Abstract
Background
Hepatic artery infusion chemotherapy (HAI) has been proposed as a valuable adjunct for multimodal therapy of primary and secondary liver malignancies. This review provides an overview of the currently available evidence of HAI, taking into account tumor response and long-term oncologic outcome.
Summary
In colorectal liver metastases (CRLM), HAI in combination with systemic therapy leads to high response rates (85–90%) and conversion to resectablity in primary unresectable disease in up to 50%. HAI in combination with systemic therapy in CRLM in the adjuvant setting shows promising long-term outcomes with up to 50% 10-year survival in a large, non-randomized single-center cohort. For hepatocellular carcinoma patients, response rates as high as 20–40% have been reported for HAI and long-term outcomes compare well to other therapies. Similarly, survival for patients with unresectable intrahepatic cholangiocarcinoma 3 years after treatment with HAI is reported as high as 34%, which compares well to trials of systemic therapy where 3-year survival is usually below 5%. However, evidence is mainly limited by highly selected, heterogenous patient groups, and outdated chemotherapy regimens. The largest body of evidence stems from small, often non-randomized cohorts, predominantly from highly specialized single centers.
Key Message
In well-selected patients with primary and secondary liver malignancies, HAI might improve response rates and, possibly, long-term survival. Results of ongoing randomized trials will show whether a wider adoption of HAI is justified, particularly to increase rates of resectability in advanced malignant diseases confined to the liver.
Keywords: Hepatic artery infusion chemotherapy, Hepatocellular carcinoma, Colorectal liver metastases, Conversion therapy, Cholangiocarcinoma
Introduction
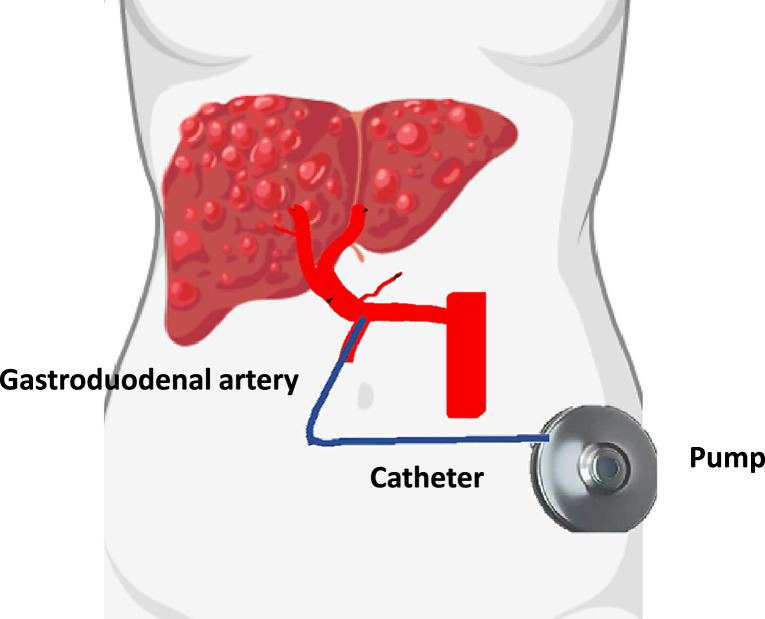
The liver has the unique anatomic configuration of a dual blood supply over the portal vein (PV) and the hepatic artery (HA), an important consideration for any systemic treatment of liver tumors [1]. Primary and secondary tumors derive their blood supply mainly from the arterial-rather than the portal system and are therefore susceptible to intra-arterial chemotherapy [2]. Tumor growth is reduced by inhibition of angiogenesis and induction of tumor cell apoptosis [3–5]. Hepatic artery infusion chemotherapy (HAI) is applied by an implantable pump with a catheter inserted into the gastroduodenal artery (GDA). The chemotherapy dosage in the tumor is increased up to 400-fold compared to systemic treatment, while the normal hepatocytes receive a lower dose due to the different perfusion pattern. Due to the high first-pass effect of drugs in the liver, fewer systemic side effects occur [6]. Implantation of a HAI pump is a standardized procedure that can nowadays be performed minimally invasive either laparoscopically or via robotic approach [7–9].
Colorectal cancer is the fourth leading cancer worldwide in terms of incidence with over 1.9 million new cases in 2020 [10]. Colorectal liver metastases (CRLM) are the main prognostic driver. Approximately 25% of the patients present with synchronous liver metastases, while another 25% will develop metachronous CRLM at a later stage [11]. Only 20–30% of those CRLM are resectable upon first detection; therefore, a multimodal treatment approach is crucial and involves both systemic and local therapies. Current systemic chemotherapy (SCT) regimens combine two to three agents, i.e., 5-Flurouracil (5-FU), leucovorin, and/or oxaliplatin or irinotecan. Furthermore, modern chemotherapy combined with biological agents has increased response rates to 60–70%. Ultimately and including downstaging by HAI, up to 40% of initially unresectable CRLM can be converted to resectable disease, leading to a prognosis that is comparable to upfront resectable CRLM [12, 13].
On the other hand, primary liver cancer is less common with 0.9 million new cases worldwide in 2020 [14]. Hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCC) are the most frequent primary liver tumors and often diagnosed in an intermediate or advanced stage. In this subgroup of patients, current treatment recommendations include transarterial chemoembolization (TACE), selective internal radiotherapy (SIRT), and systemic therapy [15, 16].
Similar to CRLM, primary liver tumors are amenable to intra-arterial therapy due to their supplying arteries, the so-called feeding vessels. Especially the management of CCC, a heterogenous and difficult to diagnose entity, is challenging due to often advanced disease stage at the time of diagnosis [17]. Multimodal treatment for downstaging is of paramount importance to convert unresectable to resectable disease in primary liver cancer [18].
Current indications for HAI are (1) downstaging of unresectable tumors, (2) adjuvant treatment after resection, or (3) a palliative therapeutic approach. HAI is usually combined with SCT. In this article, we provide a review of the highest level of evidence on the current application of HAI for primary and secondary liver tumors. The evidence level was appraised by the Centre for Evidence-Based Medicine (http://www.cebm.net).
Indication and Contraindication
For the preoperative work-up, multiple considerations have to be carefully taken into account when assessing patients’ eligibility for HAI.
Arterial Perfusion Anatomy
A prerequirement is the single arterial inflow to the liver without inflow to adjacent organs. Furthermore, a patent GDA with an adequate diameter is required to deliver the agent to the HA. High-quality preoperative imaging studies are mainly derived from CT-angiography while catheter-based angiography is rarely performed. Images need to be available preoperatively for the evaluation of the vessel anatomy, anomalies, and patency. Aberrant, replaced or accessory vessel configurations need to be identified [19].
