Abstract
Background:
Deficient sleep is implicated in nicotine dependence as well as depressive and anxiety disorders. The hypothalamus regulates the sleep-wake cycle and supports motivated behavior, and hypothalamic dysfunction may underpin comorbid nicotine dependence, depression and anxiety. We aimed to investigate whether and how the resting state functional connectivities (rsFCs) of the hypothalamus relate to cigarette smoking, deficient sleep, depression and anxiety.
Methods:
We used the data of 64 smokers and 198 age- and sex-matched adults who never smoked, curated from the Human Connectome Project. Deficient sleep and psychiatric problems were each assessed with Pittsburgh Sleep Quality Index (PSQI) and Achenbach Adult Self-Report. We processed the imaging data with published routines and evaluated the results at a corrected threshold, all with age, sex, and the severity of alcohol use as covariates.
Results:
Smokers vs. never smokers showed poorer sleep quality and greater severity of depression and anxiety. In smokers only, the total PSQI score, indicating more sleep deficits, was positively associated with hypothalamic rsFCs with the right inferior frontal/insula/superior temporal and postcentral (rPoCG) gyri. Stronger hypothalamus-rPoCG rsFCs were also associated with greater severity of depression and anxiety in smokers but not never smokers. Additionally, in smokers, the PSQI score completely mediated the relationships of hypothalamus-rPoCG rsFCs with depression and anxiety severity.
Conclusions:
These findings associate hypothalamic circuit dysfunction to sleep deficiency and severity of depression and anxiety symptoms in adults who smoke. Future studies may investigate the roles of the hypothalamic circuit in motivated behaviors to better characterize the inter-related neural markers of smoking, deficient sleep, depression and anxiety.
Keywords: Smoking, fMRI, Hypothalamic functional connectivity, Deficient sleep, Depression, Anxiety
1. Introduction
Individuals who smoke are at greater risk of experiencing deficient sleep (Brook et al., 2015; Cohrs et al., 2014; Kashyap et al., 2001), depression, anxiety, fatigue, and concentration difficulties (Hahad et al., 2022; Patten et al., 2000). Numerous studies have investigated the neural correlates of deficient sleep (Bai et al., 2022; Fjell et al., 2021; Liu et al., 2019; Ma et al., 2020). For instance, poorer sleep quality was related to faster thinning of the right lateral temporal cortex in a longitudinal study of aging (Fjell et al., 2021). Higher functional connectivity within the somatomotor cortices was associated with poorer sleep quality in young males (Bai et al., 2022). Importantly, deficient sleep reduced smoking cessation success and predicted relapse (Fucito et al., 2014; Peters et al., 2011), indicating the importance of understanding the neural mechanisms of deficient sleep in people who smoke.
The hypothalamus regulates the sleep-wake cycle and body clock (Mignot et al., 2002; Szymusiak et al., 2007). Studies have characterized changes in hypothalamic resting state functional connectivities (rsFCs) in relation to sleep-wake transition (Boes et al., 2018; Jiang et al., 2021) and to sleep deficiency and deprivation (Ding et al., 2021; Krause et al., 2017; Qi et al., 2021). For instance, patients with insomnia disorder vs. healthy controls showed stronger hypothalamic rsFCs with bilateral medial frontal cortex and left pallidum and weaker rsFCs with right inferior temporal cortex (Ding et al., 2021). Healthy adult males showed elevated hypothalamic rsFCs with bilateral thalamus and anterior cingulate cortex, right amygdala, and insula and reduced rsFCs with the middle frontal gyrus after 36 h of sleep deprivation, as compared to rested wakefulness (Qi et al., 2021).
Interacting with the striatum, thalamus, midbrain and brainstem nuclei, orbitofrontal cortex, cingulum, and temporal cortices, the hypothalamus responds to stress (Bains et al., 2015; Bao et al., 2008) and supports motivated behaviors (Kullmann et al., 2014; Le et al., 2020). Hypothalamic dysfunction has been linked to smoking (Rohleder and Kirschbaum, 2006) and mental conditions including depression and anxiety (Bao and Swaab, 2019; Tanaka et al., 2000), which are frequently comorbid with deficient sleep. For example, an earlier study provided evidence for the roles of the cholinergic neurons and nicotinic receptors within the hypothalamus in manifesting sleep and emotional symptoms resulting from chronic stress (Balkan and Pogun, 2018). Cigarette smoking attenuated hypothalamic–pituitary–adrenal axis responses to psychological stress (al’Absi, 2006; Rohleder and Kirschbaum, 2006). Individuals with depression and anxiety demonstrated altered hypothalamic–pituitary–adrenal responses to stress (Frankiensztajn et al., 2020). Dysfunction of the hypothalamic-pituitary-thyroid axis has likewise been associated with anxiety, with blunted thyroid-stimulating hormone responses to thyrotropin-releasing hormone in those self-reporting higher levels of anxiety (Fischer and Ehlert, 2018). In mice, environmental stressors elicited acute and prolonged activities in glutamatergic neurons of the medial preoptic area of the hypothalamus, which mediated the expression of anxiety-like behaviors (Zhang et al., 2021a). Other animal studies implicated the hypothalamic circuits in stress-induced physical problems, including bone loss, elevated adiposity and inflammation, and diminished immune responses (Turner et al., 2020; Yang et al., 2020).
However, it remains unknown whether or how hypothalamic connectivities are altered in relation to deficient sleep, depression and anxiety in people who smoke (Hilbert et al., 2014; Wang et al., 2019). Understanding the links among hypothalamic circuit dysfunction, deficient sleep, depression and anxiety may help unraveling the etiological processes of the comorbidities. Using the data curated from the Human Connectome Project (HCP), we examined how hypothalamic rsFCs were associated with deficient sleep in two groups of participants – adults who smoked and who never smoked. We hypothesized that smokers but not never smokers would show altered hypothalamic rsFCs in association with deficient sleep as well as higher severity of depression and anxiety symptoms. We further performed mediation analyses to characterize the inter-relationships among sleep deficits, hypothalamic rsFCs, depression and anxiety.
2. Methods
2.1. Dataset subjects and assessments
In the S1200 release (see https://www.humanconnectome.org/study/hcp-young-adult/document/1200-subjects-data-release for details), the HCP contains a total of 330 participants reported lifetime history of smoking – smoking cigarettes on at least 100 separate occasions, without severe neurodevelopmental, neuropsychiatric, or neurologic disorders. Nicotine use disorder severity was assessed with the Fagerström Test for Nicotine Dependence (FTND), with a score ranging from 0 to 10 and a higher score indicating a more severe disorder (Heatherton et al., 1991). In the HCP, FTND scores >6 were re-coded as 6 (n = 26). The current study included 83 people who smoked and reported an FTND score >3 (indicative of dependence; de Meneses-Gaya et al., 2009). However, 19 of the 83 individuals were excluded from further analyses because of questionable image quality or poor image segmentation (n = 10), missing cardiac or respiratory data (n = 3), or excessive head movements during resting state functional MRI (rsfMRI) scans (n = 6; see details in “2.2 MRI data acquisition and preprocessing”). Therefore, the smoker group comprised 64 participants (28 women). A total of 635 participants reported never smoking, of whom 464 completed MR scans (both T1 and rsfMRI) and clinical assessments. One-hundred-and-ten never smokers were excluded because of missing cardiac or respiratory data (n = 20) or excessive head movements during rsfMRI scans (n = 90). We selected the largest possible number of adults from the remaining 354 never smokers that matched smokers in mean age and sex composition (Table 1). The final sample size of never smokers was 198 (74 women).
Table 1.
Demographic and clinical measures and statistics of ANOVA/t-test.
| Smokers |
Never smokers |
F values |
|||||
|---|---|---|---|---|---|---|---|
| Men (n = 36) | Women (n = 28) | Men (n = 124) | Women (n = 74) | G × S | G main | S main | |
| Age | 29.7 ± 2.8 | 30.4 ± 3.1 | ± 3.5 | 31.0 ± 2.5 | 3.79 | 0.52 | 12.6 |
| PC1 | 1.0 ± 0.9 | −0.03 ± 0.9 | 0.003 ± 1.0 | −0.5 ± 0.7 | 4.72 | 34.3 | 36.9 |
| FTND | 4.6 ± 0.8 | 5.1 ± 0.9 | / | / | / | / | a2.18 |
| Illic | 2.1 ± 1.9 | 1.5 ± 1.6 | 0.3 ± 0.8 | 0.2 ± 0.7 | 1.03 | 51.3 | 0.01 |
| Marij | 3.8 ± 1.5 | 2.5 ± 1.8 | 0.9 ± 1.3 | 0.8 ± 1.3 | 5.66 | 80.7 | 1.46 |
| PSQI | 5.0 ± 2.9 | 6.2 ± 4.3 | 4.2 ± 2.6 | 4.6 ± 2.9 | 1.50 | 4.01 | 3.64 |
| Depr | 5.5 ± 4.0 | 4.7 ± 4.6 | 3.5 ± 3.4 | 3.7 ± 3.6 | 0.87 | 7.75 | 0.86 |
| Anxi | 5.0 ± 3.2 | 4.7 ± 3.2 | 3.1 ± 2.4 | 3.6 ± 3.0 | 0.71 | 12.6 | 0.002 |
Note: Values are mean ± SD. F values reflect the interaction and main effects of between-subject ANOVA.
