012;31:610–4. [DOI] [PubMed] [Google Scholar]
- [19].Dik P, Klijn AJ, van Gool JD, et al. Early start to therapy preserves kidney function in spina bifida patients. Eur Urol 2006;49:908–13. [DOI] [PubMed] [Google Scholar]
- [20].Veenboer PW, Bosch JL, van Asbeck FW, et al. Upper and lower urinary tract outcomes in adult myelomeningocele patients: a systematic review. PloS One 2012;7:e48399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bauer SB, Nijman RJ, Drzewiecki BA, et al. International Children’s Continence Society standardization report on urodynamic studies of the lower urinary tract in children. Neurourol Urodyn 2015;34:640–7. [DOI] [PubMed] [Google Scholar]


