Abstract
Declines in physiological function with aging are strongly linked to age-related diseases. Lifelong voluntary aerobic exercise (LVAE) preserves physiological function with aging, possibly by increasing cellular quality control processes, but the circulating molecular transducers mediating these processes are incompletely understood. The plasma metabolome may predict biological aging and is impacted by a single bout of aerobic exercise. Here, we conducted an ancillary analysis using plasma samples, and physiological function data, from previously reported studies of LVAE in male C57BL/6N mice randomized to LVAE (wheel running) or sedentary (SED) (n = 8–9/group) to determine if LVAE alters the plasma metabolome and whether these changes correlated with preservation of physiological function with LVAE. Physical function (grip strength, coordination, and endurance) was assessed at 3 and 18 months of age; vascular endothelial function and the plasma metabolome were assessed at 19 months. Physical function was preserved (%decline; mean ± SEM) with LVAE vs SED (all p < 0.05)—grip strength, 0.4 ± 1.7% vs 12 ± 4.0%; coordination, 10 ± 4% vs 73 ± 10%; endurance, 1 ± 15% vs 61 ± 5%. Vascular endothelial function with LVAE (88.2 ± 2.0%) was higher than SED (79.1 ± 2.5%; p = 0.03) and similar to the young controls (91.4 ± 2.9%). Fifteen metabolites were different with LVAE compared to SED (FDR < 0.05) and correlated with the preservation of physiological function. Plasma spermidine, a polyamine that increases cellular quality control (e.g., autophagy), correlated with all assessed physiological indices. Autophagy (LC3A/B abundance) was higher in LVAE skeletal muscle compared to SED (p < 0.01) and inversely correlated with plasma spermidine (r = − 0.5297; p = 0.054). These findings provide novel insight into the circulating molecular transducers by which LVAE may preserve physiological function with aging.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-024-01062-x.
Keywords: Aging, Physical function, Skeletal muscle, Endothelial function, Metabolomics, Spermidine, Autophagy
Introduction
The number of mid-life/older adults is expected to double by the year 2050 [1], and advancing age is the strongest independent risk factor for numerous diseases and disorders [2]. With advancing age, overall physiological function declines, as indicated by reductions in physical function, including skeletal muscle strength [3], coordination [4], and endurance [5], as well as vascular endothelial function [6]. Functional declines are strongly related to increased risk for the development of functional limitations, disabilities, and cardiovascular (CVD) and other chronic diseases of aging [7–9].
Cross-sectional studies of aerobic exercise (AE)-trained mid-life/older adults suggest that AE preserves physiological function into late life [10, 11] and AE interventions in mid-life/older adults can improve age-related physiological function [12–14]. Moreover, we have previously shown that AE performed over the lifespan can preserve skeletal muscle strength, coordination, endurance, and vascular endothelial function [15, 16]; however, the mechanisms mediating these responses are not completely understood.
It is not feasible to propose a longitudinal, lifelong AE study in humans; therefore, the implementation of lifelong voluntary AE (LVAE) in a translational preclinical model of aging is the most effective approach to study the direct effect of LVAE on the preservation of physiological function. Moreover, the use of a preclinical model of LVAE affords the opportunity to interrogate mechanisms mediating the preservation of physiological function with LVAE that are otherwise challenging or impossible to obtain in humans.
There is growing interest in the molecular transducers mediating the physiological benefits of AE [17, 18] and especially lifelong AE. These molecular transducers, and the mechanisms by which they impart their benefits, could provide novel insight into viable therapeutic targets for preserving physiological function throughout the lifespan. Because AE has pleiotropic physiological benefits that result in favorable changes systemically, assessing alterations to the circulating milieu in response to LVAE in relation to the preservation of physiological function represents a promising investigative approach [17]. Changes in the plasma metabolome are predictive of aging [19], and furthermore, acute AE influences the plasma metabolome [20, 21]. Therefore, assessing the plasma metabolome to capture underlying changes in metabolism that may support the preservation of physiological function in response to LVAE is an important first step in determining a mechanism by which LVAE preserves physiological function with aging [19, 22].
To address this research gap, we performed an ancillary analysis of plasma collected from mice during previously completed parent studies [15, 16] at 19 months of age after LVAE or sedentary (SED) conditions to determine the relations among LVAE, the plasma metabolome, and previously reported indices of physiological function [15, 16]. Based on our findings, we subsequently performed a molecular analysis in skeletal muscle samples from the same mice to gain insight into a potential mechanism by which LVAE may influence metabolism to preserve physiological function with aging. Taken together, our findings suggest that differences in the plasma metabolome with LVAE may act as molecular transducers to preserve physiological function with aging.
Methods
Ethical approval
All experiments were approved by the University of Colorado (CU) Boulder Institutional Animal Care and Use Committee (protocol no. 2539) and adhered to all guidelines set forth in The Guide for the Care and Use of Laboratory Animals (2016).
Overall study design and experimental animals
Twenty-six male C57BL/6N mice (Charles River, Wilmington, MA) were studied. Only male mice were studied, as male, but not female, mice of this strain consistently exhibit the key features of age-related physical [15, 23–26] and vascular [27–29] dysfunction as humans. All experimental animals were obtained at 8–10 weeks of age and allowed to acclimate to our facilities for at least 2 weeks prior to the beginning of study procedures. This cohort was assigned to the two treatment groups described below and followed over time. Mice were singly housed in standard cages in the CU Boulder vivarium, maintained on a 12:12 h light/dark cycle, and permitted ad libitum access to chow and drinking water. Daily animal health checks were performed by laboratory or institutional laboratory animal staff, under the supervision of the institutional veterinarian.
Treatment groups and diet
At 3 months of age, mice were assigned based on and to control for body weight to one of two groups: (1) LVAE (voluntary wheel running) or (2) SED (no running wheel) (n = 8–9 per group). Because the measure of vascular endothelium-dependent dilation is a terminal measure, a young reference group (6 months of age; n = 9) [16] was also included to assess the effect of LVAE on this measure with aging. The chow was an irradiated, fixed, and open rodent chow (Inotiv/Envigo 7917), and the drinking water consisted of Boulder, CO municipal tap water that underwent reverse osmosis and chlorination.
Voluntary aerobic exercise and assessment of physiological function
Mice assigned to LVAE were provided with ad libitum access to a running wheel in their home cage (Ware Pet Products small flying saucer, product number 03281); mice were placed in specialized cages (Lafayette Instruments, Lafayette, IN, USA) equipped with running wheels attached to electronic monitoring software (Activity Wheel Monitoring, Lafayette, IN) to enable the quantification of running volume [30–33]. Running distance was recorded for a continuous 72-h period at 6 and 19 months of age (see “Results”). All mice assigned to LVAE in this study performed LVAE until 19 months of age, at which time they were euthanized to assess vascular endothelial function. Prior to euthanasia, at 3 and 18 months of age, these same mice were assessed for physical function. Vascular endothelial function was assessed at 19 months of age given the time required to assess physical function (i.e., assessments were made from 18 to 19 months of age). A detailed description of the study design and intervention can be found in previously published manuscripts [15, 16].
