Abstract
Persistent alphavirus infections in synovial and neural tissues are believed to be associated with chronic arthritis and encephalitis, respectively, and represent likely targets for CD8+ αβ cytotoxic T lymphocytes (CTL). Here we show that the capsid protein is a dominant target for alphavirus-specific CTL in BALB/c mice and that capsid-specific CTL from these mice recognize an H-2Kd restricted epitope, QYSGGRFTI. This epitope lies in the highly conserved region of the capsid protein, and QYSGGRFTI-specific CTL were cross reactive across a range of Old World alphaviruses. In vivo the acute primary viraemia of these highly cytopathic viruses was unaffected by QYSGGRFTI-specific CTL. However, in vitro these CTL were able to completely clear virus from macrophages persistently and productively infected with the arthrogenic alphavirus Ross River virus.
Alphaviruses (family Togaviridae) are a group of arthropod-borne, positive-strand RNA viruses found throughout the New and Old Worlds. Several alphaviruses are pathogenic in humans (27), with Old World alphaviruses, which include Ross River (RR) virus (17), Chikungunya virus (7, 31), o’nyong-nyong virus (2, 58), Sindbis virus, and Okelbo virus (65), being principally associated with fever and acute and chronic arthritis/arthralgia. Infection with New World alphaviruses, which include the eastern, Venezuelan, and western equine encephalitis viruses, can result in severe and often fatal acute encephalitis (12, 68). The encephalitis viruses and Getah virus are also important pathogens of horses and wildlife (10, 30).
Following a primary alphavirus infection, there is a transient viremia which lasts for several days (57, 61), with viral clearance believed to be mediated principally by antibodies (70) and alpha/beta interferon (IFN-αβ) (25). Development of murine CD8 cytotoxic T-lymphocyte (CTL) activity correlates with viral clearance from the peripheral blood (5, 54). However, CTL are believed not to be important mediators of protection against acute viremia in alphavirus or other cytopathic viral infections (29). Neutralizing antibodies may play the dominant role in resolving acute togavirus viremias, but there are several reports which suggest that they are unable to clear residual persistent togavirus infections (i) from humans infected with rubella and suffering from arthritis and late-onset rubella syndrome (9, 66); (ii) from mouse brains infected with Bebaru virus, Sindbis virus, or Semliki Forest virus (3, 23, 37); (iii) from birds infected with Western encephalitis virus (56); and (iv) from macrophages infected with RR virus in vitro (41). Residual alphavirus infections persisting in certain tissue sites after the initial viremia has abated not only are likely to be important for mediating pathologic changes but also may become targets for CTL (23, 53, 62). The potential role and mechanisms used by CTL in the clearance of cytopathic viruses remain largely unresolved.
Murine Semliki Forest virus and Sindbis virus infections have been extensively studied as models of viral encephalitis. Both viruses are capable of persisting in vivo (4, 37), and CTL-mediated lysis of persistently infected neural tissues results in neuropathologic changes in the Semliki Forest virus model (62). RR virus, the etiological agent of epidemic polyarthritis (EPA), an acute and chronic disease (17) which affects up to 7,800 Australians annually (11), has also been shown to persist in vitro in muscle cells (14), fibroblasts (28), and macrophages (40). Although, persistence of RR virus in EPA patients has yet to be demonstrated, persistent infections are believed to be responsible for the pathogenesis of many chronic infectious arthritides (9, 34, 48, 52), with many arthrogenic organisms (including RR virus) (40) capable of persisting in macrophages (16, 24, 26, 49, 50, 59). Ineffectual clearance of persistent virus by poor or impaired CTL activity has been associated with the pathologic changes caused by a number of organisms including caprine arthritis virus (38), measles virus (51, 67), and rubella virus (66). CTL activity has also been associated with pathogen clearance and recovery in Lyme arthritis (8) and prevention of persistence in Theiler’s virus infections (13). Interestingly, the predominance of CD4 lymphocytes in the mononuclear synovial effusions of chronic EPA patients contrasts with the almost exclusive CD8+ lymphocyte infiltrate found in skin rashes of EPA patients who made early and complete recoveries (18, 19). These observations may suggest that the minority of individuals who develop chronic disease following RR virus infection (17) fail to generate RR virus-specific CTL and are unable to clear persistent virus.
The presence of alphavirus-specific CTL have been reported to depend on the presence of H-2Dk, with mice bearing other alleles failing to generate significant CTL responses (45, 46). The targets of CTL activity may also be restricted to sequences that are conserved between different alphaviruses, since murine CTL raised against one alphavirus cross-react with other serologically distinct alphaviruses (47, 54). Clearly, if CTL are required for clearance of persistent alphavirus infections, an HLA-restricted or otherwise restricted ability to generate alphavirus-specific CTL may have important implications for human pathogenesis.
The reported role of CTL in the pathogenesis of alphavirus encephalitis (62) and the potential importance of CTL in the clearance of persistent arthrogenic alphavirus infections prompted a search for the target of alphavirus-specific CTL. By using a new in vitro CTL restimulation method, alphavirus-specific CTL were demonstrated in mice with both H-2k and H-2d backgrounds. The capsid protein emerged as the dominant target of these CTL, and the target epitope was localized to the conserved region of this protein. CTL specific for this epitope were cross-reactive and were generated in mice infected with a panel of different Old World alphaviruses. Although capsid-specific CTL induced by vaccination did not affect the acute alphavirus viremia in vivo, these CTL were capable of completely clearing a persistent productive RR virus infection from macrophages in vitro.
MATERIALS AND METHODS
Alphaviruses and viral titer determinations.
Virus stocks were prepared in tissue culture as described previously (40) with Vero cells, except for Barmah Forest virus and RR virus (T48), which were prepared with HeLa cells. Viral titers were determined by using 10-fold serial dilutions in quadruplicate on Vero cells and were expressed as log10 50% cell culture infectivity dose (CCID50) (40). All virus stocks were determined to be mycoplasma free (39). Semliki Forest virus was kindly supplied by P. Hertzog (Monash University, Victoria, Australia), Barmah Forest virus and Getah virus were supplied by B. Kay (Queensland Institute of Medical Research), Sindbis virus was supplied by R. Hall (University of Queensland, Queensland, Australia), and Chikungunya virus was supplied by J. Aaskov (Queensland University of Technology).
Mice and viral infection.
Female 6- to 8-week-old C3H (H-2k) and BALB/c (H-2d) mice (Animal Resource Centre, Perth, Australia) were infected by intraperitoneal injection with 300 CCID50 of each alphavirus (except Sindbis virus, for which 600 CCID50 was used) and/or 5 × 107 PFU of each recombinant vaccinia virus (rVV), both diluted in 500 μl of phosphate-buffered saline. Alphavirus viremias in all alphavirus-infected animals were confirmed by CCID50 determination in blood taken by tail bleeds on day 2 postinfection.
Generation of alphavirus-specific CTL effectors.
