Abstract
Colitis, a subtype of inflammatory bowel disease (IBD), is a multifactorial disorder characterized by chronic inflammation of the colon. Among various experimental models used in the study of IBD, the chemical colitogenic dextran sulfate sodium (DSS) is most commonly employed to induce colitis in vivo. In the search for new therapeutic strategies, Fisetin, a flavonoid found in many fruits and vegetables, has recently garnered attention for its senolytic properties. Female mice were administered 2.5% DSS in sterile drinking water and were subsequently treated with Fisetin or vehicle by oral gavage. DSS significantly upregulated beta-galactosidase activity in colonic proteins, while Fisetin remarkably inhibited its activity to baseline levels. Particularly, qPCR revealed that the senescence and inflammation markers Vimentin and Ptgs2 were elevated by DSS exposure with Fisetin treatment inhibiting the expression of p53, Bcl2, Cxcl1, and Mcp1, indicating that the treatment reduced senescent cell burden in the DSS targeted intestine. Alongside, senescence and inflammation associated miRNAs miR-149-5p, miR-96-5p, miR-34a-5p, and miR-30e-5p were significantly inhibited by DSS exposure and restored by Fisetin treatment, revealing novel targets for the treatment of IBDs. Metagenomics was implemented to assess impacts on the microbiota, with DSS increasing the prevalence of bacteria in the phyla Bacteroidetes. Meanwhile, Fisetin restored gut health through increased abundance of Akkermansia muciniphila, which is negatively correlated with senescence and inflammation. Our study suggests that Fisetin mitigates DSS-induced colitis by targeting senescence and inflammation and restoring beneficial bacteria in the gut indicating its potential as a therapeutic intervention for IBDs.
Keywords: Fisetin, Dextran sodium sulfate (DSS), Senescence, Microbiota, Inflammatory bowel disease (IBD), miRNAs
Introduction
Inflammatory bowel diseases (IBDs) are a group of idiopathic disorders consisting of two clinical subtypes: Crohn’s disease and ulcerative colitis [1, 2]. IBDs are suspected to arise due to hyperactive, aberrant innate immune responses that are propagated along the intestinal epithelium, resulting in sustained inflammation, dysbiosis of the microbial flora, and severe tissue damage [1, 3]. More alarmingly, they are a gateway to more severe health complications ranging from colon cancer to extra-intestinal illnesses including psoriasis, metabolic syndrome, rheumatoid arthritis, and Alzheimer’s disease and dementia [4–9]. As such, safeguarding gut health by finding an effective treatment for IBDs is crucial to overall well-being.
Ongoing research efforts seek to better understand the underlying etiology of IBDs to uncover novel therapeutic strategies. Namely, one emerging area of interest is investigating the role of cellular senescence in different diseases associated with increased inflammation [10, 11]. Research shows that chronic inflammation is associated with the accumulation of nonproliferative, anti-apoptotic senescent cells [11, 12]. These senescent cells are distinguished by the senescent-associated secretory phenotype (SASP) which causes them to produce and secrete pro-inflammatory signals that then disrupt the function of neighboring cells, driving the onset of age-related diseases [13]. In particular, preliminary data reveals that senescent cell populations have been found in the stem and transient amplifying intestinal compartments of patients with Crohn’s disease [14]. To combat this, our study aims to explore the therapeutic efficacy of Fisetin, a naturally occurring flavonoid that is abundant in fruits and vegetables and has recently gained considerable scientific interest [15]. Particularly, Fisetin has been regarded as an anti-aging intervention for its ability to act as a potent senolytic agent, targeting and removing populations of senescent cells and thereby extending both the lifespan and healthspan in mice [16, 17]. Hence, this raises compelling questions about the potential of Fisetin as a novel treatment option for Crohn’s disease and/or ulcerative colitis.
In our study, we employed the dextran sulfate sodium (DSS)–induced colitis model, which is most prevalent in vivo and is widely recognized for its simplicity and many shared features with human IBD [2, 18]. DSS is known to induce colitis by disrupting the gut epithelium thereby initiating an inflammatory cascade in response to persistent DNA damage and subsequently increasing the permeability of bacterial pathogens [19]. However, the detailed molecular mechanism of DSS-induced colitis remains unclear. In the present study, we aimed to explore the effects of DSS-induced colitis on cellular senescence and the impact of the senolytic agent Fisetin as a potential therapeutic agent for IBDs. Specifically, we assessed whether DSS influences the expression of critical mRNAs that are implicated in cellular senescence, the SASP, and inflammatory processes integral to colitis. Alongside, we investigated the effects of DSS and Fisetin on noncoding RNAs (miRNAs) that act as post-transcriptional switches to regulate these mRNAs to elucidate novel biomarkers and targets for advanced molecular therapeutic intervention. Lastly, we scrutinized the effects of DSS and Fisetin on the gut microbiota, aiming to discern whether Fisetin can ameliorate DSS-induced dysbiosis and restore the microflora through reducing DSS-induced intestinal cellular senescence.
By exploring formative molecular changes caused by DSS and how these are potentially mitigated by Fisetin, this research seeks to delineate the therapeutic promise of Fisetin for colitis. The findings of the study could revolutionize our understanding of senescence in IBD and could pave the way for novel, noninvasive therapeutic strategies for colitis. Thus, the relevance of this research lies not only in its potential to advance our understanding of the pathogenesis of DSS-induced colitis but also in identifying efficacious treatment modalities, thereby contributing to improved prognosis for colitis patients along with improved quality of life through prevention of extra-intestinal systemic age-related diseases. Finally, these findings can lead researchers towards initiating new clinical trials with senolytic treatments in populations affected by IBDs, especially given that these treatment approaches are already being tested in clinical settings [17, 20].
Methods
Experimental animals
All procedures in this study were approved by the University of Central Florida’s Institutional Animal Care and Use Committee. Mice used in the study were wild-type control animals from our Ames dwarf colony. Mice (n = 8–11; 8–12 months old; females) were housed in a pathogen-free, temperature and light-controlled environment with unlimited access to food (Rodent Laboratory Chow 5001) and water. Of note, water valve lixits were removed for the course of the experiment to ensure animals were drinking dextran sodium sulfate (DSS)–infused water for induction of colitis.
Experimental design
Animals were randomly divided into control, DSS, and DSS + Fisetin experimental groups. To induce colitis, adult mice were administered 2.5% (w/v) of 40 kDa DSS (TdBLabs Consultancy, Uppsala, Sweden) in sterile drinking water for 7 days. Animal weights were recorded weekly for the duration of the experiment and fecal samples were aseptically collected for subsequent microbiota analysis. Alongside, animals were closely monitored for physical features of disease including rectal bleeding, stool consistency, and fecal blood. After 7 days, DSS-treated water was removed from the cages and animals were given a 1-week recovery period. Thereafter, senolytic intervention was introduced in the DSS + Fisetin group with gavage needle used to achieve precise dosing of 100 mg/kg of Fisetin (INDOFINE Chemical Group, Hillsborough, New Jersey, USA) in 100–150 μL diluent daily for a total of 3 days. Dosing was determined based on previously conducted in vivo studies [16]. Control animals received an equal volume of the vehicle diluent consisting of 60% Phosal 50 PG (Lipoid LLC, Newark, New Jersey, USA), 30% polyethylene glycol 400 (Sigma-Aldrich, Darmstadt, Germany), and 10% ethanol (Sigma-Aldrich, Darmdstat, Germany). These procedures were repeated for two cycles in total. Following the second cycle, Fisetin was administered for a third time prior to tissue collection. Human dose equivalent is 8 mg/kg, with clinical trials employing 20 mg/kg Fisetin orally over two to three consecutive days every month and microcrystalline cellulose administered as placebo [21, 22]. Mice were anesthetized under 2.5% isoflurane to collect blood via cardiac puncture and were subsequently euthanized by cervical dislocation. Colons were collected and snap-frozen in liquid nitrogen and placed at – 80 °C for subsequent storage. In addition, fecal pellets were collected from each part of the intestine (duodenum, jejunum, ileum, cecum, and colon).
RNA extraction and cDNA synthesis
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, California, USA) according to the manufacturer’s protocol. Samples were further purified using lithium chloride to remove traces of DSS which can inhibit qPCR [23]. cDNA was then generated for miRNA from 10 ng of total RNA using the TaqMan Advanced miRNA cDNA synthesis kit (Applied Biosystems, Carlsbad, California, USA). cDNA templates were diluted 1:10 for downstream assessment by qPCR. Alongside, cDNA was also synthesized for mRNA from 1 µg of total RNA using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, California, USA) and diluted 1:5 for downstream assessment by qPCR.
