Abstract
Objectives:
Abuse of opiates, cocaine, and lipophilic inhalants (e.g., toluene) can damage brain myelin and cause acute toxic leukoencephalopathy (TL), but little is known about recovery or prognosis in this condition. In light of the ongoing opiate epidemic in the United States, it is important to understand the natural history of patients who have acute neurological complications from illicit drug exposure. Our aim was to conduct a scoping review of the literature regarding prognosis in described cases of substance abuse-related TL.
Methods:
A strategic search of PubMed, Ovid, Cumulative Index to Nursing, and Allied Health Literature (CINAHL) databases yielded adult cases of acute TL from opiates, cocaine, or inhalants. Cases and case series were eligible for inclusion if they described acute leukoencephalopathy with a clear temporal association with opiate, cocaine, or inhalant abuse. Inclusion was contingent on availability of clinical descriptions until death or ≥4 weeks follow-up with neuroimaging consistent with TL.
Results:
Among 52 cases from 14 articles, 21 (40.4%) individuals died with mean time to death of 28.2 days; with mean follow-up of 12.8 months, 10 (19.2%) survived with no recovery, 17 (32.7%) had partial recovery, and 4 (7.7%) individuals had full recovery.
Conclusion:
Substance abuse-related acute TL often has a poor prognosis, but partial or even full recovery is possible in a subgroup of individuals over months to years.
Keywords: leukoencephalopathy, leukotoxin, substance abuse disorder, opiate, cocaine, inhalant
1. Introduction
Toxic leukoencephalopathy (TL) is a neurologic disorder in which the white matter of the brain is damaged by a leukotoxic substance. This syndrome can present with a wide variety of manifestations including mild cognitive dysfunction, dementia, severe disturbances of consciousness (i.e., stupor, coma), and death.1 The lipophilicity of leukotoxins is thought to play a key role in the myelin injury that characterizes TL, and precipitants of the syndrome include common substances of abuse including opiates, cocaine, and, more classically, the inhalant toluene.2,3 At present, opiate addiction is highly prevalent and a well-publicized cause of TL, especially by inhalation of combusted vapors, known as “chasing the dragon.”4 Neuroimaging with magnetic resonance imaging (MRI) and neuropathological studies of TL have documented preferential white matter injury with relative preservation of cortical and subcortical gray matter.4–6 The impact of leukotoxins on myelinated tracts in the cerebral hemispheres is thought to be primarily responsible for the cognitive sequalae of TL, and the syndrome serves as a prototype for a broad spectrum of white matter disorders with both acute and chronic neurobehavioral manifestations.
Despite recognition of the wide spectrum of TL sequelae, little is known regarding long-term prognosis of individual cases. A dose-response relationship between leukotoxic substance exposure and clinical severity is likely;1 however, the duration and extent of exposure are typically unknown, and descriptions of follow-up among affected individuals, many of whom have chronic exposure, is limited.2 Case studies of substance abuse-related acute TL provide an opportunity to assess prognosis from the onset of exposure to the ultimate clinical outcome.
Given recent findings suggesting that regression of vascular white matter lesions on MRI can occur with concomitant clinical improvement,7 we were curious whether and to what degree improvement in clinical status and white matter involvement might be possible with TL. Consistent with this notion, reports of patients who have recovered from TL suggest that resolution of toxic white matter lesions is indeed possible.6 These observations suggest that white matter is plastic and dynamic, with the potential for recovery after toxin withdrawal. Although the mechanisms of white matter regeneration have yet to be fully elucidated, remyelination by oligodendrocytes likely plays a key role.8,9 There is also evidence to suggest that a mechanism of leukotoxic damage in TL is direct injury to oligodendrocytes, whereby the maintenance and repair of myelin are disrupted. Understanding the details of pathophysiology has enormous importance for enhancing neural resiliency, recovery, and reversibility of this condition. Studies using mouse models show there are several possible pathways for myelin recovery following leukotoxin exposure.10–12 The potential for recovery from leukotoxic brain injury has important implications for clinical practice, including providing key information for medical decision-making during acute management of this condition.
Currently, a paucity of literature exists on the epidemiology of substance abuse-related acute TL. Given the ongoing opiate crisis in the US, with increased prevalence of heroin use, a nearly 10-fold increase in deaths from non-medical use and overdose of synthetic opioids (i.e., fentanyl) in recent years,13,14 and increased substance abuse with the COVID-19 pandemic,15 the prevalence of this complication can be expected to increase. A summary of current knowledge on prognosis and recovery in substance abuse-related acute TL is therefore needed to help guide neurologists, critical care specialists, patients, and their families not only for direct patient care but also for goals of care discussions. However, no large cohort study of acute TL related to substance abuse exists to permit the assessment of prognosis. Accordingly, we aimed to conduct a scoping review of case reports including case series as an approach to comprehensively explore the available evidence on clinical course in substance abuse-related acute TL.16,17
2. Methods
2.1. Data Sources & Search Strategy –
We conducted a scoping review of the substance abuse-related acute TL literature. Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Review (PRISMA-ScR) guidelines were followed. This study was not registered on PROSPERO as it did not meet criteria for registration. Studies of case reports and case series describing substance abuse-related acute TL with exposures to opiates, cocaine, or inhalants were sought by searches of three databases (PubMed, Cumulative Index to Nursing and Allied Health Literature/CINAHL), and Ovid). We used combinations of keywords related to TL (toxic*, leukoencephalopathy*, leukotoxin*), illicit drug abuse (substance use*, substance-related disorder*), opiates (heroin*, opiate*, fentanyl*), cocaine*, and inhalants (inhalant*, toluene*). These terms were supplemented with scanning of article reference lists and correspondence with authors as needed.
