Abstract
Repetitive negative thinking (RNT) is a transdiagnostic symptom associated with poor outcomes in major depressive disorder (MDD). MDD is characterized by altered interoception, which has also been associated with poor outcomes. The present study investigated whether RNT is directly associated with altered interoceptive processing. Interoceptive awareness toward the heart and stomach was probed on the Visceral Interoceptive Attention (VIA) task with fMRI in MDD individuals who were propensity-matched on the severity of depression and anxiety symptoms and relevant demographics but different in RNT intensity (High RNT [H-RNT, n = 48] & Low RNT [L-RNT, n = 49]), and in matched healthy volunteers (HC, n = 27). Both H-RNT and L-RNT MDD individuals revealed reduced stomach interoceptive processing compared to HC in the left medial frontal region and insular cortex (H-RNT: β = −1.04, L-RNT: β = −0.97), perirhinal cortex (H-RNT: β = −0.99, L-RNT: β = −1.03), and caudate nucleus (H-RNT: β = −1.06, L-RNT: β = −0.89). However, H-RNT was associated with decreased right medial temporal lobe activity including the hippocampus and amygdala during stomach interoceptive trials (β = −0.61) compared to L-RNT. Insular interoceptive processing was similar in H-RNT and LRNT participants (β = −0.07, p = 0.92). MDD individuals with high RNT exhibited altered gastric interoceptive responses in brain areas that are important for associating the information with specific contexts and emotions. Attenuated interoceptive processing may contribute to RNT generation, non-adaptive information processing, action selection, and thus poor treatment outcome.
1. Introduction
Depression is the main mental health cause of disability-adjusted life years, affecting mostly women and individuals in their maximum productivity years (GBD Mental Disorders Collaborators, 2019; Kalin, 2020). This public health burden results in part from the fact that current psychopharmacology and psychotherapy approaches result in only about half of treated major depressive disorder (MDD) patients achieving full remission (Cascalenda et al., 2002; Nemeroff, 2020). Therefore, there is a crucial need to identify neural processes that can be targeted to improve outcomes. MDD seems to result from a heterogeneous mixture of dysfunctions in cognitive, affective, and behavioral processes (Conroy and Holtzheimer, 2021). Current efforts seeking to target circumscribed brain circuits purported to underlie depression as a full syndrome have met with relatively disappointing results, including failed randomized, double-blind clinical trials of deep-brain stimulation (Dougherty, 2018). However, approaches involving the careful parsing of depression symptom dimensions and the neural circuits subserving them may hold promise as an intermediate step in developing new antidepressant techniques (Conroy and Holtzheimer, 2021; Leaver et al., 2022).
Repetitive negative thinking (RNT) is a transdiagnostic manifestation commonly referred to as rumination in MDD, and it displays trait-like characteristics, contributing to poor outcomes in this disorder (Ehring and Watkins, 2008). RNT often remains as a residual symptom after first-line treatments, is associated with greater risk of recurrence and suicide, and therefore is a symptom that could be potentially targeted to alleviate treatment-resistant MDD (Wahl et al., 2019; Guinjoan et al., 2021; Shihata et al., 2021). Yet, the neural mechanisms of RNT in depression are not well understood. In a recent study, we found that RNT was not linked to abnormalities in reward circuits in MDD (Park et al., 2022), in contrast with extant literature supporting reward circuit dysfunction in obsessive-compulsive disorder, which is epitomized by prominent RNT. Instead, RNT in MDD was associated with abnormal fear learning neural signatures during both fear conditioning and extinction (Park et al., 2022). Fear processing is intimately related to interoceptive feedback (Etkin and Wager, 2007; Klein et al., 2021), and therefore these findings prompted the question of whether central processing deficits associated with RNT, are related to abnormal central processing of interoceptive information. Thus, we hypothesized that interoceptive deficits previously observed in MDD (Paulus and Stein, 2006; Simmons et al., 2012; Khalsa and Lapidus, 2016; Burrows et al., 2022; Teed et al., 2022) would have a relationship with RNT intensity in this disorder.
Interoception involves the experience of self, including the level of awareness of one’s internal organs such as the heart and stomach (Ginzburg et al., 2014; Khalsa et al., 2018; Todd et al., 2020). Dysfunction of interoception is evident in individuals with depression and anxiety (Paulus and Stein, 2006; Simmons et al., 2012; Khalsa and Lapidus, 2016; Burrows et al., 2022; Teed et al., 2022). In fact, it has been found to be dysregulated in MDD and there is evidence that such abnormalities in interoception also contribute to poor outcomes (Avery et al., 2014; Eggart and Valdés-Stauber, 2021). Notably, interoception sensitivity towards stomach sensations is influenced by positive physiological experiences as well as psychological experiences with depression and anxiety in healthy individuals (Ginzburg et al., 2014; Todd et al., 2020). In addition, the domain-specificity in neural processing suggests that interoceptive processing in the brain could work as modular processing for a specific organ (Spunt and Adolphs, 2017; Khalsa et al., 2018). These findings suggest the possibility that interoceptive processing in MDD with RNT may be aberrant and that such differences in the processing may not necessarily be uniform across sensations rooted in different organs. However, due to the paucity of the studies examining RNT and interoception, it is unknown whether the association between RNT and interoception is aberrant in the clinical population of MDD and whether the association would reveal a specificity in neural processing.
Interceptive processing may interact with RNT through the inability to disengage negative affective valence involved in self-related processing. At the same time, interoception and RNT may rely on common information processing resources. RNT and depression are known for their association with poor attention regulation (Hirsch and Mathews, 2012; Fox et al., 2015; Keller et al., 2019; Burgoyne and Engle, 2020). While conscious interoceptive processing requires focusing on a target stimulus (i.e., internal organs) and inhibiting the processing of irrelevant stimulus (i.e., external stimuli), RNT is noted for the impairment of the ability to disengage from negative emotional processing (see Burgoyne and Engle, 2020, for review). Despite potential links between aberrant interoception and RNT, the relationship between these two important dimensions of depression was explored exclusively with healthy populations to the extent of our knowledge (Lackner and Fresco, 2016; Li et al., 2022).
In the present study, we examined the association between RNT and interoceptive processing in the brain using the visceral interoceptive attention task (VIA, Simmons et al., 2012). We employed a propensity matching approach to an MDD clinical cohort with varying levels of RNT (High-RNT and Low-RNT) who were similar in regards to depression and anxiety symptom severity as well as demographic characteristics, along with a matched sample of healthy individuals. We hypothesized attenuated interoceptive processing in RNT, considering that RNT would recruit the neural resources involved in interoceptive processing including self-processing and attention regulation under the assumption of limited neural and cognitive resources. However, we noted that RNT-related aberrant interoception would not necessarily be uniform across different sensations. Thus, we predicted that stomach interoceptive processing would likely be associated with high levels of RNT due to its relationship with emotionally salient processing (Ginzburg et al., 2014; Todd et al., 2020) and the impairment of RNT in emotional learning (Li et al., 2022). We also tested heart interoception as this internal organ is the most frequently associated with interoceptive deficits in depression (e.g., Arias et al., 2020), so as to test the prediction that interoceptive abnormalities are organ-specific in this disorder (Khalsa et al., 2018). Further, we expected negative associations between neural activity for interoceptive processing and the severity of RNT. Whereas intuitively higher RNT could be associated with heightened interoceptive abilities, most literature on depression, in general, tends to reveal deficits in diverse aspects of interoception in this disorder (Quadt et al., 2018). Yet, the relationship between RNT as a symptom of depression and interoception remains largely unsettled, and characterizing this association was one of the motivations of this study. In this regard, we expected negative associations between neural activity for interoceptive processing and the severity of RNT.
2. Method
2.1. Participants
The participants were drawn from the first half of the Tulsa-1000 cohort, a naturalistic study that aimed to longitudinally follow 1000 individuals with mood, anxiety, substance use, and/or eating disorders, as well as healthy comparison (HC) individuals, all of whom were deeply phenotyped for a variety of dimensions of psychopathology and neural structure and functioning (see Victor et al., 2018; Burrows et al., 2022 for details). The full eligibility criteria for the study are described in Supplemental Information. All procedures were approved by the Western Institutional Review Board. Participants provided written informed consent and received financial compensation for their participation.
