Abstract
Kaposi’s sarcoma-associated herpesvirus (KSHV) (also called human herpesvirus 8) is a novel gammaherpesvirus strongly implicated in the pathogenesis of Kaposi’s sarcoma. Although virions can be produced in high yield from latently infected B-cell lines treated with phorbol esters, little is known about the infectivity of such virus, and efficient serial propagation of KSHV has been problematic. Here we report on the infectivity of KSHV produced from phorbol-induced BCBL-1 cells, employing an assay based on the detection of a spliced late mRNA by a sensitive reverse transcriptase PCR (RT-PCR) method. The results of this study confirm previous observations that 293 cells are susceptible to viral infection; however, infection with BCBL-1-derived virus is inefficient and the pattern of viral gene expression in infected cells may not fully reproduce that of authentic lytic infection. In keeping with this finding, serial propagation of BCBL-1-derived virus could not be demonstrated on 293 cells. Eleven of 38 other cell lines tested also supported KSHV infection, as judged by this RT-PCR assay, including cells of B-cell, endothelial, epithelial, and fibroblastic origin; however, in all cases, infection proceeded at or below the levels observed in 293 cells.
Kaposi’s sarcoma-associated herpesvirus (KSHV), also called human herpesvirus 8, is a novel human gammaherpesvirus that is tightly linked to several human diseases. As implied by its name, evidence of viral infection is found in virtually all cases of Kaposi’s sarcoma (KS), (reference 14 and references therein), and several lines of evidence point to a key etiologic role in this disease. KSHV infection precedes clinical KS, is highly associated with increased KS risk in all populations thus far studied (15, 16, 30), and targets the endothelial (spindle) cell thought to be the prime determinant of KS tumorigenesis (7, 27). In addition, viral genomes are regularly found in primary effusion lymphoma (PEL) (a rare, AIDS-related B-cell neoplasm) (9) and in a second lymphoproliferative disorder, multicentric Castleman’s disease (26); more indirect links to multiple myeloma have recently been suggested (22, 24).
Attempts to cultivate KSHV in vitro have met with mixed success. B-cell lines derived from PEL are latently infected with KSHV (10, 21). One such line, BCBL-1, has been extensively characterized in our laboratory (20, 21). Unlike many PEL-derived lines, it harbors KSHV in the absence of concomitant Epstein-Barr virus (EBV) infection; viral genomes are maintained in the nucleus as circular episomes whose expression is highly attenuated (20, 21). Treatment of these cells with phorbol esters results in dramatic induction of lytic replication, with 20 to 30% of the cells displaying evidence of late gene expression and with the accumulation of large quantities of morphologically correct virions in the culture medium (21). Several other PEL cell lines with similar properties have since been described (2, 13), though other widely used lines (e.g., BC-1) (10) appear to harbor rearranged genomes that do not support virion production or do so very poorly (18, 19). These B-cell lines have been enormously useful in KSHV research, allowing experimental study of latent and lytic infection as well as serving as sources of viral antigens for serologic testing. However, they do not allow analysis of viral infectivity. Since all cells in the culture are already infected and since most cells die following induction (either from viral cytopathic effect or from phorbol ester toxicity), there is little opportunity to observe horizontal spread of infection in such cultures. Attempts to transmit infection to other cell lines by inoculation with B-cell-derived materials have been made but have not yielded a clear picture. Infection has generally been assayed by PCR for viral DNA. Using this assay, several groups have described limited or transient transfer to several recipient lines (12, 29), but interpretation has been complicated by the fact that this assay does not reliably distinguish authentic infection from persistence of viral DNA sequences derived from the inoculum. For example, material derived from BC-1 cells, a line whose rearranged genome is likely to be too large to permit efficient encapsidation, has been reported to be infectious in Raji cells (EBV-positive Burkitt’s lymphoma line) by this criterion (19). More recently, Foreman et al. (12) have examined the spread of KSHV isolates from primary explants of KS tissue to cultured cell monolayers, again using DNA PCR to assay the transfer. They showed that infection could be transferred to 293 cells, albeit inefficiently, and reported that serial transmission from 293 cells was observable. Based in part on their findings, we have explored the infectivity of KSHV derived from the PEL cell line BCBL-1, using a reverse transcriptase PCR (RT-PCR) assay that reliably distinguishes authentic infection from persistence of the inoculum. Here we show that BCBL-1-derived virus will reproducibly infect 293 cells and a variety of other cell lines but that infection is both inefficient and abortive, in that the products of infection cannot sustain further transmission of infection.
MATERIALS AND METHODS
ORF29 RT-PCR assay.
Two primers at nucleotide position 53915 (29A [GCA CGT AGC CAA CTC CGT G]) and nucleotide position 50345 (29B GCA GGA AAC TCG TGG AGC G) spanning both sides of open reading frame 29A (ORF29A) and ORF29B were synthesized to characterize the splice donor and acceptor sites of this gene. Total RNA from BCBL-1 cells and from KS biopsy material was extracted by using RNazol (Tel-Test, Inc., Friendswood, Tex.) according to the supplier’s protocol. Total RNA (500 ng) was reverse transcribed by using 200 U of Moloney murine leukemia virus RT (Gibco BRL) in a total volume of 20 μl containing 125 μM dATP, dGTP, and dTTP, 20 U of RNasin (Promega), and 120 pmol of random hexanucleotide primers (Boehringer Mannheim). After incubation at 42°C for 35 min, the reaction was stopped by heating to 95°C for 5 min. To each RT reaction mixture, 80 μl of a PCR mix containing 10× PCR buffer, 100 pmol of each primer, and 5 U of Taq polymerase (Perkin Elmer) was added. Each reaction mixture was overlaid with 50 μl of mineral oil prior to amplification for 30 cycles of PCR (1 cycle consists of 30 s at 94°C, 1 min at 58°C, and 1 min 30 s at 72°C) (6). Amplification products were directly ligated into pCRII (Invitrogen, San Diego, Calif.), and derived clones were sequenced by using Sequenase (Amersham) according to the supplier’s protocols. For all infectivity testings, 42 cycles of PCR (1 cycle consists of 30 s at 94°C, 1 min at 58°C, and 1 min 30 s at 72°C) were performed. Amplification products were electrophoresed in 1.5% agarose gels, and DNA was transferred to nylon membranes (Hybond-N). Filters were then hybridized to probe generated by PCR using a nested pair of ORF29-specific oligonucleotides at nucleotide position 50366 (29Bi [CTG ACG AGT TCA CGG ATG]) and 53815 (29Ai [TAC ACG CGA CCC GGA GGA]) at 65°C in Church buffer (11). Probes were 32P labeled by using a Random DNA Prime kit from Amersham.
