Abstract
We examined the phenotype and function of cells infiltrating the central nervous system (CNS) of mice persistently infected with Theiler’s murine encephalomyelitis virus (TMEV) for evidence that viral antigens are presented to T cells within the CNS. Expression of major histocompatibility complex (MHC) class II in the spinal cords of mice infected with TMEV was found predominantly on macrophages in demyelinating lesions. The distribution of I-As staining overlapped that of the macrophage marker sialoadhesin in frozen sections and coincided with that of another macrophage/microglial cell marker, F4/80, by flow cytometry. In contrast, astrocytes, identified by staining with glial fibrillary acidic protein, rarely expressed detectable MHC class II, although fibrillary gliosis associated with the CNS damage was clearly seen. The costimulatory molecules B7-1 and B7-2 were expressed on the surface of most MHC class II-positive cells in the CNS, at levels exceeding those found in the spleens of the infected mice. Immunohistochemistry revealed that B7-1 and B7-2 colocalized on large F4/80+ macrophages/microglia in the spinal cord lesions. In contrast, CD4+ T cells in the lesions expressed mainly B7-2, which was found primarily on blastoid CD4+ T cells located toward the periphery of the lesions. Most interestingly, plastic-adherent cells freshly isolated from the spinal cords of TMEV-infected mice were able to process and present TMEV and horse myoglobin to antigen-specific T-cell lines. Furthermore, these cells were able to activate a TMEV epitope-specific T-cell line in the absence of added antigen, providing conclusive evidence for the endogenous processing and presentation of virus epitopes within the CNS of persistently infected SJL/J mice.
Theiler’s murine encephalomyelitis virus (TMEV) is a picornavirus that induces a lifelong persistent central nervous system (CNS) infection leading to a chronic CNS demyelinating disease when inoculated intracerebrally into susceptible strains of mice. Infected mice develop progressive symptoms of gait disturbance, spastic hind limb paralysis, and urinary incontinence (39), histologically related to perivascular and parenchymal mononuclear cell infiltration and demyelination of white matter tracts within the spinal cord (8, 9, 38). Several lines of evidence have demonstrated that demyelination is immunologically mediated. These include the ability of nonspecific immunosuppression with cyclophosphamide (37), antithymocyte serum (36), and anti-CD4 or anti-major histocompatibility complex (MHC) class II monoclonal antibodies (MAbs) (14, 16, 63) to inhibit or prevent disease and the ability of TMEV-specific tolerance to prevent induction of disease (28). In the highly susceptible SJL/J mouse strain, current evidence indicates that the myelin damage is initiated by TMEV-specific CD4+ T cells targeting virus antigen (16, 28, 45, 46, 54), while the chronic stage of the disease also involves CD4+ myelin epitope-specific T cells primed via epitope spreading (48). Thus, the immune response itself may be deleterious to CNS function, as exemplified in humans by multiple sclerosis (MS), for which TMEV infection serves as a model.
The identity of the cells responsible for initiating and sustaining immune responses in the CNS remains controversial. The CNS lacks normal lymphatic circulation and tissue and is shielded from the systemic circulation by a specialized continuous vascular endothelium (6). There are specialized cells within the CNS with the potential to present antigens to T cells. In vitro, astrocytes (11, 59) and microglia (3, 13), particularly when treated with gamma interferon (IFN-γ), are capable of expressing MHC class II and presenting antigens to T cells. However, studies such as these have relied on the ability to isolate and continuously culture cells from neonatal or embryonic brain and have assumed that such cells are representative of the adult populations in vivo. Antigen presentation by neonatal cells in long-term culture may not faithfully reproduce the in vivo state in adult animals, as the ability of microglia directly isolated from adult rats to present myelin basic protein (MBP) to T-cell lines in vitro was found to differ from that of neonatally derived microglia (12). In addition, studies using allogeneic bone marrow chimeras between strains of mice or rats have generally supported the idea that cells of hematopoietic origin, i.e., microglia and macrophages, are the principal antigen-presenting cells (APCs) in the CNS active during the initiation of experimental autoimmune encephalomyelitis (EAE) (20, 22, 50). Although they are much more abundant than microglia, astrocytes are less potent when inducing EAE in chimeras (50).
The role of antigen presentation in the CNS during TMEV-induced demyelination has not been addressed directly. We previously showed that a relatively large fraction of the CD4+, but not CD8+, T cells isolated from the spinal cords of TMEV-infected mice expressed high-affinity interleukin-2 (IL-2) receptor (IL-2R), a marker of recent T-cell activation. In addition, TMEV-specific CD4+ T cells could be demonstrated in the spinal cord infiltrates of TMEV-infected mice (54). This finding raises the possibility that T cells are locally activated within the target tissue and participate directly in the pathogenesis of disease. Macrophages (5, 41, 56), astrocytes (7, 56), and oligodendroglia (55, 56) in TMEV-infected mice contain virus and conceivably could present viral antigens to pathogenic CD4+ T cells within the CNS. Isolated microglia (34) and astrocytes (17) have been shown to support persistent viral infection in vitro, and astrocytes derived from neonatal mice have been shown to present TMEV to T cells in vitro (2). To examine whether CNS cells present viral antigens and participate in the pathogenesis of TMEV-induced demyelination, the expression of MHC class II and B7 costimulatory molecules was examined in detail. Based on our previous results showing that a large proportion of CD4+ T cells isolated from the CNS of TMEV-infected mice bear markers of recent activation, we also asked if mononuclear cells isolated from the CNS of TMEV-infected mice were capable of presenting viral antigens leading to the functional activation of Th1 lines in vitro.
MATERIALS AND METHODS
Mice.
Six- to seven-week-old female SJL/J mice were purchased from the Jackson Laboratory (Bar Harbor, Maine). Mice were maintained on standard laboratory chow and water ad libitum in accordance with institutional guidelines.
Antibodies.
Anti-B7-1 (clone 16.10.A1) and anti-B7-2 (clone 1G10) were supplied by Jeffrey Bluestone, University of Chicago, or purchased from Pharmingen (San Diego, Calif.). Anti-glial fibrillary acidic protein (GFAP) (clone G-A-5) was purchased from Boehringer Mannheim, Indianapolis, Ind. MAbs directed against the macrophage markers F4/80 and sialoadhesin (clone 3d6.112) were purchased from Caltag (South San Francisco, Calif.) and Serotec (Harlan Bioproducts for Science, Indianapolis, Ind.), respectively. Anti-I-As (clone MKS4) was purified from culture supernatants by using a protein G-Sepharose column (Pierce Chemical Co., Rockford, Ill.). Avidin-R–phycoerythrin (A-PE) was obtained from Molecular Probes (Eugene, Oreg.). Isotype controls of irrelevant specificity, conjugated to fluorescein isothiocyanate (FITC), PE, or biotin as appropriate, were purchased from Pharmingen. For flow cytometry, all antibodies were titrated by using SJL/J spleen cell suspensions. Second antibodies for immunohistology against rat immunoglobulin G (IgG) and mouse IgG1, IgG2a, IgG2b, and IgG3 conjugated to biotin or FITC were purchased from Caltag. Unconjugated, purified mouse myeloma proteins (IgG1, IgG2a, IgG2b, and IgG3) were purchased from Serotec (Harlan).
Peptides.
The VP274–86 peptide (QEAFSHIRIPLPH) corresponding to regions of the VP2 viral capsid protein was synthesized by using a RaMPS multiple-peptide synthesis system (NEN-Dupont, Wilmington, Del.). The amino acid composition was confirmed by the Northwestern University Biotechnology Center.
Virus.
The BeAn 8386 strain of TMEV is a tissue culture-adapted strain that has been plaque purified and passaged in BHK-21 cells grown in Dulbecco’s modified Eagle’s medium (DMEM) (40). Working stocks of virus were purified by polyethylene glycol precipitation of total BHK-21 cell lysates, sonication in the presence of sodium dodecyl sulfate, and centrifugation over successive sucrose and CsSO4 gradients.
Virus inoculation and disease scores.
Mice were anesthetized with methoxyflurane (Pitman-Moore, Mundelein, Ill.) and incubated in the right cerebral hemisphere with 2.9 × 106 PFU of TMEV in 30 μl of DMEM. Mice were examined two to three times per week for the development of chronic gait abnormalities and spastic paralysis indicative of demyelination (35). The clinical disease endpoint is a reliable marker for severe demyelination (4). Disease incidence approaches 100% in mice infected when 7 to 9 weeks old (58). About 35 days postinfection (p.i.), SJL/J mice develop a waddling gait without loss of tail tone. The symptoms of the affected mice progress over the next 4 to 8 weeks to spastic hind limb paralysis and eventually urinary incontinence with total paralysis. Scores were assigned on a three-point scale: 0 (asymptomatic), 1 (waddling gait), 2 (spastic paralysis with impaired righting), and 3 (urinary incontinence and/or total paralysis).
