Abstract
Background
Although previous studies have reported an association between multimorbidity and frailty, its direction and mechanism remain unclear. This study aimed to investigate the direction of this association, as well as the role of depression among older Europeans.
Methods
We used a cross-lagged panel design to evaluate the temporal relationship between multimorbidity and frailty and the role of depression. Multimorbidity status was assessed by the self-reporting of 14 chronic diseases. Frailty was assessed based on the frailty phenotype. The European-Depression Scale (EURO-D) was used to assess depression.
Results
There was a bidirectional relationship between frailty and multimorbidity. More severe multimorbidity predicted greater frailty (β = 0.159; p < .001) and vice versa (β = 0.107; p < .001). All paths from multimorbidity to frailty were stronger than the paths from frailty to multimorbidity (b1–a1: β = 0.051; p < .001). Likewise, early multimorbidity change was a significant predictive factor for late frailty change (β = 0.064; p < .001) and vice versa (β = 0.048; p < .001). Depression in Wave 5 (T5) mediated the association between frailty in Wave 4 (T4) and multimorbidity in Wave 6 (T6; indirect effect: β = 0.004; bootstrap 95% confidence interval: 0.003, 0.006).
Conclusions
A positive, bidirectional association was observed between multimorbidity and frailty. Depression may be a potential cause of an increased risk of multimorbidity later in life in frail older adults. Early monitoring of frailty and depression may slow the progression of multimorbidity, thereby interrupting the vicious cycle.
Keywords: Bidirectional association, Depression, Frailty, Mediation, Multimorbidity
Multimorbidity is defined as the co-occurrence of multiple diseases in a person, and affects a large proportion of older people (1,2). A previous study showed that 33.76% of people aged ≥50 were affected by multimorbidity in Europe (3). Undoubtedly, this has a significant negative impact on both individuals and society. For example, 96% of the annual U.S. health care cost in 2002 was attributed to multiple chronic conditions; premature death, impaired function, and poor quality of life were often associated with these conditions (4,5). Therefore, identifying modifiable risk factors for multimorbidity is particularly important for its prevention, identification, and management.
Frailty, a dynamic state that can change or be reversed, is currently receiving extensive attention (6). It is characterized by a decrease in the function of several organs in the body’s physiological system, which reduces resilience to stressors and increases vulnerability to stress (7,8). Although a correlation between frailty and multimorbidity has been previously reported, several questions remain unanswered. First, most prior studies have been cross-sectional in design, which has led to uncertainty regarding the direction (9,10). Second, although some studies have used prospective cohorts to explore the relationship between frailty and multimorbidity, these were single-directional, and their findings were inconsistent. For example, a few studies have reported frailty as a predictor of multimorbidity (11,12). By contrast, others have shown that the participants with multimorbidity were more likely to experience frailty during later follow-ups (13). Third, although a systematic review suggested there may be a bidirectional association between multimorbidity and frailty, no studies have tested this bidirectional association in the same population simultaneously (14). Therefore, given the previous inconsistent findings, it is worthwhile to explore the bidirectional association between frailty and multimorbidity by using cross-lagged panel models with longitudinal data, which may provide a scientific basis for clinicians to better treat patients and prevent or delay the onset of both multimorbidity and frailty.
Furthermore, there is little research on the mechanisms and pathways underlying the relationship between frailty and multimorbidity. A systematic review has revealed a bidirectional association between frailty and depression (15), which may be attributed to common pathophysiological mechanisms, such as oxidative stress, chronic inflammation, and mitochondrial dysfunction (16). Prior depression has also been widely reported to increase the risk of future multimorbidity, while multimorbidity at baseline increases subsequent depression (17). Inflammatory biomarkers, such as interleukin-6 and C-reactive protein, may be the intermediate bridge in this relationship (18,19). Taking into consideration the association between frailty, depression, and multimorbidity, depression may mediate the bidirectional relationship between frailty and multimorbidity. However, this hypothesis has not yet been tested empirically.
Therefore, based on the Survey of Health, Ageing and Retirement in Europe (SHARE), the aims of this study were (i) to investigate the existence of a bidirectional relationship between frailty and multimorbidity, (ii) to explore the existence of a bidirectional relationship between changes in frailty and multimorbidity, and (iii) to better evaluate whether depression is a potential mediator of this bidirectional relationship.
Method
Study Design and Participants
The current study used data from Waves 4 (2011), 5 (2013), and 6 (2015) of SHARE, which included 22 786 respondents. SHARE is a representative prospective study with more than 1.4 million participants aged ≥50 years from 27 European countries and Israel. Longitudinal data were collected every 2–3 years starting in 2004. Trained investigators conducted face-to-face interviews using computer-assisted personal interviewing technology. The Internal Review Board of the University of Mannheim and the Ethics Committee of the Max Planck Society approved SHARE. The participants were required to provide verbal informed consent prior to each interview (20). Additional details regarding SHARE are available at http://www.share-project.org.