Preparation of inoculum and infections.
BCBL-1 cells were induced by treatment with tetradecanoyl phorbol acetate (TPA) as previously described (21). KSHV virions were pelleted and concentrated in three steps. First, induced-cell cultures were centrifuged for 5 min at 4,000 rpm, and supernatants were transferred to fresh tubes and centrifuged for 30 min at 4,000 rpm. KSHV virions were then pelleted from these cell-free supernatants by ultracentrifugation for 2 h at 15,000 rpm. Viral pellets were resuspended in 1/30 of the original volume using RPMI 1640 without fetal calf serum (FCS). To prepare virions from uninduced BCBL-1 cells, the TPA induction step was omitted. For most infectivity assays, 1 ml of inoculum (which equals 30 ml of supernatant) was added to monolayers (30 to 50% confluency) or to suspension cultures at a density of 2 × 105 to 5 × 105 cells/ml. After 8 h, cells were washed with phosphate-buffered saline and overlaid with fresh medium. In experiments in which TPA was used, virus-inoculated cells were treated 8 to 12 h prior to harvest with TPA at a concentration of 10 ng/ml. Total RNA was extracted 48 to 96 h after inoculation. To prepare inocula from cell extracts, TPA-induced or uninduced BCBL-1 cells were lysed by three cycles of freezing and thawing. These lysates were centrifuged at 10,000 × g for 10 min and finally filtered through a 0.45-μm-pore-size membrane.
Analysis of infected 293 cell expression pattern.
Total RNA was extracted with RNazol (Tel-Test, Inc.) according to the supplier’s protocol, and poly(A)+ RNA was selected by using the Oligotex mRNA Kit (Qiagen). To generate radiolabeled cDNA probes, 100 ng of poly(A)+ RNA from either TPA-induced BCBL-1 cells or KSHV-infected 293 cells was reverse transcribed by using 200 U of Moloney murine leukemia virus RT in a total volume of 50 μl containing 0.5 mM dATP, dGTP, and dTTP, and 0.1 mM dCTP, 50 μCi (1 Ci = 37 Gbq) of [α-32P]dCTP, 50 U of RNasin (Promega) and about 50 pmol of random hexanucleotide primers (Boehringer Mannheim). The reaction mixture was incubated at 37°C for 1 h, and the reaction was stopped by heating to 95°C for 5 min. The labeled cDNA probes were hybridized to filters containing cloned KSHV DNA at 65°C overnight in Church hybridization buffer (11). To produce filters, nine lambda clones containing ca. 120 kbp of KSHV DNA were digested with SalI or SacI and 2 to 4 μg of each clone was loaded on a 0.8% agarose gel, separated by electrophoresis, and transferred to nylon membranes (Hybond-N).
Cell cultures.
All Burkitt’s lymphoma cell lines (BL30, BL41, BL41B95-8, Namalwa, Ramos, Raji, Daudi, Loukes, IB4, and BJAB) were a kind gift of Eliott Kieff and were grown in RPMI 1640 supplemented with 10% FCS, 0.05 mM 2-mercaptoethanol, 1 mM sodium pyruvate, and 2 mM l-glutamine. BPH-1, ND-1, and Ln-Cap were grown in RPMI 1640 supplemented as described above but with only 5% FCS. 293 cells, Vero, BHK-21, OMK637, and COS-7 cells were obtained from the American Type Culture Collection (ATCC) and were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% FCS and 2 mM l-glutamine. A549 and CHELI (kindly provided by E. Lenette) cells were cultured in DMEM supplemented with 10% FCS, 2 mM l-glutamine and antibiotics. CEMx174, U937, HCT-8, HT29, 343MG, and HUf23 cells were cultured in RPMI 1640 medium supplemented with 10% FCS, 2 mM glutamine, and antibiotics. The endothelial cells HUVEC, CNVEC, AEC, BB19, and 181GB1-4 were transformed with the E6 and E7 genes of human papillomavirus (HPV) type 16. These transformed endothelial cells (kindly provided by Ashlee Moses and Jay Nelson) were cultured in Endothelial Cell Growth Medium (Clonetics Corporation), which was supplemented with G418 (200 μg/ml). Primary neonatal and adult capillary endothelial cell cultures (kindly provided by Scott Heron) were also grown in Endothelial Cell Growth Medium (Clonetics Corporation). FaDu, RPMI2650, and SCC15 were obtained from ATCC and cultured according to ATCC instructions.
Patient KSHV isolates and coculture with 293 cells.
KS biopsies were previously quick-frozen in liquid nitrogen. Biopsy tissue was cocultivated with subconfluent 293 cells in six-well plates. Supernatant was harvested at 3 and 7 days, and cells were passaged every 7 days. Virus-containing supernatants were filtered through a 0.45-μm-pore-size filter before storage at −70°C or reinfection of 293 cells. For passage of cell-free virus supernatant, filtered stocks were added to freshly trypsinized 293 cells. The medium was changed after the cells were allowed to adhere overnight.
