Abstract
The genus Pestivirus of the family Flaviviridae comprises three established species, namely, bovine viral diarrhea virus (BVDV), classical swine fever virus (CSFV), and border disease virus from sheep (BDV). In this study, we report the first complete nucleotide sequence of BDV, that of strain X818. The genome is 12,333 nucleotides long and contains one long open reading frame encoding 3,895 amino acids. The 5′ noncoding region (NCR) of BDV X818 consists of 372 nucleotides and is thus similar in length to the 5′ NCR reported for other pestiviruses. The 3′ NCR of X818 is 273 nucleotides long and thereby at least 32 nucleotides longer than the 3′ NCR of pestiviruses analyzed thus far. Within the 3′ NCR of BDV X818, the sequence motif TATTTATTTA was identified at four locations. The same repeat was found at two or three locations within the 3′ NCR of different CSFV isolates but was absent in the 3′ NCR of BVDV. Analysis of five additional BDV strains showed that the 3′ NCR sequences are highly conserved within this species. Comparison of the deduced amino acid sequence of X818 with the ones of other pestiviruses allowed the prediction of polyprotein cleavage sites which were conserved with regard to the structural proteins. It has been reported for two BVDV strains that cleavage at the nonstructural (NS) protein sites 3/4A, 4A/4B, 4B/5A, and 5A/5B is mediated by the NS3 serine protease and for each site a conserved leucine was found at the P1 position followed by either serine or alanine at P1′ (N. Tautz, K. Elbers, D. Stoll, G. Meyers, and H.-J. Thiel, J. Virol. 71:5415–5422, 1997; J. Xu, E. Mendez, P. R. Caron, C. Lin, M. A. Murcko, M. S. Collett, and C. M. Rice, J. Virol. 71:5312–5322). Interestingly, P1′ of the predicted NS5A/5B cleavage site of BDV is represented by an asparagine residue. Transient expression studies demonstrated that this unusual NS5A/5B processing site is efficiently cleaved by the NS3 serine protease of BDV.
Border disease virus (BDV) belongs to the genus Pestivirus, which also comprises bovine viral diarrhea virus (BVDV) and classical swine fever virus (CSFV). The genera Pestivirus and Flavivirus and the hepatitis C virus group are included in the family Flaviviridae (51). BDV is the causative agent of an important congenital disease of sheep and is probably distributed worldwide (see reference 29 for a review). Intrauterine infections during early pregnancy can cause fetal death and abortions. Alternatively, intrauterine infections during the first half of gestation can result in lambs which show tremor, ataxia, hairy fleece, brain malformations, and poor growth. Affected lambs, termed hairy shakers, are persistently infected and play an important role in virus transmission (29, 46). In addition, a syndrome similar to mucosal disease of cattle has been described for sheep infected with ruminant pestiviruses (1, 30).
The genomes of pestiviruses are positive-strand RNAs, usually with a length of 12.3 kb. The genomic RNA contains one continuous long open reading frame (ORF) flanked by 5′ and 3′ noncoding regions (NCR) (13, 14, 16, 23, 24, 28, 34). The ORF is translated into a hypothetical polyprotein of about 3,900 amino acids (aa). Generally, 11 to 13 pestiviral proteins can be found as products of polyprotein processing that is mediated by viral and host cell proteases (see reference 25 for a review). In the polyprotein, the mature viral proteins are arranged in the following order (from the N to C terminus): Npro, C, Erns, E1, E2, p7, NS2-3, (NS2), (NS3), NS4A, NS4B, NS5A, NS5B. The first protein of the polyprotein, a nonstructural autoprotease (Npro), is followed by the structural proteins C, Erns, E1, and E2. The remaining proteins are presumably nonstructural (NS). Processing at the cleavage sites NS3/4A, NS4A/4B, NS4B/5A, and NS5A/5B has been shown to be mediated by the NS3 serine protease (45, 55, 56).
For pestiviruses and hepatitis C viruses (HCV), it has been reported that the 5′ NCR contains an internal ribosome entry site (IRES) for translation initiation (32, 35, 50). Taking into account our knowledge about other positive-strand RNA viruses, the 5′ and 3′ sequences of pestiviral RNAs probably harbor additional specific signals for viral replication, transcription, and translation (11, 42, 53). Both primary and secondary structures can serve as signals recognized by specific replicase and translation complexes. So far, little is known about the function of the 3′ NCR of pestiviruses and HCV.
Comparative sequence analyses of pestiviruses from cattle, sheep, and pigs led to the identification of four distinct genotypes (2, 47). This is in contrast to the currently used nomenclature that considers only the three members mentioned above. Furthermore, several studies have shown that pestiviruses are not strictly host specific but may infect many species within the Artiodactyla (4, 29). To reconsider the taxonomy of pestiviruses, it has been proposed to term BVDV type 1 (BVDV-1) pestivirus type 1, CSFV pestivirus type 2, “true” BDV pestivirus type 3, and BVDV-2 pestivirus type 4 (2). A pestivirus isolate from a giraffe apparently represents the first member of a fifth genotype (4).
Complete genomic sequences are available for BVDV-1 (13, 14, 16, 24), BVDV-2 (34), and CSFV (23, 27, 28, 36). In this study, we report the first entire genomic sequence of BDV by using our pestivirus type 3 reference strain, X818 (5).
MATERIALS AND METHODS
Cells and viruses.
MDBK and BHK-21 cells were obtained from the American Type Culture Collection (Rockville, Md.). Cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum. BDV strains X818 (5), L83-84 (5), Cumnock (3), Moredun (3, 48), V-TOB (4, 40), and Frijters (4, 52) as well as ovine genotype 1 pestivirus R2727 (5, 10) have been described previously. The modified vaccinia virus Ankara expressing the T7 polymerase (MVA-T7pol) was kindly provided by G. Sutter (Institute of Molecular Virology, GSF-Centre for Environmental and Health Research, Oberschleissheim, Germany) (43).
Infection of cells.
Supernatants and lysates of infected cells were combined and used for infection of MDBK cells. Material for infection was prepared by freezing and thawing cultures 48 h postinfection and then stored at −70°C. A multiplicity of infection of about 0.1 was used for infections.
Oligonucleotides.
Oligonucleotides were purchased from MWG Biotech GmbH (Ebersberg, Germany). Sequences of oligonucleotides and their positions in the genomic sequence of BDV X818 (in parentheses) are as follows: Ol 100, CATGCCCWYAGTAGGACTAGC (97–117); Ol 200R, GGGCATGCCCTCGTCCAC (243–226); Ol 380R, AACTCCATGTGCCATGTACAG (380–360); Ol 10770, AGTGATACAGTACCCAGAGG (10758–10777); Ol 11500, GAGAAAAGCCTAGGAGAG (11266–11283), Ol 11900, TGCAAAGTGGAGGCCATAC (11888–11906); Ol 12000, ATTGGGACCCATAGTCAACG (11985–12004); Ol 12050R, TTCGGGCCCCATCCCTCTACCAACCAGG (12057–12039); and Ol 12200R, CMYMCAGCTAAAGTGCT (12283–12267). Primers Ol 100, Ol 200R, Ol 380R, Ol 11500, and Ol 12200R were designed by using published sequences of BVDV-1 strains NADL (13), Osloss (14), and CP7 (24), BVDV-2 strain 890 (34), and CSFV strains Alfort-T (23) and Brescia (28). Other primers were derived from the X818 sequence. The sequence of primer Ol BDV8R has been published previously (2).