The present study focused on individuals with MDD and healthy volunteers. For diagnosing MDD, the Mini International Neuropsychiatric Inventory (MINI) in the DSM-IV or 5 was used. Core symptom, duration, and functional impact are identical in both systems and therefore no significant variation in the depression diagnosis assignment was expected (American Psychiatric Association, 2013). Furthermore, MINI-based diagnosis was followed by clinical case conferences with a board-certified psychiatrist for diagnostic verification in instances where clarification was needed. All participants completed self-report measures including the Ruminative Responses Scale (RRS) and the World Health Organization Disability Assessment Schedule (WHODAS) for measuring RNT and functional disability, respectively. The original 22-item RRS was employed to quantify the intensity of RNT (Nolen--Hoeksema and Morrow, 1991). Individuals with MDD (n = 100) were propensity-matched using the following procedure. An initial pool of 235 participants with MDD was divided into high and low rumination (H-RNT, L-RNT) based on a median split of RRS score = 57. Fifty random participants were selected from the H-RNT, and then a matching set of 50 were selected from the L-RNT based on based on age, depressive symptom severity (PHQ-9), and anxiety symptom severity (OASIS) using the MatchIt library. Then, out of an initial sample of 59 HCs, 30 were selected to match the selected MDD participants on gender, age, income, and employment status. After removing participants with missing or poor-quality VIA imaging data, the present study was based on 124 participants (48 H-RNT MDD, 49 L-RNT MDD, & 27 HC). The demographic and clinical information of participants is displayed in Table 1. The final sample of MDD participants with either High or Low RNT was demographically and clinically similar to the original sample of all MDD participants in the initial half of the T1000 study (not shown). Four HC were taking medications with potential psychotropic effects or psychiatric indications, for non psychiatric symptoms. These included as-needed over-the-counter muscle relaxants, and beta-blockers for blood pressure control. Both MDD groups were matched for exposure to psychotropic medications so reported results were not controlled for this variable.
Table 1.
Demographic and clinical characteristics.
| HC (n = 27) | Low RNT (n = 49) | High RNT (n = 48) | p | |
|---|---|---|---|---|
| Age | 31.7 ± 10.0 | 34.8 ± 11.3 | 34.3 ± 12.5 | 0.52 |
| Female, n (%) | 16 (59.3) | 36 (73.5) | 35 (72.9) | 0.38 |
| Ethnicity, n (%) | 0.32 | |||
| Asian | 1 (3.7) | 1 (2.0) | 0 (0) | |
| Black | 0 (0) | 4 (8.2) | 6 (12.5) | |
| Hispanic | 1 (3.7) | 2 (4.1) | 0 (5.5) | |
| Native American | 2 (7.4) | 7 (14.3) | 10 (20.8) | |
| White | 23 (85.2) | 34 (69.4) | 30 (62.5) | |
| Other | 0 (0) | 1 (1.0) | 2 (4.2) | |
| Education, n (%) | 0.58 | |||
| No High School | 0 (0) | 1 (2.0) | 4 (8.3) | |
| High School | 4 (14.8) | 8 (16.3) | 7 (14.6) | |
| Some College | 10 (37.0) | 21 (42.9) | 20 (41.7) | |
| College or Higher | 13 (48.1) | 19 (38.8) | 17 (35.4) | |
| BMI | 27.67 (6.37) | 28.28 (5.53) | 28.30 (5.52) | 0.88 |
| Employed, n (%) | 20 (76.9) | 28 (62.2) | 30 (63.8) | 0.41 |
| Income ($) | $47,020 (38,385) | $51,681 (46,291) | $39,577 (35,235) | 0.42 |
| Psychotropic Medication, n (%) | 4 (14.8) | 30 (61.2) HC | 30 (62.5) HC | <0.001 |
| OASIS | 1.15 (1.61) | 9.63 (3.10) HC | 10.35 (3.47) HC | <0.001 |
| PHQ-9 | 0.89 (1.48) | 13.69 (3.58) HC | 14.27 (4.38) HC | <0.001 |
| RRS | 29.15 (6.76) | 47.8 (5.94)HC, H–RNT | 64.6 (6.10)HC, L–RNT | <0.001 |
| WHODAS | 13.48 (3.07) | 23.47 (7.0)HC | 26.12 (8.21)HC | <0.001 |
Abbreviations: HC, Healthy Control; H-RNT, High repetitive negative thinking; L-RNT, Low repetitive negative thinking; MDD, major depressive disorder; OASIS, Overall Anxiety Severity and Impairment Scale; PHQ-9, Patient Health Questionnaire; RRS, Ruminative Response Scale; WHODAS, World Health Organization Disability Assessment Schedule.
2.2. Task
The VIA paradigm is an established imaging task in which participants are instructed to selectively attend to the sensations originating from specific internal organs as a way of identifying neural activity supporting interoceptive processing (Nolen-Hoeksema and Morrow, 1991; Simmonset al., 2012; Averyet al., 2014; Burrows et al., 2022; Victor et al., 2018). The version of the task employed consisted of two conditions, interoception and exteroception. The interoception condition was split into heart and stomach interoceptive trials. An interoception trial consisted of a word (‘HEART’ or ‘STOMACH’) presented in black letters on a white background for 10 s. Participants were instructed to focus attention on the intensity of visceral sensations experienced from the organ indicated by the presented word during the trial. In the exteroception condition, the word ‘TARGET’ in black was presented on a white background. During an exteroceptive target trial, the color of the word periodically changed from black to a shade of gray, and participants were instructed to focus on the intensity of the color change. Each type of trial (Heart, Stomach, & Target) was presented 12 times, for a total of 36 trials. To ensure selectively attending to the stimulus during trials, participants were instructed to rate the intensity of the visceral sensation (heart, stomach) or the intensity of the color change (target) on a scale from 0 (no visceral sensation/color change) to 6 (extremely intense visceral sensation/color change) on half of the trials, resulting in 18 ratings. The inter-trial interval was jittered between 2.5 s and 15 s. Participants performed the task in the scanner for two runs, 6 min for each run.
2.3. Imaging analysis
Imaging data acquisition and preprocessing are described in the Supplemental Information. For data analysis, three main events corresponding to the type of trial were constructed at the subject level: Heart-Interoceptive, Stomach-Interoceptive, and Exteroceptive trials. The BOLD signal was convolved with a delta function spanning the 10 s trial duration from the onset of the trial. The rating period was also modeled with a 5 s regressor following the duration. Nuisance regressors included the first four polynomial terms and the six motion parameters. The contrasts for visceral interoceptive processing (Heart interoception: Heart-Interoceptive trials vs. Exteroceptive trials, Stomach interoception: Stomach-Interoceptive trials vs. Exteroceptive trials) were constructed as within-subjects. Then, a multivariate ANCOVA (3dMVM) was modeled with group (H-RNT, L-RNT, & HC) and interoception (Heart interoception, Stomach interoception) for finding neural correlates associated with interoception and RNT, with covariates of age and sex, in the whole-brain functional analysis. A cluster-extent threshold of α < .01 (k > 203) was used in conjunction with a voxel-wise threshold of p < 0.005. Significant clusters were further examined with extracted beta coefficients following a-priori comparisons between groups, with Bonferroni corrections. The correlations between RRS scores and beta coefficients in suprathreshold clusters were also examined to see the association between RNT severity and neural activity supporting interoceptive attentional processing. In addition, behavioral data on intensity rating were also examined analogous to imaging analysis (p < 0.01).
3. Results
3.1. Participant characteristics
Participants did not differ across age, sex, ethnicity, BMI, education, income, and employment, but as expected, healthy control individuals differed from MDD participants in exposure to psychotropic medications, measures of depression, anxiety, RNT intensity, and disability, whereas all groups differed in the intensity of RNT (Table 1).