Induction and scoring of relapsing (R-EAE).
Female SJL/J mice (8 to 10 weeks) were immunized with a peptide (PLP139–151) corresponding to the dominant encephalitogenic epitope of proteolipid protein (43). Each mouse received 0.1 ml of emulsion subcutaneously in three sites on the shaved flank, containing 50 nmol of PLP139–151 and 200 μg of Mycobacterium tuberculosis H37Ra (Difco, Detroit, Mich.). Initial clinical signs of paralysis are usually observed between 10 and 14 days postimmunization. Scores were assigned on a five-point scale: 0 (asymptomatic), 1 (loss of tail tone), 2 (ataxic gait), 3 (hind limb weakness), 4 (total paralysis of both hind limbs), and 5 (death).
Isolation of CNS-infiltrating mononuclear cells and splenocytes.
Mice were anesthetized with methoxyflurane and perfused through the left ventricle with phosphate-buffered saline (PBS) until the effluent ran clear. Spinal cords were extruded by flushing the vertebral canal with PBS and then were rinsed in PBS. Spleens were removed from the same mice and placed in PBS. Tissues were forced through 100-mesh stainless steel screens to give a single-cell suspension. Erythrocytes in spleen cell preparations were lysed by hypotonic shock in Tris-NH4Cl, and the cells were washed and resuspended in isotonic buffered saline containing 0.1% NaN3 (IBS; Baxter Diagnostics, Inc., McGaw Park, Ill.) and 1.0% normal goat serum (NGS; Pel-Freez, Rogers, Ark.). The spinal cord homogenate was resuspended in 30% Percoll (Pharmacia, Piscataway, N.J.), divided into tubes (equivalent to four to five spinal cords per tube), and underlaid with 70% Percoll. The gradients were centrifuged at 500 × g and 24°C for 20 min. CNS mononuclear cells were collected from the 30%/70% interface, washed, and resuspended in IBS-NGS.
A modified procedure was used to isolate cells from the CNS for use in culture experiments. Spinal cords were removed as described above except that all solutions and instruments were kept sterile throughout. The spinal cords were placed in DMEM supplemented with 5% fetal bovine serum, 2 mM l-glutamine, 100 μg of streptomycin per ml and 100 U of penicillin per ml (DMEM-5; Sigma Chemical Co., St. Louis, Mo.) containing 300 U of type IV clostridial collagenase (Worthington Biochemical Corp., Freehold, N.J.) per ml and minced with sterilized razor blades or scissors in 10-cm-diameter plastic petri dishes (10 to 15 spinal cords per dish). The pieces were digested at 37°C and triturated approximately every 30 min for 2 to 3 h until completely homogeneous. The suspension was centrifuged at low speed, the supernatant was discarded, and the mononuclear cells were isolated by centrifugation over 30%/70% discontinuous Percoll gradients. To remove and isolate macrophages and activated microglia, the cells recovered from the gradients were resuspended in DMEM-5 and allowed to adhere to 10-cm-diameter plastic tissue culture dishes for 1 to 2 h in a 37°C humidified CO2 incubator. The nonadherent cells were removed, and the dishes were rinsed with warm medium. The adherent cells were then removed by scraping and washing the dishes with cold balanced salt solution.
Flow cytometry.
The cells to be stained were resuspended in IBS-NGS. For anti-B7-1 and anti-B7-2 antibodies, the cells to be stained were incubated first in normal mouse serum and anti-FcRgII/III (2.4G2) hybridoma supernatant. The cells (0.5 × 106 to 1 × 106) were incubated with a predetermined concentration of biotinylated MAb for 30 min at 4°C, washed in IBS-NGS, incubated with A-PE and the appropriate FITC-conjugated MAb for 30 min at 4°C in the dark, washed again, and resuspended in 1 ml of IBS containing 0.1 μg of propidium iodide per ml. Data collection and analysis were performed on a FACScan flow cytometer (Becton Dickinson, Mountain View, Calif.) with Cellquest software. Nonspecific staining was determined by incubating cells with A-PE alone or with directly labeled, isotype-matched control antibodies.
Preparation, storage, and sectioning of tissues.
Mice were anesthetized and perfused with PBS through the left ventricle. Spinal cords were removed by dissection, and 2- to 3-mm pieces were immediately frozen in OCT (Miles Laboratories, Elkhart, Ind.) in a liquid nitrogen-cooled isopentane bath. The blocks were stored in plastic bags to prevent dehydration at −80°C. Sections (5 μm) were cut on a Reichert-Jung Cryocut 1800 cryostat (Leica Instruments, Deerfield, Ill.), mounted on poly-l-lysine- or gelatin-coated slides, and air dried.
Immunofluorescence.
All steps were performed at room temperature. Sections were fixed in acetone for 10 min and rehydrated in PBS. Nonspecific staining was blocked by incubation in 5% NGS and 0.2% fish skin gelatin (Sigma) in PBS for 30 to 60 min; primary antibodies diluted in blocking solution were overlaid on the slides for 1 to 2 h; and secondary antibodies conjugated with biotin, FITC or Texas red were overlaid on the slides for 1 h. Slides that received biotin-conjugated second antibodies were overlaid with avidin-Texas red or avidin-FITC. Between incubations, the slides were washed three times with PBS for 5 to 10 min. The sections were examined by epifluorescence on an Opti-Phot microscope (Nikon, Melville, N.Y.).
Immunohistochemistry.
Sections were cut, air dried, and fixed in acetone at −20°C. The slides were stained with biotin-conjugated anti-F4/80 (Caltag), anti-CD4 (L3T4), anti-B7-1 (16-10A1), and/or anti-B7-2 (GL1) (Pharmingen). Slides were stained sequentially by using an NEN Life Science Products tyramide signal amplification-direct kit according to the manufacturer’s instructions. Slides were coverslipped with Vectashield mounting medium (Vector Laboratories, Burlingame, Calif.) and viewed by epifluorescence using a chroma triple-band filter (Chroma Technology Corp., Brattleboro, Vt.).
Maintenance of T-cell lines.
T-cell line sTV1, specific for TMEV VP274–86, and a T-cell line specific for horse myoglobin (hMyo) were maintained in DMEM supplemented with 10% fetal bovine serum 2 mM l-glutamine, 100 μg of streptomycin per ml, and 100 U of penicillin per ml (DMEM-10; Sigma). T cells (4 × 105) were stimulated in 2-ml cultures with irradiated (3,000 R) normal spleen cells (3 × 106) and UV-inactivated TMEV lysate (50 μl) or hMyo (5 μM). T-cell blasts were isolated on Ficoll-Histopaque (Pharmacia, Piscataway, N.J.) by centrifugation at 500 × g and 24°C for 15 min. The blasts (0.5 × 106 to 1 × 106) were expanded in 24-well plates by using human recombinant IL-2 (10 to 20 U/ml) for 5 to 7 days before being restimulated.
Antigen presentation assays.
The plastic-adherent fraction of isolated CNS-infiltrating mononuclear cells was assayed for the ability to stimulate long-term, antigen-specific T-cell lines. Adherent cells (4 × 103 to 1 × 105 per well) isolated from spinal cords of symptomatic mice with TMEV infection or R-EAE were cultured with (i) the sTV1 line (2 × 104 per well) in the presence or absence of UV-inactivated TMEV (5 μg/ml) or VP274–86 peptide (0.5 μM) or (ii) the hMyo-specific line (2 × 104 per well) in the presence or absence of hMyo (10 μM). Depending on the number of cells recovered, duplicate or triplicate cultures were used. In all experiments, U-bottom 96-well tissue culture plates were used to maximize cell contact and the medium (DMEM-5) was supplemented with aminoguanidine (1 mM) to suppress nitric oxide synthetase activity. Proliferative responses were determined by [3H]TdR (0.1 μCi/well) incorporation during the final 24 h of the 66-h culture period. Cultures were harvested on 96-well filter plates (Uni-plates; Packard Instrument Co., Meriden, Conn.) for liquid scintillation counting, and the results are expressed as change in counts per minute (Δcpm), calculated as follows: Δcpm = mean cpm of stimulated cultures − mean cpm of control cultures.
Statistical analyses.
Differences in T-cell proliferation were analyzed by a one-tailed Student t test assuming equal variances. Values of P < 0.05 were considered statistically significant.
RESULTS
MHC class II is expressed predominantly by CNS-infiltrating macrophages in TMEV-induced demyelinating disease.