Independent-sample t value is presented for FTND in smokers only. G: group; S: sex; PC1: first principal component of drinking metrics; FTND: Fagerström Test for Nicotine Dependence; Illic: times of non-marijuana illicit drug use, including cocaine, hallucinogens, opiates, sedatives, or other; Marij: times of marijuana use; PSQI: Pittsburgh Sleep Quality Index; Depr: Depressive Problems; Anxi: Anxiety Problems. Bold F values represent significant effects at p < 0.05/28 = 0.0018 (correction for multiple comparisons).
Participants were assessed with the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA), an instrument designed to evaluate physical, psychological, and social manifestations of alcoholism and related disorders (Bucholz et al., 1994). As in our previous study (Li et al., 2022), we performed a principal component analysis on the 15 interrelated drinking metrics and used the first principal component (PC1) with an eigenvalue >7 that accounted for 49.75% of the variance to represent the severity of alcohol use. Smokers vs. never smokers showed significantly higher PC1 values (Table 1), suggesting more severe drinking. Thus, to control for the effects of alcohol use, we included drinking PC1 as a covariate in all analyses. Participants also reported how many times they had used marijuana (never used = 0; 1–5 = 1; 6–10 = 2; 11–25 = 3; 26–50 = 3; 51–100 = 3; 101–999 = 4; and >1000 = 5) and non-marijuana illicit drugs, including cocaine, hallucinogens, opiates, sedatives, or others [never used = 0; 1–2 times = 1; 3–10 times = 2; 11–25 times = 3; 26–100 times = 4 (for males); >25 times = 4 (for females); and >100 times = 5 (for males only)]. The sex-specific scoring for drug consumption aligned with evidence suggesting that women compared to men tend to use illicit drugs at lower doses and frequencies (Becker and Hu, 2008; Roth et al., 2004).
Participants also completed the Achenbach Adult Self Report (ASR; Achenbach and Rescorla, 2003). We used ASR raw scores in our analyses. The ASR includes 8 syndromal scales (Anxious/Depressed Syndromes; Withdrawn Syndromes; Somatic Complaints; Thought Problems; Attention Problems; Aggressive Behaviors; Rule-breaking Behaviors; and Intrusive Problems) and 6 DSM-oriented scales (Depressive; Anxiety; Somatic; Avoidant Personality; Attention Deficit/Hyperactivity; and Antisocial Personality Problems), with each scored: 0-Not True, 1-Somewhat or Sometimes True, and 2-Very True or Often True. In addition to these item scores, ASR provided 8 additional raw scores: Inattention and Hyperactivity/Impulsivity Subscales, Other Problems, Critical Items, Internalizing Problems (Anxious/Depressed Syndromes, Withdrawn Syndromes, and Somatic Complaints), Externalizing Problems (Aggressive Behaviors, Rule-breaking Behaviors, and Intrusive Problems), TAO sum (Thought Problems, Attention Problems, and Other Problems), and Total Problems (all problems). Thus, there were a total of 22 ASR measures, with higher scores indicating greater severity of symptoms. We focused on depression and anxiety in the current analyses; however, to facilitate future studies, we conducted the analyses for all ASR measures and included the results in the Supplement. The ASR also provided T scores adjusted for age and sex, with T > 69 indicating a clinical diagnosis. In our sample, 4.7%/3.5% and 7.8%/2.5% of smokers/never-smokers met the criteria for depression and anxiety disorders, respectively.
Participants were assessed with the Pittsburgh Sleep Quality Index (PSQI), to evaluate sleep quality and pattern, including subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction (Buysse et al., 1989). Each PSQI question was rated from 0 = no difficulty to 3 = severe difficulty, with the total score ranging from 0 to 21 and a score >5 indicating clinically significant sleep deficiency (Chiu and Hsu, 2016). Approximately 37.5% smokers and 23.7% never smokers had a PSQI total score >5.
2.2. MRI data acquisition and preprocessing
All imaging data were acquired on a customized Siemens 3T Skyra with a standard 32-channel Siemens receiver head coil and a body transmission coil. T1-weighted high-resolution structural images were acquired using a 3D MPRAGE sequence with 0.7 mm isotropic resolution (FOV = 224 mm, matrix = 320, 256 sagittal slices, TR = 2400 ms, TE = 2.14 ms, TI = 1000 ms, FA = 8°) and were used to register resting state fMRI (rsfMRI) data to a standard brain space. The rsfMRI data were collected in two sessions, using gradient-echo echo-planar imaging (EPI) with 2.0 mm isotropic resolution (FOV = 208 × 180 mm, matrix = 104 × 90, 72 slices, TR = 720 ms, TE = 33.1 ms, FA = 52°, multi-band factor = 8). Physiological data (i.e., cardiac and respiratory signals) associated with each fMRI scan were also acquired, using a standard Siemens pulse oximeter placed on a digit and a respiratory belt placed on the abdomen. These physiological signals were sampled equally at 400 Hz (~288 samples per frame). More details of the data collection procedures can be found in the HCP S1200 Release Reference Manual. Within each session, oblique axial acquisitions alternated between phase encoding in a right-to-left direction in one run and phase encoding in a left-to-right direction in the other run. Each run lasted 14.4 min (1200 frames).
We preprocessed the first session (two runs: left-to-right and right-to-left) of rsfMRI data with SPM8 as described earlier (Chen et al., 2022a; Chen and Li, 2023). Images of each participant were first realigned (motion corrected), and a mean functional image volume was constructed from the realigned image volumes. These mean images were co-registered with the high-resolution structural MPRAGE image and then segmented for normalization with affine registration followed by nonlinear transformation. The normalization parameters determined for the structural volume were then applied to the corresponding functional image volumes for each participant. Afterwards, the images were smoothed with a Gaussian kernel of 4 mm at Full Width at Half Maximum. White matter and cerebrospinal fluid signals, whole-brain mean signal, and physiological signals were regressed out to reduce spurious BOLD variances and to eliminate cardiac-and respiratory-related artifacts. A temporal band-pass filter (0.009 Hz <f< 0.08 Hz) was also applied to the time course to obtain low-frequency fluctuations, as in our prior work (Zhang and Li, 2018).
Lastly, to further eliminate global motion-related artifacts, a “scrubbing” method was applied. Specifically, frame-wise displacement given by was computed for every time point t, where and are the translational and rotational movements, respectively (Power et al., 2012). Moreover, the root mean square variance of the differences (DVARS) in % BOLD intensity I(t) between consecutive time points across brain voxels, was computed as: , where the brackets indicate the mean across brain voxels. Following previous HCP studies (Chen et al., 2021b; Li et al., 2019), we marked volumes with FD >0.2 mm or DVARS >75 as well as one frame before and two frames after these volumes as outliers (censored frames). Un-censored segments of data lasting fewer than five contiguous volumes were also labeled as censored (Li et al., 2019). A total of 6 smokers and 90 never smokers who had both BOLD runs with more than half of the frames scrubbed were removed from further analyses.
2.3. Whole-brain hypothalamic rsFCs
The mask of the hypothalamus was obtained from the Automated Anatomic Labeling atlas (Tzourio-Mazoyer et al., 2002) and used as the seed region (Fig. 2). We computed the whole-brain hypothalamic rsFCs by averaging the BOLD time courses of each voxel and estimating the correlation coefficient between the average time course of all voxels of the seed and the time courses of all other voxels of the brain for individual participants. The correlation matrix was further Fisher’s z-transformed into z score maps.
Fig. 2.
