Abstract
Aims
Atrial fibrillation (AF) symptom relief is a primary indication for catheter ablation, but AF symptom resolution is not well characterized. The study objective was to describe AF symptom documentation in electronic health records (EHRs) pre- and post-ablation and identify correlates of post-ablation symptoms.
Methods and results
We conducted a retrospective cohort study using EHRs of patients with AF (n = 1293), undergoing ablation in a large, urban health system from 2010 to 2020. We extracted symptom data from clinical notes using a natural language processing algorithm (F score: 0.81). We used Cochran’s Q tests with post-hoc McNemar’s tests to determine differences in symptom prevalence pre- and post-ablation. We used logistic regression models to estimate the adjusted odds of symptom resolution by personal or clinical characteristics at 6 and 12 months post-ablation. In fully adjusted models, at 12 months post-ablation patients, patients with heart failure had significantly lower odds of dyspnoea resolution [odds ratio (OR) 0.38, 95% confidence interval (CI) 0.25–0.57], oedema resolution (OR 0.37, 95% CI 0.25–0.56), and fatigue resolution (OR 0.54, 95% CI 0.34–0.85), but higher odds of palpitations resolution (OR 1.90, 95% CI 1.25–2.89) compared with those without heart failure. Age 65 and older, female sex, Black or African American race, smoking history, and antiarrhythmic use were also associated with lower odds of resolution of specific symptoms at 6 and 12 months.
Conclusion
The post-ablation symptom patterns are heterogeneous. Findings warrant confirmation with larger, more representative data sets, which may be informative for patients whose primary goal for undergoing an ablation is symptom relief.
Keywords: Atrial fibrillation, Catheter ablation, Natural language processing, Signs and symptoms
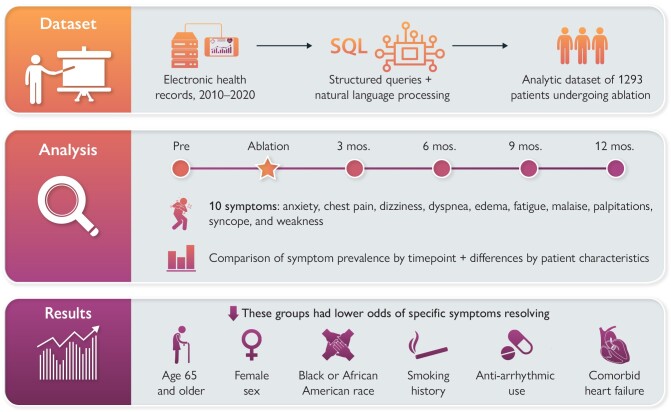
Graphical Abstract
Graphical Abstract.
Novelty.
In this study of nearly 1300 electronic health records of patients with atrial fibrillation undergoing de novo catheter ablation, the majority of patients continue to experience symptoms post-ablation.
There is significant variability in the specific symptoms that resolve by personal and clinical characteristics with patients who are 65 and older, female sex, Black or African American race, smoking history, antiarrhythmic use, and comorbid heart failure having lower odds of resolution of specific symptoms at 6 and 12 months post-ablation.
Robust natural language processing methods for extracting the symptom information from electronic health records may add important evidence in cases when patient-reported outcomes data are sparse or only available in small samples.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia.1 Adults living with AF report a variety of physical and psychological symptoms, including dyspnoea, chest pain, fatigue, anxiety, and palpitations, which limit daily functioning and impact health-related quality of life (HRQoL).2 Catheter ablation is a minimally invasive, percutaneous procedure for symptomatic patients with AF when symptoms and/or heart rhythm are not well controlled by medications, or medications cause intolerable side effects.3
Symptom relief is one of the primary indications for performing an ablation4 and is considered by patients to be more important than rhythm control.5 However, the impact of ablation on AF symptoms has not been well described. To date, most data about AF symptoms post-ablation originate from clinical trials whose primary endpoints are medically focused (e.g. mortality, stroke incidence), with HRQoL measured as a secondary endpoint.6–8 The diverse range of symptoms that patients with AF experience, including mental health symptoms like anxiety, is generally not measured.9 Moreover, differences in trajectories of symptom relief post-ablation, or in individual symptom burden based on personal and clinical characteristics, have not been well described.6,7
Because symptoms are a pre-eminent indication for undergoing ablation, healthcare professionals commonly document AF symptoms before and after ablation in clinical notes in electronic health records (EHRs). Secondary data reuse from EHRs may be an instrument for understanding symptom patterns following catheter ablation. Information stored in encounter notes (e.g. ‘Patient presents with fatigue’) can be parsed into quantitative data (e.g. ‘fatigue = 1’), using techniques such as natural language processing (NLP). Natural language processing is a suite of automated methods used to organize and evaluate the information contained in unstructured clinical notes. Natural language processing is increasingly being used to conduct cardiovascular research and to evaluate patient symptoms.10,11 To improve understanding of post-ablation symptom patterns using real-world, routinely collected clinical data, we aimed to extract and describe AF symptom documentation in EHRs before and after de novo catheter ablation for AF, and to identify personal and clinical characteristics associated with post-ablation symptom resolution.
Methods
Study design
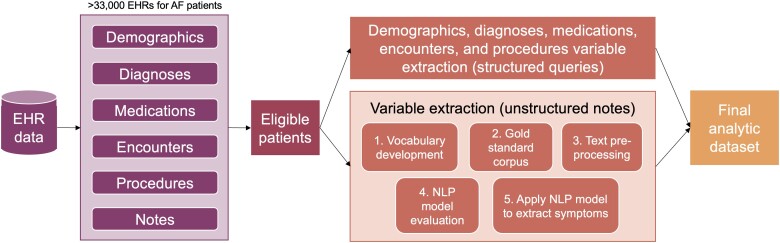
We conducted a retrospective cohort study of patients with AF undergoing catheter ablation using data from EHRs at a large, urban academic medical centre in New York City. Data from EHRs exist in structured fields (e.g. patient demographics, comorbidities, encounters, procedures, medications, vital signs, and laboratory results) and unstructured formats (e.g. encounter notes). Both structured and unstructured data were used in this analysis. A description of the steps undertaken to query, clean, and aggregate EHR data in this study is provided in Figure 1 and described in detail below. This study was approved by the Weill Cornell Medicine Institutional Review Board. The investigation conforms with the principles outlined in the Declaration of Helsinki.
Figure 1.
Diagram of electronic health record (EHR) data extraction for atrial fibrillation patients at our institution.