To explore cross-lag relationships with temporal changes, respondents who participated in 3 repeated measures, including frailty, multimorbidity, and depression, were included in our study. Of the 40 499 participants who completed the survey at all stages (Waves 4, 5, and 6), 5 750 were excluded due to missing information regarding age, or because they were under 50 years of age; 5 940 were excluded due to incomplete multimorbidity information; 5 367 were excluded due to failure to collect frailty information; and 513 were excluded due to incomplete information regarding depression. In addition, 143 individuals were excluded due to the absence of covariate information. In the end, 22 786 participants were included in the cross-lagged analysis.
Frailty Assessment
Frailty was assessed using the frailty phenotype proposed by Fried et al. (21). However, the definitions of the 5 dimensions were manipulated based on the contents of the SHARE questionnaire. Therefore, we defined frailty variables based on those selected in previous studies (Supplementary Table S1) (22,23). The points for each criterion were totaled, and the total score ranged from 0 to 5 point(s). To describe the baseline characteristics, participants were also classified as not frail, prefrail, or frail. When 3 or more of these criteria were met, the participants were considered frail. They were considered prefrail if they met 1 or 2 criteria and robust if they did not meet any of the criteria.
Multimorbidity Assessment
In this study, multimorbidity was expressed as a continuous variable (2,24), in order to indicate its severity and the cumulative effect of chronic diseases. Participants were categorized by their number of chronic diseases into groups of 0, 1, 2, 3, and 4 or more. Fourteen diseases from SHARE were determined by asking the following question: “Have you ever been diagnosed with the following conditions by a doctor?” These chronic diseases included heart problems, high blood pressure or hypertension, high blood cholesterol, stroke, diabetes or high blood sugar, chronic lung disease, arthritis or rheumatism, cancer or malignant tumors, stomach or duodenal ulcers, Parkinson’s disease, cataracts, hip or femoral fractures, other fractures, and serious memory impairment. In addition, to demonstrate the baseline characteristics of participants, we defined multimorbidity as the presence of ≥2 chronic diseases (25).
Depression Assessment
The EURO-D 12-item scale was used to assess depression in SHARE, including depressive symptoms, crying, appetite, guilt, loss of interest, pessimism, sleep problems, wishing for death, irritability, fatigue, reduced ability to concentrate, and capacity to enjoy things over the last month. The variable was dichotomous, with yes counting as 1 point and no as 0. The lowest score was 0, and the highest score was 12; the higher the score, the more severe the depression level (26,27).
Assessment of Covariates
Basic demographic information (age [50–64, 65–79, and ≥80], sex [male and female], level of education [low, medium, and high], country [Mediterranean and non-Mediterranean], and retirement status [retired and not retired]) and health-related influences (smoking status [nonsmoker, ex-smoker, and current smoker] and drinking status [not at all in the last 3 months, less than once a month, and at least once a month]) were adjusted. According to the International Standard Classification of Education Degrees, education levels were assessed as low, medium, and high, corresponding to 0–2, 3–4, and 5–6 in that order (28). With regards to the country information, we followed former studies that divided respondents’ countries into Mediterranean (Spain, France, Italy, and Slovenia) and non-Mediterranean (Sweden, Denmark, Germany, Belgium, Switzerland, the Czech Republic, Estonia, and Austria) (29). In addition, disability was measured by 7 items from the instrumental activities of daily living. The 7 items included using a map to get around in a strange place, preparing a hot meal, shopping for groceries, making a telephone call, taking medications, doing work around the house or garden, and managing money. Each item was coded as 1 = “difficulty with activity” or 0 = “no difficulty with activity.” In this study, disability was defined as a dichotomous variable: no disability and disability (at least 1 difficulty) (30).
Statistical Analysis
The baseline characteristics are presented as frequencies (percentages) for all categorical variables and as means and standard deviations for all numerical variables. For numerical and categorical variables, parametric p values were calculated using analysis of variance and Chi-square tests, respectively. The Pearson correlation test was used to examine the correlations between frailty, multimorbidity, and depression at the 3 time points.