3.2. Behavioral results
Table 2 shows mean ratings of sensation intensity for Heart- and Stomach-Interoceptive trials and ratings of intensity of color change for Exteroceptive trials by group. Intensity ratings differed by trials with Greenhouse-Geisser correction, F[1.998, 241.578] = 20.72, p < 0.001, in the order of Heart (mean = 3.58 [SE = 0.13]) < Stomach (mean = 3.86 [SE = 0.14]) < Exteroceptive Target (mean = 4.49 [SE = 0.09]). However, intensity ratings did not differ by group, F[3.995, 241.70] = 1.31, p = 0.27: Heart-Interoceptive, F[2, 121] = 0.54, p = 0.58; Stomach-Interoceptive, F[2, 121] = 1.51, p = 0.23, and Exteroceptive trials, F [2, 121] = 0.68, p = 0.51.
Table 2.
Mean ratings of intensity (SDs) by group.
| Heart-interoceptive | Stomach-interoceptive | Target-exteroceptive | |
|---|---|---|---|
| HC | 3.40 (1.25) | 3.50 (1.51) | 4.56 (0.92) |
| Low RNT | 3.75 (1.48) | 3.99 (1.32) | 4.35 (0.81) |
| High RNT | 3.58 (1.46) | 4.09 (1.54) | 4.54 (1.01) |
Abbreviations: HC, Healthy Control; High RNT, High repetitive negative thinking; Low RNT, Low repetitive negative thinking.
3.3. fMRI results
Five regions revealed the effects of RNT in interoceptive processing: left medial frontal region from the operculum to the caudate, right medial temporal lobe from the hippocampus extending to the amygdala and the entorhinal cortex, left perirhinal cortex/parahippocampal cortex, left caudate nucleus/basal ganglia, and right mid occipital gyrus (Table 3). Follow-up analysis revealed that all of these regions exhibited Group effects in the Stomach condition but not in the Heart condition. As shown in Fig. 1, post-hoc pairwise comparisons on beta coefficients after Bonferroni corrections for multiple comparisons showed that both H-RNT and L-RNT revealed decreased activities for Stomach interoceptive processing compared to HC in the left medial frontal cortex extending to left insula and operculum (panel A, H-RNT: β = −1.04, 95% CI[−1.58,−0.51], L-RNT: β = −0.97, 95% CI[−1.50, −0.43]), left perirhinal cortex (panel B, H-RNT: β = −0.99, 95% CI[−1.52,−0.45], L-RNT: β = −1.03, 95% CI[−1.56,−0.50]), and left caudate nucleus (panel C, H-RNT: β = −1.06, 95% CI[−1.60,−0.53], L-RNT: β = −0.89, 95% CI[−1.43,−0.36]).
Table 3.
Group effects in Stomach interoceptive processing.
| Group effects | Peak | Voxels | Regions | Statistics | ||
|---|---|---|---|---|---|---|
| H-RNT vs. HC & L-RNT vs. HC | −19 | 27 | 17 | 347 | L frontal gyrus/insula/operculum | F[2121] = 12.76 |
| −35 | −21 | −29 | 303 | L perirhinal cortex/parahippocampal cortex | F[2121] = 11.25 | |
| −17 | −11 | 31 | 290 | L caudate nucleus/basal ganglia | F[2121] = 12.86 | |
| L-RNT vs, HC | 29 | −81 | 17 | 208 | R mid occipital gyrus | F[2121] = 12.39 |
| H-RNT vs, L-RNT & H-RNT vs. HC | 25 | −13 | −29 | 309 | R hippocampus/amygdala/entorhinal cortex | F[2121] = 7.21 |
Fig. 1.
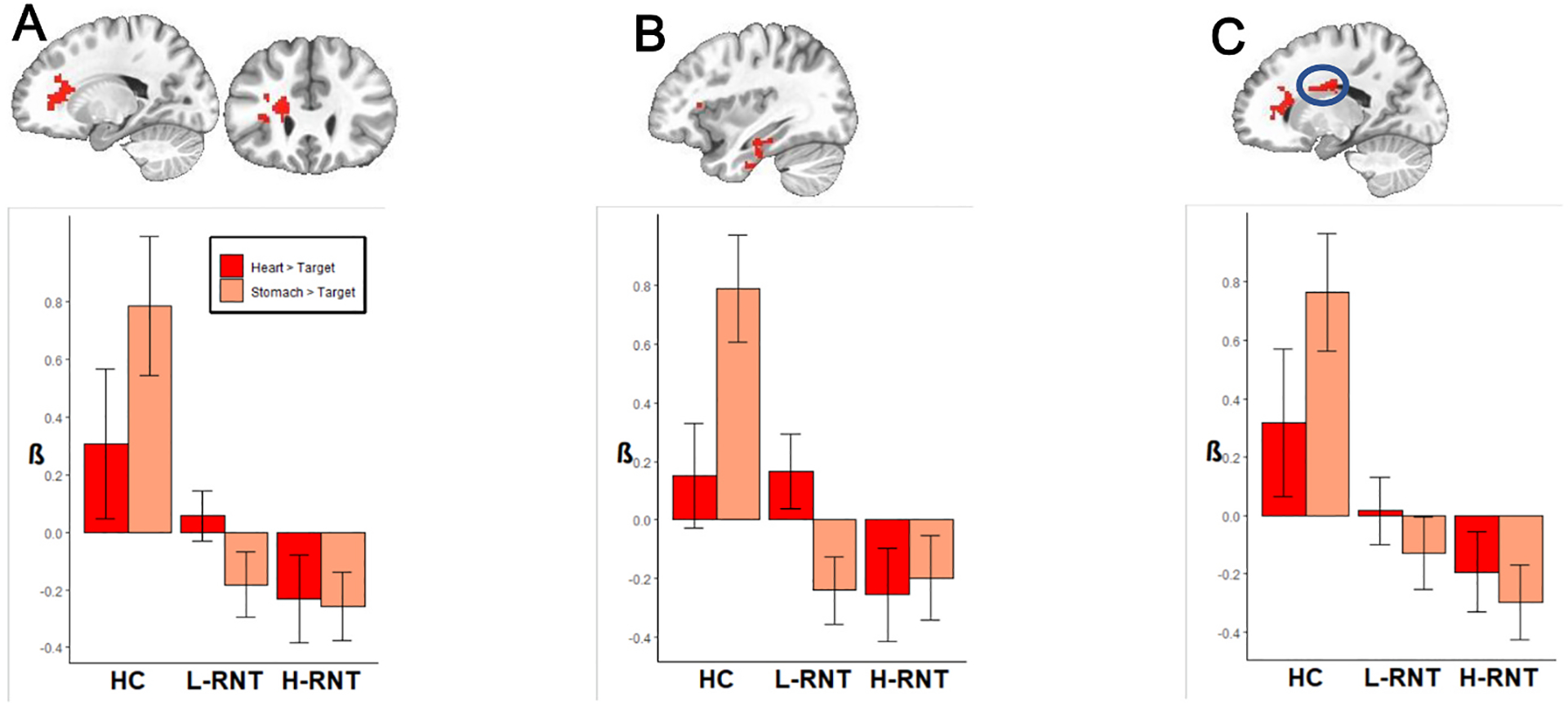
Brain regions showing the depression effects (H-RNT > HC, L-RNT > HC) in stomach interoceptive processing. A: L medial frontal cluster including the insula, B: L perirhinal/entorhinal cortex, C: L caudate nucleus.