2.2. Eligibility & Study Selection –
Case reports and case series were included if they met the following minimum criteria: 1) age ≥ 18 years old; 2) diagnosis of TL with an explicitly defined temporal association with reported opiate, cocaine, or inhalant use; 3) MRI description of findings consistent with TL, either in the pretreatment or early treatment phases, defined by the presence of FLAIR abnormalities in the periventricular and supraventricular white matter6,18; 4) available description of the clinical course defined as time from presentation to death or ≥4 weeks of follow-up; 5) initial presentation in an inpatient setting; and 5) CAse REports (CARE) guidelines met for preparation of case reports for publication.19 Descriptions of patients’ age and available demographics, agents abused (including mode of ingestion), clinical presentation, imaging findings, laboratory testing, recovery time, and treatments were collected from studies that met inclusion criteria when available.
Studies were excluded if: 1) the MRI description of findings were not suggestive of TL (e.g., cortical edema or other cortical involvement); 2) the report described infectious disorders, hypoxic-ischemic injury, trauma, neoplasia, leukodystrophies, or potential confounding medical conditions (i.e., acute hepatitis, acute renal failure); and/or 3) the report described clinical findings indicating TL without acute or subacute onset.
2.3. Data Collection –
For every eligible study, the following information was extracted and included in a standardized table: number of cases, age, sex, substance of abuse, clinical presentation, MRI findings, clinical course, and time to death or time to longest follow-up. The CARE checklist was used for critical appraisal of studies prior to data extraction.16 The information was recorded using a standard form to track case reports and case series by study. Data were extracted and cross-checked by two authors (ZAM and CMF). Discrepancies were resolved by further review and discussion (ZAM and CMF) as well as consultation with the third author (TCC). Verbatim descriptions related to imaging, treatment, clinical presentation, and sequelae were extracted for qualitative analysis. Microsoft Excel for Mac (Microsoft Office 2019, Version 16.46.21021202) was used for data management.
2.4. Analysis & Data Synthesis –
Pooled descriptive statistics of clinical data were extracted and tabulated in a standard table. Descriptive statistics were used to synthesize data provided in the literature for extracted quantitative data. Qualitative data related to descriptions of clinical presentation, course, and imaging were analyzed using a deductive approach. Synthesis and analysis of qualitative data from each study were performed using a matrix design which summarizes qualitative data in a table of columns and rows, allowing for comparison of data across predetermined domains which included imaging, treatment, clinical presentation, and sequelae.20 The primary goal of this scoping review was to report on the clinical course of substance abuse-related acute TL, most importantly morbidity and mortality.
3. Results
Of 47 articles reviewed, 14 were eligible for inclusion (see Figure 1). Seven case reports21–27 and seven case series28–34 were included in our analysis. Thirty-three studies were excluded during full-text screening and data extraction due to age ≤ 18 (N = 5) or insufficient data (N = 27), and concurrent acute hepatic failure with encephalopathy (N = 1). For two studies included in data extraction, 14 cases were excluded due to a lack of follow-up timeframe, and 31 cases were excluded because of self-described “subacute” or “chronic” presentations.
Figure 1.