Peripheral blood mononuclear cells (PBMCs) from KS patients were separated from 30-ml samples of EDTA-treated whole blood on Ficoll gradients. T cells were then depleted using CD4- and CD8-specific magnetic beads (MACS) (Miltenyi Biotech Inc). The remaining cells were cocultivated with freshly trypsinized 293 cells as described above, with and without 5 mM N-butyrate. For PCR analysis, 500 μl of filtered supernatant was pelleted at 100,000 rpm for 30 min in a Beckman Optima TLX benchtop ultracentrifuge. DNA was extracted from the pellet, and nested PCR was performed as described by Boshoff et al. (8).
RESULTS
Infectivity assay.
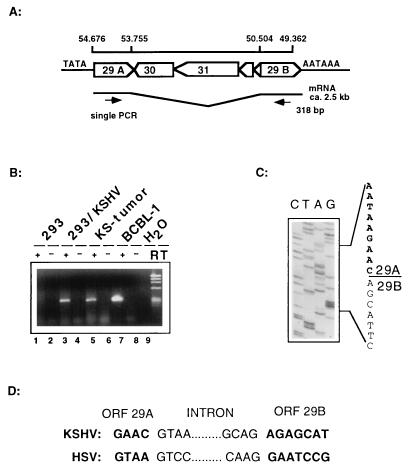
To develop a more reliable assay for KSHV infectivity, we decided to assay for the production of viral mRNA, which can occur only following viral particle uptake and nuclear delivery of the viral genome. For this purpose, we chose to employ RT-PCR, which can sensitively detect even small quantities of viral transcripts. To allow rigorous discrimination from input DNA and to provide information about the nature of the infection in the recipient cell, we selected as the RT-PCR target a gene (KSHV ORF29) which in other herpesviruses is (i) spliced and (ii) expressed only late in the lytic infection cycle, following viral DNA replication. The product of this gene is involved in DNA packaging, a step late in the capsid assembly pathway (3, 4). The recently published sequence of the KSHV genome suggested that ORF29 has two exons which are separated by a 4-kbp-long intron harboring two viral genes on the opposite strand (a similar arrangement exists in other herpesviruses, e.g., herpes simplex virus, herpesvirus saimiri, and EBV) (Fig. 1A) (23). The presence of such a large intron in ORF29 makes this gene an ideal candidate for the development of an RT-PCR assay, since the spliced RT-PCR product is smaller than any amplification product from contaminating genomic DNA. To validate this assumption, we prepared primers from regions flanking the proposed splice donor and acceptor regions (see Materials and Methods) and used them to amplify cDNA prepared from BCBL-1 cells as well as from a solid KS tumor. As shown in Fig. 1B (lanes 5 and 7), a PCR product approximately 300 bp long was produced only from RNA which had been reverse transcribed (lanes 5 and 7) but not from RNA in the absence of RT (lanes 6 and 8). To confirm its spliced nature, we cloned the amplified fragment and determined its sequence. The cloned fragment contained sequences from both exons (ORF29A and ORF29B) and mapped the splice donor to nucleotide position 53755. This splice donor was spliced to a splice acceptor site at position 50504 in the KSHV genome (Fig. 1A and C). The intron termini (5′ GT and 3′ AG) are in agreement with the conventional splicing consensus sequence and are similar to those observed in UL15, the ORF29 homolog of herpes simplex virus (Fig. 1D) (4). Therefore, ORF29 of KSHV is expressed from a spliced mRNA which is readily detectable in BCBL-1 cells and also in RNA extracted from KS biopsy material.
FIG. 1.
Mapping of the splice donor and acceptor sites of ORF29 in BCBL-1 cells and KS tumors by RT-PCR. (A) Genomic map of the region containing both exons of ORF29 divided by a 3.2-kbp region encoding four ORFs on the opposite strand. (B) Agarose gel showing the RT-PCR products. Lanes 5 and 7 show products derived from KS tumor RNA and BCBL-1 cells after 30 cycles. Lanes 1 and 2 show products derived from mock-infected or KSHV-infected 293 cells after 42 PCR cycles. As an additional control for DNA contamination, all samples were amplified in the presence (+) and absence (−) of RT. (C) After cloning of the RT-PCR product into the vector pCRII (Invitrogen), clones derived from BCBL-1 and 293 cells were sequenced and showed identical results. The sequence of the exon border between ORF29A and ORF29B is outlined. (D) Comparison of the exon and intron borders of KSHV ORF29 and herpes simplex virus (HSV) UL15; both show the conserved GT/AG major class intron consensus sequence.
Next we assessed if this ORF29-directed RT-PCR assay can be used to screen cell lines for their susceptibility to KSHV derived from TPA-induced BCBL-1 cells. 293 cells (a transformed human kidney cell line) were first chosen as a target cell line, since Foreman et al. (12) had earlier reported this cell line as susceptible to KSHV derived from KS biopsies. As inoculum, cell-free supernatants of TPA-induced BCBL-1 cells containing approximately 107 viral genome equivalents/ml were prepared as previously described (21). 293 cells were seeded into six-well plates 24 h prior to inoculation with KSHV virions. After adsorption for 8 to 12 h, monolayers were washed twice before with fresh growth medium (Materials and Methods) was laid over the monolayers. Total cellular RNA was extracted 48 to 72 h after exposure, and 0.5 μg of RNA was used for RT-PCR analysis as described in Materials and Methods. As shown in Fig. 1B, cells which were mock infected did not produce a PCR band (lanes 1 and 2), whereas 293 cells exposed to KSHV showed a band which comigrated with the PCR products obtained by amplifying cDNA from either BCBL-1 cells or a KS tumor (lanes 5 and 7). To confirm these results, these bands have also been cloned and sequenced as described earlier and showed the same sequence and splice junctions as found in BCBL-1-derived ORF29 transcripts (data not shown). These data indicate that BCBL-1-derived virus is capable of entering 293 cells, delivering its genome to the nucleus, and allowing a series of transcriptional events, including expression of genes belonging to the late phase of the lytic cycle.
