Abstract
The oyster mushroom (Pleurotus spp.) is one of the most widely cultivated mushroom species globally. The present study investigated the effect of synbiotics on the growth and quality of Pleurotus ostreatus and Pleurotus pulmonarius. Different synbiotics formulations were applied by spraying mushroom samples daily and measuring their growth parameters, yield, biological efficiency, proximate composition, mineral content, total phenolic content (TPC), and diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging activity. Results demonstrated that the most significant yield of oyster mushrooms was harvested from synbiotics sprayed with inulin and Lactobacillus casei (56.92 g). Likewise, the highest biological efficiency obtained with a similar synbiotic was 12.65%. Combining inulin and L. casei was the most effective method of improving the mushrooms’ growth performance and nutrient content in both samples. Furthermore, synbiotics that combined inulin and L. casei resulted in the highest TPC (20.550 mg gallic acid equivalent (GAE)/g dry extract (DE)) in white oyster mushrooms (P. ostreatus). In comparison, in grey mushroom (P. pulmonarius) the highest TPC was yielded by L. casei (1.098 mg GAE/g DE) followed by inulin and L. casei (1.079 mg GAE/g DE). The DPPH results indicated that the oyster mushroom could be an efficient antioxidant. The results revealed that applying synbiotics improved the mushrooms’ quality by increasing their antioxidant capacity with higher amounts of phenolic compounds and offering better health benefits with the increased levels of mineral elements. Together, these studies demonstrated the potential of using synbiotics as a biofertilizer, which is helpful for mushroom cultivation; therefore, it might solve the challenge of inconsistent quality mushroom growers face.
Keywords: Antioxidant, Biofertilizer, Growth performance, Mushroom cultivation, Pleurotus, Synbiotics
Abstract
平菇(Pleurotus spp.)是全球最广泛栽培的蘑菇品种之一。本研究旨在探讨合生元对Pleurotus ostreatus和Pleurotus pulmonarius生长和品质的影响。通过每日对蘑菇样品喷洒不同浓度的合生元,测量其生长参数、产量、生物效率、近似成分、矿物质含量、总酚含量和二苯基-1-三硝基苯肼(DPPH)自由基清除活性。结果表明,使用喷洒菊粉和干酪乳杆菌的合生元,平菇的产量最高(56.92克),生物效率也最高(12.65%)。菊粉和干酪乳杆菌的组合最为高效,可以同时提高两种样品蘑菇的生长性能和营养成分。此外在白平菇(P. ostreatus)中,菊粉和干酪乳杆菌组合的合生元可产生最高的总酚含量(20.550 mg GAE/g DE),而在灰蘑菇(P. pulmonarius)中产生最高总酚含量的是干酪乳杆菌(1.098 mg GAE/g DE),其次是菊粉和干酪乳杆菌(1.079 mg GAE/g DE)。DPPH结果表明,平菇可以作为一种有效的抗氧化剂。综上,合生元可通过增加酚类化合物的含量来提高蘑菇的抗氧化能力,并通过增加矿物质元素的含量来提供更好的健康益处,从而提高了蘑菇的品质。上述研究证明了合生元作为一种生物肥料用于蘑菇栽培的应用潜力,是帮助蘑菇种植者解决质量不稳定的一个有效方案。
Keywords: 抗氧化剂, 生物肥料, 成长表现, 蘑菇种植, 侧耳属, 合生元
1. Introduction
The global mushroom industry has expanded significantly due to health-conscious consumers’ demand for high-nutritional foods. Mushrooms are edible fungi that are appreciated as a food source and, more importantly, are also recognized as a functional food because of their nutritional value as well as their potential pharmaceutical and nutraceutical uses; for example, the oyster mushroom (Adebay and Oloke, 2017; Munir et al., 2023). The growing popularity of mushrooms as a substitution for meat, associated with the rising number of vegan populations, provides an opportunity for the mushroom industry to produce more mushroom-based products, increasing the demand for fresh mushrooms. Among approximately fourteen thousand kinds of mushrooms globally, two thousand are edible, and only around thirty-five edible species can be commercially cultivated (Bakratsas et al., 2021; Munir et al., 2021). The genus Pleurotus, commonly known as the oyster mushroom, is one of the most commercially cultivated mushrooms, possessing significant economic value as they are abundant in proteins, fibers, minerals, and vitamins and are low in fats. Pleurotus ostreatus (white oyster mushroom) and Pleurotus pulmonarius (grey oyster mushroom) are widely available due to their high nutrient content. They can grow on various lignocellulose substrates (Rahmat et al., 2020).
Mushroom cultivation has evolved from growing them on wood or logs to utilizing cultivation techniques to enhance yields and quality. The cultivation of mushrooms might be simple due to their adaptability and the availability of substrate to act as a growing bed for mushrooms. However, the production of mushrooms is affected by biological, chemical, and physical factors such as substrate composition, nitrogen source, antimicrobial agents, substrate, pH, and moisture content. The environmental factors include humidity, temperature, oxygen, and carbon dioxide (Bellettini et al., 2019). All of these factors must be considered in mushroom cultivation. The production of mushrooms can be increased when the cultivation techniques in a modern setting provide optimized conditions for mushroom growth; these include substrate sterilization, spawn preparation processes, a monitoring system for controlling environmental conditions, and a harvesting system. However, these methods are not feasible in the commercial mushroom industry as they require considerably capital and operational costs (Higgins et al., 2017).
Generally, mushroom supplementation is a practicable strategy for improving the fruiting bodies’ yield and the performance of mushroom substrate (Carrasco et al., 2018). Several studies have shown that nutritionally supplementing the mushroom blocks can improve the mushroom’s growth; for example, the use of beneficial microorganisms enhances mycelium development, as described by some authors (Kumari and Naraian, 2021). Some live organisms act as probiotics, so taking prebiotics can confer beneficial effects for mushroom health; for instance, the lactobacilli prefers the prebiotics inulin and fructooligosaccharides, whereas bifidobacteria prefers inulin, fructooligosaccharides, xylo-oligosaccharides, and galactooligosaccharides (Wilson and Whelan, 2017). A study has indicated that Lactobacillus delbrueckii and Lactobacillus alimentatus possess significant antifungal effects against the mushroom pathogens Penicillium and Aspergillus fulvous (Karami et al., 2017). Similarly, potential prebiotics like inulin, which are utilized by Lactobacillus spp., prolong the viability of the probiotics by selectively stimulating their growth, decreasing the doubling time, and improving the strains (Nagpal and Kaur, 2011). In addition, it has been reported that adding bacteria at different phases during cultivation is a promising strategy for increasing mushroom productivity. They may act directly by stimulating vegetative growth and mushroom formation or indirectly by inhibiting pathogens (Braat et al., 2022).
These findings suggest that there are some potential opportunities for synbiotics, which can have a positive influence on cultivated mushrooms by improving their physical development. However, there is a lack of studies on using prebiotics and probiotic supplementation to promote mushroom growth. The use of probiotic bacteria in plants has been widely studied, highlighting their role in agriculture, where the interaction between microorganisms and host plants can improve disease resistance and plant growth (El-Aidy et al., 2020). Therefore, applying probiotics in combination with prebiotics in mushroom cultivation might be a potential way to improve mushroom agriculture. Commercially cultivated mushrooms are considered one of the highest-valued commodities in Malaysia under Malaysia’s National Agro-Food Policy (2011‒2020). As a tropical country with high temperatures (23 to 35 ℃), high humidity (80% to 90%), and high annual rainfall, the mushroom species that can be cultivated in Malaysia are very few. Therefore, this allows this country to generate higher income from this commodity.