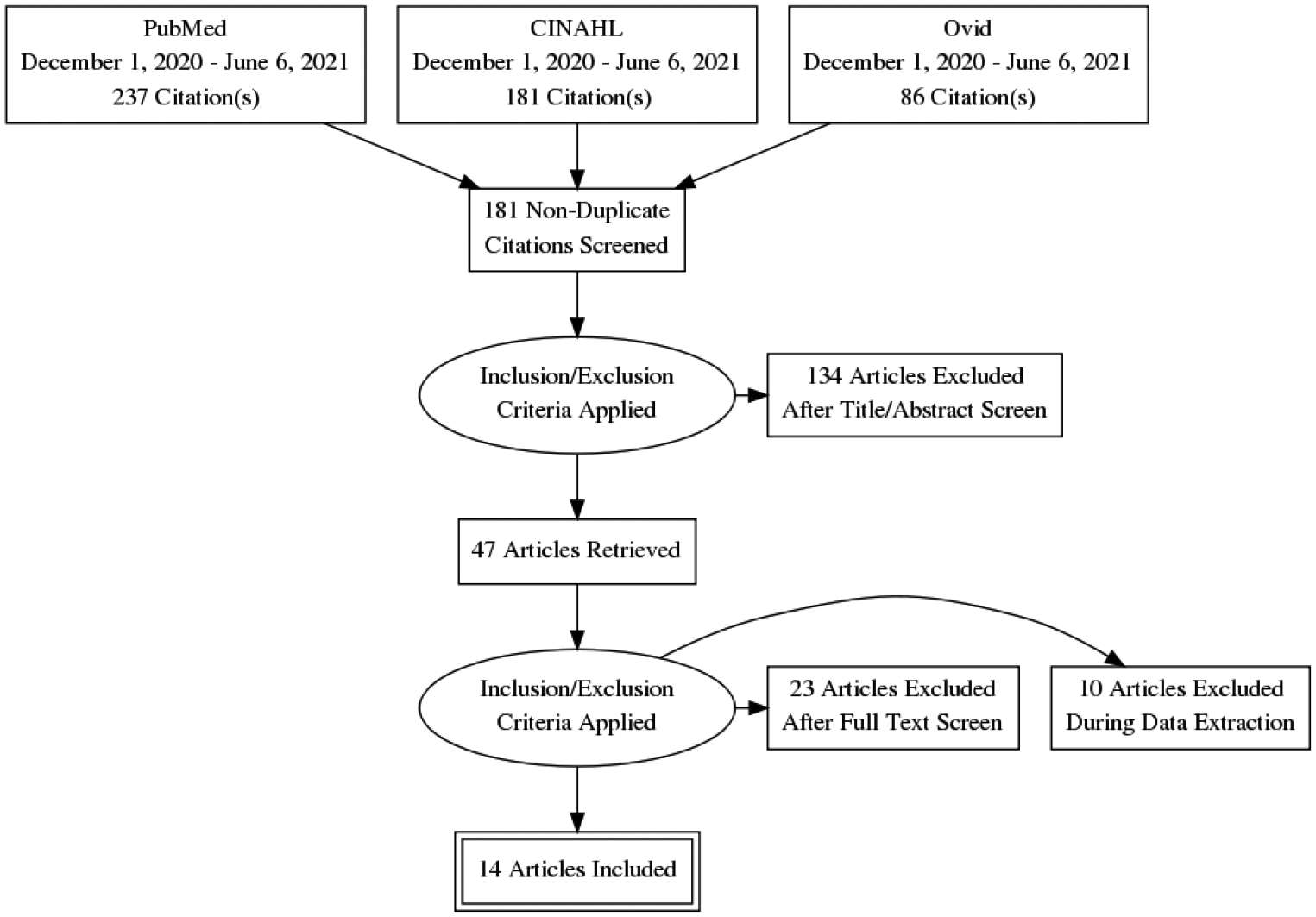
Search Strategy
Table 1 provides a summary of the data extracted from included studies. In total, 52 cases of substance abuse-related acute TL were identified with a mean age of 37.3 years (SD = 12.5). The pooled percentage of deceased patients was 40.4% (N = 21) with an average 28.2 days from presentation to death (SD = 20.6). For patients (N = 31) who were alive at the longest follow-up (12.8 months, SD=17.7), 19.2% (N = 10) had no recovery, 32.7% (N = 17) had partial recovery, and 7.7% (N = 4) had full recovery. When available, the most commonly described clinical presentations included “encephalopathy,” “altered mental status” and/or “coma” (N = 10) and new onset motor disturbances including ataxia, “posturing,” and “pyramidal signs” (N = 11).
Table 1.
Summary of Studies Included in Data Extraction and Literature Synthesis
| Article; Number of Cases | Agent(s) Abused (Mode of Ingestion) | Mean Age (years) | Clinical Presentation | Recovery Time and/or Mortality | Treatment |
|---|---|---|---|---|---|
| Achamallah et al. 201928; N=3 | Heroin (INH) | 25.0 | Acute encephalopathy and posturing | Case #1: No recovery at 2 months. Case #2: Partial recovery with persistent motor deficits at 8 months. Case #3: Full recovery at 5 years. |
Antioxidant therapy |
| Bianco et al. 201121; N=1 | Cocaine (NA) | 30.0 | Acute hemiplegia progressing to mutism over 6 months | Partial recovery at 2 years | Corticosteroids |
| Buxton, Sebastian, Clearsky 201129; N=13* | Heroin (INH) | 33.0 | Cerebellar dysfunction, ataxia, and dysarthria | Mortality of 48.1% (N=13) with average time to death of 54 days from onset | NA |
| Cartier, Gonzalez, Harlan 201530‘ N=3 | Cocaine (NA) | 25.0 | Severe, persistent headache and changes in level of consciousness | Cases #1 & #2: Death at 2 days Case #3: No recovery at 1 month |
Corticosteroids |
| Cheng, Chin, Chang 201931; N=19** | Heroin (IV) | 32.5 | Mixture of acute and delayed onset mental status changes, cerebellar ataxia, pyramidal signs, mutism, dysarthria, extrapyramidal signs, and incontinence | Cases #1 & #2: Full recovery For case series (N=17): Pooled data with mortality of 11.8% (N=2) at 4 weeks. Partial recovery 64.7% of cases (N=11). No recovery for 21.1% (N=4) |
Antioxidant therapy |
| Havé et al. 201222; N=1 | Heroin (Insufflation) | 46.0 | Acute behavioral disturbances with delirium, impaired comportment, involuntary movements, and apraxia | Partial recovery at 4 months, with persistent anosognosia, and deficits in attention, visual and verbal memory, and executive function | “Cognitive rehabilitation” |
| Holyoak et al. 201432; N=2 | Non-toluene solvents (INH), Opioids (NA) | 24.5 | Coma and pyramidal signs | Case #1: Partial recovery with persistent cognitive dysfunction at 8 months Case #2: No recovery |
Antioxidant therapy |
| Maschke et al. 199927; N=1 | Heroin, cocaine (NA) | 37.0 | Acute onset of altered mental status and mutism | Full recovery at 6 months | NA |
| Ryan et al. 200533; N=2 | Cocaine (NA) | 45.5 | Acute behavioral changes, confusion, aggression, and amnesia progressing to coma with posturing, pyrexia | Case #1: Death at 1 month Case #2 Death at 2 weeks |
Supportive care |
| Salgado et al. 201023; N=1 | Methadone (NA) | 65.0 | Acute onset pyramidal signs and coma | Partial recovery at 1 month | Supportive care |
| Torralba-Morón et al. 201734; N=3 | Heroin (NA) | 47.5 | Fluctuating consciousness, pyrexia, myoclonus, inattention, amnesia, and executive dysfunction | Case #1: Death at 40 days Cases #2 & 3: No recovery. |
Supportive care |
| van Esch et al. 201924; N=1 | Cocaine, Methadone (NA) | 54.0 | Psychosis progressing to catatonia with echolalia, stupor, and rigidity | Death at 3 months | Electroconvulsive therapy |
| Villella et al. 201025; N=1 | Heroin (IV) | 32.0 | Altered mental status, posturing, and right hemiparesis | Partial recovery at 2 years | NA |
| Weizer et al. 202026; N=1 | Heroin (IV) | 25.0 | Coma progressing to catatonia | No recovery at 31 days with persistent catatonia | NA |
Abbreviations: INH = inhalation; IV = intravenous; NA = not available.