Fig. 2 displays differences in neural processing of interoception between MDD individuals with varying levels of RNT. Individuals with H-RNT revealed diminished BOLD signals in the right medial temporal lobe including the hippocampus, amygdala, and adjacent regions during interoceptive processing focused on the stomach, compared with individuals with L-RNT (β = −0.61, 95% CI[−1.06, −.15]) and HCs (β = −1.01, 95% CI[−1.55, −.47]) (Fig. 2, panel A). A mid occipital gyrus cluster in the right hemisphere showed less activation in L-RNT compared to HC (β = −0.81, 95% CI[−1.36,−0.25]); the difference between H-RNT and L-RNT in this cluster was not significant, however (Fig. 2 panel B). We did not observe a difference between H-RNT and L-RNT in the insula (β = −0.07, p = 0.92), although decreased insula activity for stomach interoception was evident in the aforementioned left medial frontal cluster including insula in both H-RNT and L-RNT groups (Fig. 1A). As shown in Fig. 3, the correlations between RNT (RRS scores) and neural activity in these clusters demonstrated that H-RNT’s activity was more attenuated than activity in L-RNT or HC. The higher the RRS score was, the lower neural activity for stomach interoceptive attention occurred. No correlation between RRS and neural activity was found in the mid occipital cluster where only L-RNT differed from HC.
Fig. 2.
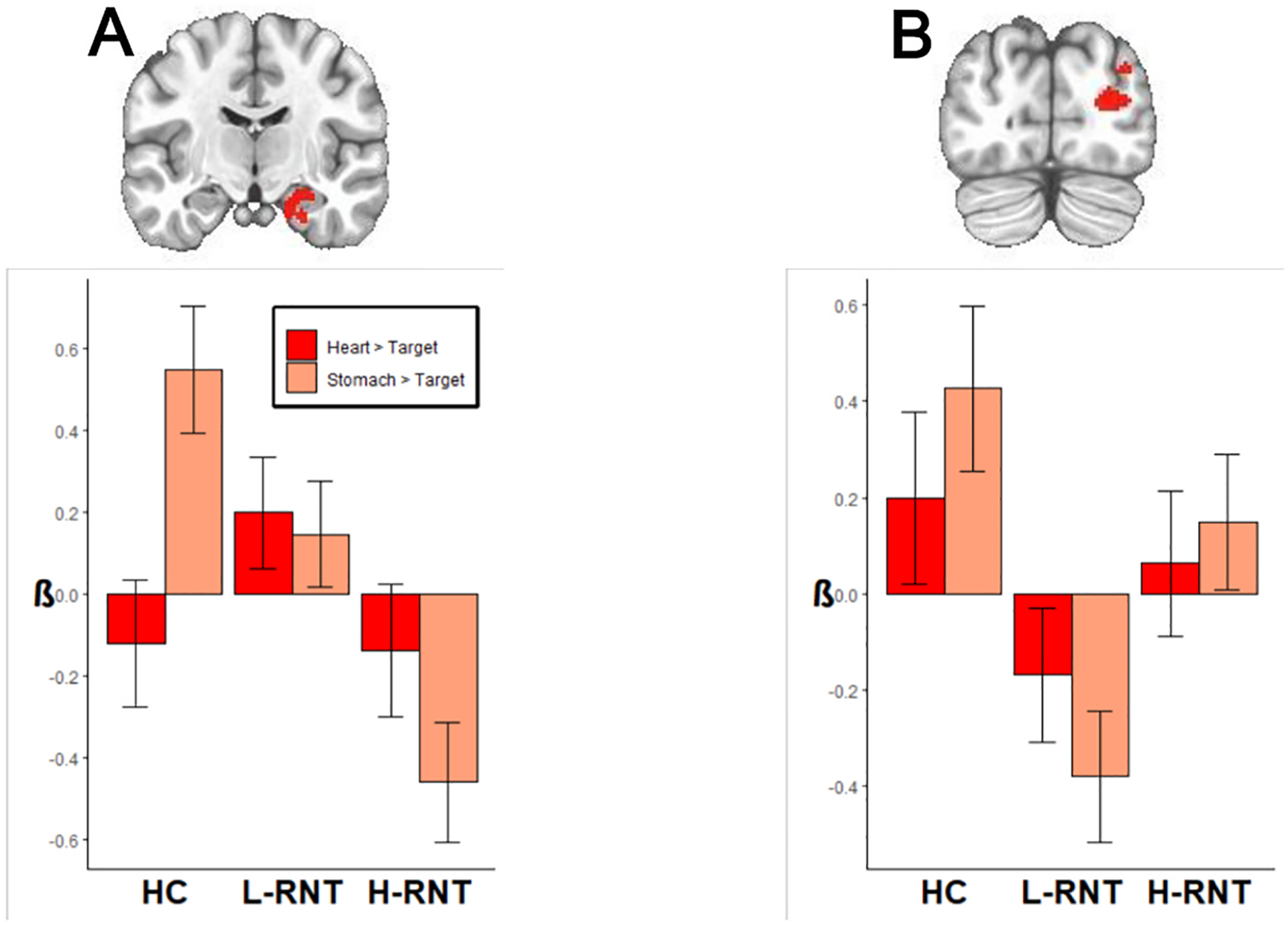
Brain regions showing high RNT intensity effects (H-RNT > L-RNT) and low-RNT group effects (L-RNT > HC). A: R hippocampus, amygdala, and adjacent medial temporal lobe. B: R occipital gyrus region.
Fig. 3.
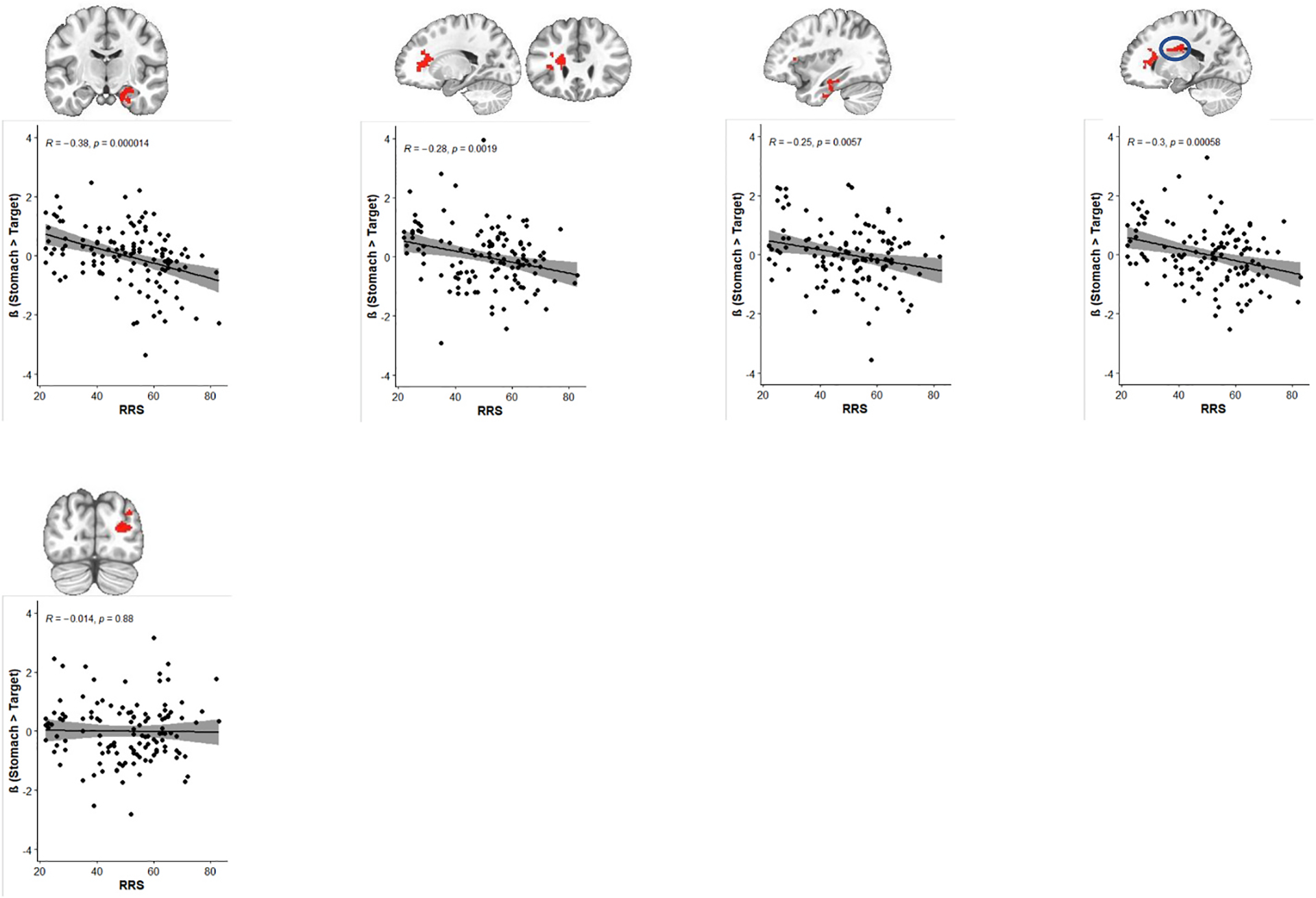
Correlations between RNT symptom severity and neural activity for stomach interoceptive processing in RNT.