Conventional cultivation involves solid-state fermentation, affecting mushroom yield and quality. Thus, the present investigation is the first attempt to identify the most suitable formulation of synbiotics for cultivating oyster mushroom species by proximate, mineral, and antioxidant analyses. The present study aimed to improve the growth performance and quality of white and grey oyster mushrooms and investigated the potential of a synbiotic formulation consisting of Lactobacillus plantarum and Lactobacillus casei as probiotics and inulin as a prebiotic. We also studied the effect of synbiotics on mushroom productivity and quality, which may help improve future commercial mushroom production.
2. Materials and methods
2.1. Sample collection and preparation
The studied oyster mushroom species, P. pulmonarius and P. ostreatus, were purchased from the local mushroom growers of Pekan Mushroom Resources SDN Company BHD (Pekan, Malaysia) and were transported to the laboratory of the Faculty of Industrial Sciences and Technology, Universiti Malaysia Pahang Al-Sultan Abdullah (UMPSA) (Gambang, Malaysia) for further experiments.
2.2. Preparation of probiotics
Pure freeze-dried starter cultures of L. plantarum and L. casei were purchased from Pekan Mushroom Resources SDN Company BHD. To obtain the active cultures, they were cultured under anaerobic conditions in sterile de Man Rogosa and Sharpe (MRS) agar media for 2 d at 37 ℃. The probiotic pellets were centrifuged at 4500g for 25 min and washed twice in sterile 0.9% (9 g/L) NaCl solution to be resuspended in MRS broth for incubation overnight. The bacteria were inoculated on an MRS agar plate and incubated anaerobically at 37 ℃ for 48 h. The L. plantarum and L. casei were indicated as positive controls C and D, respectively.
2.3. Preparation of prebiotic and synbiotics
The prebiotic used in the research was commercial inulin from Sigma Aldrich, USA. The 1% (10 g/L) inulin solution was prepared and sprayed on both mushrooms once a day (about 10‒15 mL) according to the formulation of the synbiotics. Formulations with a bacterial suspension–water volume ratio of 1:100 for each probiotic and prebiotic were prepared and stored until further use.
2.4. Experimental design
The experimental design comprised probiotics L. plantarum and L. casei mixed with prebiotic inulin. Seven different formulations were applied to cultivate both mushrooms, as shown in Table 1. Sample A had no prebiotics or probiotics added to it and served as a negative control sample. Samples B, C, and D were positive controls and consisted of inulin, L. plantarum, and L. casei, respectively. For Samples E and F, the prebiotics and probiotics were mixed at the volume ratio of 1:1. Sample G was prepared by mixing 15 mL of inulin solution, 15 mL of 1% (volume fraction) L. plantarum, and 15 mL of 1% (volume fraction) L. casei. These formulations were sprayed at 10‒15 mL/d on mushroom bag logs. The fruiting bodies of the mushrooms were harvested by hand when the mycelia of the samples were fully formed and the caps of the mushroom flattened at the edges. To evaluate the growth performance, different parameters of the harvested samples were observed and analyzed by comparing the samples with varying formulations of synbiotics. In total, 46 mushroom bags were sampled for the present study, as described below (Table 1).
Table 1.
Different formulations of synbiotics applied to the cultivation of Pleurotus ostreatus and Pleurotus pulmonarius
| Sample | Formulation of synbiotics |
|---|---|
| A (negative control) | Distilled water |
| B (positive control) | Inulin |
| C (positive control) | Lactobacillus plantarum |
| D (positive control) | Lactobacillus casei |
| E (Synbiotic 1) | L. plantarum+inulin (1:1)* |
| F (Synbiotic 2) | L. casei+inulin (1:1)* |
| G (Synbiotic 3) | L. plantarum+L. casei+inulin |
* Volume ratio.
2.5. Cultivation and harvesting of P. ostreatus and P. pulmonarius
The samples were cultivated in the laboratory at room temperature within the range of 25 to 30 ℃ and 90% humidity was maintained. The region for the mushroom cultivation was cleaned with 70% (volume fraction) ethyl alcohol before the cultivation started to prevent contamination. This research was conducted with two biological replications and three technical replications for each sample with different synbiotics formulations. Next, the fruiting bodies of the grey and white oyster mushrooms were harvested by hand when the mycelia of the samples were fully formed and the mushroom caps flattened at the edges. The harvested samples were collected and placed in several sterile zip bags with labels for further analysis.
2.6. Effects of synbiotics on the cultivation yields of P. ostreatus and P. pulmonarius
The growth of both mushroom species samples was monitored every day. The growth parameters of the harvested samples were observed and analyzed by comparing the samples with different formulations of synbiotics. The parameters included the diameter of the mushroom cap (cm), the thickness of the cap (cm), the length of the mushroom stalk (cm), and the thickness of the stalk (cm), all measured using a ruler, along with the weight of each fruiting body (g) and the number of fruiting bodies per harvest. The yield parameters were recorded, including the mushroom’s total fresh weight (g) per harvest. Further, the biological efficiency per flush of the grey and white oyster mushrooms was calculated using the equation below (Grimm et al., 2021):
| Biological efficiency=FWf.f./DWs×100%, | (1) |
where FWf.f. is the fresh weight of the first flush of the fresh fruiting bodies (g), and DWs is the dry weight of the dry substrate per bag before inoculation (g).
2.7. Proximate and mineral content analyses
The proximate composition was determined by the official methods of the Association of Official Analytical Chemists (AOAC). The contents analyzed included carbohydrates, protein, fat, fibers, ash, and moisture. Eurofins Food Testing Malaysia SDN Company BHD performed this proximate analysis per the guidelines. Further, the mineral contents in the samples were determined by the nutritional analysis. The macroelements, calcium (Ca) and sodium (Na), and the microelements, copper (Cu), iron (Fe), and zinc (Zn), as well as the heavy metal element, lead (Pb), were determined by the inductively coupled plasma optical emission spectroscopy (ICP-OES).
2.8. Preparation of mushroom extract
The collected fruiting body was washed with distilled water, dried at 40‒50 ℃ for 48‒72 h, and powdered for further use. The Soxhlet extraction was performed using 95% ethanol for 4 h and then filtered using Whatman’s filter paper. The crude extract was obtained by a rotary evaporator and was stored at 4 ℃.
2.9. Determination of total phenolic content
The total phenolic content (TPC) of the grey and white oyster mushroom extracts was determined using the Folin-Ciocalteu colorimetric method, according to Phuyal et al. (2020). To prepare samples, 5 mL of 10% (volume fraction) Folin-Ciocalteu reagent and 4 mL of 7% (0.07 g/mL) Na2CO3 were added to 1 mL of the 1 mg/mL mushroom extract. The blue-colored mixture was vortexed vigorously and was incubated for 30 min at 40 ℃ in a water bath. After incubation, the absorbance values were measured at 760 nm using a spectrophotometer. The TPC in all the extracts was calculated by using the formula:
| C=c×V/m, | (2) |
where C refers to the TPC (mg gallic acid equivalent (GAE)/g dry extract (DE)), c refers to the concentration of gallic acid obtained from the calibration curve in mg/mL, V refers to the volume of the extract in mL, and m refers to the mass of extract in g.