Regarding the most frequent substance of abuse, heroin was described in the majority of articles included (N=8 articles; 57.1%); additionally, heroin was the most common drug among cases of mortality (N=16; 76.2%), followed by cocaine (N=4; 19.0%). Evidence of polysubstance use immediately prior to acute presentation of TL was described in only one case (1.9%).
There were limited data on administered treatments; however, when therapeutic information was available, a variety of treatment approaches were described, and the most common were supportive care (N = 3), antioxidant therapy (N = 2; e.g., Vitamin C, Vitamin E, CoQ10), and corticosteroids (N = 2). Data were insufficient across studies to determine response to treatments in each case. We attempted to assess any available follow-up imaging, cerebrospinal fluid analysis, other types of testing, and patient comorbidities, but found little consistency between studies due to variability in case reporting. Several reports presented additional data related to the clinical course for those patients who had some degree of recovery with persistent cognitive impairment and/or “catatonia” the most common sequelae for survivors (N = 6) at the time of longest follow-up.
4. Discussion
Our findings show a range of outcomes ranging from full recovery to severe disease burden with high mortality. For the patients who experienced recovery, the clinical course was typically protracted over months to years. In other patients, there is high mortality most evident in the first few weeks following presentation. These observations have important implications for understanding the pathophysiology of substance abuse-related TL and the management of patients who often require long-term hospitalization with extended disability.
The prognosis of TL has been difficult to ascertain because of often incomplete exposure data and limited follow-up of patients beyond the acute or subacute period. The present study substantially addresses these deficiencies by focusing on acute TL related to substance abuse, together with its longer-term follow-up, and the clinical information available on patients from the time of TL onset until death or recovery permits a reasonable assessment of prognosis. This approach, using well-documented cases, reveals a spectrum of clinical outcomes in which 19.2% survived without recovery, 32.7% had partial recovery, and 7.7% experienced full recovery, despite a high mortality rate of 40.4%.
4.1. Axonal Integrity and Recovery
A key finding is that about 40% of patients had at least some recovery and a small portion (7.7%) recovered completely. This outcome suggests the potential for partial or complete restoration of cerebral myelin in patients with toxic white matter injury. The term TL implies selective white matter injury that is likely mediated by a stripping of myelin from the axon by the toxic substance.1 This phenomenon is most convincingly demonstrated by the inhalant toluene, a highly lipophilic solvent closely associated with TL.35 Other leukotoxins have similar effects,1 although the precise pathophysiology of myelin injury may vary depending on the specific substance involved. One well-known factor that affects prognosis is the state of the axon; outcomes in white matter disorders are worse with axonal loss, which is hypothesized to be due to destruction of the axonal scaffolding that would provide the basis for remyelination.36 Axonal integrity can be preserved in toluene leukoencephalopathy,35 which may help explain why some patients with substance abuse-related acute TL in our review recovered to a variable extent. Additionally, given the role oligodendrocytes play in remyelination and maintenance of the myelin sheath, injury to these cells may play a role in determining recovery,37 a factor that also may contribute to the variability in clinical course among our cases. Solid evidence now exists to support the notion that the prognosis of acute TL is less favorable in cases featuring axonal destruction and impaired remyelination, similar to the poor outcomes associated with axonal damage in demyelinating diseases such as multiple sclerosis38 and other white matter disorders.36 Figure 2 illustrates the mechanisms of remyelination that may be involved in recovery after TL.