4. Discussion
There were three main findings in the present study. First, MDD individuals regardless of levels of RNT showed altered central processing of stomach, but not heart, interoceptive information. Second, only MDD individuals with high RNT showed attenuated processing of stomach sensations in the right medial temporal lobe including the hippocampus, amygdala, and entorhinal cortex. Third, subjective behavioral ratings of stomach sensation intensity were greater than those of the heart, although the groups did not differ in intensity ratings. All group differences in the imaging results exhibited attenuated hemodynamic responses during attention to interoceptive signals from the stomach in depression compared to healthy individuals. The present results suggest that attenuated hippocampal and amygdala activity for gastric interoceptive signaling is associated with RNT, while overall reduced neural interoceptive processing of the stomach, including anterior insular cortex, which is pivotal in the central processing of interoceptive information (Chen et al., 2021), was linked to MDD. These findings may have a series of clinical and mechanistic implications regarding RNT generation and maintenance.
Notably, altered neural activity during interoception in suprathreshold clusters indicated changed interoception associated with depression. While this pattern of association was found in both H-RNT and L-RNT groups, neural activity during interoceptive processing showed a negative correlation with the severity of RNT. These conjoint findings revealed an impairment of neural processing of interoception in individuals with RNT in MDD in several brain regions including the anterior insular cortex. Further, the association between RNT and interoception was specific to the stomach interoception, which provides supporting evidence for neural specificity of interoception connected to different internal organs (See Khalsa et al., 2018), particularly in MDD.
Symptoms localized to the gut are extremely relevant to depression throughout the lifespan and are associated with MDD of severe and protracted course. For example, somatic complaints referred to the gut are frequent in the psychiatric assessment of individuals with MDD, and unexplained gastrointestinal symptoms account for a substantial proportion of general medicine consults of non-psychiatric patients eventually diagnosed with depression (Mussell et al., 2008; Leung et al., 2020; Arias de la Torre et al., 2021; in press Bergmans and Smith,). The present finding of abnormalities in the neural processing of interoception specific to the stomach but not the heart may reflect that the gut is structurally (Levinthal and Strick, 2020) and functionally (Rebollo and Tallon-Baudry, 2022) related to various central sensorimotor areas for somatic and autonomic responses (see Stewart et al., 2020 for other results). Additionally, the present findings expand previous observations on the relationship between RNT and interoceptive accuracy (Lackner and Fresco, 2016) and interoceptive sensitivity (Li et al., 2022) from healthy samples to MDD patient samples.
Importantly, the present findings indicate that the association between RNT and interoception is related to functions of the medial temporal lobe including the hippocampus and amygdala. The hippocampus is critically involved in the processing of contextual information (Eichenbaum and Cohen, 1988), with a role of the amygdala in emotional processing. The association between attenuated activations in the medial temporal structures and intensity of RNT during gastric interoceptive processing in MDD patients suggests a role of archicortical structures in the generation and/or maintenance of RNT, possibly through aberrant contextual information processing in conjunction with negatively biased emotion processing (Edwards-Duric et al., 2020). The relationship between RNT and interoceptive attention found in the present and previous studies with nonclinical populations, suggests that RNT symptom generation and/or maintenance incorporates faulty interoceptive feedback in a context of aberrant associative emotional learning (Levinthal and Strick, 2020; Guinjoan et al., 2021; Klein et al., 2021; Park et al., 2022).
The interaction between interoceptive disturbances, impaired fear conditioning, and repetitive negative thinking can be illustrated by burgeoning literature on the physiological and clinical effects of vagal nerve stimulation (VNS), which directly affects interoceptive signals (Klein et al., 2021). Recent evidence supports the idea that VNS owes its beneficial effect in depression to impairment of fear learning, generalization of fear extinction, or both mechanisms, in animals and humans (e.g. Burger et al., 2019a; Noble et al., 2019a,b; Souza et al., 2021a,b). In particular, VNS is purported to exert its beneficial clinical effect at least in part by facilitating emotional learning extinction, directly (Alvarez-Dieppa et al., 2016) and also as an adjuvant to exposure-based therapies (Peña et al., 2013; Noble et al., 2019b). Whereas neuro-chemical effects on the dentate gyrus through facilitated norepinephrine release from the locus coeruleus are proposed to be responsible for VNS’s treatment effects (Burger et al., 2019a), recent evidence suggests that interference with interoceptive feedback could be a reasonable complementary interpretation of the effect of VNS (Klein et al., 2021).
In addition, neural activity representing fear conditioning did not appropriately extinct but extended to the early extinction period in MDD individuals with high RNT, but not in MDD with low RNT (Park et al., in press). Taken together, the present finding of diminished hemodynamic responses in the medial temporal lobe linked to visceral interoception suggests that poor interoceptive feedback onto emotional memory systems might also interfere with normal emotional memory extinction in individuals with depression and intense RNT. Apart from VNS, other treatments useful in treatment-resistant depression have been proposed to act via interference in maladaptive emotional learning resulting from RNT (Guinjoan et al., 2021). A recent randomized controlled trial study in which VNS abated spontaneous negative thought intrusions in individuals with high worry is also in line with the interpretation that the VNS could exert its effect in treatment-resistant depression by amelioration of RNT (Burger et al., 2019b).
Altered interoceptive feedback is proposed to occur via a direct mechanism of emotional learning, as it does not interfere with attention to fearful stimuli (Verkuil and Burger, 2019). In addition, VNS has been suggested as an adjuvant to facilitate extinction in exposure-based psychotherapies (Noble et al., 2019b). RNT can be interpreted primarily as a form of abnormal emotional memory function occurring in a retrieval and reconsolidation cycle (Guinjoan et al., 2021). However, the finding that interference with visceral interoception with VNS reduces spontaneous intrusive negative thoughts (Burger et al., 2019b) also suggests that a causative role for alterations of emotional learning in the generation and maintenance of RNT cannot be ruled out.
Based on these findings and extant literature by our group and others (Oh et al., 2014; Burger et al., 2019b; Guinjoan et al., 2021; Ray et al., 2021; Park et al., 2022), we propose a potential model of RNT with abnormal emotional learning (including altered interoceptive feedback in fear learning) as a mechanism of RNT (Fig. 4). In this model, RNT would arise from an unrelenting cycle of poor interoceptive negative feedback to the acquisition of aversive memory, increased autonomic output in a pattern characteristic of such negative affective states, and again faulty interoceptive bottom-up modulatory feedback which leaves the retrieval-reconsolidation of aversive memories in a status of “free running.” The fact that antidepressant methods useful to reduce RNT act on the interoceptive feedback (VNS; Burger et al., 2019b) and reconsolidation (ECT, ketamine, and sleep deprivation; Guinjoan et al., 2021) mechanisms, reinforces the notion that abnormal emotional learning might have a role in the maintenance of RNT. Previous evidence by our group (Park et al., 2022) along with the present results also supports this view, by demonstrating faulty emotional learning extinction, and interoceptive information, respectively, in line with the notion of malfunctioning contextual processing of emotion.
Fig. 4.