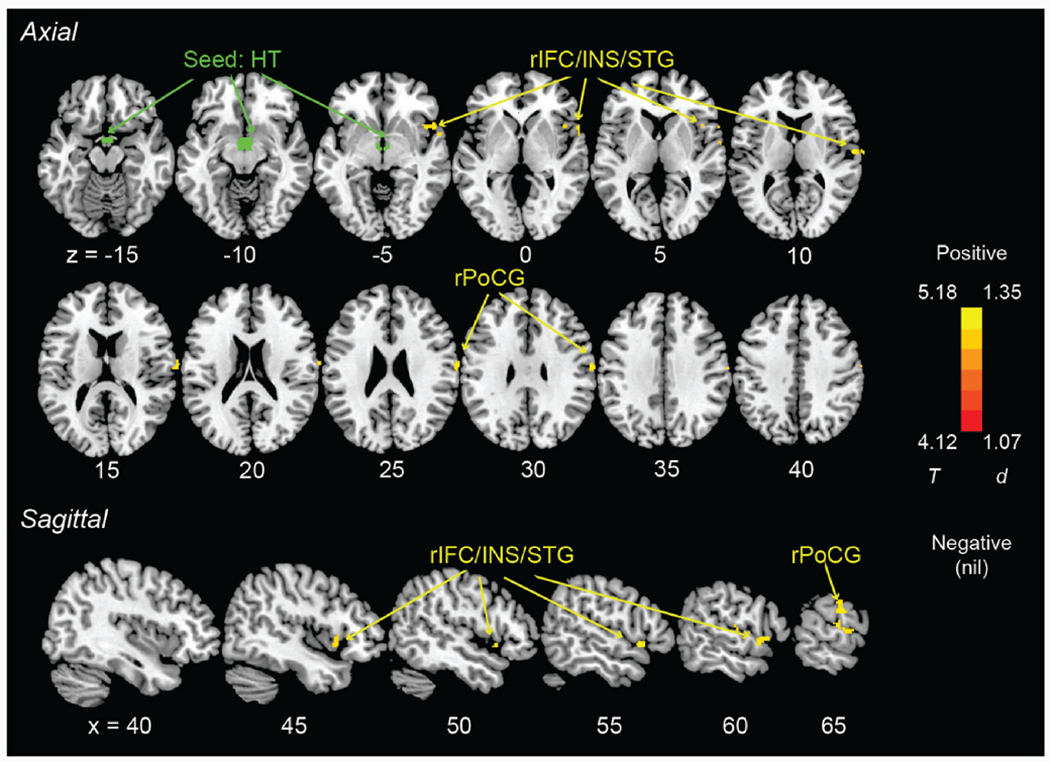
Seed of hypothalamus (green) and axial and sagittal views of clusters (warm color) showing hypothalamic rsFCs in positive correlation with PSQI score in smokers only (evaluated at voxel p < 0.001, uncorrected, in combination with a cluster p < 0.05, corrected for family-wise error), with age, sex, and drinking PC1 as covariates. Color bar shows T value and Cohen’s d. r: right; IFC: inferior frontal cortex; INS: insula; STG: superior temporal gyrus; PoCG: postcentral gyrus. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.4. Group data analyses
2.4.1. Demographic and clinical measures
We performed a group (smokers vs. never smokers) × sex (men vs. women) ANOVA on demographic and clinical measures. Drinking PC1 showed significant main and interaction effects (see Results). Women were older than men across groups (F = 12.6, p < 0.001). Thus, for all subsequent analyses, we included age and drinking PC1 (and sex) as covariates. With these covariates, we performed group × sex ANOVAs on the PSQI score and each of the 22 ASR measures and evaluated the results at a corrected p < 0.05/23 = 0.00217. We computed the coefficients of partial correlations between PSQI score and each of the 22 ASR measures with age, sex, and drinking PC1 as covariates, in smokers and never smokers separately. For the partial correlations which were significant at a corrected p < 0.05/(22 × 2) = 0.00114 for either group, we compared the correlations between the two groups with slope t tests, again with age, sex, and drinking PC1 as covariates.
2.4.2. Whole-brain analyses
We first performed a whole-brain group × sex factorial analysis, with age and drinking PC1 as covariates. The interaction term did not yield significant clusters; therefore, we combined men and women and performed all the following analyses with sex as an additional covariate. We performed whole-brain one-sample t tests for smokers and never smokers separately and a two-sample t-test (smokers vs. never smokers) on the hypothalamic rsFCs. Finally, we performed whole-brain regressions on the hypothalamic rsFCs against the PSQI score in smokers and never smokers separately, with age, sex, and drinking PC1 as covariates. All tests were evaluated at voxel p < 0.001, uncorrected, in combination with a cluster p < 0.05, corrected for family-wise error (FWE), on the basis of Gaussian random field theory, as implemented in SPM, following current reporting standards (Poldrack et al., 2008). At this threshold, one-sample t tests yielded large, continuous clusters, and thus we also evaluated one-sample t tests at voxel p < 0.05 FWE-corrected. In addition to reporting the peak voxel Z value, we computed the effect size by approximating Cohen’s d from the t-statistics, t value and degrees of freedom (df), using the expression (Howell, 2013).
2.4.3. Region-of-interest (ROI) analyses
For the ROIs identified from whole-brain regressions against the PSQI score in either smokers or never smokers, we computed the β estimates of the hypothalamic rsFCs for correlation with the PSQI score and 22 ASR measures in smokers and never smokers separately, with age, sex, and drinking PC1 as covariates. We evaluated the results at a corrected of p < 0.05/23 = 0.0022. For the correlations that were significant for either group, we also performed slope t tests to examine group differences in the correlations as in our prior work (Chen et al., 2022c; Chen and Li, 2022; Zar, 1999): where is the standard error of the difference between the regression coefficients β1 and β2. Note that the slope tests did not represent “double dipping.” We used a corrected threshold in identifying the clusters from whole-brain regressions, and those clusters that met the threshold in, for example, smokers, might have just missed the threshold in never smokers, and vice versa. Thus, slope tests were needed to confirm group differences.
2.4.4. Mediation analyses
We performed mediation analyses to examine the inter-relationships between hypothalamic rsFCs, PSQI scores, and ASR measures in smokers (no hypothalamic rsFCs were correlated with PSQI scores in never smokers; see Results). Further, given that hypothalamus-rIFC/INS/STG rsFCs were not significantly correlated with any ASR measures at the corrected p < 0.00217 (see Results), we considered only hypothalamus-rPoCG rsFC in the mediation analyses. Likewise, the ASR measures in the mediation analyses were those showing significant correlations with both hypothalamus-rPoCG rsFC and PSQI score at corrected p’s, including scores of somatic complaints, critical items, internalizing problems, total problems, depressive problems, and anxiety problems (see Results and Supplementary Results).
As with our prior work (Chen et al., 2022b), in a mediation analysis, the relation between the independent variable X and dependent variable Y, i.e. X→Y, is tested to see if the relation is significantly mediated by a variable M. The mediation test is performed by employing three regression equations:
where a represents X→M, b represents M→Y (controlling for X), c’ represents X→Y (controlling for M), and c represents X→Y. The constants i1, i2, 13 are the intercepts, and e1, e2, e3 are the residual errors. In the literature, a, b, c and c’ were referred as path coefficients or simply paths, and we followed this notation. Variable M is a mediator of the correlation X→Y if (c –c’), which is mathematically equivalent to the product of the paths a*b, is significantly different from zero (MacKinnon et al., 2007). Sobel tests were used to determine significance of mediation models (Me, 1982). If the product a*b and the paths a and b are significant, one concludes that X→Y is mediated by M. In addition, if path c’ is not significant, there is no direct connection from X to Y and that X→Y is completely mediated by M. Note that path b is the relation between Y and M, controlling for X, and should not be confused with the correlation coefficient between Y and M.
3. Results
3.1. Demographic and clinical measures
The statistics of ANOVA and t tests of age, drinking PC1, and scores of FTND, PSQI, and depression and anxiety problems are shown in Table 1. A Chi-square test of independence showed that the sex distribution did not differ between smokers and never smokers (χ2 = 0.83, p = 0.363). In group × sex ANOVAs, the group-by-sex interaction was significant for drinking PC1 (F = 4.72, p = 0.031), but not for age (F = 3.79, p = 0.053). Simple-effect analyses showed that men’s PC1 were significantly higher than women in both smokers and never smokers (p’s < 0.001); smokers vs. never smokers showed higher PC1 in both men (p < 0.001) and women (p = 0.016). In addition, female smokers showed higher FTND scores than male smokers (5.1 ± 0.9 vs. 4.6 ± 0.8, t = 2.18, p = 0.033).
With age and drinking PC1 as covariates, the group × sex interaction was not significant for illicit drug use, PSQI, depression, anxiety problems, or any other ASR measures (F’s ≤ 1.50, p’s ≥ 0.222), but was significant for marijuana use (F = 5.66, p = 0.018). In simple effects analyses, male smokers used significantly more times of marijuana as compared to female smokers (p = 0.039), but the sex difference in never smokers was not significant (p = 0.303). For the main effects, smokers showed significantly more times of illicit drug use (F = 51.26, p < 0.001), higher PSQI score (F = 4.01, p = 0.046), greater severity of depression (F = 7.75, p = 0.006) and anxiety (F = 12.61, p < 0.001) problems. Men vs. women did not show significant differences in illicit drug use, PSQI score, depression, or anxiety symptom severity (F’s ≤ 3.64, p’s ≥ 0.058). The statistics of group and sex main effects for all other ASR measures are shown in Supplementary Table S1.
Partial correlations between PSQI score and ASR measures with age, sex, and drinking PC1 as covariates in smokers and never smokers separately are shown in Supplementary Table S2. In smokers, PSQI scores were significantly correlated with scores of somatic complaints, thought problems, critical items, internalizing problems, total problems, depressive problems, anxiety problems, and somatic problems (r’s ≥ 0.42, p’s ≤ 0.00081). In never smokers, PSQI scores were significantly correlated with most ASR measures (r’s ≥ 0.26, p’s ≤ 0.00022) except for intrusive problems, avoidant personality problems, and inattention problems (r’s ≤ 0.22, p’s > 0.00225). Slope tests did not show significant differences in any of the correlations between the two groups (t’s ≤ 1.23, p’s ≥ 0.2185). For both groups, the most significant correlations were those between PSQI score and somatic complaints and depression problems.