Figure 2.
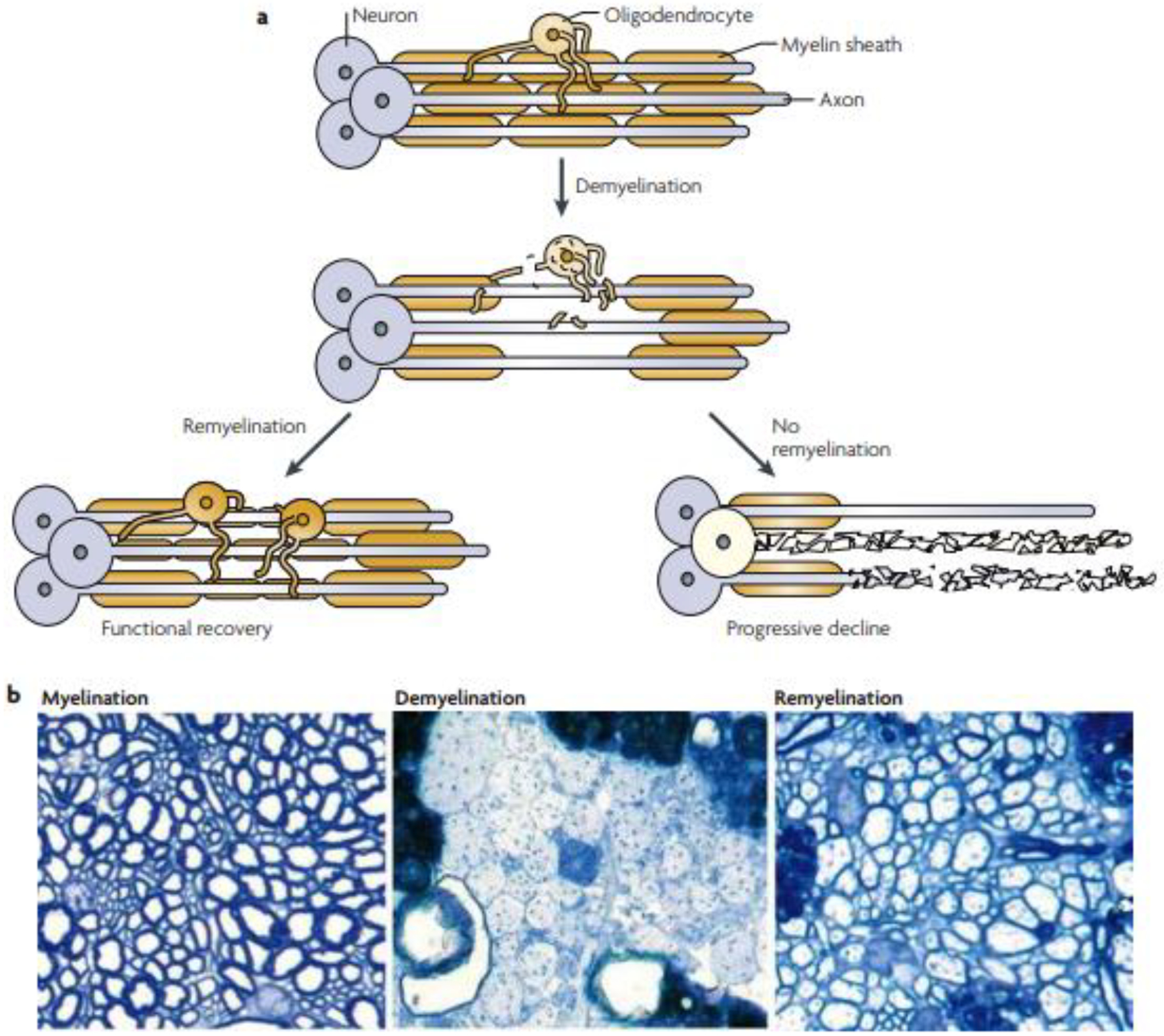
Schematic (a) and histologic (b) views of normal brain myelination, demyelination, and remyelination. Some cases of toxic leukoencephalopathy may have a better outcome because axons are preserved and remyelination can occur. Adapted from Franklin RJ, Ffrench-Constant C. 2008.39
4.2. Implications for Clinical Practice
Whereas the possibility of a favorable outcome after drug-mediated, acute TL is clinically useful, the poor prognosis observed for many patients, along with the recent rise in illicit opiate use, underscores the need for guidance in which goals of care discussions will be required. This discussion is likely to occur in the intensive care unit (ICU) setting because of the level of care required by patients with substance abuse-related TL. The severity of impairments in cognition and level of consciousness in acute TL are often striking, highlighting the importance of surrogate decision-makers. The presence of comorbid substance abuse adds another dimension to the process of counseling. Critical care providers need to be prepared to provide as much guidance as possible to aid surrogate medical decision-making. This process involves conversations designed to inform these surrogate decision-makers about various treatment options in the setting of possibly long-standing substance abuse.