Liver Function
Damage to the liver parenchyma has either already occurred or contributed to the development of a primary liver tumor, in addition the damage of past and future drug toxicities has to be anticipated. Pre-existing liver disease and portal hypertension may increase morbidity from the pump placement and also reduce tolerability of chemotherapy which reduces the therapy effect. Currently, there is no clear, evidence-based cut-off, below which the liver function is considered insufficient for HAI.
Liver function tests are manifold and generally indirectly reflect the liver function. Basic tests include excretory and synthetic function, namely bilirubin, albumin, and coagulation factors. Ascites may indicate liver dysfunction or advanced disease stages. Patients must also be able to tolerate the often simultaneously administered SCT. HAI in patients with Child-Pugh A liver disease has shown to be feasible and safe and can potentially offer a less toxic treatment option than systemic monotherapy in patients with Child-Pugh B cirrhosis [20, 21].
Surgical Technique
The implantation of a HA catheter is a complex technical procedure which requires an experienced hepatobiliary surgeon (Fig. 1). Minimally invasive and especially a robot assisted approach have been adopted, nevertheless the open approach remains a preferred technique [22]. The following steps need to be followed during the implantation of the pump:
-
1.
Exploration of the abdominal cavity to identify extrahepatic disease.
-
2.
Division of the falciform ligament and ligamentum teres.
-
3.
Definition of the arterial anatomy by identification of the right gastric artery, the common HA, and the GDA.
-
4.
Isolation of the GDA and dissection of lymphatic tissue from the hepatoduodenal ligament to gain sufficient space and to identify accessory and replaced arteries to the liver that need to be ligated to avoid extrahepatic application of chemotherapy.
-
5.
A transverse skin incision in the left middle abdomen for the subcutaneous pump.
-
6.
The catheter is inserted into the abdominal cavity and the GDA is clamped proximally and ligated distally.
-
7.
The catheter is inserted through a transverse arteriotomy until the tip of the catheter lies just outside the HA lumen, and the position of the artery is secured with a nonabsorbable suture.
-
8.
Cholecystectomy is always performed to prevent chemotherapy-induced cholecystitis.
Fig. 1.
Hepatic arterial catheter placement in the GDA.
Surgical complications comprise HA thrombosis, dissection of the HA due to implantation, catheter dislocation, catheter leakage or misperfusion, and pocket infections. Failure of the pump can occur. Further, morbidity can be caused by the application of the chemotherapy and includes chemical hepatitis, biliary sclerosis, or gastrointestinal symptoms like ulcers, gastritis, or duodenitis.
Percutaneous implantation has been described via a femoral arterial access or less often through other peripheral arteries [23–25]. The catheter is placed in the GDA and fixed with coils. The pump or port was similarly placed in the abdominal wall.
Colorectal Liver Metastasis
HAI without Systemic Therapy in Unresectable CRLM (Level of Evidence 1a)
The sole use of HAI without systemic therapy was abandoned due to the risk of extrahepatic disease progression without systemic therapy and limited benefit [26–29]. A meta-analysis compared mostly fluoropyrimidine-based HAI only with SCT for unresectable CRLM [30]. Except for one trial, the included studies were performed between 1987 and 2006. The pooled results of nine RCTs suggested a higher percentage of patients with tumor response undergoing HAI (43% vs. 20%; relative risk of downstaging: 2.10 [95% CI: 1.59–2.79; p < 0.001]). Although the authors found that HAI had a better overall survival (OS; hazard ratio [HR] 0.83 (95% CI: 0.70–0.99), p = 0.04), this finding was derived from a significant advantage against a subgroup of the control arm, in which more than 1 third of the patients had no therapy at all in the SCT group. Moreover, the analysis has to be interpreted with caution due to the significant heterogeneity among the included studies. Most of the included RCTs used fluoropyrimidine-based SCT, which is inferior to current combination therapies. Therefore, the effect of the evaluated SCT may be underestimated with regard to OS and tumor response.
The influence of the different HAI agents must also be taken into consideration. Some RCTs used floxuridine as therapeutic agent, with the advantage of higher liver extraction concentrations than 5-FU. But the high extraction rate can also be seen as a disadvantage in diseases not confined to the liver, as systemic blood concentration of floxuridine possibly is too low to treat extrahepatic metastases [31]. Lorenz and Müller [32] found a 3.2-fold higher incidence rate of extrahepatic metastases in patients with HAI-floxuridine (41%) compared with HAI-5-FU and folinic acid (13%). Another disadvantage of floxuridine is the hepatotoxicity, which can lead to primary biliary sclerosis and cholecystitis. 5.4% of patients treated with HAI-fluorodeoxyuridine (FUDR) died as a result of biliary sclerosis. In 10% of the HAI group and 5.6% of the control group, treatment was terminated due to severe toxicity.
Regarding the control SCT group, the current gold standard of 5-FU in combination with oxaliplatin and/or irinotecan and an EGFR- or VEGF-inhibitor was not part of most studies. Even though, OS rates were predominantly not different between the treatment groups, only hepatic tumor progression rates were lower in the HAI group [32].
In the SCT group severe diarrhea was the leading side effect also with dose-limiting toxicity and subsequent liver damage (e.g., biliary sclerosis). The latter can be reduced using dexamethasone. The dose-limiting toxicities in the SCT arm were diarrhea and myelosuppression.
HAI in Combination with SCT for the Treatment of Unresectable CRLM (Level of Evidence 2a)
The importance of SCT to enable resectability was emphasized by Lam and colleagues in a RCT, demonstrating response rates in patients with SCT of 64%, whereof 23% underwent curative liver resection [33]. A combined SCT and liver-directed therapy to improve response rate and progression-free survival (PFS) is an appealing treatment approach to further increase the chance of downstaging. The first study describing HAI with SCT was published in 1989 by Safi et al. [34] as phase I study comparing HAI-FUDR alone against HAI-FUDR and SCT but without a significant difference in response rates. The response rates in the CT-scan were 50% in both groups. No improvement of short-term survival was seen in either group during the 36-month follow-up; however, in patients with response to either HAI +/− chemotherapy the survival time increased significantly. RCTs in this area are rare and evidence mainly comes from a large cohort from the Memorial Sloan Kettering Cancer Center (MSKCC).
A phase II study combining HAI with SCT found the combination to be safe and effective. D’Angelica et al. [35] combined HAI-FUDR with best SCT based on prior patients’ chemotherapy history in patients with unresectable CRLM. In 76%, a tumor response was accomplished and most importantly in 47%, a conversion to resectable disease with prolonged survival was possible. Also, the combination HAI-FUDR with bevacizumab was investigated but showed an increased biliary toxicity, so the authors advised against this combination therapy. Considering surgical morbidity, 35% of the patients suffered from minor complications and only 1 patient had a major complication.
The long-term results from this MSKCC cohort including more patients described an even higher conversion to resection rate (52%) of prior unresectable CRLM. The 5-year survival rate in this single-arm study was excellent with 36% [36].