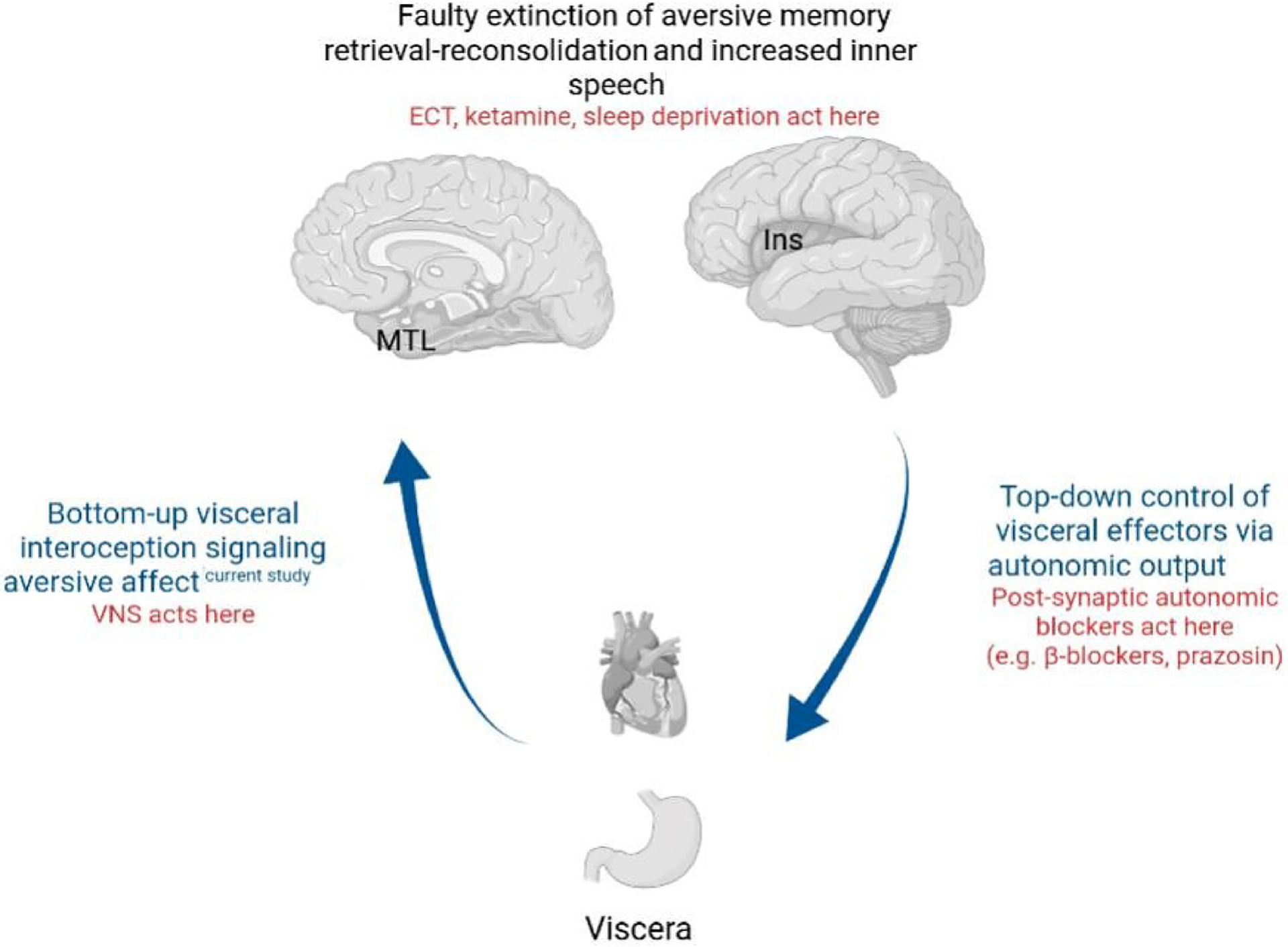
A proposed model on the role of interoceptive processing in RNT generation, incorporating bottom-up interoceptive processing, top-down autonomic nervous system output, and emotional learning abnormalities. Please see the text for details. MTL: medial temporal lobe (including parahippocampal cortex, hippocampus, and amygdala). Ins: Insular cortex. VNS: vagal nerve stimulation. ECT: electroconvulsive therapy.
This mechanistic model offers a parsimonious explanation for the purported beneficial effect of autonomic blocking agents like prazosin or β-blockers in anxiety disorders characterized by RNT, whose efficacy might respond at least in part to their peripheral activity (e.g., Giustino et al., 2016; Raskind et al., 2016; Srivastava et al., 2020; Boyce et al., 2021). While the model offers an explanation for unrelenting retrieval-reconsolidation that maintains aversive memories and negative affect, the question remains on how this model translates into intrusive and repetitive thoughts. Ray et al. (2021) recently proposed that RNT constitutes an inner-speech phenomenon that can be triggered by brain regions involved not only in the production of abnormal negative affect regulation (including insula) but also in the production of pre-articulatory speech motor responses (Ackermann and Riecker, 2004; Oh et al., 2014) that are a characteristic component of self-referential negative affect in the human species (McLaughlin et al., 2007). In theory, this phenomenon might be subserved by extensive structural and functional connectivity between the insular cortex and speech production area (i.e., Broca’s area) along with other regions related to speech (Oh et al., 2014). This model is speculative at this time and is being proposed here to inform future studies, particularly those exploring potential targets to alleviate RNT in depression. Moreover, the model does not account for the organ-specific interoceptive feedback abnormalities related to RNT, that is, why stomach, but not heart, interoception is associated with RNT. It is possible that interoceptive signaling from the gut is more specific to affect valence than heart interoception. Heart rate can increase or decrease in positive or adverse settings, depending on different circumstances. For instance, heart rate usually increases in stress in general, but decreases in fear-related freezing, in spite of both situations representing aversive affect (Hashemi et al., 2021). In addition, stomach interoceptive signaling has widespread central effects (Rebollo and Tallon-Baudry, 2022). In regard to heart interoception, not only did we not observe a clear effect of RNT intensity, but in fact differences between MDD and HC did not reach statistical significance either, as would have been expected from the literature reviewed above (Khalsa et al., 2018; Quadt et al., 2018). A number of reasons could account for this negative result. First, the sample size might have obscured meaningful differences due to insufficient statistical power. Second, the propensity matching process, designed to parse the specific effect of RNT, could result in an MDD sample which, not being naturalistic, is not fully representative of the general MDD population. These observations notwithstanding, the results confirm previous suggestions that interoceptive deficits are not necessarily widespread in depression, but instead they seem to show a degree of organ specificity. This peripheral organ specificity is however an open question that necessitates further investigation.
Behaviorally, the intensity of stomach interoception was higher than that of the heart, which is in line with the specificity of stomach interoception. In other words, MDD individuals were able to subjectively note stronger sensations from the stomach. Nonetheless, the main group difference was not significant, which may negate the possibility that subjective reports for interoception would differ by MDD groups or patients versus controls. These behavioral results are in agreement with the functional results of altered processing of stomach interoception by RNT, in that individuals tend reveal interoceptive processing more specific to the stomach.The present study has some limitations. First, it is a cross-sectional study in which associations do not permit causal inferences. Second, while the propensity matching method used for sampling herein provides a means to separate the effect of RNT from the effect of general symptom severity, it does not allow to establish continuous, population-level correlations between variables. Therefore, the nature of the association between RNT and deficits in the central processing of interoceptive information remains unsettled. Third, it was beyond the scope of the present study to delve into the specificity of gastric interoception in RNT, while modular processing of interoception by each organ would provide an account. Future studies should investigate the specificity of interoception in relation to RNT. Last, whereas the propensity matching process yielded comparable groups without statistically significant differences, HC still tended to be younger, more educated, and with a greater composition of women and White ethnicity. This might have contributed to differences between HC and MDD participants, it does not affect our interpretation of differences between individuals with MDD and high or low RNT.