If infectious virus particles were generated in these 293 cells, then it should be possible to serially passage KSHV derived from these cells. When we inoculated fresh 293 cells with supernatants of 293 cells which had scored positive in the ORF29 RT-PCR assay, we were not able to demonstrate serial passage, suggesting that infection of 293 cells was abortive (data not shown). We also did not observe any cytopathic effect in 293 cells infected with KSHV, in contrast to previously reported observations (12). We therefore decided to analyze the transcriptional program of KSHV in 293 cells in more detail.
Characterization of 293 cell infection.
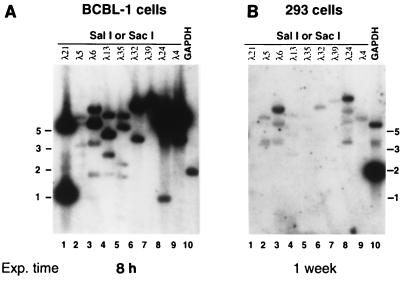
In infected 293 cells which were positive in the ORF29 RT-PCR, we were not able to detect individual viral transcripts by Northern blot analysis with virus-specific probes, including the 1.1-kb nut-1 transcript which is the most abundant RNA produced in the lytic infection cycle of KSHV (28, 31, 32). To examine further the pattern of viral transcription in 293 cells, we employed a cDNA assay we had earlier developed to study viral gene expression in BCBL cells and KS tumors. Briefly, poly(A)+ RNA was prepared from the infected cells and used as the template for randomly primed cDNA synthesis in the presence of 32P-labeled deoxynucleoside triphosphates. This labeled cDNA probe, which represents all viral and cellular RNA in the population was then used to probe a filter bearing restriction fragments spanning most of the genome of KSHV. As previously reported (21), when such an experiment is performed with RNA from TPA-induced BCBL-1 cells, strong hybridization to many bands is observed (Fig. 2A). KSHV-infected 293 cells, however, showed a different expression pattern of viral genes. Using RNA from three independent infections which scored positive in the ORF29 RT-PCR assay, only very faint signals in this cDNA hybridization assay were detected in two of these three experiments. The third assay generated stronger autoradiographic signals; results of this assay are shown in Fig. 2B. Two points are noteworthy. (i) The exposure time employed here was 20 times that in the BCBL-1 cell experiment of Fig. 2A. (ii) The pattern of positive bands is not identical to that generated in BCBL-1 cells. The most striking difference is seen in lanes 1 and 9. These lanes contain fragments corresponding to the nut-1 (T1.1) gene of KSHV, whose product accumulates to ca. 105 copies per cell in lytic infection (28, 32). As expected, a prominent band is observed in this lane with cDNA from BCBL-1 cells (Fig. 2A, lanes 1 and 9); however, no comparable signal is generated by cDNA from infected 293 cells (Fig. 2B, lanes 1 and 9). Taken together, these results show that (i) expression of KSHV genes by 293 cells is extremely inefficient compared to that generated by induction of BCBL-1 cells, with variable RNA levels hovering around the detection threshold of the cDNA hybridization assay, and that (ii) the transcriptional program in 293 cells is not identical to that observed in BCBL-1 cells or in KS lesions (32). Several possible explanations for the differences in the transcriptional program (and the failure to serially passage the virus) can be envisioned. (i) 293 cells may be only semipermissive and may support only abortive infection. (ii) KSHV virions from BCBL-1 cells may harbor mutations that affect infectivity. (iii) Cell-free virus does not efficiently infect using these cell culture conditions. (iv) The transcriptional program following de novo infection may differ from that following induction from latency.
FIG. 2.
Gene expression in BCBL-1 cells compared to that in KSHV-infected 293 cells. Phage DNA from the indicated lambda clones, which together span ca. 120 kb of the KSHV genome, was cleaved with the indicated restriction enzyme, electrophoresed through 0.8% agarose gels, stained with ethidium bromide, and transferred to duplicate nylon filters. To these filters was annealed radiolabeled cDNA probes corresponding to poly(A)+ transcripts from TPA-treated BCBL-1 cells or KSHV-infected 293 cells prepared as described in Materials and Methods. As a control for integrity of the probe as well as an index of the amount of poly(A) RNA used in the assay, a plasmid encoding glyceraldehyde phosphate dehydrogenase (GAPDH) was also electrophoresed in parallel (lane 10). Blot A was exposed for 8 h; in contrast, blot B was exposed for 1 week. Exp. time, Exposure time. (A) Hybridization of the filter with cDNA prepared from mRNA from TPA-treated BCBL-1 cells. (B) Hybridization of an identical filter with cDNA probe prepared from mRNA from KSHV-infected 293 cells. The positions of molecular size standards (in kilobases) are shown at the sides of the gels.
Cell-free virus preparations versus cellular extracts and cocultivation.