T-cell activation requires at least two signals. The first determines antigen specificity and is delivered to the T-cell receptor by a complex of peptide bound to the appropriate MHC molecule. A second or costimulatory signal, synergistic to the first, is sent to the T cell by one of a number of molecules on the APC (24), the most important of which appears to be mediated by B7-1 and B7-2, both of which bind CD28 and CTLA-4 on the T cell (26). To identify potential APC populations in the CNS of mice with TMEV-induced demyelination, we first determined which cells, if any, expressed MHC class II along with B7-1 and/or B7-2.
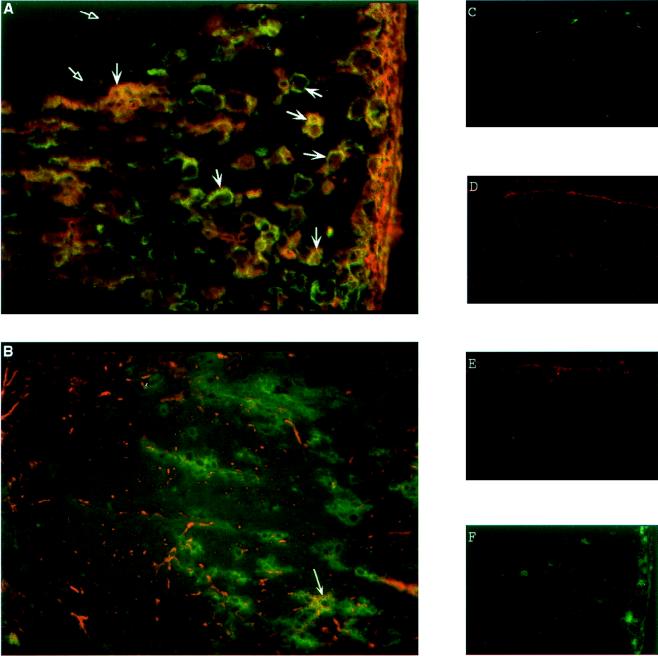
MHC class II expression was examined in frozen sections of TMEV-infected spinal cords prepared from symptomatic mice and labeled for indirect immunofluorescence. For these experiments, the macrophage marker sialoadhesin was used because it is not expressed on microglia in normal CNS tissue, except at specialized sites of blood-brain barrier permeability (52). Thus, sialoadhesin expression should identify activated macrophages and microglia in the demyelinating lesions but leave normal, resting microglia unlabeled. Antisialoadhesin strongly labeled many large cells in the chronic demyelinating lesions of TMEV-infected mice (Fig. 1A) but left adjacent, uninvolved areas unlabeled. These cells clearly made up the majority of MHC class II-positive cells in the lesions, as can be seen by the overlap of staining in Fig. 1A. Macrophages and activated microglia cannot be distinguished reliably by this type of analysis. As expected, expression of sialoadhesin (Fig. 1C) and expression of MHC class II (Fig. 1E) were essentially absent from the white matter of normal SJL/J spinal cords. Similarly, isotype-matched control staining was minimal in cords from TMEV-infected mice (Fig. 1F).
FIG. 1.
Expression of MHC class II in normal and TMEV-infected SJL/J spinal cords. Frozen tissue sections (5 μm) were prepared from spinal cords of TMEV-infected SJL/J mice (A and B). (A) Sections were labeled with MAbs specific for the macrophage marker sialoadhesin (green) and I-As (red). Expression of MHC class II largely overlapped that of sialoadhesin (large arrows), but a few singly labeled MHC class II-positive cells were found at the margins of the lesion (open arrowheads). (B) Sections were labeled with the astrocyte marker GFAP (red) and I-As (green). Overlap of labeling was observed only rarely on large ramified cells (arrow). Frozen sections (6 μm) from normal spinal cords were also examined (C to E). In the spinal cords of uninfected mice, sialoadhesin (C) and MHC class II (E) are negative except for a few cells in the meninges and vasculature, while GFAP (D) shows punctate labeling of astrocyte processes. A mouse IgG2b isotype control for I-As (F) shows hazy background over infiltrating cells and a few bright cells in the meninges of a spinal cord section from a TMEV-infected mouse. Magnification, ×200.
MHC class II is only rarely expressed on astrocytes.
The expression of MHC class II by astrocytes was examined by labeling frozen spinal cord sections for GFAP and I-As. In normal spinal cord white matter, GFAP expression was detectable at low levels, staining the many attenuated cellular processes of astrocytes distributed radially and longitudinally along the cord (Fig. 1D). In contrast, staining of large, hypertrophic astrocytes resembling fibrillary astrogliosis was seen in the spinal cords of TMEV-infected mice, often at a distance from the lesions (Fig. 1B) as has been reported for many types of CNS injury. Very little overlap in the patterns of staining was observed when MHC class II and GFAP were labeled on the same sections. An occasional cell (<1 per section examined) that appeared to be double positive for GFAP and MHC class II was seen (Fig. 1B). Confocal microscopy confirmed that the cell shown in Fig. 1B was double labeled and excluded two cells in different planes of the section. There was some background staining observed with an isotype-matched antibody control for anti-I-As (Fig. 1F); however, the rarity of GFAP/MHC class II double-positive cells and their distinctive morphology argue for the authenticity of the labeling. Overall, the results suggest that MHC class II expression is essentially absent on microglia and astrocytes in normal spinal cords, abundant on macrophages and microglia within the lesions of TMEV-infected spinal cords, and rare on reactive astrocytes in infected spinal cords (21).
Flow cytometric analysis of MHC class II and B7 costimulatory molecule expression on CNS macrophages and microglia.
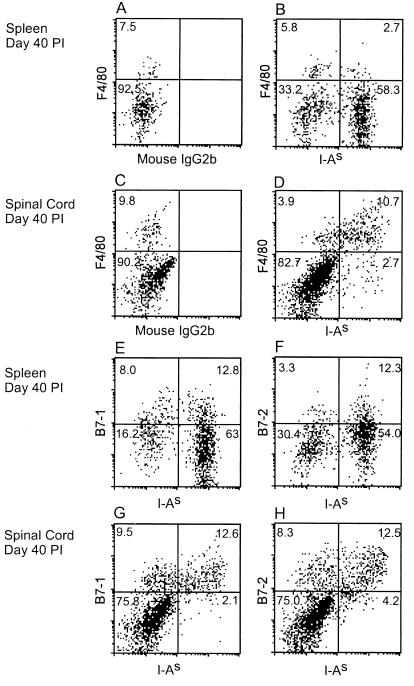
Mononuclear cells from the spinal cords of mice at 40 days after infection with TMEV were isolated by centrifugation on discontinuous Percoll gradients and analyzed by flow cytometry to quantitate the level of MHC class II and B7 costimulatory molecule expression on infiltrating macrophages and activated microglia. The cells were labeled with the MAb F4/80, which recognizes a widely expressed, macrophage lineage-specific marker of unknown function (53), and with anti-I-As. Under the microscope, the isolated macrophages and microglia were large, vacuolated, plastic-adherent cells easily distinguishable from lymphocytes (5). Electronic gates were set correspondingly to exclude the lymphocytes from analysis and focus on the properties of cells with greater size and internal complexity, i.e., those exhibiting greater forward and side scatter. At 40 days p.i., 70% of the large F4/80+ mononuclear cells isolated from spinal cords were MHC class II+ and, conversely, approximately 80% of the I-As+ cells were F4/80+ macrophages and microglia (Fig. 2D). In contrast, only 30% of F4/80+ cells in the spleens were MHC class II+ and F4/80+ cells accounted for only 4% of the I-As+ population (Fig. 2B). The numerous F4/80−, I-As+ events in the spleen, which presumably represent B cells, are present only in very small numbers (<2% of total cells) in the CNS of TMEV-infected mice (data not shown).
FIG. 2.
Flow cytometric analysis of MHC class II, B7-1, and B7-2 expression by macrophages and microglia in the spinal cords of TMEV-infected mice. Splenic and CNS mononuclear cells were isolated from TMEV-infected mice 40 days p.i. and stained with F4/80-PE, anti-B7-1-biotin, or anti-B7-2-biotin, followed by anti-I-As-FITC or control antibody, A-PE, and propidium iodide. Most MHC class II+ cells in the infected CNS were large F4/80+ macrophages and microglia (group D). In the spleen, MHC class II was expressed predominantly on F4/80− cells, most of which were probably B cells (group B). Essentially all of the MHC class II+ cells in the infected CNS expressed B7-1 and/or B7-2 (groups G and H). Most of the B7-1 and B7-2 expressing cells were also MHC class II+. The splenic MHC class II+ cells expressed B7-1 and B7-2 weakly (groups E and F). Identical results were obtained at day 23 p.i. FACS data for viable cells were collected by exclusion of propidium iodide. During analysis, lymphocytes and myelin debris were excluded by electronic gating. Numbers are the percentages of gated events in each quadrant.