The treatment of acute TL remains uncertain. Whereas anecdotal evidence can be found for the use of medications such as corticosteroids, immunomodulating therapies (e.g., intravenous immunoglobulins), or antioxidant treatment in cases of substance abuse-related acute TL, there are currently no rigorously tested treatments available to reduce morbidity or mortality in TL from any etiology. Supportive care is thus the mainstay of treatment. Unfortunately, our study shows that this approach may be inadequate to avert poor outcomes. We therefore propose that a palliative care model is warranted given the typically guarded prognosis and potential mortality. A palliative care approach would assist in addressing the many needs of patients, families, and surrogate decision makers including complex symptom management, goals of care discussions and advance care planning, psychosocial issues, and care partner and family support.
In some circumstances it may be difficult for clinicians to determine the timeline of progression in TL, presenting a challenge in distinguishing acute versus subacute or chronic cases. This information, which may be obscured by deficient information on length and intensity of exposure to various substances, clearly influences medical decision-making and management. While the clinical history is imperative for determining a plausible timeline, neuroimaging may also play a role. One possibility for establishing acuity is the use of diffusion-weighted imaging (DWI) sequences on MRI. Cases of acute TL are more likely to have abnormal DWI compared to suspected subacute or chronic cases.6,37 The presence of reduced diffusion may also be a marker of the potential for clinical and radiologic reversibility, and may represent a potential biomarker for prognosis in TL. While DWI is not always immediately available across all healthcare settings, it should be considered in the evaluation of cases of suspected acute TL.
Given the often dire prognosis of acute TL, a clear need exists to address substance abuse as a potential cause. Substance abuse-related disorders carry a high burden of psychosocial distress that further highlights the value of an interdisciplinary, comprehensive approach, bringing together providers across critical care, addiction, and mental health providers. Although caring for patients with substance abuse often involves treating episodic deteriorations in health, this care also requires an intersecting and interacting network of social support and education. Recent success has been seen for programs worldwide that view substance abuse as a medical rather than a legal problem, with harm reduction approaches yielding beneficial outcomes including reductions in substance use.40 The opiate epidemic in the US has ignited the debate over reassessing the ways in which public policy addresses substance abuse, and currently there is a call for developing a network within healthcare to address this issue.41
4.3. Limitations
Our study has several limitations. First and foremost is the reliance on non-standardized case descriptions that provide only a general appreciation of the clinical features of substance abuse-related acute TL, which is the result of the scoping review approach using previously published case reports and case series. The majority of cases in our literature review used history provided by patients, or through collateral interviewing of friends and family, to determine the relationship between substances used and onset of neurologic symptoms. It is therefore difficult to determine with full certainty the timing of opiate, cocaine, or inhalant abuse in relation to the onset of TL symptoms and signs. Moreover, there were insufficient data to describe potential confounders such as ingestion of multiple illicit substances, use of prescription drugs, and medical and neurologic comorbidities in some cases. We also cannot exclude that some cases may have had exposure to other substances that were not reported, drugs not captured on routine drugs of abuse screens, or cutting agents. While it has been hypothesized that agents used to cut illicit opiates and heroin (e.g., levamisole)42 are implicated in the pathophysiology of some cases of acute TL, the leukotoxic capacity of a variety of opiates, cocaine, and inhalants is well-documented; we attempted to control for this confounder through a careful review of each case and case series. We were also limited in our ability to examine the effects of the healthcare setting on recovery or mortality, as there is the potential for reporting bias reflecting the possibility that tertiary and/or academic medical centers may see more severe cases than community-based and non-academic medical centers.
Limited data also precluded determinations of patient demographics, disease characteristics, or specific treatment plans (i.e., supportive/comfort care) that might impact clinical outcomes. Prospective cohort studies are needed to address these limitations, and such studies would benefit from inclusion of standardized clinical and neuroimaging assessments, confirmatory blood or urine testing to seek evidence of recently ingested substances as possible precipitants of TL, and collection of data relevant to other potential confounders, including comorbid medical conditions and other concurrent medication use or drugs of abuse. Finally, future studies should examine patient outcome measures relevant to prognosis such as those used in other studies examining TL (e.g., modified Rankin score),37 which were unavailable across cases and case series included in our review.
5. Conclusions
Substance abuse-related acute TL is a clinically dramatic and often fatal disorder that shows no signs of abating given the ongoing opioid epidemic. Patients typically present with severe cognitive impairments that may or may not be reversible. Prognosis is variable, ranging from death to full recovery, and likely reflects the degree of myelin damage in relation to axonal loss. The limited pharmacologic treatments available have not shown promise for preventing death or enhancing recovery. A collaborative, team-based approach is warranted in view of the protracted and complex clinical course for patients that ultimately die and survivors alike, and palliative care can be very helpful in many cases. Care beyond the ICU setting in substance abuse-related acute TL requires incorporating families, nursing, social work, and other allied staff in the care of these patients. In particular, decisions about long-term care in patients lacking medical decision-making capacity highlights the necessity for recurrent conversations regarding goals of care. Our study thus provides data that may assist clinicians in empowering patients’ families and surrogate decision-makers with the knowledge to inform these decisions. Finally, the substantial number of patients who experience some degree of recovery suggests that detailed study of white matter restoration may yield important pathophysiological clues that may improve the care of TL in all its forms, as well as a wide range of other white matter disorders.