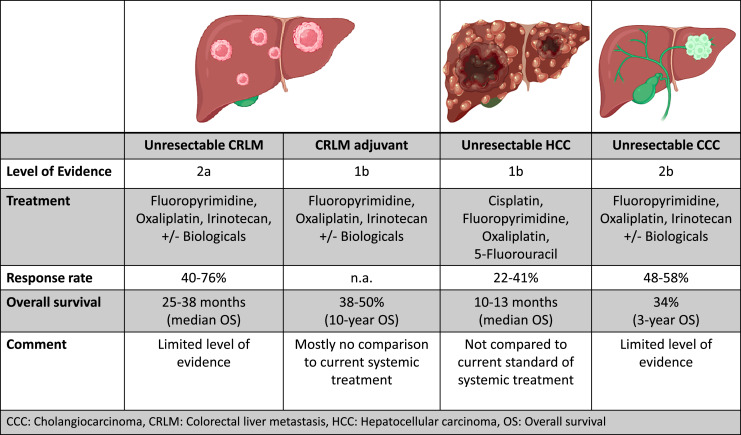
The application of irinotecan, oxaliplatin, and 5-FU in combination with intravenous cetuximab achieved response rates of 41% with an OS of 25.5 months. About 30% of patients accomplished resectability [37–40]. Kemeny et al. [41] reported even better numbers, 92% response rate and a resectability rate of 47% comparing HAI-5-FU and SCT with oxaliplatin and irinotecan (Fig. 2) [42]. An overview of studies is shown in Table 1.
Fig. 2.
Summary of the application of hepatic arterial infusion chemotherapy with clinical outcomes.
Table 1.
Studies assessing the outcome of HAI
| Author | Year | Country | Patients, n | Treatment (HAI) | OS | Response rate, % | Complete resection, % | Study design |
|---|---|---|---|---|---|---|---|---|
| Focan et al. [39] 1999 | 1999 | Belgium | 56 | FUDR + SCT 5-FU with different infusion rates | n.a | 38–48 | 22–27 | RCT |
| Carnaghi et al. [40] 2007 | 2007 | Italy | 39 | 5-FU + SCT oxaliplatin and folinic acid | 21 months | 41 | 21 | Single-arm phase II |
| Boige et al. [42] 2008 | 2008 | France | 44 | Oxaliplatin + SCT 5-FU and leucovorin | 16 months | 87 | 18 | Prospective |
| Kemeny et al. [41] 2009 | 2009 | US | 49 | Floxuridine and dexamethasone + SCT oxaliplatin and irinotecan | 39.8 months | 92 | 47 | Phase I |
| D’Angelica et al. [35] 2015 | 2015 | US | 49 | FUDR + SCT depending on history + bevacizumab | 38 months | 76 | 47 | Single-arm phase II |
| Levi et al. [37] 2016 | 2016 | France | 64 | Irinotecan, oxaliplatin, 5-FU + SCT cetuximab | 25.5 months | 41 | 30 | Single-arm phase II |
| Pak et al. [36] 2018 | 2018 | US | 64 | FUDR + SCT depending on history + bevacizumab | 38 months | 73 | 52 | Single-arm phase II |
HAI, hepatic arterial infusion chemotherapy; SCT, systemic chemotherapy; US, United States; FUDR, fluorodeoxyuridine, 5-FU, 5-FU, n.a. not applicable.
Adjuvant Therapy of CRLM (Level of Evidence 1b)
A recent meta-analysis included eight RCTs and 652 patients [43]. The pooled HR for OS was 0.91 (95% CI: 0.72–1.14) and therefore showed no difference in patients with or without HAI. Half of the studies investigated SCT as control group, whereas the other half compared to no treatment. When adding non-randomized studies, there was a benefit of HAI in terms of OS (HR: 0.77; 95% CI: 0.64–0.93). Important for the interpretation of the results, only two RCTs were published after 2010 and only two studies included more than 100 patients.
In further subgroup analyses including non-randomized studies, a benefit was predominantly shown using floxuridine (HR: 0.76, 95% CI: 0.62–0.94) and the combination of HAI with adjuvant SCT (HR: 0.75, 95% CI: 0.59–0.90). A major limitation of the included studies is once again the heterogeneity in disease burden, response-, and resectability assessment. SCT regimens are mainly a combination of 5-FU, oxaliplatin and irinotecan, but various new biologicals may be added. With modern agents applied through HAI, response rates of CRLM increase. The opportunity to initiate HAI in patients who progress under SCT even after multiple lines of treatment is a promising expansion of life-prolonging therapeutic options in a challenging to treat advanced disease.
Again, the largest study evaluating outcomes in this setting comes from MSKCC. In patients who underwent complete CRLM resection with HAI, 10-year OS was 51% compared to 31% in patients receiving SCT only [44]. The 10-year follow-up was updated from their institutional database and improved to an 57% OS [45]. Of note, few centers have published more than an initial series of their programme and consensus and benchmarks that should be achieved in terms of patient outcome are lacking.
Ongoing studies like the French multicenter combined phase II and III PACHA-01 trial (NCT02494973) include patients with high-risk disease, defined as at least four surgically or interventionally treated CRLM [46]. Patients in the interventional arm receive HAI with oxaliplatin and 5-FU, those in the control arm FOLFOX. The trial is designed to assess the efficacy after curative-intent surgery on RFS at 18 months. If 39% (21 of 54) of the patients show no sign of hepatic recurrence, the trial will be continued to a phase III to demonstrate superiority of the HAI arm in terms of 3-year RFS. Results are expected in 2028. Furthermore, the multicenter phase III PUMP trial is currently conducted in the Netherlands (Netherlands Trial Register 7493) and compares HAI combined with systemic 5-FU to no therapy after resection of CRLM [47]. Patients with low-risk disease, corresponding to a clinical risk score of 0–2 are enrolled and the primary endpoint is PFS. An overview of ongoing studies is shown in Table 2.
Table 2.