T-cell costimulatory signals delivered by B7 molecules on APCs are recognized as important regulators of the immune response in a number of experimental systems, including R-EAE, autoimmune diabetes, and allogeneic transplant rejection (33, 49). Therefore, the expression of B7-1 and B7-2 was examined by two-color flow cytometry analysis of the spinal cord-infiltrating mononuclear cells in TMEV-infected mice. The analysis revealed MHC class II expression essentially congruent with that of either B7-1 or B7-2 at 40 days p.i. (Fig. 2G and H). It was not possible to determine directly if the B7-1 and B7-2 populations overlapped or were mutually exclusive, but the combined percentages of the two markers equaled more than 100%, implying simultaneous expression on I-As+ cells. Therefore, it can be inferred that the large F4/80+ cells in the CNS of TMEV-infected mice express MHC class II and one or both B7 molecules. As noted, the spleens of infected mice contained F4/80+ cells that were both MHC class II positive and negative. The splenic MHC class II+ cells expressed B7-1 along a continuum from positive to negative (Fig. 2E) and appeared to be weakly B7-2 positive (Fig. 2F), at expression levels lower than those in the spinal cord infiltrates.
Additional experiments (not shown) confirmed these results and also showed that B7-1- and B7-2-expressing macrophages and microglia increase in number in the CNS with time following infection. Furthermore, B7-1+ macrophages became the dominant population relative to B7-2 by 63 days p.i., as measured by the parameters of percentage, mean fluorescence intensity, and number per cord. As discussed below, a shift toward increased B7-1 expression may be functionally relevant to the onset of epitope spreading after the accumulation of CNS damage as is apparent in relapsing R-EAE in the SJL/J mouse (49). Interestingly, we have recently shown that myelin-specific CD4+ autoimmune responses arise during the chronic phase of TMEV-induced demyelination (48), and this response temporally corresponds to the shift toward increased B7-1 expression on CNS macrophages and microglia in virus-infected mice.
Immunohistochemical localization of B7-1 and B7-2 in the CNS of TMEV-infected mice.
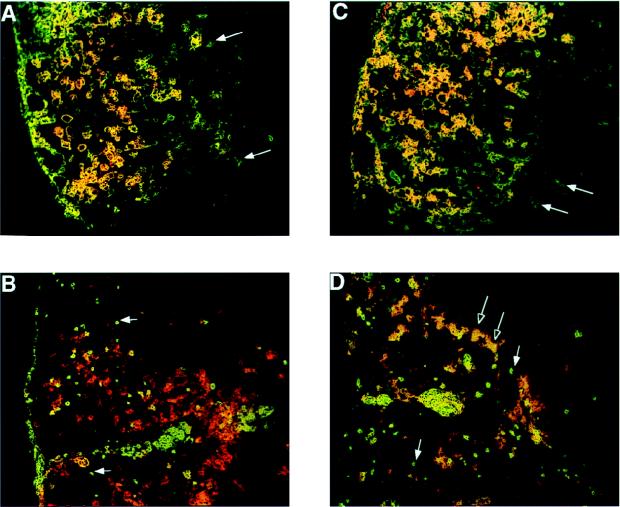
The expression of costimulatory molecules was examined in situ by immunohistochemistry to gain an understanding of the relationship between these cells and the lesions in TMEV-infected spinal cords. Symptomatic SJL/J mice 45 days p.i. were sacrificed by total-body perfusion with cold PBS, the spinal cords were removed by dissection, and frozen sections (5 to 6 μm) were stained for B7-1 and B7-2 expression in combination with either CD4 or F4/80. Isotype-matched antibody controls were processed in parallel and showed no background staining (not shown). B7-1 was expressed primarily on the large, F4/80+ cells that dominate the center of the lesions (Fig. 3A). The appearance of the cells was identical to that of sialoadhesin-positive cells seen in previous analyses (Fig. 1A). B7-1 did not colocalize to the CD4+ T cells (Fig. 3B). In contrast, B7-2 was located on a wider variety of cells, including the F4/80+ macrophages and microglia (Fig. 3C) and a subpopulation of CD4+ T cells, mainly the larger, CD4+ blast cells located at the lesion margins (Fig. 3D). The spatial location of B7-1 and B7-2 staining may have bearing on the temporal course of lesion development in relation to costimulatory molecule expression as discussed below. Overall, the appearance of B7-1 and B7-2 staining confirms our flow cytometry results and directly demonstrates the association of these costimulatory molecules with macrophages and T cells within the demyelinating lesions.
FIG. 3.
Demonstration of B7-1 and B7-2 in the spinal cords of TMEV-infected mice by immunohistochemistry. Frozen sections (5 to 6 μm) of SJL/J spinal cord were prepared 45 days p.i. and stained sequentially for F4/80 or CD4 (green), followed by B7-1 or B7-2 (red). B7-1 (A) and B7-2 (C) colocalize on large F4/80+ macrophages and microglia with the exception of a few F4/80+ microglia at the edges of the lesions (arrows). B7-1 and B7-2 do not colocalize to small CD4+ T cells in the central part of the lesions (B and D, filled arrows), but a subpopulation of large, blast-like CD4+ T cells at the lesion margins express B7-2 (D, open arrows).
Endogenous presentation of viral antigens by mononuclear cells isolated from the spinal cords of chronically infected mice.
The presence of numerous macrophages and microglia expressing readily detectable levels of MHC class II and costimulatory molecules in the spinal cords of TMEV-infected mice suggested that these cells may serve as functional APCs. Furthermore, we had previously reported that CNS mononuclear cells isolated by Percoll density gradient centrifugation and glass adherence contained infectious virus, demonstrating that viral antigens are present in the major MHC class II-expressing population (5, 41). Thus, experiments were designed to directly test the antigen presenting function of the CNS-infiltrating macrophages and microglia. Spinal cord-infiltrating mononuclear cells were enriched by adherence to plastic tissue culture dishes at 37°C, and the adherent cells were examined for the ability to activate a long-term, TMEV-specific Th1 line in vitro. The TMEV-specific T-cell line, sTV1, was derived from TMEV-primed SJL/J lymph node cells (16) and has been characterized extensively. The cell line is specific for amino acids 74 to 86 of the viral capsid protein VP2, the immunodominant T-cell epitope in SJL/J mice (15). In agreement with its functional capacity to augment virus-specific delayed-type hypersensitivity (DTH) in vivo (16), tissue culture supernatants taken 24 and 48 h after stimulation with TMEV in vitro revealed this line to be a Th1 cell, i.e., producing IL-2 and IFN-γ but not IL-4 (data not shown).
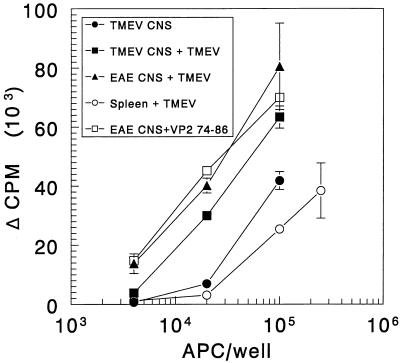
Plastic-adherent, spinal cord-infiltrating mononuclear cells were isolated from mice with TMEV-induced demyelination (mean score = 1.7, 83 days p.i.) or with R-EAE (mean score = 1.7, 29 days postimmunization). The latter were used as a control because they could not carry infectious TMEV or viral antigens into culture yet were qualitatively similar in phenotype to the TMEV-derived mononuclear cells. Although the CNS-infiltrating cells were harvested somewhat later following the onset of disease in order to optimize cell recovery, expression of MHC class II and costimulatory molecules was not markedly different from that in Fig. 1 to 3. As noted, B7-1 expression increases relative to B7-2 gradually as disease progresses. The potential APCs were irradiated and used to present TMEV (5 μg/ml) or VP274–86 (0.5 μM) to the sTV1 T-cell line. Cultures of normal, irradiated SJL/J splenic APCs were established in parallel. As seen in Fig. 4, mononuclear cells isolated from spinal cords of mice with R-EAE were able to process and present UV-inactivated TMEV and directly present the VP274–86 determinant in a dose-dependent manner to the sTV1 Th1 line but failed to activate the virus-specific T-cell line in the absence of added antigen (this is the background subtracted from all of the groups containing CNS-derived APCs). Similarly, TMEV-pulsed mononuclear cells isolated from the spinal cords of TMEV-infected mice also stimulated dose-dependent proliferation of the sTV1 line. Most significantly, CNS APCs from the virus-infected mice were able to activate the VP274–86-specific Th1 line in the absence of added virus or peptide. In this experiment, splenic APCs were not as efficient as CNS-derived APCs at presenting virus or peptide. Thus, mononuclear cells isolated from the spinal cords of mice with R-EAE- or TMEV-induced demyelination can effectively process and present antigen to a virus-specific T-cell line in vitro. These results indicate that there is a significant amount of processed, MHC class II-associated VP274–86 peptide endogenously present on the mononuclear cells recovered from the CNS of SJL/J mice infected with TMEV almost 3 months previously, as these cells were able to stimulate T-cell proliferation in the absence of exogenously added virus.