For anchored reverse transcription (RT)-PCR, RNA oligonucleotide Ol LIG (5′ CAUCUCGAUGCUCGACCUCUC 3′) and the corresponding antisense oligonucleotide Ol G31 (5′ GCGGATCCGAGAGGTCGAGCATCGAGATG 3′) were used; within the sequence of Ol G31, the region complementary to Ol LIG is underlined. To prevent intramolecular and intermolecular ligation, the 3′ ends of Ol LIG were blocked by the addition of an amino group (57).
RT-PCR.
RNA from pestivirus-infected cells was prepared by using either the RNeasy total RNA kit (Qiagen GmbH, Hilden, Germany) or the RNA Extraction Kit (Pharmacia Biotech) as recommended by the supplier. RT-PCR was done as described previously (4).
Molecular cloning of the genomic sequence.
cDNA synthesis, establishment and screening of lambda ZAPII library, and cloning of cDNA fragments encompassing nucleotides 1300 to 11500 of the BDV X818 genome have been described previously (5). The remaining part of the genome was cloned after RT-PCR by using the TA cloning kit (Invitrogen, De Schelp, The Netherlands). Prior to cloning, the cDNA fragments obtained after RT-PCR were separated by agarose gel electrophoresis and purified by using the Qiaex DNA purification kit (Qiagen). For amplification of the genomic regions encompassing nucleotides 100 to 1400 and 11280 to 12260 (numbers according to the obtained nucleotide sequence of BDV X818), primer pairs Ol BDV8R/Ol 100 and Ol 12200R/Ol 11500 were used, respectively.
Analysis of the 5′ and 3′ sequences.
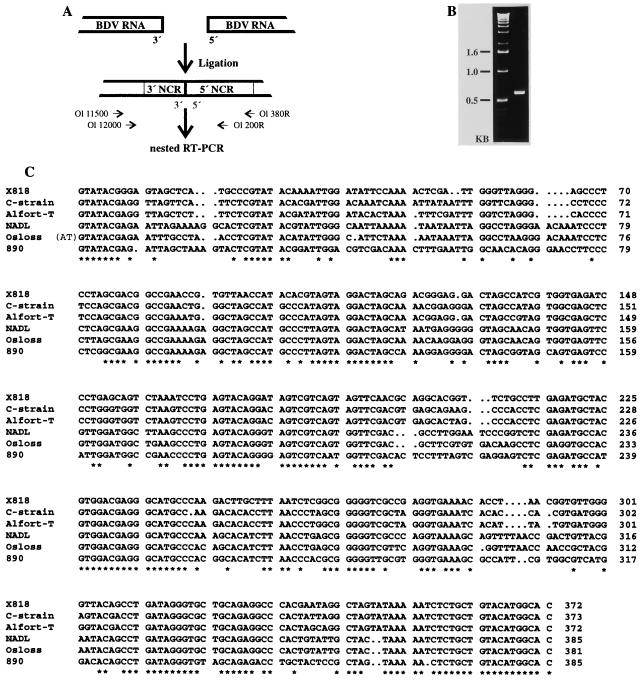
For determination of the 5′ and 3′ sequences, both an RNA ligation method and an RNA oligonucleotide ligation method were employed. For these analyses, genomic RNA was prepared from 500 ml of supernatant of infected cells. After clarification of the supernatant at 2,500 × g for 30 min at +4°C, virions were pelleted by centrifugation at 100,000 × g for 4 h at +4°C. The viral pellet was resuspended in 400 μl of lysis buffer, and RNA was prepared by using the RNA Extraction Kit (Pharmacia Biotech). Two micrograms of RNA was ligated by using 20 U of T4 RNA ligase (New England Biolabs) in a reaction mixture containing 50 mM Tris-HCl (pH 7.8), 10 mM MgCl2, 1 mM β-mercaptoethanol, 1 mM ATP, and 16 U of RNase inhibitor (RNasin; Promega) for 5 h at 37°C in a volume of 10 μl. After phenol-chloroform extraction and ethanol precipitation, the pellet was resuspended in 10 μl of diethylpyrocarbonate-treated H2O. A 2.5-μl volume of this solution was used for RT-PCR with primers Ol 380R and Ol 11500. A second, nested PCR was performed with primers Ol 200R and Ol 12000 (see Fig. 1).
FIG. 1.
5′ sequences of BDV X818. (A) Experimental strategy. After ligation of the 5′ and 3′ ends of the viral RNA, RT-PCR was performed with primers Ol 380R and Ol 11500. Two microliters of the amplification product was used for a second, nested PCR with primers Ol 200R and Ol 12000. (B) PCR product obtained after nested PCR. (C) Alignment of the 5′ NCR sequences of the four pestivirus genotypes. For BDV X818, the consensus sequence was determined from six independent clones. Other sequences were extracted from the GenBank/EMBL database (CSFV C strain [27], CSFV Alfort-T [26], BVDV-1 NADL [13], BVDV-1 Osloss [14], BVDV-2 890 [34]). Conserved nucleotides are indicated with an asterisk.
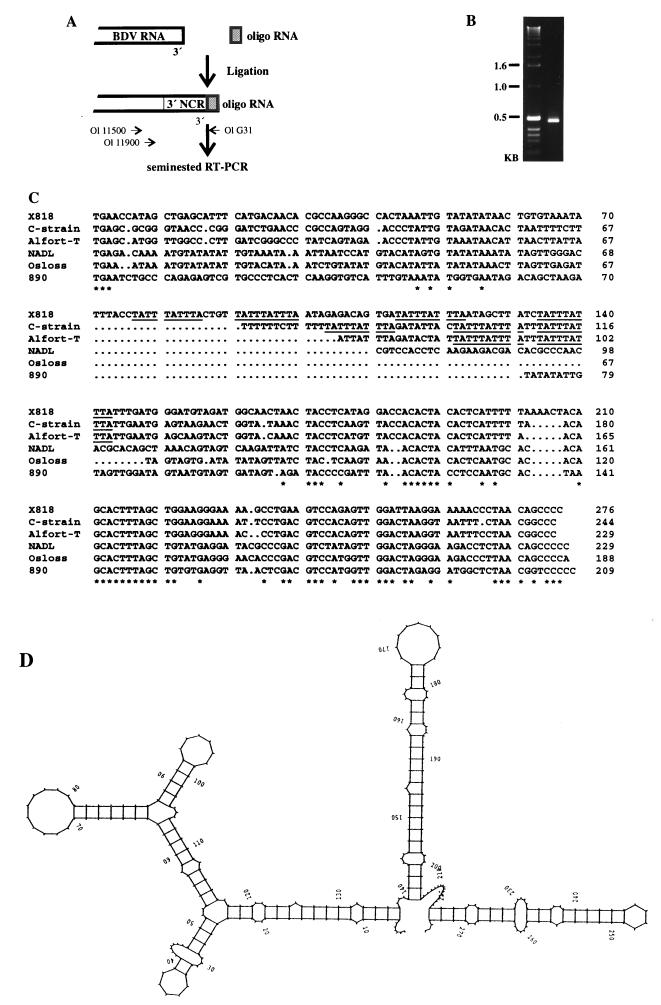
For analysis of the 3′ sequences, 20 ng of RNA oligonucleotide Ol LIG was ligated to 2 μg of RNA with 20 U of T4 RNA ligase (New England Biolabs) by using the same reaction conditions as those described above. After phenol-chloroform extraction and ethanol precipitation, RT-PCR analysis was performed with primer Ol G31, which is complementary to the sequence of Ol LIG, and sense primer Ol 11500. A second, seminested PCR was performed with primers Ol G31 and Ol 11900 (see Fig. 2).