3.2. Hypothalamic rsFCs: whole-brain analyses
The results of whole-brain one-sample t tests are shown for smokers and never smokers in Fig. 1A and B. respectively. The clusters are summarized in Supplementary Table S3. In smokers, we found positive hypothalamic rsFCs with bilateral orbitofrontal cortex, superior frontal gyri, anterior cingulate cortex, insula, middle temporal gyri, hippocampus, amygdala, and caudate, and negative rsFCs with bilateral superior and inferior parietal gyri, superior occipital gyri, supplementary motor areas, and left thalamus. In never smokers, the hypothalamus showed more extensive connectivities encompassing the frontal, parietal, temporal, and occipital cortices, insula, cerebellum, and subcortical regions. However, with age, sex, and drinking PC1 as covariates, a whole-brain t-test of smokers vs. never smokers did not show significant differences in hypothalamic rsFCs.
Fig. 1.
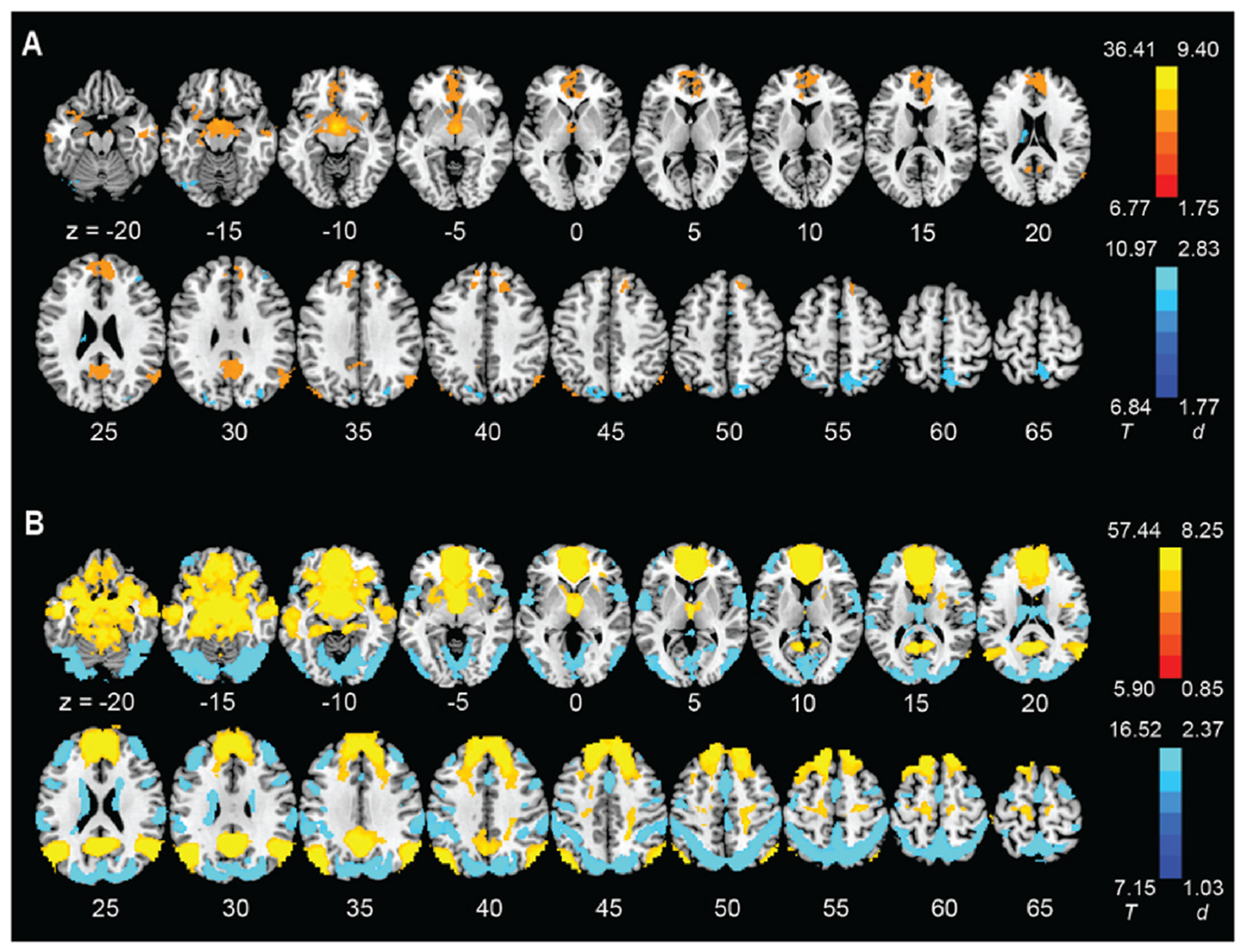
Whole-brain resting state connectivity of the hypothalamus (one-sample t-test, evaluated at voxel p < 0.05 corrected for family-wise error) in (A) smokers and (B) never smokers. Color bars show voxel T value and Cohen’s d. Warm/cool color: positive/negative resting state functional connectivity. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
With the same covariates, we performed whole-brain regressions of the hypothalamic rsFCs against the PSQI score in smokers and never smokers separately. We identified a cluster (size k = 86, MNI coordinates x = 54, y = 16, z = −4, peak Z = 4.28) encompassing the right inferior frontal cortex, insula, and superior temporal gyrus (rIFC/INS/STG) and a cluster (size k = 160, MNI coordinates x = 62, y = −12, z = 28, peak Z = 4.45) in the right postcentral gyrus (rPoCG) showing hypothalamic rsFCs in positive correlation with PSQI score in smokers (Fig. 2). We did not find any hypothalamic rsFCs in significant correlation with PSQI score in never smokers. In order to control for the effects of drug use history, we performed the whole-brain regression of the hypothalamic rsFCs against PSQI in smokers, with non-marijuana illicit drug use and marijuana use as additional covariates. The results remained largely the same (Supplementary Fig. S1).
3.3. Correlations of hypothalamic rsFCs with clinical characteristics: ROI analyses
The partial correlations of hypothalamic rsFCs with the PSQI score and all ASR measures for smokers and never smokers separately and results of slope tests are shown in Supplementary Table S4. The p value was set at 0.05/(23 × 4) = 0.00054348, to correct for multiple comparisons. The PSQI score was strongly correlated with hypothalamus-rIFC/INS/STG rsFCs (r = 0.54, p = 0.000007) and hypothalamus-rPoCG rsFC (r = 0.54, p = 5.5948E-8) in smokers, as expected, but not in never smokers (r = 0.12, p = 0.110 and r = −0.05, p = 0.478). The scores of Depression and Anxiety Problems were significantly correlated with hypothalamus-rPoCG rsFC (Fig. 3) but not with hypothalamus-rIFC/INS/STG rsFC (Fig. 4) in smokers, at the corrected threshold, and none of these correlations were significant in never smokers. Slope tests showed that these correlations were significantly stronger in smokers than in never smokers (t’s ≥ 2.98, p’s ≤ 0.0032). FTND scores were not significantly correlated with either hypothalamus-rIFC/INS/STG rsFC (r = −0.04, p = 0.744) or hypothalamus-rPoCG rsFC (r = −0.02, p = 0.907) in smokers.
Fig. 3.