Briefly, RR virus-specific effectors were generated by restimulating splenocytes in vitro with RR virus-infected, 48-h thioglycolate-induced peritoneal macrophages (effector-to-stimulator ratio, ≈20:1) for 5 days, and the effectors were used in standard 6-h 51Cr release assays against the target cells listed below. The medium used throughout was determined to be endotoxin free (<0.01 ng/ml) by the method of Sweet and Hume (63) and contained bicarbonate-buffered RPMI 1640 (Gibco), 10% fetal calf serum (PA Biologicals), 2 mM glutamine (Sigma), 10 mM HEPES (Sigma), 5 × 10−5 M β-mercaptoethanol (Sigma), 100 μg of streptomycin per ml, and 100 IU of penicillin per ml (CSL Australia). The thioglycolate-induced peritoneal macrophages were seeded at 4 × 105/24-well plate in serum-free RPMI 1640, and the nonadherent cells were removed after 6 h at 37°C. After a 48-h culture in medium, nonadherent cells were again removed by two washes and the remaining cells were infected with RR virus at a multiplicity of infection (MOI) of 2 for 2 h followed by the addition of splenocytes (5 × 106/24-well plate). (Fresh adherent murine splenocytes or thioglycolate-elicited macrophages, or the same cells cultured for 1 day, were inefficiently infected by RR virus, as determined by an indirect fluorescent-antibody assay (IFA) 24 h postinfection, and failed to restimulate CTL. In contrast, after 2 days in culture, ≈5 to 10% of thioglycolate-elicited macrophages could be infected with RR virus [data not shown].)
QYSGGRFTI-specific effectors were generated by adding 2 μg of QYSGGRFTI (Chiron Mimotopes, Melbourne, Australia) per ml to splenocytes (5 × 105/24-well plate) in 1 ml of medium. Another 1 ml of medium was added on day 3, and the cells were used as effectors in standard 6-h 51Cr release assays on day 5.
A CTL line specific for QYSGGRFTI was developed from QYSGGRFTI-specific effectors and maintained by weekly restimulation with QYSGGRFTI-sensitized (2 μg/ml for 1 h at 37°C), γ-irradiated (8,000 rads), and washed P815 cells (effector-to-stimulator ratio, ca. 30:1). A control BALB/c murine cytomegalovirus-specific CTL line recognizing YPHFMPTNL (60) (kindly supplied by S. Elliott [Queensland Institute of Medical Research]) was maintained in the same way. Both T-cell lines were maintained in T-cell medium, which is the medium described above supplemented with 1 mM pyruvate (ICN, Irvine, Calif.), 1% nonessential amino acid (ICN), and 20 U of recombinant interleukin-2 IL-2 (kindly provided by Cetus Corp., Emeryville, Calif.).
Preparation of target cells. (i) RR virus-infected cells.
L929 (H-2k) and BALB/c 3T3 (H-2d) fibroblasts (CSL) and RAW264.7 cells were cultured in endotoxin-free medium. Cells were scraped or aspirated from tissue culture flasks, pipetted to disperse cell clumps, and pelleted by centrifugation (170 × g for 5 min). The pelleted cells were infected with RR virus (MOI = 10) for 1 h, 51Cr was added for a further 1 h at 37°C in ≈100 μl of medium, and the mixture was washed twice in medium. Infection of macrophages and fibroblast lines with RR virus was strongly inhibited by endotoxin contamination in the medium and trypsin preparations (data not shown).
(ii) Peptide-sensitized target cells.
P815 cells and RAW264.7 cells were sensitized with synthetic QYSGGRFTI peptide (10 μg/ml) (Chiron Mimotopes, Melbourne, Australia) for 1 h at 37°C prior to 51Cr labelling for 1 h and washed twice. The target cells used for the 20-mer peptide net were 51Cr-labelled P815 target cells incubated in 96-round-well plates with each 20-mer peptide (50 μg/ml) for 2 h at 37°C in 50 μl followed by the addition of effectors in 150 μl.
(iii) VV-infected target cells.
P815 cells were infected with rVV (MOI = 10) overnight prior to 51Cr labelling and washing.
Construction of rVV coding for the nonstructural proteins and capsid.
rVV were constructed against each cytoplasmic alphavirus protein, the capsid, and the four nonstructural proteins (NSP). cDNA was prepared from 4 × 106 NB5092 RR virus (15)-infected Vero cells 8 h postinfection (MOI = 2). RNA was extracted with the total RNA isolation reagent (Advanced Technologies, London, United Kingdom) as specified by the manufacturer. The RNA was reverse transcribed at room temperature for 20 min and then at 40°C for 1 h in a 20-μl reaction mixture containing 1 μg of RNA, 1 μg of random hexamer primers (Boehringer, Mannheim, Germany), 1 μl of 10 mM deoxynucleoside triphosphates (Promega, Madison, Wis.), and the following from the Superscript II RT kit (Gibco BRL, Gaithersburg, Md.): 4 μl of 5× first-strand buffer, 2 μl of 0.1 M dithiothreitol, and 1 μl of reverse transcriptase. Following hydrolysis of the RNA with 6 N NaOH, the cDNA was purified by QIAquick-spin PCR purification (Qiagen, Hilden, Germany) and eluted in 50 μl. PCR was performed in a 20-μl reaction volume containing 1 μl of cDNA and 1 μl of each forward and reverse primer (10 μM) (listed below). Each PCR mixture contained 0.4 μl (2 U/μl) of DyNAZyme II DNA polymerase (Finnzymes Oy, Espoo, Finland), 2 μl of 10× PCR buffer II (Finnzymes), and 0.5 μl of deoxynucleoside triphosphates. PCR was performed with a GeneAmp PCR system 9600 with a 5-min denaturation at 95°C followed by 30 cycles of 94°C for 45 s (denaturation) and a 10-min final extension with the following annealing and extension times each protein: capsid, 52°C for 45 s and 72°C for 60 s; NSP1, 55°C for 45 s and 72°C for 90 s; NSP2, 60°C for 45 s and 72°C for 3 min; NSP3, 58°C for 45 s and 72°C for 90 s; NSP4, 55°C for 45 s and 72°C for 90 s.
The primers used contained an ATA cap, a BamHI restriction site (replaced with a HindIII site for NSP3), and a start codon before 18 to 20 bp of coding sequence for each protein. The reverse primers contained 16 to 20 bp of coding sequence followed by a stop codon, a SalI site (replaced with an EcoRI site for NSP2 and NSP3), and an ATA cap. The primer sequences used were ATAGGATCCATGAAGGTCACTGTAGATGTTG, NSP1 (forward); ATAGTCGACCTATGCTCCGGCGCGGTACGTCA, NSP1 (reverse); ATAGGATCCATGGGGGTAGTGGAGACACCCAG, NSP2 (forward); ATAGAATTCCTAGCATCCGGCTGTGTGTAGCC, NSP2 (reverse); ATAAAGCTTATGGCACCCTCATACCGTGTGCG, NSP3 (forward); ATAGAATTCCTACGCCCCCGCTCTGCTTAGTC, NSP3 (reverse); ATAGGATCCATGTACATCTTCTCGTCTGATAC, NSP4 (forward); ATAGTCGACCTATTTAGGACCGCCGTAG, NSP4 (reverse); ATAGGATCCATGAATTACATACCAACCC, CAP (forward); and ATAGTCGACCTACCACTCTTCGGTTCCTTC, CAP (reverse). The PCR products were resolved on a 1% agarose gel, excised, and purified with Wizard PCR Preps (Promega). NSP1, NSP2, and NSP4 were cloned into pGEM-T, and capsid and NSP3 were blunt-end cloned into EcoRV-cut Bluescript II KS+. Plasmids were used to transform SURE competent cells (Stratagene, La Jolla, Calif.), clones were subjected to minipreps by alkaline lysis, and inserts were checked by restriction digestion and confirmed by PCR. After sequencing, the correct clones were cut with BamHI-SalI (for NSP1, NSP4, and capsid), BamHI-EcoRI (for NSP 2), or HindIII-EcoRI (for NSP3) and cloned into the VV shuttle vector PBCB06. The rVV were then constructed as described previously (64). Expression of the recombinant NSPs by each rVV was demonstrated by Western blot analysis on rVV-infected BHK cells with polyclonal anti-NSP1, anti-NSP2, anti-NSP3, and anti-NSP4 sera raised against the related proteins from Sindbis virus (32, 33) and kindly supplied by T. Ahola (Institute of Biotechnology, University of Helsinki, Helsinki, Finland). Expression of the capsid by rVV was similarly demonstrated with polyclonal anti-RR virus sera (kindly supplied by J. Aaskov and was reactive against all the structural proteins (data not shown). The rVV coding for ovalbumin was kindly supplied by F. Carbone (Monash Medical School, Melbourne, Australia).