Ongoing randomized controlled trials assessing the effect of HAI on CRLM registered on clinicaltrials.gov
| NCT | Acronym | Size, n | Intervention (HAI) | Control | Efficacy endpoint | Phase | Country |
|---|---|---|---|---|---|---|---|
| NCT05103020 | 100 | Oxaliplatin + iv FOLFIRI + bevacizumab or cetuximab | FOLFIRI+ bevacizumab or cetuximab | Curative-intent resection rate, OS, PFS, ORR | 2 | Korea | |
| NCT04898504 | EXCALIBUR1+2 | 45 | 5-FU | Arm 2: transplant | OS, QoL | 2 | Norway |
| Arm 3: 2nd line iv chemotherapy | |||||||
| NCT02494973 | PACHA-01 | 220 | Oxaliplatin, iv 5-FU + LV5 | mFOLFOX6 | RFS | 2/3 | France |
| NCT02885753 | OSCAR | 348 | Oxaliplatin, iv 5-FU + LV5 + panitumumab or Bevacizumab | FOLFOX + panitumumab or bevacizimab iv mFOLFIRINOX + panitumumab or bevacizimab | PFS | 3 | France |
| Oxaliplatin, iv FOLFIRI + panitumumab or bevacizumab | |||||||
| NCT02102789 | 142 | FUDR + iv mFOLFOX6 | mFOLFOX6 | R0 rate, ORR, R0/R1 resection, RFS, OS, PFS | 3 | China | |
| NCT01312857 | 75 | FUDR + iv FOLFIRI + panitumumab | FUDR + FOLFIRI | RFS, OS | 2 | US | |
| NCT00200200 | 73 | Bevacizumab 5-FU + iv oxaliplatin + LV5 | 5-FU + irinotecan + LV5 | PFR, OS | 2 | US | |
| NCT03500874 | 288 | FUDR + iv FOLFOX | FOLFOX | RFS, OS | 3 | China | |
| NCT03678428 | 160 | Oxaliplatin FUDR + iv irinotecan | FOLFOXIRI | ORR, R0 rate, tumor regression, PFS, RFS, OS | 3 | China |
Primary Liver Tumors
Hepatocellular Carcinoma (Level of Evidence 1b)
Management of HCC is guided by the 2022 updated BCLC algorithm [48]. Surgical resection is only offered to patients with small HCC, without macrovascular invasion and preserved liver function. Transplantation is mainly reserved for patients with HCC within the Milan criteria or adapted versions in most countries [49].
For intermediate or advanced-stage HCC, there are several options to deliver local and systemic therapies. Transarterial therapies play a role in the management of HCC outside the transplant criteria or as bridging procedure. Besides catheter-based embolization such as TACE or locally delivered radiation, HAI is part of multimodal therapy options in this algorithm. In practice, HAI is mainly combined with systemic therapy since HAI alone did not result in a survival benefit [50].
The efficacy and safety of a combination of sorafenib and HAI have been assessed in multiple RCTs [51–54]. Ikeda et al. [51] demonstrated three-fold higher response rates (7.3% vs. 21.7%, p = 0.09) and a better median survival when patients were treated with sorafenib and HAI Cisplatin compared to sorafenib alone (10.6 vs. 8.7 months, HR: 0.68; 95% CI: 0.44–1.05; p = 0.073). Similarly, in a randomized multicentre setting He et al. [52] demonstrated a much higher response rate (40.8% vs. 2.5%, p < 0.001) and an almost twice as long OS (13.4 vs. 7.1 months, HR: 0.35; 95% CI: 0.26–0.48; p < 0.001) when HAI with FOLFOX was added to sorafenib versus sorafenib alone for patients with HCC and PV invasion. Conversely, the largest randomized multicentre phase III trial, including 206 patients, found no significant difference in OS when combining sorafenib with HAI administering low-dose cisplatin and FU (11.5 vs. 11.8 months, p = 0.955) [53]. Another small randomized trial including patients with major PV thrombosis showed higher response rates in combination therapy of sorafenib and oxaliplatin/5-FU HAI versus sorafenib alone (41% vs. 3%, p < 0.001) [54]. Also, the median OS was 16.3 months with sorafenib plus HAI and 6.5 months with sorafenib alone (HR: 0.28; 95% CI: 0.15, 0.53; p < 0.001). In these mentioned trials, overall adverse event rates were similar in the HAI and the control arm. Catheter-related complications were low, with a reported incidence of 6–12%. Importantly, only one reoperation was required for an adverse event across the four RCTs. While RCTs demonstrated the feasibility of HAI, the added survival benefit of combining sorafenib was inconsistent. Ultimately, a confirmatory large multicentre RCT is needed to assess the efficacy of HAI in HCC. Table 3 lists ongoing randomized studies registered on clinicaltrials.gov.
Table 3.
Ongoing randomized controlled trials assessing the effect of HAI on HCC identified on clinicaltrials.gov
| NCT | Size, n | Intervention (HAI) | Control | Efficacy endpoint | Phase | Country |
|---|---|---|---|---|---|---|
| NCT03949231 | 200 | PD-1/PDL1 inhibitor | PD-1/PDL1 inhibitor | OS, PFS | 3 | China |
| NCT04777942 | 320 | Neoadjuvant TACE-HAI + surgery | Surgery | PFS, OS | – | China |
| NCT04424043 | 280 | Neoadjuvant TACE-HAI + surgery | Surgery | PFS, OS | – | China |
| NCT03780049 | 304 | FOLFOX + H101 (recombinant human type-5 adenovirus) | FOLFOX | OS, PFS | 3 | China |
| NCT03009461 | 64 | FOLFOX + Sorafenib | Sorafenib | OS | 2 | China |
| NCT05231382 | 426 | Raltitrexed, oxaliplatin (SALOX) | FOLFOX | PFS, OS, Objective response | 3 | China |
| NCT03591705 | 240 | TACE + FOLFOX | FOLFOX | PFS, OS, Downstaging rate | China | |
| NCT04181931 | 320 | nTACE-HAI (FOLFOX) + surgery | Surgery | PFS, OS | China | |
| NCT04178642 | 138 | Idarubicin-lipiodol | No adjuvant therapy | RFS, OS | 2 | France |
| NCT04595864 | 40 | Neoadjuvant mFOLFOX6 | Surgery | OS, RFS, PFS | 3 | China |
| NCT04947826 | 100 | FOLFOX + HLX10 (PD-1 antibody) + HLX04 (VEGF antibody) | FOLFOX | ORR, PFS, OS, TTR | 2 | China |
| NCT05313282 | 140 | mFOLFOX7 + apatinib + camrelizumab | Apatinib + camrelizumab | PFS, OS, TTR, DOR, ORR, DCR | 3 | China |
| NCT03469479 | 252 | FOLFOX | Surgery | OS, DFS | 3 | China |
| NCT05263219 | 230 | FOLFOX + DEB-TACE | FOLFOX | OS, ORR, DCR, PFS, conversion rate to resection | 3 | China |
| NCT03722498 | 100 | FOLFOX | Sorafenib | PFS, OS, ORR | 2 | China |
| NCT04667351 | 400 | Oxaliplatin, leucovorin, and 1,200 mg/m2 5-FU | Oxaliplatin, leucovorin, and 2,400 mg/m2 5-FU | PFS, OS, ORR, DCR | 3 | China |
| NCT05007587 | 60 | mFOLFOX + lenvatinib | Oxaliplatin/raltitrexed + lenvatinib | ORR, DCR, OS, PFS | 1 | China |
| NCT05198609 | 214 | FOLFOX + camrelizumab + oral apatinib | Camrelizumab + oral apatinib | OS, PFS, TTR, DOR, ORR | 3 | China |
| NCT05171166 | 156 | FOLFOX + donafenib + TACE | Donafenib + TACE or DEB-TACE | PFS, OS, ORR, DCR | 2/3 | China |
| NCT04687163 | 400 | FOLFOX 130 mg/m2 + sorafenib or lenvatinib | FOLFOX 85 mg/m2 + sorafenib or lenvatinib | OS, PFS, ORR, TTR | 3 | China |
Cholangiocellular Carcinoma (Level of Evidence 2b)
Patients with CCC often present with advanced disease, and survival is limited. For intrahepatic CCC (iCCC) in early stages, surgery can improve 5-year survival rates by up to 40% [55]. The role of transplantation remains controversial in unresectable disease as selection criteria are currently not well defined. Moreover, while some advanced have been made in systemic treatment for patients with advanced iCCC and other biliary tract cancers, median survival with the most active regimen is still only just above 12 months [51]. Local treatment modalities such as radio- and chemoembolization or HAI are appealing treatment options when the tumor is confined to the liver.