Cohort creation
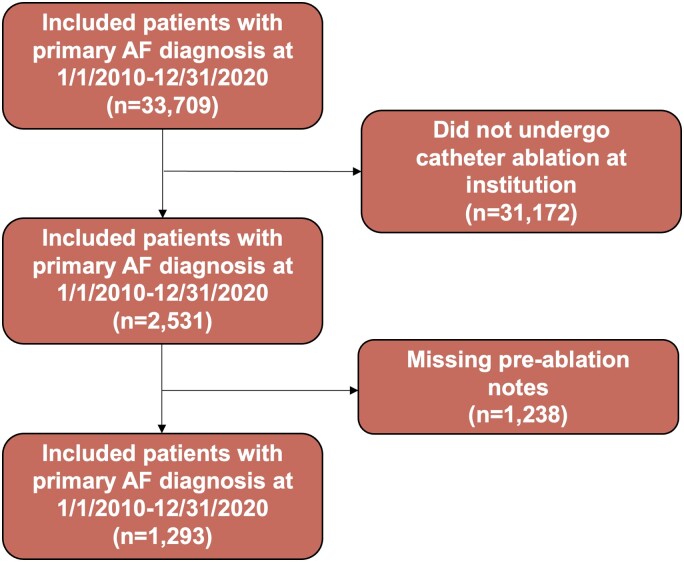
Electronic health record data at our institution adhere to the Observational Medical Outcomes Partnership (OMOP) Common Data Model, a standardized set of tables and variables that are commonly used for storing and aggregating EHR data across sites.12,13 The clinical research informatics team at our institution created an OMOP instance, containing structured and unstructured EHR data for patients with a primary diagnosis of paroxysmal AF identified using international classification of diseases (ICD)-9 and ICD-10 codes for at least one visit at our institution between 1 January 2010 and 31 December 2020. From this instance, we created a cohort of patients based on the following eligibility criteria: (i) underwent de novo catheter ablation for the treatment of AF, determined using current procedural terminology codes, (ii) age 18 or older, and (iii) at least one encounter note available within 30 days of the date of the ablation.
Structured data
We extracted demographics, comorbid diagnoses, and medications for patients in the cohort from structured fields. Demographic information included gender, race, ethnicity, and age at the time of the ablation calculated using the date of birth and the date of ablation. We used ICD-9 and ICD-10 billing codes to identify patients diagnosed with comorbid heart failure (systolic or diastolic), hypertension, stroke, transient ischaemic attack, thromboembolism, vascular disease, and diabetes. Using these variables together with age and gender, we calculated the CHA2DS2-VASC (congestive heart failure, hypertension, age, diabetes mellitus, prior stroke or TIA or thromboembolism, vascular disease, age, sex category) score, a measure of stroke risk in AF, using established scoring criteria.14 A CHA2DS2-VASC score of two or higher is considered a moderate-to-high stroke risk and an indication to start anticoagulation. We summarized CHA2DS2-VASC as both a continuous variable and categorical (score of ³2 vs. <2). We used smoking and alcohol use documentation to create a binary variable characterizing patients as ever having been smokers or ever having admitted to alcohol use (smoker vs. non-smoker, denies vs. admits alcohol use). We did not constrain billing codes, smoking status, or alcohol status to specific dates because of documentation idiosyncrasies based on visit type.15,16
We characterized inpatient or outpatient medications according to three categories relevant to catheter ablation for AF: (i) antiarrhythmic agents including amiodarone, dofetilide, dronedarone, flecainide, and propafenone, (ii) rate control medications including atenolol, diltiazem, metoprolol, atenolol, and verapamil, and (iii) anticoagulant medications including apixaban, dabigatran, edoxaban, rivaroxaban, and warfarin. Medication records included prescription refills, single medication administrations (primarily in inpatient visits), and the medication list that is manually maintained by clinicians. Only oral medications were included because they are more likely to reflect a patient’s outpatient regimen compared with injections, which are typically administered during inpatient encounters or for short periods of time. We used the start dates and, when available, end dates of each medication record to confirm the patient was taking the medication at specific times of interest during the study period (e.g. pre-ablation, 3, 6, 9, and 12 months post-ablation). Approximately, half of all medication records in our data set had start dates but no end dates. These medication records were included if the start date was within 1 year prior to the ablation year.
As a social determinant of health measure, we determined the social deprivation index (SDI) for each patient using zip codes of home addresses from the demographics table. Social deprivation index is a composite measure of neighbourhood poverty created from seven demographic variables collected from the American Community Survey.17 The variables include the per cent of individuals in a neighbourhood living in poverty, <12 years of education, single-parent households, living in a rented housing unit, living in an overcrowded housing unit, households without a car, and non-employed adults under 65 years of age. Social deprivation index is calculated at the census tract level and ranges from 1 (least disadvantaged) to 100 (most disadvantaged). In New York City, where this study was conducted and where poverty varies dramatically within relatively small geographical areas, SDI is a more sensitive indicator of socioeconomic status than county- or state-level measures.18 For this study, we accessed data sets containing SDI scores for every census tract in the USA, which are made freely available by the creators of the index.17 We determined the SDI of each patient’s community by mapping the zip code with census tracts and subsequently with SDI.
Unstructured data
We undertook a multistep process to extract symptom information from clinical notes using NLP. We extracted 10 AF symptoms determined through consultation with cardiologists, registered nurses with cardiology expertise, and the literature: anxiety, chest pain, dizziness, dyspnoea, lower extremity oedema, fatigue, malaise, palpitations, syncope, and weakness. We used the NLP software, NimbleMiner,19 and closely followed the methods outlined in prior NLP-based symptom science research conducted with this tool.20 The NLP model performance on our corpus of notes was as follows: precision = 0.732, recall = 0.926, F score = 0.807. Details regarding our NLP methods are provided in Supplementary material.
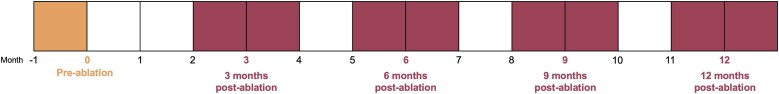
After applying the NLP model to extract AF symptoms, we grouped symptoms based on the timeframe that the notes were published (Figure 2). This generated a data set indicating whether each symptom was present or absent in the notes at each time point for each patient: pre-ablation, 3, 6, 9, and 12 months post-ablation. If multiple notes were available from a time point, all were analysed, and any mention of a symptom was retained. For example, if two notes were available during the 3-month time point, one describing palpitations and the other not, palpitations were considered endorsed at that time point.
Figure 2.
Symptom time points pre- and post-ablation used in the statistical analysis.
Statistical analysis
Data from structured fields and unstructured fields (extracted using NLP) were merged into a single analytic data set. In addition to evaluating each symptom separately, we created a composite variable reflecting whether the patient had any of the 10 symptoms at each time point. We conducted basic descriptive statistics to describe the demographic and clinical characteristics of the sample as well as symptom prevalence at each time point. We used Cochran’s Q tests with post-hoc McNemar’s tests to determine whether the prevalence of symptoms differed significantly across time points. Significance was determined using the Benjamini–Hochberg procedure for multiple comparisons correction. Only patients contributing data at each time point (n = 207) were included in the statistical comparisons of prevalence to meet the assumption of the Cochran’s Q test that the sample sizes are equal over time. We then used bivariate logistic regression models to estimate the unadjusted odds of symptom prevalence by a range of personal or clinical characteristics at 6 and 12 months post-ablation. We chose these time points because they clear the 3-month post-ablation ‘blanking period’, and align with endpoints previously reported in clinical trials.8,21 All patients contributing data at 6 months (n = 651) and 12 months (n = 523) were included in the respective models.