Synthetic peptide immunization.
Peptide immunization was performed as described previously (60). Briefly, mice received a single subcutaneous injection of 10 μg of QYSGGRFTI and 0.25 μg of tetanus toxoid formulated as a water-in-oil emulsion with the adjuvant Montanide ISA 720 (SEPPIC, Paris, France).
Coculture of CTL lines and persistently infected macrophages.
RAW264.7 cells persistently and productively infected with RR virus T48 (RAW/RRv-PER) have been described previously (40, 41) and were maintained in endotoxin-free medium without mercaptoethanol. This persistent culture, like many other persistent productive cytopathic infections in vitro (41), relied on autocrine IFN-αβ to limit viral replication and thereby prevent overt cytopathic effect of the entire culture. A complete change of medium of RAW/RRv-PER cells removed the autocrine IFN-αβ and resulted in 100% of the cells becoming productively infected and being killed by the virus. A 50% medium change avoided this problem (data not shown); thus, the medium used to establish the coculture experiments always contained 50% of the 3- to 5-day tissue culture supernatants from RAW/RRv-PER cells. This IFN-αβ rich conditioned medium was UV irradiated (40) to remove viable RR virus. To set up the coculture RAW/RRv-PER cells were first washed three times to remove free RR virus and were resuspended in the UV-irradiated conditioned medium. At the time of the experiment, ≈5% of these cells were positive for RR virus by immunofluorescence (see Fig. 8A). The washed RAW/RRv-PER cells were then placed into six replicate wells in a 96-flat-well plate (105 cells/well in 100 μl of conditioned medium) with either (i) the QYSGGRFTI-specific CTL line or (ii) the control CTL line (both at 2 × 105 cells/well in 100 μl of T-cell medium) or (iii) 100 μl of T-cell medium alone. Supernatants (100 μl) were collected at the indicated time points and replaced with 50% UV-irradiated conditioned medium–50% T-cell medium. The viral titers in these supernatants were determined by a CCID50 assay with Vero cells.
FIG. 8.
Immunofluorescent antibody staining of persistently and productively infected macrophages (RAW/RRv-PER) before (A) and 30 days after (B) coculture with QYSGGRFTI-specific CTL. Approximately 250 RAW264 cells are present in each field. Bar, 100 μm.
Indirect immunofluorescence was performed as described previously (40) with a rabbit polyclonal anti-RR virus antibody (a kind gift from J. Aaskov).
RESULTS
RR virus-specific CTL are generated in both H-2d and H-2k mice.
Previous reports have suggested that the CTL response to alphaviruses was restricted to H-2k mice (45, 46). To determine whether the ability to generate alphavirus-specific CTL was major histocompatibility complex (MHC) restricted, BALB/c (H-2d) and C3H/HeJ (H-2k) mice were infected with RR virus and their splenocytes were restimulated in vitro with autologous RR virus-infected, thioglycolate-elicited macrophages. These macrophages were first cultured for 2 days, and then 5 to 10% of them could be infected with RR virus. Effectors from C3H/HeJ (H-2k) mice were capable of specifically killing RR virus-infected H-2k but not H-2d target cells, and effectors from BALB/c (H-2d) mice killed RR virus-infected H-2d but not H-2k targets (Fig. 1). Thus by using the new restimulation method, MHC-restricted CTL activity specific for RR virus was demonstrated in both C3H/HeJ and BALB/c mice.
FIG. 1.
Lysis of RR virus-infected cells by effectors from RR virus-infected mice. C3H (H-2k) mice (n = 4) and BALB/c (H-2d) mice (n = 4) were infected with RR virus. After 35 days, splenocytes from each strain of mice were separately pooled and restimulated in vitro with RR virus-infected, autologous 48-h thioglycolate-elicited peritoneal macrophages. The resulting effectors were used against both uninfected and RR virus-infected 3T3 fibroblasts (H-2d; solid squares) and L929 target cells (H-2k; open squares). % RR virus specific lysis refers to the percent lysis obtained from RR virus-infected targets minus the percent lysis obtained from uninfected targets. E:T ratio, effector-to-target ratio.
Significant nonspecific cytotoxic activity was observed when restimulated splenocytes from mice sacrificed within the first few weeks postinfection were used (data not shown). The efficient lysis of YAC 1 cells by these effectors (data not shown) indicated that this activity was largely due to NK cells, consistent with previous reports (1). Specific MHC-restricted CTL activity was more clearly apparent when splenocytes were taken at day 35 postinfection (Fig. 1).
The capsid protein was the dominant target of RR virus-specific CTL.
To determine the target antigen of RR virus-specific CTL, rVV coding for each of the cytoplasmic RR antigens (NSP1 through NSP4 and capsid) were constructed. Separate groups of mice were immunized with each rVV plus a control rVV coding for ovalbumin (rVV.ovalbumin). After 6 weeks, all the mice were infected with RR virus and their viremias were monitored daily by tail bleeds. There was no significant difference in the level or longevity of RR virus viremias between (i) mice immunized with any of the rVV constructs expressing RR virus antigens and (ii) control animals, which had received no previous rVV or the control rVV.ovalbumin (data not shown).
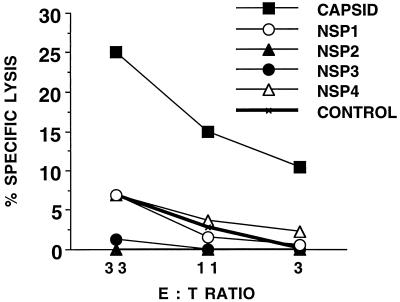
Splenocytes from all animals, which were immunized with each rVV and then RR virus, were restimulated in vitro with RR virus-infected macrophages and used as effectors against autologous target cells infected with the immunizing rVV. Figure 2 clearly illustrates that the capsid protein, but not the NSPs, represented a major target for RR virus-specific CTL.
FIG. 2.