FIG. 4.
CNS-derived mononuclear cells from TMEV-infected mice endogenously present viral epitopes to the VP274–86-specific sTV1 T-cell line. CNS-derived, plastic-adherent mononuclear cells were prepared from symptomatic TMEV-infected SJL/J mice (n = 23, mean clinical score = 1.7) or from mice with R-EAE (n = 24, mean clinical score = 1.7). Various numbers (4 × 103 to 2.5 × 105) of the CNS APC populations were cultured with 2 × 104 sTV1 cells in the presence and absence of UV-inactivated TMEV virions (5 μg/ml) or VP274–86 (0.5 μM). The cultures were pulsed with [3H]TdR at 42 h and harvested 24 h thereafter. For CNS-derived APCs, the background subtracted was the counts per minute obtained from the culture of the same number of R-EAE-derived CNS APC in the absence of antigen.
To test the specificity of the endogenous stimulation, a long-term T-cell line was prepared from SJL/J lymph node cells primed with hMyo. The fine specificity of the T-cell response to myoglobins by B10.S mice (H-2s) has been well characterized (1). After confirming the specificity for hMyo, the line was found to secrete IFN-γ (2.8 × 104 ± 1,500 pg/ml at 72 h after stimulation) and IL-2 (4,264 ± 257 pg/ml at 72 h) but not IL-4 (68 ± 257 pg/ml at 72 h) and was therefore a Th1-type line. Plastic-adherent, infiltrating mononuclear cells were prepared from the spinal cords of TMEV-infected mice (mean score = 1.9, 80 days p.i.) or mice with R-EAE (mean score = 1.2, 18 days postimmunization). Once again the R-EAE cells provided a virus-negative control. These cells were irradiated and used to present TMEV (5 μg/ml) or hMyo (10 μM) to the sTV1 or hMyo-specific T-cell line, respectively. Normal, irradiated, splenic APCs were tested in parallel cultures. The results at the optimal concentrations of CNS-derived APCs (4 × 104/well) and splenic APCs (4 × 105/well) are shown in Fig. 5. Irradiated, splenic APCs induced significant proliferation of both the hMyo-specific Th1 line (group B) and the TMEV-specific sTV1 line (group D) when pulsed with the relevant antigen. hMyo-pulsed CNS-derived mononuclear cells from TMEV-infected mice induced significant proliferation of the hMyo-specific Th1 line (group F) but failed to activate these cells in the absence of added hMyo (group E). Similarly, CNS mononuclear cells derived from mice with R-EAE stimulated proliferation of the sTV1 line when pulsed with TMEV (group H) but failed to activate these cells in the absence of added antigen (group G). However, CNS mononuclear cells derived from TMEV-infected mice activated sTV1 cells in both the absence (group I) and the presence (group J) of added TMEV. The absence of background proliferation by the hMyo T-cell line confirms that the proliferation of sTV1 in response to mononuclear cells from the spinal cords of TMEV-infected mice is virus specific and not the result of a nonspecific T-cell stimulus delivered by these APCs.
FIG. 5.
Specificity of endogenous antigen presentation by CNS-infiltrating mononuclear cells to TMEV-specific (sTV1) and hMyo-specific T-cell lines. Plastic-adherent, CNS-infiltrating mononuclear cells prepared from mice with TMEV (n = 30, mean score = 1.9) and R-EAE (n = 29, mean score = 1.2) were cultured with sTV1 or hMyo-specific Th1 cells (2 × 104) with UV-inactivated TMEV (BeAn; 5 μg/ml) or hMyo (10 μM), respectively. The results are expressed as the counts per minute in the absence (open bars) or presence (filled bars) of added antigen. Responses of cultures of CNS-derived APC from TMEV-infected mice should be compared to those obtained from the culture of R-EAE-derived CNS APC cultured in the absence of TMEV (group G).
DISCUSSION
In this study, we examined the phenotype and function of cells infiltrating the CNS of mice infected with TMEV for evidence that viral antigens are presented to T cells within the CNS. By FACS and immunohistochemistry, expression of MHC class II in the spinal cords of infected mice was found predominantly on macrophages and microglia in demyelinating lesions. In addition, these MHC class II-bearing macrophages expressed B7-1 and B7-2 costimulatory molecules at levels exceeding those found in the spleens of infected mice. Immunohistochemistry revealed B7-1 expressed predominantly on large cells in spinal cord lesions that were probably infiltrating macrophages, while B7-2 was expressed both on F4/80+ macrophages and microglia and on a subpopulation of CD4+ T cells. Most interestingly, plastic-adherent F4/80+, I-As+ cells freshly isolated from the spinal cords of TMEV-infected mice were able to process and present TMEV and hMyo to antigen-specific T-cell lines and activate a TMEV VP274–86-specific T-cell line in the absence of added antigen. This finding provides conclusive evidence for the endogenous processing and presentation of virus epitopes within the CNS of persistently infected SJL/J mice.
In contrast to macrophages and microglia, astrocytes rarely expressed detectable MHC class II, although fibrillary gliosis associated with the CNS damage was clearly seen (Fig. 1B). Astrocytes are abundant glial cells that are responsive to CNS injury. It is significant, therefore, that I-As labeling in general did not overlap that of GFAP in spinal cord sections from persistently infected mice. There is ample evidence that astrocytes in vitro can present antigens, including myelin epitopes, to T cells, especially after upregulation of surface MHC expression by IFN-γ treatment (51, 59). Borrow and Nash (2) showed that cultured neonatal astrocytes treated with IFN-γ could present TMEV to antigen-specific T cells in vitro. Moreover, the ability of astrocytes derived from different strains of mice to upregulate MHC class II expression correlated with their susceptibility to TMEV-induced demyelination (2). Superficially, these results implicate astrocytes in the induction of demyelination. However, activated astrocytes have been reported to suppress T-cell responses in vitro (42), and studies using chimeric animals suggest that nonhematopoietic cells are very inefficient at inducing EAE (50). In contrast, MHC compatibility solely among hematopoetic cells is sufficient for EAE induction (20, 22, 50). It has been reported that microglia are irradiation resistant and are replaced slowly or not at all in bone marrow chimeric animals (20). Thus, in bone marrow chimeric animals, microglia are mostly of the recipient H-2 type, suggesting that even microglia may not be necessary for the induction of EAE. Our results do not directly address the antigen-presenting function of astrocytes in mice with ongoing TMEV-induced demyelinating disease. It is possible that the few astrocytes which express I-As are an extraordinarily potent APC population or that expression levels of MHC class II (below the limit of detection by immunohistochemistry) are sufficient for astrocytes to participate in activation of CNS-infiltrating T cells as suggested by in vitro antigen presentation studies (51, 59).
While studies using chimeric animals support the importance of hematopoeitic cells, including macrophages and microglia, in the induction of EAE, differentiating the roles of these two populations in CNS inflammation has proven difficult because of their common origin and similar phenotypes. A range of macrophage lineage markers including F4/80, MOMA-2, and Mac-1 have been found on microglia in normal and inflamed CNS tissue (53). While these are found on many types of macrophages including microglia (53), sialoadhesin expression is limited to infiltrating macrophages plus microglia exposed to serum factors by compromise of the blood-brain barrier (52). Thus, in the normal CNS parenchyma, sialoadhesin distinguishes resting microglia from macrophages, whereas at sites of inflammation some microglia might also be induced to express sialoadhesin. Our results show the expression of MHC class II predominantly on sialoadhesin-positive cells which, therefore, are most likely macrophages and microglia involved in the lesion. The few sialoadhesin-negative cells that appeared to be I-As+ at the edges of the lesions (Fig. 1A) are possibly activated microglia. Functionally, the results of Ford et al. (12) indicated that FACS-sorted rat microglia (CD11b/c+ CD45low) were poor at presenting guinea pig MBP to an MBP-specific T-cell line, as measured by [3H]TdR uptake or IL-2 production. Conversely, CD11b/c+ CD45high macrophages from normal rat brains were functional APCs able to stimulate maximal proliferation and IL-2 production. These results suggest that the major population of APCs, even in the normal CNS, is composed of a few infiltrating macrophages which may correspond to the radiation-sensitive, perivascular macrophages and microglia previously described (20, 25) or to other transient macrophages such as those in the choroid plexus (25, 53).