Finally, we conducted an analysis to evaluate the randomness of missing notes among all patients undergoing ablation at our institution. We compared the age, gender, race, ethnicity, and SDI between all patients undergoing ablation, those with pre-ablation notes available (and thus included in our analytic data set), and those with post-ablation notes available, using χ2 and t-tests for categorical and continuous data, respectively.
Results
Description of the sample
A total of 1293 patients were included in this analysis. The exclusion cascade is shown in Figure 3. The demographic and clinical characteristics of the sample are described in Table 1. The mean age of the sample was 65.5 (SD 12.6) years, approximately one-third were female, and two-thirds were White. Approximately 22% lived in a neighbourhood that is considered socially deprived.17 Nearly half were prescribed an antiarrhythmic medication and three-quarters were prescribed a rate control medication pre-ablation. Nearly half (45%) of the sample had a comorbid diagnosis of heart failure.
Figure 3.
Exclusion cascade of patients included in the final analytic sample.
Table 1.
Patient characteristics (n = 1293)
| Age (65 and older) | 738 (57.1) |
| Gender (female) | 455 (35.2) |
| Race | |
| Asian | 59 (4.6) |
| Black or African American | 68 (5.3) |
| Not reported | 303 (23.4) |
| Other racea | 113 (8.7) |
| White | 750 (58.0) |
| Ethnicity (Hispanic/Latino) | 55 (4.3) |
| Living in socially deprived neighbourhoodb | 278 (21.5) |
| Prescribed antiarrhythmic medication | 577 (44.6) |
| Prescribed rate control medication | 981 (75.9) |
| Prescribed anticoagulant medication | 921 (71.2) |
| Alcohol use history | 710 (54.9) |
| Smoking history | 324 (25.1) |
| CHA2DS2-VASC score ≥2c | 983 (76.0) |
| Comorbid heart failure | 575 (44.5) |
The values are given as n (%).
aIncludes a small number of American Indian or Alaska Nation (n = 2) and Native Hawaiian or other Pacific Islander (n = 3).
bMeasured using social deprivation index (SDI) which is a composite measure used to quantify the socioeconomic variation in health outcomes. The patient’s zip code was used to correlate to an area-level deprivation calculated based on seven demographic characteristics collected in the American Community Survey. An SDI score above 90 is considered a socially deprived area.17
cCHA2DS2-VASC is a measure of stroke risk in AF. A score ≥2 is considered a moderate-to-high stroke risk and an indication to start anticoagulation.14
Patterns of symptom prevalence over time
Patterns of symptom prevalence pre-ablation and 3, 6, 9, and 12 months post-ablation are displayed in Table 2, and pairwise comparisons are in Supplementary material online, Table S1. Almost all (96%) patients had documentation of any AF symptoms pre-ablation, while the remainder were asymptomatic. The most prevalent symptoms documented pre-ablation were dyspnoea (64%), oedema (62%), palpitations (57%), and fatigue (49%). Most patients continued to have documented symptoms at each time point post-ablation (91–95%). There was a significant change in the proportion of patients with documented anxiety from 6 months (41%) to 12 months (36%). There was a significant change in the proportion of patients with documented weakness from pre-ablation (15%) to all time points post-ablation (20–22%). There were no other significant differences in symptom prevalence across time points.
Table 2.
Symptom prevalence by time point among patients undergoing catheter ablationa
| Pre-ablation, n = 1293, n (%) | 3 months, n = 822, n (%) | 6 months, n = 651, n (%) | 9 months, n = 471, n (%) | 12 months, n = 523, n (%) | P-valueb | |
|---|---|---|---|---|---|---|
| Any symptoms | 1246 (96) | 769 (94) | 592 (91) | 449 (95) | 495 (95) | 0.47 |
| Anxiety | 444 (34) | 312 (38) | 264 (41) | 204 (43) | 190 (36) | 0.009 |
| Chest pain | 551 (43) | 351 (43) | 278 (43) | 220 (47) | 218 (42) | 0.29 |
| Dizziness | 569 (44) | 346 (42) | 276 (42) | 198 (42) | 211 (40) | 0.75 |
| Fatigue | 637 (49) | 386 (47) | 291 (45) | 223 (47) | 243 (46) | 0.36 |
| Malaise | 104 (8) | 77 (9) | 63 (10) | 46 (10) | 55 (11) | 0.12 |
| Palpitations | 736 (57) | 453 (55) | 358 (55) | 245 (52) | 294 (56) | 0.89 |
| Dyspnoea | 823 (64) | 474 (58) | 379 (58) | 301 (64) | 300 (57) | 0.74 |
| Oedema | 796 (62) | 473 (58) | 355 (55) | 290 (62) | 299 (57) | 0.99 |
| Syncope | 332 (26) | 226 (28) | 202 (31) | 145 (31) | 146 (28) | 0.06 |
| Weakness | 194 (15) | 182 (22) | 132 (20) | 103 (22) | 111 (21) | 0.006 |
Bold values indicate statistical significance at P < 0.05 and correspond to footnote ‘b’.
aThe prevalence is calculated as the proportion of patients with symptoms among those contributing data at each time point.
bSignificant at P < 0.05. Significance is determined using Cochran’s Q tests.
Associations between patient characteristics and symptom resolution
At 6 months post-ablation, in fully adjusted models, patients 65 and older had significantly lower odds of palpitations resolution [odds ratio (OR) 0.58, 95% confidence interval (CI) 0.40–0.85] compared with patients under 65 (Table 3). Females had significantly lower odds of anxiety resolution (OR 0.46, 95% CI 0.31–0.69), dizziness resolution (OR 0.65, 95% CI 0.45–0.96), and palpitations resolution (OR 0.42, 95% CI 0.29–0.61) compared with males. Asian patients had significantly higher odds of anxiety resolution (OR 4.70, 95% CI 1.36–29.60) compared with White patients. Black or African American patients had significantly lower odds of malaise resolution (OR 0.17, 95% CI 0.04–0.91) compared with White patients. Patients living in socially deprived neighbourhoods had significantly higher odds of chest pain resolution (OR 1.73, 95% CI 1.06–2.92) compared with those not living in social-deprived neighbourhoods. Patients with an active antiarrhythmic prescription at 6 months had significantly lower odds of dizziness resolution (OR 0.61, 95% CI 0.41–0.93) and syncope resolution (OR 0.55, 95% CI 0.34–0.91) compared with those with no prescription. Patients with a history of alcohol use had significantly higher odds of weakness resolution (OR 1.87, 95% CI 1.01–3.46) compared with those with no history. Patients with a smoking history had significantly lower odds of oedema resolution (OR 0.60, 95% CI 0.42–0.87) compared with those with no history. Patients with comorbid heart failure had significantly lower odds of chest pain resolution (OR 0.51, 95% CI 0.33–0.78), fatigue resolution (OR 0.46, 95% CI 0.30–0.69), dyspnoea resolution (OR 0.41, 95% CI 0.28–0.59), and oedema resolution (OR 0.33, 95% CI 0.23–0.48), but significantly higher odds of palpitations resolution (OR 1.90, 95% CI 1.30–2.81), compared with those without heart failure.