FIG. 2.
3′ sequences of BDV X818. (A) Experimental strategy. After ligation of an RNA oligonucleotide (Ol LIG) to the 3′ end of the viral RNA, RT-PCR was performed with primer Ol G31, which is complementary to Ol LIG, and primer Ol 11500. Two microliters of the amplification product was used for a second, seminested PCR with primers Ol G31 and Ol 11900. (B) PCR product obtained after seminested PCR. (C) Alignment of the 3′ NCR sequences of the four pestivirus genotypes. For BDV X818, the consensus sequence was determined from 12 clones obtained after ligation with an RNA oligonucleotide and from six clones obtained after ligation of the 5′ and 3′ ends of viral RNA (see also Fig. 1). Other sequences were extracted from the GenBank/EMBL database. Each sequence starts with the translational stop codon. Conserved nucleotides are indicated with an asterisk. The repeat sequence motif TATTTATTTA identified within the 3′ NCR of BDV X818, as well as CSFV C strain and Alfort-T, is underlined. (D) Predicted secondary structure of BDV X818 3′ NCR. Modeling was performed with the computer program RNAFOLD. The numbers correspond to those of the sequence shown in panel C.
Nucleotide sequencing and sequence analysis.
Dideoxy sequencing (38) of double-stranded DNA templates was carried out with the T7 polymerase sequencing kit in the presence of [α-35S]dATP (Pharmacia LKB). All sequences were determined by sequencing both complementary strands of at least two independent cDNA clones. In the case of nucleotide differences, the consensus sequence was obtained by sequencing a third independent clone. Computer analysis of sequence data was performed by using HUSAR (DKFZ, Heidelberg, Germany), which provides the GCG (17) and PHYLIP (19) software packages. Multiple sequence alignments of the DNA and deduced amino acid sequences were generated with the GCG programs PILEUP and CLUSTAL.
Transient expression with the T7 vaccinia virus system.
BHK-21 cells (5 × 105 per 3.5-cm-diameter dish) were infected with the recombinant T7 vaccinia virus MVA-T7pol at a multiplicity of infection of 10 (43). After 1 h of incubation at 37°C, the cells were washed twice with medium lacking fetal calf serum. Subsequently, cells were transfected with 2.0 μg of plasmid DNA by using Superfect reagent (Qiagen). After 3 h of incubation at 37°C, the supernatant was replaced with medium containing 10% fetal calf serum and the cells were incubated overnight at 37°C. Finally, the cells were washed with phosphate-buffered saline and stored at −20°C.
Construction of T7 expression plasmids.
All T7 expression plasmids were based on vector pCITE (Invitrogen). To establish a construct for expression of NS3, NS4A, and NS4B of BDV X818, a BamHI-SacI fragment encompassing nucleotides 5121 to 8435 of the X818 sequence was derived from cDNA clone X9 (5) and cloned into pCITE-2c. The resulting plasmid is termed pX818-NS34AB. As a first step to generate the construct encoding NS5AB, a cDNA fragment encompassing nucleotides 8431 to 11224 of the X818 sequence was isolated by digestion of the X818-specific cDNA clone X2 (5) with SacI and XhoI. This cDNA fragment was cloned into pCITE-2a, resulting in plasmid pCITE-NS5AB*. To obtain the C-terminal part of the NS5AB gene, a cDNA fragment was generated by RT-PCR using primers Ol 10770 and Ol 12050R and subsequently cloned into pCRII (Invitrogen). From the resulting plasmid, the X818-specific insert was isolated after digestion with EcoRI and ApaI and cloned into pSecTagA (Invitrogen; precut with EcoRI and ApaI) to fuse the 3′ end of the NS5AB gene with a sequence encoding a 12-residue epitope from the human c-myc gene product together with a six-His tag. Subsequently, an NheI/BclI fragment was obtained and cloned into pCITE-NS5AB*. The resulting plasmid, pCITE-NS5AB, encodes the complete NS5AB together with the C-terminally fused immunological marker.
Immunoblotting.
Infected MDBK cells were lysed 48 h postinfection, while lysis of BHK-21 cells was performed 16 h after transfection. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (8% polyacrylamide) and immunoblotting were carried out as described before (3).
Nucleotide sequence accession numbers.
Sequence data from this article have been deposited in EMBL and GenBank data libraries and assigned the following accession numbers: AF037405 (complete nucleotide sequence of BDV X818) and AF037406 to AF037411 (3′ NCR of pestivirus strains L83-84, Moredun, Cumnock, V-TOB, Frijters, and R2727, respectively).
RESULTS
Determination of the 3′ and 5′ sequences of the genome of BDV X818.
To determine the 5′ and 3′ sequences of the X818 genome, an RNA ligation method was used as the first step. After ligation of viral genomic RNA prepared from the supernatant of infected cells, a nested RT-PCR assay was performed. This amplification produced a DNA fragment of the expected size (about 600 bp), which was subsequently subjected to sequence analysis (Fig. 1). The consensus sequence was obtained from six independent clones and compared with the respective sequences of other pestiviruses. This kind of analysis does not allow determination of the border between 5′ and 3′ ends of the genomic RNA. The exact positions of the 5′ and 3′ ends were determined by ligation of an RNA oligonucleotide, Ol LIG, to the 3′ end of the viral genomic RNA and subsequent RT-PCR (Fig. 2). First-strand cDNA synthesis was carried out with primer Ol G31, complementary to the oligonucleotide extension. After cloning of the amplified cDNA, 12 independent clones were obtained and subjected to nucleotide sequence analysis.
Analysis of 5′ NCR.
The 5′ NCR of X818 is 372 nucleotides long, while those of CSFV, BVDV-1, and BVDV-2 have lengths between 372 and 385 nucleotides (13, 14, 16, 26–28, 34, 36). An alignment of the 5′ NCR of the four pestivirus genotypes, including BVDV-1, CSFV, BVDV-2, and BDV X818, allowed the identification of both highly conserved and highly variable regions (Fig. 1C). The first 7 nucleotides of the pestivirus 5′ NCR and 18 nucleotides preceding the initiation AUG are identical for all pestiviruses, suggesting a strong functional pressure. The 5′ NCR of pestiviruses contains an IRES element for initiation of viral protein synthesis (32, 35). Secondary-structure analysis of the 5′ NCR of BVDV-1 and CSFV predicted a series of stem-loop structures (15). It has been reported that secondary structure rather than merely a linear nucleotide sequence functions as the pestiviral IRES. Similar analyses performed with the 5′ NCR of BDV X818 suggest that the secondary structure of this region is conserved for all pestiviruses (data not shown).
Analysis of 3′ NCR.