The capsid protein is the dominant target for RR-specific CTL. BALB/c mice (n = 2) were infected separately with rVV coding for NSP1, NSP2, NSP3, NSP4, capsid, and ovalbumin (CONTROL). Six weeks later, all the mice were infected with RR virus, and 14 days later splenocytes from each pair of mice were pooled and restimulated in vitro as for Fig. 1. The resulting effectors were used against uninfected P815 cells and P815 cells infected with the same rVV with which the mice were first infected. Thus, the capsid lysis values were obtained with rVV.capsid-infected target cells and effectors from mice which were infected first with rVV.capsid and then RR virus. This protocol was designed to boost in vivo any RR virus-specific CTL generated by the rVVs, prior to further RR virus-specific restimulation in vitro (see Fig. 4B). [Effectors generated from animals infected with RRV alone failed to show convincing lysis of any of the rVVs (data not shown).] % Specific lysis refers to percent lysis of rVV-infected P815 cells minus percent lysis of uninfected P815 cells (lysis values for the latter did not exceed 5%). E:T ratio, effector-to-target ratio.
Definition of the capsid protein epitope recognized by RR virus-specific CTL.
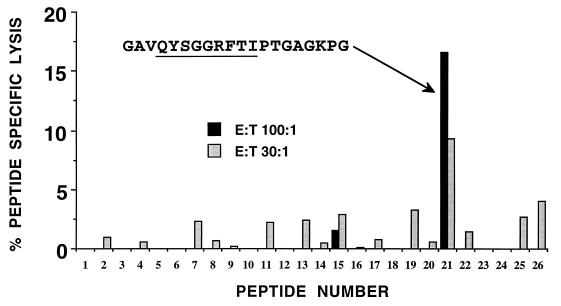
To further define the target of the capsid-specific CTL demonstrated in Fig. 2, CTL raised in the same way were used against target cells sensitized with an overlapping peptide set representing the sequence of capsid protein of RR virus. A single peptide emerged as the dominant target of the CTL (Fig. 3). This 20-mer peptide contained a 9-mer, QYSGGRFTI, which conformed to the MHC motif of H-2Kd (anchor residues underlined). No other sequences within this peptide conformed to the H-2Kd, H-2Dd, or H-2Ld binding motifs (55).
FIG. 3.
Localization of the capsid epitope with an overlapping peptide set. Capsid-specific effectors generated as described for Fig. 2 were used against an overlapping peptide set of the capsid protein (20-mers overlapping by 10). Initially an effector-to-target ratio of 30:1 in duplicate was used, and all peptides giving a lysis value above 2.5% were retested the next day at an effector-to-target ratio of 100:1. The underlined sequence represents a potential epitope that would conform to the H-2Kd binding motif.
To confirm that QYSGGRFTI represented the epitope recognized by RR virus-specific CTL, splenocytes from RR virus-infected mice were restimulated in vitro with synthetic QYSGGRFTI peptide and the effectors were used against (i) QYSGGRFTI-sensitized target cells, (ii) target cells infected with RR virus, and (iii) rVV virus coding for the capsid protein (rVV.capsid). Splenocyte effectors from RR virus-infected mice showed high levels of specific activity against QYSGGRFTIsensitized and rVV.capsid-infected target cells (Fig. 4A). This activity was enhanced when mice were first infected with rVV.capsid and 4 weeks later with RR virus (Fig. 4B), illustrating that RR virus infection boosted a QYSGGRFTI-specific response induced by rVV.capsid. A CTL line generated by weekly restimulations with peptide-sensitized irradiated P815 cells showed very high killing of peptide, RR virus, and capsid target cells (Fig. 4C). This line was >95% CD8+ CD4− CD3+ by fluorescence-activated cell sorting (data not shown).
FIG. 4.
Confirmation of QYSGGRFTI as the target of RR virus-specific CTL. Splenocytes were restimulated in vitro with 2 μg of QYSGGRFTI per ml and were used as effectors against QYSGGRFTI-sensitized (solid triangles), RR virus-infected (solid circles), rVV.capsid-infected (solid squares), and control (open circles and open squares) target cells. Effector cells were restimulated splenocytes from mice infected with RR virus 14 days previously (A), restimulated splenocytes from mice infected with rVV.capsid and 4 weeks later with RR virus and sacrificed 14 days thereafter (B), or a CTL line generated by weekly restimulations of the cells in panel A with peptide-sensitized irradiated P815 cells (C). E:T ratio, effector-to-target ratio.
Lysis of RAW264 cells infected with RR virus was always lower than that of peptide-sensitized or rVV.capsid-infected targets (Fig. 4A and B) but became highly significant when the CTL line was used as an effector (Fig. 4C). These lower lysis values for RR virus-infected RAW264 can be explained in part by the fact that only ≈60% of RAW264 cells can be infected with RR virus (as determined by IFA) whereas ≈100% of P815 cells were infected by the VV (data not shown) and a similar percentage of cells would be sensitized with peptide. In addition, the lower lysis levels for peptide-sensitized RAW264 than for peptide-sensitized P815 (Fig. 5) suggest that the macrophage line may also be generally less susceptible to CTL lysis.
FIG. 5.
QYSGGRFTI represents a cross-reactive epitope recognized by CTL from mice infected with different alphaviruses. Mice (BALB/c, n = 3) were infected with each alphavirus (SF, Semliki Forest virus; CHIK, Chikungunya virus; GET, Getah virus; SIN, Sindbis virus; BF, Barmah Forest virus). After 14 days, splenocytes were restimulated with peptide as for Fig. 4 and used against QYSGGRFTI-sensitized (solid squares and solid triangles), RR virus-infected (solid circles), and control (open squares and open circles) target cells. E:T ratio, effector-to-target ratio.
These experiments confirmed that QYSGGRFTI was an epitope from the capsid protein recognized by RR virus-specific CTL.
QYSGGRFTI represented a cross-reactive CTL epitope.
Previous reports have demonstrated that CTL from alphaviruses cross-react (47, 54). To determine whether QYSGGRFTI represented a cross-reactive alphavirus CTL epitope, BALB/c mice were infected with a panel of alphaviruses. Splenocytes harvested from these animals were restimulated in vitro with QYSGGRFTI peptide and were used as effectors against peptide-sensitized and RR virus-infected target cells. (Peptide restimulation generated a much lower level of NK activity in vitro; thus, splenocytes from animals infected for 12 to 14 days, rather than 35 days, could be used for these experiments [data not shown]). For all the alphaviruses tested, except Barmah Forest virus, infected animals produced CTL specific for QYSGGRFTI. In addition, QYSGGRFTI-specific CTL from animals infected with the different alphaviruses were capable of killing RR virus-infected cells, illustrating that they were cross-reactive (Fig. 5). The Barmah Forest virus capsid protein contains two substitutions in this epitope region (Table 1), which is likely to explain the lack of recognition of QYSGGRFTI.
TABLE 1.
Comparison of the epitope sequence for all known alphavirus capsid sequences
| Alphavirus | Sequencea |
|---|---|
| RR T48 | QYSGGRFTI |
| RR NB | QYSXGRFTI |
| Sindbis | QYSGGRFTI |
| Semliki Forest | QYSGGRFTI |
| Chikungunya | QYSGGRFTI |
| O’nyong-nyong | QYSGGRFTI |
| Ockelbo | QYSGGRFTI |
| Aura | QFSGGRFTI |
| Barmah Forest | QFSNGRFTI |
| Western equine encephalitisb | QYENGRFTV |
| Venezuelan equine encephalitisb | QYENGRFTV |
| Eastern equine encephalitisb | QYENNRFTV |
Sequences obtained from blast@ncbi.nlm.nih.gov. Underlined residues indicate changes. Y (or F) and I (or L, V) are the anchor residues for H-2Kd. X = G in RR virus NB (44a). The Getah virus capsid sequence is not available.