Table 3.
Fully adjusted associations between patient characteristics and resolution of any symptoms and specific symptoms 6 months post-ablation among patients with the symptom pre-ablation, n = 651, odds ratios (ORs), and 95% confidence intervals (95% CIs)
| Characteristic | Any symptoms | Anxiety | Chest pain | Dizziness | Dyspnoea | Oedema |
|---|---|---|---|---|---|---|
| Age (ref: under 65, n = 255) | 1.16 (0.63–2.09) | 0.95 (0.61–1.47) | 0.96 (0.63–1.46) | 1.16 (0.78–1.75) | 0.86 (0.60–1.25) | 1.00 (0.69–1.47) |
| Gender (ref: Male, n = 408) | 0.78 (0.41–1.42) | 0.46 (0.31–0.69) | 0.71 (0.48–1.06) | 0.65 (0.45–0.96) | 0.82 (0.57–1.17) | 1.00 (0.69–1.45) |
| Race (ref: White, n = 393) | ||||||
| Asian (n = 34) | 0.92 (0.21–2.90) | 4.70 (1.36–29.60) | 0.75 (0.33–1.87) | 0.65 (0.30–1.55) | 0.87 (0.41–1.91) | 0.52 (0.23–1.16) |
| Black/African American (n = 40) | 0.71 (0.16–2.24) | 1.05 (0.49–2.41) | 0.74 (0.36–1.61) | 0.60 (0.30–1.24) | 1.10 (0.53–2.33) | 0.48 (0.23–1.00) |
| Ethnicity (ref: not Hispanic/Latino, n = 616) | 1.48 (0.32–5.16) | 0.42 (0.19–1.00) | 0.47 (0.21–1.12) | 0.71 (0.31–1.75) | 0.55 (0.24–1.24) | 0.58 (0.24–1.37) |
| Neighbourhood SDIa (ref: not deprived, n = 477) | 1.10 (0.56–2.07) | 1.07 (0.68–1.75) | 1.73 (1.06–2.92) | 1.46 (0.93–2.37) | 1.09 (0.73–1.65) | 1.02 (0.68–1.54) |
| Antiarrhythmic rxb (ref: no rx, n = 503) | 1.65 (0.89–3.00) | 0.94 (0.59–1.52) | 1.15 (0.73–1.86) | 0.61 (0.41–0.93) | 0.87 (0.59–1.30) | 1.17 (0.78–1.76) |
| Rate control rxb (ref: no rx, n = 347) | 1.13 (0.64–1.98) | 0.68 (0.45–1.02) | 0.75 (0.51–1.12) | 0.79 (0.54–1.16) | 0.91 (0.64–1.29) | 0.80 (0.56–1.13) |
| Alcohol history (ref: no history, n = 224) | 0.91 (0.51–1.67) | 1.07 (0.70–1.62) | 0.91 (0.61–1.37) | 0.80 (0.54–1.18) | 1.28 (0.89–1.83) | 1.19 (0.83–1.71) |
| Smoking history (ref: no history, n = 407) | 1.06 (0.58–1.89) | 0.74 (0.49–1.13) | 0.88 (0.58–1.34) | 0.72 (0.48–1.07) | 0.71 (0.50–1.02) | 0.60 (0.42–0.87) |
| Heart failure (ref: no heart failure, n = 251) | 0.96 (0.52–1.76) | 0.68 (0.44–1.04) | 0.51 (0.33–0.78) | 0.76 (0.51–1.13) | 0.41 (0.28–0.59) | 0.33 (0.23–0.48) |
| Fatigue | Malaise | Palpitations | Syncope | Weakness | ||
| Age (ref: under 65, n = 255) | 0.94 (0.63–1.41) | 0.94 (0.32–2.90) | 0.58 (0.40–0.85) | 0.75 (0.47–1.22) | 0.70 (0.37–1.34) | |
| Gender (ref: Male, n = 408) | 0.94 (0.63–1.39) | 2.42 (0.73–11.10) | 0.42 (0.29–0.61) | 0.65 (0.41–1.03) | 0.55 (0.30–1.00) | |
| Race (ref: White, n = 393) | ||||||
| Asian (n = 34) | 1.16 (0.50–3.03) | 0.43 (0.07–8.34) | 1.12 (0.51–2.61) | 0.98 (0.36–3.46) | 1.59 (0.43–10.30) | |
| Black/African American (n = 40) | 0.94 (0.46–1.98) | 0.17 (0.04–0.91) | 0.72 (0.35–1.51) | 0.59 (0.27–1.40) | 0.73 (0.28–2.15) | |
| Ethnicity (ref: not Hispanic/Latino, n = 616) | 1.82 (0.70–5.71) | NA | 0.89 (0.39–2.14) | 1.17 (0.42–4.20) | NA | |
| Neighbourhood SDIa (ref: not deprived, n = 477) | 1.23 (0.79, 1.97) | 1.13 (0.35, 5.05) | 1.13 (0.74, 1.75) | 0.89 (0.54, 1.51) | 1.23 (0.60, 2.80) | |
| Antiarrhythmic rxb (ref: no rx, n = 503) | 0.66 (0.44–1.02) | 0.87 (0.29–3.23) | 0.92 (0.61–1.42) | 0.55 (0.34–0.91) | 0.59 (0.32–1.13) | |
| Rate control rxb (ref: no rx, n = 347) | 1.05 (0.72–1.53) | 0.50 (0.17–1.41) | 0.73 (0.50–1.04) | 0.64 (0.41–1.01) | 0.62 (0.33–1.14) | |
| Alcohol history (ref: no history, n = 224) | 1.19 (0.81–1.76) | 0.72 (0.22–2.11) | 1.41 (0.97–2.05) | 0.63 (0.38–1.02) | 1.87 (1.01–3.46) | |
| Smoking history (ref: no history, n = 407) | 0.84 (0.56–1.26) | 0.46 (0.16–1.30) | 1.05 (0.71–1.55) | 1.03 (0.64–1.68) | 0.59 (0.32–1.11) | |
| Heart failure (ref: no heart failure, n = 251) | 0.46 (0.30–0.69) | 1.18 (0.39–3.66) | 1.90 (1.30–2.81) | 0.84 (0.51–1.35) | 0.53 (0.27–1.02) |
Bold values indicate statistical significance at P < 0.05.
aSocial deprivation index (SDI) measured at the census tract level. SDI <90 is considered not deprived.
bRx = active prescription at 6 months post-ablation, per electronic health records.