Highlights.
The clinical course in acute toxic leukoencephalopathy (TL) due to substance abuse is variable with a high rate of mortality or lasting disability.
Across cases in the literature, the majority of cases of acute TL are associated with opiate abuse.
A subgroup of patients experience partial or full recovery, suggesting that leukotoxic injury may be reversible in some cases.
Recovery is often protracted for survivors of acute TL, an important feature of this condition that may influence shared medical decision-making between clinicians, patients’ families, and surrogate decision makers.
Funding:
This work was supported by the National Institutes of Health (#5T32AG044296)
References
- 1.Filley CM, Kleinschmidt-DeMasters BK. Toxic leukoencephalopathy. N Engl J Med. 2001;345(6):425–432. [DOI] [PubMed] [Google Scholar]
- 2.Filley CM, McConnell BV, Anderson CA. The Expanding Prominence of Toxic Leukoencephalopathy. J Neuropsychiatry Clin Neurosci. 2017;29(4):308–318. [DOI] [PubMed] [Google Scholar]
- 3.Kriegstein AR, Shungu DC, Millar WS, et al. Leukoencephalopathy and raised brain lactate from heroin vapor inhalation (“chasing the dragon”). Neurology. 1999;53(8):1765. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett E, Mikulis D. Chasing “chasing the dragon” with MRI: leukoencephalopathy in drug abuse. The British journal of radiology. 2005;78(935):997–1004. [DOI] [PubMed] [Google Scholar]
- 5.Offiah C, Hall E. Heroin-induced leukoencephalopathy: characterization using MRI, diffusion-weighted imaging, and MR spectroscopy. Clinical radiology. 2008;63(2):146–152. [DOI] [PubMed] [Google Scholar]
- 6.McKinney AM, Kieffer SA, Paylor RT, SantaCruz KS, Kendi A, Lucato L. Acute Toxic Leukoencephalopathy: Potential for Reversibility Clinically and on MRI With Diffusion-Weighted and FLAIR Imaging. American Journal of Roentgenology. 2009;193(1):192–206. [DOI] [PubMed] [Google Scholar]
- 7.Al-Janabi OM, Bauer CE, Goldstein LB, et al. White Matter Hyperintensity Regression: Comparison of Brain Atrophy and Cognitive Profiles with Progression and Stable Groups. Brain Sci. 2019;9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fünfschilling U, Supplie LM, Mahad D, et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485(7399):517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan GJ, Manesh SB, Hilton BJ, Assinck P, Plemel JR, Tetzlaff W. The fate and function of oligodendrocyte progenitor cells after traumatic spinal cord injury. Glia. 2020;68(2):227–245. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava T, Diba P, Dean JM, et al. A TLR/AKT/FoxO3 immune tolerance–like pathway disrupts the repair capacity of oligodendrocyte progenitors. The Journal of Clinical Investigation. 2018;128(5):2025–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Preston M, Gong X, Su W, et al. Digestion products of the PH20 hyaluronidase inhibit remyelination. Ann Neurol. 2013;73(2):266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sloane JA, Batt C, Ma Y, Harris ZM, Trapp B, Vartanian T. Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc Natl Acad Sci U S A. 2010;107(25):11555–11560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volkow ND, Blanco C. The changing opioid crisis: development, challenges and opportunities. Mol Psychiatry. 2021;26(1):218–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han B, Compton WM, Jones CM, Cai R. Nonmedical Prescription Opioid Use and Use Disorders Among Adults Aged 18 Through 64 Years in the United States, 2003–2013. Jama. 2015;314(14):1468–1478. [DOI] [PubMed] [Google Scholar]
- 15.Ornell F, Moura HF, Scherer JN, Pechansky F, Kessler FHP, von Diemen L. The COVID-19 pandemic and its impact on substance use: Implications for prevention and treatment. Psychiatry Res. 2020;289:113096–113096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. International Journal of Social Research Methodology. 2005;8(1):19–32. [Google Scholar]
- 17.Peters MDJ, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. JBI Evidence Implementation. 2015;13(3):141–146. [DOI] [PubMed] [Google Scholar]
- 18.Özütemiz C, Roshan SK, Kroll NJ, et al. Acute Toxic Leukoencephalopathy: Etiologies, Imaging Findings, and Outcomes in 101 Patients. AJNR American journal of neuroradiology. 2019;40(2):267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. 2017;89:218–235. [DOI] [PubMed] [Google Scholar]
- 20.Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855–866. [DOI] [PubMed] [Google Scholar]