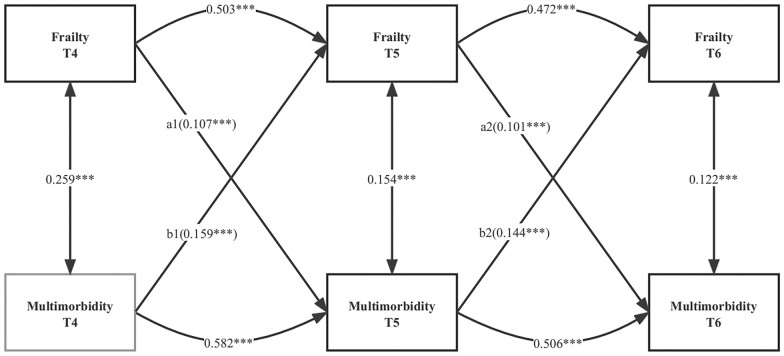
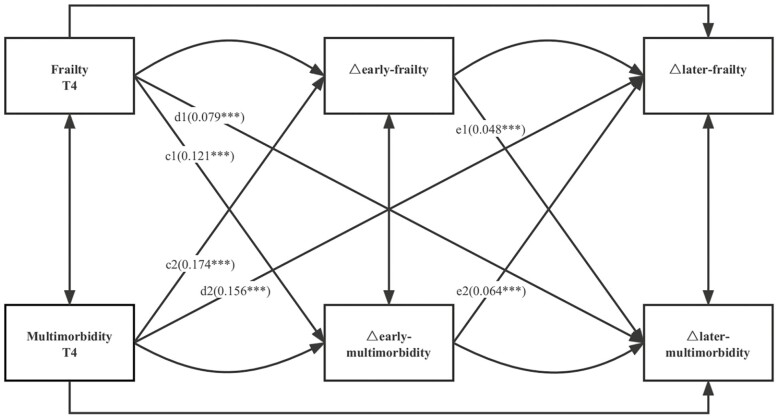
The cross-lagged panel model is a common method for examining the reciprocal relationships between variables in longitudinal data (31). In a 3-wave repeated-measures sample, the first cross-lagged structural equation model was constructed to examine the bidirectional relationship between frailty and multimorbidity (Figure 1). The cross-lagged effects of frailty on multimorbidity (a1 and a2) and of multimorbidity on frailty (b1 and b2) were explored in this model. A full adjustment was made for all covariates, including age, sex, country, educational level, smoking and drinking status, and retirement status. In addition, subgroup analyses for age and sex were performed to determine how frailty and multimorbidity might be affected by age and sex. In the second cross-lagged structural equation model, the association between changes in frailty and changes in multimorbidity was measured to investigate the potential dynamic relationships (Figure 2). In this model, the cross-lagged effect of frailty or multimorbidity at baseline on early and late change in other variable (c1 and c2; d1 and d2) and the early change in frailty or multimorbidity on late change in other variable (e1 and e2) were explored. Based on previous studies, early changes in frailty and multimorbidity were defined as the differences between the measured values of Waves 5 and 4, and the late changes in frailty and multimorbidity were defined as the differences between the measured values of Waves 6 and 5 (32,33).
Figure 1.
Standardized path diagram of the cross-lagged model for frailty and multimorbidity; Survey of Health, Ageing, and Retirement in Europe (N = 22 786). ***p < .001; **p < .01; *p < .05; T4, Wave 4; T5, Wave 5; T6, Wave 6; model adjusted for age, sex, country, level of education, retirement status, and smoking and drinking status in Wave 4.
Figure 2.
Standardized path diagram of the cross-lagged model for changes in frailty and multimorbidity; Survey of Health, Ageing, and Retirement in Europe (N = 22 786). ***p < .001; **p < .01; *p < .05; T4, Wave 4; T5, Wave 5; T6, Wave 6; model adjusted for age, sex, country, level of education, retirement status, and smoking and drinking status in Wave 4; △early frailty = T5 frailty − T4 frailty; △later frailty = T6 frailty − T5 frailty; △early multimorbidity = T5 multimorbidity − T4 multimorbidity; △later multimorbidity = T6 multimorbidity − T5 multimorbidity.