At 12 months post-ablation, in fully adjusted models, patients 65 and older had significantly lower odds of palpitations resolution (OR 0.60, 95% CI 0.39–0.91) compared with those under 65 (Table 4). Females had significantly lower odds of anxiety resolution (OR 0.53, 95% CI 0.32–0.86) and palpitations resolution (OR 0.42, 95% CI 0.28–0.63) compared with males. Black or African American patients had significantly lower odds of syncope resolution (OR 0.30, 95% CI 0.12–0.84) compared with White patients. Patients with an active antiarrhythmic prescription at 12 months had significantly lower odds of weakness resolution (OR 0.42, 95% CI 0.19–0.98) compared with those with no prescription. Patients with an alcohol use history had significantly higher odds of weakness resolution (OR 3.10, 95% CI 1.46–6.86) compared with those with no history. Patients with a smoking history had significantly lower odds of dyspnoea resolution (OR 0.62, 95% CI 0.42–0.93) and fatigue resolution (OR 0.54, 95% CI 0.35–0.83) compared with those with no history. Patients with heart failure had significantly lower odds of dyspnoea resolution (OR 0.38, 95% CI 0.25–0.57), oedema resolution (OR 0.37, 95% CI 0.25–0.56), and fatigue resolution (OR 0.54, 95% CI 0.34–0.85), but higher odds of palpitations resolution (OR 1.90, 95% CI 1.25–2.89) compared with those without heart failure.
Table 4.
Fully adjusted associations between patient characteristics and resolution of any symptoms and specific symptoms 12 months post-ablation among patients with the symptom pre-ablation, n = 523, odds ratios (ORs), and 95% confidence intervals (95% CIs)
| Characteristic | Any symptoms | Anxiety | Chest pain | Dizziness | Dyspnoea | Oedema |
|---|---|---|---|---|---|---|
| Age (ref: under 65, n = 207) | 1.16 (0.57–2.32) | 1.48 (0.87–2.61) | 0.93 (0.58–1.49) | 1.38 (0.85–2.28) | 0.69 (0.46–1.04) | 1.12 (0.74–1.69) |
| Gender (ref: Male, n = 330) | 0.70 (0.33–1.40) | 0.53 (0.32–0.86) | 0.78 (0.50–1.24) | 0.65 (0.41–1.02) | 0.71 (0.48–1.06) | 1.03 (0.70–1.52) |
| Race (ref: White, n = 323) | ||||||
| Asian (n = 26) | NA | 1.39 (0.46–6.06) | 1.30 (0.47–4.60) | 0.97 (0.35–3.45) | 1.35 (0.57–3.48) | 0.39 (0.17–0.91) |
| Black/African American (n = 32) | 0.44 (0.07–1.62) | 1.15 (0.45–3.59) | 0.61 (0.28–1.41) | 0.56 (0.25–1.36) | 0.88 (0.40–1.94) | 0.90 (0.42–1.96) |
| Ethnicity (ref: not Hispanic/Latino, n = 495) | 1.28 (0.18–5.77) | 0.63 (0.24–1.90) | 0.57 (0.23–1.50) | 1.12 (0.44–3.32) | 0.66 (0.27–1.62) | 0.49 (0.20–1.22) |
| Neighbourhood SDIa (ref: not deprived, n = 370) | 1.52 (0.73–3.04) | 1.19 (0.68–2.19) | 1.37 (0.82–2.40) | 0.98 (0.60–1.65) | 1.16 (0.75–1.83) | 1.04 (0.68–1.61) |
| Antiarrhythmic rxb (ref: no rx, n = 400) | 0.96 (0.41–2.08) | 0.89 (0.49–1.69) | 1.04 (0.59–1.92) | 0.58 (0.35–1.00) | 1.08 (0.67–1.77) | 1.06 (0.66–1.72) |
| Rate control rxb (ref: no rx, n = 246) | 1.63 (0.85–3.18) | 0.73 (0.44–1.19) | 0.65 (0.42–1.02) | 0.83 (0.53–1.32) | 0.97 (0.66–1.44) | 0.71 (0.48–1.04) |
| Alcohol history (ref: no history, n = 183) | 1.33 (0.67–2.79) | 0.85 (0.50–1.40) | 1.23 (0.78–1.93) | 0.77 (0.48–1.23) | 1.39 (0.94–2.06) | 1.10 (0.75–1.63) |
| Smoking history (ref: no history, n = 316) | 0.79 (0.39–1.55) | 0.65 (0.40–1.08) | 0.80 (0.50–1.28) | 0.72 (0.45–1.15) | 0.62 (0.42–0.93) | 0.70 (0.47–1.03) |
| Heart failure (ref: no heart failure, n = 192) | 1.09 (0.55–2.21) | 0.73 (0.43–1.20) | 0.75 (0.46–1.19) | 0.97 (0.60–1.54) | 0.38 (0.25–0.57) | 0.37 (0.25–0.56) |
| Fatigue | Malaise | Palpitations | Syncope | Weakness | ||
| Age (ref: under 65, n = 207) | 1.15 (0.73–1.84) | 1.51 (0.42–6.22) | 0.60 (0.39–0.91) | 1.07 (0.58–2.04) | 1.35 (0.61–3.19) | |
| Gender (ref: Male, n = 330) | 0.76 (0.49–1.17) | 2.60 (0.62–18.00) | 0.42 (0.28–0.63) | 0.77 (0.42–1.41) | 0.85 (0.40–1.83) | |
| Race (ref: White, n = 323) | ||||||
| Asian (n = 26) | 1.72 (0.63–6.02) | NA | 1.31 (0.52–3.82) | 1.56 (0.30–28.6) | NA | |
| Black/African American (n = 32) | 0.98 (0.47–2.20) | NA | 0.56 (0.26–1.23) | 0.30 (0.12–0.84) | 0.36 (0.12–1.21) | |
| Ethnicity (ref: not Hispanic/Latino, n = 495) | 1.13 (0.41–3.65) | 0.26 (0.03–5.81) | 0.62 (0.26–1.50) | 0.71 (0.25–2.37) | 3.20 (0.57–60.90) | |
| Neighbourhood SDIa (ref: not deprived, n = 370) | 0.82 (0.51–1.34) | 0.78 (0.21–3.67) | 0.68 (0.44–1.07) | 0.74 (0.40–1.42) | 0.77 (0.35–1.82) | |
| Antiarrhythmic rxb (ref: no rx, n = 400) | 1.02 (0.60–1.78) | 0.41 (0.11–1.96) | 1.17 (0.69–2.02) | 0.75 (0.38–1.59) | 0.42 (0.19–0.98) | |
| Rate control rxb (ref: no rx, n = 246) | 0.83 (0.54–1.28) | 0.71 (0.20–2.90) | 0.69 (0.46–1.05) | 0.53 (0.29–0.95) | 0.93 (0.44–2.05) | |
| Alcohol history (ref: no history, n = 183) | 1.31 (0.84–2.03) | 1.00 (0.25–3.51) | 1.28 (0.85–1.93) | 0.51 (0.26–0.96) | 3.10 (1.46–6.86) | |
| Smoking history (ref: no history, n = 316) | 0.54 (0.35–0.83) | 1.45 (0.39–6.97) | 0.82 (0.54–1.26) | 0.70 (0.39–1.27) | 0.54 (0.25–1.16) | |
| Heart failure (ref: no heart failure, n = 192) | 0.54 (0.34–0.85) | 1.30 (0.36–4.91) | 1.90 (1.25–2.89) | 1.13 (0.60–2.10) | 0.71 (0.31–1.57) |
Bold values indicate statistical significance at P < 0.05.
aSocial deprivation index (SDI) measured at the census tract level. SDI <90 is considered not deprived.
bRx = active prescription at 6 months post-ablation, per electronic health records.