New world alphaviruses.
These experiments demonstrated that CTL specific for QYSGGRFTI were generated in BALB/c mice infected with a number of different alphaviruses, illustrating that this epitope was broadly cross-reactive across serologically distinct alphaviruses.
CTL specific for QYSGGRFTI or capsid did not affect RR virus acute viremia.
CTL are believed not to mediate protection against an acute alphavirus infection (3, 29). To test this contention directly, mice (n = 5) were immunized with (i) rVV.capsid or (ii) a peptide formulation comprising synthetic QYSGGRFTI peptide and tetanus toxoid emulsified in Montanide ISA 720, a water-in-oil formulation which has been shown previously to induce protective CTL (60). Both peptide- and rVV.capsid-immunized animals produced significant levels of QYSGGRFTI-specific CTL (data not shown), but when they were challenged with RR virus, no significant change in the course or magnitude of the viremia could be demonstrated (Fig. 6). (rVV.capsid-immunized animals developed only very weak antibody responses against capsid [data not shown]). These experiments suggest that capsid-specific CD8 CTL did not mediate significant protective activity against an acute alphavirus viremia in mice.
FIG. 6.
CTL specific for QYSGGRFTI or capsid do not affect acute alphavirus viremia. Mice (BALB/c, n = 6) were immunized with rVV.capsid (rVV.CAPSID) or rVV.ovalbumin (rVV.CONTROL) or a synthetic peptide vaccine formulation containing QYSGGRFTI and tetanus toxoid emulsified in Montanide ISA 720 (QYSGGRFTI/TT/M720) or the same formulation without peptide (CONTROL). Three weeks later, the animals were challenged with RR virus. Viremia was monitored by titer determination in blood samples taken by daily tail bleeding.
QYSGGRFTI-specific CTL could clear RR virus from macrophage cultures.
We have previously shown that RR virus can establish a persistent productive infection in the macrophage line RAW264.7 and have postulated that these cultures (RAW/RRv-PER) represent an in vitro model of the behavior of RR virus in the arthritic joints of patients with chronic EPA (40, 41). These persistent cultures are similar to many persistent cytopathic viral infections in vitro (20). The cultures contained a small fluctuating percentage of cells continuously undergoing cytopathic infection, and the production of autocrine IFN-αβ protected the majority of cells from CPE (reference 20 and data not shown). To determine whether QYSGGRFTI-specific CTL could clear virus from RAW/RRv-PER cultures, the QYSGGRFTI specific CTL line (Fig. 4C) was cocultured with RAW/RRv-PER cells and the amount of virus produced in the supernatant was monitored over time. A control CTL line and medium controls were set up in parallel. At 15 days after the coculture was established, no more infectious virus could be detected from any of the six replicate wells containing QYSGGRFTI-specific CTL, whereas RR virus titers from medium control cultures and RAW/RRv-PER–control CTL-line cocultures were unaffected (Fig. 7).
FIG. 7.
CTL specific for QYSGGRFTI can clear RR virus from persistently and productively infected macrophages. The CTL line specific for QYSGGRFTI (Fig. 4C) [CTL(QYSGGRFTI)] and a control cell line specific for a murine cytomegalovirus epitope [CTL (CONTROL)] and medium alone (MEDIUM) were separately cocultured with washed RAW264 macrophages persistently and productively infected with RR virus (RAW/RRv-PER). Supernatants from these cocultures were taken at the indicated time points and assayed for RR virus titers. ∗, No virus was detected in supernatants taken from all six replicates of CTL QYSGGRFTI plus RAW/RRv-PER on these days.
To further illustrate the removal of virus-infected cells from the persistently infected RAW264 cultures by the QYSGGRFTI-specific CTL, IFA was performed on the persistently infected cultures before and 30 days after addition of the CTL. About 5% of the RAW264 cells were IFA positive in the persistently infected cultures; however, 30 days after addition of the QYSGGRFTI-specific CTL, no IFA-positive cells could be found (Fig. 8).
This experiment illustrated that alphavirus-specific CTL were extremely efficient at clearing the highly cytopathic alphavirus RRV from these persistent and productively infected macrophages.
DISCUSSION
This report identifies the capsid protein as a dominant target of alphavirus-specific CTL, describes the first characterization of an alphavirus-specific CD8 CTL epitope, and shows that an alphavirus-specific CTL can clear a cytopathic infection from a persistent and productively infected macrophage culture. The alphavirus-specific CTL epitope identified here, QYSGGRFTI, was H-2Kd restricted and localizes to the conserved region of the capsid protein (6), a region believed to be involved in binding to the surface glycoprotein E2 (35). The epitope sequence QYSGGRFTI was also conserved across RR, Sindbis, Semliki Forest, Getah, and Chikungunya viruses (Table 1), and this conservation correlated with the observed cross-reactivity of CTL from mice infected with these viruses. The conservation and cross-reactivity did not extend to Barmah Forest virus, nor would it be expected to extend to the encephalitis viruses, since all these viruses have one or more nonconservative substitutions in the epitope region (Table 1). However, all the aligned capsid sequences listed may nevertheless represent H-2Kd-restricted CTL epitopes for each respective alphavirus, since the anchor residues required for H-2Kd binding (Y or F at position 2, and I, L, or V at position 9) (55) are conserved in all the sequences. Based on recent sequence analysis, alphaviruses have been partitioned into two major evolutionary groups, which reflect their geographic localization to the Old and New Worlds. The human clinical syndromes caused by alphaviruses associate with these groupings, with Old World viruses being principally associated with fever and polyarthritis and New World viruses causing mainly encephalitis (27). The cross-reactivity described in this paper is thus restricted to the Old World arthrogenic alphaviruses.
The capsid protein was identified as a dominant target for class I-restricted alphavirus-specific CTL. The capsid is also a dominant target of rubella virus-specific CTL (42). The rubella virus, a Rubivirus, shares membership of Togaviridae with alphaviruses based on similarities in their genomic organization and replication strategies. CTL specific for the alphavirus NSPs could not be demonstrated in this study but may exist at low precursor frequency and/or in other mouse strains (21). The very low level and restricted period of NSP synthesis in alphavirus-infected cells (43, 69) may, however, preclude these proteins from becoming significant targets of CTL activity. In comparison, the capsid protein is synthesized in large amounts throughout the infection period, with nucleocapsids even being detectable by electron microscopy (36). In contrast, NSPs are the dominant target of CTL in flavivirus infections (44) but flavivirus NSPs are synthesized in readily detectable amounts throughout the much longer infectious cycle of these notably less cytopathic arboviruses (69). Whether the dominant target of alphavirus-specific CTL in humans is the same as that seen in BALB/c mice remains to be established. If the target of anti-alphavirus CTL activity was confined to the capsid protein in humans, this might restrict the ability of certain individuals to generate CTL, since the capsid is a small (32-kDa) protein. The RNA binding half of this protein may also contain fewer CTL epitopes, since this poorly conserved region contains a high percentage of prolines and basic amino acids (6) and fewer large hydrophobic residues, which are often required as MHC anchor residues (55).