Physical function testing, consisting of forelimb grip strength, coordination, and endurance, was performed as previously described by our laboratory [15, 23–26] at 3 and 18 months of age in the same animals. The use of rota-rod to assess coordination and endurance is a traditional and established method [34–36]. The assessment of coordination and endurance via the rota-rod is considered an important component of overall motor function testing [37]. Carotid artery endothelial function [vascular endothelium-dependent dilation (EDD), peak EDD (%)] was also assessed via pressure myography, as we have previously described [27, 28, 32, 38]. The assessment of vascular endothelial function was conducted immediately post-euthanasia at 6 and 19 months of age. For a detailed description of the physiological function assessments please see “Supplemental Methods”.
All testing sessions occurred in the afternoon hours of the light cycle (11 am to 5 pm), and each test occurred at the same time of day with little variation within or across cohorts. The experimental apparati were cleaned with ethanol between the testing of each mouse. Body weight was not observed to be different at either 3 or 18 months of age (see “Results”); therefore, indices of physical function are reported in absolute values.
Plasma collection and metabolomics
Whole blood was collected via cardiac puncture with a heparin-coated needle at the time of euthanasia as performed previously by our laboratory [25, 39]. Blood was centrifuged at 1000 × g for 10 min and plasma was separated and immediately snap-frozen in liquid nitrogen. We performed a targeted metabolomics analysis on plasma from LVAE and SED animals as previously described [25, 39] and briefly summarized below.
Plasma metabolomics analysis
Plasma aliquots were thawed on ice; then, 20 µL was diluted with 480 µL of ice-cold methanol/acetonitrile/water (5/3/2). Extractions were performed and resulting samples were analyzed using a 5-min C18 gradient on a Thermo Vanquish-Q Exactive system (San Jose, CA, USA), as previously described [40].
Lipidomics sample preparation
Samples were thawed on ice, and 10 µL aliquots were diluted with 90 µL of ice-cold methanol. Suspensions were vortexed and placed at − 20 °C for 30 min. Insoluble material was removed by centrifugation at 12,000 g for 10 min at 4 °C. Supernatants were isolated and diluted 1:1 (v/v) with 10 mMol/L ammonium acetate for analysis by ultra-high pressure LC–MS (UHPLC-MS) [41].
UHPLC-MS lipid analysis
Samples were analyzed on a Thermo Vanquish UHPLC system (San Jose, CA, USA) coupled online to a Thermo Q Exactive mass spectrometer (Bremen, Germany). Lipids were resolved over a Waters ACQUITY HSS T3 column (2.1 × 150 mm, 1.8 µm) using an aqueous phase (A) of 25% acetonitrile and 5 mM ammonium acetate and a mobile phase (B) of 50% isopropanol, 45% acetonitrile, and 5 mMol/L ammonium acetate. Lipids were eluted from the column using the solvent gradient as follows: 0–1 min, 25% B and 0.3 mL/min; 1–2 min, 25–50% B and 0.3 mL/min; 2–8 min, 50–90% B and 0.3 mL/min; 8–10 min, 90–99% B and 0.3 mL/min; 10–14 min, hold at 99% B and 0.3 mL/min; 14–14.1 min, 99–25% B and 0.3 mL/min; 14.1–16.9 min, hold at 25% B and 0.4 mL/min; 16.9–17 min, hold at 25% B and resume flow of 0.3 mL/min. The mass spectrometer was operated in negative ion mode, scanning in Full MS mode (2 µ-scans) from 150 to 1500 m/z at 70,000 resolution, with 4 kV spray voltage, 45 sheath gas, and 15 auxiliary gas. Samples were analyzed in randomized order with a technical mixture injected incrementally to qualify instrument performance. Acquired data was then converted from.raw to.mzXML file format using the RawConverter. Metabolites were assigned using Maven (Princeton, NJ, USA) [42, 43].
Metabolic pathway analysis
Kyoto Encyclopedia of Genes and Genomes (KEGG) metabolic enrichment analysis was performed using the Metaboanalyst 5.0 software [44]. Metabolites meeting both parameters of false discovery rate (FDR)-adjusted p-value < 0.05 and Log2 Fold Change ± 1 relative to SED were considered significant and utilized for KEGG enrichment analysis. Pathways were considered significant if they met an FDR-adjusted p-value of < 0.2. A total of 105 metabolites were identified in our targeted analysis. The FDR-adjusted p-value and Log2 Fold change values for each analyzed metabolite are reported in the Supplemental Table. For a more detailed description of the processing and analysis of the raw metabolomic values please see the “Statistical Analysis” section below.
Cell culture
Human aortic endothelial cells (HAECs; Promocell) were cultured in EGM-2 complete media (Lonza) at 37 °C and 5% CO2 as previously descibed [45]. To validate the specificity of our LC3A/B antibody to increases in autophagy, we used a well-established method whereby cells were treated for 24 h with increasing concentrations of chloroquine diphosphate salt (Sigma) [46, 47] (Supplemental Figure. 1). HAECs were washed twice with HBSS and lysed in a radio-immunoprecipitation (RIPA) buffer. Lysates were sonicated at 20% amplitude for 30 s. Lysates were then clarified and measured for protein content as described above. We validated the LC3A/B antibody in HAECs as these are the cells we have readily available in our laboratory.
Mouse quadricep protein quantification
Quadriceps tissue was mechanically homogenized using a Bullet Blender in a RIPA lysis buffer (Millipore) supplemented with complete protease and phosphatase inhibitor cocktails (Roche). Lysates were clarified via centrifugation at 10,000 × rpm for 10 min at 4 °C. Protein content was quantified using a bicinchoninic acid assay (Thermo Fisher). The abundance of LC3A/B (cell signaling #12741) was measured by the Jess, a capillary-based automated western blot quantitative analyzer (Biotechne). Lysates were loaded at 0.32 mg/mL and probed with an antibody diluted at 1:50. A RePlex protocol was performed according to the manufacturer’s instructions to subsequently probe the capillaries for total protein content (Biotechne). Peak area quantification was performed using the Compass software (Biotechne), and the peak area of the protein of interest was normalized to the total protein area. One sample in the LVAE group was removed due to a technical error indicated by the Jess. We performed this measure in SED and LVAE quadriceps skeletal muscle only as aortic lysates/tissue no longer remained from the original study. The full total protein and protein of interest (LC3A/B) blots to quantify relative LC3A/B in skeletal muscle (Fig. 5A) can be found in Supplemental Figure. 2.
Fig. 5.