5. Conclusion
The present study shows that RNT in MDD was associated with faulty central processing of gastric, but not cardiac, interoception. Further, RNT intensity was linked to attenuated neural processing of gastric interoceptive information in the medial temporal lobe, a region critical for contextual and emotional information processing. These findings suggest aberrant feedback on negative emotional information, leading to deficient contextual processing of information in RNT. Whether the observed faulty central processing of interoceptive information related to RNT in MDD is amenable to interventions for alleviation of RNT, remains open to further investigation.
Supplementary Material
Acknowledgements
This work has been supported in part by the William K. Warren Foundation, and the National Institute of General Medical Sciences Center Grant Award Number 1P20GM121312. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The funder had no role in study design, in the collection, analysis, and interpretation of data, in the writing of the manuscript, or in the decision to submit the paper for publication.
The ClinicalTrials.gov identifier for the clinical protocol associated with data published in the current paper is NCT02450240, “Latent Structure of Multi-level Assessments and Predictors of Outcomes in Psychiatric Disorders” (https://clinicaltrials.gov/ct2/show/NCT02450240).
Footnotes
Author statement
The manuscript “Attenuated interoceptive processing in individuals with major depressive disorder and high repetitive negative thinking,” revised according to feedback from you and the Reviewers, has been approved by all authors, and all funding sources have been acknowledged.
Declaration of competing interest
Dr. Paulus is an advisor to Spring Care, Inc., a behavioral health startup, and he has received royalties for an article about methamphetamine in UpToDate. All other authors report no biomedical financial interests or potential conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpsychires.2022.10.020.
References
- Ackermann H, Riecker A, 2004. The contribution of the insula to motor aspects of speech production: a review and a hypothesis. Brain Lang. 89, 320–328. [DOI] [PubMed] [Google Scholar]
- Alvarez-Dieppa AC, Griffin K, Cavalier S, Mcintyre CK, 2016. Vagus nerve stimulation enhances extinction of conditioned fear in rats and modulates Arc protein, CaMKII, and GluN2B-containing NMDA receptors in the basolateral amygdala. Neural Plast., 4273280 10.1155/2016/4273280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Highlights of Changes from DSM-IV-TR to DSM-5. https://www.psychiatry.org/File%20Library/Psychiatrists/Practice/DSM/APA_DSM_Changes_from_DSM-IV-TR_-to_DSM-5.pdf. (Accessed 23 August 2022).
- Arias JA, Williams C, Raghvani R, Aghajani M, Baez S, Belzung C, Booij L, Busatto G, Chiarella J, Fu CH, Ibanez A, Liddell BJ, Lowe L, Penninx BWJH, Rosa P, Kemp AH, 2020. The neuroscience of sadness: a multidisciplinary synthesis and collaborative review. Neurosci. Biobehav. Rev 111, 199–228. [DOI] [PubMed] [Google Scholar]
- Arias de la Torre J, Ronaldson A, Prina M, Matcham F, Pinto Pereira SM, Hatch SL, et al. , 2021. Depressive symptoms during early adulthood and the development of physical multimorbidity in the UK: an observational cohort study. Lancet Healthy Longev 2 (12), e801–e810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery JA, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, Simmons WK, 2014. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol. Psychiatr 76, 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmans RS, Smith J, (in press). Associations of mental health and chronic physical illness during childhood with major depression in later life. Aging Ment. Health doi: 10.1080/13607863.2021.1958143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce TG, Ballone NT, Certa KM, Becker MA, 2021. The use of beta-adrenergic receptor antagonists in psychiatry: a review. J. Acad. Consult. Liaison Psychiatry 62 (4), 404–412. [DOI] [PubMed] [Google Scholar]
- Burger AM, Van Diest I, Van der Does W, Korbee JN, Waziri N, Brosschot JF, et al. , 2019a. The effect of transcutaneous vagus nerve stimulation on fear generalization and subsequent fear extinction. Neurobiol. Learn. Mem 161, 192–201. [DOI] [PubMed] [Google Scholar]
- Burger AM, Van der Does W, Thayer JF, Brosschot JF, Verkuil B, 2019b. Transcutaneous vagus nerve stimulation reduces spontaneous but not induced negative thought intrusions in high worriers. Biol. Psychol 142, 80–89. [DOI] [PubMed] [Google Scholar]
- Burgoyne AP, Engle RW, 2020. Attention control: a cornerstone of higher-order cognition. Curr. Dir. Psychol. Sci 29 (6), 624–630. 10.1177/0963721420969371. [DOI] [Google Scholar]
- Burrows KP, DeVille DC, Cosgrove KT, Kuplicki RT, , Tulsa 1000 Investigators, Paulus MP, Aupperle R, Khalsa SS, Stewart JL, 2022. Impact of serotonergic medication on interoception in major depressive disorder. Biol. Psychol 169, 108286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascalenda N, Perry JC, Looper K, 2002. Remission in major depressive disorder: a comparison of pharmacotherapy, psychotherapy, and control conditions. Am. J. Psychiatr 159 (8), 1354–1360. [DOI] [PubMed] [Google Scholar]
- Chen WG, Schloesser D, Arensdorf AM, Simmons JM, Cui C, Valentino R, et al. , 2021. The emerging science of interoception: sensing, integrating, interpreting, and regulating signals within the self. Trends Neurosci. 44 (1), 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy SK, Holtzheimer PE, 2021. Neuromodulation strategies for the treatment of depression. Am. J. Psychiatr 178, 1082–1088. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, 2018. Deep brain stimulation: clinical applications. Psychiatr. Clin 41 (3), 385–394. [DOI] [PubMed] [Google Scholar]
- Edwards-Duric J, Stevenson RJ, Francis HM, 2020. The congruence of interoceptive predictions and hippocampal-related memory. Biol. Psychol 155, 107929. [DOI] [PubMed] [Google Scholar]
- Eggart M, Valdés-Stauber J, 2021. Can changes in multidimensional self-reported interoception be considered as outcome predictors in severely depressed patients? A moderation and mediation analysis. J. Psychosom. Res 141, 110331 10.1016/j.jpsychores.2020.110331. [DOI] [PubMed] [Google Scholar]
- Ehring T, Watkins ER, 2008. Repetitive negative thinking as a transdiagnostic process. Int. J. Cognit. Ther 1 (3), 192–205. [Google Scholar]
- Eichenbaum H, Cohen NJ, 1988. Representation in the hippocampus: what do hippocampal neurons code? Trends Neurosci. 11, 244–248. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD, 2007. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatr 164 (10), 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E, Dutton K, Yates A, Georgiou GA, Mouchlianitis E, 2015. Attentional control and suppressing negative thought intrusions in pathological worry. Clin. Psychol. Sci 3 (4), 593–606. 10.1177/2167702615575878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2019 Mental Disorders Collaborators, 2022. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatr. 9 (2), 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzburg K, Tsur N, Barak-Nahum A, Defrin R, 2014. Body awareness: differentiating between sensitivity to and monitoring of bodily signals. J. Behav. Med 37 (3), 564–575. [DOI] [PubMed] [Google Scholar]
- Giustino TF, Fitzgerald PJ, Maren S, 2016. Revisiting propranolol and PTSD: memory erasure or extinction enhancement? Neurobiol. Learn. Mem 130, 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinjoan SM, Bär KJ, Camprodon JA, 2021. Cognitive effects of rapid-acting treatments for resistant depression: just adverse, or contributing to clinical efficacy? J. Psychiatr. Res 140, 512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi MM, Zhang W, Kaldewaij R, Koch SBJ, Smit A, Figner B, et al. , 2021. Human defensive freezing: associations with hair cortisol and trait anxiety. Psychoneuroendocrinology 133, 105417. [DOI] [PubMed] [Google Scholar]