The rise and fall of a primary alphavirus viremia appears to be largely unaffected in perforin and FasL knockout mice (29), suggesting that CTL lytic mediators do not play a significant role in controlling these cytopathic viruses. The inability of rVV.capsid- and peptide vaccine-induced alphavirus-specific CTL to significantly affect the levels of virus in the blood during the primary infection adds to this data, indicating that neither CTL lytic mediators nor CTL-derived cytokines (5, 22) play a significant protective role at this stage of an alphavirus infection. However, both the explosive nature of the primary viremia and the insensitivity of measuring CTL activity through blood viremia monitoring may contribute to this apparent lack of CTL efficacy against RR virus. The cell in vitro which gives rise to the RR viremia is not known, although many cell types might contribute and the infection is likely to spread to many tissues in the body (14, 19, 28, 40, 61, 62). Only a very substantial CTL number might be able to kill the large number of cells (which would probably be infected during the primary viremia) within the short period before virus production begins within each cell. Even if the CTL activity was able to kill or prevent virus production by 90% of these infected cells, the blood viremia would only change by 1 log unit, an insignificant change given the mouse-to-mouse variation (Fig. 6).
In contrast to the observations about CTL activity during the primary viremia, the clear demonstration that QYSGGRFTI-specific CTL were capable of completely clearing RR virus from persistently and productively infected macrophage cultures suggests that CTL can be extremely effective against RR virus infections. The mechanisms used by the RR virus-specific CTL to clear the persistent infections from the macrophage cultures have not been characterized but may involve both direct lysis and secretion of antiviral cytokines, principally IFN-γ (22). Alphavirus infections are well known to be highly sensitive to the antiviral activity of IFNs (25). The testable implication of this work is that the small percentage of individuals who develop chronic arthritis following an alphavirus infection (17, 27, 31, 65) have a persisting synovial infection because they fail to generate significant anti-alphavirus CTL activity. These results suggest that CTL may generally be extremely effective at controlling cytopathic viruses in restricted tissue microenvironments where high concentrations of CTL-derived antiviral cytokines might accumulate (5, 22) and/or high effector-to-target ratios might be achieved.
ACKNOWLEDGMENTS
M.L.L. and L.M. contributed equally to this work.
This work was supported financially by the following Australian organizations: The National Health and Medical Research Council, the Australian Centre for International and Tropical Health and Nutrition, and the Queensland Health Arbovirus Research Fund.
We thank K. W. Sproat and B. E. H. Coupar (CSIRO, Australian Animal Health Laboratory, Geelong, Victoria, Australia) for help with construction of the recombinant vaccinia viruses and N. Kienzle, S. Burrows, and S. Elliott (Queensland Institute of Medical Research) for helpful technical and scientific discussions.
REFERENCES
- 1.Aaskov J G, Dalglish D A, Harper J J, Douglas J F, Donaldson M D, Hertzog P J. Natural killer cells in viral arthritis. Clin Exp Immunol. 1987;68:23–32. [PMC free article] [PubMed] [Google Scholar]
- 2.Adebajo A O. Rheumatic manifestations of tropical diseases. Curr Opin Rheumatol. 1996;8:85–89. doi: 10.1097/00002281-199601000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Amor S, Scallan M F, Morris M M, Dyson H, Fazakerley J K. Role of immune responses in protection and pathogenesis during Semliki Forest virus encephalitis. J Gen Virol. 1996;77:281–291. doi: 10.1099/0022-1317-77-2-281. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson T, Barrett A D, Mackenzie A, Dimmock N J. Persistence of virulent Semliki Forest virus in mouse brain following co-inoculation with defective interfering particles. J Gen Virol. 1986;67:1189–1194. doi: 10.1099/0022-1317-67-6-1189. [DOI] [PubMed] [Google Scholar]
- 5.Blackman M J, Morris A G. Gamma interferon production and cytotoxicity of spleen cells from mice infected with Semliki Forest virus. J Gen Virol. 1984;65:955–961. doi: 10.1099/0022-1317-65-5-955. [DOI] [PubMed] [Google Scholar]
- 6.Boege U, Wengler W, Wengler G, Wittmann-Liebold B. Primary structure of the core proteins of the alphaviruses Semliki Forest virus and Sindbis virus. Virology. 1981;113:293–303. doi: 10.1016/0042-6822(81)90156-2. [DOI] [PubMed] [Google Scholar]
- 7.Brighton S W. Chloroquine phosphate treatment of chronic Chikungunya arthritis. An open pilot study. S Afr Med J. 1984;66:217–218. [PubMed] [Google Scholar]
- 8.Busch D H, Jassoy C, Brinckmann U, Girschick H, Huppertz H I. Detection of Borrelia burgdorferi-specific CD8+ cytotoxic T cells in patients with Lyme arthritis. J Immunol. 1996;157:3534–3541. [PubMed] [Google Scholar]
- 9.Chantler J K, Tingle A J, Petty R E. Persistent rubella virus infection associated with chronic arthritis in children. N Engl J Med. 1985;313:1117–1123. doi: 10.1056/NEJM198510313131803. [DOI] [PubMed] [Google Scholar]
- 10.Clark G G, Dein F J, Crabbs C L, Carpenter J W, Watts D M. Antibody response of sandhill and whooping cranes to an eastern equine encephalitis virus vaccine. J Wildl Dis. 1987;23:539–544. doi: 10.7589/0090-3558-23.4.539. [DOI] [PubMed] [Google Scholar]
- 11.Curran M, Harvey B, Crerar S, Oliver G, D’Sousza R, Myint H, Rann C, Andrews R. Australia’s notifiable diseases status, 1996. Commun Dis Intell. 1996;21:281–307. doi: 10.33321/cdi.1997.21.52. [DOI] [PubMed] [Google Scholar]
- 12.Deresiewicz R L, Thaler S J, Hsu L, Zamani A A. Clinical and neuroradiographic manifestations of eastern equine encephalitis. N Engl J Med. 1997;336:1867–1874. doi: 10.1056/NEJM199706263362604. [DOI] [PubMed] [Google Scholar]
- 13.Dethlefs S, Brahic M, Larsson-Sciard E L. An early, abundant cytotoxic T-lymphocyte response against Theiler’s virus is critical for preventing viral persistence. J Virol. 1997;71:8875–8878. doi: 10.1128/jvi.71.11.8875-8878.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eaton B T, Hapel A J. Persistent noncytolytic togavirus infection of primary mouse muscle cells. Virology. 1976;72:266–271. doi: 10.1016/0042-6822(76)90329-9. [DOI] [PubMed] [Google Scholar]
- 15.Faragher S G, Meek A D, Rice C M, Dalgarno L. Genome sequences of a mouse-avirulent and a mouse-virulent strain of Ross River virus. Virology. 1988;163:509–526. doi: 10.1016/0042-6822(88)90292-9. [DOI] [PubMed] [Google Scholar]