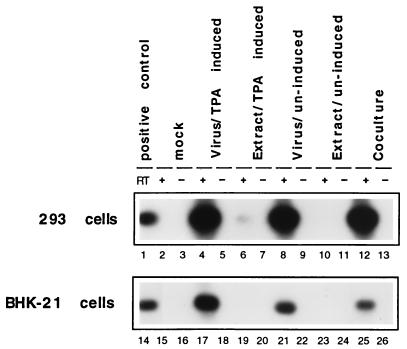
To address the possibility that cell-associated rather than cell-free virus might be the more infectious inoculum, we cocultivated 293 and BCBL-1 cells and also prepared inocula from extracts from BCBL-1 cells, by using techniques similar to those described by Foreman et al. (12). We inoculated 293 cells either with virion particles purified from the media of TPA-induced BCBL-1 cells or from extracts prepared by freeze-thaw cycles from induced and uninduced BCBL-1 cells. In addition, we tested supernatants of uninduced BCBL-1 cells and also performed coculture experiments with these cells. Total RNA and supernatants were harvested 72 h after exposure, and the RT-PCR assay for ORF29 mRNA was performed. To increase sensitivity, we blotted the PCR products and hybridized them to a probe spanning the splice sites of ORF29 (see Materials and Methods). The experimental results (Fig. 3) clearly demonstrate that cell-free virus derived from TPA-induced or uninduced BCBL-1 cells is much more infectious than extracts prepared from these cells (Fig. 3, compare lanes 4 and 8 to lanes 6 and 10). Cocultivation of BCBL-1 and 293 cells seems to be as efficient as cell-free virus preparations; however, in this assay we cannot exclude the possibility that some of the signal is due to contamination with input BCBL-1 cells, which can stick to the 293 monolayer. The lower panel of Fig. 3 shows a similar experiment with BHK-21 (baby hamster kidney cells) which are also infectible with KSHV, though at a lower level than 293 cells. In BHK-21 cells, we were not able to detect any signal by using cell extracts as the inoculum (Fig. 3, lanes 19 and 23). However, cell-free virus of either TPA-induced or uninduced BCBL-1 cells lead to a detectable signal (lanes 17 and 21). This result clearly shows that cell-free virus preparations of KSHV are more infectious than cellular extracts prepared from BCBL-1. The observation that supernatants from uninduced BCBL-1 cells have infectious material is not surprising, since 1 to 3% of the cells in these cultures spontaneously enter the lytic cycle. The fact that cell-free virus of both TPA-induced and uninduced cells give rise to comparable signals (Fig. 3, lanes 4, 8, 17, and 21) suggest that the virus titer might not be the limiting factor in the infectivity of KSHV for 293 and BHK-21 cells.
FIG. 3.
Cell-free virus is more infectious than inocula prepared from extracts. ORF29-specific RT-PCR products after 42 cycles of PCR were electrophoresed on a 0.8% agarose gel and transferred to a nylon membrane. Filters were hybridized to an ORF29-specific probe as described in Materials and Methods. As additional control for contamination, all samples were amplified in the presence (+) and absence (−) of RT. 293 and BHK-21 cells were infected with (i) cell-free virus from TPA-induced (lanes 4, 5, 17, and 18) or uninduced (lanes 8, 9, 21, and 22) BCBL-1 cells or (ii) with extracts prepared from TPA-induced (lanes 6, 7, 19, and 20) or uninduced (lanes 10, 11, 23, and 24) BCBL-1 cells. 293 and BHK-21 cells were seeded at 4 × 105 cells/well in six-well plates. Monolayers were then incubated for 8 h with either cell-free virions concentrated from 10 ml of supernatant (TPA induced and uninduced) or extracts prepared from 10 ml of BCBL-1 cells at a density of 5 × 105 cells/ml (TPA-induced and uninduced) as described in Materials and Methods. Monolayers were washed three times and overlaid with fresh medium, and total RNA was harvested 72 h later. In addition, 293 and BHK-21 cells were cocultivated with 106 BCBL-1 cells for 24 h (lanes 12, 13, 25, and 26). As a positive control, a diluted RT-PCR amplification product derived from BCBL-1 RNA was loaded in lanes 1 and 14.
BCBL-1-derived KSHV virions versus virus preparations obtained from KS biopsies.
The fact that we could not observe serial transmission of BCBL-1-derived KSHV virions in 293 cells raised the possibility that such virus stocks might harbor mutations reducing infectivity, despite the efficient production of morphologically correct virions. Such infectivity defects have been reported for virus from EBV-infected Burkitt’s lymphoma cell lines (17). Accordingly, we determined whether KSHV isolates from other sources might be more readily serially passaged. We examined four primary KSHV isolates that had been obtained from KS patients; two isolates were obtained by cocultivating the PBMCs from KS patients with 293 cells and two isolates were obtained by inoculating 293 cells with KS biopsy material as described in Materials and Methods. Supernatants of these cocultures were passaged several times on 293 cells and tested positive for the presence of viral genomes by DNA-based nested PCR. When cell-free supernatants of these cultures were used to infect fresh 293 cells, no signals were generated in the ORF29 RT-PCR assay (Fig. 4). This result suggests that the block to serial transmission of KSHV in 293 cells is not unique to BCBL-1-derived virus.
FIG. 4.
KSHV isolates from four different patients behave like BCBL-1-derived virus in the ORF29-directed infectivity assay. Fresh 293 cells were incubated with 293 cell supernatants from serially passaged KSHV isolates. All supernatants which have been serially passaged up to three times scored positive in a nested DNA PCR assay (Materials and Methods). Inoculation and RT-PCR assay was done as described in the legend to Fig. 3. None of these isolates scored positive even after prolonged exposure times (lanes 1 to 10).
Screening of additional cell lines for KSHV susceptibility.