A Assessment of LC3A/B (marker of autophagy) protein normalized to total protein in quadriceps skeletal muscle after sedentary (SED) and lifelong voluntary aerobic exercise (LVAE) conditions in 19-month-old male mice (N = 7/condition). Representative LC3A/B protein content and total protein for each SED and LVAE are provided. Student’s t-test; **p < 0.01. AU arbitrary units. B Spearman correlation between LC3A/B abundance in quadriceps skeletal muscle and circulating spermidine in 19-month-old male mice following SED (black) and LVAE (gold) (N = 7/condition)
Statistical analyses
For plasma metabolomics data, we tested raw values for normality by using the Shapiro–Wilk test. When one or both groups were nonparametric, comparisons were performed using the Mann–Whitney U-test. For parametric data, equality of variances was tested using the F-test, and groups with unequal variances were tested using Welch’s modified T-test. Groups with equal variances were tested using the student’s t-test. We utilized the Grubbs test to identify outliers (α = 0.05). Raw p-values were then adjusted for false discovery rate (FDR) using the Benjamani–Hochberg test. Of the reported significant metabolites [meeting both parameters of FDR-adjusted p-value < 0.05 and Log2 Fold Change ± 1 (relative to SED)] in Fig. 1C, outliers were identified and removed in the same LVAE mouse for malate, fumarate, and eicosatetraenoic acid. An outlier was also identified and removed in a single SED mouse for spermidine. All metabolomic data are expressed relative to the mean or median (as appropriate) SED value for that metabolite. Changes in indices of physical function were analyzed utilizing two-way repeated-measure ANOVAs, with main effects of group (SED vs LVAE) and time [baseline (3 months) vs 19 months]. Peak EDD was analyzed using a one-way ANOVA with Tukey’s multiple comparisons test. Relations between abundance of significant plasma metabolites and indices of physiological function were analyzed by Pearson correlation. Distributions were tested for normality using the Shapiro–Wilk test. When one or both groups were nonparametric, correlations were performed using the Spearman statistic. Protein abundance of LC3A/B was tested using the student’s t-test. The Grubbs test identified an outlier for LC3A/B within the LVAE group (N = 7/condition). Relations between circulating spermidine and skeletal muscle LC3A/B were analyzed by Spearman correlation. Distributions using the Shapiro–Wilk normality test were found to be significantly skewed (p = 0.0002). Statistics and visual and graphical representations of data were calculated and created using the Graph Pad Prism version 8 (Graph Pad Software, La Jolla, CA).
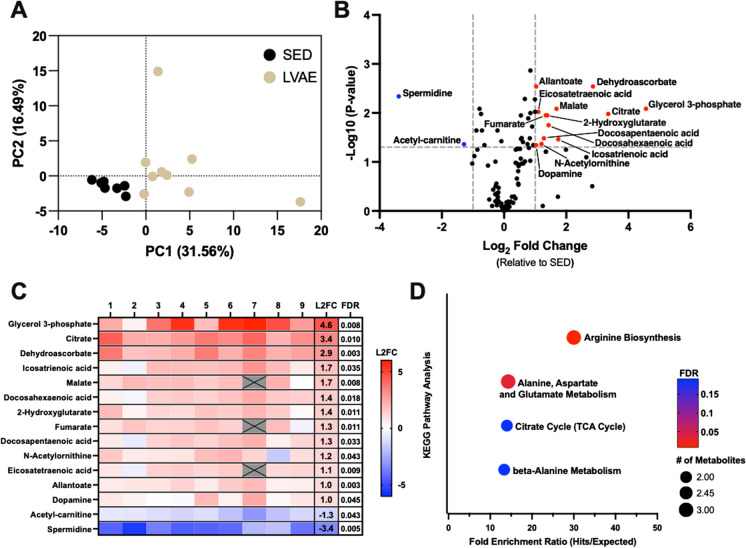
Fig. 1.
A Principal component analysis performed on raw metabolomics data in sedentary (SED) and lifelong voluntary aerobic exercise (LVAE) groups. B Volcano plot illustrating the impact of LVAE on plasma metabolites. Significance was considered to meet both parameters of a false discovery rate (FDR)-adjusted p-value (Benjamani–Hochberg) < 0.05 and Log2 Fold change (L2FC) ± 1 (relative to SED). Thirteen metabolites were found higher (red) and two lower (blue) with LVAE. C Heat map of individual changes for each of the 15 significantly altered plasma metabolites observed with LVAE normalized to SED (red = higher in LVAE; blue = lower in LVAE). LVAE group changes normalized to SED for each metabolite are shown in the L2FC column. FDR-adjusted p-values are reported in the last column. Gray boxes with X’s indicate identified and removed outliers. D KEGG enrichment analysis performed with Metaboanalyst using the 15 significant metabolites shown in “C”. Circle size represents the number of metabolite hits; the color of circles represents FDR-adjusted p-value; fold enrichment ratio is the number of metabolite hits within a pathway relative to expected metabolite hits by random chance. All pathways met an FDR-adjusted p-value < 0.2
Results
Running distances with LVAE and body weight characteristics
At 6 months of age, LVAE mice ran an average of 10,927 ± 1546 m/day. At 19 months of age (~ 60 years of age in humans), LVAE mice ran an average of 4,113 ± 791 m/day (mean ± SEM). The age-related decline in voluntary wheel running distance is consistent with prior observations [48, 49]. At the time of randomization (3 months), the average body weight was similar between SED (26.88 g ± 1.1) and LVAE (28.9 g ± 1.26) (p = 0.25). At 19 months of age, SED (28.44 g ± 0.92) and LVAE (28.35 g ± 1.18) body weight remained similar between groups (p = 0.82).
LVAE alters the plasma metabolome
To determine if LVAE modulated the plasma metabolome, we quantified plasma abundance of 105 metabolites in 19-month-old male mice after SED or LVAE conditions. Principal component analysis indicated a differential abundance of plasma metabolites following SED vs LVAE conditions (Fig. 1A). In-depth interrogation of this phenotype uncovered 15 individual metabolites to be different with LVAE (FDR-adjusted p < 0.05 and Log2FC ± 1); 13 metabolites were higher (in red) following LVAE, and 2 were lower (in blue). The largest increase and decrease in abundance of individual plasma metabolites with LVAE was glycerol-3-phopshate and spermidine, respectively (Fig. 1B and C).
To better understand the metabolic pathways and physiological processes associated with the changes in the 15 individual metabolites, we performed KEGG enrichment analysis using Metaboanalyst and found that four metabolic pathways were involved (all FDR-adjusted p-value < 0.2) (Fig. 1D). The identified pathways were associated with the regulation of nitric oxide signaling (arginine biosynthesis), skeletal muscle and central nervous system energy production (alanine, aspartate, and glutamate metabolism), aerobic energy production (citrate cycle), and exercise capacity and muscular endurance (beta-alanine metabolism).
LVAE preserves physical and vascular endothelial functions
For this ancillary analysis, we utilized previously measured indices of physical function (e.g., grip strength, coordination, and endurance) at 3 months and 18 months of age and vascular endothelial function at 19 months of age, as it is a terminal measure, in mice from the parent preclinical studies [15, 16]. Only mice that had all physiological function indices assessed and plasma metabolomics quantified were included. Since body weight was not different at either the 3- or 18-month age time points, all indices of physiological function have been reported in absolute values.