Analysis of biomolecular markers of senescence and inflammation
qPCR was conducted for miRNAs and mRNAs of interest on a QuantStudio 7 thermocycler (ThermoFisher) for 40 cycles. For miRNA targets, reactions were prepared using 5 µL of Taqman Fast Advanced Master Mix (Applied Biosystems, Vilnius, Lithuania), 0.5 µL Taqman Advanced miRNA assay (Applied Biosystems, Pleasanton, California, USA), 2 µL of nuclease-free water, and 2.5 µL of cDNA template for a total volume of 10 µL. miR-16-5p was used as a housekeeping control. For mRNA targets, reactions were prepared using 5 µL of FAST SYBR Green Master Mix (Applied Biosystems, Vilnius, Lithuania), 12.6 µL of nuclease-free water, 0.2 µL of 10 µM reverse and forward primers, and 2 µL of cDNA template for a total volume of 20 µL. Beta-2-microglobulin (β2M) was used as housekeeping control. Primer sequences were as follows: β2M (Fwd 5′–AAGTATACTCACGCCACCCA–3′ and Rev 5′–CAGGCGTATGTATCAGTCTC–3′), p16Ink4a (Fwd 5′-CCCAACGCCCCGAACT-3′ and Rev 5′-GCAGAAGAGCTGCTACGTGAA-3′), p21Cip1 (Fwd 5′-GTCAGGCTGGTCTGCCTCCG-3′ and Rev 5′-CGGTCCCGTGGACAGTGAGCAG-3′), p38 (Fwd 5′-TGGAAGAGCCTGACCTATGA-3′ and Rev 5′-GACACACACACACACACAAAC-3′), p53 (Fwd 5′-TCACAGTCGGATATCAGCCT-3′ and Rev 5′-ACACTCGGAGGGCTTCACTT-3′), Il1β (Fwd 5′-TCCTGTGTGATGAAAGACGGCAC-3′ and Rev 5′-GTGCTGATGTACCAGTTGGGGAAC-3′), Mcp1 (Fwd 5′-GCATCCACGTGTTGGCTCA-3′ and Rev 5′-CTCCAGCCTACTCATTGGGATCA-3′), Cxcl1 (Fwd 5′-ACCCGCTCGCTTCTCTGT-3′ and Rev 5′-AAGGGAGCTTCAGGGTCAAG-3′), Lmnb1 (Fwd 5′-GGGAAGTTTATTCGCTTGAAGA and Rev 5′-ATCTCCCAGCCTCCCATT), Ptgs2 (Fwd 5′-TGGTGCCTGGTCTGATGATG–3′ and Rev 5′–GCAATGCGGTTCTGATACTG–3′), Nfkb (Fwd 5′-GACCACTGCTCAGGTCCACT-3′ and Rev 5′-TGTCACTATCCCGGAGTTCA-3′), Stat3 (Fwd 5′-AGAAAGTGTCCTACAAGGGCGAC-3′ and Rev 5′-CACGAAGGCACTCTTCATTAAGTTT-3′), Bcl2 (Fwd 5′-TGGGATGCCTTTGTGGAACT-3′ and Rev 5′-GAGACAGCCAGGAGAAATCA-3′), and Ccnd1 (Fwd 5′-GCAGAAGGAGATTGTGCCATCC-3′ and Rev 5′-AGGAAGCGGTCCAGGTAGTTCA-3′). Relative expression of each target miRNA and mRNA was measured using the 2−ΔΔCT method.
Protein extraction and beta-galactosidase protein assay
Protein extraction was completed using the TRIzol method according to the manufacturer’s directions (Invitrogen Corporation). Specifically, the interphase and organic phase were separated from the aqueous phase to conduct protein extraction. Protein pellets were solubilized at 50 °C, then suspended in a solution of 1:1 1% SDS and 8 M urea, and sonicated. Thereafter, samples were centrifuged at 10,000 rcf for 10 min at 4 °C and the jelly-like pellet was redissolved in the solution with insoluble fragments separated and supernatant collected in a new tube and stored at – 80 °C [24]. Protein concentrations were quantified using the bicinchoninic acid (BCA) assay (Thermo Scientific, Rockford, Illinois, USA). Thereafter, the Mammalian β-Galactosidase Assay Kit (Thermo Scientific, Waltham, Massachusetts, USA) was used to assess senescence in the protein samples. Fifty microliters of protein homogenates was mixed with 50 µL of beta-galactosidase reagent and incubated for 30 min at 37 °C in a dry incubator. Absorbance was then recorded in a spectrophotometer at a wavelength of 405 nm.
Bioinformatics and statistical analysis of biomolecular data
Considering our relative small data sets with some outliers, we decided to perform nonparametric multiple pairwise Dunn’s test to compare the fold change of each targeted mRNA and miRNA, as well as beta-galactosidase protein activity, among different treatments. In the analysis, we selected Bonferroni adjustment to control for overall type I error < 0.05. The fold changes of mRNAs and miRNAs were calculated and normalized using the 2−∆∆Ct method, in which − ∆∆Ct was calculated as − ∆∆Ct = average (∆Ctcontrol) − ∆Ctsample and ∆Ct = Cttarget mRNA/miRNA − Ct housekeeping mRNA/miRNA. To evaluate the effects of DSS and Fisetin on body weight loss over time (by week), we applied a linear mixed effect model in which the effects of time and treatment were set as a fixed effect while individual identity (baseline intercept) was set as a random effect. All the above analyses were conducted using Stata/MP 15.1.
Metagenomic DNA extraction
Total DNA from the mouse feces were extracted using QIAamp PowerFecal Pro DNA Kit (Qiagen, USA) according to manufacturer’s instructions. The yield and quality of the community DNA samples were checked on 0.8% agarose gel, and DNA concentration was measured using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, USA). All the extracted DNA samples were stored at – 20 °C until further processing.
Preparation of 16S rRNA gene amplicon libraries and sequencing
The microbiome communities were analyzed using 16S rRNA gene amplicon sequencing. PCR amplification was performed using the primer set 515F (5′ CCTACGGGNGGCWGCAG 3′) and 806R (5′ GACTACHVGGGTATCTAATCC 3′) that selectively amplified the V4 region of the 16S rRNA gene. Amplified and uniquely barcoded amplicons were purified using AMPure® magnetic purification beads (Agencourt, Beckman Coulter, CA, USA) and quantified in a Qubit-3 fluorimeter (Invitrogen, Carlsbad, CA, USA). Template and library preparation was carried out according to the manufacturer’s protocol (Illumina, USA). The sequencing of multiplexed 16S rDNA amplicon libraries was performed on the Illumina Miseq platform.
Bioinformatics and statistical analysis of microbiome data
The sample-specific demultiplexed data was used for the taxonomic classification of the reads using QIIME 2 (Bolyen E, 2019). Denoising and preprocessing of the sequence reads were carried out using the QIIME 2 pipeline. Taxonomy assignment to the Amplicon Sequence Variants (ASVs) was done using the SILVA database release 132 (Quast et al. 2013). Alpha diversity analysis was done using microbiome analysts (Chong et al. 2020). Further preprocessing and filtering of the data included exclusion of ASVs belonging to chloroplast, mitochondria, and unclassified ASVs at phylum level were filtered out. The representation of bacterial major taxa and statistical analysis of taxa across the groups was estimated and plotted using GraphPad Prism (Prism 9.5.0). Principal component analysis (PCA)–based beta-diversity was performed using ggplot2 package. The shared and unique species across the groups were done using a web-based tool InteractiVenn (Heberle et al., 2015).
Results
5% DSS exposure reduces body weight and induces rectal bleeding in mice
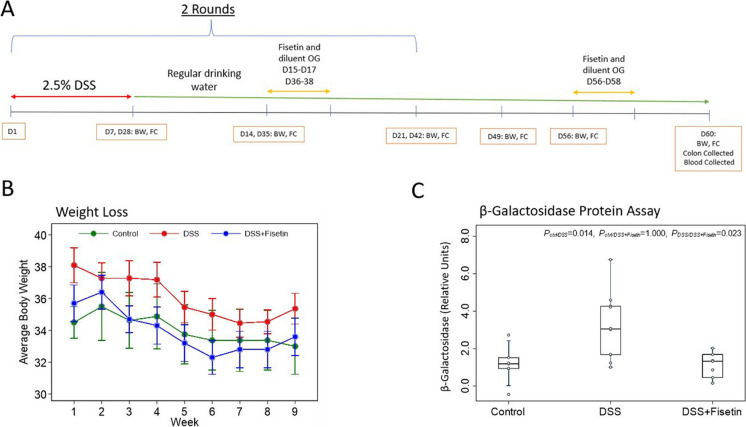
To monitor physical changes after exposure to DSS-supplemented water, body weights were recorded and fecal samples were collected weekly (Fig. 1A). DSS prompted a reduction in body weight, with exposed animals exhibiting rectal bleeding (p = 0.148) (Fig. 1B, Table 1).
Fig. 1.
DSS decreases body weight and significantly increases the expression of senescent marker β-galactosidase in colonic proteins of female wild-type mice. A Timeline of the study, conducted over 60 days, with body weight (BW) recorded and fecal collection (FC) performed weekly for the duration of the study. B Changes in average body weight recorded weekly for the duration of the experiment (mean ± SEM). C Boxplot showing the comparison of β-galactosidase among different treatments. Nonparametric multiple pairwise Dunn’s test was performed to evaluate the effects of the different treatments on the expression of β-galactosidase with Bonferroni adjustment applied to control for type I error
Table 1.