To search for cell lines that might be more permissive for KSHV infection, we examined a total of 38 cultured cell lines and primary cells of different origin for KSHV susceptibility, using the ORF29-specific RT-PCR assay. Cell lines or primary cells tested for KSHV susceptibility are listed in Table 1, which also indicates their cell type of origin (where known). Based on the known phylogeny of KSHV as a gammaherpesvirus, and given its association with B-cell lymphomas, considerable emphasis was placed on infection of B cells, including numerous B-cell lines (either positive or negative for EBV infection) and also primary, CD19-positive B cells isolated from human peripheral blood. A complete summary of the results of this infectivity testing is presented in Table 1, and selected examples of these assays are shown in Fig. 5. Since ORF29 is a lytic gene, most tested cells were examined in the presence and absence of TPA; however, this treatment did not increase signal strength in any of the cell lines which tested positive for ORF29 transcripts (e.g., Fig. 5, lanes 10, 12, 24, and 26). Of the B-cell lines tested, only primary B-cells (CD19-positive PBMCs) supported KSHV infection, as has previously been reported by Blackbourn et al. (5). We also tested many endothelium-derived cell populations, given the known presence of viral DNA in KS spindle cells, which are thought to be of endothelial lineage (7, 27). Several established endothelial lines, whether derived from large vessels (e.g., HUVEC) or capillaries (e.g., HMEC) were nonpermissive. However, primary neonatal microvascular endothelial cells did support viral entry and transcription in this assay (Fig. 5, lanes 10 and 12); BB19 and 181GB1-4 cells, two human brain endothelial lines, were also susceptible at very low levels. Other positive cell lines included Ln-Cap, a line derived from cancerous human prostatic epithelium, and—surprisingly—two cell lines of nonhuman origin, owl monkey kidney cells (OMK), a line used for the growth of the related herpesvirus saimiri, and BHK-21 cells (Fig. 3 and Table 1). In summary, these data show that several cell lines are partially susceptible to KSHV infection. However, none of the 11 cell lines or primary cells seemingly permissive for KSHV were more susceptible than 293 cells.
TABLE 1.
Summary of results of infectivity assays using BCBL-1-derived KSHV virion preparations
| Cell origin | Cell line or type (description) | ORF29 RT-PCR resultc |
|---|---|---|
| Lymphoid | BL30a (EBV-negative Burkitt’s lymphoma) | − |
| BL41a (EBV-negative Burkitt’s lymphoma) | − | |
| Ramosa (EBV-negative Burkitt’s lymphoma) | − | |
| BJABa (EBV-negative Burkitt’s lymphoma) | − | |
| Louckesa (EBV-negative Burkitt’s lymphoma) | − | |
| Daudia (EBV-positive Burkitt’s lymphoma) | − | |
| BL41B95-8a (EBV-positive Burkitt’s lymphoma) | − | |
| Rajia (EBV-positive Burkitt’s lymphoma) | − | |
| IB4a (EBV positive) | − | |
| Namalwaa (EBV-positive Burkitt’s lymphoma) | − | |
| CEMx174 (B-cell–T-cell hybrid) | − | |
| U937 (monocyte derived) | − | |
| CD19+ B cellsa,b (primary B cells) | + | |
| Endothelial | HUVECa (human vascular endothelial cells [HPV transformed]) | − |
| HMECa (human microvascular endothelial cells [HPV transformed]) | − | |
| AEC (human aortic endothelial cells [HPV transformed]) | − | |
| BB19 (human brain endothelial cells [HPV transformed]) | + | |
| 181GB1-4 (human brain endothelial cell [HPV transformed]) | + | |
| Primary neonatal capillary endothelial cellsa | + | |
| Primary adult capillary endothelial cellsa | − | |
| Mixed primary adult capillary endothelial cellsa | − | |
| Epithelial | HCT-8 (human rectal epithelial [adenocarcinoma]) | − |
| HT29 (colon epithelial [adenocarcinoma]) | − | |
| 343MG (brain epithelial) | − | |
| OMK637 (owl monkey kidney) | + | |
| 293a (human kidney) | + | |
| Fibroblastoid | HUf23 (foreskin fibroblastoid cells) | − |
| Veroa (African green monkey kidney) | − | |
| COS-7 (African green monkey kidney) | − | |
| BHK-21a (baby hamster kidney) | + | |
| Prostate cancer-derived lines | BPH-1a | − |
| ND-1a | − | |
| Ln-Capa | + | |
| Other cancer-derived lines | A549 (human lung carcinoma) | + |
| CHELI (Chediak-Higachi syndrome) | + | |
| FaDu (squamous cell carcinoma, pharynx) | − | |
| RPMI2650 (squamous cell carcinoma, nasal septum) | − | |
| SCC15 (squamous cell carcinoma, tongue) | + |
Cells were tested in the absence and presence of TPA to induce lytic growth from a possibly latent stage of infection.
Prepared and cultured by the method of Blackbourn et al. (5).
+, positive; −, negative.
FIG. 5.
Screening for KSHV-susceptible cell lines. ORF29 RT-PCR after inoculation of cell-free virus concentrated from supernatants of TPA-induced BCBL-1 cells. A summary of all lines tested is given in Table 1. Assays were performed as described in the legend to Fig. 3. Endo, endothelial cells.
DISCUSSION
We have established a reliable and sensitive RT-PCR assay to screen for KSHV-susceptible cell lines and/or primary cell populations. The selection of a spliced gene (ORF29) as the RT-PCR target avoids possible confusion from contaminating input DNA, since the PCR product from the spliced gene will be 3.2 kb smaller than that derived from the genomic KSHV DNA (Fig. 1). The sensitivity of the assay allows detection of this presumably late mRNA in solid KS tumors where we have previously demonstrated that at least 1 to 5% of the cells are expressing lytic genes (27). We used this assay (i) to evaluate the infectivity of BCBL-1-derived KSHV virion preparations and (ii) to identify cell lines which are susceptible to KSHV. Using supernatants from TPA-induced BCBL-1 cells, we could readily infect 293 cells, as judged by our RT-PCR assay. Clearly then, 293 cells must possess at least one functional receptor or entry mechanism for KSHV, though the possibility that other, perhaps more-efficient, entry pathways exist is by no means excluded. In herpes simplex virus, for example, multiple entry pathways, based in part upon different viral envelope proteins, are known to exist (25). Analysis of the viral transcription pattern in infected 293 cells revealed major differences compared to those of TPA-induced BCBL-1 cells and KS tumors (Fig. 3); nut-1, for example, a transcript which is highly expressed in BCBL-1 and BC-1 cells and in KS tumors, was not detected in 293 cells (Fig. 2, lanes 1 and 9) (32). Although it is formally possible that the transcription patterns in cells of epithelial origin differ from that in either endothelial or lymphoblastoid origins, the observed transcription pattern in 293 cells is more suggestive of an abortive infection. This interpretation is in agreement with the lack of serial passaging of the virus in 293 cells and the absence of observable cytopathic effects that had been reported by others (12).