- 16.Fish K N, Britt W, Nelson J A. A novel mechanism for persistence of human cytomegalovirus in macrophages. J Virol. 1996;70:1855–1862. doi: 10.1128/jvi.70.3.1855-1862.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser J R. Epidemic polyarthritis and Ross River virus disease. Clin Rheum Dis. 1986;12:369–388. [PubMed] [Google Scholar]
- 18.Fraser J R, Becker G J. Mononuclear cell types in chronic synovial effusions of Ross River virus disease. Aust N Z J Med. 1984;14:505–506. doi: 10.1111/j.1445-5994.1984.tb03629.x. [DOI] [PubMed] [Google Scholar]
- 19.Fraser J R, Ratnamohan V M, Dowling J P, Becker G J, Varigos G A. The exanthem of Ross River virus infection: histology, location of virus antigen and nature of inflammatory infiltrate. J Clin Pathol. 1983;36:1256–1263. doi: 10.1136/jcp.36.11.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman R M, Ramseur J M. Mechanisms of persistent infections by cytopathic viruses in tissue culture. Arch Virol. 1979;60:83–103. doi: 10.1007/BF01348025. [DOI] [PubMed] [Google Scholar]
- 21.Gorrell M D, Lemm J A, Rice C M, Griffin D E. Immunization with nonstructural proteins promotes functional recovery of alphavirus-infected neurons. J Virol. 1997;71:3415–3419. doi: 10.1128/jvi.71.5.3415-3419.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guidotti L G, Chisari F V. To kill or to cure: options in host defense against viral infection. Curr Opin Immunol. 1996;8:478–483. doi: 10.1016/s0952-7915(96)80034-3. [DOI] [PubMed] [Google Scholar]
- 23.Hapel A J. The protective role of thymus-derived lymphocytes in arbovirus-induced meningoencephalitis. Scand J Immunol. 1975;4:267–278. doi: 10.1111/j.1365-3083.1975.tb02626.x. [DOI] [PubMed] [Google Scholar]
- 24.Hober D, Andreoletti L, Shen L, Copin M C, Desmidt A, Wattre P. Coxsackievirus B3-induced chronic myocarditis in mouse: use of whole blood culture to study the activation of TNF alpha-producing cells. Microbiol Immunol. 1996;40:837–845. doi: 10.1111/j.1348-0421.1996.tb01149.x. [DOI] [PubMed] [Google Scholar]
- 25.Hwang S Y, Hertzog P J, Holland K A, Sumarsono S H, Tymms M J, Hamilton J A, Whitty G, Bertoncello I, Kola I. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons alpha and beta and alters macrophage responses. Proc Natl Acad Sci USA. 1995;92:11284–11288. doi: 10.1073/pnas.92.24.11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Itescu S. Rheumatic aspects of acquired immunodeficiency syndrome. Curr Opin Rheumatol. 1996;8:346–353. doi: 10.1097/00002281-199607000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Johnston R E, Peters C J. Alphaviruses. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. p. 843. [Google Scholar]
- 28.Journeaux S F, Brown W G, Aaskov J G. Prolonged infection of human synovial cells with Ross River virus. J Gen Virol. 1987;68:3165–3169. doi: 10.1099/0022-1317-68-12-3165. [DOI] [PubMed] [Google Scholar]
- 29.Kagi D, Hengartner H. Different roles for cytotoxic T cells in the control of infections with cytopathic versus noncytopathic viruses. Curr Opin Immunol. 1996;8:472–477. doi: 10.1016/s0952-7915(96)80033-1. [DOI] [PubMed] [Google Scholar]
- 30.Kamada M, Ando Y, Fukunaga Y, Kumanomido T, Imagawa H, Wada R, Akiyama Y. Equine Getah virus infection: isolation of the virus from racehorses during an enzootic in Japan. Am J Trop Med Hyg. 1980;29:984–988. doi: 10.4269/ajtmh.1980.29.984. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy A C, Fleming J, Solomon L. Chikungunya viral arthropathy: a clinical description. J Rheumatol. 1980;7:231–236. [PubMed] [Google Scholar]
- 32.Kujala P, Rikkonen M, Ahola T, Kelve M, Saarma M, Kaariainen L. Monoclonal antibodies specific for Semliki Forest virus replicase protein nsP2. J Gen Virol. 1997;78:343–351. doi: 10.1099/0022-1317-78-2-343. [DOI] [PubMed] [Google Scholar]
- 33.Laakkonen P, Hyvonen M, Peranen J, Kaariainen L. Expression of Semliki Forest virus nsP1-specific methyltransferase in insect cells and in Escherichia coli. J Virol. 1994;68:7418–7425. doi: 10.1128/jvi.68.11.7418-7425.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lahesmaa R, Shanafelt M C, Steinman L, Peltz G. Immunopathogenesis of human inflammatory arthritis: lessons from Lyme and reactive arthritis. J Infect Dis. 1994;170:978–985. doi: 10.1093/infdis/170.4.978. [DOI] [PubMed] [Google Scholar]
- 35.Lee S, Owen K E, Choi H K, Lee H, Lu G, Wengler G, Brown D T, Rossmann M G, Kuhn R J. Identification of a protein binding site on the surface of the alphavirus nucleocapsid and its implication in virus assembly. Structure. 1996;4:531–541. doi: 10.1016/s0969-2126(96)00059-7. [DOI] [PubMed] [Google Scholar]
- 36.Lehane M J, Leake C J. A kinetic and ultrastructural comparison of alphavirus infection of cultured mosquito and vertebrate cells. J Trop Med Hyg. 1982;85:229–238. [PubMed] [Google Scholar]
- 37.Levine B, Hardwick J M, Griffin D E. Persistence of alphaviruses in vertebrate hosts. Trends Microbiol. 1994;2:25–28. doi: 10.1016/0966-842x(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 38.Lichtensteiger C A, Cheevers W P, Davis W C. CD8+ cytotoxic T lymphocytes against antigenic variants of caprine arthritis-encephalitis virus. J Gen Virol. 1993;74:2111–2116. doi: 10.1099/0022-1317-74-10-2111. [DOI] [PubMed] [Google Scholar]
- 39.Linn M L, Bellett A J D, Parsons P G, Suhrbier A. Complete removal of mycoplasma from viral preparations using solvent extraction. J Virol Methods. 1995;52:51–54. doi: 10.1016/0166-0934(94)00136-5. [DOI] [PubMed] [Google Scholar]
- 40.Linn M L, Aaskov J, Suhrbier A. Antibody dependent enhancement and persistence in macrophages of an arbovirus associated with arthritis. J Gen Virol. 1996;77:407–412. doi: 10.1099/0022-1317-77-3-407. [DOI] [PubMed] [Google Scholar]
- 41.Linn M L, Suhrbier A. Persistence of Ross River virus in macrophages. Arbovirus Res Aust. 1997;7:153–159. [Google Scholar]
- 42.Lovett A E, Hahn C S, Rice C M, Frey T K, Wolinsky J S. Rubella virus-specific cytotoxic T-lymphocyte responses: identification of the capsid as a target of major histocompatibility complex class I-restricted lysis and definition of two epitopes. J Virol. 1993;67:5849–5858. doi: 10.1128/jvi.67.10.5849-5858.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin J H, Weir R C, Dalgarno L. Replication of standard and defective Ross River virus in BHK cells: patterns of viral RNA and polypeptide synthesis. Arch Virol. 1979;61:87–103. doi: 10.1007/BF01320594. [DOI] [PubMed] [Google Scholar]
- 44.Mathew A, Kurane I, Rothman A L, Zeng L L, Brinton M A, Ennis F A. Dominant recognition by human CD8+ cytotoxic T lymphocytes of dengue virus nonstructural proteins NS3 and NS1.2a. J Clin Invest. 1996;98:1684–1691. doi: 10.1172/JCI118964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44a.Mateo, L. Unpublished information.