Summary of linear mixed effect model analysis evaluating the fixed effects of week and treatment on the average body weight loss with individual identity (baseline intercept) set as random effect
| Effect | Coefficient | SE | Z | p-value |
|---|---|---|---|---|
| Fixed effect | ||||
| Week | − 0.39 | 0.06 | − 6.91 | < 0.001 |
| Treatment | ||||
| Control | Reference (0) | |||
| DSS | 2.03 | 1.43 | 1.42 | 0.156 |
| DSS + Fisetin | − 0.06 | 1.46 | − 0.04 | 0.965 |
| Intercept | 35.99 | 1.12 | 32.01 | < 0.001 |
| Random effect | ||||
| Individual identify | ||||
| SD (intercept) | 2.98 | 0.42 | < 0.001 |
Molecular responses to DSS and fisetin in mice with colitis
The β-galactosidase senescent protein assay showed significantly higher expression of β-galactosidase in the DSS group compared to the control (p < 0.014) and a significant decrease in the DSS + Fisetin group relative to the DSS-exposed animals (p < 0.023) (Fig. 1C).
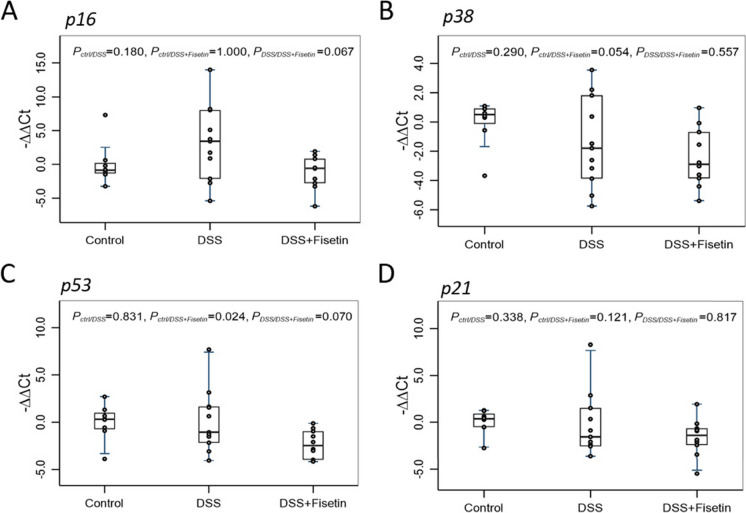
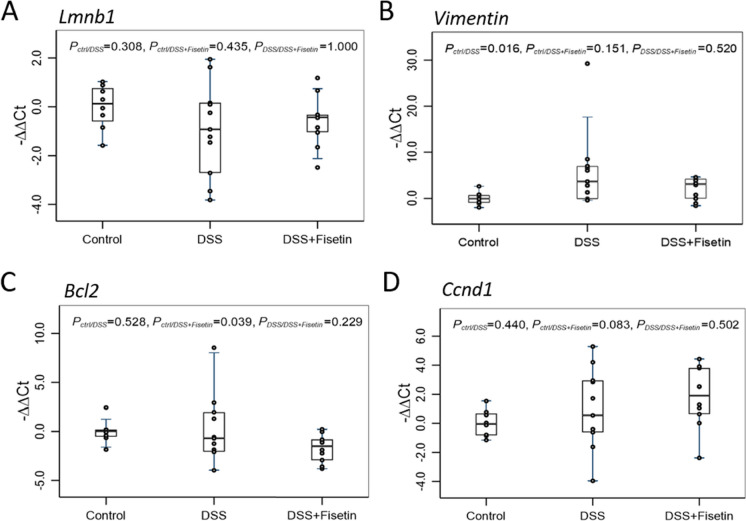
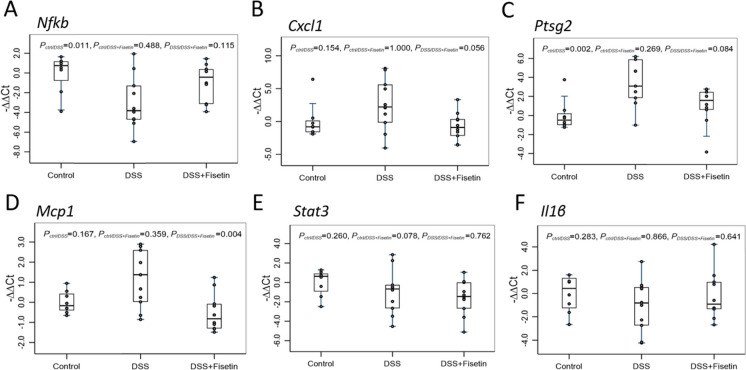
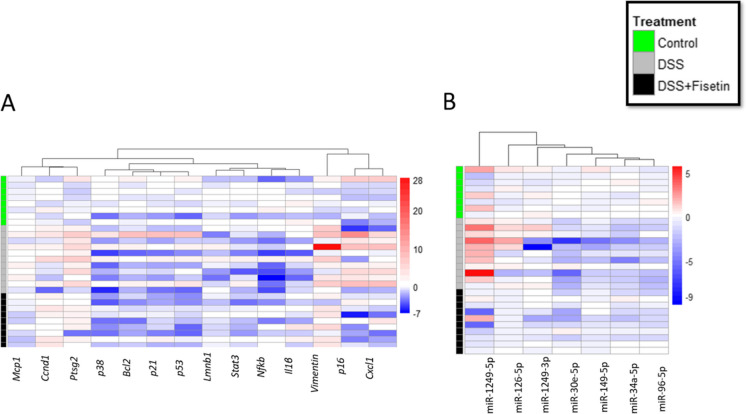
Using qPCR, we then measured the expression of p16Ink4a, p38, p53, and p21Cip1 (cell cycle regulators); Lmnb1, Vimentin, Bcl2, and Ccnd1 (markers of senescence and apoptosis); and Nfkb, Cxcl, Ptgs2, Mcp1, Stat3, and Il1β (markers of the senescent-associated secretory phenotype [SASP]) to investigate senescent cell burden and inflammation in a mouse-model of DSS-induced colitis (Figs. 2, 3, and 4). Heatmap was generated to demonstrate changes in these mRNAs for each sample across all groups (Fig. 6A).
Fig. 2.
Boxplot of fold changes of cell cycle checkpoint mRNAs (n = 8–11 per group). A p16 gene expression, B p38 gene expression, C p53 gene expression, and D p21 gene expression in female wild-type mice treated with DSS and DSS + Fisetin. The 2−ΔΔCt method was used to calculate and normalize the gene expression, in which − ∆∆Ct was calculated as − ∆∆Ct = average (∆Ct control) − ∆Ct sample, and ∆Ct = Ct target gene − Ct housekeeping gene. The housekeeping gene was β2M. Nonparametric multiple pairwise Dunn’s test was performed to evaluate the differential gene expression among different treatments with Bonferroni adjustment applied to control for type I error
Fig. 3.
Boxplot of fold changes of senescence and apoptosis mRNAs (n = 8–11 per group). A Lmnb1 gene expression, B Vimentin gene expression, C Bcl2 gene expression, and C Ccnd1 gene expression in female wild-type mice treated with DSS and DSS + Fisetin. The 2−ΔΔCt method was used to calculate and normalize the gene expression, in which − ∆∆Ct was calculated as − ∆∆Ct = average (∆Ctcontrol) − ∆Ctsample and ∆Ct = Cttarget gene − Cthousekeeping gene. The housekeeping gene was β2M. Nonparametric multiple pairwise Dunn’s test was performed to evaluate the differential gene expression among different treatments with Bonferroni adjustment applied to control for type I error
Fig. 4.
Boxplot of fold changes of SASP mRNAs (n = 8–11 per group). A Nfkb gene expression, B Cxcl1 gene expression, C Ptgs2 gene expression, D Mcp1 gene expression, E Stat3 gene expression, and F Il1β gene expression in female wild-type mice treated with DSS and DSS + Fisetin. The 2−ΔΔCt method was used to calculate and normalize the gene expression, in which − ∆∆Ct was calculated as − ∆∆Ct = average (∆Ctcontrol) − ∆Ctsample and ∆Ct = Cttarget gene − Cthousekeeping gene. The housekeeping gene was β2M. Nonparametric multiple pairwise Dunn’s test was performed to evaluate the differential gene expression among different treatments with Bonferroni adjustment applied to control for type I error
Fig. 6.
Heat map showing the fold changes in each sample using − ∆∆Ct, normalized based on average of control samples. Blue indicates downregulation, and red indicates upregulation. Groups are separated along the y-axis, with control samples marked in green (n = 8), DSS in grey (n = 11), and DSS + Fisetin in black (n = 9)
Our qPCR analyses of cell cycle checkpoint regulators revealed a trend towards upregulation of p16Ink4a in the DSS group compared to the control (p = 0.180), while DSS + Fisetin normalized p16Ink4a back to basal levels (p = 0.067) (Fig. 2A). p38 mRNA levels were downregulated in the DSS- (p = 0.054) treated colons compared to the control (Fig. 2B). Further, p53 mRNA levels were significantly downregulated in the DSS + Fisetin (p = 0.024) group compared to the control, with a downward trend also noted against the DSS-exposed animals (p = 0.07) (Fig. 2C). Additionally, Fisetin appeared to upregulate p21Cip1 when compared with DSS group alone (p = 0.121) (Fig. 2D).