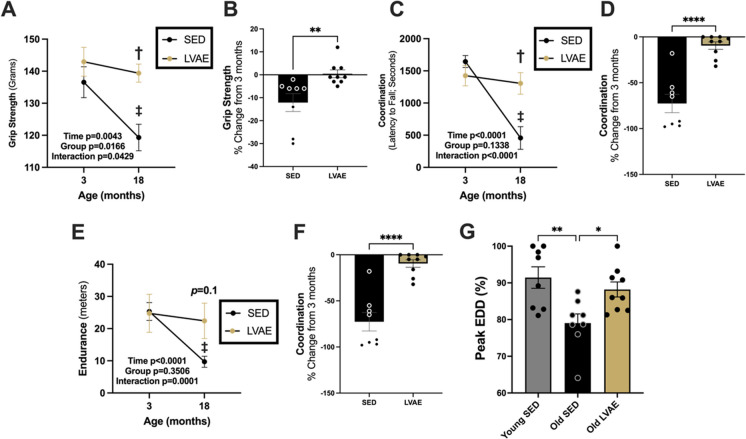
At 3 months of age, there were no differences between groups in grip strength (SED, 136.6 ± 4.8 g (mean ± SEM); LVAE, 142.9 ± 4.5 g; p = 0.48; Fig. 2A), coordination (SED, 1645 ± 92.3 s; LVAE, 1426 ± 159.0 s; p = 0.54; Fig. 2C), or endurance (SED, 25.33 ± 2.8 m; LVAE, 24.8 ± 5.9 m; p = 0.99; Fig. 2E); however, at 18 months of age, grip strength (SED, 119.3 ± 4.1 g; LVAE, 139.4 ± 2.8 g; p = 0.003; Fig. 2A) and coordination (SED, 456.8 ± 176.4 s; LVAE, 1306 ± 167.6 s; p = 0.001; Fig. 2C) were significantly lower in SED compared to LVAE; similarly, endurance tended to be lower at 18 months of age (SED, 9.7 ± 1.727 m; LVAE, 22.4 ± 5.5 m; p = 0.112; Fig. 2E). Within group, grip strength (SED, − 17.25 ± 6.0 g, p = 0.003; LVAE, − 3.56 ± 2.42 g, p = 0.66; Fig. 2A), coordination (SED, − 1188.13 ± 184.3 s, p < 0.0001; LVAE, − 120.4 ± 51.03 s, p = 0.58; Fig. 2C), and endurance (SED, -15.625 ± 2.0 m, p < 0.0001; LVAE, − 2.33 ± 1.63 m, p = 0.38; Fig. 2E) significantly declined in SED from 3 to 18 months of age, whereas physical function was preserved with aging following LVAE. Between groups, we found that the relative change in physical function from 3 to 18 months of age was greater in SED compared to LVAE: grip strength, − 12 ± 4.0% vs − 0.4 ± 1.7% (p = 0.0074; Fig. 2B); coordination, − 73 ± 10% vs − 10 ± 4% (p < 0.0001; Fig. 2D); and endurance, − 61 ± 5% vs − 1 ± 15% (p = 0.0024; Fig. 2F). Lastly, vascular endothelial function, measured as a percent of peak EDD, was higher in 19-month-old mice following LVAE (88.2 ± 2.0%) compared to SED (79.1 ± 2.5%) (p = 0.03) and similar to a young 6-month-old sedentary male mice reference group previously published in the parent study [16] (91.4 ± 2.9%) (p = 0.63) (Fig. 2G).
Fig. 2.
Assessment of physical function (A grip strength; C coordination; E endurance) in male mice at 3 months and then at 18 months of age following sedentary (SED, black) or lifelong voluntary aerobic exercise (LVAE, gold). P < 0.05 compared with (‡) 3 months within groups and (†) between groups at 18 months. Two-way repeated measures ANOVA. Percent (%) change in physical function (B grip strength; D coordination; F endurance) at 18 months of age compared to 3 months of age in SED and LVAE. Unpaired t-test. G Vascular endothelial function assessed as percent (%) peak endothelium-dependent dilation (EDD) following SED or LVAE at 19 months of age (Old SED, Old LVAE) or in a Young SED reference group at 6 months of age. The Young SED reference group was previously published in the parent study [16] and repurposed here to perform this specific analysis. One-way ANOVA, Tukey’s post-hoc. *p < 0.05; **p < 0.01; ****p < 0.0001. All data mean ± SEM
Abundance of plasma metabolites correlate with preservation of physiological function with LVAE
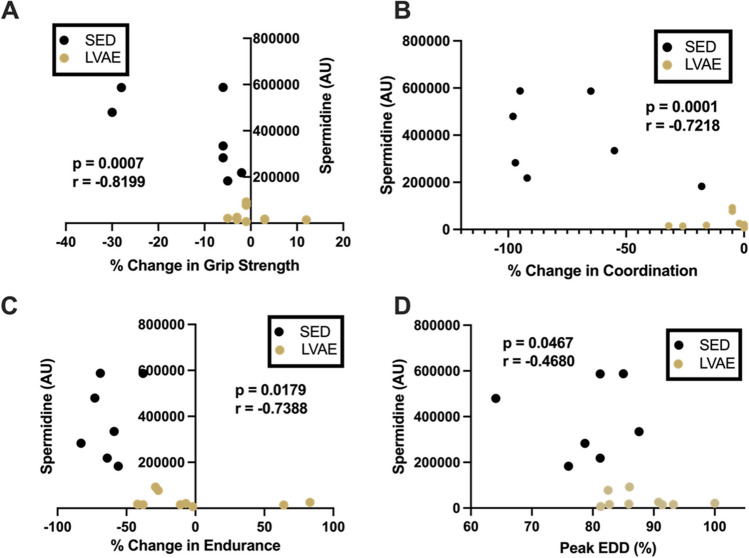
We next examined if the 15 individual plasma metabolites that differed with LVAE were correlated with changes, or lack thereof, in physical function with aging or vascular endothelial function in late life. Of the 15 metabolites, 10 correlated (all p < 0.05) with 1 or more of the indices of physiological function (grip strength, 4 metabolites; coordination, 10 metabolites; endurance, 5 metabolites, and peak EDD, 1 metabolite) (Fig. 3). One metabolite, spermidine (a polyamine implicated in the modulation of autophagy [50] and angiogenesis [51]), was the only metabolite to correlate with all 4 indices of physiological function (Fig. 4A–D).
Fig. 3.

Significant Pearson or Spearman correlations between abundance of significant plasma metabolites and (1) percentage change in indices of physical function (grip strength, coordination, endurance) or (2) vascular endothelial function [percent (%) peak endothelium-dependent dilation (EDD)]. Numbers and color gradients correspond with r values. *p < 0.05; **p < 0.01; ***p < 0.001
Fig. 4.
Spearman correlations between plasma spermidine and percentage change in indices of physical function (A grip strength; B coordination; C endurance) and D vascular endothelial function [percent (%) peak endothelium-dependent dilation (EDD)]. Sedentary (black, SED); lifelong voluntary aerobic exercise (gold, LVAE). AU arbitrary units
LVAE increases markers of autophagy in skeletal muscle
To determine if reductions in circulating spermidine with LVAE may be associated with higher levels of autophagy (levels of skeletal muscle autophagy decline with sedentary aging [52, 53] and can be rescued with exercise in older adults [54]), we quantified protein abundance of LC3A/B, an established marker of autophagy [55, 56], in quadriceps skeletal muscles collected after SED or LVAE (n = 7/condition). We chose quadriceps skeletal muscle as they are heavily engaged during voluntary wheel running. LC3A/B was ~ 30% higher in quadriceps skeletal muscle from LVAE (17.5 ± 1.1 AU) compared to SED (13.7 ± 0.5 AU) (p = 0.0092) (Fig. 5A). There was a trend toward a moderate inverse correlation between abundance of LC3A/B in quadriceps skeletal muscle and circulating concentrations of spermidine (r = − 0.5297; p = 0.0544) (Fig. 5B).