2.10. Determination of DPPH radical scavenging assay
The experiment was performed in triplicate per the previously described protocol (Elmastas et al., 2007). One milliliter of 0.004% (volume fraction) diphenyl-1-picryl-hydrazyl (DPPH) solution was added to 3 mL of the ascorbic acid (0.0067, 0.0133, 0.0200, 0.0267, 0.0333, and 0.0667 mg/mL) solutions and shaken vigorously. After incubation at room temperature for 30 min, the mixture was immediately measured at 517 nm using a spectrophotometer. The procedure was repeated for the different concentrations of grey and white mushroom extracts. The capability to scavenge the DPPH radical was calculated using the formula below:
| DPPH radical scavenging activity=(1-A 1)/A 0×100%, | (3) |
where A 0 was the absorbance of the control reaction, and A 1 was the absorbance in the presence of the mushroom extract sample. A concentration versus percentage of inhibition graph was plotted to calculate the 50% inhibitory concentration (IC50) values.
2.11. Statistical analysis
This experiment was performed in two biological replications by cultivating two batches of mushrooms with three replicates for each measurement and analysis, and the values were represented as average values along with their standard deviation. The results were analyzed statistically using Microsoft Excel to determine the average value and standard deviation. Furthermore, GraphPad Prism software (Version 10.0.0 153) was used to perform a one-way analysis of variance (ANOVA) and a principal component analysis (PCA) to identify differences among groups. A Tukey’s post-hoc multi-comparison test was applied to examine significant differences between treatments. P<0.05 was considered statistically significant.
3. Result and discussion
3.1. Effects of synbiotics on the growth of P. ostreatus and P. pulmonarius
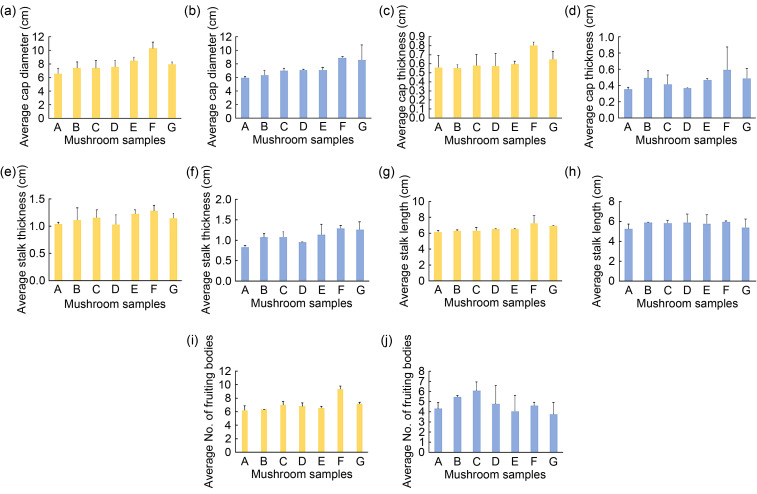
The sizes of seven mushroom samples of P. ostreatus and P. pulmonarius were analyzed to compare their growing performance according to the measurement of the fruiting bodies. Several measurements were collected from two batches of mushroom samples based on cap diameter, cap thickness, stalk thickness, stalk length, and average number of fruiting bodies. The summarized average results obtained from the triplicate samples of two batches of P. ostreatus and P. pulmonarius mushroom samples are shown in Fig. 1. In terms of P. ostreatus cap diameter, it was evident that the mushroom sample F, with the use of formulated synbiotics (a watering solution of prebiotics and L. casei), achieved the highest average cap diameter and its respective value was 10.35 cm. However, sample A, without any supplementation of prebiotics or probiotics, obtained the lowest average cap diameter of 6.54 cm (P<0.05, Fig. 1a). The cap diameter of P. pulmonarius indicated that the combination of inulin and L. casei is capable of improving the diameter of the mushroom cap (Fig. 1b). In addition, the average P. pulmonarius cap thickness of the samples was different according to the order: F>G&B>E>C>D>A. Next, the highest average cap thickness of P. pulmonarius was that of sample F (0.60 cm), which was sprayed with inulin and L. casei, while sample A, which was sprayed with distilled water, had the lowest (0.36 cm) (P<0.05; Fig. 1d). Additionally, the P. ostreatus mushroom samples A, B, C, and D achieved lower values in terms of average cap thickness. They were arranged in ascending order as B, A, D, and C, recording 0.55, 0.56, 0.57, and 0.58 cm, respectively (Fig. 1c). In the synbiotics mushroom samples E and G, the average cap thickness of sample G was greater than that of sample E. Overall, the study showed that the cap diameter and thickness of sample F gained the highest values.
Fig. 1. Effects of synbiotics on growth of Pleurotus ostreatus and Pleurotus pulmonarius. The average cap diameters of P. ostreatus (a) and P. pulmonarius (b); the average cap thicknesses of P. ostreatus (c) and P. pulmonarius (d); the average stalk thicknesses of P. ostreatus (e) and P. pulmonarius (f); the average stalk lengths of P. ostreatus (g) and P. pulmonarius (h); the average numbers of fruiting bodies of P. ostreatus (i) and P. pulmonarius (j) from two batches of mushroom samples using different formulated synbiotic solutions. Data are expressed as mean±standard deviation, n=3. A: distilled water; B: prebiotics (inulin); C: probiotic Lactobacillus plantarum; D: probiotic Lactobacillus casei; E: Synbiotics 1 (L. plantarum+prebiotics); F: Synbiotics 2 (L. casei+prebiotics); G: Synbiotics 3 (L. casei+L. plantarum+prebiotics).
A similar study showed that the involvement of bacteria can promote the growth and metabolism of mushrooms as the community structure bacteria in the substrates reflect the mushrooms’ growth and nutrient utilization status (Rohani et al., 2022). In addition, this previous study showed the positive effect of inulin on mushroom growth and the increase in the survival rate of probiotics such as Lactobacillus acidophilus (Zarenejad et al., 2012). Therefore, the results suggest that the inulin can improve the growth and increase the survival rate of Lactobacillus sp., thus improving the community structure bacteria in the substrate and the cap diameter and thickness of the P. pulmonarius.