High-level evidence for HAI in iCCA is scarce. In a single-arm phase 2 trial of HAI-FUDR combined with systemic gemcitabine and oxaliplatin, 38 patients with initially unresectable tumors demonstrated a radiologic response rate of 58% and disease control in 86%. The effect was independent of lymph node status. Four patients (11%) even were converted to a resectable stage [56].
Long-term data from prospective studies have also shown excellent tumor control in patients with unresectable iCCC treated with HAI with 48% of patients showing partial response as per RECIST criteria and 3-year survival of almost 40% without surgical resection [57–59]. Kasai and colleagues reported a case series of 20 patients with advanced iCCC with a 60% response rate when patients underwent combined therapy with 5-FU and pegylated interferon with HAI. However, 25% of the patients in this cohort already had lung metastasis [60]. Ghingirelli reported a disease control rate of 91% in a series of 12 patients treated with a combination of gemcitabine and oxaliplatin [61].
The latest published bi-institutional study including 268 patients from the Netherlands and the MSKCC reported a longer survival in a HAI cohort as compared to a cohort treated with systemic gemcitabine and cisplatin (OS at 3 years 34.3% in the HAI group vs. 3.5% in the SCT only group) [62]. The adjusted HR for HAI was 0.27 (95% CI: 0.19–0.39). Of note, all patients receiving HAI were treated at MSKCC whereas the SCT only group comes from two Dutch centers. This design impairs comparability of the groups and increases the risk of unmeasured confounding.
Treatment of histologically proven multifocal iCCC with HAI-5-FU in 141 patients was compared to resection (R) in 178 patients in a retrospective multicentre cohort [63]. Eligible patients treated over a 18-year period had a higher tumor burden in the HAI group with regard to bilobar disease (HAI: 88.0% vs. R: 34.3%), median tumor size (HAI: 8.4 cm vs. R: 7.0 cm), and proportion of patients with 4 or more lesions (HAI: 67% vs. R: 24%). The 30-day mortality in the resection group was eight times higher in the resection group (HAI: 0.8% vs. R: 6.2%), while the median OS was comparable (HAI: 20 months vs. R: 19 months). Based on these results, the indication for resection of multifocal iCCC needs reconsideration when no survival benefit can be achieved as compared to HAI; however, the risk of surgical resection was significantly higher.
Unresectable iCCC have been treated with intra-arterial floxuridine and a favorable 3-year OS of 39.5% compared to SCT (0%) was achieved in a meta-analysis of 4 studies and 144 patients, including the abovementioned phase II trial by Cercek et al. [56, 64]. Local therapy in this setting is especially appealing because patients usually die from biliary obstruction and liver failure rather than a systemic disease progression. To further assess the benefit of HAI in addition to systemic therapy with gemcitabine and oxaliplatin in patients with unresectable iCCC, a multicentre phase II RCT is currently recruiting patients (NCT04891289). CCC in other locations has rarely been treated with HAI. A phase II trial conducted in China assessed tumor response in 37 patients with advanced perihilar CCC. The administered HAI consisted of oxaliplatin and FUDR, maintenance therapy was oral capecitabine [23]. The overall achieved response rate was 68% and the disease control rate was 89%. Table 4 lists ongoing randomized studies registered on clinicaltrials.gov.
Table 4.
Ongoing randomized controlled trials assessing the effect of HAI on CCC identified on clinicaltrials.gov
| NCT | Size, n | Intervention (HAI) | Control | Efficacy endpoint | Phase | Country |
|---|---|---|---|---|---|---|
| NCT04891289 | 164 | FUDR + Gemcitabine/oxaliplatin | Gemcitabine/oxaliplatin | PFS, OS | 2 | USA |
| NCT03771846 | 188 | FOLFIRINOX | Gemcitabine/oxaliplatin | OS, PFS, TTP | 3 | China |
| NCT01862315 | 55 | FUDR + gemcitabine/oxaliplatin | Gemcitabine/oxaliplatin | PFS, response in MRI | 2 | USA |
| NCT04961970 | 18 | FOLFOX | Gemcitabine/cisplatin | OS, PFS, TTP | 3 | China |
OS, overall survival; PFS, progression-free survival; TTP, time to progression.
HAI and Interventional Oncology Therapies
Thermal Ablation
To treat small-volume disease, the sequential combination of thermal ablation with either radiofrequency (RFA) or microwave ablation (MWA) was assessed in patients with CRLM and HCC. For CRLM, the largest series comes from MSKCC including 286 patients with 415 CRLM. Interestingly, there were no biliary complications in HAI-naive patients, versus 11% in HAI patients. Predictors for biliary complications were biliary dilatation, bevacizumab therapy and an ablation margin >10 mm. Therefore, the authors advocate for an ablation margin between 6 and 10 mm with a resulting 76% local tumor control rate and improved biliary complication incidence (4%) [65].
In a randomized study, Oyama et al. [66] compared HAI with RFA ablation with RFA only in patients with early stage HCC. The potential advantage of the combined approach is the destruction of tumor tissue with the prevention of recurrence by HAI. Although only including 70 patients, the intrahepatic recurrence-free survival was better in the combination group compared to ablation alone (HR: 0.468; 95% CI: 0.235–0.896; p = 0.022). The primary endpoint, recurrence-free survival at 3 years, was 54% in the combination group versus 34% with ablation alone; however, this 20% difference was not statistically significant (HR: 0.597, 95% CI: 0.320–1.091; p = 0.094) [66].
For advanced HCC, Liu et al. [67] compared HAI in combination with lenvatinib to HAI, lenvatinib and MWA in 150 consecutive patients. The group including MWA showed longer median OS (>30 months vs. 13.6 months; p = 0.010), longer PFS (12.8 vs. 5.6 months; p < 0.001), and longer intrahepatic PFS (14.6 vs. 6.8 months; p = 0.002). Only 1 patient developed bilioma after thermal ablation [67]. The combination of HAI and thermal ablation mainly lacks high-quality trials; therefore, the next step should be to collect more reliable and solid evidence in subsequent prospective studies.