Discussion
This study showed that (1) the direct effect of LVAE on the composition of the plasma metabolome, (2) changes in the plasma metabolome with LVAE are associated with LVAE-mediated preservation of physiological function in aged mice, and (3) LVAE reduced circulating concentrations of spermidine and stimulated autophagy in skeletal muscle following LVAE. These findings are the first to identify plasma metabolites that may be circulating molecular transducers by which LVAE confers its beneficial effects on physiological function with aging and may potentially do so by increasing levels of autophagy in skeletal muscle.
Of the 105 metabolites evaluated in our targeted plasma metabolomics analysis, 15 were different (13 increased; 2 decreased) following LVAE. Unbiased enrichment analysis elucidated that these 15 metabolites are implicated in pathways strongly associated with the modulation of our assessed indices of physiological function [e.g., arginine biosynthesis (nitric oxide signaling) and citrate cycle (AE-related energy production)]. These findings provided initial evidence that the general changes we observed in the plasma metabolome with LVAE are possibly circulating molecular transducers by which LVAE preserves physiological function with aging. These findings may also indicate the potential pathways by which habitual exercise training over the lifespan may impart its beneficial effects on physiological function with aging at the molecular level. As such, these pathways could be thought of as putative therapeutic targets to promote healthy aging in humans.
Some of the individual metabolites we found to be altered in response to LVAE (e.g., spermidine) have been shown to beneficially modulate physiological function with aging in human and preclinical studies following exogenous supplementation [27, 51, 57]. The present study sought to interrogate changes in endogenous concentrations of plasma metabolites with LVAE and their relation with the preservation of physiological function with aging. These indices of physiological function are biomedically relevant as the human corollaries of each index are strong independent predictors for risk of future disability, dependence, and/or cardiovascular disease and overall mortality [58–61]. Together, we found that the changes in 10 of the 15 circulating metabolites altered with LVAE correlated with the preservation of physiological function following LVAE in at least one of our assessed indices. Relations between changes in the circulating milieu and primary outcome measures are more robust in this study compared to previous investigations in humans (5–7 relations) performed by our laboratory [62–64]. These findings suggest that changes in individual circulating metabolites important to the modulation of our assessed indices of physiological function may also serve as predictive biomarkers of the preservation of the same indices following LVAE.
One of the metabolites found to be changed with LVAE, spermidine, correlated with all four indices of physiological function. The observed relation between abundance of spermidine and the relative changes of physiological function with SED or LVAE with aging are clustered in a manner expected if we studied differences in young and old cohorts. This clustering may exhibit the impact of LVAE on the plasma metabolome (e.g., spermidine) and its capacity to act as a molecular transducer mediating the beneficial effects of LVAE in similar-aged male mice.
Spermidine is a polyamine known for its role in regulating autophagy (by inhibiting the acetyltransferase, EP3000, a main inhibitor of autophagy) [50, 65] and, more recently, angiogenesis [51]. With healthy aging, circulating [50, 66] and skeletal muscle [67, 68] levels of spermidine and skeletal muscle autophagy [52, 53] decline in association with impairments in physiological function. Following acute exercise, abundance of spermidine also declines in circulation [20] and skeletal muscle (beyond the decline observed with aging alone) [67] in young endurance-trained humans and older rats with high aerobic capacity, respectively. But unlike primary aging, skeletal muscle autophagy is increased in this setting [54, 69] as it is required for skeletal muscle adaptations to exercise [69]. Although not measured in these studies, it is feasible that changes in the levels of spermidine and autophagy with exercise are possibly in response to increased uptake and enhanced utilization of intramuscular spermidine to promote a greater, exercise-induced need for autophagy. However, little is known about the uptake and utilization of spermidine by mammalian skeletal muscle [70], and it is a topic that requires further investigation [70].
As such, we hypothesized that lower circulating spermidine with LVAE may suggest higher levels of skeletal muscle autophagy compared to SED to support adaptations to LVAE [69]. Indeed, we found that abundance of LC3A/B (a marker of autophagy) [55, 56] was higher in the skeletal muscle of mice that underwent LVAE compared to SED. As stated above, autophagy is an established aging hallmark [71] and is generally reduced with sedentary aging. This lowers the capacity of aged skeletal muscle to remove and recycle damaged organelles and structures. This disrupts cellular homeostasis which contributes to physiological dysfunction with aging. With lifelong aerobic exercise in mice, autophagy is higher in skeletal muscle, suggesting more effective cellular quality control within skeletal muscle that likely promotes preservation of physiological function with aging. There was also a trend toward a moderate inverse correlation between plasma spermidine and skeletal muscle autophagy. These findings supported our hypothesis and suggested that lower circulating abundance of spermidine following LVAE may be associated with levels of autophagy in skeletal muscle following LVAE in mice. Recent studies show that supplementation with spermidine can promote angiogenesis [51] and improve endothelial function in old mice [72]. Although untested in this current study, it is plausible that in addition to higher levels of autophagy in skeletal muscle, LVAE-mediated reductions in circulating spermidine may also contribute to angiogenesis (a well-known adaptation to AE [73]) and preserved vascular endothelial function with aging by maintaining higher levels of autophagy.
In addition to spermidine, several other metabolites found to be altered with LVAE have previously been implicated in favorable modulation of physiological function. The largest magnitude change in our data set was an increase in circulating glycerol-3-phosphate. Greater biosynthesis of glycerol-3-phosphate restores NAD+ bioavailability, which is reduced with aging [74, 75], by exploiting the glycerol-3-phosphate shuttle at the level of the mitochondria to improve mitochondrial function in a model of accelerated mitochondrial aging [76] (e.g., mitochondrial complex I dysfunction [77]). Increased circulating omega-3 fatty acid docosahexaenoic acid was also observed with LVAE. Docosahexaenoic acid affects cell physiology by improving membrane structure and function and subsequent cellular signaling [78]. Supplementation with docosahexaenoic acid has improved skeletal muscle aging by upregulating autophagy in a mouse model of sarcopenia [79], improved cognitive function [80], and reduced circulating inflammatory markers [57] in older adults. Many other LVAE-mediated changes in circulating metabolites (e.g., citrate, malate, fumarate) support citrate cycle–related [81] energy production needed to execute LVAE or related to nitric oxide signaling to modulate vascular function [82, 83] (e.g., N-acetylornithine). Taken together, changes in these circulating metabolites with LVAE may play a contributing role in the pleiotropic and systemic favorable effects of AE on physiological function with aging.