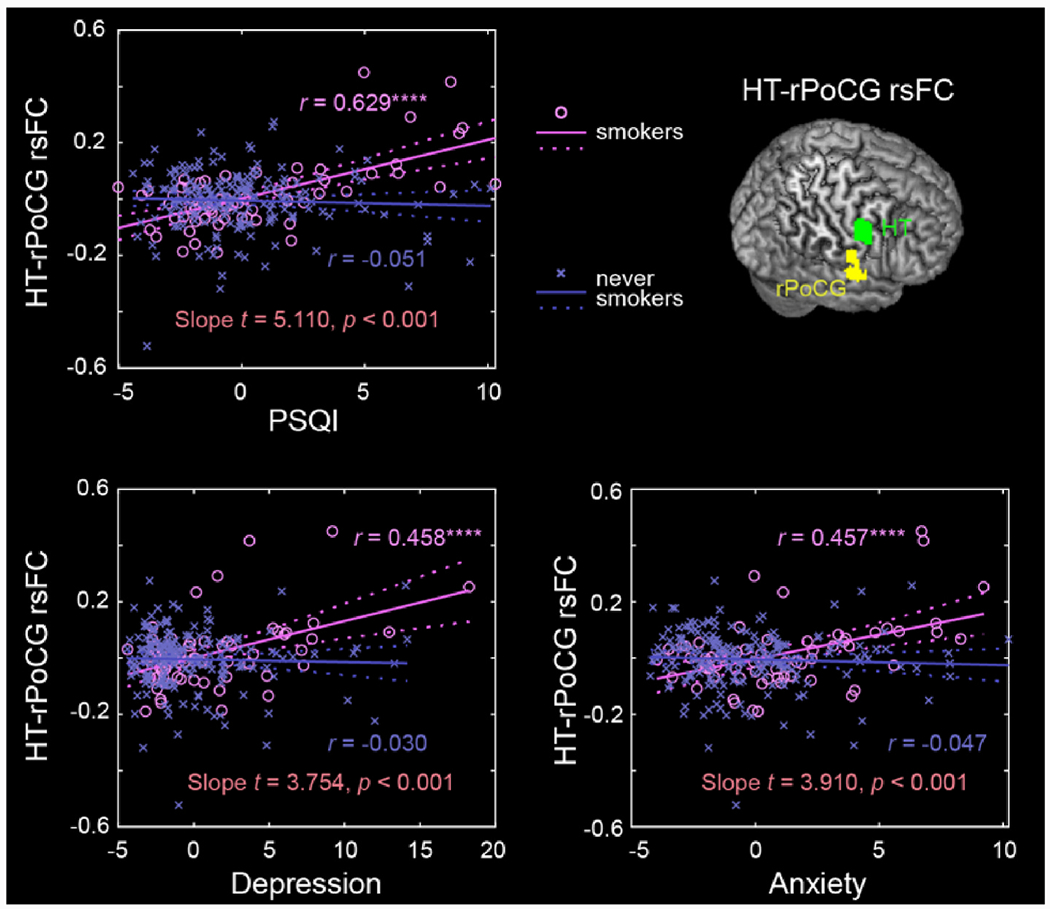
Scatter plots to show the correlations of hypothalamus-rPoCG rsFC with PSQI, Depression and Anxiety Problems, with age, sex, and drinking PC1 as covariates for smokers and never smokers separately. Residuals are presented here. Solid lines represent the regressions. The r values are marked with ****p < 0.05/(23 × 2 × 2) = 0.00054 (correction for multiple comparisons). See Supplementary Table S3 for details.
Fig. 4.
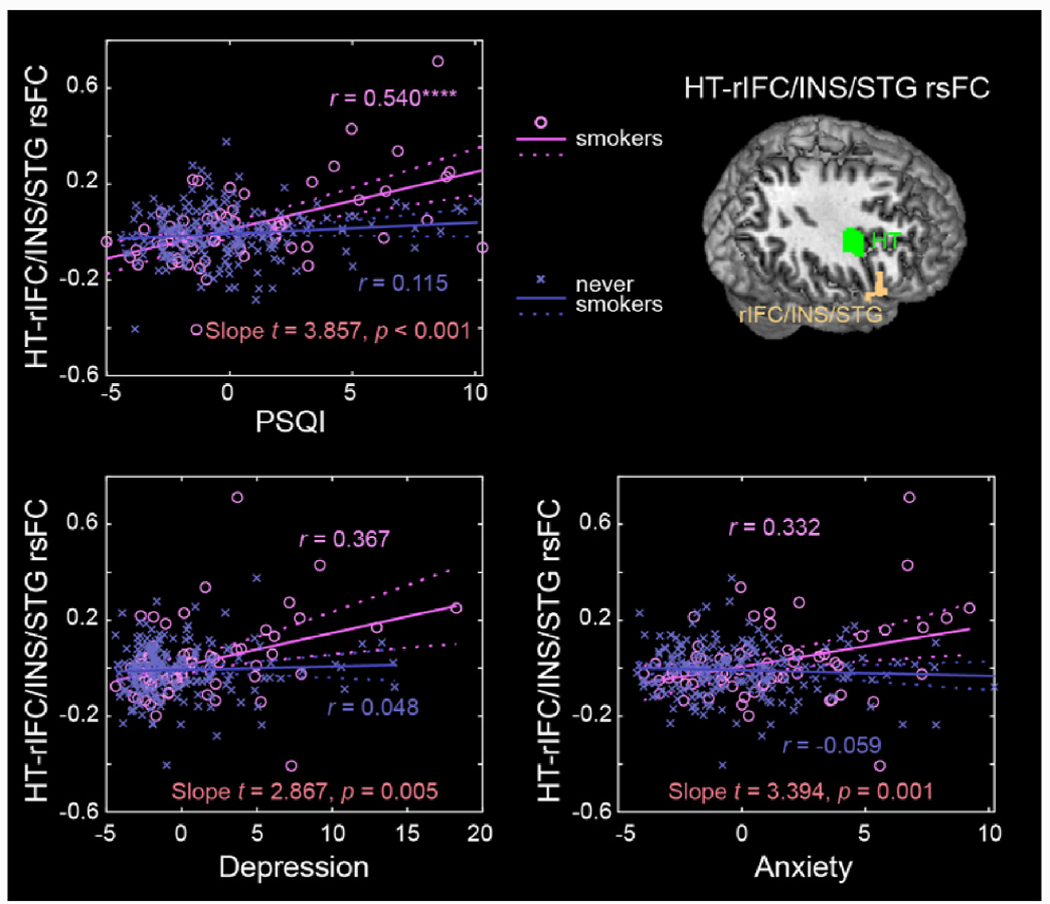
Scatter plots to show the correlations of hypothalamus-rIFC/INS/STG rsFC with PSQI, Depression and Anxiety Problems, with age, sex, and drinking PC1 as covariates for smokers and never smokers separately. Residuals are presented here. Solid lines represent the regressions. The r values are marked with ****p < 0.05/(23 × 2 × 2) = 0.00054 (correction for multiple comparisons). See Supplementary Table S3 for details.
3.4. Mediation models
As shown in Fig. 5, PSQI score completely mediated the pathways from hypothalamus-rPoCG rsFC to depression and anxiety in smokers at a corrected threshold. Besides, the hypothalamus-rPoCG rsFC fully mediated the pathway from anxiety to PSQI score. The statistics of all 36 mediation models are summarized in Supplementary Table S5.
Fig. 5.
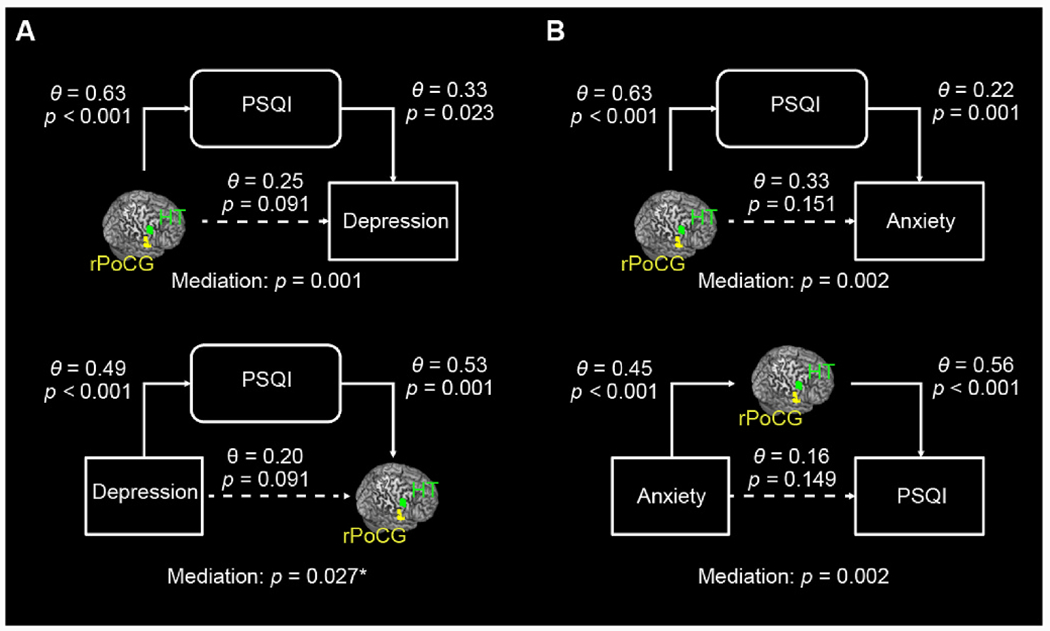
Full mediation models for (A) Hypothalamus-rPoCG rsFC, PSQI, and Depression Problems, and (B) Hypothalamus-rPoCG rsFC, PSQI, and Anxiety Problems in smokers. For each, the mediation effects were assessed for all six models and evaluated at a corrected threshold p = 0.05/6 = 0.0083. The Θ values are standardized coefficients. The mediation p values are for the path “a × b” (see Methods). *Not significant at the corrected threshold. The statistics of all mediation models are summarized in Supplementary Table S4.