Our findings confirm that 293 cells are at least semipermissive for KSHV infection in that they support the entry of virions and limited transcription of viral DNA. Although infection of 293 is inefficient, both with BCBL-1 virus and with primary isolates, it is highly reproducible and could serve as the basis for assays of infectivity and neutralization with materials from clinical specimens. In fact, one such use has already been reported: KSHV-infected subjects frequently display virions in their saliva, and these virions can infect 293 cells with efficiencies roughly similar to those found here (29). Importantly, these primary salivary isolates also could not be serially propagated on 293 cells and showed no cytopathic effect on these cells (29), again suggesting that 293 cells are not fully permissive to the KSHV lytic cycle. Although our results and those of Vieira et al. (29) differ from those of Foreman et al. (12), who reported serial transmission of KSHV in 293 cultures, we note that some of these differences may relate to the use of different viral isolates, different lots of 293 cell lines, and possibly to subtle differences in in vitro culture conditions.
Testing BCBL-1-derived virus preparations on a variety of cell lines and/or primary cells of different origin (Table 1 and Fig. 3) indicates that cells of fibroblastic, epithelial, endothelial, or lymphoid origin appear to support limited KSHV infection; notably, even cells of hamster or simian origin were sensitive to low-level infection. In agreement with the fact that the mass of KS tumor cells consists of spindle cells believed to be of endothelial origin, it is also noteworthy that two different endothelial cell lines (BB19 and 181GB1-4) as well as primary neonatal capillary endothelial cells were infectible. In addition, we confirm with our assay that CD19+ primary B cells of healthy donors can be infected (5); this cell population has been shown to be infected in KS patients in vivo by DNA-based PCR assays (1). However, we emphasize that all of these in vitro infections proceed extremely inefficiently, in general at or below the level seen in 293 cells, and it seems likely that many of these are abortive infections as well, though we have not tested supernatants from most of these cells for serial transmission. Clearly, there remains a need for the identification of cell lines supporting more robust replication of this emerging pathogen. Such cell culture systems will be crucial for a better understanding of the life cycle and pathogenesis of KSHV.
ACKNOWLEDGMENTS
We thank Simon Hayward, Scott Heron, Eliott Kieff, E. Lenette, Ashlee Moses, Jay Nelson, and Marc Shuman for providing cell lines and primary cell cultures.
D.B. was supported in part by the University of California Universitywide AIDS Research Program (UARP). R.R. is a fellow of the Leukemia Society of America. This work was supported by the Howard Hughes Medical Institute. This work was also supported by UARP grant R95-SF-088 and by the Medical Research Council.
REFERENCES
- 1.Ambroziak J A, Blackbourn D J, Herndier B G, Glogau R G, Gullett J H, McDonald A R, Lennette E T, Levy J A. Herpes-like sequences in HIV-infected and uninfected Kaposi’s sarcoma patients. Science. 1995;268:582–583. doi: 10.1126/science.7725108. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 2.Arvanitakis L, Mesri E A, Nador R G, Said J W, Asch A S, Knowles D M, Cesarman E. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood. 1996;88:2648–2654. [PubMed] [Google Scholar]
- 3.Baines J D, Poon A P, Rovnak J, Roizman B. The herpes simplex virus 1 UL15 gene encodes two proteins and is required for cleavage of genomic viral DNA. J Virol. 1994;68:8118–8124. doi: 10.1128/jvi.68.12.8118-8124.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baines J D, Roizman B. The cDNA of UL15, a highly conserved herpes simplex virus 1 gene, effectively replaces the two exons of the wild-type virus. J Virol. 1992;66:5621–5626. doi: 10.1128/jvi.66.9.5621-5626.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackbourn D J, Ambroziak J, Lennette E, Adams M, Ramachandran B, Levy J A. Infectious human herpesvirus 8 in a healthy North American blood donor. Lancet. 1997;349:609–611. doi: 10.1016/S0140-6736(96)10004-0. [DOI] [PubMed] [Google Scholar]
- 6.Blackbourn D J, Chuang L F, Sutjipto S, Killam K F, Jr, McCready P M, Doi R H, Li Y, Chuang R Y. Detection of simian immunodeficiency virus RNA from infected rhesus macaques by the polymerase chain reaction. J Virol Methods. 1992;37:109–117. doi: 10.1016/0166-0934(92)90038-f. [DOI] [PubMed] [Google Scholar]
- 7.Boshoff C, Schulz T F, Kennedy M M, Graham A K, Fisher C, Thomas A, McGee J O, Weiss R A, O’Leary J J. Kaposi’s sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat Med. 1995;1:1274–1278. doi: 10.1038/nm1295-1274. [DOI] [PubMed] [Google Scholar]
- 8.Boshoff C, Talbot S, Kennedy M, O’Leary J, Schulz T, Chang Y. HHV8 and skin cancers in immunosuppressed patients. Lancet. 1996;347:338–339. doi: 10.1016/s0140-6736(96)90524-3. . (Letter.) (Erratum, 348:138.) [DOI] [PubMed] [Google Scholar]
- 9.Cesarman E, Chang Y, Moore P S, Said J W, Knowles D M. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 10.Cesarman E, Moore P S, Rao P H, Inghirami G, Knowles D M, Chang Y. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi’s sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood. 1995;86:2708–2714. [PubMed] [Google Scholar]