On the other hand, the average thickness of the stalk in sample F in the grey mushroom was the highest, 1.29 cm, while the average thickness of the stalk in the negative control sample was the lowest, 0.83 cm (P<0.05; Fig. 1f). Sample F also showed the highest average length of the stalk among the samples of 6.02 cm, while the negative control sample showed the lowest average length of the stalk at 5.33 cm (P<0.05; Fig. 1h). However, the white mushroom samples B, C, and D recorded lower values of average stalk thickness as compared to the synbiotics mushroom samples of E, G, and F (P<0.05; Fig. 1e). The average stalk length of mushroom samples arranged in ascending order was B and C, followed by D, recording 6.29, 6.37, and 6.55 cm, respectively (P<0.05; Fig. 1g). The synbiotics mushroom sample G achieved a higher average stalk length than sample E; they were 6.96 and 6.59 cm, respectively. From the overall results, the data suggested that cultivating mushrooms supplemented with prebiotics and probiotics leads to larger fruiting bodies. This is because the mushroom samples that grew with a formulated watering solution of prebiotics and probiotics alone exhibited a bigger cap diameter and longer stalk than the control mushroom samples. The fruitification of Pleurotus mushrooms depends not only on the substrate itself but also on the interactions between the bacteria and substrate, potentially stimulating the growth and formation of fruiting bodies (Kertesz and Thai, 2018). Remarkably, the plant growth-promoting bacteria in nitrogen fixation and the secretion of phytohormone can promote plant growth (di Benedetto et al., 2017). The research also supports probiotics’ effect on plant growth, which positively impacts the plant via compositional changes in the resident rhizosphere microbiome (Hu et al., 2021). The growth and composition of strawberries were also positively affected by the probiotic bacteria of Bacillus in the study by Rahman et al. (2018). In addition, the grey oyster mushrooms were cultivated in an open area with good aeration and an average concentration of carbon dioxide, so they did not need to develop long stalks to grab more oxygen for growth. This is because high concentrations of carbon dioxide will only lead to the development of the stalk and the failure to develop caps (O'Keefe, 1998). Therefore, this is also an essential factor to be considered in the cultivation of mushrooms, as if the cap fails to develop, mushroom growers will face great losses. In addition, oyster mushrooms with more oversized caps and shorter stalks are considered better quality as the stalks contain more insoluble dietary fiber than the caps (Synytsya et al., 2008). Following this report, our study results exhibit a better performance of mushroom caps than stalks for both samples; this may be due to the synbiotics, which enhance the utilization of the substrates by the mushroom, resulting in larger cap sizes.
Fig. 1i shows the maximum number of fruiting bodies (9.33) in sample F of white mushrooms. Mushroom samples A, B, E, and D produced approximately six fruiting bodies, where the average numbers recorded were 6.17, 6.33, 6.50, and 6.84, respectively. The average numbers of fruiting bodies of samples C and G were slightly higher at 7.0 and 7.17, respectively. The synbiotics sample E does not show a better yield of the average number of fruiting bodies than the others that used prebiotics or probiotics as the watering solution. However, only 1 and 2 fruiting bodies differ from synbiotics samples G and F. Indeed, the difference between the average number of fruiting bodies of all seven mushroom samples is insignificant. This result suggests that supplementing with prebiotics, probiotics, or synbiotics during mushroom cultivation does not notably increase the number of fruiting bodies. Nonetheless, synbiotics of L. casei still demonstrated a more significant effect than the others on fruiting body formation. Fig. 1j shows that sample C, sprayed with only L. casei, recorded the highest number of fruiting bodies, with 6 per harvest (P<0.05). The number of fruiting bodies in sample F (5 fruiting bodies) was lower than that of sample C, but it gained the highest weight of fruiting bodies per harvest. This can be explained by the low number of fruiting bodies of grey oyster mushrooms, meaning that they can utilize the substrates sufficiently to grow better as the nutrients will not disperse to develop more fruiting bodies. A study also showed similar results, in which the highest weight of fruiting bodies gained the least number of fruiting bodies per harvest (Das et al., 2015). Therefore, the synbiotics improve the number of fruiting bodies per harvest and the other growth parameters of the grey oyster mushroom. By comparing the results of the negative control samples with those of sample F, it can be proved that the synbiotics formulation with the combination of inulin and L. casei improves the growth parameters of the grey oyster mushrooms. This is because L. casei can stimulate the mycelial growth of the grey oyster mushroom and enhance the mushroom fruitification (Kertesz and Thai, 2018). In addition, a prior study showed that probiotics can also improve the growth and yield of plants and suppress some diseases at an adequate amount (Rahman et al., 2018).
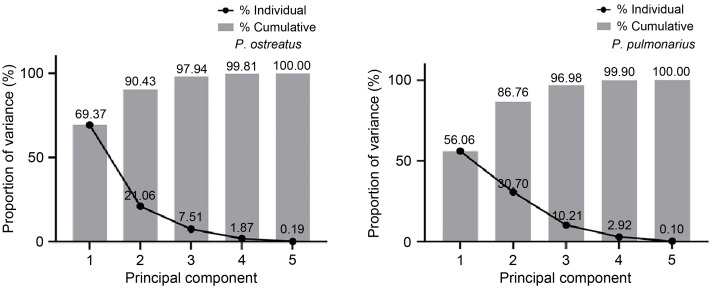
PCA was used to understand the importance of each variable and its independent contribution to mushroom growth. Fig. 2 shows that the cap diameter contributes the most to the overall variance, explaining 69.37% of the variability in P. ostreatus and 56.06% of the variability in P. pulmonarius. However, variables such as cap thickness, stalk length, stalk thickness, and average fruiting bodies have less influence on data variability. The overall PCA suggests that the majority of variance can be effectively captured by the first two principal components (PC1 and PC2) for both the mushroom species. These observations align with the growth parameters studied above, suggesting that synbiotic formulations have a more significant effect on cap diameter growth as compared to other variables. The synbiotics formulation of inulin and L. casei is more suitable for cultivating grey oyster mushrooms, as this formulation provides the best growth parameters and statistical analysis (Fig. 2).
Fig. 2. Principal component analysis of mushroom growth variables. The graph displays the proportion of variance by each individual variable: 1, cap diameter; 2, cap thickness; 3, stalk thickness; 4, stalk length; and 5, average fruiting bodies.
3.2. Effects of synbiotics on yield and biological efficiency
This study determined the yield and biological efficiency of the first flush grey oyster mushroom from two biological replications. Each sample was performed in three replicates. The fresh weight of the fruiting bodies was measured to determine the biological efficiency of the first flush of the grey oyster mushroom. A higher value of biological efficiency indicates better growth of the mushroom. Based on the results in Table 2, the largest yield of oyster mushrooms was harvested from sample F (56.92 g), which was sprayed with inulin and L. casei, while the negative control sample showed the lowest yield (34.45 g). Similarly, the highest biological efficiency was obtained from sample F (12.65%), while the lowest was observed in the negative control sample (7.66%). This indicates that the growing performance of oyster growth and yield in the sawdust substrate was minimal. This might be explained by the fact that the lignocellulosic components in the sawdust contain little protein and are, therefore, insufficient for the growth of mushrooms (Girmay et al., 2016).
Table 2.
Total yield and biological efficiency of the first flush of Pleurotus ostreatus and Pleurotus pulmonarius with different synbiotics formulations
| Sample | Total weight per harvest (g) | Biological efficiency of first flush (%) | ||
|---|---|---|---|---|
| P. ostreatus | P. pulmonarius | P. ostreatus | P. pulmonarius | |
| A | 34.45±6.66 | 10.18±0.18 | 7.66±1.58 | 10.14±0.35 |
| B | 51.61±5.03 | 11.78±0.95 | 11.47±1.12 | 11.55±0.72 |
| C | 56.31±1.70 | 11.97±0.43 | 12.51±0.38 | 12.34±0.03 |
| D | 39.39±4.86 | 14.89±1.08 | 8.75±1.08 | 12.67±0.19 |
| E | 39.19±9.35 | 14.39±2.29 | 8.71±2.08 | 14.71±0.22 |
| F | 56.92±5.85 | 22.84±0.90 | 12.65±1.30 | 19.92±1.97 |
| G | 53.29±1.53 | 14.47±0.18 | 11.84±0.34 | 18.15±1.45 |
A‒G formulations as mentioned in Table 1.