As we have stated, aerobic exercise and/or spermidine supplementation have been found to enhance autophagy to improve physiological function or cellular quality control, namely in preclinical models [27, 69]. To translate these promising findings, cross-sectional studies that compare the circulating metabolome between lifelong aerobic exercise–trained older adults (men and women) to sedentary older adults are warranted. Another option would be to perform intervention studies that investigate chronic supplementation with a metabolite found altered in the cross-sectional study or to assess the circulating metabolome following an aerobic exercise intervention in older adults to determine if the circulating metabolome can be similarly altered with chronic aerobic exercise later in life. These are prudent next steps in this line of research as lifelong human aerobic exercise studies are not feasible.
Experimental considerations
Our study was conducted in only male mice, as male, but not female, mice of this strain consistently exhibit the key features of age-related physical [15, 23–26] and vascular [27–29] dysfunction as humans. Also, we utilized heparin to perform blood collection. Importantly, studies that compared different methods of blood collection prior to the analysis of the metabolome indicated that plasma collected with heparin and serum-based collection methods resulted in similar metabolomic profiles [84, 85]. Also, we only interrogated changes in the plasma metabolome with LVAE. Future studies should investigate the relation between LVAE and changes in the plasma proteome or consider changes in the epigenome and transcriptome within skeletal muscle or the vasculature. Lastly, we did not determine the role of spermidine uptake and utilization by skeletal muscle to facilitate increased skeletal muscle autophagy with LVAE. Future studies should assess mechanistically this relation with LVAE.
Conclusions
Here, we demonstrate that LVAE alters the plasma metabolome and that changes in the plasma metabolome with LVAE are associated with LVAE-mediated preservation of physiological function. Furthermore, our findings indicate that preservation of physiological function with LVAE is associated with a reduction in circulating abundance of spermidine, which may promote increased autophagy in skeletal muscle. These novel observations suggest that changes in plasma metabolites with LVAE may contribute as molecular transducers by which AE beneficially modulates physiological function with aging.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplemental Figs. 1 and 2, the complete list of assessed plasma metabolites (and fold change and FDR-adjusted p-values), and the supplemental methods can be found here: https://doi.org/10.6084/m9.figshare.23699265. (PDF 323 KB)
Acknowledgements
We thank Jill Miyamoto-Ditmon for her assistance with data collection.
Author contribution
K.O.M., G.S.M., and Z.S.C. conceived and designed the research, performed experiments, analyzed data, interpreted results of experiments, prepared figures, drafted the manuscript, edited and revised the manuscript, and approved the final version of the manuscript. R.A.G. and M.C.Z. conceived and designed the research, performed experiments, analyzed data, interpreted results of experiments, edited and revised the manuscript, and approved the final version of the manuscript. K.R.L. performed experiments, edited and revised the manuscript, and approved the final version of the manuscript. A.D. and J.A.R. performed experiments, edited and revised the manuscript, and approved the final version of the manuscript. M.J.R. edited and revised the manuscript and approved the final version of the manuscript. D.R.S. conceived and designed research, acquired funding, interpreted the results of experiments, edited and revised the manuscript, and approved the final version of the manuscript.
Funding
This work was supported by the National Institutes of Health Grants R01HL107120 (DRS), 5T32KD007135 (KOM & ZSC), F31AG047784 (RGR), K01DK115524 (MJR), F32HL167552 (KOM), and F32HL151022 & K99HL159241 (ZSC) and the American Heart Association grants 23POST1025630 (KOM) (10.58275/AHA.23POST1025630.pc.gr.161298) and 23CDA1056582 (MJR) (10.58275/AHA.23CDA1056582.pc.gr.168037).
Data availability
Data will be made available upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Kevin O. Murray and Grace S. Maurer are co-first authors.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Federal Interagency Forum on Aging-Related Statistics. U.S. Government Bookstore https://bookstore.gpo.gov/agency/federal-interagency-forum-aging-related-statistics.
- 2.Olshansky SJ, Goldman DP, Zheng Y, Rowe JW. Aging in America in the twenty-first century: demographic forecasts from the MacArthur Foundation Research Network on an Aging Society. Milbank Q. 2009;87:842–862. doi: 10.1111/j.1468-0009.2009.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Celis-Morales CA, et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ. 2018;361:k1651. doi: 10.1136/bmj.k1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tieland M, Trouwborst I, Clark BC. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle. 2018;9:3–19. doi: 10.1002/jcsm.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bemben MG. Age-related alterations in muscular endurance. Sports Med. 1998;25:259–269. doi: 10.2165/00007256-199825040-00004. [DOI] [PubMed] [Google Scholar]
- 6.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 2011;120:357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rantanen T, et al. Midlife muscle strength and human longevity up to age 100 years: a 44-year prospective study among a decedent cohort. Age (Dordr) 2012;34:563–570. doi: 10.1007/s11357-011-9256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy BK, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray KO, Mahoney SA, Venkatasubramanian R, Seals DR, Clayton ZS. Aging, aerobic exercise, and cardiovascular health: Barriers, alternative strategies and future directions. Exp Gerontol. 2023;173:112105. doi: 10.1016/j.exger.2023.112105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierce GL, et al. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell. 2011;10:1032–1037. doi: 10.1111/j.1474-9726.2011.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci (Lond) 2011;120:13–23. doi: 10.1042/CS20100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeSouza CA, et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.CIR.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 14.Matsubara T, et al. Aerobic exercise training increases plasma Klotho levels and reduces arterial stiffness in postmenopausal women. Am J Physiol Heart Circ Physiol. 2014;306:H348–355. doi: 10.1152/ajpheart.00429.2013. [DOI] [PubMed] [Google Scholar]
- 15.Clayton ZS, et al. Lifelong physical activity attenuates age- and Western-style diet-related declines in physical function and adverse changes in skeletal muscle mass and inflammation. Exp Gerontol. 2022;157:111632. doi: 10.1016/j.exger.2021.111632. [DOI] [PubMed] [Google Scholar]
- 16.Gioscia-Ryan RA, et al. Lifelong voluntary aerobic exercise prevents age- and Western diet- induced vascular dysfunction, mitochondrial oxidative stress and inflammation in mice. J Physiol. 2021;599:911–925. doi: 10.1113/JP280607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanford JA, et al. Molecular transducers of physical activity consortium (MoTrPAC): mapping the dynamic responses to exercise. Cell. 2020;181:1464–1474. doi: 10.1016/j.cell.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Group MS, et al. Temporal dynamics of the multi-omic response to endurance exercise training across tissues. 2023. bioRxiv 2022.09.21.508770.