Radioembolization
In a prospective phase I, study from MSKCC SIRT using yttrium-90 microspheres was evaluated as a salvage therapy for liver-predominant CRLM in 19 patients with progression after HAI and SCT. In this heavily pretreated population, median local PFS and OS after SIRT were 5.2 months and 14.9 months. Similar results could be achieved in a retrospective analysis on 103 CRLM patients from MSKCC with a local PFS of 4 months (95% CI: 3.3–4.8) and OS of 11.3 months (95% CI: 7.9–15.1). In multivariate analysis number of extrahepatic disease sites, carcinoembryonic antigen level, albumin, tumor differentiation level, and sum of the two largest tumor diameters were independently associated with OS [68, 69].
Conclusion
HAI might improve response rates, resectability, and long-term survival in well selected patients with primary and secondary liver malignancies. The combination of SCT with HAI is especially promising for conversion therapy of initially unresectable tumors. Other areas of interest consist of the adjuvant setting in patients with high-risk liver disease or the palliative setting for liver-only disease with the potential to minimize systemic adverse events. However, high-level evidence incorporating up-to date systemic treatment is limited, and future studies should focus on homogenous patient selection and standardized response evaluation.
Conflict of Interest Statement
The authors have no conflicts of interest or financial ties to disclose.
Funding Sources
There was no funding for this study.
Author Contributions
C.K., S.S., and P.C.M. performed literature search, drafted the manuscript, and created tables and figures; P.D. created figure and critical revision of the manuscript; V.H., U.K. G.F.H., A.T.B. B.P.M., and O.K. performed literature search and critical revision of the manuscript.
Funding Statement
There was no funding for this study.
References
- 1. Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009;89(4):1269–339. [DOI] [PubMed] [Google Scholar]
- 2. Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30(5):969–77. [PMC free article] [PubMed] [Google Scholar]
- 3. Sperling J, Schäfer T, Benz-Weißer A, Ziemann C, Scheuer C, Kollmar O, et al. Hepatic arterial infusion but not systemic application of cetuximab in combination with oxaliplatin significantly reduces growth of CC531 colorectal rat liver metastases. Int J Colorectal Dis. 2013;28(4):555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sperling J, Schäfer T, Ziemann C, Benz-Weiber A, Kollmar O, Schilling MK, et al. Hepatic arterial infusion of bevacizumab in combination with oxaliplatin reduces tumor growth in a rat model of colorectal liver metastases. Clin Exp Metastasis. 2012;29(2):91–9. [DOI] [PubMed] [Google Scholar]
- 5. Sperling J, Ziemann C, Gittler A, Benz-Weißer A, Menger MD, Kollmar O. Hepatic arterial infusion of temsirolimus inhibits tumor growth of colorectal rat liver metastases even after a growth stimulating procedure like liver resection. J Surg Res. 2013;185(2):587–94. [DOI] [PubMed] [Google Scholar]
- 6. Ensminger WD, Gyves JW. Clinical pharmacology of hepatic arterial chemotherapy. Semin Oncol. 1983;10(2):176–82. [PubMed] [Google Scholar]
- 7. Cheng J, Hong D, Zhu G, Swanstrom LL, Hansen PD. Laparoscopic placement of hepatic artery infusion pumps: technical considerations and early results. Ann Surg Oncol. 2004;11(6):589–97. [DOI] [PubMed] [Google Scholar]
- 8. Qadan M, D’Angelica MI, Kemeny NE, Cercek A, Kingham TP. Robotic hepatic arterial infusion pump placement. HPB. 2017;19(5):429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sinn M, Nicolaou A, Ricke J, Podrabsky P, Seehofer D, Gebauer B, et al. Interventionally implanted port catheter systems for hepatic arterial infusion of chemotherapy in patients with primary liver cancer: a phase II-study (NCT00356161). BMC Gastroenterol. 2013;13:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72(2):338–44. gutjnl-2022-327736. [DOI] [PubMed] [Google Scholar]
- 11. Pozzo C, Barone C, Kemeny NE. Advances in neoadjuvant therapy for colorectal cancer with liver metastases. Cancer Treat Rev. 2008;34(4):293–301. [DOI] [PubMed] [Google Scholar]
- 12. Bolhuis K, Kos M, van Oijen MGH, Swijnenburg RJ, Punt CJA. Conversion strategies with chemotherapy plus targeted agents for colorectal cancer liver-only metastases: a systematic review. Eur J Cancer. 2020;141:225–38. [DOI] [PubMed] [Google Scholar]
- 13. Popescu I, Alexandrescu ST. Surgical options for initially unresectable colorectal liver metastases. HPB Surg. 2012;2012:454026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77(6):1598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the barcelona-2000 EASL conference. European association for the study of the liver. J Hepatol. 2001;35(3):421–30. [DOI] [PubMed] [Google Scholar]
- 16. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68(2):723–50. [DOI] [PubMed] [Google Scholar]
- 17. Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Riby D, Mazzotta AD, Bergeat D, Verdure L, Sulpice L, Bourien H, et al. Downstaging with radioembolization or chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2020;27(10):3729–37. [DOI] [PubMed] [Google Scholar]
- 19. Sharib JM, Creasy JM, Wildman-Tobriner B, Kim C, Uronis H, Hsu SD, et al. Hepatic artery infusion pumps: a surgical toolkit for intraoperative decision-making and management of hepatic artery infusion-specific complications. Ann Surg. 2022;276(6):943–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abdelmaksoud AHK, Abdelaziz AO, Nabeel MM, Hamza I, Elbaz TM, Shousha HI, et al. Hepatic arterial infusion chemotherapy in the treatment of advanced hepatocellular carcinoma with portal vein thrombosis: a case-control study. Clin Radiol. 2021;76(9):709.e1–709.e6. [DOI] [PubMed] [Google Scholar]
- 21. Shao YY, Wang SY, Lin SM; Diagnosis Group; Systemic Therapy Group . Management consensus guideline for hepatocellular carcinoma: 2020 update on surveillance, diagnosis, and systemic treatment by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J Formos Med Assoc. 2021;120(4):1051–60. [DOI] [PubMed] [Google Scholar]
- 22. Judge SJ, Ghalambor T, Cavnar MJ, Lidsky ME, Merkow RP, Cho M, et al. Current practices in hepatic artery infusion (HAI) chemotherapy: an international survey of the HAI consortium research network. Ann Surg Oncol. 2023;30(12):7362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang X, Hu J, Cao G, Zhu X, Cui Y, Ji X, et al. Phase II study of hepatic arterial infusion chemotherapy with oxaliplatin and 5-fluorouracil for advanced perihilar cholangiocarcinoma. Radiology. 2017;283(2):580–9. [DOI] [PubMed] [Google Scholar]