Surprisingly, Lmnb1 mRNA levels were not influenced by DSS exposure or by Fisetin treatment (Fig. 3A). Additionally, Vimentin was significantly upregulated in the DSS-exposed animals (p = 0.016), but was not restored to basal levels by Fisetin treatment. Bcl2 mRNA levels were significantly downregulated in the DSS + Fisetin (p = 0.039) group relative to control (Fig. 3B). Concurrently, Ccnd1 mRNA levels demonstrated an upward trend in the DSS + Fisetin group compared to the control group (p = 0.083).
Lastly, qPCR analyses revealed modulation of markers associated with inflammation and the SASP in the colons of animals exposed to DSS and treated with Fisetin. Notably, Nfkb was significantly downregulated in the DSS-exposed animals (p = 0.011) compared to the control animals with an upward trend noted against mice administered Fisetin treatment (p = 0.115) (Fig. 4A). Cxcl1, another inflammatory mediator, showed an upward trend in the DSS group (p = 0.154) relative to the control animals and was subsequently downregulated by Fisetin treatment (p = 0.056) (Fig. 4B). Ptgs2 was significantly upregulated in the DSS group relative to the control animals (p < 0.002) and subsequently downregulated in the DSS + Fisetin group against the DSS-exposed animals although not reaching statistical significance (p = 0.084) (Fig. 4C). Furthermore, Mcp1 was not significantly altered in the DSS group but showed an increasing trend relative to the control animals (p = 0.167) and was significantly downregulated in the DSS + Fisetin group relative to the DSS-exposed animals (p = 0.004) (Fig. 4D). Stat3 appeared to be downregulated in the DSS + Fisetin (p = 0.078) group relative to the control animals (Fig. 4E). Il1β did not reveal any statistically significant trends, but appeared to be downregulated in the DSS-treated animals against the control group (p = 0.283) (Fig. 4F).
Assessment of senescence and inflammation associated miRNAs in the colons of DSS-exposed and fisetin-treated mice
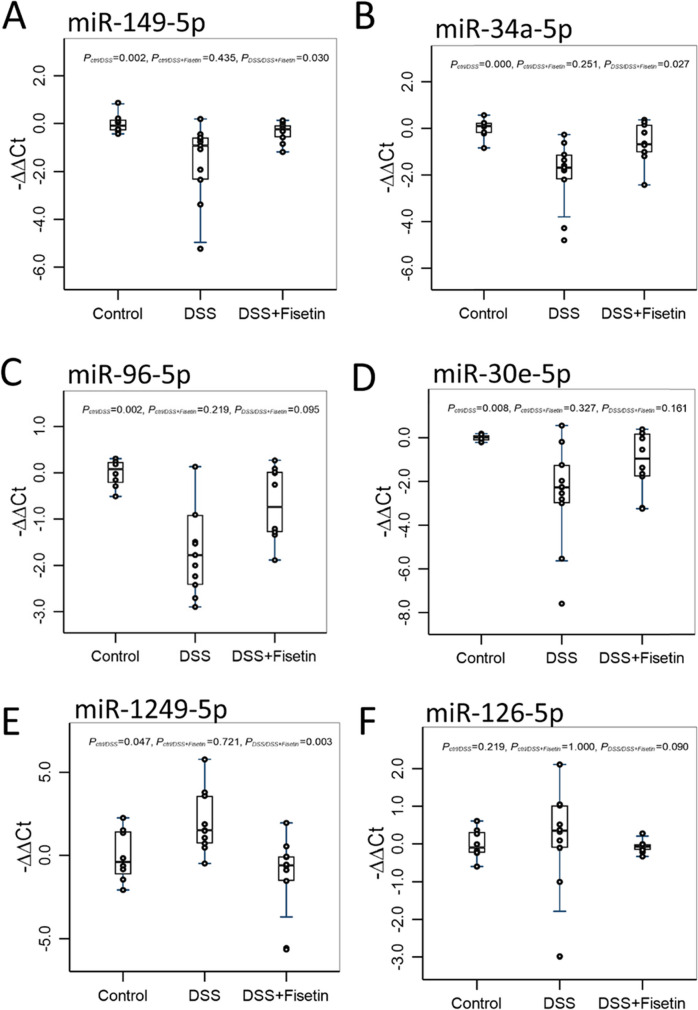
We selected a panel of 15 miRNAs to further investigate the biomolecular changes in response to DSS exposure and Fisetin treatment. Our findings elucidate that DSS significantly inhibits the expression of miR-149-5p (p = 0.002), miR-96-5p (p = 0.002), miR-34a-5p (p = 0.000), and miR-30e-5p (p = 0.008) in the colons of mice with DSS-induced colitis and that Fisetin treatment was able to significantly restore the expression of miR-149-5p (p = 0.03) and miR-34a-5p (p = 0.027) (Fig. 5A–D). Both miR-96-5p and miR-30e-5p demonstrated an upward trend in the Fisetin-treated animals relative to the DSS-exposed animals (p = 0.095 and p = 0.161, respectively) (Fig. 5C, D). These miRNAs have been shown to have implications in cellular senescence and inflammation. Alongside, we also observed that miR-1249-5p was significantly upregulated in the DSS-treated animals (p = 0.047) and significantly downregulated in the Fisetin-treated animals relative to the DSS-exposed animals (p = 0.003) (Fig. 5E). Lastly, an upward trend was noted for miR-126-5p in the DSS-exposed animals relative to the control animals (p = 0.219), with a downward trend also noted for the Fisetin-treated animals relative to the DSS-exposed animals (p = 0.090) (Fig. 5F). Heatmap was generated to demonstrate changes in these miRNAs for each sample across all groups (Fig. 6B).
Fig. 5.
Boxplot of fold changes of differentially expressed miRNAs in the colons of female wild-type mice treated with DSS and DSS + Fisetin (n = 8–11 per group). A miR-149-5p expression, B miR-34a-5p expression, C miR-96-5p expression, D miR-30e-5p, E miR-1249-5p expression, and F miR-126-5p expression. The 2−ΔΔCt method was used to calculate and normalize the gene expression, in which − ∆∆Ct was calculated as − ∆∆Ct = average (∆Ctcontrol) − ∆Ctsample and ∆Ct = Cttarget gene − Cthousekeeping gene. The housekeeping was miR-16. Nonparametric multiple pairwise Dunn’s test was performed to evaluate the differential gene expression among different treatments with Bonferroni adjustment applied to control for type I error
Senolytic treatment with fisetin modulates the intestinal microbiota in normal mice with DSS-induced colitis
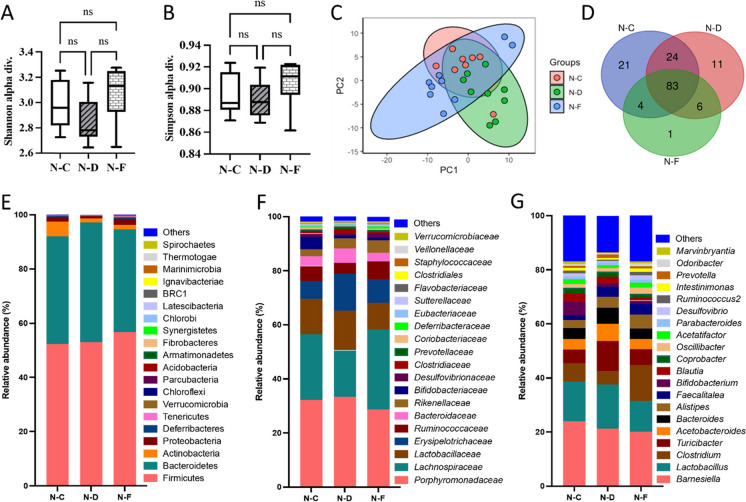
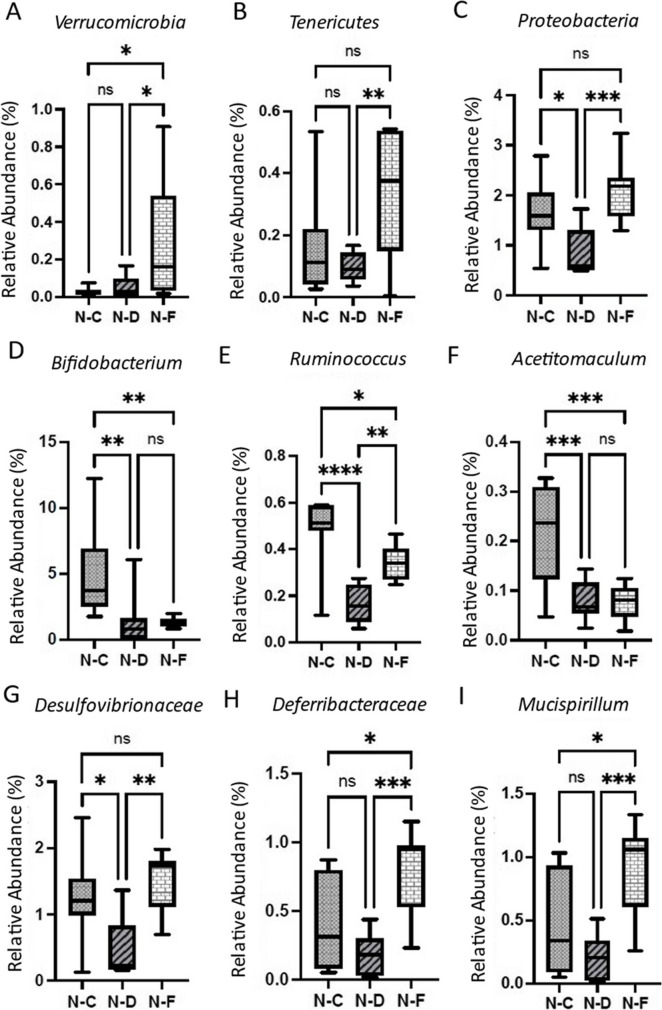
Analysis of Shannon and Simpson alpha diversities did not show a remarkable difference between the control, DSS, and DSS + Fisetin groups (Fig. 7A, B). However, there was a trend seen with decreased microbial diversity in the DSS-treated colon fecal samples relative to the control and DSS + Fisetin-treated animals (Fig. 7A, B). Particularly, analysis of bacterial genera revealed 21 distinct genera in the control group, 11 distinct genera in the DSS-treated group, and 1 distinct genus in the DSS + Fisetin-treated group (Fig. 7D). Fisetin-treated animals were uniquely characterized by high levels of Akkermansia muciniphila. Relative abundance plots reveal that both DSS and Fisetin altered the gut phyla (Fig. 7E–G). Importantly, DSS increased the production of Bacteroidetes while Fisetin appeared to stimulate the population of Firmicutes in the colon (Fig. 7E). Interestingly, DSS appeared to increase the production of Clostridiaceae, Erysipelotrichaceae, and Turicibacter while decreasing the abundance of Lachnospiraceae (Fig. 7F, G). In particular, Fisetin appeared to significantly increase the prevalence of Verrucomicrobia, Tenericutes, Proteobacteria, Desulfovibrionaceae, Deferribacteraceae, Ruminococcus, and Mucispirillum relative to the DSS-treated animals (Fig. 8). A summary figure of the proposed mechanism by which fisetin mediates DSS-induced colitis is illustrated in Fig. 9.