There are several implications of these findings for the pathogenesis of TMEV-induced demyelination. First, the events initiating demyelination following TMEV infection need not involve presentation by macroglial or microglial cells since there are CD45high Mac-1+ macrophages present in the normal adult CNS that are efficient at presenting antigens. Moreover, because intracerebral inoculation of TMEV produces a transient peripheral viremia, specific immune responses might arise first outside the CNS. This possibility is consistent with our previous results in which induction of peripheral, virus-specific tolerance inhibited the development of virus-specific DTH and proliferative responses and protected mice from developing demyelination (27, 28). Second, once the virus is cleared from the periphery and CNS inflammation is established, antigen presentation could occur on persistently infected macrophages in the CNS, sustaining disease progression. This possibility is consistent with the location of MHC class II- and sialoadhesin-positive, F4/80+ macrophages and microglia and with results showing that the major burden of viral antigens is in infiltrating macrophages (5, 41, 56). Antigen presentation on persistently infected astrocytes might also occur but probably would not be necessary for the progression of disease. Third, the greater capacity for antigen presentation by macrophages than by microglia (12) indirectly suggests that the endogenous presentation of viral antigens that we observed most likely occurred on infiltrating macrophages and not microglia or astrocytes in the absence of IFN-γ pretreatment. Previous work by Sedgwick et al. (57) showed that the distinctive CD45low CD11b/c+ microglial cell population is found in rats with JHM coronavirus encephalomyelitis along with a large influx of CD45high macrophages; however, the antigen-presenting ability of these cells when sorted from inflammatory lesions was not tested. It will be interesting to see if the distinction between macrophages and microglia based on CD45 expression levels holds in normal and TMEV-infected SJL/J mice and whether these cell types differ in the ability to be persistently infected and to process and present viral antigen. While published studies of microglial APC function have used cells cultured from brain tissue (19, 57), we have been unable to derive sufficient microglia directly from naive SJL/J spinal cords to directly compare the APC functions of naive and activated infiltrating macrophages and microglia.
The expression of costimulatory molecules by CNS-infiltrating macrophages is further evidence that these cells are the primary APCs in the CNS during TMEV infection. By FACS (Fig. 2), the majority of I-As+ cells were F4/80+ and also B7-1+ or B7-2+. This finding was supported by immunohistochemical analyses (Fig. 2) which revealed the colocalization of both B7-1 and B7-2 to large F4/80+ cells. The temporal regulation of B7 expression on the cell surface has not been fully resolved for all cell types. Some groups report that B7-2 is rapidly induced on the surface of activated B cells whereas B7-1 peaks 3 to 4 days later (32), while others report similarity between the kinetics of B7-1 and B7-2 increases on lipopolysaccharide or anti-IgD-dextran-activated B cells (18). Curiously, CD4+ T cells in the lesions expressed predominantly B7-2, especially on larger blast-like cells at the margins of the lesions, suggesting that these cells were activated more recently than the T cells dominating the lesion center.
The comparison of MHC class II and B7 expression between TMEV-induced demyelination and R-EAE is important in relation to our recent data on the manipulation of B7 molecules in vivo during R-EAE in the SJL/J mouse (49). As in TMEV-infected mice, F4/80+ cells expressed high levels of MHC class II, B7-1, and B7-2. Likewise, B7-1 became the dominant costimulatory molecule, as its expression increased relative to that of B7-2 over time in mice with R-EAE. In vivo administration of MAbs against B7-1 or B7-2 has revealed that these molecules have distinct functions in the induction and progression of EAE (29, 49). Moreover, blockade of anti-B7-1 with Fab fragments during disease remission inhibited subsequent disease relapses by preventing activation of T cells specific for endogenous myelin epitopes (i.e., epitope spreading) which play a major role in mediating the pathogenesis of R-EAE relapses (44, 49). We have recently shown the development of DTH and proliferative responses to multiple encephalitogenic myelin epitopes in the spleens and lymph nodes of TMEV-infected SJL/J mice during the chronic course of disease (47, 48). Thus, the relative increase in B7-1 on F4/80+ cells that appears to be important to disease progression in R-EAE may be similarly important in the progression of TMEV-induced demyelination and moreover may serve as a target for immunotherapy. Experiments are ongoing to clarify the role of costimulation in endogenous antigen presentation, epitope spreading, and the progression of disease. Preliminary studies indicate that endogenous presentation of TMEV epitopes by CNS-infiltrating cells is B7 dependent, as proliferation is blocked by CTLA-4 Ig (unpublished data).
In addition to astrocytes, activated microglia, and infiltrating macrophages, there are other candidate APCs within the CNS that require brief consideration. Antigen-specific B cells are especially efficient APCs (31) and could participate in the activation of T cells within the CNS. Previous FACS experiments quantitating the B-cell-restricted CD45 isoform B220 showed that a very small fraction of the mononuclear cell infiltrates in TMEV-infected spinal cords, usually less than 1%, were B cells (data not shown). In our analyses of MHC class II expression, a small number of F4/80−, MHC class II+ events were detected among the cells isolated from the spinal cords (Fig. 2), perhaps representing small numbers of MHC class II+ B cells. Because of their very small numbers in the CNS, however, it was impractical to directly isolate and test the APC function of the B220+ B cells. Thus, it remains possible that CNS-resident B cells contribute APC function to the disease process.
Perhaps the most interesting observation is the ability of plastic-adherent cells isolated from the spinal cords of mice infected with TMEV several months previously to endogenously activate TMEV-specific T cells by presenting viral epitopes originating in vivo (Fig. 4 and 5). The antigen specificity of this activation was confirmed by the inability of analogous cells isolated from the spinal cords of mice with R-EAE to activate virus-specific T cells in the absence of added virus. Furthermore, TMEV-derived CNS APC did not stimulate T-cell lines specific for hMyo (Fig. 5) without the addition of exogenous, specific antigen. It is unlikely that a significant source of viral antigen came from processing and presentation of debris from infected cells during the APC isolation procedure, especially since the majority of the viral antigen (9, 41) and infectious virus (5) is already present in the infiltrating macrophage population. In this regard, incorporation of splenic APC into dissociated spinal cord homogenates prepared from naive mice does not result in activation of a highly sensitive PLP139–151-specific T-cell line when these cells are subjected to the identical Percoll gradient, plastic adherence isolation procedure and tested for APC activity (data not shown).
The finding that cells isolated from the CNS of TMEV-infected mice contain and present viral antigens to T cells ex vivo is significant to understanding the pathogenesis of MS for several reasons. First, HLA class II has been observed by a number of investigators in lesions from patients with M5 (23, 60, 62). The positive cells have been variously identified as macrophages, astrocytes, and endothelial cells. Expression of B7 molecules in MS lesions was shown recently by three different laboratories (10, 64, 65). Several types of cells in MS lesions, therefore, are equipped to fully activate T cells within the CNS. Second, IL-2R-bearing cells have been observed in the lesions (23), as have products of activated T cells, including IFN-γ (61) and IL-2 (23). This finding further suggests local antigen presentation. Third, the epidemiology of MS strongly suggests a role for an infectious agent, perhaps a virus, that is widespread, chronic, and usually subclinical (30). Presentation within the CNS of viral antigens (leading to bystander demyelination), of neuroantigens cross-reactive with viral antigens (molecular mimicry), and of neuroantigens liberated by virus-induced CNS damage (epitope spreading) are all possible mechanisms by which pathogenic immune reactions could be initiated by viruses within the CNS. Our results directly demonstrate that sufficient viral antigen was present in the APC population within the CNS of TMEV-infected mice to activate a sensitive, virus-specific T-cell line. Thus, a chronic CNS infection seems mechanistically plausible as an initiating event in the etiology of MS. Finally, our results may be important in considering new therapies for MS. The phenomenon of epitope spreading may make the goal of antigen-specific therapy for MS elusive. Nevertheless, if epitope spreading proves to be as important to the progression of MS as it appears to be in R-EAE (44), and perhaps in TMEV infection (48), effective molecules to target might be B7-1 and/or B7-2. A cautious approach will be required, nonetheless, as current data seem to indicate that the functions of these molecules shift from acute to chronic disease from possibly ameliorative to potentially harmful.