In addition, the sawdust substrate must undergo a period of composting to break down the cellulose and lignin components of the wood so that the substances that are essential for the development of mushroom mycelium can be released (Raman et al., 2022). Furthermore, a study showed that lactic acid bacteria (LAB) can promote plant growth, stimulate roots and shoots, and accelerate soil organic content (Hoa et al., 2015). Adding synbiotics might aid in this process and result in better yield and biological efficiency. Therefore, the synbiotics formulation that contains L. casei and inulin is suitable for improving the cultivation of grey oyster mushrooms. Additionally, when comparing the total fresh weight per harvest, sample F showed a higher total fresh weight per harvest than in other studies (Fan et al., 2000); these were 42.4 g and 38.76 g. Thus, L. casei and inulin might be the best synbiotic formulations to cultivate P. pulmonarius as these synbiotics not only improve the growth parameters of the mushroom but also improve the yield of the mushroom, according to this research.
The lowest biological efficiency in the white mushroom samples (Table 2) was obtained from control sample A, which recorded 10.14%, followed by 11.55% in sample B. There was an increase in the biological efficiency in samples C, D, and E, which recorded 12.34%, 12.67%, and 14.71%, respectively. The biological efficiency obtained from sample G increased significantly to 18.15% compared to control samples. The highest biological efficiency was recorded at 19.92% by sample F. Biological efficiency is defined as the mushroom’s ability to come to fruition through bioconversion of the substrate to fruiting bodies (Fan et al., 2000). By comparing the biological efficiency with the average mushroom yield, the mushroom’s biological efficiency corresponds to the mushroom yield. The higher the yield of mushroom samples, the higher the biological efficiency. An increase in the biological efficiency indicates that more substrates are being used to form fruiting bodies, resulting in a greater yield. Our data follow the claim that biological efficiency depends on the mushroom yield (Oladipo et al., 2020). The increase in biological efficiency is primarily related to the microbial community. Research on P. ostreatus revealed that inoculating Pseudomonas spp. to mycelium enhanced primordia formation and the development of basidiome. The authors’ findings showed the vital role of bacteria in mushroom growth performance by inducing fruitification (Oladipo et al., 2020).
3.3. Proximate, nutritional, and heavy metal analyses
Various mushrooms can grow in hot, humid, and wet regions. Since the weather and environment of Malaysia are favorable to the growth of P. ostreatus and P. pulmonarius, they are two of the most cultivated edible mushrooms in Malaysia. Mushrooms are a healthy food source rich in protein, carbohydrates, minerals, and vitamins (Hasan and Aunsary, 2020). This study also focused on the effects of synbiotics on the nutritional values of the P. ostreatus and P. pulmonarius. This analysis studied moisture, ash, protein, fat, and crude fiber contents, as shown in Table 3.
Table 3.
Proximate analysis of the fruiting body Pleurotus ostreatus and Pleurotus pulmonarius in samples A and F
| Mushroom sample | Parameter (%, mass fraction) | ||||
|---|---|---|---|---|---|
| Moisture | Crude protein | Crude fiber | Crude fat | Ash | |
| P. ostreatus (A) | 75.16 | 3.90 | 0.13 | 1.88 | |
| P. ostreatus (F) | 85.50 | 2.80 | 1.70 | 0.30 | 1.20 |
| P. pulmonarius (A) | 87.30 | 2.00 | 0.06 | 1.24 | |
| P. pulmonarius (F) | 90.10 | 3.30 | 0.20 | 1.40 | 1.80 |
A and F formulations as mentioned in Table 1.
According to the test method of AOAC, the proximate analysis was performed to compare the moisture, protein, fiber, fat, and ash contents of normal white mushroom sample A with those of synbiotics mushroom sample F. Sample F achieved 85.5% moisture content, which was higher than that of control sample A (75.16%) (Table 3). However, it is still an acceptable range as the moisture content of fresh mushrooms is generally 85% to 95%, according to Kumar et al. (2013). Nevertheless, the moisture content of grey mushroom sample F was also higher than that of the negative sample, i.e., 90.10% and 87.30%, respectively (Table 3). However, the crude protein content for both samples of F was relatively lower, with 2.80% and 3.30% for white and grey mushrooms, respectively, which was not significantly different. However, mushrooms’ slightly lower protein content might be because the samples involved in the analysis were not fresh enough, and protein leeching occurred during the browning reactions (Tolera and Abera, 2017).
This study also focused on the effects of the synbiotics on the mineral content of both mushroom samples using the ICP-OES method. In a mineral analysis of P. ostreatus, our results followed those of Lesa et al. (2022), who claimed that the major constituents of the mineral element in fruiting bodies are potassium (K), Na, magnesium (Mg), and Ca, while minor constituents include Fe, Zn, selenium (Se), and manganese (Mn). Both normal sample A and synbiotics sample F contained microelements that can be arranged from higher to lower concentrations as follows: K>Na>Mg>Ca>Fe>Zn>Mn>Se. Synbiotics sample F recorded 21 778.37 mg/kg of K and 783.23 mg/kg of Na, whereas typical sample A recorded 14 524.45 mg/kg of K and 737.49 mg/kg of Na (Table 4). The K and Na contents of synbiotics sample F were higher than those of standard sample A. Na and K are critical for maintaining an osmotic balance between cells and intestinal fluid in animal systems. The high amount of Na and K indicated that oyster mushrooms can be beneficial for lowering blood pressure, minimizing the risk of osteoporosis, and maintaining bone health (Elkanah et al., 2022). The K level was particularly high concerning Na, considered a nutritional advantage, as previous findings support that a high K-to-Na ratio can lower blood pressure and reduce cardiovascular disease (Kogure et al., 2021). In addition, the mineral contents in P. pulmonarius were determined to include six minerals: Ca, Cu, Fe, Na, Pb, and Zn. The macroelements (Ca and Na), microelements (Cu, Fe, and Zn), and heavy metal element (Pb) of the negative control sample and the best sample, sample F, which was cultivated with L. casei and inulin, were determined and are recorded in Table 4.
Table 4.
Mineral contents and heavy metal analyses of the fruiting body mushroom samples A and F in Pleurotus ostreatus and Pleurotus pulmonarius
| Mushroom sample | Mineral and heavy metals (mg/kg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu | K | Cd | Pb | Fe | Na | Ca | Zn | Mg | Se | Mn | |
| P. ostreatus (A) | 1.00 | 14 524.45 | 0.02 | 0.31 | 18.57 | 737.49 | 237.98 | 6.63 | 302.99 | 0.11 | 1.48 |
| P. ostreatus (F) | 1.54 | 21 778.37 | 0.05 | 0.30 | 17.38 | 783.23 | 248.00 | 11.15 | 301.52 | 0.10 | 1.49 |
| P. pulmonarius (A) | 0.08 | 5.69 | 37.48 | 1.87 | 0.57 | ||||||
| P. pulmonarius (F) | 0.92 | 1076.85 | 0.05 | 0.19 | 20.42 | 49.65 | 46.48 | 4.70 | 137.21 | 0.02 | 0.71 |
A and F formulations as mentioned in Table 1.