- 19.Johnson LC, et al. The plasma metabolome as a predictor of biological aging in humans. Geroscience. 2019;41:895–906. doi: 10.1007/s11357-019-00123-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabone M, et al. The effect of acute moderate-intensity exercise on the serum and fecal metabolomes and the gut microbiota of cross-country endurance athletes. Sci Rep. 2021;11:3558. doi: 10.1038/s41598-021-82947-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morville T, Sahl RE, Moritz T, Helge JW, Clemmensen C. Plasma metabolome profiling of resistance exercise and endurance exercise in humans. Cell Rep. 2020;33:108554. doi: 10.1016/j.celrep.2020.108554. [DOI] [PubMed] [Google Scholar]
- 22.Lewis GD, et al. Metabolic signatures of exercise in human plasma. Sci Transl Med. 2010;2:33ra37. doi: 10.1126/scitranslmed.3001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Justice JN, et al. Sodium nitrite supplementation improves motor function and skeletal muscle inflammatory profile in old male mice. J Appl Physiol. 2015;1985(118):163–169. doi: 10.1152/japplphysiol.00608.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Justice JN, et al. Battery of behavioral tests in mice that models age-associated changes in human motor function. Age (Dordr) 2014;36:583–592. doi: 10.1007/s11357-013-9589-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballak DB, et al. Short-term interleukin-37 treatment improves vascular endothelial function, endurance exercise capacity, and whole-body glucose metabolism in old mice. Aging Cell. 2020;19:e13074. doi: 10.1111/acel.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunt VE, et al. Suppression of trimethylamine N-oxide with DMB mitigates vascular dysfunction, exercise intolerance, and frailty associated with a Western-style diet in mice. J Appl Physiol. 2022;133:798–813. doi: 10.1152/japplphysiol.00350.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaRocca TJ, Gioscia-Ryan RA, Hearon CM, Seals DR. The autophagy enhancer spermidine reverses arterial aging. Mech Ageing Dev. 2013;134:314–320. doi: 10.1016/j.mad.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sindler AL, et al. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell. 2011;10:429–437. doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleenor BS. Large elastic artery stiffness with aging: novel translational mechanisms and interventions. Aging Dis. 2013;4:76–83. [PMC free article] [PubMed] [Google Scholar]
- 30.Durrant JR, et al. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-β1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol. 2010;588:3971. doi: 10.1113/jphysiol.2010.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lesniewski LA, et al. Aging compounds western diet-associated large artery endothelial dysfunction in mice: prevention by voluntary aerobic exercise. Exp Gerontol. 2013;48:1218–1225. doi: 10.1016/j.exger.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gioscia-Ryan RA, et al. Late-life voluntary wheel running reverses age-related aortic stiffness in mice: a translational model for studying mechanisms of exercise-mediated arterial de-stiffening. GeroScience. 2021;43:423–432. doi: 10.1007/s11357-020-00212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deacon RM. Measuring motor coordination in mice. J Vis Exp. 2013;(75):e2609. [DOI] [PMC free article] [PubMed]
- 35.Jones BJ, Roberts DJ. The quantiative measurement of motor inco-ordination in naive mice using an acelerating rotarod. J Pharm Pharmacol. 1968;20:302–304. doi: 10.1111/j.2042-7158.1968.tb09743.x. [DOI] [PubMed] [Google Scholar]
- 36.Dunham NW, Miya TS. A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc Am Pharm Assoc. 1957;46:208–209. doi: 10.1002/jps.3030460322. [DOI] [PubMed] [Google Scholar]
- 37.Shiotsuki H, et al. A rotarod test for evaluation of motor skill learning. J Neurosci Methods. 2010;189:180–185. doi: 10.1016/j.jneumeth.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 38.Gioscia-Ryan RA, et al. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol. 2014;592:2549–2561. doi: 10.1113/jphysiol.2013.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clayton ZS, et al. Doxorubicin-induced oxidative stress and endothelial dysfunction in conduit arteries is prevented by mitochondrial-specific antioxidant treatment. JACC CardioOncol. 2020;2:475–488. doi: 10.1016/j.jaccao.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gehrke S, et al. Red blood cell metabolic responses to torpor and arousal in the hibernator arctic ground squirrel. J Proteome Res. 2019;18:1827–1841. doi: 10.1021/acs.jproteome.9b00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Alessandro A, et al. Effects of aged stored autologous red blood cells on human plasma metabolome. Blood Adv. 2019;3:884–896. doi: 10.1182/bloodadvances.2018029629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nemkov T, Hansen KC, D’Alessandro A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun Mass Spectrom. 2017;31:663–673. doi: 10.1002/rcm.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clasquin MF, Melamud E, Rabinowitz JD. LC-MS data processing with MAVEN: a metabolomic analysis and visualization engine. Curr Protoc Bioinforma Chapter 14, Unit14.11. 2012. [DOI] [PMC free article] [PubMed]
- 44.Pang Z, et al. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021;49:W388–W396. doi: 10.1093/nar/gkab382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murray KO, et al. Chronic mitochondria antioxidant treatment in older adults alters the circulating milieu to improve endothelial cell function and mitochondrial oxidative stress. Am J Physiol-Heart Circ Physiol. 2023;325:H187–H194. doi: 10.1152/ajpheart.00270.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mauthe M, et al. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy. 2018;14:1435–1455. doi: 10.1080/15548627.2018.1474314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Redmann M, et al. Inhibition of autophagy with bafilomycin and chloroquine decreases mitochondrial quality and bioenergetic function in primary neurons. Redox Biol. 2017;11:73–81. doi: 10.1016/j.redox.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown M, Ross TP, Holloszy JO. Effects of ageing and exercise on soleus and extensor digitorum longus muscles of female rats. Mech Ageing Dev. 1992;63:69–77. doi: 10.1016/0047-6374(92)90017-8. [DOI] [PubMed] [Google Scholar]
- 49.White Z, et al. Voluntary resistance wheel exercise from mid-life prevents sarcopenia and increases markers of mitochondrial function and autophagy in muscles of old male and female C57BL/6J mice. Skelet Muscle. 2016;6:45. doi: 10.1186/s13395-016-0117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madeo F, Bauer MA, Carmona-Gutierrez D, Kroemer G. Spermidine: a physiological autophagy inducer acting as an anti-aging vitamin in humans? Autophagy. 2018;15:165–168. doi: 10.1080/15548627.2018.1530929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueno D, et al. Spermidine improves angiogenic capacity of senescent endothelial cells, and enhances ischemia-induced neovascularization in aged mice. Sci Rep. 2023;13:8338. doi: 10.1038/s41598-023-35447-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aman Y, et al. Autophagy in healthy aging and disease. Nat Aging. 2021;1:634–650. doi: 10.1038/s43587-021-00098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rubinsztein DC, Mariño G, Kroemer G. Autophagy and Aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 54.Fry CS, et al. Skeletal muscle autophagy and protein breakdown following resistance exercise are similar in younger and older adults. J Gerontol A Biol Sci Med Sci. 2013;68:599–607. doi: 10.1093/gerona/gls209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanida I, Ueno T, Kominami E. LC3 and Autophagy. Methods Mol Biol. 2008;445:77–88. doi: 10.1007/978-1-59745-157-4_4. [DOI] [PubMed] [Google Scholar]