In six clones obtained after ligation of an RNA oligonucleotide, the synthetic oligonucleotide was preceded by the sequence 5′ …ACAGCCCC 3′, while in other clones, either two or five C residues were found; in one case, the terminal sequence was 5′ …ACAGCCCT 3′. With respect to the number of C residues at the 3′ end, heterogeneity was also observed in the sequences of clones obtained after 3′-5′ ligation of genomic RNA. Again, the sequence 5′ …ACAGCCCC 3′ was observed most frequently. In other cases, three, five, or seven C residues were found at the 3′ end. Accordingly, the 3′ end of the genome of BDV X818 is obviously not represented by a single sequence but shows considerable heterogeneity. This is similar to the situation reported for BVDV-1 and CSFV strains (16, 26).
For BVDV-1 strains NADL and SD-1 and for most CSFV strains, the 3′ NCR has been reported to be approximately 226 nucleotides long (13, 16, 26, 28, 36), while those of BVDV-1 strain Osloss (14) and BVDV-2 strain 890 (34) comprise only 185 and 206 nucleotides, respectively. The 3′ NCR of CSFV C strain consists of 241 nucleotides (27). Surprisingly, the 3′ NCR of BDV X818 contains 273 nucleotides and is thus at least 32 nucleotides longer than the 3′ NCR of any pestivirus analyzed thus far (Fig. 2C). Comparison of the 3′ NCR nucleotide sequences of selected pestivirus strains showed that a highly variable region following the UGA translational termination codon leads to the size heterogeneity, while the most-3′ 70 nucleotides form a conserved element with a predicted stem-loop structure for the last 56 to 60 bases (Fig. 2D). Interestingly, the sequence motif TATTTATTTA was identified at four different locations within the variable part of the 3′ NCR of BDV X818. This motif can be also found at two or three corresponding regions within the 3′ NCR of CSFV C strain and Alfort-T, while it is absent in the 3′ NCR of BVDV-1 and BVDV-2 strains (Fig. 2). In addition to this sequence motif, other AT-rich stretches can be found in the variable region of pestivirus 3′ NCR. The nucleotide sequences and locations of these stretches, however, differed for the four established pestivirus genotypes.
3′ NCR sequences from other BDV isolates.
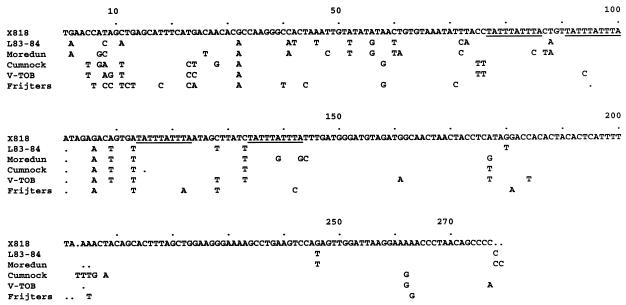
To investigate whether size and sequence characteristics of the 3′ NCR of X818 are unique for this strain or represent a common feature of this species, we determined the 3′ NCR sequences of additional BDV isolates from sheep. The resulting comparative sequence analysis showed that the 3′ NCR of BDV strains L83-84, Moredun, and Cumnock consist of about 273 nucleotides and that the respective sequences are most similar to the one of BDV X818 (Fig. 3). The repeat motif TATTTATTTA identified in the X818 3′ NCR sequence was found at four locations in the 3′ NCR of strains L83-84 and Cumnock, while it is present twice in the corresponding region of strain Moredun.
FIG. 3.
Comparison of the 3′ NCR sequence of BDV X818 with the ones of other BDV strains. The corresponding nucleotide sequences of the 3′ NCR of ovine strains L83-84, Moredun, and Cumnock, bovine strain V-TOB, and porcine strain Frijters were determined from at least three independent clones obtained after ligation with an RNA oligonucleotide and subsequent seminested RT-PCR (for experimental strategy, see also the legend to Fig. 2). Each sequence starts with the translational stop codon. Only differences from the BDV X818 sequence are indicated. The repeat sequence motif TATTTATTTA is underlined.
While most BDV isolates were obtained from sheep, other members of the Artiodactyla have also been found to harbor these viruses (4). To determine whether length and sequence of the 3′ NCR can be connected with host origin of pestivirus isolates, porcine BDV strain Frijters and bovine BDV strain V-TOB were analyzed. Again, the 3′ NCR of these porcine and bovine BDV strains comprise about 273 nucleotides with two or three copies of the above-mentioned repeat sequence motif (Fig. 3). In addition, BVDV-1 strain R2727 isolated from sheep was also included in this study. The results indicated that the 3′ NCR of BVDV-1 strain R2727 comprises 224 nucleotides and lacks the repeat sequence identified for BDV (data not shown). To assess the degree of variation, the nucleotide sequence identities of 3′ NCR between the investigated strains and single BVDV-1, BVDV-2, and CSFV strains were calculated. With respect to the complete 3′ NCR, the nucleotide sequence identities among all investigated BDV strains are greater than 90%, but those between BDV and other pestivirus isolates are less than 80%. Taken together, our data demonstrate that length and sequence of the 3′ NCR, including the presence of repeat sequence motifs, are highly conserved among the analyzed ovine, porcine, and bovine BDV strains.
Sequence analysis of the ORF and processing of the polyprotein.
The genome of BDV X818 is 12,333 nucleotides long. Accordingly, the length of the genomic RNA determined by nucleotide sequencing is in good agreement with the size of RNA demonstrated before by Northern blotting (5). Analysis of the nucleotide sequence of BDV X818 revealed a single, continuous ORF originating at nucleotide 373 and terminating at nucleotide 12060. The ORF consists of 11,688 nucleotides encoding 3,895 aa. The complete nucleotide sequence of BDV X818 was compared to the ones of other pestiviruses, and coding regions of X818 could be readily aligned (data not shown).
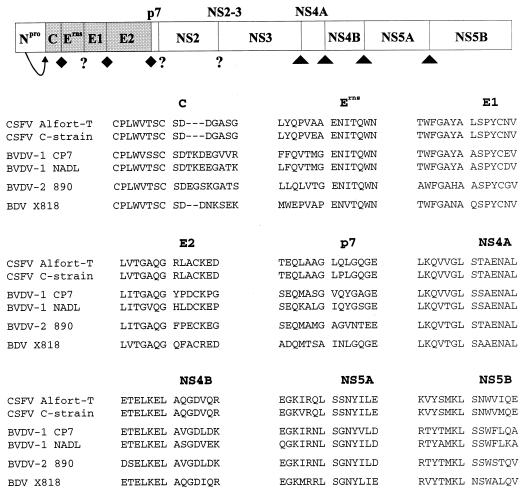
Processing of the polyprotein into the mature viral polypeptides requires several cleavages performed by viral and cellular proteases (12, 18, 37, 41, 45, 54–56). Studies on processing of the pestiviral polyprotein included determination of the N termini of individual viral proteins. With respect to the structural proteins, the N termini of C, Erns, E1, and E2 have been determined for CSFV (37, 41), while the N termini of the nonstructural proteins p7, NS4A, NS4B, NS5A, and NS5B have been reported for BVDV-1 (18, 45, 56). Thus, the N termini of NS2 and NS3 have not been directly determined for any pestivirus; however, there is evidence that the N terminus of NS2-3 (NS2) is located near aa 1130 (18) and that aa 1590 can represent the N terminus of NS3 (numbers refer to the genomic sequence of BVDV-1 strain SD-1 [16]); the latter assumption applies to certain cytopathogenic BVDV-1 strains (25, 56). Alignments of the BDV X818 polyprotein sequence with respective sequences of other pestiviruses revealed a high degree of homology at the predicted cleavage sites, suggesting that processing of the polyprotein occurs at conserved locations (Fig. 4). Using these cleavage sites to define the boundaries of each protein, we calculated the identities between X818 virus protein sequence and the respective sequences of other selected pestiviruses. The greatest amino acid sequence identity was found with CSFV. The most conserved pestiviral proteins are represented by NS3 and NS4A, while E2, p7, NS2, and NS5A appear to be least conserved (Table 1).