According to the results in Table 4, all the elements in sample F were recorded in higher amounts than the negative control in P. pulmonarius. The amount of the elements differed according to this order: Na>Fe>Ca>Zn>Cu for the negative control sample and Na>Ca>Fe>Zn>Cu>Pb for sample F, which was cultivated with inulin and L. casei. Comparing the two samples, both indicated that Na was the most abundant, while the heavy metal Pb was the least abundant element in both species. Though the crude fiber, fat, or ash content in both samples did not show much variation, the overall study showed that the symbiotic formulations can increase both mushrooms’ macroelements, microelements, and heavy metals. Therefore, synbiotics can be a booster or fertilizer of mushrooms to improve their chemical composition. In addition, this can also help mushroom growers cultivate high-quality oyster mushrooms to gain more profits.
3.4. Total phenolic content and antioxidant analysis
In this study, the TPC of the mushroom extract from all samples was determined using the Folin-Ciocalteu method based on previous research (Phuyal et al., 2020). A series of gallic acid standard stock solutions (0, 25, 50, 75, and 100 µg/mL) were prepared and used to plot the calibration curve. The absorbance values for each concentration were recorded to obtain the calibration curve for grey and white oyster mushrooms. From this calibration curve, the concentration of the phenolic content in the mushroom extracts was calculated using the linear equation, where y refers to the absorbance value and x refers to the concentration of gallic acid in mg/mL. Thus, each sample’s concentration was calculated and shown in Table 5.
Table 5.
Total phenolic concentration (TPC) of mushroom samples
| Mushroom sample | TPC (mg GAE/g DE) | |
|---|---|---|
| P. ostreatus | P. pulmonarius | |
| A | 10.570±0.769 | 0.475±0.109 |
| B | 17.314±0.407 | 0.903±0.037 |
| C | 13.457±0.262 | 0.508±0.058 |
| D | 13.225±0.622 | 1.098±0.030 |
| E | 12.915±0.178 | 0.817±0.197 |
| F | 20.550±0.341 | 1.079±0.020 |
| G | 19.388±0.262 | 0.898±0.027 |
A‒G formulations as mentioned in Table 1. GAE: gallic acid equivalent; DE: dry extract.
Based on the results, as shown in Table 5, the TPC is different between all the mushroom extracts obtained from different mushroom samples. Sample F in white oyster mushroom had the highest, recorded as 20.550 mg GAE/g DE. It was approximately double the TPC in sample A (10.570 mg GAE/g DE). Another synbiotics mushroom extract, sample G, recorded a higher phenolic content of 19.388 mg GAE/g DE. However, sample E contained 12.915 mg GAE/g DE, slightly higher than mushrooms without supplementation. There was an increased TPC in mushrooms cultivated with prebiotics or probiotics alone. The TPC of mushroom extracts B, C, and D was 17.314, 13.457, and 13.225 mg GAE/g DE, respectively. Similarly, for grey mushroom samples, the highest phenolic content was found in sample D (1.098 mg GAE/g DE), followed by sample F (1.079 mg GAE/g DE). In contrast, the lowest phenolic content was observed in sample A, which was the negative control (0.475 mg GAE/g DE). The order of the TPC in the samples, from highest to lowest, was D>F>B>G>E>C>A. If comparing the probiotics used, L. casei produced better results than L. plantarum, which only showed 0.817 mg GAE/g DE in sample E (inulin+L. plantarum) and 0.508 mg GAE/g DE in sample C (L. plantarum). This indicates that L. casei can increase the amount of TPC in P. pulmonarius. This result suggested that supplemented prebiotics combined with microbes provide a synbiotic effect that can be more effective than either microbes or prebiotics alone (Ananthan et al., 2021). In contrast, the combined effect in synbiotic extract E was not better than probiotics or prebiotics alone. This may be because the L. plantarum does not interact closely with the prebiotic inulin from chicory as the efficiency of a prebiotic in synbiotics depends upon its ability to be selectively fermented by specific microorganisms (Saminathan et al., 2011).
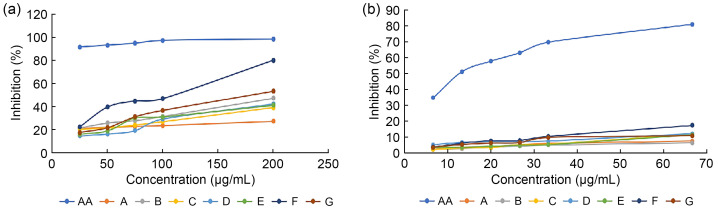
To determine the antioxidant activity of the mushroom extracts, DPPH radical scavenging was used. This study determined the antioxidant activity of P. pulmanarius cultivated with different synbiotic formulations. Ascorbic acid was used as a reference to investigate the DPPH radical scavenging activity. A series of ascorbic acid standard solutions (0.0067, 0.0133, 0.0200, 0.0267, 0.0333, and 0.0667 mg/mL) were prepared, and the absorbance readings were measured at 517 nm. Each mushroom extract of the mushroom samples was analyzed in triplicate. Fig. 3a (P. ostreatus) and Fig. 3b (P. pulmonarius) show the ability of the ascorbic acid and different mushroom extracts at various concentrations to scavenge the free radical of DPPH by plotting the graph of the percentage of inhibition against concentration. The results were then further analyzed to determine the concentrations of the mushroom extracts and ascorbic acid needed to inhibit 50% of the free radicals (IC50). Based on the results, the radical scavenging activity of both the mushroom extracts increased with concentration as an increasing trend was observed from the chart. Following the analysis of TPC, the highest DPPH scavenging activity was found in synbiotics mushroom extract F, followed by extract G and prebiotics extract B.
Fig. 3. DPPH radical scavenging activity of Pleurotus ostreatus extracts at different concentrations (25‒200 μg/mL) (a) and Pleurotus pulmonarius extracts at different concentrations (5‒70 μg/mL) (b) cultivated with different synbiotics formulations. A‒G formulations as mentioned in Table 1. AA: ascorbic acid; DPPH: diphenyl-1-picryl-hydrazyl.
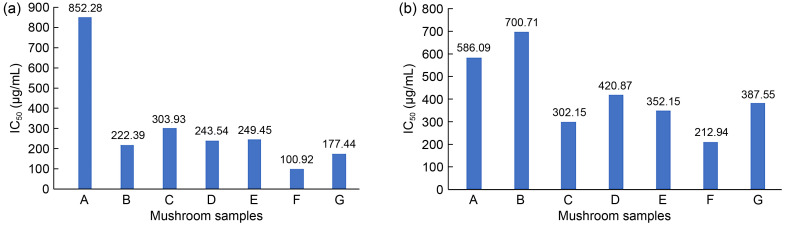
Furthermore, Fig. 4a shows the mushroom extract required to scavenge 50% of DPPH free radicals for investigating the antioxidant activity in white oyster mushrooms. The higher the value of IC50, the higher the amount of sample extract required to be involved in free radical scavenging activity. Higher antioxidant activity will result in a lower value of IC50 (Jin et al., 2018). Since ascorbic acid is a potent antioxidant, the analysis showed that only a deficient concentration of ascorbic acid, 0.0002 μg/mL, was required to reduce 50% of free DPPH free radical. In contrast, a total of 852.28 μg/mL was required by mushroom extract A to be involved in 50% of DPPH radical scavenging activity in P. ostreatus. It was noted that the value of IC50 dropped to 303.93 μg/mL in mushroom extract C. The value of IC50 dropped to 300 μg/mL in mushroom extracts E, D, and B, which recorded IC50 values of 249.45, 243.54, and 222.39 μg/mL, respectively. Mushroom extract F established the highest antioxidant activity, recording the lowest IC50 of 100.92 μg/mL. The antioxidant activity of synbiotics G was also high, achieving an IC50 of 177.44 μg/mL. All these results showed that the mushroom could act as an antioxidant to neutralize the free radicals of DPPH. Supplementing prebiotics, probiotics, and synbiotics increased mushroom extracts’ TPC and antioxidant activity. The reason for the improvement is that the chemical composition of the substrate on which oyster mushrooms grow significantly impacts the matured fruiting bodies. The fruiting bodies can efficiently absorb bioactive substances, such as polyphenols, flavonoids, alkaloids, and tannins, and all of these compounds contribute to the establishment of high antioxidant properties (Jin et al., 2018).