Fig. 7.
Changes in the microbiota in response to DSS exposure and Fisetin treatment in the colons of female wild-type mice. y-axis represents treatment condition, with N–C (normal-control), N-D (normal-DSS), and N-F (normal-Fisetin) (n = 8–11 per group). A Shannon diversity of the microbiota, B Simpson diversity of the microbiota, C principal component analysis (PCA), D Venn diagram of distinct bacterial genera, E relative abundance of bacterial phyla, F relative abundance of distinct bacterial families, and G relative abundance of distinct bacterial classes
Fig. 8.
Boxplots showing changes in relative abundance of bacterial populations in response to DSS exposure and Fisetin treatment in the colons of female wild-type mice. y-axis represents treatment condition, with N–C (normal-control), N-D (normal-DSS), and N-F (normal-Fisetin). Relative abundance of A Verrucomicrobia, B Tenericutes, C Proteobacteria, D Bifidobacterium, E Ruminococcus, F Acetitomaculum, G Desulfovirionaceae, H Deferribacteraceae, and I Mucispirillum in female wild-type mice treated with DSS and DSS + Fisetin. (*p < 0.05, **p < 0.01, ***p < 0.001)
Fig. 9.
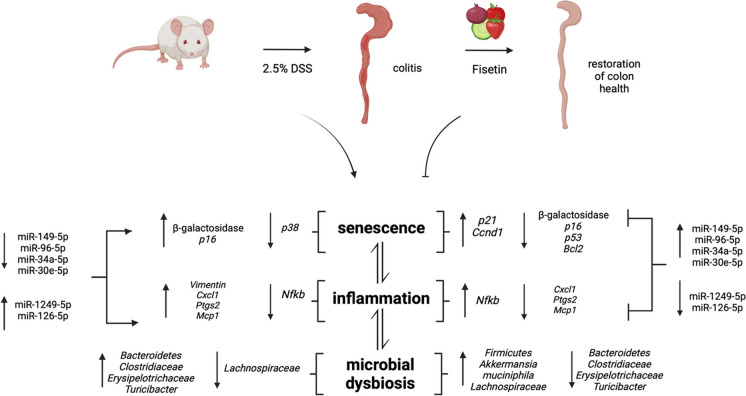
Proposed mechanism by which DSS induces colitis and fisetin mediates restoration of colon health. DSS insult results in the accumulation of senescent cells which release SASP factors that propagate inflammation and influence neighboring cells. DSS insult also triggers inflammation which causes senescence. As a result, the biomolecular landscape is altered with changes in key mRNAs involved with senescent and inflammatory processes alongside colitis inducing miRNA signatures. Microbial dysbiosis further produces a pro-inflammatory microenvironment. Fisetin inhibits senescence and inflammation and colitis inducing miRNA signatures. Restoration of the microbiota by Fisetin treatment particularly results in increased abundance of Akkermansia mucinphila, a bacteria that is negatively correlated with senescence and inflammation. Created with BioRender.com
Discussion
Our study elucidates the multifaceted impact of Fisetin on a mouse model of DSS-induced colitis, with a particular focus on molecular markers associated with cellular senescence and the SASP. In this study, we observed that exposure to DSS resulted in body weight reduction and rectal bleeding in mice, which are key features of IBD. Importantly, our study highlights that DSS exposure induces cellular senescence in the gut, which aligns with earlier findings demonstrating elevated expression of senescent markers in Crohn’s patients [14].
In particular, the major marker of senescence, β-galactosidase, indicated that DSS strongly activates cellular senescence in intestinal tissue, while treatment with Fisetin was effective in reducing senescence burden after chemical insult normalizing it to the levels observed in vehicle-treated animals. Coupled with these findings, the upward trend of p16Ink4a in the DSS-treated animals is pivotal to our understanding of the molecular mechanisms underlying colitis and may point to increased cellular senescence, revealing that DSS pushes colonic cells towards a senescent state. Similarly, the downward trend of p16Ink4a in the DSS + Fisetin group suggests that Fisetin inhibits cellular senescence caused by DSS exposure [25, 26]. Additionally, other mRNAs involved with regulating senescence, inflammation, and the SASP including molecular markers p21Cip1, p38, and p53 displayed differential expression patterns in response to DSS and DSS + Fisetin treatments, providing insight into the underlying mechanisms of cellular senescence and inflammation. Particularly, p38 was downregulated in the DSS group indicating weakened stress response or anti-inflammatory activity due to overstimulation of immune signaling by DSS [25]. In addition, p53 was significantly downregulated in the Fisetin-treated animals against the control animals and demonstrated a downward trend against the DSS-exposed animals showing a plausible way of counteracting cellular senescence [25]. Interestingly, p21Cip1 exhibited a downward trend in both the DSS and DSS + Fisetin groups compared to the control group, suggesting impaired cellular repair attributed to inflammatory stress due to DSS exposure. Alongside, treatment with Fisetin appeared to have a slight upward trend relative to the DSS-treated animals suggesting reparative processes potentially re-established following senolytic treatment [25]. This also elucidates that Fisetin may be preventing the pro-cancer potential of DSS by encouraging defensive cell cycle arrest mechanisms [25], warranting further investigation with a longer treatment duration. Collectively, these findings indicate that Fisetin is exerting a complex regulatory effect on central cell cycle associated mRNAs demonstrating its utility as a therapy for colitis.
Additional analysis of Lmnb1, Vimentin, Bcl2, and Ccnd1 provides more insight into the molecular mechanisms of DSS and Fisetin in the context of senescence. Specifically, Lmnb1 does not show any statistically significant changes in both the DSS and DSS + Fisetin-treated groups which may indicate that DSS induces senescence and Fisetin mitigates senescence independent of Lmnb1. Given the trends observed, this finding may also be attributed to the duration of the study, with decrease in expression of Lmnb1 in DSS-exposed animals and increase in expression of Lmnb1 due to Fisetin possible over a longer treatment regimen. However, Vimentin, an intermediate filament protein, was significantly upregulated by DSS exposure supporting previous findings suggesting that its production is linked to increased bacterial infiltration due to suppression of reactive oxygen species (ROS), intestinal fibrosis, senescence, and impaired immune response [27–29]. Additionally, the anti-apoptotic Bcl2 is also significantly downregulated in the DSS + Fisetin group, which might indicate senescence cell clearance as a byproduct of Fisetin treatment. Lastly, the upward trend of Ccnd1 in the DSS + Fisetin group relative to the control group adds an interesting angle to this study, especially in the scope of aging, senescence, and inflammation. Ccnd1 regulates cell cycle progression and its upregulation in the Fisetin-treated animals indicates that Fisetin may be counteracting cell death as demonstrated by loss of Lmnb1 and Bcl2 in response to DSS exposure or that it may be acting to repair damaged cells [12]. Of note, loss of Ccnd1 is associated with cellular senescence suggesting that Fisetin thwarts the pro-senescent effects of DSS while also counteracting its potential pro-cancer properties. We aimed to understand how these mRNAs interplay with markers of inflammation and SASP to provide a more comprehensive view of how Fisetin regulates DSS-induced colitis.