- 56.Koukourakis MI, et al. Autophagosome Proteins LC3A, LC3B and LC3C Have Distinct Subcellular Distribution Kinetics and Expression in Cancer Cell Lines. PLoS One. 2015;10:e0137675. doi: 10.1371/journal.pone.0137675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tan A, Sullenbarger B, Prakash R, McDaniel JC. Supplementation with eicosapentaenoic acid and docosahexaenoic acid reduces high levels of circulating proinflammatory cytokines in aging adults: A randomized, controlled study. Prostaglandins Leukot Essent Fatty Acids. 2018;132:23–29. doi: 10.1016/j.plefa.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yeboah J, Crouse JR, Hsu F-C, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 59.Sasaki H, Kasagi F, Yamada M, Fujita S. Grip Strength Predicts Cause-Specific Mortality in Middle-Aged and Elderly Persons. Am J Med. 2007;120:337–342. doi: 10.1016/j.amjmed.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 60.Clausen JSR, Marott JL, Holtermann A, Gyntelberg F, Jensen MT. Midlife Cardiorespiratory Fitness and the Long-Term Risk of Mortality. J Am Coll Cardiol. 2018;72:987–995. doi: 10.1016/j.jacc.2018.06.045. [DOI] [PubMed] [Google Scholar]
- 61.Dunsky A. The Effect of Balance and Coordination Exercises on Quality of Life in Older Adults: A Mini-Review. Front Aging Neurosci. 2019;11:318. doi: 10.3389/fnagi.2019.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson LC, et al. Amino acid and lipid associated plasma metabolomic patterns are related to healthspan indicators with ageing. Clin Sci (Lond) 2018;132:1765–1777. doi: 10.1042/CS20180409. [DOI] [PubMed] [Google Scholar]
- 63.DeVan AE, et al. Effects of sodium nitrite supplementation on vascular function and related small metabolite signatures in middle-aged and older adults. J Appl Physiol. 2016;120:416. doi: 10.1152/japplphysiol.00879.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Santos-Parker JR, Santos-Parker KS, McQueen MB, Martens CR, Seals DR. Habitual aerobic exercise and circulating proteomic patterns in healthy adults: relation to indicators of healthspan. J Appl Physiol. 2018;1985(125):1646–1659. doi: 10.1152/japplphysiol.00458.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pietrocola F, et al. Spermidine induces autophagy by inhibiting the acetyltransferase EP300. Cell Death Differ. 2015;22:509–516. doi: 10.1038/cdd.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pucciarelli S, et al. Spermidine and Spermine Are Enriched in Whole Blood of Nona/Centenarians. Rejuvenation Res. 2012;15:590–595. doi: 10.1089/rej.2012.1349. [DOI] [PubMed] [Google Scholar]
- 67.Zhuang H, et al. Interactive effects of aging and aerobic capacity on energy metabolism–related metabolites of serum, skeletal muscle, and white adipose tissue. GeroScience. 2021;43:2679–2691. doi: 10.1007/s11357-021-00387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uchitomi R, et al. Metabolomic Analysis of Skeletal Muscle in Aged Mice. Sci Rep. 2019;9:10425. doi: 10.1038/s41598-019-46929-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lira VA, et al. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J. 2013;27:4184. doi: 10.1096/fj.13-228486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hofer SJ, et al. Mechanisms of spermidine-induced autophagy and geroprotection. Nat Aging. 2022;2:1112–1129. doi: 10.1038/s43587-022-00322-9. [DOI] [PubMed] [Google Scholar]
- 71.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: An expanding universe. Cell. 2023;186:243–278. doi: 10.1016/j.cell.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 72.LaRocca TJ, et al. Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol. 2012;590:3305–3316. doi: 10.1113/jphysiol.2012.229690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Laughlin MH, Roseguini B. Mechanisms for exercise training-induced increases in skeletal muscle blood flow capacity: differences with interval sprint training versus aerobic endurance training. J Physiol Pharmacol. 2008;59:71–88. [PMC free article] [PubMed] [Google Scholar]
- 74.Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22:119–141. doi: 10.1038/s41580-020-00313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhasin S, Seals D, Migaud M, Musi N, Baur JA. Nicotinamide Adenine Dinucleotide in Aging Biology: Potential Applications and Many Unknowns. Endocr Rev. 2023;bnad019. 10.1210/endrev/bnad019. [DOI] [PMC free article] [PubMed]
- 76.Liu S, Fu S, Wang G, Cao Y, Li L, Li X, Yang J, Li N, Shan Y, Cao Y, Ma Y, Dong M, Liu Q, Jiang H. Glycerol-3-phosphate biosynthesis regenerates cytosolic NAD+ to alleviate mitochondrial disease. Cell Metab. 2021;33(10):1974–1987. doi: 10.1016/j.cmet.2021.06.013. [DOI] [PubMed] [Google Scholar]
- 77.Lenaz G, et al. Mitochondrial complex I defects in aging. Mol Cell Biochem. 1997;174:329–333. doi: 10.1023/A:1006854619336. [DOI] [PubMed] [Google Scholar]
- 78.Calder PC. Docosahexaenoic acid. Ann Nutr Metab. 2016;69:8–21. doi: 10.1159/000448262. [DOI] [PubMed] [Google Scholar]
- 79.Lee JH, Jeon JH, Lee MJ. Docosahexaenoic acid, a potential treatment for sarcopenia, modulates the ubiquitin–proteasome and the autophagy–lysosome systems. Nutrients. 2020;12:2597. doi: 10.3390/nu12092597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA. DPA and DHA Front Aging Neurosci. 2015;7:52. doi: 10.3389/fnagi.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Howarth KR, LeBlanc PJ, Heigenhauser GJF, Gibala MJ. Effect of endurance training on muscle TCA cycle metabolism during exercise in humans. J Appl Physiol. 2004;1985(97):579–584. doi: 10.1152/japplphysiol.01344.2003. [DOI] [PubMed] [Google Scholar]
- 82.Tsukiyama Y, Ito T, Nagaoka K, Eguchi E, Ogino K. Effects of exercise training on nitric oxide, blood pressure and antioxidant enzymes. J Clin Biochem Nutr. 2017;60:180–186. doi: 10.3164/jcbn.16-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Higashi Y, et al. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects. Circulation. 1999;100:1194–1202. doi: 10.1161/01.CIR.100.11.1194. [DOI] [PubMed] [Google Scholar]
- 84.Sotelo-Orozco J, Chen S-Y, Hertz-Picciotto I, Slupsky CM. A comparison of serum and plasma blood collection tubes for the integration of epidemiological and metabolomics data. Front Mol Biosci. 2021;8. [DOI] [PMC free article] [PubMed]
- 85.Yu Z, Kastenmüller G, He Y, Belcredi P, Möller G, Prehn C, Mendes J, Wahl S, Roemisch-Margl W, Ceglarek U, Polonikov A, Dahmen N, Prokisch H, Xie L, Li Y, Wichmann HE, Peters A, Kronenberg F, Suhre K, Adamski J, Illig T, Wang-Sattler R. Differences between human plasma and serum metabolite profiles. PLoS One. 2011;6(7):e21230. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figs. 1 and 2, the complete list of assessed plasma metabolites (and fold change and FDR-adjusted p-values), and the supplemental methods can be found here: https://doi.org/10.6084/m9.figshare.23699265. (PDF 323 KB)
Data Availability Statement
Data will be made available upon reasonable request.