- 24. Arai Y, Takeuchi Y, Inaba Y, Yamaura H, Sato Y, Aramaki T, et al. Percutaneous catheter placement for hepatic arterial infusion chemotherapy. Tech Vasc Interv Radiol. 2007;10(1):30–7. [DOI] [PubMed] [Google Scholar]
- 25. Deschamps F, Elias D, Goere D, Malka D, Ducreux M, Boige V, et al. Intra-arterial hepatic chemotherapy: a comparison of percutaneous versus surgical implantation of port-catheters. Cardiovasc Intervent Radiol. 2011;34(5):973–9. [DOI] [PubMed] [Google Scholar]
- 26. Kemeny NE, Niedzwiecki D, Hollis DR, Lenz HJ, Warren RS, Naughton MJ, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol. 2006;24(9):1395–403. [DOI] [PubMed] [Google Scholar]
- 27. Mocellin S, Pilati P, Lise M, Nitti D. Meta-analysis of hepatic arterial infusion for unresectable liver metastases from colorectal cancer: the end of an era? J Clin Oncol. 2007;25(35):5649–54. [DOI] [PubMed] [Google Scholar]
- 28. Datta J, Narayan RR, Kemeny NE, D’Angelica MI. Role of hepatic artery infusion chemotherapy in treatment of initially unresectable colorectal liver metastases: a review. JAMA Surg. 2019;154(8):768–76. [DOI] [PubMed] [Google Scholar]
- 29. Sperling J, Brandhorst D, Schäfer T, Ziemann C, Benz-Weißer A, Scheuer C, et al. Liver-directed chemotherapy of cetuximab and bevacizumab in combination with oxaliplatin is more effective to inhibit tumor growth of CC531 colorectal rat liver metastases than systemic chemotherapy. Clin Exp Metastasis. 2013;30(4):447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhao J, Zheng Y, Liu T, Chang J, Shan H, Cong K. Comparison between fluoropyrimidine-hepatic arterial infusion and systemic chemotherapy for unresectable liver metastases: a protocol for systematic review and meta-analysis based on 16 observational studies. Medicine. 2021;100(41):e27483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kerr DJ, McArdle CS, Ledermann J, Taylor I, Sherlock DJ, Schlag PM, et al. Intrahepatic arterial versus intravenous fluorouracil and folinic acid for colorectal cancer liver metastases: a multicentre randomised trial. Lancet. 2003;361(9355):368–73. [DOI] [PubMed] [Google Scholar]
- 32. Lorenz M, Müller HH. Randomized, multicenter trial of fluorouracil plus leucovorin administered either via hepatic arterial or intravenous infusion versus fluorodeoxyuridine administered via hepatic arterial infusion in patients with nonresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2000;18(2):243–54. [DOI] [PubMed] [Google Scholar]
- 33. Lam VW, Spiro C, Laurence JM, Johnston E, Hollands MJ, Pleass HCC, et al. A systematic review of clinical response and survival outcomes of downsizing systemic chemotherapy and rescue liver surgery in patients with initially unresectable colorectal liver metastases. Ann Surg Oncol. 2012;19(4):1292–301. [DOI] [PubMed] [Google Scholar]
- 34. Safi F, Bittner R, Roscher R, Schuhmacher K, Gaus W, Beger GH. Regional chemotherapy for hepatic metastases of colorectal carcinoma (continuous intraarterial versus continuous intraarterial/intravenous therapy). Results of a controlled clinical trial. Cancer. 1989;64(2):379–87. [DOI] [PubMed] [Google Scholar]
- 35. DʼAngelica MI, Correa-Gallego C, Paty PB, Cercek A, Gewirtz AN, Chou JF, et al. Phase II trial of hepatic artery infusional and systemic chemotherapy for patients with unresectable hepatic metastases from colorectal cancer: conversion to resection and long-term outcomes. Ann Surg. 2015;261(2):353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pak LM, Kemeny NE, Capanu M, Chou JF, Boucher T, Cercek A, et al. Prospective phase II trial of combination hepatic artery infusion and systemic chemotherapy for unresectable colorectal liver metastases: long term results and curative potential. J Surg Oncol. 2018;117(4):634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lévi FA, Boige V, Hebbar M, Smith D, Lepère C, Focan C, et al. Conversion to resection of liver metastases from colorectal cancer with hepatic artery infusion of combined chemotherapy and systemic cetuximab in multicenter trial OPTILIV. Ann Oncol. 2016;27(2):267–74. [DOI] [PubMed] [Google Scholar]
- 38. Boilève A, De Cuyper A, Larive A, Mahjoubi L, Najdawi M, Tazdait M, et al. Hepatic arterial infusion of oxaliplatin plus systemic chemotherapy and targeted therapy for unresectable colorectal liver metastases. Eur J Cancer. 2020;138:89–98. [DOI] [PubMed] [Google Scholar]
- 39. Focan C, Levi F, Kreutz F, Focan-Henrard D, Lobelle JP, Adam R, et al. Continuous delivery of venous 5-fluorouracil and arterial 5-fluorodeoxyuridine for hepatic metastases from colorectal cancer: feasibility and tolerance in a randomized phase II trial comparing flat versus chronomodulated infusion. Anticancer Drugs. 1999;10(4):385–92. [DOI] [PubMed] [Google Scholar]
- 40. Carnaghi C, Santoro A, Rimassa L, Doci R, Rosati R, Pedicini V, et al. The efficacy of hybrid chemotherapy with intravenous oxaliplatin and folinic acid and intra-hepatic infusion of 5-fluorouracil in patients with colorectal liver metastases: a phase II study. Invest New Drugs. 2007;25(5):479–85. [DOI] [PubMed] [Google Scholar]
- 41. Kemeny NE, Melendez FD, Capanu M, Paty PB, Fong Y, Schwartz LH, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2009;27(21):3465–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Boige V, Malka D, Elias D, Castaing M, De Baere T, Goere D, et al. Hepatic arterial infusion of oxaliplatin and intravenous LV5FU2 in unresectable liver metastases from colorectal cancer after systemic chemotherapy failure. Ann Surg Oncol. 2008;15(1):219–26. [DOI] [PubMed] [Google Scholar]
- 43. Buisman FE, Filipe WF, Galjart B, Grünhagen DJ, Homs MYV, Moelker A, et al. Adjuvant intra-arterial chemotherapy for patients with resected colorectal liver metastases: a systematic review and meta-analysis. HPB Oxf. 2022;24(3):299–308. [DOI] [PubMed] [Google Scholar]