Next, we observed downregulation of fundamental inflammatory markers such as Nfkb in the DSS-treated animals which we postulate may be a compensatory response by the cells to minimize excessive inflammation. Interestingly, our results also reveal that treatment with Fisetin appeared to show an upward trend in the expression of Nfkb which suggests that it may be eliciting an immune response to counteract inflammation and thus also suggesting impaired immune function as a result of DSS exposure. The time course of this study should be extended in the future to further understand these findings. Cxcl1, a chemokine that recruits immune cells to sites of inflammation, and Ptgs2, responsible for generating prostaglandins, were upregulated in the DSS group and downregulated in the DSS + Fisetin-treated group [30, 31]. This may indicate that Fisetin has an anti-inflammatory role to diminish inflammation resulting from colitis by eliminating senescent cells that secrete the SASP. Similarly, Mcp1 was also downregulated in the DSS + Fisetin-treated animals implying that it may exert anti-inflammatory effects by impeding monocyte recruitment to the site of inflammation [13]. Interestingly, Stat3 was downregulated in both the DSS and DSS + Fisetin-treated groups demonstrating that DSS impairs cell survival and propagates excessive inflammatory processes which drive counteracting anti-inflammatory responses to maintain intestinal health. This is consistent with our findings regarding the expression of p38 which was also downregulated and is a known activator of Stat3 [32]. Overall, DSS appears to induce senescence thereby increasing SASP production in the colon with Fisetin successfully modulating these effects through effective elimination of senescent cells, reducing SASP production and secretion, and thus reducing local inflammation. The complex actions of Fisetin warrant further investigation in the context of senescence and inflammation in IBDs with a longer treatment duration necessary to further understand these findings.
Alongside, we investigated the miRNAs that are involved with regulating senescence-associated mRNAs. miRNAs are small, endogenous molecules roughly 20 nucleotides in length that work by regulating post-transcriptional processes by binding to complementary mRNAs and degrading them or preventing their translation into functional proteins [18]. As such, miRNAs can be used as potent tools for the diagnosis and prognosis of various diseases and hold robust therapeutic potential [33]. The miRNAs that were significantly differentially expressed in response to DSS exposure (miR-149-5p, miR-96-5p, miR-34a-5p, and miR-30e-5p) have implications in regulating mRNAs involved cellular senescence and inflammation. Particularly, current literature indicates that DSS-induced colitis is exacerbated in mice that are deficient in miR-149-5p and that inhibition of this miRNA is also evident in the peripheral blood and colonic tissue of patients with Crohn’s disease thus highlighting its central role in the pathogenesis of IBDs [34, 35]. Particularly, miR-149 deficiency results in increased expression of inflammation-associated mRNAs including Ptgs2, Cxcl1, Tgfb, Icam1, and Il6 [32]. Further investigations have demonstrated that Mycobacterium bovis infection and lipopolysaccharide stimulation can decrease the expression of miR-149 in murine macrophages, revealing its importance in regulating immune function [36]. Restoration of this miRNA by Fisetin indicates its unique function in modulation of dysbiosis, inflammation, and senescence in the colon and reveals a novel method to target this miRNA to treat colitis. The mechanism by which miR-96-5p, miR-34a-5p, and miR-30e-5p influence IBDs needs to be further elucidated as they may be useful diagnostic and prognostic markers. Alongside, the restoration of these miRNAs by Fisetin indicates that they may also be promising therapeutic targets for colitis. Additionally, the significant upregulation of miR-1249-5p in the DSS-exposed animals and its significant restoration in the Fisetin-treated animals demonstrate another robust biomarker for colitis and therapeutic target for Fisetin. Alongside, miR-126-5p also shows promise as a prognostic and diagnostic tool with an upward trend noted in the DSS-treated animals and a downward trend noted in the Fisetin-treated animals. These findings require further functional studies in the future.
Lastly, because dysbiosis is a hallmark of IBDs and a gateway to extra-intestinal manifestations including metabolic syndrome and Alzheimer’s disease, discovering new therapeutic methods to restore microbial balance is imperative not only to improving enteric immune function but also for overall well-being [9, 37–39]. Since Fisetin is classified as a plant flavonoid, its anti-oxidative properties may be useful for improving the resident microbial composition of the gut thereby mitigating IBDs by exerting immunomodulatory effects to regulate intestinal homeostasis [40]. Our analysis reveals that the relative abundance of dominant pathogenic and healthy bacterial phyla was influenced by Fisetin treatment. Assessment of the gut microbiota indicates that DSS may be causing dysbiosis by increasing the population of pathogenic bacteria in large due to increased cellular senescence, production, and secretion of SASPs, thus promoting an inflammatory environment. Consistent with previous studies, our study demonstrates that DSS exposure results in increased Bacteroidetes and decreased Firmicutes [41]. Particularly, in our model of DSS-induced colitis, we observed an increase in abundance of Clostridiaceae, Hungatella, and Mycoplasma. We also observed a decrease in Lachnospiraceae, a bacteria known for its anti-inflammatory properties, and an increase observed following treatment with Fisetin. Alongside, we discovered that Fisetin can increase the relative abundance of Firmicutes and Verrucomicrobia thereby improving microbial imbalance. Particularly, Firmicutes is involved with having anti-inflammatory effects and is important to the management of colitis [42]. Verrucomicrobia is known to generate mucin-degrading enzymes that break down the mucus layer that lines the gut, which helps to maintain a healthy gut barrier function [43]. This breakdown of mucus allows other beneficial bacteria to access nutrients and establish a balanced microbial community in the gut. Additionally, Verrucomicrobia has been associated with the production of short-chain fatty acids (SCFAs), particularly acetate. SCFAs are important energy sources for the cells lining the colon and have been linked to various health benefits, such as reducing inflammation and promoting gut motility [44]. At the genus level, we found that the beneficial gut bacterium Akkermansia mucinphila was uniquely expressed in the Fisetin-treated samples [41]. Akkermansia mucinphila has garnered significant attention in recent years due to its potential beneficial effects on health. Research has suggested that high levels of Akkermansia mucinphila are associated with a healthy gut and improved metabolic health and that supplementation with Akkermansia mucinphila can promote healthy aging [45]. Previous studies from our lab have demonstrated that dasatinib and quercetin senolytic treatment increases the abundance of Akkermansia mucinphila in the intestines of aged mice and that high levels of these taxa are negatively correlated with markers of senescence and inflammation [46]. It has been found to play a role in maintaining the integrity of the gut barrier, preventing leaky gut, promoting mucus production, and modulating the immune response [43–45]. Studies have shown that individuals with obesity, type 2 diabetes, and inflammatory bowel disease often have lower levels of Akkermansia muciniphila [47]. This has led to investigations into the potential therapeutic applications of this bacterium. Animal studies have demonstrated that supplementation with Akkermansia muciniphila can improve metabolic parameters, reduce inflammation, and protect against diet-induced obesity [47]. Although Akkermansia muciniphila shows promise, it is important to note that the research is still in its early stages, and more studies are needed to fully understand its mechanisms and potential therapeutic applications. Additionally, factors such as diet, lifestyle, and host genetics can influence the abundance of Akkermansia muciniphila in the gut, highlighting the complexity of its interactions within the microbiome.
Further, Ruminococcus bacterium was significantly downregulated in the DSS-exposed animals and significantly upregulated by Fisetin treatment. Ruminococcus is known for its ability to break down complex carbohydrates, such as cellulose and hemicellulose, that are present in plant-based foods [48]. The fermentation of these complex carbohydrates by Ruminococcus and other bacteria in the gut leads to the production of SCFAs, such as acetate, propionate, and butyrate [48]. SCFAs provide an energy source for the cells lining the colon and have various health benefits, including supporting gut barrier function, reducing inflammation, and regulating metabolism [49]. Moreover, Ruminococcus species play an important role in maintaining a balanced gut microbiome. They are considered key members of the microbial community and contribute to the overall stability and diversity of the gut ecosystem. They help create an environment that is less favorable for the growth of harmful bacteria, promoting gut health [50]. Interestingly, alterations in the abundance or diversity of Ruminococcus species have been associated with certain gut disorders [50]. For example, reduced levels of Ruminococcus have been observed in individuals with IBD and irritable bowel syndrome (IBS) [50, 51]. This suggests that Ruminococcus may have a protective role in these conditions, and its depletion may contribute to gut dysbiosis and disease development. We also observed significantly higher relative abundance of Mucispirillum. While the role of this bacterium in the gut microbiome is still not fully understood, studies suggest that they may have a symbiotic relationship with the host. They may contribute to the maintenance of gut homeostasis by interacting with the mucus layer and participating in the degradation of mucin. Mucispirillum bacteria have been found to possess various enzymes involved in mucin degradation, allowing them to break down complex carbohydrates present in the mucin molecules. Overall, previous studies from our lab have shown that increased abundance of Verrucomicrobia, Akkermansia, and Ruminococcus in the colon due to senolytic treatment is negatively correlated with senescence and SASP-associated markers such as p16, p21, Cxcl1, Mcp1, Il1β, Tnfα, and Il6 [46].