FIG. 4.
Pestivirus polyprotein and alignment of sequences flanking the predicted cleavage sites. The diagram at the top shows the location of structural proteins (shaded boxes) and nonstructural proteins (open boxes). Proteases involved in processing include viral N-terminal autoprotease Npro, cellular signal peptidase (⧫), and viral NS2-3 (NS3) serine protease (▴). The N termini of structural proteins C, Erns, E1, and E2 have been determined for CSFV Alfort-T (37, 41), while those of the nonstructural proteins p7, NS4A, NS4B, NS5A, and NS5B have been determined for BVDV-1 strains CP7 and NADL (18, 45, 56). The alignment of sequences around the predicted processing sites include deduced amino acid sequences of CSFV Alfort-T (26), CSFV C strain (27), BVDV-1 CP7 (24), BVDV-1 NADL (13), BVDV-2 890 (34), and BDV X818 (present study).
TABLE 1.
Amino acid identity between proteins of BDV X818 and other pestivirusesa
| Virus and strain | Amino acid identity (%)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Npro | C | Erns | E1 | E2 | p7 | NS2b | NS3 | NS4A | NS4B | NS5A | NS5B | |
| CSFV Alfort-T | 76.2 | 74.0 | 81.1 | 86.7 | 66.8 | 81.3 | 69.1 | 94.4 | 89.1 | 83.5 | 62.2 | 78.4 |
| CSFV Brescia | 72.6 | 79.0 | 79.3 | 86.2 | 65.7 | 81.3 | 70.6 | 94.1 | 90.6 | 84.4 | 62.2 | 78.9 |
| BVDV-1 NADL | 70.8 | 71.6 | 76.7 | 75.4 | 59.4 | 54.7 | 61.2 | 91.8 | 89.1 | 74.0 | 58.3 | 71.0 |
| BVDV-1 CP7 | 72.0 | 70.6 | 77.1 | 73.8 | 58.8 | 46.9 | 59.9 | 91.8 | 89.1 | 73.7 | 57.7 | 72.1 |
| BVDV-2 890 | 69.0 | 70.6 | 78.0 | 78.5 | 55.6 | 59.4 | 56.2 | 91.2 | 87.5 | 74.6 | 56.9 | 72.1 |
Termini of the individual proteins are as indicated in Fig. 4. The assumed N termini of NS2-3 (NS2) and NS3 are described in the text.
Insertions of cellular and viral sequences within the NS2 genes of BVDV-1 NADL, BVDV-1 CP7, and BVDV-2 890 were deleted for this comparison.
Generation of the nonstructural proteins NS4A, NS4B, NS5A, and NS5B is mediated by the pestiviral NS3 (NS2-3) serine protease. Determination of the four respective cleavage sites of two BVDV-1 strains has led to the conclusion that a conserved leucine residue is found at the P1 position followed by either serine or alanine at the P1′ position (45, 56). Comparison of the deduced amino acid sequences around the cleavage site between NS5A and NS5B revealed, however, a remarkable difference between BDV strain X818 and other pestiviruses. For X818, the leucine at the P1 position is followed by an asparagine residue at P1′, while all other pestiviruses have a serine at this location (Fig. 4). Processing in the NS5AB region of X818 was therefore of particular interest.
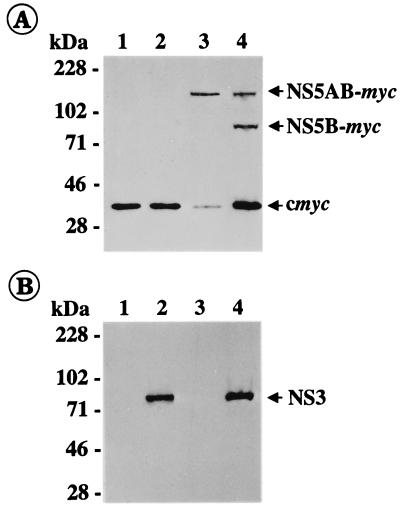
Since NS5A and NS5B of BDV X818 could not be identified by using the CSFV- and BVDV-specific antisera available to us, the genomic region encoding the proteins NS3, NS4A, NS4B, NS5A, and NS5B as well as smaller parts of this region were expressed in the T7 vaccinia virus system. For detection of NS5AB and the cleavage product NS5B, we added an immunological marker to the C terminus of NS5AB by fusing the NS5AB gene with a sequence encoding a 12-residue epitope from the human c-myc gene product. The presence of NS5AB and any release of NS5B were monitored by immunoblotting with an anti-myc monoclonal antibody. Expression of a cDNA fragment encompassing the genomic region encoding NS5AB resulted in detection of a protein with an apparent molecular mass of about 140 kDa. Coexpression of this NS5AB construct together with a second cDNA fragment encoding NS3, NS4A, and NS4B allowed us to demonstrate that NS5AB of BDV X818 is efficiently cleaved by the NS3 serine protease (Fig. 5). The apparent molecular size of the NS5B-myc fusion protein is 83 kDa and thus similar to that of NS5B, as shown for BVDV-1 NADL (12) and as deduced for other pestiviruses. Accordingly, the BDV NS3 proteinase mediates cleavage at a processing site where asparagine is present at the P1′ position.
FIG. 5.
Immunoblot analysis of BDV X818 nonstructural proteins. After infection with vaccinia virus MVA-T7pol, BHK-21 cells were transfected with pX818-NS34AB (lane 2), pX818-NS5AB (lane 3), or a combination of both (lane 4). BHK-21 cells infected with MVA-T7pol but not transfected served as a control (lane 1). Cells were lysed 16 h posttransfection or postinfection, and the samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (8% polyacrylamide) under reducing conditions, transferred to nitrocellulose, and incubated with anti-myc (A) or anti-NS3 (B) monoclonal antibody. The sizes of marker proteins are indicated on the left. The positions of c-myc (A) and X818-specific proteins NS5AB-myc (A), NS5B-myc (A), and NS3 (B) are marked with arrows.
DISCUSSION
Complete genomic sequences are known for all pestivirus species except BDV. In the present study, we report the entire genomic sequence of our BDV reference strain, X818. The genome of X818 virus comprises 12,333 nucleotides and contains one long ORF flanked by NCR. The polyprotein encoded by this ORF consists of 3,895 aa, which is very similar to the length of the polyprotein of other pestiviruses. Pestiviruses encode four structural and seven to nine nonstructural proteins. The N termini of most mature pestiviral proteins have been determined for either BVDV-1 or CSFV (18, 37, 41, 45, 56). Comparison of the BDV X818 polyprotein sequence with the respective sequences of the other three pestivirus species demonstrated a high degree of homology at the predicted cleavage sites (Fig. 4). Analysis of proteins encoded by BDV X818 including immunoblot and immunoprecipitation assays with various CSFV- and BVDV-specific antisera resulted in identification of Npro, C, Erns, E1, E2, NS2-3, NS3, NS4A, and NS4B (5, 6). In general, the apparent molecular weights of these BDV proteins were very similar to the ones described for CSFV and BVDV-1. The identification of BDV X818-encoded proteins together with the predictions of conserved cleavage sites strongly suggest that the polyprotein of BDV is processed in a manner very similar to the ones of other pestivirus species.