Fig. 4. IC50 of DPPH radical scavenging activity of the Pleurotus ostreatus (a) and Pleurotus pulmonarius (b) mushroom extracts cultivated with different synbiotics formulations. A‒G formulations as mentioned in Table 1. IC50: 50% inhibitory concentration; DPPH: diphenyl-1-picryl-hydrazyl.
4. Conclusions
In this study, white and grey oyster mushrooms were grown with prebiotics, probiotics, and synbiotics to investigate their effects on the growing performance of the mushrooms. This study showed that all the formulated watering solutions of prebiotics, probiotics, and synbiotics could influence the growth of oyster mushrooms, as a bigger size and greater yield were obtained from synbiotics mushroom samples. In addition, supplementing synbiotics can improve the polyphenol content and antioxidant activity of white and grey oyster mushrooms. The greater the amount of polyphenol in the mushrooms, the greater the mushroom’s capacity to scavenge free radicals. The effect of synbiotics is not evident in the proximate analysis, as there was no huge difference in the macronutrient content between the synbiotics sample and the standard sample. Yet, our results still suggested that the synbiotics can improve the quality of oyster mushrooms by increasing the levels of certain minerals in the nutritional analysis; higher levels of K, Na, Ca, Zn, and Mn can offer better health benefits to the consumer. The white mushroom also showed an increase of heavy metals in the synbiotic samples; however, these were still at levels that are safe to consume. Furthermore, supplementing L. plantarum with inulin and probiotics does not significantly improve the growing performance of white and grey oyster mushrooms. The poor effect of the synbiotics might be because of the low efficiency of the prebiotics in selectively fermenting the L. plantarum; a further in vivo study is required to investigate their compatibility. To conclude, the formulated synbiotics watering solution can be applied as a potential biofertilizer to improve the growing performance of white and grey oyster mushrooms. L. casei and inulin can be considered a potential synbiotic combination because of their extraordinary effect on mushroom growth better than the other formulated synbiotics.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
This work was supported Acknowledgement:by the Universiti Malaysia Pahang Al-Sultan Abdullah (UMPSA) (Nos. RDU223010 and PDU223211).
Author contributions
Ang Shu XUAN and Ng Zhi XIN performed the experiments and analyzed the data. Reshma PATIL and Aizi Nor Mazila RAMLI offered suggestions, designed the research, and wrote the manuscript. Nur Izyan Wan AZELEE and Prakash BHUYAR revised the manuscript. All authors have read and approved the final manuscript, and therefore, take responsibility for the integrity of the study.
Compliance with ethics guidelines
Reshma PATIL, Aizi Nor Mazila RAMLI, Ang Shu XUAN, Ng Zhi XIN, Nur Izyan Wan AZELEE, and Prakash BHUYAR declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
Data availability statement
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Adebay EA, Oloke JK, 2017. Oyster mushroom (Pleurotus species); a natural functional food. J Microbiol Biotechnol Food Sci, 7(3): 254-264. 10.15414/jmbfs.2017/18.7.3.254-264 [DOI] [Google Scholar]
- Ananthan SV, Ahmad N, Noor SH, et al. , 2021. Formulation of plant nutrient with synbiotic enhancement. J Chem Eng Ind Biotechnol, 7(2): 11-14. 10.15282/jceib.v7i2.3747 [DOI] [Google Scholar]
- Bakratsas G, Polydera A, Katapodis P, et al. , 2021. Recent trends in submerged cultivation of mushrooms and their application as a source of nutraceuticals and food additives. Future Foods, 4: 100086. 10.1016/j.fufo.2021.100086 [DOI] [Google Scholar]
- Bellettini MB, Fiorda FA, Maieves HA, et al. , 2019. Factors affecting mushroom Pleurotus spp. Saudi J Biol Sci, 26(4): 633-646. 10.1016/j.sjbs.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braat N, Koster MC, Wösten HAB, 2022. Beneficial interactions between bacteria and edible mushrooms. Fungal Biol Rev, 39: 60-72. 10.1016/j.fbr.2021.12.001 [DOI] [Google Scholar]
- Carrasco J, Zied DC, Pardo JE, et al. , 2018. Supplementation in mushroom crops and its impact on yield and quality. AMB Express, 8: 146. 10.1186/s13568-018-0678-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das N, Mishra S, Biswas L, et al. , 2015. Comparative study of five Pleurotus species cultivated in warm temperature on non-sterilized rice straw. Emirates J Food Agric, 27(5): 749-755. 10.9755/ejfa.2015.04.107 [DOI] [Google Scholar]
- di Benedetto NA, Corbo MR, Campaniello D, et al. , 2017. The role of plant growth promoting bacteria in improving nitrogen use efficiency for sustainable crop production: a focus on wheat. AIMS Microbiol, 3(3): 413-434. 10.3934/microbiol.2017.3.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Aidy F, Abdalla M, El-Sawy SA, et al. , 2020. Role of plant probiotics, sucrose and silicon in the production of tomato (Solanum lycopersicum L.) seedlings under heat stress in a greenhouse. Appl Ecol Environ Res, 18(6): 7685-7701. 10.15666/aeer/1806_76857701 [DOI] [Google Scholar]
- Elkanah FA, Oke MA, Adebayo EA, 2022. Substrate composition effect on the nutritional quality of Pleurotus ostreatus (MK751847) fruiting body. Heliyon, 8(11): e11841. 10.1016/J.HELIYON.2022.E11841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmastas M, Isildak O, Turkekul I, et al. , 2007. Determination of antioxidant activity and antioxidant compounds in wild edible mushrooms. J Food Comp Anal, 20(3-4): 337-345. 10.1016/j.jfca.2006.07.003 [DOI] [Google Scholar]
- Fan L, Pandey A, Mohan R, et al. , 2000. Use of various coffee industry residues for the cultivation of Pleurotus ostreatus in solid state fermentation. Acta Biotechnol, 20(1): 41-52. 10.1002/abio.370200108 [DOI] [Google Scholar]
- Girmay Z, Gorems W, Birhanu G, et al. , 2016. Growth and yield performance of Pleurotus ostreatus (Jacq. Fr.) Kumm (oyster mushroom) on different substrates. AMB Express, 6: 87. 10.1186/s13568-016-0265-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm A, Eilertsen L, Chen F, et al. , 2021. Cultivation of Pleurotus ostreatus mushroom on substrates made of cellulose fibre rejects: product quality and spent substrate fuel properties. Waste Biomass Valor, 12(8): 4331-4340. 10.1007/s12649-020-01311-y [DOI] [Google Scholar]