Interestingly, we also observed that Proteobacteria was more abundant in the Fisetin-treated animals. While some Proteobacteria are associated with IBDs, we believe that the abundance of this bacterium in the Fisetin-treated animals may be attributed to some species within the class Deltaproteobacteria which produce beneficial metabolites that maintain gut function [42]. Specifically, resident hydrogen sulfide–producing bacterium Desulfovibrionaceae was significantly upregulated in the DSS + Fisetin group. Studies have shown that hydrogen sulfide reconstitutes the intestinal mucosa, reduces intestinal inflammation, and has protective effects against stress-induced cellular senescence due to its anti-oxidative properties [52–57]. Further, Deferribacteraceae was also more abundant in the Fisetin-treated animals. Members of the Deferribacteraceae family are known for their ability to utilize iron as an energy source [58, 59]. They are anaerobic bacteria, meaning they thrive in environments with low oxygen levels, such as the gut. Deferribacteraceae bacteria have also been found to produce SCFAs as a byproduct of their fermentation processes [58, 59]. Moreover, some studies have suggested a potential link between Deferribacteraceae and the metabolism of mucin, the glycoprotein component of mucus produced by the gut lining, which is important for maintaining gut health and preventing certain gut disorders [59]. All in all, analysis of the microbiota indicates that DSS causes intestinal dysbiosis and that Fisetin may be restoring intestinal health by increasing the abundance of bacteria involved with modulation of senescence and inflammation, making it a promising therapeutic intervention for the management of IBDs.
In this study, we underscore the therapeutic potential of Fisetin in a model of DSS-induced colitis. Importantly, we reveal critical information on the pathophysiology of colitis while also offering preliminary evidence on the therapeutic potential of Fisetin to target senescent intestinal cells. In particular, Fisetin appears to significantly influence the expression of various molecular markers implicated in DSS-induced senescence and inflammation while also improving the microbiota. Our study reveals that DSS insult initiates a cascade of cellular events that lead to the accumulation of senescent cells. In turn, these cells release SASP factors to exacerbate inflammation and influence neighboring cells. Concurrently, DSS induces inflammation to promote cellular senescence thereby altering the biomolecular landscape. This pro-inflammatory milieu drives senescence and generates DNA damage, thereby activating cell cycle arrest proteins that are crucial to cellular repair mechanisms. Particularly, DSS insult is characterized by changes in key mRNAs central to senescence and inflammatory processes, alongside miRNA signatures conducive to colitis. Microbial dysbiosis is another factor that aggravates the pro-inflammatory environment. Fisetin intercepts by inhibiting cellular senescence, decreasing inflammation, reversing miRNA expression, and restoring healthy microbiota. Previous studies have shown that Fisetin also exerts anti-inflammatory activity in colitis through suppression of Nfkb which may regulate the expression of pro-inflammatory markers such as Ptgs2 [60], representing potential alternative pathways aside from directly targeting and removing senescent cells. Remarkably, the efficacy of Fisetin to restore the gut microbiota, particularly the increased relative abundance of Akkermansia mucinphila, is notable as this microbe is inversely correlated with senescence and inflammation [46]. This initial data indicates that targeting senescent cells in IBDs should be a promising avenue of interest for researchers and clinicians. Considering that Fisetin is a naturally occurring compound with low toxicity, it is a very attractive candidate for the treatment of IBDs compared to currently available synthetic drugs which may cause drug resistance and relapse of symptoms. The clinical application of Fisetin requires a deeper understanding of how genetic diversity and lifestyle behaviors may influence its therapeutic potential, how it interacts with existing synthetic drugs, and how it influences different clinical subtypes of IBDs. Alongside, further investigations are necessary to determine its interactions with the complex human gut microbiome. Our study elucidates the molecular impacts of Fisetin and lays the foundation for future studies. These studies are necessary to validate and understand some of our nuanced findings and to determine the long-term impacts of Fisetin treatment on colitis.
Acknowledgements
The authors acknowledge the support from the USF Center for Microbiome Research and its core facilities for performing microbiome sequencing and bioinformatics support.
Author contribution
SAA, DC, AS, SAN, HY, and MMM conceptualized the project. SAA, DC, XS, AS, SAN, HY, and MMM developed the methodology. SAA, DC, SN, SS, DNG, AG, MS, BR, BMZ, and MAAM performed the experiments. MMM, HY, SN, BR, AG, MS, SAA acquired funding. HY, SAN, and MMM supervised the project. SAA wrote the original draft of the manuscript. All authors were responsible for editing and review of the manuscript.
Funding
We would like to thank the National Institutes of Health (R56 AG074499, R56AG069676, R56AG064075, RF1AG071762, R21AG072379, U01AG076928, R21DE032197), the Department of Defense (W81XWH-18-PRARP AZ180098), the Ed and Ethel Moore Alzheimer’s Disease Research Program of the Florida Department of Health (22A17) for Drs. Masternak and Yadav groups, the Florida Legislative Grant 2014–2022 for Drs. Masternak and Naser groups, the European Commission program HORIZON 2020-MSCA-RISE, Marie Sklodowska-Curie Staff Actions for MMM, BR, AG, MS, and the Richard Tucker Gerontology Applied Research Grant sponsored by the Learning Institute for Elders at the University of Central Florida for the support for Sarah Ashiqueali.
Declarations
Conflict of interest
Dr. Yadav is co-founder and chief scientific officer of the Postbiotics Inc, but has no conflict in the work presented in this manuscript. Other authors declare that no competing interests exist related to this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369(9573):1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 2.Perse M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol. 2012;2012:718617. doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Head K, Jurenka J. Inflammatory bowel disease part i: ulcerative colitis – pathophysiology and conventional and alternative treatment options. Altern Med Rev. 2003;8:247–283. [PubMed] [Google Scholar]
- 4.Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154(12):1417–1423. doi: 10.1001/jamadermatol.2018.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saccon TD, et al. Plasma miRNA profile of Crohn’s disease and rheumatoid arthritis patients. Biology (Basel) 2022;11:4. doi: 10.3390/biology11040508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang B, et al. Inflammatory bowel disease is associated with higher dementia risk: a nationwide longitudinal study. Gut. 2021;70(1):85–91. doi: 10.1136/gutjnl-2020-320789. [DOI] [PubMed] [Google Scholar]
- 7.Yorulmaz E, et al. Metabolic syndrome frequency in inflammatory bowel diseases. Saudi J Gastroenterol. 2011;17(6):376–382. doi: 10.4103/1319-3767.87177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, et al. The risk of rheumatoid arthritis among patients with inflammatory bowel disease: a systematic review and meta-analysis. BMC Gastroenterol. 2020;20(1):192. doi: 10.1186/s12876-020-01339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh H, et al. Gastro-intestinal and oral microbiome signatures associated with healthy aging. Geroscience. 2019;41(6):907–921. doi: 10.1007/s11357-019-00098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faye A. Colombel, J-F, Aging and IBD: a new challenge for clinicians and researchers. Inflamm Bowel Dis. 2022;28:126–132. doi: 10.1093/ibd/izab039. [DOI] [PubMed] [Google Scholar]
- 11.Kirkland JL, Tchkonia T. Cellular senescence: a translational perspective. EBioMedicine. 2017;21:21–28. doi: 10.1016/j.ebiom.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumari R, Jat P. Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front Cell Dev Biol. 2021;9:645593. doi: 10.3389/fcell.2021.645593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tchkonia T, et al. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123(3):966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, et al. Senescent stem and transient amplifying cells in Crohn’s disease intestine. Inflamm Bowel Dis. 2020;26(2):e8–e9. doi: 10.1093/ibd/izz295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirkland JL, Tchkonia T. Senolytic drugs: from discovery to translation. J Intern Med. 2020;288(5):518–536. doi: 10.1111/joim.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yousefzadeh MJ, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28. doi: 10.1016/j.ebiom.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Justice JN, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. doi: 10.1016/j.ebiom.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chassaing B, et al. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:15 251–1-5 25 14. doi: 10.1002/0471142735.im1525s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitajima S, Takuma S, Morimoto M. Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Exp Anim. 1999;48:137–143. doi: 10.1538/expanim.48.137. [DOI] [PubMed] [Google Scholar]