- 45.Muellbacher A, Blanden R V. Murine cytotoxic T-cell response to alphavirus is associated mainly with H-2Dk. Immunogenetics. 1978;7:551–561. doi: 10.1007/BF01844044. [DOI] [PubMed] [Google Scholar]
- 46.Muellbacher A, Blanden R V. H-2-linked control of cytotoxic T-cell responsiveness to alphavirus infection. Presence of H-2Dk during differentiation and stimulation converts stem cells of low responder genotype to T cells of responder phenotype. J Exp Med. 1979;49:786–790. doi: 10.1084/jem.149.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muellbacher A, Marshall I D, Blanden R V. Cross-reactive cytotoxic T cells to alphavirus infection. Scand J Immunol. 1979;10:291–296. doi: 10.1111/j.1365-3083.1979.tb01353.x. [DOI] [PubMed] [Google Scholar]
- 48.Musiani M, Zerbini M, Gentilomi G, Plazzi M, Gallinella G, Venturoli S. Parvovirus B19 clearance from peripheral blood after acute infection. J Infect Dis. 1995;172:1360–1363. doi: 10.1093/infdis/172.5.1360. [DOI] [PubMed] [Google Scholar]
- 49.Nanagara R, Li F, Beutler A, Hudson A, Schumacher H R., Jr Alteration of Chlamydia trachomatis biologic behavior in synovial membranes. Suppression of surface antigen production in reactive arthritis and Reiter’s syndrome. Arthritis Rheum. 1995;38:1410–1417. doi: 10.1002/art.1780381008. [DOI] [PubMed] [Google Scholar]
- 50.Narayan O, Zink M C, Gorrell M, McEntee M, Sharma D, Adams R. Lentivirus induced arthritis in animals. J Rheumatol Suppl. 1992;32:25–32. [PubMed] [Google Scholar]
- 51.Niewiesk S, Brinckmann U, Bankamp B, Sirak S, Liebert U G, ter Meulen V. Susceptibility to measles virus-induced encephalitis in mice correlates with impaired antigen presentation to cytotoxic T lymphocytes. J Virol. 1993;67:75–81. doi: 10.1128/jvi.67.1.75-81.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nocton J J, Dressler F, Rutledge B J, Rys P N, Persing D H, Steere A C. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;330:229–234. doi: 10.1056/NEJM199401273300401. [DOI] [PubMed] [Google Scholar]
- 53.Oldstone M B. How viruses escape from cytotoxic T lymphocytes: molecular parameters and players. Virology. 1997;234:179–185. doi: 10.1006/viro.1997.8674. [DOI] [PubMed] [Google Scholar]
- 54.Peck R, Brown A, Wust C J. In vitro heterologous cytotoxicity by T effector cells from mice immunized with Sindbis virus. J Immunol. 1979;123:1763–1766. [PubMed] [Google Scholar]
- 55.Rammensee H G, Friede T, Stevanoviic S. MHC ligands and peptide motifs: first listing. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 56.Reeves W C, Hutson G A, Bellamy R E, Scrivani R P. Chronic latent infections of birds with western equine encephalomyelitis virus. Proc Soc Exp Biol Med. 1958;97:733–736. doi: 10.3181/00379727-97-23862. [DOI] [PubMed] [Google Scholar]
- 57.Rosen L, Gubler D J, Bennett P H. Epidemic polyarthritis (Ross River) virus infection in the Cook Islands. Am J Trop Med Hyg. 1981;30:1294–1302. doi: 10.4269/ajtmh.1981.30.1294. [DOI] [PubMed] [Google Scholar]
- 58.Rwaguma E B, Lutwama J J, Sempala S D, Kiwanuka N, Kamugisha J, Okware S, Bagambisa G, Lanciotti R, Roehrig J T, Gubler D J. Emergence of epidemic O’nyong-nyong fever in southwestern Uganda, after an absence of 35 years. Emerg Infect Dis. 1997;3:77. doi: 10.3201/eid0301.970112. . (Letter.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarmiento R E, Tirado R, Gomez B. Reinfection-induced increase of rubella persistently infected cells in a macrophage-like cell line. Virus Res. 1997;50:15–22. doi: 10.1016/s0168-1702(97)00048-8. [DOI] [PubMed] [Google Scholar]
- 60.Scalzo A A, Elliott S L, Cox J, Gardner J, Moss D J, Suhrbier A. Induction of protective cytotoxic T cells to murine cytomegalovirus by using a nonapeptide and a human-compatable adjuvant (Montanide ISA 720) J Virol. 1995;69:1306–1309. doi: 10.1128/jvi.69.2.1306-1309.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seay A R, Griffin D E, Johnson R T. Experimental viral polymyositis: age dependency and immune responses to Ross River virus infection in mice. Neurology. 1981;31:656–660. doi: 10.1212/wnl.31.6.656. [DOI] [PubMed] [Google Scholar]
- 62.Subak-Sharpe I, Dyson H, Fazakerley J. In vivo depletion of CD8+ T cells prevents lesions of demyelination in Semliki Forest virus infection. J Virol. 1993;67:7629–7633. doi: 10.1128/jvi.67.12.7629-7633.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sweet M J, Hume D A. RAW264 macrophages stably transfected with an HIV-1 LTR reporter gene provide a sensitive bioassay for analysis of signalling pathways in macrophages stimulated with lipopolysaccharide, TNF-alpha or taxol. J Inflamm. 1995;45:126–135. [PubMed] [Google Scholar]
- 64.Thomson S, Khanna R, Gardner J, Burrows S R, Coupar B, Moss D J, Suhrbier A. Minimal epitopes expressed in a recombinant polyepitope protein are processed and presented to CD8+ cytotoxic T cells: implications for vaccine design. Proc Natl Acad Sci USA. 1995;92:5845–5849. doi: 10.1073/pnas.92.13.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vene S, Franzen C, Niklasson B. Development of specific antibody patterns and clinical symptoms following Ockelbo virus infection. Arch Virol. 1994;134:61–71. doi: 10.1007/BF01379107. [DOI] [PubMed] [Google Scholar]
- 66.Verder H, Dickmeiss E, Haahr S, Kappelgaard E, Leerboy J, Moller-Larsen A, Nielsen H, Platz P, Koch C. Late-onset rubella syndrome: coexistence of immune complex disease and defective cytotoxic effector cell function. Clin Exp Immunol. 1986;63:367–375. [PMC free article] [PubMed] [Google Scholar]
- 67.Wakefield A J, Sim R, Akbar A N, Pounder R E, Dhillon A P. In situ immune responses in Crohn’s disease: a comparison with acute and persistent measles virus infection. J Med Virol. 1997;51:90–100. doi: 10.1002/(sici)1096-9071(199702)51:2<90::aid-jmv2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 68.Weaver S C, Salas R, Rico-Hesse R, Ludwig G V, Oberste M S, Boshell J, Tesh R B. Re-emergence of epidemic Venezuelan equine encephalomyelitis in South America. VEE Study Group. Lancet. 1996;348:436–440. doi: 10.1016/s0140-6736(96)02275-1. [DOI] [PubMed] [Google Scholar]
- 69.Westaway E G. Proteins specified by group B togaviruses in mammalian cells during productive infections. Virology. 1973;51:454–465. doi: 10.1016/0042-6822(73)90444-3. [DOI] [PubMed] [Google Scholar]
- 70.Yu S, Aaskov J G. Development of a candidate vaccine against Ross River virus infection. Vaccine. 1994;12:1118–1124. doi: 10.1016/0264-410x(94)90182-1. [DOI] [PubMed] [Google Scholar]