Evaluation of data missingness
Among the 2531 patients with a documented ablation at the institution, 1293 (51.1%) had pre-ablation notes and were included in our analytic data set, and 1053 (41.6%) had any post-ablation notes. Compared with all patients undergoing ablations, patients with pre-ablation notes were significantly older, fewer were males, and fewer were non-White (see Supplementary material online, Table S2). Compared with all patients undergoing ablations, patients with any post-ablation notes were significantly older, fewer were females, and fewer were non-White. There were no differences by ethnicity or SDI in either comparison.
Discussion
In this analysis of EHRs of nearly 1300 patients with AF undergoing catheter ablation, we found that the majority of patients continue to experience AF symptoms per EHRs, but that there is significant variability in the specific symptoms that resolve by personal and clinical characteristics. Importantly, age 65 and older, female sex, Black or African American race, smoking history, antiarrhythmic use, and comorbid heart failure are associated with lower odds of resolution of specific symptoms at 6 and 12 months. Importantly, nuances regarding the data sources, including the lack of characterization of subtle changes in symptom burden and potential differences in clinician perception of patient symptoms, exist which may have influenced these results. Therefore, these findings warrant confirmation in future investigations with larger, more representative data sets. Nonetheless, this study raises questions about whether specific subgroups of patients with AF symptoms will experience symptom relief post-ablation. Providing patients with realistic expectations of symptom relief post-ablation is an important component of building trust and conducting high-quality shared decision-making around catheter ablation.
A 2016 meta-analysis22 and multiple recent clinical trials8,21 and registry studies23 have confirmed that catheter ablation produces lasting improvements in quality of life at 12 months, and even 2 years,24 post-ablation. At the same time, multiple studies have also acknowledged differences in symptom outcomes post-ablation. Qualitative and survey studies alike show that many patients report fatigue25 and increased anxiety26,27 persisting for several months post-ablation, which they report is upsetting for patients who hoped for faster recoveries after the procedure. Similarly, we found that most patients continued to report at least one symptom post-ablation and that the prevalence of patients with documented anxiety symptoms increased incrementally throughout the post-ablation period, before falling back to baseline at 12 months. This is consistent with prior reports of anxiety about AF recurrence post-ablation.26,28 Given that anxiety and negative affect can exacerbate other AF symptoms and lead to recurrence,7,29 addressing post-ablation anxiety should be a priority.
Our findings regarding differences in symptoms by gender and antiarrhythmic use are corroborated by prior studies. A 2-year observational cohort of over 10 000 patients with AF from the ORBIT-AF registry reported significant differences in the types and severity of AF symptoms by gender, but this was not focused on the ablation period.23 A separate observational cohort following patients with AF 2 years post-ablation reported that patients on continued anticoagulant and/or antiarrhythmic medication had higher odds of AF symptoms.24 We also uncovered associations between Black/African American race and post-ablation symptoms that have not been previously reported to our knowledge, but our finding was limited by the small proportion of patients identified as this race and high amount of missingness of this variable in EHRs. A previous study suggests patients with missing race data are more likely to represent non-White individuals.30 Finally, in our study, heart failure was strongly associated with differences in symptom prevalence. The American Heart Association, American College of Cardiology, and Heart Rhythm Society (AHA/ACC/HRS) guidelines for AF support catheter ablation for symptomatic patients with comorbid heart failure because of new evidence, suggesting it is associated with lower hospitalization and mortality rates.31 Our study suggests heart failure patients may experience relief of some symptoms but not all, and that symptoms that are more directly correlated with heart failure itself (dyspnoea, oedema) may persist post-ablation.
Findings from this study and other investigations of post-ablation symptoms may be informative to patients whose primary goal for undergoing ablation is symptom reduction or resolution. The AHA/ACC/HRS guidelines for AF support catheter ablations for the treatment of symptomatic paroxysmal AF in the setting of refractory AF or intolerance to antiarrhythmic medications.4 Symptom control is also a primary goal for patients who opt for ablation.5 In practice, the decision to undergo ablation requires a nuanced balance of benefits, including the potential to relieve symptoms, and complications, including the high frequency of recurrence and need for repeat ablation.32,33 Shared decision-making, supported by high-quality decision aids, could improve patient guidance as they weigh risks and benefits.34,35 However, a recent study demonstrated that only one in five patients with AF engaged in shared decision-making around rhythm control strategies, and over half did not understand what different rhythm control options were available to them.36 Thus, while shared decision-making interventions and decision aids supporting anticoagulation decisions for AF have proliferated in recent years,37–45 there remains a lack of attention on AF symptom and rhythm management.35 Once generated, high-quality evidence about post-ablation symptom outcomes could be presented to patients to facilitate shared decision-making.
As symptoms drive poor quality of life among patients with cardiovascular disease, comparisons of different methods for collecting high-quality evidence about symptoms should be prioritized. Symptom mining from EHRs to conduct symptom science work is still in its nascent stages, and the accuracy and usefulness of the information retrieved could be improved.11 For example, in this study, we were only able to characterize symptoms by their presence or absence; in reality, symptom burden is a continuum. More subtle changes in symptom burden (e.g. improvement but not altogether resolved) could potentially be captured with more sophisticated, machine-learning-based NLP techniques, but nonetheless relies on clinicians to document these changes. Symptom documentation in EHRs is conducted by clinicians, who may de-prioritize certain symptoms, misunderstand patient reports, or conduct incomplete symptom assessments.
Nonetheless, real-world evidence from EHRs may offer benefits over patient-reported outcomes in clinical trials and disease registries, which are typically time and resource intensive to conduct,46 may include younger, healthier, predominantly White populations due to trial eligibility criteria and patient mistrust of researchers,47 and often face significant challenges with missing longitudinal patient-reported outcomes data.8 In this single-site study, we were able to access over 33 000 EHRs and ultimately study the symptoms of ∼1300 patients across 10 years, with significantly less time and resource utilization compared with a clinical trial of the same size. With use of common data models such as OMOP, aggregation of records across multiple institutions is possible. Given these potential benefits, EHRs may provide a complementary mechanism for studying symptoms when clinical trials are infeasible. Future studies comparing patient self-reported symptoms to those documented in EHRs by clinicians will illuminate the degree to which symptom documentation in EHRs reflects patients’ true symptom status.