- 21.Bianco F, Iacovelli E, Tinelli E, Lepre C, Pauri F. Recurrent leukoencephalopathy in a cocaine abuser. Neurotoxicology. 2011;32(4):410–412. [DOI] [PubMed] [Google Scholar]
- 22.Havé L, Drouet A, Lamboley JL, et al. [Toxic leucoencephalopathy after use of sniffed heroin, an unrecognized form of beneficial evolution]. Rev Neurol (Paris). 2012;168(1):57–64. [DOI] [PubMed] [Google Scholar]
- 23.Salgado RA, Jorens PG, Baar I, Cras P, Hans G, Parizel PM. Methadone-induced toxic leukoencephalopathy: MR imaging and MR proton spectroscopy findings. AJNR Am J Neuroradiol. 2010;31(3):565–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Esch AMJ, Fest A, Hoffland BS, et al. Toxic Leukoencephalopathy Presenting as Lethal Catatonia. J Addict Med. 2019;13(3):241–244. [DOI] [PubMed] [Google Scholar]
- 25.Villella C, Iorio R, Conte G, Batocchi AP, Bria P. Toxic leukoencephalopathy after intravenous heroin injection: a case with clinical and radiological reversibility. J Neurol. 2010;257(11):1924–1926. [DOI] [PubMed] [Google Scholar]
- 26.Weitzer D, Shmuts R, Khan M. Altered Mental Status: A Case Report of Toxic Leukoencephalopathy Following Heroin Exposure. J Addict Med. 2020;14(6):e375–e377. [DOI] [PubMed] [Google Scholar]
- 27.Maschke M, Fehlings T, Kastrup O, Wilhelm HW, Leonhardt G. Toxic leukoencephalopathy after intravenous consumption of heroin and cocaine with unexpected clinical recovery. J Neurol. 1999;246(9):850–851. [DOI] [PubMed] [Google Scholar]
- 28.Achamallah N, Wright RS, Fried J. Chasing the wrong dragon: A new presentation of heroin-induced toxic leukoencephalopathy mimicking anoxic brain injury. J Intensive Care Soc. 2019;20(1):80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buxton JA, Sebastian R, Clearsky L, et al. Chasing the dragon - characterizing cases of leukoencephalopathy associated with heroin inhalation in British Columbia. Harm Reduct J. 2011;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cartier RL, González LD, Harán DJ. [Fatal toxic leukoencephalopathy associated with consumption of pasta base of cocaine: Report of three cases]. Rev Med Chil. 2015;143(11):1484–1489. [DOI] [PubMed] [Google Scholar]
- 31.Cheng MY, Chin SC, Chang YC, et al. Different routes of heroin intake cause various heroin-induced leukoencephalopathies. J Neurol. 2019;266(2):316–329. [DOI] [PubMed] [Google Scholar]
- 32.Holyoak AL, Trout MJ, White RP, Prematuranga S, Senthuran S. Toxic leukoencephalopathy in the intensive care unit. Anaesth Intensive Care. 2014;42(6):782–788. [DOI] [PubMed] [Google Scholar]
- 33.Ryan A, Molloy FM, Farrell MA, Hutchinson M. Fatal toxic leukoencephalopathy: clinical, radiological, and necropsy findings in two patients. J Neurol Neurosurg Psychiatry. 2005;76(7):1014–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torralba-Morón Á, Ortiz-Imedio J, Morales-Conejo M, Ruiz-Morales J, Guerra-Vales JM. Delayed Leukoencephalopathy: Three Case Reports and a Literature Review. Eur J Case Rep Intern Med. 2017;4(2):000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg NL, Kleinschmidt-DeMasters BK, Davis KA, Dreisbach JN, Hormes JT, Filley CM. Toluene abuse causes diffuse central nervous system white matter changes. Annals of Neurology. 1988;23(6):611–614. [DOI] [PubMed] [Google Scholar]
- 36.Medana IM, Esiri MM. Axonal damage: a key predictor of outcome in human CNS diseases. Brain. 2003;126(Pt 3):515–530. [DOI] [PubMed] [Google Scholar]
- 37.Özütemiz C, Roshan SK, Kroll NJ, et al. Acute Toxic Leukoencephalopathy: Etiologies, Imaging Findings, and Outcomes in 101 Patients. American Journal of Neuroradiology. 2019;40(2):267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kornek B, Lassmann H. Axonal pathology in multiple sclerosis. A historical note. Brain Pathol. 1999;9(4):651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9(11):839–855. [DOI] [PubMed] [Google Scholar]
- 40.Logan DE, Marlatt GA. Harm reduction therapy: a practice-friendly review of research. J Clin Psychol. 2010;66(2):201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.U.S. Department of Health and Human Services (HHS) OotSG. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. https://addiction.surgeongeneral.gov/sites/default/files/surgeon-generals-report.pdf. Published 2016. Accessed December 10, 2021, 2021. [PubMed]
- 42.Gilbert JW Re: Xu N, Zhou W, Shuy L, Zhou G, Zhang N. Clinical and MRI characteristics of Levamisole-induced leukoencephalopathy in 16 patients. J Neuroimaging 2009;13:326–331. J Neuroimaging. 2011;21(2):e188. [DOI] [PubMed] [Google Scholar]