ACKNOWLEDGMENTS
This work was supported by PHS grants NS-23349 and NS-21913 from NIH and by a Howard Hughes predoctoral fellowship (J.G.P.).
REFERENCES
- 1.Berkower I, Buckenmeyer G K, Gurd F R, Berzofsky J A. A possible immunodominant epitope recognized by murine T lymphocytes immune to different myoglobins. Proc Natl Acad Sci USA. 1982;79:4723–4727. doi: 10.1073/pnas.79.15.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borrow P, Nash A A. Susceptibility to Theiler’s virus-induced demyelinating disease correlates with astrocyte class II induction and antigen presentation. Immunology. 1992;76:133–139. [PMC free article] [PubMed] [Google Scholar]
- 3.Cash E, Rott O. Microglial cells qualify as the stimulators of unprimed CD4+ and CD8+ T lymphocytes in the central nervous system. Clin Exp Immunol. 1994;98:313–318. doi: 10.1111/j.1365-2249.1994.tb06143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clatch R J, Melvold R W, Dal Canto M C, Miller S D, Lipton H L. The Theiler’s murine encephalomyelitis virus (TMEV) model for multiple sclerosis shows a strong influence of the murine equivalents of HLA-A, B, and C. J Neuroimmunol. 1987;15:121–135. doi: 10.1016/0165-5728(87)90087-7. [DOI] [PubMed] [Google Scholar]
- 5.Clatch R J, Miller S D, Metzner R, Dal Canto M C, Lipton H L. Monocytes/macrophages isolated from the mouse central nervous system contain infectious Theiler’s murine encephalomyelitis virus (TMEV) Virology. 1990;176:244–254. doi: 10.1016/0042-6822(90)90249-q. [DOI] [PubMed] [Google Scholar]
- 6.Cserr H F, Knopf P M. Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: a new view. Immunol Today. 1992;13:507–512. doi: 10.1016/0167-5699(92)90027-5. [DOI] [PubMed] [Google Scholar]
- 7.Dal Canto M C. Uncoupled relationship between demyelination and primary infection of myelinating cells in Theiler’s virus encephalomyelitis. Infect Immun. 1982;35:1133–1138. doi: 10.1128/iai.35.3.1133-1138.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dal Canto M C, Barbano R L. Immunocytochemical localization of MAG, MBP and P0 protein in acute and relapsing demyelinating lesions of Theiler’s virus infection. J Neuroimmunol. 1985;10:129–140. doi: 10.1016/0165-5728(85)90003-7. [DOI] [PubMed] [Google Scholar]
- 9.Dal Canto M C, Lipton H L. Ultrastructural immunohistochemical localization of virus in acute and chronic demyelinating Theiler’s virus infection. Am J Pathol. 1982;106:20–29. [PMC free article] [PubMed] [Google Scholar]
- 10.De Simone R, Giampaolo A, Giometto B, Gallo P, Levi G, Peschle C, Aloisi F. The costimulatory molecule B7 is expressed on human microglia in culture and in multiple sclerosis acute lesions. J Neuropathol Exp Neurol. 1995;54:175–187. doi: 10.1097/00005072-199503000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Fontana A, Fierz W, Wekerle H. Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature. 1984;307:273–276. doi: 10.1038/307273a0. [DOI] [PubMed] [Google Scholar]
- 12.Ford A L, Goodsall A L, Hickey W F, Sedgwick J D. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol. 1995;154:4309–4321. [PubMed] [Google Scholar]
- 13.Frei K, Siepl C, Groscurth P, Bodmer S, Schwerdel C, Fontana A. Antigen presentation and tumor cytotoxicity by interferon-gamma-treated microglial cells. Eur J Immunol. 1987;17:1271–1278. doi: 10.1002/eji.1830170909. [DOI] [PubMed] [Google Scholar]
- 14.Friedmann A, Frankel G, Lorch Y, Steinman L. Monoclonal anti-I-A antibody reverses chronic paralysis and demyelination in Theiler’s virus-infected mice: critical importance of timing of treatment. J Virol. 1987;61:898–903. doi: 10.1128/jvi.61.3.898-903.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerety S J, Clatch R J, Lipton H L, Goswami R G, Rundell M K, Miller S D. Class II-restricted T cell responses in Theiler’s murine encephalomyelitis virus-induced demyelinating disease. IV. Identification of an immunodominant T cell determinant on the N-terminal end of the VP2 capsid protein in susceptible SJL/J mice. J Immunol. 1991;146:2401–2408. [PubMed] [Google Scholar]
- 16.Gerety S J, Rundell M K, Dal Canto M C, Miller S D. Class II-restricted T cell responses in Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease. VI. Potentiation of demyelination with and characterization of an immunopathologic CD4+ T cell line specific for an immunodominant VP2 epitope. J Immunol. 1994;152:919–929. [PubMed] [Google Scholar]
- 17.Graves M C, Bologa L, Siegel L, Londe H. Theiler’s virus in brain cell cultures: lysis of neurons and oligodendrocytes and persistence in astrocytes and macrophages. J Neurosci Res. 1986;15:491–501. doi: 10.1002/jnr.490150406. [DOI] [PubMed] [Google Scholar]
- 18.Hathcock K S, Laszlo G, Pucillo C, Linsley P, Hodes R J. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med. 1994;180:631–640. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Havenith C E G, Askew D, Walker W S. Mouse resident microglia: isolation and characterization of immunoregulatory properties with naive CD4+ and CD8+ T cells. Glia. 1998;22:348–359. [PubMed] [Google Scholar]
- 20.Hickey W F, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 21.Hickey W F, Osborn J P, Kirby W M. Expression of Ia molecules by astrocytes during acute experimental allergic encephalomyelitis in the Lewis rat. Cell Immunol. 1985;91:528–535. doi: 10.1016/0008-8749(85)90251-5. [DOI] [PubMed] [Google Scholar]
- 22.Hinrichs D J, Wegmann K W, Dietsch G N. Transfer of experimental allergic encephalomyelitis to bone marrow chimeras. Endothelial cells are not a restricting element. J Exp Med. 1987;166:1906–1911. doi: 10.1084/jem.166.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofman F M, von Hanwehr R I, Dinarello C A, Mizel S B, Hinton D, Merrill J E. Immunoregulatory molecules and IL 2 receptors identified in multiple sclerosis brain. J Immunol. 1986;136:3239–3245. [PubMed] [Google Scholar]
- 24.Jenkins M K. The ups and downs of costimulation. Immunity. 1995;1:443–446. doi: 10.1016/1074-7613(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 25.Jordan F L, Thomas W E. Brain macrophages: a question of origin and interrelationship. Brain Res Rev. 1988;13:165–178. doi: 10.1016/0165-0173(88)90019-7. [DOI] [PubMed] [Google Scholar]
- 26.June C H, Bluestone J A, Nadler L M, Thompson C B. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 27.Karpus W J, Peterson J D, Miller S D. Anergy in vivo: down-regulation of antigen-specific CD4+ Th1 but not Th2 cytokine responses. Int Immunol. 1994;6:721–730. doi: 10.1093/intimm/6.5.721. [DOI] [PubMed] [Google Scholar]
- 28.Karpus W J, Pope J G, Peterson J D, Dal Canto M C, Miller S D. Inhibition of Theiler’s virus-mediated demyelination by peripheral immune tolerance induction. J Immunol. 1995;155:947–957. [PubMed] [Google Scholar]
- 29.Kuchroo V K, Das M P, Brown J A, Ranger A M, Zamvil S S, Sobel R A, Weiner H L, Nabavi N, Glimcher L H. B7-1 and B7-2 costimulatory molecules differentially activate the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 30.Kurtzke J F. Epidemiologic evidence for multiple sclerosis as an infection. Clin Microbiol Rev. 1993;6:382–427. doi: 10.1128/cmr.6.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lanzavecchia A. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu Rev Immunol. 1990;8:773–793. doi: 10.1146/annurev.iy.08.040190.004013. [DOI] [PubMed] [Google Scholar]
- 32.Lenschow D J, Su G H, Zuckerman L A, Nabavi N, Jellis C L, Gray G S, Miller J, Bluestone J A. Expression and functional significance of an additional ligand for CTLA-4. Proc Natl Acad Sci USA. 1993;90:11054–11058. doi: 10.1073/pnas.90.23.11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lenschow D J, Walunas T L, Bluestone J A. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 34.Levy M, Aubert C, Brahic M. Theiler’s virus replication in brain macrophages cultured in vitro. J Virol. 1992;66:3188–3193. doi: 10.1128/jvi.66.5.3188-3193.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipton H L, Dal Canto M C. Chronic neurologic disease in Theiler’s virus infection of SJL/J mice. J Neurol Sci. 1976a;30:201–207. doi: 10.1016/0022-510x(76)90267-7. [DOI] [PubMed] [Google Scholar]