For pestiviruses and HCV, cleavage at the processing sites NS3/4A, NS4A/4B, NS4B/5A, and NS5A/5B is mediated by the NS3 serine proteinase. Analysis of two BVDV-1 strains revealed that a leucine is present at the P1 position, followed by either serine or alanine at the P1′ position (45, 56). Comparison of sequences around the four predicted cleavage sites showed that P1 and P1′ are highly conserved among all BVDV-1, BVDV-2, and CSFV isolates. Similar to NS3 of pestiviruses, the NS3 proteinase of HCV also prefers amino acids with short side chains at the P1′ position of the respective cleavage sites (33). Remarkably, P1′ of the predicted NS5A/5B processing site of BDV X818 is represented by asparagine (Fig. 4). Accordingly, it was interesting to determine whether NS5AB of BDV X818 is cleaved at the predicted processing site by the NS3 proteinase. In this context, it is noteworthy that NS5 of members of the genus Flavivirus is not further processed (33). Since we failed to detect NS5A and NS5B of BDV X818 by using the CSFV- and BVDV-specific antisera available to us, the NS5AB gene of BDV X818 was expressed as a fusion protein encompassing an immunological marker at its C terminus. Coexpression of this modified NS5AB together with BDV NS3, NS4A, and NS4B demonstrated that NS5AB of BDV X818 is efficiently cleaved by the NS3 serine protease; the released NS5B has the expected size of about 80 kDa (Fig. 5). Analysis of the X818 polyprotein sequence revealed that L-S(A) dipeptides are at least 120 amino acids apart from the proposed site; accordingly, cleavage of NS5AB at such sites would result in calculated molecular sizes for NS5B below 70 kDa or above 90 kDa. Thus, our data strongly suggest that the NS3 serine protease of BDV can mediate cleavage at a site where asparagine is present at P1′. Interestingly, for another BDV strain (BD-31), asparagine is also found at the P1′ position of the predicted NS5A/5B processing site (56). The presence of asparagine at this position may represent a common feature of BDV, and it can be speculated that the BDV NS3 proteinase may differ from the one of other pestiviruses. However, experiments with NS3 of BVDV-1 showed that NS5AB of BDV X818 is also efficiently cleaved by the NS3 proteinase of BVDV-1 (6).
Establishment of the entire genomic sequence of BDV X818 enables comparative analyses of complete sequences of the four pestivirus species and thereby reconsideration of pestivirus taxonomy. The overall nucleotide sequence identities between BDV X818 and BVDV-1, BVDV-2, or CSFV strains are less than 72%. Phylogenetic analysis on the basis of complete nucleotide and polyprotein sequences including BDV X818, several BVDV-1 and CSFV strains, as well as BVDV-2 isolate 890 showed that pestiviruses segregated into four major genotypes, which is consistent with the previously reported groupings of pestiviruses identified by comparison of partial sequences (references 2, 4, and 47 and data not shown).
The 5′ NCR of BDV X818 is 372 nucleotides long and with regard to length and sequence most similar to the one of CSFV. Pestiviruses and HCV have relatively long 5′ NCR encompassing 372 to 385 and 341 to 344 nucleotides, respectively. In contrast, the 5′ NCR of members of the genus Flavivirus consists of 95 to 132 bases (33). The genomic RNA of flaviviruses has a type I cap at its 5′ end (m7GpppAmp), while pestiviruses and HCV lack a 5′ cap structure (15). For the latter two, an IRES which mediates cap-independent translation of the long ORF has been described (32, 35, 50). With respect to length, predicted secondary structure, and function of the 5′ NCR, pestiviruses and HCV appear similar to each other but are remarkably different from flaviviruses.
Complete 3′ NCR sequences have been established for several BVDV-1 and CSFV strains as well as for BVDV-2 strain 890 (13, 14, 16, 26–28, 34, 36). For the majority of CSFV and BVDV-1 isolates, the 3′ NCR comprises about 226 nucleotides, while the 3′ NCR of BVDV-1 Osloss, BVDV-2 890, and CSFV C strain consist of 185, 206, and 241 nucleotides, respectively. Our finding that the 3′ NCR of X818 and other BDV isolates are about 273 nucleotides long and thus at least 32 nucleotides longer than the ones of other pestiviruses was unexpected. Comparative analysis of the 3′ NCR of pestiviruses demonstrated that size heterogeneity is due to regions of variable length following the UGA termination codon. Variation in length has also been described for the 3′ NCR of flaviviruses and HCV. For both, the respective heterogeneity is also located in a variable region following the translational stop codon (21, 22, 44, 49, 57). For BDV strain X818, a repeat sequence (TATTTATTTA) was identified at four locations within the variable region of the 3′ NCR; two to four copies of this sequence were also found within the 3′ NCR of five other analyzed BDV strains (Fig. 2 and 3). Comparative analyses showed that the same repeat is also present within the 3′ NCR of CSFV strains but is absent in the 3′ NCR of BVDV-1 and BVDV-2. The significance of these repeats remains to be investigated. The availability of infectious pestivirus cDNA clones will allow insertion or deletion of such repeat sequences and subsequent studies of the resulting phenotype.
The most-3′ 70 nucleotides of pestiviral genomes form a highly conserved element, of which the last 56 to 60 nucleotides are predicted to form a stable stem-loop structure (Fig. 2). For HCV, the secondary structure of the conserved 98-base element at the 3′ end has been recently determined by using enzymatic and chemical probing techniques. Accordingly, this conserved region of HCV genome contains stem-loops but may also form alternative structures (9, 20). For pestiviruses, similar analyses are awaited and will provide a solid basis for studying the function of the 3′ NCR. Genomic replication of positive-strand RNA viruses is initiated from the 3′-end region where specific replication mechanisms involve interaction of the RNA genome with viral and cellular factors. The high conservation of primary and predicted secondary structure for the 3′ 70 nucleotides suggests the presence of recognition signals for viral and cellular proteins. For West Nile virus and HCV, several cellular proteins that bind to the 3′ stem-loop structure of the RNA genome have been recently described (7, 8, 20). In addition, interaction of cellular proteins with 3′ and 5′ regions has been reported for other RNA viruses (11, 42, 53). Future studies on pestivirus replication will include the analysis of viral and cellular proteins that interact with the 3′ and 5′ regions of the genome.
ACKNOWLEDGMENTS
We thank Norbert Tautz for critical reading of the manuscript.
This study was supported by Intervet International BV (project 75/73.1808.720).
ADDENDUM IN PROOF
J. F. Ridpath et al. (Virus Res. 50:237–243, 1997) have recently reported the genomic sequence of BDV strain BD31. This sequence comprises 12,268 nucleotides, while the sequence of BDV X818 is 12,333 nucleotides long. According to our comparison of the BD31 sequence with complete pestivirus sequences including BDV X818, the BD31 sequence is missing at least 8 nucleotides at the 5′ end and 48 nucleotides at the 3′ end.