- 20.Nambiar A, et al. Senolytics dasatinib and quercetin in idiopathic pulmonary fibrosis: results of a phase I, single-blind, single-center, randomized, placebo-controlled pilot trial on feasibility and tolerability. EBioMedicine. 2023;90:104481. doi: 10.1016/j.ebiom.2023.104481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullen M, et al. Fisetin attenuates cellular senescence accumulation during culture expansion of human adipose-derived stem cells. Stem Cells. 2023;41(7):698–710. doi: 10.1093/stmcls/sxad036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viennois E, Tahsin A, Merlin D. Purification of Total RNA from DSS-treated Murine Tissue via Lithium Chloride Precipitation. Bio Protoc. 2018;8(9):e2829. 10.21769/BioProtoc.2829. [DOI] [PMC free article] [PubMed]
- 24.Simões AE, Pereira DM, Amaral JD, Nunes AF, Gomes SE, Rodrigues PM, Lo AC, D'Hooge R, Steer CJ, Thibodeau SN, Borralho PM, Rodrigues CM. Efficient recovery of proteins from multiple source samples after TRIzol(®) or TRIzol(®)LS RNA extraction and long-term storage. BMC Genomics. 2013;14:181. 10.1186/1471-2164-14-181. [DOI] [PMC free article] [PubMed]
- 25.Mijit M, et al. Role of p53 in the regulation of cellular senescence. Biomolecules. 2020;10:3. doi: 10.3390/biom10030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valieva Y, et al. Senescence-associated beta-galactosidase detection in pathology. Diagnostics (Basel) 2022;12:10. doi: 10.3390/diagnostics12102309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mor-Vaknin N, et al. Murine colitis is mediated by vimentin. Sci Rep. 2013;3:1045. doi: 10.1038/srep01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andoh A, Nishida A. Molecular basis of intestinal fibrosis in inflammatory bowel disease. Inflamm Intest Dis. 2023;7(3–4):119–127. doi: 10.1159/000528312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frescas D, et al. Senescent cells expose and secrete an oxidized form of membrane-bound vimentin as revealed by a natural polyreactive antibody. Proc Natl Acad Sci U S A. 2017;114(9):E1668–E1677. doi: 10.1073/pnas.1614661114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coppe JP, et al. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolodkin-Gal D, et al. Senolytic elimination of Cox2-expressing senescent cells inhibits the growth of premalignant pancreatic lesions. Gut. 2022;71(2):345–355. doi: 10.1136/gutjnl-2020-321112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meng A, Zhang X, Shi Y. Role of p38 MAPK and STAT3 in lipopolysaccharide-stimulated mouse alveolar macrophages. Exp Ther Med. 2014;8(6):1772–1776. doi: 10.3892/etm.2014.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tüfekci KU, Oner MG, Meuwissen RL, Genç S. The role of microRNAs in human diseases. Methods Mol Biol. 2014;1107:33–50. 10.1007/978-1-62703-748-8_3. [DOI] [PubMed]
- 34.Feng Q, Li Y, Zhang H, Wang Z, Nie X, Yao D, Han L, Chen WD, Wang YD. Deficiency of miRNA-149-3p shaped gut microbiota and enhanced dextran sulfate sodium-induced colitis. Mol Ther Nucleic Acids. 2022;30:208–25. 10.1016/j.omtn.2022.09.018. [DOI] [PMC free article] [PubMed]
- 35.Peck BC, et al. MicroRNAs classify different disease behavior phenotypes of Crohn’s disease and may have prognostic utility. Inflamm Bowel Dis. 2015;21(9):2178–2187. doi: 10.1097/MIB.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu G, et al. MicroRNA-149 negatively regulates TLR-triggered inflammatory response in macrophages by targeting MyD88. J Cell Biochem. 2014;115(5):919–927. doi: 10.1002/jcb.24734. [DOI] [PubMed] [Google Scholar]
- 37.Santana PT, et al. Dysbiosis in inflammatory bowel disease: pathogenic role and potential therapeutic targets. Int J Mol Sci. 2022;23:7. doi: 10.3390/ijms23073464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coman V, Vodnar DC. Gut microbiota and old age: modulating factors and interventions for healthy longevity. Exp Gerontol. 2020;141:111095. doi: 10.1016/j.exger.2020.111095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, et al. Age-related compositional changes and correlations of gut microbiome, serum metabolome, and immune factor in rats. Geroscience. 2021;43(2):709–725. doi: 10.1007/s11357-020-00188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Catalkaya G, et al. Interaction of dietary polyphenols and gut microbiota: microbial metabolism of polyphenols, influence on the gut microbiota, and implications on host health. Food Frontiers. 2020;1(2):109–133. doi: 10.1002/fft2.25. [DOI] [Google Scholar]
- 41.Yang B, et al. Lactobacillus ruminis alleviates DSS-induced colitis by inflammatory cytokines and gut microbiota modulation. Foods. 2021;10:6. doi: 10.3390/foods10061349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stojanov S, Berlec A, Strukelj B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020;8:11. doi: 10.3390/microorganisms8111715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geerlings SY, et al. Akkermansia muciniphila in the Human gastrointestinal tract: when, where, and how? Microorganisms. 2018;6:3. doi: 10.3390/microorganisms6030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JG, et al. Impact of short-chain fatty acid supplementation on gut inflammation and microbiota composition in a murine colitis model. J Nutr Biochem. 2022;101:108926. doi: 10.1016/j.jnutbio.2021.108926. [DOI] [PubMed] [Google Scholar]
- 45.van der Lugt B, et al. Akkermansia muciniphila ameliorates the age-related decline in colonic mucus thickness and attenuates immune activation in accelerated aging Ercc1 (-/Delta7) mice. Immun Ageing. 2019;16:6. doi: 10.1186/s12979-019-0145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saccon TD, et al. Senolytic combination of dasatinib and quercetin alleviates intestinal senescence and inflammation and modulates the gut microbiome in aged mice. J Gerontol A Biol Sci Med Sci. 2021;76(11):1895–1905. doi: 10.1093/gerona/glab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodrigues VF, et al. Akkermansia muciniphila and gut immune system: a good friendship that attenuates inflammatory bowel disease, obesity, and diabetes. Front Immunol. 2022;13:934695. doi: 10.3389/fimmu.2022.934695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Portincasa P, et al. Gut microbiota and short chain fatty acids: implications in glucose homeostasis. Int J Mol Sci. 2022;23:3. doi: 10.3390/ijms23031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.den Besten G, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagao-Kitamoto H, Kamada N. Host-microbial cross-talk in Inflammatory Bowel Disease. Immune Netw. 2017;17(1):1–12. doi: 10.4110/in.2017.17.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schirmer M, et al. Microbial genes and pathways in inflammatory bowel disease. Nat Rev Microbiol. 2019;17(8):497–511. doi: 10.1038/s41579-019-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Latorre E, Torregrossa R, Wood ME, Whiteman M, Harries LW. Mitochondria-targeted hydrogen sulfide attenuates endothelial senescence by selective induction of splicing factors HNRNPD and SRSF2. Aging (Albany NY). 2018;10:1666–81. 10.18632/aging.101500. [DOI] [PMC free article] [PubMed]
- 53.Wallace JL, et al. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology. 2009;137(2):569–78, 578 e1. doi: 10.1053/j.gastro.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Qin M, et al. Hydrogen sulfide protects against DSS-induced colitis by inhibiting NLRP3 inflammasome. Free Radic Biol Med. 2019;137:99–109. doi: 10.1016/j.freeradbiomed.2019.04.025. [DOI] [PubMed] [Google Scholar]
- 55.Blachier F, et al. Production of hydrogen sulfide by the intestinal microbiota and epithelial cells and consequences for the colonic and rectal mucosa. Am J Physiol Gastrointest Liver Physiol. 2021;320(2):G125–G135. doi: 10.1152/ajpgi.00261.2020. [DOI] [PubMed] [Google Scholar]
- 56.Motta JP, et al. Hydrogen sulfide protects from colitis and restores intestinal microbiota biofilm and mucus production. Inflamm Bowel Dis. 2015;21(5):1006–1017. doi: 10.1097/MIB.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 57.Perridon BW, Leuvenink HGD, Hillebrands J-L, van Goor H, Bos EM. The role of hydrogen sulfide in aging and age-related pathologies. Aging. 2016;8(10):2264–89. 10.18632/aging.101026. [DOI] [PMC free article] [PubMed]
- 58.Yang F, et al. Association of fecal microbiota with irritable bowel syndrome-diarrhea and effect of traditional chinese medicine for its management. Gastroenterol Res Pract. 2021;2021:7035557. doi: 10.1155/2021/7035557. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Herp S, et al. Mucispirillum schaedleri antagonizes salmonella virulence to protect mice against colitis. Cell Host Microbe. 2019;25(5):681–694 e8. doi: 10.1016/j.chom.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 60.Sahu BD, Kumar JM, Sistla R. Fisetin, a dietary flavonoid, ameliorates experimental colitis in mice: relevance of NF-kappaB signaling. J Nutr Biochem. 2016;28:171–182. doi: 10.1016/j.jnutbio.2015.10.004. [DOI] [PubMed] [Google Scholar]