- 36.Lipton H L, Dal Canto M C. Theiler’s virus-induced demyelination: prevention by immunosuppression. Science. 1976b;192:62–64. doi: 10.1126/science.176726. [DOI] [PubMed] [Google Scholar]
- 37.Lipton H L, Dal Canto M C. Contrasting effects of immunosuppression on Theiler’s virus infection in mice. Infect Immun. 1977;15:903–909. doi: 10.1128/iai.15.3.903-909.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipton H L, Dal Canto M C. Susceptibility of inbred mice to chronic central nervous system infection by Theiler’s murine encephalomyelitis virus. Infect Immun. 1979;26:369–374. doi: 10.1128/iai.26.1.369-374.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lipton H L, Gonzalez-Scarano F. Central nervous system immunity in mice infected with Theiler’s virus. I. Local neutralizing antibody response. J Infect Dis. 1978;137:145–151. doi: 10.1093/infdis/137.2.145. [DOI] [PubMed] [Google Scholar]
- 40.Lipton H L, Melvold R. Genetic analysis of susceptibility to Theiler’s virus-induced demyelinating disease in mice. J Immunol. 1984;132:1821–1825. [PubMed] [Google Scholar]
- 41.Lipton H L, Twaddle G, Jelachich M L. The predominant virus antigen burden is present in macrophages in Theiler’s murine encephalomyelitis virus-induced demyelinating disease. J Virol. 1995;69:2525–2533. doi: 10.1128/jvi.69.4.2525-2533.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsumoto Y, Ohmori K, Fujiwara M. Immune regulation by brain cells in the central nervous system: microglia but not astrocytes present myelin basic protein to encephalitogenic T cells under in vivo-mimicking conditions. Immunology. 1992;76:209–216. [PMC free article] [PubMed] [Google Scholar]
- 43.McRae B L, Kennedy M K, Tan L J, Dal Canto M C, Miller S D. Induction of active and adoptive chronic-relapsing experimental autoimmune encephalomyelitis (EAE) using an encephalitogenic epitope of proteolipid protein. J Neuroimmunol. 1992;38:229–240. doi: 10.1016/0165-5728(92)90016-e. [DOI] [PubMed] [Google Scholar]
- 44.McRae B L, Vanderlugt C L, Dal Canto M C, Miller S D. Functional evidence for epitope spreading in the relapsing pathology of EAE in the SJL/J mouse. J Exp Med. 1995;182:75–85. doi: 10.1084/jem.182.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller S D, Clatch R J, Pevear D C, Trotter J L, Lipton H L. Class II-restricted T cell responses in Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease. I. Cross-specificity among TMEV substrains and related picornaviruses, but not myelin proteins. J Immunol. 1987;138:3776–3784. [PubMed] [Google Scholar]
- 46.Miller S D, Gerety S J, Kennedy M K, Peterson J D, Trotter J L, Tuohy V K, Waltenbaugh C, Dal Canto M C, Lipton H L. Class II-restricted T cell responses in Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease. III. Failure of neuroantigen-specific immune tolerance to affect the clinical course of demyelination. J Neuroimmunol. 1990;26:9–23. doi: 10.1016/0165-5728(90)90115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller S D, McRae B L, Vanderlugt C L, Nikcevich K M, Pope J G, Pope L, Karpus W J. Evolution of the T cell repertoire during the course of experimental autoimmune encephalomyelitis. Immunol Rev. 1995;144:225–244. doi: 10.1111/j.1600-065x.1995.tb00071.x. [DOI] [PubMed] [Google Scholar]
- 48.Miller S D, Vanderlugt C L, Begolka W S, Pao W, Yauch R L, Neville K L, Katz-Levy Y, Carrizosa A, Kim B S. Persistent infection with Theiler’s virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- 49.Miller S D, Vanderlugt C L, Lenschow D J, Pope J G, Karandikar N J, Dal Canto M C, Bluestone J A. Blockade of CD28/B7-1 interaction prevents epitope spreading and clinical relapses of murine EAE. Immunity. 1995;3:739–745. doi: 10.1016/1074-7613(95)90063-2. [DOI] [PubMed] [Google Scholar]
- 50.Myers K J, Dougherty J P, Ron Y. In vivo antigen presentation by both brain parenchymal cells and hematopoietically derived cells during the induction of experimental autoimmune encephalomyelitis. J Immunol. 1993;151:2252–2260. [PubMed] [Google Scholar]
- 51.Nikcevich K M, Gordon K B, Tan L, Hurst S D, Kroepfl J F, Gardinier M, Barrett T A, Miller S D. Interferon-gamma activated primary murine astrocytes express B7 costimulatory molecules and prime naive antigen-specific T cells. J Immunol. 1997;158:614–621. [PubMed] [Google Scholar]
- 52.Perry V H, Crocker P R, Gordon S. The blood-brain barrier regulates the expression of a macrophage sialic acid-binding receptor on microglia. J Cell Sci. 1992;101:201–207. doi: 10.1242/jcs.101.1.201. [DOI] [PubMed] [Google Scholar]
- 53.Perry V H, Hume D A, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15:313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- 54.Pope J G, Karpus W J, Vanderlugt C L, Miller S D. Flow cytometric and functional analyses of CNS-infiltrating cells in SJL/J mice with Theiler’s virus-induced demyelinating disease: evidence for a CD4+ T cell-mediated pathology. J Immunol. 1996;156:4050–4058. [PubMed] [Google Scholar]
- 55.Rodriguez M, Leibowitz J L, Lampert P W. Persistent infection of oligodendrocytes in Theiler’s virus-induced encephalomyelitis. Ann Neurol. 1983;13:426–433. doi: 10.1002/ana.410130409. [DOI] [PubMed] [Google Scholar]
- 56.Rossi C P, Delcroix M, Huitinga I, McAllister A, Van Rooijen N, Claassen E, Brahic M. Role of macrophages during Theiler’s virus infection. J Virol. 1997;71:3336–3340. doi: 10.1128/jvi.71.4.3336-3340.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sedgwick J D, Schwender S, Imrich H, Dörries R, Butcher G W, ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci USA. 1991;88:7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steiner C M, Rozhon E J, Lipton H L. Relationship between host age and persistence of Theiler’s virus in the central nervous system of mice. Infect Immun. 1984;43:432–434. doi: 10.1128/iai.43.1.432-434.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tan L J, Gordon K B, Mueller J P, Matis L A, Miller S D. Presentation of proteolipid protein epitopes and B7-1-dependent activation of encephalitogenic T cells by IFN-γ-activated SJL/J astrocytes. J Immunol. 1998;160:4271–4279. [PubMed] [Google Scholar]
- 60.Traugott U. Multiple sclerosis: relevance of class I and class II MHC-expressing cells to lesion development. J Neuroimmunol. 1987;16:283–302. doi: 10.1016/0165-5728(87)90082-8. [DOI] [PubMed] [Google Scholar]
- 61.Traugott U, Lebon P. Demonstration of alpha, beta, and gamma interferon in active chronic multiple sclerosis lesions. Ann NY Acad Sci. 1988;540:309–311. doi: 10.1111/j.1749-6632.1988.tb27083.x. [DOI] [PubMed] [Google Scholar]
- 62.Traugott U, Reinherz E L, Raine C S. Multiple sclerosis: distribution of T cell subsets within active chronic lesions. Science. 1983;219:308–310. doi: 10.1126/science.6217550. [DOI] [PubMed] [Google Scholar]
- 63.Welsh C J, Tonks P, Nash A A, Blakemore W F. The effect of L3T4 T cell depletion on the pathogenesis of Theiler’s murine encephalomyelitis virus infection in CBA mice. J Gen Virol. 1987;68:1659–1667. doi: 10.1099/0022-1317-68-6-1659. [DOI] [PubMed] [Google Scholar]
- 64.Williams K, Ulvestad E, Antel J P. B7/BB-1 antigen expression on adult human microglia studied in vitro and in situ. Eur J Immunol. 1994;24:3031–3037. doi: 10.1002/eji.1830241217. [DOI] [PubMed] [Google Scholar]
- 65.Windhagen A, Newcombe J, Dangond F, Strand C, Woodroofe M N, Cuzner M L, Hafler D A. Expression of costimulatory molecules B7-1 (CD80), B7-2 (CD80), and interleukin 13 cytokine in multiple sclerosis lesions. J Exp Med. 1995;182:1985–1996. doi: 10.1084/jem.182.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]