4. Discussion
We presented, to the best of our knowledge, the first evidence of altered hypothalamic connectivities associated with deficient sleep in people who smoke. Stronger rsFCs of hypothalamus-rIFC/INS/STG and hypothalamus-rPoCG were associated with poorer sleep quality in smokers but not never smokers. Also confirmed by slope tests, higher hypothalamus-rPoCG rsFC was correlated with more depression and anxiety problems in smokers but not never smokers. Deficient sleep completely mediated the relationships of hypothalamus-rPoCG rsFC with depression and anxiety problems unidirectionally. We discuss the main findings below.
4.1. Hypothalamic connectivities and deficient sleep in smokers
Although we did not identify significant group differences in hypothalamic connectivities, we observed that the hypothalamus was broadly connected with the frontoparietal and default mode networks as well as limbic structures in both smokers and never smokers, indicating a key role of the hypothalamus in regulating cognition, emotion, and motivation (Pop et al., 2018). Never smokers showed more robust and widespread hypothalamic connectivity, potentially because of the larger sample size of this group.
Smokers relative to never smokers showed more sleep, depression, and anxiety problems, in accord with prior findings (Jaehne et al., 2009, 2012). We observed higher hypothalamus-rIFC/INS/STG rsFCs in positive correlation with the PSQI score, suggesting vulnerability in hypothalamic-fronto/insular/temporal cortical connectivities to deficient sleep, in smokers. The right IFC, supporting response inhibition and attentional control (Chao et al., 2009; Forstmann et al., 2008; Hampshire et al., 2010; Li et al., 2006), has been linked to deficient sleep. Lower gray matter volumes of bilateral IFC were associated with greater sleep fragmentation in old adults (Lim et al., 2016). Relative to healthy controls, patients with obstructive sleep apnea showed markedly higher amplitude of low-frequency fluctuation in the right IFC, insula, cingulate and paracingulate gyri, and left superior frontal gyrus (Kang et al., 2020). Moreover, sleep deprivation was associated with deficits in bilateral IFC activation in relation to inhibitory control (Zhao et al., 2019). Our findings of hypothalamus-rIFC hyperconnectivity associated with worse PSQI score may reflect altered neural processes, potentially in functional compensation, for vigilance and attention control in smokers.
The insula, as a key structure of the interoceptive network and with reciprocal anatomical connections with the hypothalamus (Allen et al., 1991), has been implicated in regulating sleep and wakefulness (Elvsashagen et al., 2019; Krause et al., 2017). Smaller gray matter volumes of the right insula were noted in individuals with more deficient sleep (Sung et al., 2020). In an animal study, as compared to controls, rats with lesions in the anterior insula demonstrated lower wakefulness and more frequent rapid eye movement sleep and non-rapid eye movement sleep (Chen et al., 2016). It is less clear about why right STG dysfunction manifests in deficient sleep. Patients with obstructive sleep apnea vs. health controls showed significantly higher rsFCs between the right hippocampus and STG (Zhou et al., 2020). A meta-analysis demonstrated higher STG responses to psychosocial stress (Kogler et al., 2015). More studies are needed to investigate the roles of the STG potentially in relation to memory and emotional dysfunction in deficient sleep.
4.2. Hypothalamus-rPoCG rsFC, deficient sleep, depression and anxiety
Smokers also showed higher hypothalamus-rPoCG rsFC in relation to higher PSQI score. Sleep deprivation resulted in regional activity changes in the PoCG, the primary somatosensory cortex that processes body sensations (Chen et al., 2018). Previous studies have reported both volumetric and functional PoCG markers of deficient sleep (Bai et al., 2022; Chen et al., 2016; Joo et al., 2013; Sung et al., 2020). For instance, lower gray matter volumes of the PoCG were related to the severity of deficient sleep in otherwise-healthy adolescents (Sung et al., 2020) and adult patients with insomnia (Joo et al., 2013). Enhanced functional connectivities within the somatosensory network were associated with poorer sleep quality (Bai et al., 2022). A recent within-subject study showed significantly higher regional homogeneity of rPoCG following 36 h of sleep deprivation, as compared to a state without sleep deprivation (Chen et al., 2023). According to a model of insomnia (Riemann et al., 2010), higher connectivity between the hypothalamus and rPoCG in smokers may reflect a hyperarousal state, whereby excessive somatosensory information processing leads to deficient sleep (Scammell et al., 2017).
We found that the PSQI sleep score fully mediated the relationships between hypothalamus-rPoCG rsFC and depression and anxiety problems, suggesting that changes in hypothalamic-somatosensory network function may impact sleep quality and sequentially lead to depression and anxiety, in smokers. We also found the mediating roles of hypothalamus-rPoCG rsFC in the relationship between anxiety and deficient sleep. Although we highlighted the findings of mediation analyses for depression and anxiety, across the ASR measures that showed a significant correlation with both total PSQI scores and hypothalamus-rPoCG rsFC, the models of hypothalamus-somatosensory cortical connectivity → sleep disturbance → clinical comorbidities showed significant and complete mediation in most cases (Supplementary Table S4). Note that the findings of mediation analyses do not suggest causality but rather highlight an important role of deficient sleep in inter-relating brain functional changes and emotional symptoms in adults who smoke, as has also been demonstrated for alcohol use disorders (Zhang et al., 2021b). These findings suggest that treatments to improve deficient sleep may benefit individuals with nicotine and potentially other substance use disorders.
4.3. Limitations and conclusions
We consider some limitations for the current study. First, as described in the Methods and noted too in our prior study (Chen et al., 2021a), the HCP comprised only 83 smokers with an FTND score >3 and those with FTND scores >6 were re-coded as 6 (n = 26). This could potentially explain why we did not observe a significant correlation between FTND and PSQI scores. The sample size of smokers was relatively small which may lead to inflated effect sizes. Studies of a larger sample of individuals with more severe nicotine dependence are warranted to replicate the current findings. Further, e-cigarette use (not evaluated in HCP) is common in youth (Park-Lee et al., 2022) and needs to be considered for its impact on sleep, too. Second, drinking influences multiple sleep indices and comorbid alcohol and nicotine misuse may have interacting effects on hypothalamic functions. Although we have controlled for the effects of alcohol use by including drinking PC1 as a covariate, we cannot completely rule out the effects of alcohol use severity. Third, the HCP did not contain any objective measures of sleep quality such as polysomnography or actigraphy and the current findings were based solely on subjective reports. Moreover, longitudinal studies are needed to establish the causal links of altered hypothalamic circuit functions, deficient sleep, and nicotine use as well as its psychiatric comorbidities.
Finally, it is important to highlight a few potential issues concerning image preprocessing and seed selection. We applied smoothing before computing hypothalamic rsFCs and thus may have incorporated signals from adjacent ventricles in the mask. Afterwards, we regressed out the global signals to eliminate the nuisance effects. However, this may lead to anti-correlated resting state networks in functional connectivity analyses (Murphy et al., 2009). Additionally, we applied a band-pass filter to obtain low-frequency signals; however, high frequency noise may not be completely eliminated due to aliasing (Rachid et al., 2013). Lastly, the hypothalamus comprises functional subregions. For instance, medial and lateral subregions of hypothalamus showed different functional networks in both neurotypical individuals (Kullmann et al., 2014) and those with cocaine dependence (Zhang et al., 2018). More studies are warranted to distinguish subregional hypothalamic connectivities in link with sleep disturbances in smokers.
In conclusion, we associated altered hypothalamic connectivities with deficient sleep in adults who smoke. Elevated hypothalamic functional connectivity with the somatosensory cortex was related to worse sleep quality and greater severity of depression and anxiety symptoms in smokers. These preliminary findings characterize a neural marker of deficient sleep in cigarette smokers and may contribute to studies of the pathophysiology of nicotine use disorders and of treatment for smoking cessation.
Supplementary Material
Acknowledgements
This study is supported by National Institutes of Health (NIH) grant DA051922. The NIH is otherwise not responsible for the conceptualization of the study, data collection and analysis, or in the decision in publishing the results.
Footnotes
CRediT authorship contribution statement
Yu Chen: Writing – review & editing, Writing – original draft, Visualization, Software, Methodology, Formal analysis, Data curation, Conceptualization. Shefali Chaudhary: Writing – review & editing, Writing – original draft, Formal analysis. Guangfei Li: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Lisa M. Fucito: Writing – review & editing, Writing – original draft, Methodology. Jinbo Bi: Writing – review & editing, Writing – original draft. Chiang-Shan R. Li: Writing – review & editing, Writing – original draft, Supervision, Methodology, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynirp.2024.100200.
Data availability, Ethics declarations, and consent to participate
We have obtained permission from the Human Connectome Project (HCP) to use the Open and Restricted Access data for the current study. Data were provided by the WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. The HCP young-adult data is publicly available at https://www.humanconnectome.org/study/hcp-young-adult/.
Data availability
Data will be made available on request.