- Hirsch CR, Mathews A, 2012. A cognitive model of pathological worry. Behav. Res. Ther 50 (10), 636–646. 10.1016/j.brat.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, 2020. The critical relationship between anxiety and depression. Am. J. Psychiatr 177 (5), 365–367. [DOI] [PubMed] [Google Scholar]
- Keller AS, Leikauf JE, Holt-Gosselin B, Staveland BR, Williams LM, 2019. Paying attention to attention in depression. Transl. Psychiatry 9 (1), 279. 10.1038/s41398-019-0616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Lapidus RC, 2016. Can interoception improve the pragmatic search for biomarkers in psychiatry? Front. Psychiatr 7, 121. 10.3389/fpsyt.2016.00121.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Adolphs R, Cameron OG, Critchley HD, Davenport PW, Feinstein JS, et al. , 2018. Interoception and mental health: a roadmap. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3 (6), 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AS, Dolensek N, Weiand C, Gogolla N, 2021. Fear balance is maintained by bodily feedback to the insular cortex in mice. Science 374 (6570), 1010–1015. [DOI] [PubMed] [Google Scholar]
- Lackner RJ, Fresco DM, 2016. Interaction effect of brooding rumination and interoceptive awareness on depression and anxiety symptoms. Behav. Res. Ther 85, 43–52. 10.1016/j.brat.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver AM, Espinoza R, Wade B, Narr KL, 2022. Parsing the network mechanisms of electroconvulsive therapy. Biol. Psychiatr 10.1016/j.biopsych.2021.11.016 (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J, Fan VS, Mahadevan R, 2020. How do different chronic condition comorbidities affect changes in depressive symptoms of middle aged and older adults? J. Affect. Disord 272, 46–49. [DOI] [PubMed] [Google Scholar]
- Levinthal DJ, Strick PL, 2020. Multiple areas of the cerebral cortex influence the stomach. Proc. Natl. Acad. Sci. U.S.A 117 (23), 13078–13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Qin F, Liu J, Luo Q, Zhang Y, Hu J, et al. , 2022. An insula-based network mediates the relation between rumination and interoceptive sensibility in the healthy population. J. Affect. Disord 299, 6–11. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Borkovec TD, Sibrava NJ, 2007. The effects of worry and rumination on affect states and cognitive activity. Behav. Ther 38, 23–38. [DOI] [PubMed] [Google Scholar]
- Mussell M, Kroenke K, Spitzer RL, Williams JBW, Herzog W, Loewe B, 2008. Gastrointestinal symptoms in primary care: prevalence and association with depression and anxiety. J. Psychosom. Res 64 (6), 605–612. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, 2020. The state of our understanding of the pathophysiology and optimal treatment of depression: glass half full or half empty? Am. J. Psychiatr 177 (8), 671–685. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Meruva VB, Hays SA, Rennaker RL, Kilgard MP, McIntyre CK, 2019a. Vagus nerve stimulation promotes generalization of conditioned fear extinction and reduces anxiety in rats. Brain Stimul. 12 (1), 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble LJ, Souza RR, McIntyre CK, 2019b. Vagus nerve stimulation as a tool for enhancing extinction in exposure-based therapies. Psychopharmacology (Berl) 236 (1), 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J, 1991. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: the 1989 Loma Prieta earthquake. J. Pers. Soc. Psychol 61 (1), 115–121. [DOI] [PubMed] [Google Scholar]
- Oh A, Duerden EG, Pang EW, 2014. The role of the insula in speech and language processing. Brain Lang. 135, 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Kirlic N, Kuplicki R, Tulsa 1000 investigators, Paulus M, Guinjoan SM, 2022. Neural processing dysfunctions during fear learning but not reward-related processing characterize depressed individuals with high levels of repetitive negative thinking. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 7 (7), 716–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stein MB, 2006. An insular view of anxiety. Biol. Psychiatr 60, 383–387. [DOI] [PubMed] [Google Scholar]
- Peña DF, Engineer ND, McIntyre CK, 2013. Rapid remission of conditioned fear expression with extinction training paired with vagus nerve stimulation. Biol. Psychiatr 73, 1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadt L, Critchley HD, Garfinkel SN, 2018. The neurobiology of interoception in health and disease. Ann. N. Y. Acad. Sci 1428, 112–128. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Millard SP, Petrie EC, Peterson K, Williams T, Hoff DJ, et al. , 2016. Higher pretreatment blood pressure is associated with greater posttraumatic stress disorder symptom reduction in soldiers treated with prazosin. Biol. Psychiatr 80 (10), 736–742. [DOI] [PubMed] [Google Scholar]
- Ray D, Bezmaternykh D, Melnikov M, Friston KJ, Das M, 2021. Altered effective connectivity in sensorimotor cortices is a signature of severity and clinical course in depression. Proc. Natl. Acad. Sci. U.S.A 118 (40), e2105730118 10.1073/pnas.2105730118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebollo I, Tallon-Baudry C, 2022. The sensory and motor components of the cortical hierarchy are coupled to the rhythm of the stomach during rest. J. Neurosci 10.1523/JNEUROSCI.1285-21.2021 (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihata S, Johnson AR, Erceg-Hurn DM, McEvoy PM, 2021. Measurement invariance of disorder-specific and transdiagnostic measures of repetitive negative thinking. Assessment 10731911211028657. 10.1177/10731911211028657. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Avery JA, Barcalow JC, Bodurka J, Drevets WC, Bellgowan P, 2012. Keeping the body in mind: insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Hum. Brain Mapp 34, 2944–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza RR, Robertson NM, McIntyre CK, Rennaker RL, Hays SA, Kilgard MP, 2021a. Vagus nerve stimulation enhances fear extinction as an inverted-U function of stimulation intensity. Exp. Neurol 341, 113718 10.1016/j.expneurol.2021.113718. [DOI] [PubMed] [Google Scholar]
- Souza RR, Oleksiak CR, Tabet MN, Rennaker RL, Hays SA, Kilgard MP, et al. , 2021b. Vagus nerve stimulation promotes extinction generalization across sensory modalities. Neurobiol. Learn. Mem 181, 107425 10.1016/j.nlm.2021.107425. [DOI] [PubMed] [Google Scholar]
- Spunt RP, Adolphs R, 2017. A new look at domain specificity: insights from social neuroscience. Nat. Rev. Neurosci 18, 559–567. [DOI] [PubMed] [Google Scholar]
- Srivastava AA, Opler DJ, 2020. Clinical use of prazosin in a patient with acute stress disorder: a case report. J. Psychiatr. Pract 26 (3), 246–248. [DOI] [PubMed] [Google Scholar]
- Teed AR, Feinstein JS, Puhl M, Lapidus RC, Upshaw V, Kuplicki RT, Bodurka J, Ajijola OA, Kaye WH, Thompson WK, Paulus MP, Khalsa SS, 2022. Association of generalized anxiety disorder with autonomic hypersensitivity and blunted ventromedial prefrontal cortex activity during peripheral adrenergic stimulation: a randomized clinical trial. JAMA Psychiatr, e214225 10.1001/jamapsychiatry.2021.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J, Aspell JE, Barron D, Toh EKL, Zahari HS, Khatib NAM, Laughton R, Swami V, 2020. Greater gastric interoception is associated with more positive body image: evidence from adults in Malaysia and the United Kingdom. Body Image 34, 101–111. [DOI] [PubMed] [Google Scholar]
- Verkuil B, Burger AM, 2019. Transcutaneous vagus nerve stimulation does not affect attention ot fearful faces in high worriers. Behav. Res. Ther 113, 25–31. [DOI] [PubMed] [Google Scholar]
- Victor TA, Khalsa SS, Simmons WK, Feinstein JS, Savitz J, Aupperle RL, et al. , 2018. Tulsa 1000: a naturalistic study protocol for multilevel assessment and outcome prediction in a large psychiatric sample. BMJ Open 8 (1), e016620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl K, Ehring T, Kley H, Lieb R, Meyer A, Kordon A, et al. , 2019. Is repetitive negative thinking a transdiagnostic process? A comparison of key processes of RNT in depression, generalized anxiety disorder, obsessive-compulsive disorder, and community controls. J. Behav. Ther. Exp. Psychiatr 64, 45–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