Additional limitations of this study include that it was a single-arm study without a comparator cohort (e.g. patients with AF on medications alone). Additionally, we observed missing data across the peri- and post-ablation periods, which may have been biased by age, gender, and race. In particular, patients in this sample were significantly older than the broader population of patients undergoing ablation at our institution. This missing datum probably reflects a mixture of both true missingness (inconsistent attendance at follow-up appointments and/or follow-up outside the institution) and documentation bias (some symptoms not documented and some notes not stored in the institution’s OMOP instance). Similarly, data for certain variables may have been underreported and dates associated with the data may have been incorrect in some cases; this was particularly true for medication administrations (which lacked end dates) and smoking and alcohol use, which were historical variables and not representative of active use at each time point.
Implications for practice
This analysis of symptom information documented in institutional EHRs suggests that symptom resolution post-ablation varies by specific symptoms and specific patient characteristics. Specifically, we noted trends in characteristics such that patients with comorbid heart failure, age 65 and older, female sex, Black or African American race, smoking history, and antiarrhythmic use may be less likely to experience resolution of specific symptoms 6 and 12 months post-ablation. While warranting confirmation in future research, this information may be informative for practice, as it could facilitate high-quality shared decision-making among patients and clinicians considering catheter ablation to treat AF symptoms.
Supplementary Material
Contributor Information
Meghan Reading Turchioe, Columbia University School of Nursing, 560 W. 168th Street, New York, NY 10032, USA.
Alexander Volodarskiy, Department of Cardiology, NewYork-Presbyterian Queens Hospital, 56-45 Main St, Queens, NY 11355, USA; Department of Population Health Sciences, Weill Cornell Medicine, 402 E 67th St, New York, NY 10065, USA.
Winston Guo, Department of Population Health Sciences, Weill Cornell Medicine, 402 E 67th St, New York, NY 10065, USA.
Brittany Taylor, Columbia University School of Nursing, 560 W. 168th Street, New York, NY 10032, USA.
Mollie Hobensack, Columbia University School of Nursing, 560 W. 168th Street, New York, NY 10032, USA.
Jyotishman Pathak, Department of Population Health Sciences, Weill Cornell Medicine, 402 E 67th St, New York, NY 10065, USA.
David Slotwiner, Department of Cardiology, NewYork-Presbyterian Queens Hospital, 56-45 Main St, Queens, NY 11355, USA; Department of Population Health Sciences, Weill Cornell Medicine, 402 E 67th St, New York, NY 10065, USA.
Conclusion
This study demonstrated that post-ablation symptom patterns are heterogeneous. Though confirmation with larger, more representative data sets is needed, these findings may be informative for patients whose primary goal for undergoing an ablation is symptom relief.
Author contributions
M.R.T. conceptualized the study, acquired funding, collected and aggregated the data, completed the data analysis, and drafted the original manuscript. M.R.T., A.V., W.G., B.T., and M.H. completed the data curation (annotating the gold standard corpus). J.P. provided methodological guidance and supervision. D.S. provided clinical domain-specific guidance and supervision. A.V., W.G., B.T., M.H., J.P., and D.S. provided critical feedback on the data analysis and reviewed and edited the manuscript.
Supplementary material
Supplementary material is available at European Journal of Cardiovascular Nursing online.
Funding
This research was supported by the National Institute of Nursing Research of the NIH under a research grant (R00NR019124; PI: M.R.T.) and training grant (T32NR007969; supporting M.H.).
Data availability
The data underlying this article cannot be shared publicly due to the privacy of individuals who participated in the study, as determined by the Weill Cornell Medicine Institutional Review Board. The data will be shared on reasonable request with the corresponding author.
Ethics approval
This study was approved by the Weill Cornell Medicine Institutional Review Board.
References
- 1. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart Disease and Stroke Statistics-2022 Update: a report from the American Heart Association. Circulation 2022;145:e153–e639. [DOI] [PubMed] [Google Scholar]
- 2. Verma A, Champagne J, Sapp J, Essebag V, Novak P, Skanes A, et al. Discerning the incidence of symptomatic and asymptomatic episodes of atrial fibrillation before and after catheter ablation (DISCERN AF): a prospective, multicenter study. JAMA Intern Med 2013;173:149–156. [DOI] [PubMed] [Google Scholar]
- 3. Prystowsky EN, Padanilam BJ, Fogel RI. Treatment of atrial fibrillation. JAMA 2015;314:278–288. [DOI] [PubMed] [Google Scholar]
- 4. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, et al. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society J Am Coll Cardiol 2014;64:2246–2280. [DOI] [PubMed] [Google Scholar]
- 5. Sultan A, Lüker J, Andresen D, Kuck KH, Hoffmann E, Brachmann J, et al. Predictors of atrial fibrillation recurrence after catheter ablation: data from the German Ablation Registry. Sci Rep 2017;7:16678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rienstra M, Lubitz SA, Mahida S, Magnani JW, Fontes JD, Sinner MF, et al. Symptoms and functional status of patients with atrial fibrillation: state of the art and future research opportunities. Circulation 2012;125:2933–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heidt ST, Kratz A, Najarian K, Hassett AL, Oral H, Gonzalez R, et al. Symptoms in atrial fibrillation: a contemporary review and future directions. J Atr Fibrillation 2016;9:1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH, et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schnabel RB, Pecen L, Rzayeva N, Lucerna M, Purmah Y, Ojeda FM, et al. Symptom burden of atrial fibrillation and its relation to interventions and outcome in Europe. J Am Heart Assoc 2018;7:e007559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reading Turchioe M, Volodarskiy A, Pathak J, Wright DN, Tcheng JE, Slotwiner D. Systematic review of current natural language processing methods and applications in cardiology. Heart 2021;108:909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koleck TA, Dreisbach C, Bourne PE, Bakken S. Natural language processing of symptoms documented in free-text narratives of electronic health records: a systematic review. J Am Med Inform Assoc 2019;26:364–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. OMOP common data model . https://www.ohdsi.org/data-standardization/the-common-data-model/ (29 May 2022).
- 13. Hripcsak G, Duke JD, Shah NH, Reich CG, Huser V, Schuemie MJ, et al. Observational Health Data Sciences and Informatics (OHDSI): opportunities for observational researchers. Stud Health Technol Inform 2015;216:574–578. [PMC free article] [PubMed] [Google Scholar]
- 14. Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 15. Lyons AM, Dimas J, Richardson SJ, Sward K. Assessing EHR data for use in clinical improvement and research. Am J Nurs 2022;122:32–41. [DOI] [PubMed] [Google Scholar]
- 16. Weiskopf NG, Weng C. Methods and dimensions of electronic health record data quality assessment: enabling reuse for clinical research. J Am Med Inform Assoc 2013;20:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Social Deprivation Index (SDI) . https://www.graham-center.org/maps-data-tools/social-deprivation-index.html (31 May 2022).