- 44. Srouji R, Narayan R, Boerner T, Buisman F, Seier K, Gonen M, et al. Addition of adjuvant hepatic artery infusion to systemic chemotherapy following resection of colorectal liver metastases is associated with reduced liver-related mortality. J Surg Oncol. 2020;121(8):1314–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ecker BL, Shin P, Saadat LV, Court CM, Balachandran VP, Chandwani R, et al. Genomic stratification of resectable colorectal liver metastasis patients and implications for adjuvant therapy and survival. Ann Surg. 2022;275(2):371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Goéré D, Pignon JP, Gelli M, Elias D, Benhaim L, Deschamps F, et al. Postoperative hepatic arterial chemotherapy in high-risk patients as adjuvant treatment after resection of colorectal liver metastases - a randomized phase II/III trial - PACHA-01 (NCT02494973). BMC Cancer. 2018;18(1):787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Buisman FE, Homs MYV, Grünhagen DJ, Filipe WF, Bennink RJ, Besselink MGH, et al. Adjuvant hepatic arterial infusion pump chemotherapy and resection versus resection alone in patients with low-risk resectable colorectal liver metastases - the multicenter randomized controlled PUMP trial. BMC Cancer. 2019;19(1):327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tschuor C, Ferrarese A, Kuemmerli C, Dutkowski P, Burra P, Clavien PA, et al. Allocation of liver grafts worldwide - is there a best system? J Hepatol. 2019;71(4):707–18. [DOI] [PubMed] [Google Scholar]
- 50. Kondo M, Morimoto M, Kobayashi S, Ohkawa S, Hidaka H, Nakazawa T, et al. Randomized, phase II trial of sequential hepatic arterial infusion chemotherapy and sorafenib versus sorafenib alone as initial therapy for advanced hepatocellular carcinoma: SCOOP-2 trial. BMC Cancer. 2019;19(1):954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ikeda M, Shimizu S, Sato T, Morimoto M, Kojima Y, Inaba Y, et al. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: randomized phase II trial. Ann Oncol. 2016;27(11):2090–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. He M, Li Q, Zou R, Shen J, Fang W, Tan G, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5(7):953–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kudo M, Ueshima K, Yokosuka O, Ogasawara S, Obi S, Izumi N, et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3(6):424–32. [DOI] [PubMed] [Google Scholar]
- 54. Zheng K, Zhu X, Fu S, Cao G, Li WQ, Xu L, et al. Sorafenib plus hepatic arterial infusion chemotherapy versus sorafenib for hepatocellular carcinoma with major portal vein tumor thrombosis: a randomized trial. Radiology. 2022;303(2):455–64. [DOI] [PubMed] [Google Scholar]
- 55. Marcus R, Christopher W, Keller J, Nassoiy S, Chang SC, Goldfarb M, et al. Systemic therapy is associated with improved oncologic outcomes in resectable stage II/III intrahepatic cholangiocarcinoma: an examination of the national cancer database over the past decade. Cancers. 2022;14(17):4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cercek A, Boerner T, Tan BR, Chou JF, Gönen M, Boucher TM, et al. Assessment of hepatic arterial infusion of floxuridine in combination with systemic gemcitabine and oxaliplatin in patients with unresectable intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2020;6(1):60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Konstantinidis IT, Do RK, Gultekin DH, Gönen M, Schwartz LH, Fong Y, et al. Regional chemotherapy for unresectable intrahepatic cholangiocarcinoma: a potential role for dynamic magnetic resonance imaging as an imaging biomarker and a survival update from two prospective clinical trials. Ann Surg Oncol. 2014;21(8):2675–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jarnagin WR, Schwartz LH, Gultekin DH, Gönen M, Haviland D, Shia J, et al. Regional chemotherapy for unresectable primary liver cancer: results of a phase II clinical trial and assessment of DCE-MRI as a biomarker of survival. Ann Oncol. 2009;20(9):1589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kemeny NE, Schwartz L, Gönen M, Yopp A, Gultekin D, D’Angelica MI, et al. Treating primary liver cancer with hepatic arterial infusion of floxuridine and dexamethasone: does the addition of systemic bevacizumab improve results? Oncology. 2011;80(3–4):153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kasai K, Kooka Y, Suzuki Y, Suzuki A, Oikawa T, Ushio A, et al. Efficacy of hepatic arterial infusion chemotherapy using 5-fluorouracil and systemic pegylated interferon α-2b for advanced intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2014;11:3638–45. [DOI] [PubMed] [Google Scholar]
- 61. Ghiringhelli F, Lorgis V, Vincent J, Ladoire S, Guiu B. Hepatic arterial infusion of gemcitabine plus oxaliplatin as second-line treatment for locally advanced intrahepatic cholangiocarcinoma: preliminary experience. Chemotherapy. 2013;59(5):354–60. [DOI] [PubMed] [Google Scholar]
- 62. Franssen S, Holster JJ, Jolissaint JS, Nooijen LE, Cercek A, D’Angelica MI, et al. Gemcitabine with cisplatin versus hepatic arterial infusion pump chemotherapy for liver-confined unresectable intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2024;31(1):115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Franssen S, Soares KC, Jolissaint JS, Tsilimigras DI, Buettner S, Alexandrescu S, et al. Comparison of hepatic arterial infusion pump chemotherapy vs resection for patients with multifocal intrahepatic cholangiocarcinoma. JAMA Surg. 2022;157(7):590–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Holster JJ, El Hassnaoui M, Franssen S, Ijzermans JNM, de Jonge J, Mostert B, et al. Hepatic arterial infusion pump chemotherapy for unresectable intrahepatic cholangiocarcinoma: a systematic review and meta-analysis. Ann Surg Oncol. 2022;29(9):5528–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kurilova I, Bendet A, Petre EN, Boas FE, Kaye E, Gonen M, et al. Factors associated with local tumor control and complications after thermal ablation of colorectal cancer liver metastases: a 15-year retrospective cohort study. Clin Colorectal Cancer. 2021;20(2):e82–95. [DOI] [PubMed] [Google Scholar]
- 66. Oyama A, Nouso K, Yoshimura K, Morimoto Y, Nakamura S, Onishi H, et al. Randomized controlled study to examine the efficacy of hepatic arterial infusion chemotherapy with cisplatin before radiofrequency ablation for hepatocellular carcinoma. Hepatol Res. 2021;51(6):694–701. [DOI] [PubMed] [Google Scholar]
- 67. Liu Y, Qiao Y, Zhou M, Guo J, Lin Y, Li W, et al. Efficacy and safety of hepatic arterial infusion chemotherapy combined with lenvatinib and sequential ablation in the treatment of advanced hepatocellular carcinoma. Cancer Med. 2023;12(5):5436–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sofocleous CT, Garcia AR, Pandit-Taskar N, Do KG, Brody LA, Petre EN, et al. Phase I trial of selective internal radiation therapy for chemorefractory colorectal cancer liver metastases progressing after hepatic arterial pump and systemic chemotherapy. Clin Colorectal Cancer. 2014;13(1):27–36. [DOI] [PubMed] [Google Scholar]
- 69. Kurilova I, Beets-Tan RGH, Flynn J, Gönen M, Ulaner G, Petre EN, et al. Factors affecting oncologic outcomes of 90y radioembolization of heavily pre-treated patients with colon cancer liver metastases. Clin Colorectal Cancer. 2019;18(1):8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]