References
- Achenbach TM, Rescorla LA, 2003. Manual for the ASEBA Adult Forms & Profiles. University of Vermont, Burlington, VT. [Google Scholar]
- al’Absi M, 2006. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int. J. Psychophysiol 59, 218–227. [DOI] [PubMed] [Google Scholar]
- Allen GV, Saper CB, Hurley KM, Cechetto DF, 1991. Organization of visceral and limbic connections in the insular cortex of the rat. J. Comp. Neurol 311, 1–16. [DOI] [PubMed] [Google Scholar]
- Bai Y, Tan J, Liu X, Cui X, Li D, Yin H, 2022. Resting-state functional connectivity of the sensory/somatomotor network associated with sleep quality: evidence from 202 young male samples. Brain Imag. Behav 16, 1832–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bains JS, Wamsteeker Cusulin JI, Inoue W, 2015. Stress-related synaptic plasticity in the hypothalamus. Nat. Rev. Neurosci 16, 377–388. [DOI] [PubMed] [Google Scholar]
- Balkan B, Pogun S, 2018. Nicotinic cholinergic system in the hypothalamus modulates the activity of the hypothalamic neuropeptides during the stress response. Curr. Neuropharmacol 16, 371–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao AM, Meynen G, Swaab DF, 2008. The stress system in depression and neurodegeneration: focus on the human hypothalamus. Brain Res. Rev 57, 531–553. [DOI] [PubMed] [Google Scholar]
- Bao AM, Swaab DF, 2019. The human hypothalamus in mood disorders: the HPA axis in the center. IBRO Rep. 6, 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Hu M, 2008. Sex differences in drug abuse. Front. Neuroendocrinol 29, 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes AD, Fischer D, Geerling JC, Brass J, Saper CB, Fox MD, 2018. Connectivity of sleep-and wake-promoting regions of the human hypothalamus observed during resting wakefulness. Sleep 41, zsy108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook JS, Zhang C, Rubenstone E, Brook DW, 2015. Insomnia in adults: the impact of earlier cigarette smoking from adolescence to adulthood. J. Addiction Med 9, 40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger, Schuckit MA, 1994. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J. Stud. Alcohol 55 (2), 149–158. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ, 1989. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr. Res 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Chao HH, Luo X, Chang JL, Li CR, 2009. Activation of the pre-supplementary motor area but not inferior prefrontal cortex in association with short stop signal reaction time–an intra-subject analysis. BMC Neurosci. 10, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Gong X, Wang L, Xu M, Zhong X, Peng Z, Song T, Xu L, Lian J, Shao Y, Weng X, 2023. Altered postcentral connectivity after sleep deprivation correlates to impaired risk perception: a resting-state functional magnetic resonance imaging study. Brain Sci. 13, 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Qi X, Zheng J, 2018. Altered regional cortical brain activity in healthy subjects after sleep deprivation: a functional magnetic resonance imaging study. Front. Neurol 9, 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MC, Chiang WY, Yugay T, Patxot M, Ozcivit IB, Hu K, Lu J, 2016. Anterior insula regulates multiscale temporal organization of sleep and wake activity. J. Biol. Rhythm 31, 182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chaudhary S, Wang W, Li CR, 2021a. Gray matter volumes of the insula and anterior cingulate cortex and their dysfunctional roles in cigarette smoking. Addiction Neurosci. 1, 100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Dhingra I, Chaudhary S, Fucito L, Li CR, 2022a. Overnight abstinence is associated with smaller secondary somatosensory cortical volumes and higher somatosensory-motor cortical functional connectivity in cigarette smokers. Nicotine Tob. Res 24, 1889–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Dhingra I, Le TM, Zhornitsky S, Zhang S, Li CR, 2022b. Win and loss responses in the monetary incentive delay task mediate the link between depression and problem drinking. Brain Sci. 12, 1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Ide JS, Li CS, Chaudhary S, Le TM, Wang W, Zhornitsky S, Zhang S, Li CR, 2022c. Gray matter volumetric correlates of dimensional impulsivity traits in children: sex differences and heritability. Hum. Brain Mapp 43, 2634–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Li CR, 2022. Striatal gray matter volumes, externalizing traits, and N-back task performance: an exploratory study of sex differences using the human connectome project data. J. Exp. Psychopathol 13, 1–11. [Google Scholar]
- Chen Y, Li CR, 2023. Overnight abstinence, ventrostriatal-insular connectivity, and tridimensional personality traits in cigarette smokers. J. Integr. Neurosci 22, 1–8. [DOI] [PubMed] [Google Scholar]
- Chen Y, Li G, Ide JS, Luo X, Li CR, 2021b. Sex differences in attention deficit hyperactivity symptom severity and functional connectivity of the dorsal striatum in young adults. Neuroimage: Rep. 1, 100025. [Google Scholar]
- Chiu N-Y, Hsu W-Y, 2016. Sleep disturbances in methadone maintenance treatment (MMT) patients. In: Neuropathology of Drug Addictions and Substance Misuse. Elsevier, pp. 608–615. [Google Scholar]
- Cohrs S, Rodenbeck A, Riemann D, Szagun B, Jaehne A, Brinkmeyer J, Grander G, Wienker T, Diaz-Lacava A, Mobascher A, Dahmen N, Thuerauf N, Kornhuber J, Kiefer F, Gallinat J, Wagner M, Kunz D, Grittner U, Winterer G, 2014. Impaired sleep quality and sleep duration in smokers-results from the German Multicenter Study on Nicotine Dependence. Addiction Biol. 19, 486–496. [DOI] [PubMed] [Google Scholar]
- de Meneses-Gaya C, Zuardi AW, de Azevedo Marques JM, Souza RM, Loureiro SR, Crippa JAS, 2009. Psychometric qualities of the Brazilian versions of the Fagerström test for nicotine dependence and the heaviness of smoking index. Nicotine Tob. Res 11, 1160–1165. [DOI] [PubMed] [Google Scholar]
- Ding S, Gao L, Kukun H, Ai K, Zhao W, Xie C, Wang Y, 2021. Novel neuroimaging biomarker for sleep quality in insomnia disorder: a hypothalamus resting state study. Front. Neurosci 15, 634984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvsashagen T, Mutsaerts HJ, Zak N, Norbom LB, Quraishi SH, Pedersen PO, Malt UF, Westlye LT, van Someren EJ, Bjornerud A, Groote IR, 2019. Cerebral blood flow changes after a day of wake, sleep, and sleep deprivation. Neuroimage 186, 497–509. [DOI] [PubMed] [Google Scholar]
- Fischer S, Ehlert U, 2018. Hypothalamic-pituitary-thyroid (HPT) axis functioning in anxiety disorders. A systematic review. Depress. Anxiety 35, 98–110. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Sorensen O, Amlien IK, Bartres-Faz D, Brandmaier AM, Buchmann N, Demuth I, Drevon CA, Duzel S, Ebmeier KP, Ghisletta P, Idland AV, Kietzmann TC, Kievit RA, Kuhn S, Lindenberger U, Magnussen F, Macia D, Mowinckel AM, Nyberg L, Sexton CE, Sole-Padulles C, Pudas S, Roe JM, Sederevicius D, Suri S, Vidal-Pineiro D, Wagner G, Watne LO, Westerhausen R, Zsoldos E, Walhovd KB, 2021. Poor self-reported sleep is related to regional cortical thinning in aging but not memory decline-results from the lifebrain Consortium. Cerebr. Cortex 31, 1953–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Jahfari S, Scholte HS, Wolfensteller U, van den Wildenberg WP, Ridderinkhof KR, 2008. Function and structure of the right inferior frontal cortex predict individual differences in response inhibition: a model-based approach. J. Neurosci 28, 9790–9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankiensztajn LM, Elliott E, Koren O, 2020. The microbiota and the hypothalamus-pituitary-adrenocortical (HPA) axis, implications for anxiety and stress disorders. Curr. Opin. Neurobiol 62, 76–82. [DOI] [PubMed] [Google Scholar]
- Fucito LM, Redeker NS, Ball SA, Toll BA, Ikomi JT, Carroll KM, 2014. Integrating a behavioural sleep intervention into smoking cessation treatment for smokers with insomnia: a randomised pilot study. J. Smok. Cessat 9, 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahad O, Beutel M, Gilan DA, Michal M, Schulz A, Pfeiffer N, Konig J, Lackner K, Wild P, Daiber A, Munzel T, 2022. The association of smoking and smoking cessation with prevalent and incident symptoms of depression, anxiety, and sleep disturbance in the general population. J. Affect. Disord 313, 100–109. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM, 2010. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage 50, 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO, 1991. The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addiction 86, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Hilbert K, Lueken U, Beesdo-Baum K, 2014. Neural structures, functioning and connectivity in Generalized Anxiety Disorder and interaction with neuroendocrine systems: a systematic review. J. Affect. Disord 158, 114–126. [DOI] [PubMed] [Google Scholar]
- Howell DC, 2013. Statistical Methods for Psychology, eighth ed. Cengage Learning. [Google Scholar]
- Jaehne A, Loessl B, Bárkai Z, Riemann D, Hornyak M, 2009. Effects of nicotine on sleep during consumption, withdrawal and replacement therapy. Sleep Med. Rev 13, 363–377. [DOI] [PubMed] [Google Scholar]
- Jaehne A, Unbehaun T, Feige B, Lutz UC, Batra A, Riemann D, 2012. How smoking affects sleep: a polysomnographical analysis. Sleep Med. 13, 1286–1292. [DOI] [PubMed] [Google Scholar]