- 18. Zhang Y, Zhang Y, Sholle E, Abedian S, Sharko M, Turchioe MR, et al. Assessing the impact of social determinants of health on predictive models for potentially avoidable 30-day readmission or death. PLoS One 2020;15:e0235064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Topaz M, Murga L, Bar-Bachar O, McDonald M, Bowles K. Nimbleminer: an open-source nursing-sensitive natural language processing system based on word embedding. Comput Inform Nurs 2019;37:583–590. [DOI] [PubMed] [Google Scholar]
- 20. Koleck TA, Tatonetti NP, Bakken S, Mitha S, Henderson MM, George M, et al. Identifying symptom information in clinical notes using natural language processing. Nurs Res 2021;70:173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blomström-Lundqvist C, Gizurarson S, Schwieler J, Jensen SM, Bergfeldt L, Kennebäck G, et al. Effect of catheter ablation vs antiarrhythmic medication on quality of life in patients with atrial fibrillation: the CAPTAF randomized clinical trial. JAMA 2019;321:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim YG, Shim J, Choi J-I, Kim Y-H. Radiofrequency catheter ablation improves the quality of life measured with a short form-36 questionnaire in atrial fibrillation patients: a systematic review and meta-analysis. PLoS One 2016;11:e0163755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piccini JP, Simon DN, Steinberg BA, Thomas L, Allen LA, Fonarow GC, et al. Differences in clinical and functional outcomes of atrial fibrillation in women and men: two-year results from the ORBIT-AF Registry. JAMA Cardiol 2016;1:282–291. [DOI] [PubMed] [Google Scholar]
- 24. Wokhlu A, Monahan KH, Hodge DO, Asirvatham SJ, Friedman PA, Munger TM, et al. Long-term quality of life after ablation of atrial fibrillation the impact of recurrence, symptom relief, and placebo effect. J Am Coll Cardiol 2010;55:2308–2316. [DOI] [PubMed] [Google Scholar]
- 25. Wood KA, Barnes AH, Paul S, Hines KA, Jackson KP. Symptom challenges after atrial fibrillation ablation. Heart Lung 2017;46:425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reading M, Baik D, Beauchemin M, Hickey KT, Merrill JA. Factors influencing sustained engagement with ECG self-monitoring: perspectives from patients and health care providers. Appl Clin Inform 2018;9:772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koleck TA, Mitha SA, Biviano A, Caceres BA, Corwin EJ, Goldenthal I, et al. Exploring depressive symptoms and anxiety among patients with atrial fibrillation and/or flutter at the time of cardioversion or ablation. J Cardiovasc Nurs 2020;36:470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Risom SS, Zwisler A-D, Thygesen LC, Svendsen JH, Berg SK. High readmission rates and mental distress 1 yr after ablation for atrial fibrillation or atrial flutter: a nationwide survey. J Cardiopulm Rehabil Prev 2019;39:33–38. [DOI] [PubMed] [Google Scholar]
- 29. Efremidis M, Letsas KP, Lioni L, Giannopoulos G, Korantzopoulos P, Vlachos K, et al. Association of quality of life, anxiety, and depression with left atrial ablation outcomes. Pacing Clin Electrophysiol 2014;37:703–711. [DOI] [PubMed] [Google Scholar]
- 30. Sholle ET, Pinheiro LC, Adekkanattu P, Davila MA, Johnson SB, Pathak J, et al. Underserved populations with missing race ethnicity data differ significantly from those with structured race/ethnicity documentation. J Am Med Inform Assoc 2019;26:722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation 2019;140:e125–e151. [DOI] [PubMed] [Google Scholar]
- 32. Chun KRJ, Perrotta L, Bordignon S, Khalil J, Dugo D, Konstantinou A, et al. Complications in catheter ablation of atrial fibrillation in 3,000 consecutive procedures. JACC Clin Electrophysiol 2017;3:154–161. [DOI] [PubMed] [Google Scholar]
- 33. Corrado D, Zorzi A. Risk of catheter ablation for atrial fibrillation. JACC Clin Electrophysiol 2017;3:1434–1436. [DOI] [PubMed] [Google Scholar]
- 34. Stacey D, Légaré F, Lewis K, Barry MJ, Bennett CL, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2017;4:CD001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seaburg L, Hess EP, Coylewright M, Ting HH, McLeod CJ, Montori VM. Shared decision making in atrial fibrillation: where we are and where we should be going. Circulation 2014;129:704–710. [DOI] [PubMed] [Google Scholar]
- 36. Ali-Ahmed F, Pieper K, North R, Allen LA, Chan PS, Ezekowitz MD, et al. Shared decision-making in atrial fibrillation: patient-reported involvement in treatment decisions. Eur Heart J Qual Care Clin Outcomes 2020;6:263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Coylewright M, Holmes DR Jr. Caution regarding government-mandated shared decision making for patients with atrial fibrillation. Circulation 2017;135:2211–2213. [DOI] [PubMed] [Google Scholar]
- 38. Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision making. Patient Educ Couns 2014;94:291–309. [DOI] [PubMed] [Google Scholar]
- 39. Clifford AM, Ryan J, Walsh C, McCurtin A. What information is used in treatment decision aids? A systematic review of the types of evidence populating health decision aids. BMC Med Inform Decis Mak 2017;17:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Noseworthy PA, Brito JP, Kunneman M, Hargraves IG, Zeballos-Palacios C, Montori VM, et al. Shared decision-making in atrial fibrillation: navigating complex issues in partnership with the patient. J Interv Card Electrophysiol 2018;56:159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Siebenhofer A, Ulrich L-R, Mergenthal K, Berghold A, Pregartner G, Kemperdick B, et al. Primary care management for patients receiving long-term antithrombotic treatment: a cluster-randomized controlled trial. PLoS One 2019;14:e0209366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zeballos-Palacios CL, Hargraves IG, Noseworthy PA, Branda ME, Kunneman M, Burnett B, et al. Developing a conversation aid to support shared decision making: reflections on designing anticoagulation choice. Mayo Clin Proc 2019;94:686–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Man-Son-Hing M, Gage BF, Montgomery AA, Howitt A, Thomson R, Devereaux PJ, et al. Preference-based antithrombotic therapy in atrial fibrillation: implications for clinical decision making. Med Decis Making 2005;25:548–559. [DOI] [PubMed] [Google Scholar]
- 44. Thomson R, Robinson A, Greenaway J, Lowe P; DARTS Team . Development and description of a decision analysis based decision support tool for stroke prevention in atrial fibrillation. Qual Saf Health Care 2002;11:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thomson R, Parkin D, Eccles M, Sudlow M, Robinson A. Decision analysis and guidelines for anticoagulant therapy to prevent stroke in patients with atrial fibrillation. Lancet 2000;355:956–962. [DOI] [PubMed] [Google Scholar]
- 46. Speich B, von Niederhäusern B, Schur N, Hemkens LG, Fürst T, Bhatnagar N, et al. Systematic review on costs and resource use of randomized clinical trials shows a lack of transparent and comprehensive data. J Clin Epidemiol 2018;96:1–11. [DOI] [PubMed] [Google Scholar]
- 47. Scharff DP, Mathews KJ, Jackson P, Hoffsuemmer J, Martin E, Edwards D. More than Tuskegee: understanding mistrust about research participation. J Health Care Poor Underserved 2010;21:879–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals who participated in the study, as determined by the Weill Cornell Medicine Institutional Review Board. The data will be shared on reasonable request with the corresponding author.