REFERENCES
- 1.Barlow R M, Gardiner A C, Nettleton P F. The pathology of a spontaneous and experimental mucosal disease in sheep recovered from clinical border disease. J Comp Pathol. 1983;93:451–461. doi: 10.1016/0021-9975(83)90032-4. [DOI] [PubMed] [Google Scholar]
- 2.Becher P, König M, Paton D, Thiel H-J. Further characterization of border disease virus isolates: evidence for the presence of more than three species within the genus pestivirus. Virology. 1995;209:200–206. doi: 10.1006/viro.1995.1243. [DOI] [PubMed] [Google Scholar]
- 3.Becher P, Meyers G, Shannon A D, Thiel H-J. Cytopathogenicity of border disease virus is correlated with integration of cellular sequences into the viral genome. J Virol. 1996;70:2992–2998. doi: 10.1128/jvi.70.5.2992-2998.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becher P, Orlich M, Shannon A D, Horner G, Kőnig M, Thiel H-J. Phylogenetic analysis of pestiviruses from domestic and wild ruminants. J Gen Virol. 1997;78:1357–1366. doi: 10.1099/0022-1317-78-6-1357. [DOI] [PubMed] [Google Scholar]
- 5.Becher P, Shannon A D, Tautz N, Thiel H-J. Molecular characterization of border disease virus, a pestivirus from sheep. Virology. 1994;198:542–551. doi: 10.1006/viro.1994.1065. [DOI] [PubMed] [Google Scholar]
- 6.Becher, P., and H.-J. Thiel. Unpublished data.
- 7.Blackwell J L, Brinton M A. Translation elongation factor-1 alpha interacts with the 3′ stem-loop region of West Nile virus genomic RNA. J Virol. 1997;71:6433–6444. doi: 10.1128/jvi.71.9.6433-6444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackwell J L, Brinton M A. BHK cell proteins bind to the 3′ stem-loop structure of the West Nile virus genome RNA. J Virol. 1995;69:5650–5658. doi: 10.1128/jvi.69.9.5650-5658.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blight K J, Rice C M. Secondary structure determination of the conserved 98-base sequence at the 3′ terminus of hepatitis C virus genome RNA. J Virol. 1997;71:7345–7352. doi: 10.1128/jvi.71.10.7345-7352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brockman S, Wood L, Edwards S, Harkness J W. Selection of an appropriate pestivirus strain for border disease serodiagnosis. Vet Rec. 1988;122:586–587. doi: 10.1136/vr.122.24.586. [DOI] [PubMed] [Google Scholar]
- 11.Buck K W. Comparison of the replication of positive-stranded RNA viruses of plants and animals. Adv Virus Res. 1996;47:159–251. doi: 10.1016/S0065-3527(08)60736-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collett M S, Larson R, Belzer S, Retzel E. Proteins encoded by bovine viral diarrhea virus: the genome organization of a pestivirus. Virology. 1988;165:200–208. doi: 10.1016/0042-6822(88)90673-3. [DOI] [PubMed] [Google Scholar]
- 13.Collett M S, Larson R, Gold C, Strick D, Anderson D K, Purchio A F. Molecular cloning and nucleotide sequence of the pestivirus bovine viral diarrhea virus. Virology. 1988;165:191–199. doi: 10.1016/0042-6822(88)90672-1. [DOI] [PubMed] [Google Scholar]
- 14.de Moerlooze L, Lecomte C, Brown-Shimmer S, Schmetz D, Guiot C, Vandenbergh D, Allaer D, Rossius M, Chappuis G, Dina D, Renard A, Martial J A. Nucleotide sequence of the bovine viral diarrhoea virus Osloss strain: comparison with related viruses and identification of specific DNA probes in the 5′ untranslated region. J Gen Virol. 1993;74:1433–1438. doi: 10.1099/0022-1317-74-7-1433. [DOI] [PubMed] [Google Scholar]
- 15.Deng R, Brock K V. 5′ and 3′ untranslated regions of pestivirus genome: primary and secondary structure analyses. Nucleic Acids Res. 1993;21:1949–1957. doi: 10.1093/nar/21.8.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng R, Brock K V. Molecular cloning and nucleotide sequence of a pestivirus genome, noncytopathogenic bovine viral diarrhea virus strain SD-1. Virology. 1992;191:867–879. doi: 10.1016/0042-6822(92)90262-n. [DOI] [PubMed] [Google Scholar]
- 17.Devereux J, Haeberli P, Smithies O A. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elbers K, Tautz N, Becher P, Rümenapf T, Thiel H-J. Processing in the pestivirus E2-NS2 region: identification of the nonstructural proteins p7 and E2p7. J Virol. 1996;70:4131–4135. doi: 10.1128/jvi.70.6.4131-4135.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felsenstein J. PHYLIP (Phylogeny Inference Package), 3.5c ed. Seattle: Department of Genetics, University of Washington; 1993. [Google Scholar]
- 20.Ito T, Lai M M C. Determination of the secondary structure of and cellular protein binding to the 3′ untranslated region of the hepatitis C virus RNA genome. J Virol. 1997;71:8698–8706. doi: 10.1128/jvi.71.11.8698-8706.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolykhalov A A, Feinstone S M, Rice C M. Identification of a highly conserved sequence element at the 3′ terminus of hepatitis C virus genome RNA. J Virol. 1996;70:3363–3371. doi: 10.1128/jvi.70.6.3363-3371.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandl C W, Kunz C, Heinz F X. Presence of (poly)A in a flavivirus: significant differences between the 3′ noncoding regions of the genomic RNAs of tick-borne encephalitis virus strains. J Virol. 1991;65:4070–4077. doi: 10.1128/jvi.65.8.4070-4077.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyers G, Rümenapf T, Thiel H-J. Molecular cloning and nucleotide sequence of the genome of hog cholera virus. Virology. 1989;171:555–567. doi: 10.1016/0042-6822(89)90625-9. [DOI] [PubMed] [Google Scholar]
- 24.Meyers G, Tautz N, Becher P, Thiel H-J, Kümmerer B. Recovery of cytopathogenic and noncytopathogenic bovine viral diarrhea viruses from cDNA constructs. J Virol. 1996;70:8606–8613. doi: 10.1128/jvi.70.12.8606-8613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyers G, Thiel H-J. Molecular characterization of pestiviruses. Adv Virus Res. 1996;47:53–118. doi: 10.1016/s0065-3527(08)60734-4. [DOI] [PubMed] [Google Scholar]
- 26.Meyers G, Thiel H-J, Rümenapf T. Classical swine fever virus: recovery of infectious viruses from cDNA constructs and generation of recombinant cytopathogenic defective interfering particles. J Virol. 1996;70:1588–1595. doi: 10.1128/jvi.70.3.1588-1595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moormann R J M, van Gennip H G P, Miedema G K W, Hulst M M, van Rijn P A. Infectious RNA transcribed from an engineered full-length cDNA template of the genome of a pestivirus. J Virol. 1996;70:763–770. doi: 10.1128/jvi.70.2.763-770.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moormann R J M, Warmerdam P A M, Van der Meer B, Schaaper W M M, Wensvoort G, Hulst M M. Molecular cloning and nucleotide sequence of hog cholera virus strain brescia and mapping of the genomic region encoding envelope glycoprotein E1. Virology. 1990;177:184–198. doi: 10.1016/0042-6822(90)90472-4. [DOI] [PubMed] [Google Scholar]