- Jiang J, Zou G, Liu J, Zhou S, Xu J, Sun H, Zou Q, Gao JH, 2021. Functional connectivity of the human hypothalamus during wakefulness and nonrapid eye movement sleep. Hum. Brain Mapp 42, 3667–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo EY, Noh HJ, Kim JS, Koo DL, Kim D, Hwang KJ, Kim JY, Kim ST, Kim MR, Hong SB, 2013. Brain gray matter deficits in patients with chronic primary insomnia. Sleep 36, 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D, Qin Z, Wang W, Zheng Y, Hu H, Bao Y, Bao H, 2020. Brain functional changes in Tibetan with obstructive sleep apnea hypopnea syndrome: a resting state fMRI study. Medicine (Baltim.) 99, e18957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap R, Hock LM, Bowman TJ, 2001. Higher prevalence of smoking in patients diagnosed as having obstructive sleep apnea. Sleep Breath. 5, 167–172. [DOI] [PubMed] [Google Scholar]
- Kogler L, Muller VI, Chang A, Eickhoff SB, Fox PT, Gur RC, Derntl B, 2015. Psychosocial versus physiological stress - meta-analyses on deactivations and activations of the neural correlates of stress reactions. Neuroimage 119, 235–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause AJ, Simon EB, Mander BA, Greer SM, Saletin JM, Goldstein-Piekarski AN, Walker MP, 2017. The sleep-deprived human brain. Nat. Rev. Neurosci 18, 404–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S, Heni M, Linder K, Zipfel S, Haring HU, Veit R, Fritsche A, Preissl H, 2014. Resting-state functional connectivity of the human hypothalamus. Hum. Brain Mapp. 35, 6088–6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TM, Liao DL, Ide J, Zhang S, Zhornitsky S, Wang W, Li CR, 2020. The interrelationship of body mass index with gray matter volume and resting-state functional connectivity of the hypothalamus. Int. J. Obes 44, 1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Chen Y, Chaudhary S, Tang X, Li CSR, 2022. Loss and frontal striatal reactivities characterize alcohol use severity and rule-breaking behavior in young adult drinkers. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 7 (10), 1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CR, Huang C, Constable RT, Sinha R, 2006. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J. Neurosci 26, 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Kong R, Liégeois R, Orban C, Tan Y, Sun N, Holmes AJ, Sabuncu MR, Ge T, Yeo BTT, 2019. Global signal regression strengthens association between resting-state functional connectivity and behavior. Neuroimage 196, 126–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AS, Fleischman DA, Dawe RJ, Yu L, Arfanakis K, Buchman AS, Bennett DA, 2016. Regional neocortical gray matter structure and sleep fragmentation in older adults. Sleep 39, 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Cai W, Zhao M, Cai W, Sui F, Hou W, Wang H, Yu D, Yuan K, 2019. Reduced resting-state functional connectivity and sleep impairment in abstinent male alcohol-dependent patients. Hum. Brain Mapp 40, 4941–4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Wu D, Mai Y, Xu G, Tian J, Jiang G, 2020. Functional connectome fingerprint of sleep quality in insomnia patients: individualized out-of-sample prediction using machine learning. Neuroimag. Clin 28, 102439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS, 2007. Mediation analysis. Annu. Rev. Psychol 58, 593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Me S, 1982. Asymptotic intervals for indirect effects in structural equation models. Socio. Methodol 290–312. [Google Scholar]
- Mignot E, Taheri S, Nishino S, 2002. Sleeping with the hypothalamus: emerging therapeutic targets for sleep disorders. Nat. Neurosci 5, 1071–1075. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA, 2009. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 44, 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park-Lee E, Ren C, Cooper M, Cornelius M, Jamal A, Cullen KA, 2022. Tobacco product use among middle and high school students—United States, 2022. MMWR (Morb. Mortal. Wkly. Rep.) 71, 1429–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten CA, Choi WS, Gillin JC, Pierce JP, 2000. Depressive symptoms and cigarette smoking predict development and persistence of sleep problems in US adolescents. Pediatrics 106, e23 e23. [DOI] [PubMed] [Google Scholar]
- Peters EN, Fucito LM, Novosad C, Toll BA, O’Malley SS, 2011. Effect of night smoking, sleep disturbance, and their co-occurrence on smoking outcomes. Psychol. Addict. Behav 25, 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Fletcher PC, Henson RN, Worsley KJ, Brett M, Nichols TE, 2008. Guidelines for reporting an fMRI study. Neuroimage 40, 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop MG, Crivii C, Opincariu I, 2018. Anatomy and Function of the Hypothalamus. Hypothalamus in Health and Diseases. IntechOpen. [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE, 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Li BZ, Zhang Y, Pan B, Gao YH, Zhan H, Liu Y, Shao YC, Zhang X, 2021. Altered hypothalamic functional connectivity following total sleep deprivation in young adult males. Front. Neurosci 15, 688247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachid M, Pamarti S, Daneshrad B, 2013. Filtering by aliasing. IEEE Trans. Signal Process 61, 2319–2327. [Google Scholar]
- Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, Nissen C, 2010. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med. Rev 14, 19–31. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Kirschbaum C, 2006. The hypothalamic-pituitary-adrenal (HPA) axis in habitual smokers. Int. J. Psychophysiol 59, 236–243. [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME, 2004. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci. Biobehav. Rev 28, 533–546. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Arrigoni E, Lipton JO, 2017. Neural circuitry of wakefulness and sleep. Neuron 93, 747–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung D, Park B, Kim SY, Kim BN, Park S, Jung KI, Kim J, Park MH, 2020. Structural alterations in large-scale brain networks and their relationship with sleep disturbances in the adolescent population. Sci. Rep 10, 3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymusiak R, Gvilia I, McGinty D, 2007. Hypothalamic control of sleep. Sleep Med. 8, 291–301. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Yoshida M, Emoto H, Ishii H, 2000. Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: basic studies. Eur. J. Pharmacol 405, 397–406. [DOI] [PubMed] [Google Scholar]
- Turner AI, Smyth N, Hall SJ, Torres SJ, Hussein M, Jayasinghe SU, Ball K, Clow AJ, 2020. Psychological stress reactivity and future health and disease outcomes: a systematic review of prospective evidence. Psychoneuroendocrinology 114, 104599. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M, 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289. [DOI] [PubMed] [Google Scholar]
- Wang D, Xue SW, Tan Z, Wang Y, Lian Z, Sun Y, 2019. Altered hypothalamic functional connectivity patterns in major depressive disorder. Neuroreport 30, 1115–1120. [DOI] [PubMed] [Google Scholar]
- Yang F, Liu Y, Chen S, Dai Z, Yang D, Gao D, Shao J, Wang Y, Wang T, Zhang Z, 2020. A GABAergic neural circuit in the ventromedial hypothalamus mediates chronic stress-induced bone loss. J. Clin. Invest 130, 6539–6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH, 1999. Biostatistical Analysis. Pearson Education India. [Google Scholar]
- Zhang G, Shen L, Tao C, Jung A-H, Peng B, Li Z, Zhang LI, Tao HW, 2021a. Medial preoptic area antagonistically mediates stress-induced anxiety and parental behavior. Nat. Neurosci 24, 516–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Tomasi D, Manza P, Shokri-Kojori E, Demiral SB, Feldman DE, Kroll DS, Biesecker CL, McPherson KL, Wang GJ, Wiers CE, Volkow ND, 2021b. Sleep disturbances are associated with cortical and subcortical atrophy in alcohol use disorder. Transl. Psychiatry 11, 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li CR, 2018. Ventral striatal dysfunction in cocaine dependence – difference mapping for subregional resting state functional connectivity. Transl. Psychiatry 8, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wang W, Zhornitsky S, Li CR, 2018. Resting state functional connectivity of the lateral and medial hypothalamus in cocaine dependence: an exploratory study. Front. Psychiatr 9, 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Zhang X, Fei N, Zhu Y, Sun J, Liu P, Yang X, Qin W, 2019. Decreased cortical and subcortical response to inhibition control after sleep deprivation. Brain Imag. Behav 13, 638–650. [DOI] [PubMed] [Google Scholar]
- Zhou L, Liu G, Luo H, Li H, Peng Y, Zong D, Ouyang R, 2020. Aberrant hippocampal network connectivity is associated with neurocognitive dysfunction in patients with moderate and severe obstructive sleep apnea. Front. Neurol 11, 580408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We have obtained permission from the Human Connectome Project (HCP) to use the Open and Restricted Access data for the current study. Data were provided by the WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. The HCP young-adult data is publicly available at https://www.humanconnectome.org/study/hcp-young-adult/.
Data will be made available on request.