- 11.Church G M, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foreman K E, Friborg J, Jr, Kong W P, Woffendin C, Polverini P J, Nickoloff B J, Nabel G J. Propagation of a human herpesvirus from AIDS-associated Kaposi’s sarcoma. N Engl J Med. 1997;336:163–171. doi: 10.1056/NEJM199701163360302. [DOI] [PubMed] [Google Scholar]
- 13.Gaidano G, Pastore C, Gloghini A, Capello D, Tirelli U, Saglio G, Carbone A. Microsatellite instability in KSHV/HHV-8 positive body-cavity-based lymphoma. Hum Pathol. 1997;28:748–750. doi: 10.1016/s0046-8177(97)90187-8. [DOI] [PubMed] [Google Scholar]
- 14.Ganem D. KSHV and Kaposi’s sarcoma: the end of the beginning? Cell. 1997;91:157–160. doi: 10.1016/s0092-8674(00)80398-0. [DOI] [PubMed] [Google Scholar]
- 15.Gao S J, Kingsley L, Li M, Zheng W, Parravicini C, Ziegler J, Newton R, Rinaldo C R, Saah A, Phair J, Detels R, Chang Y, Moore P S. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2:925–928. doi: 10.1038/nm0896-925. [DOI] [PubMed] [Google Scholar]
- 16.Kedes D H, Operskalski E, Busch M, Kohn R, Flood J, Ganem D. The seroepidemiology of human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat Med. 1996;2:918–924. doi: 10.1038/nm0896-918. . (Erratum, 2:1041.) [DOI] [PubMed] [Google Scholar]
- 17.Kieff E. Epstein-Barr virus and its replication. In: Knipe D M, Fields B N, Howley P M, Channock R M, Melnick J L, Monath T P, Roizman B, editors. Field’s virology. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2343–2396. [Google Scholar]
- 18.Miller G, Heston L, Grogan E, Gradoville L, Rigsby M, Sun R, Shedd D, Kushnaryov V M, Grossberg S, Chang Y. Selective switch between latency and lytic replication of Kaposi’s sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J Virol. 1997;71:314–324. doi: 10.1128/jvi.71.1.314-324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore P S, Gao S J, Dominguez G, Cesarman E, Lungu O, Knowles D M, Garber R, Pellett P E, McGeoch D J, Chang Y. Primary characterization of a herpesvirus agent associated with Kaposi’s sarcomae. J Virol. 1996;70:549–558. doi: 10.1128/jvi.70.1.549-558.1996. . (Erratum, 70:9083.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renne R, Lagunoff M, Zhong W, Ganem D. The size and conformation of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) DNA in infected cells and virions. J Virol. 1996;70:8151–8154. doi: 10.1128/jvi.70.11.8151-8154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 22.Rettig M B, Ma H J, Vescio R A, Pold M, Schiller G, Belson D, Savage A, Nishikubo C, Wu C, Fraser J, Said J W, Berenson J R. Kaposi’s sarcoma-associated herpesvirus infection of bone marrow dendritic cells from multiple myeloma patients. Science. 1997;276:1851–1854. doi: 10.1126/science.276.5320.1851. [DOI] [PubMed] [Google Scholar]
- 23.Russo J J, Bohenzky R A, Chien M C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Said J W, Rettig M R, Heppner K, Vescio R A, Schiller G, Ma H J, Belson D, Savage A, Shintaku I P, Koeffler H P, Asou H, Pinkus G, Pinkus J, Schrage M, Green E, Berenson J R. Localization of Kaposi’s sarcoma-associated herpesvirus in bone marrow biopsy samples from patients with multiple myeloma. Blood. 1997;90:4278–4282. [PubMed] [Google Scholar]
- 25.Sears A E, Roizman B. Cell-specific selection of mutants of a herpes simplex virus recombinant carrying deletions. Virology. 1985;145:176–180. doi: 10.1016/0042-6822(85)90213-2. [DOI] [PubMed] [Google Scholar]
- 26.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d’Agay M F, Clauvel J P, Raphael M, Degos L, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- 27.Staskus K A, Zhong W, Gebhard K, Herndier B, Wang H, Renne R, Beneke J, Pudney J, Anderson D J, Ganem D, Haase A T. Kaposi’s sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J Virol. 1997;71:715–719. doi: 10.1128/jvi.71.1.715-719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun R, Lin S F, Gradoville L, Miller G. Polyadenylated nuclear RNA encoded by Kaposi sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1996;93:11883–11888. doi: 10.1073/pnas.93.21.11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vieira J, Huang M L, Koelle D M, Corey L. Transmissible Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in saliva of men with a history of Kaposi’s sarcoma. J Virol. 1997;71:7083–7087. doi: 10.1128/jvi.71.9.7083-7087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitby D, Howard M R, Tenant-Flowers M, Brink N S, Copas A, Boshoff C, Hatzioannou T, Suggett F E, Aldam D M, Denton A S, et al. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi’s sarcoma. Lancet. 1995;346:799–802. doi: 10.1016/s0140-6736(95)91619-9. [DOI] [PubMed] [Google Scholar]
- 31.Zhong W, Ganem D. Characterization of ribonucleoprotein complexes containing an abundant polyadenylated nuclear RNA encoded by Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) J Virol. 1997;71:1207–1212. doi: 10.1128/jvi.71.2.1207-1212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong W, Wang H, Herndier B, Ganem D. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc Natl Acad Sci USA. 1996;93:6641–6646. doi: 10.1073/pnas.93.13.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]