- 29.Nettleton P F. Pestivirus infections in ruminants other than cattle. Rev Sci Tech Off Int Epizoot. 1990;9:131–150. doi: 10.20506/rst.9.1.485. [DOI] [PubMed] [Google Scholar]
- 30.Nettleton P F, Gilmour J S, Herring J A, Sinclair J A. The production and survival of lambs persistently infected with border disease virus. Comp Immunol Microbiol Infect Dis. 1992;15:179–188. doi: 10.1016/0147-9571(92)90091-5. [DOI] [PubMed] [Google Scholar]
- 31.Paton D J. Pestivirus diversity. J Comp Pathol. 1995;112:215–236. doi: 10.1016/s0021-9975(05)80076-3. [DOI] [PubMed] [Google Scholar]
- 32.Poole T L, Wang C, Popp R A, Potgieter L N D, Siddiqui A, Collett M S. Pestivirus translation occurs by internal ribosome entry. Virology. 1995;206:750–754. doi: 10.1016/s0042-6822(95)80003-4. [DOI] [PubMed] [Google Scholar]
- 33.Rice C M. Flaviviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 931–959. [Google Scholar]
- 34.Ridpath J F, Bolin S R. The genomic sequence of a virulent bovine viral diarrhea virus (BVDV) from the type 2 genotype: detection of a large genomic insertion in a noncytopathic BVDV. Virology. 1995;212:39–46. doi: 10.1006/viro.1995.1451. [DOI] [PubMed] [Google Scholar]
- 35.Rijnbrand R, van der Straaten T, van Rijn P A, Spaan W J M, Bredenbeek P J. Internal entry of ribosomes is directed by the 5′ noncoding region of classical swine fever virus and is dependent on the presence of an RNA pseudoknot upstream of the initiation codon. J Virol. 1997;71:451–457. doi: 10.1128/jvi.71.1.451-457.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruggli N, Tratschin J-D, Mittelholzer C, Hofmann M A. Nucleotide sequence of classical swine fever virus strain Alfort/187 and transcription of infectious RNA from stably cloned full-length cDNA. J Virol. 1996;70:3478–3487. doi: 10.1128/jvi.70.6.3478-3487.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rümenapf T, Unger G, Strauss J H, Thiel H-J. Processing of the envelope glycoproteins of pestiviruses. J Virol. 1993;67:3288–3295. doi: 10.1128/jvi.67.6.3288-3294.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 40.Snowdon W A. Mucosal disease: its incidence and diagnosis in Australia. Bull Off Int Epizoot. 1973;79:529–542. [Google Scholar]
- 41.Stark R, Meyers G, Rümenapf T, Thiel H-J. Processing of pestivirus polyprotein: cleavage site between autoprotease and nucleocapsid protein of classical swine fever virus. J Virol. 1993;67:7088–7095. doi: 10.1128/jvi.67.12.7088-7095.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strauss J H, Strauss E G. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutter G, Ohlmann M, Erfle V. Non-replicating vaccinia vector efficiently expresses bacteriophage T7 RNA polymerase. FEBS Lett. 1995;371:9–12. doi: 10.1016/0014-5793(95)00843-x. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka T, Kato N, Cho M-J, Sugiyama K, Shimotono K. Structure of the 3′ terminus of the hepatitis C virus genome. J Virol. 1996;70:3307–3312. doi: 10.1128/jvi.70.5.3307-3312.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tautz N, Elbers K, Stoll D, Meyers G, Thiel H-J. Serine protease of pestiviruses: determination of cleavage sites. J Virol. 1997;71:5415–5422. doi: 10.1128/jvi.71.7.5415-5422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terpstra C. Border disease: virus persistence, antibody response and transmission studies. Res Vet Sci. 1981;30:185–191. [PubMed] [Google Scholar]
- 47.Tijssen P, Pellerin C, Lecomte J, Van Den Hurk J. Immunodominant E2 (gp53): sequences of highly virulent bovine viral diarrhea group II viruses indicate a close relationship to a subgroup of border disease viruses. Virology. 1996;217:356–361. doi: 10.1006/viro.1996.0123. [DOI] [PubMed] [Google Scholar]
- 48.Vantsis J T, Barlow R M, Fraser J, Rennie J C, Mould D L. Experiments in border disease. VIII. Propagation and properties of a cytopathic virus. J Comp Pathol. 1976;86:111–120. doi: 10.1016/0021-9975(76)90035-9. [DOI] [PubMed] [Google Scholar]
- 49.Wallner G, Mandl C W, Kunz C, Heinz F X. The flavivirus 3′-noncoding region: extensive size heterogeneity independent of evolutionary relationships among tick-borne encephalitis virus. Virology. 1995;213:169–178. doi: 10.1006/viro.1995.1557. [DOI] [PubMed] [Google Scholar]
- 50.Wang C, Sarnow P, Siddique A. Translation of human hepatitis C virus RNA in cultured cells is mediated by an internal ribosome binding mechanism. J Virol. 1993;67:3338–3344. doi: 10.1128/jvi.67.6.3338-3344.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wengler G, Bradley D W, Collett M S, Heinz F X, Schlesinger R W, Strauss J H. Flaviviridae. In: Murphy F A, Fauquet C M, Bishop D H L, Ghabrial S A, Jarvis A W, Martelli G P, Mayo M A, Summers M D, editors. Virus taxonomy. Sixth Report of the International Committee on Taxonomy of Viruses. Springer-Verlag, Vienna, Austria. 1995. [Google Scholar]
- 52.Wensvoort G, de Smith H, Terpstra C. A non-CSFV pestivirus is currently circulating among a number of Dutch pig herds and causing false-positive CSFV-serology. Report on Meeting of the National Swine Fever Laboratories. Commission of the European Communities, Brussels, Belgium. 1994. [Google Scholar]
- 53.Wimmer E, Hellen C U, Cao X. Genetics of poliovirus. Annu Rev Genet. 1993;27:353–436. doi: 10.1146/annurev.ge.27.120193.002033. [DOI] [PubMed] [Google Scholar]
- 54.Wiskerchen M, Belzer S K, Collett M S. Pestivirus gene expression: the first protein product of the bovine viral diarrhea virus large open reading frame, P20, possesses proteolytic activity. J Virol. 1991;65:4508–4514. doi: 10.1128/jvi.65.8.4508-4514.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wiskerchen M A, Collett M S. Pestivirus gene expression: protein p80 of bovine viral diarrhea virus is a proteinase involved in polyprotein processing. Virology. 1991;184:341–350. doi: 10.1016/0042-6822(91)90850-b. [DOI] [PubMed] [Google Scholar]
- 56.Xu J, Mendez E, Caron P R, Lin C, Murcko M A, Collett M S, Rice C M. Bovine viral diarrhea NS3 serine proteinase: polyprotein cleavage sites, cofactor requirements, and molecular model of an enzyme essential for pestivirus replication. J Virol. 1997;71:5312–5322. doi: 10.1128/jvi.71.7.5312-5322.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamada N, Tanihara K, Takada A, Yorihuzi T, Tsutsumi M, Shimomura T, Tsuji T, Date T. Genetic organization and diversity of the 3′ noncoding region of the hepatitis C virus genome. Virology. 1996;223:255–261. doi: 10.1006/viro.1996.0476. [DOI] [PubMed] [Google Scholar]