- Hasan SMN, Aunsary MN, 2020. Impact of supplanting of flour with mushroom powder on nutritional composition and sensory attributes of cookies. Malays J Halal Res, 3(2): 43-49. 10.2478/mjhr-2020-0008 [DOI] [Google Scholar]
- Higgins C, Margot H, Warnquist S, et al. , 2017. Mushroom cultivation in the developing world: a comparison of cultivation technologies. IEEE Global Humanitarian Technology Conference, p.1-7. 10.1109/GHTC.2017.8239314 [DOI] [Google Scholar]
- Hoa HT, Wang CL, Wang CH, 2015. The effects of different substrates on the growth, yield, and nutritional composition of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology, 43(4): 423-434. 10.5941/MYCO.2015.43.4.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Yang TJ, Friman VP, et al. , 2021. Introduction of probiotic bacterial consortia promotes plant growth via impacts on the resident rhizosphere microbiome. Proc Royal Soc B, 288(1960): 20211396. 10.1098/rspb.2021.1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin ZQ, Li YL, Ren JH, et al. , 2018. Yield, nutritional content, and antioxidant activity of Pleurotus ostreatus on corncobs supplemented with herb residues. Mycobiology, 46(1): 24-32. 10.1080/12298093.2018.1454014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karami S, Roayaei M, Zahedi E, et al. , 2017. Antifungal effects of Lactobacillus species isolated from local dairy products. Int J Pharm Investig, 7(2): 77. 10.4103/jphi.JPHI_9_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz MA, Thai M, 2018. Compost bacteria and fungi that influence growth and development of Agaricus bisporus and other commercial mushrooms. Appl Microbiol Biotechnol, 102(4): 1639-1650. 10.1007/s00253-018-8777-z [DOI] [PubMed] [Google Scholar]
- Kogure M, Nakaya N, Hirata T, et al. , 2021. Sodium/potassium ratio change was associated with blood pressure change: possibility of population approach for sodium/potassium ratio reduction in health checkup. Hypertens Res, 44(2): 225-231. 10.1038/s41440-020-00536-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Singh M, Singh G, 2013. Effect of different pretreatments on the quality of mushrooms during solar drying. J Food Sci Technol, 50(1): 165-170. 10.1007/s13197-011-0320-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S, Naraian R, 2021. Enhanced growth and yield of oyster mushroom by growth‐promoting bacteria Glutamicibacter arilaitensis MRC119. J Basic Microbiol, 61(1): 45-54. 10.1002/jobm.202000379 [DOI] [PubMed] [Google Scholar]
- Lesa KN, Khandaker MU, Mohammad Rashed Iqbal F, et al. , 2022. Nutritional value, medicinal importance, and health-promoting effects of dietary mushroom (Pleurotus ostreatus). J Food Qual, 2022: 2454180. 10.1155/2022/2454180 [DOI] [Google Scholar]
- Munir N, Xiang TC, Bhuyar P, et al. , 2021. Effective microbes (EM) and their potential on mushroom commercialization in Malaysia. Maejo Int J Energy Environ Commun, 3(3): 45-55. 10.54279/mijeec.v3i3.246955 [DOI] [Google Scholar]
- Munir N, Ramli ANM, Norsazali NFS, et al. , 2023. Valorization of agro-industrial waste for the advancement of mushrooms and their production yield. Biomass Convers Biorefin, in press. 10.1007/s13399-023-04773-x [DOI] [Google Scholar]
- Nagpal R, Kaur A, 2011. Synbiotic effect of various prebiotics on in vitro activities of probiotic lactobacilli. Ecol Food Nutr, 50(1): 63-68. 10.1080/03670244.2011.539161 [DOI] [PubMed] [Google Scholar]
- O'Keefe JM, 1998. Review of research. J Catholic Educ, 2(2): 235-236. 10.15365/joce.0202132013 [DOI] [Google Scholar]
- Oladipo AD, Adegboyega DA, Osunlaja OA, et al. , 2020. Comparative yield and biological efficiency of oyster mushroom (Pleurotus ostreatus) cultivated on sawdust of some selected tree species. J Res Forestry Wildl Environ, 12(3): 216-222. [Google Scholar]
- Phuyal N, Jha PK, Raturi PP, et al. , 2020. In vitro antibacterial activities of methanolic extracts of fruits, seeds, and bark of Zanthoxylum armatum DC. J Trop Med, 2020: 2803063. 10.1155/2020/2803063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M, Sabir AA, Mukta JA, et al. , 2018. Plant probiotic bacteria Bacillus and Paraburkholderia improve growth, yield and content of antioxidants in strawberry fruit. Sci Rep, 8: 2504. 10.1038/s41598-018-20235-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmat, Rahim I, Putera MI, et al. , 2020. Growth and production of white oyster mushroom (Pleurotus ostreatus) by adding coconut water to agricultural waste as a carbon source media. IOP Conf Ser Earth Environ Sci, 575: 012090. 10.1088/1755-1315/575/1/012090 [DOI] [Google Scholar]
- Raman J, Kim JS, Choi KR, et al. , 2022. Application of lactic acid bacteria (LAB) in sustainable agriculture: advantages and limitations. Int J Mol Sci, 23(14): 7784. 10.3390/ijms23147784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohani MF, Islam SM, Hossain MK, et al. , 2022. Probiotics, prebiotics and synbiotics improved the functionality of aquafeed: upgrading growth, reproduction, immunity and disease resistance in fish. Fish Shellfish Immunol, 120: 569-589. 10.1016/j.fsi.2021.12.037 [DOI] [PubMed] [Google Scholar]
- Saminathan M, Sieo CC, Kalavathy R, et al. , 2011. Effect of prebiotic oligosaccharides on growth of Lactobacillus strains used as a probiotic for chickens. Afr J Microbiol Res, 5(1): 57-64. 10.5897/AJMR10.700 [DOI] [Google Scholar]
- Synytsya A, Míčková K, Jablonský I, et al. , 2008. Mushrooms of genus Pleurotus as a source of dietary fibres and glucans for food supplements. Czech J Food Sci, 26(6): 441-446. 10.17221/1361-CJFS [DOI] [Google Scholar]
- Tolera KD, Abera S, 2017. Nutritional quality of oyster mushroom (Pleurotus ostreatus) as affected by osmotic pretreatments and drying methods. Food Sci Nutr, 5(5): 989-996. 10.1002/fsn3.484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B, Whelan K, 2017. Prebiotic inulin‐type fructans and galacto‐oligosaccharides: definition, specificity, function, and application in gastrointestinal disorders. J Gastroenterol Hepatol, 32(S1): 64-68. 10.1111/jgh.13700 [DOI] [PubMed] [Google Scholar]
- Zarenejad F, Yakhchali B, Rasooli I, 2012. Evaluation of indigenous potent mushroom growth promoting bacteria (MGPB) on Agaricus bisporus production. World J Microbiol Biotechnol, 28(1): 99-104. 10.1007/s11274-011-0796-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article does not contain any studies with human or animal subjects performed by any of the authors.