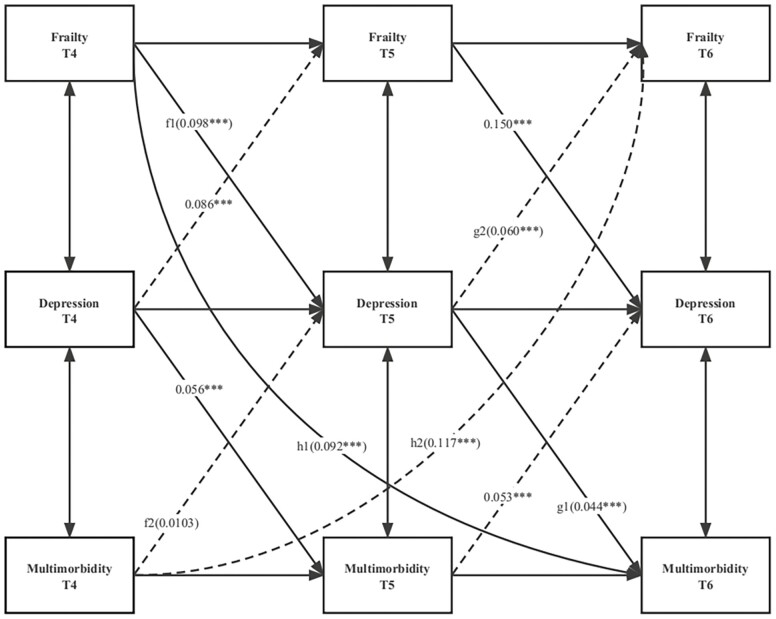
Subsequently, to evaluate the potential mediating role of depression (f1 × g1 and f2 × g2) on the bidirectional relationship between frailty and multimorbidity, a cross-lagged mediating model was developed (34) (Figure 3). Zero was not included in the 95% bootstrap confidence interval (CI) for significant indirect effects, which was assessed using the biased bootstrap method (5 000 draws) (35). Standardized path coefficients and 95% CIs were calculated and compared to determine which path predicted a greater effect. The significance of the differences in the standardized path coefficients was tested using the Sobel test.
Figure 3.
Standardized path diagram of the cross-lagged mediation model; Survey of Health, Ageing, and Retirement in Europe (N = 22 786). ***p < .001; **p < .01; *p < .05; T4, Wave 4; T5, Wave 5; T6, Wave 6; model adjusted for age, sex, country, level of education, retirement status, and smoking and drinking status in Wave 4.
Five model fit indices were used to assess the rationality of the model: comparative fit index (CFI), incremental fit index (IFI), goodness-of-fit index (GFI), standardized root mean square residual (SRMR), and root mean square error of approximation (RMSEA). A model was considered acceptable when the CFI, IFI, and GFI were higher than or equal to 0.9 and when the RMSEA and SRMR were less than or equal to 0.08 (33,36).
We performed several sensitivity analyses to examine the robustness of the findings. First, we repeated the main analyses using another frailty measure: the frailty index. Details on the frailty index are described in Supplementary Material and Supplementary Table S2. Second, to account for the missing data, we used multiple imputation to impute missing data on covariates and repeated the main analysis. Third, we repeated the main analysis using data from Waves 2 (2007), 4 (2011), and 6 (2015) of SHARE to avoid the impact of the length of the time interval on the association. Fourth, we constructed a cross-lagged model that assumed that the cross-lagged path coefficients were equal across 3 waves (a1 = a2; b1 = b2) to explore the temporal relationship between frailty and multimorbidity. Fifth, given the overlap in the variables defining frailty and depression, we further excluded 2 items (appetite and fatigue) related to frailty from the 12 items defining depression and repeated the mediation analyses. Sixth, considering the effect of disability on frailty (37), depression (38), and multimorbidity (1), analyses stratified according to disability (no disability/disability) was conducted to examine the potential moderating role of disability on the relationship between frailty, multimorbidity, and depression.
SAS (version 9.4; SAS Institute, Cary, NC) was used for statistical analyses. AMOS (version 28.0; IBM, Armonk, NY) was used to analyze the cross-lagged panel models. Statistics were considered significant if the p value was less than or equal to .05.
Results
Characteristics and Correlations at Baseline
Table 1 shows the baseline characteristics of the participants. There were 22 786 participants, of which 12 853 (56.41%) were female, and the majority of the participants (n = 11 404, 50.05%) were aged ≥65 years. When compared to nonfrail individuals, those who were frail were more likely to be older, female, from the Mediterranean areas, with a lower educational level, retired, and suffering from numerous chronic diseases, and they were less likely to smoke and drink alcohol. Furthermore, the baseline (Wave 4) characteristics described according to different disease counts showed similar results (Supplementary Table S3). Meanwhile, we also found that the prevalence of multimorbidity in the frail population was 76.3%, and the prevalence of frailty in the multimorbid population was 13.2%, which was similar to results reported of a previous study (14).
Table 1.
Baseline Characteristics of the Study Population by Frailty Status
| Characteristics | Total (n = 22 786) | Frail Status | p Value | ||
|---|---|---|---|---|---|
| Robust (n = 11 750) | Prefrail (n = 9 392) | Frail (n = 1 644) | |||
| Age, n (%) | <.001 | ||||
| 50–64 | 11 382 (49.95) | 6 510 (55.40) | 4 421 (47.07) | 451 (27.43) | |
| 65–79 | 9 641 (42.31) | 4 764 (40.54) | 4 081 (43.45) | 796 (48.42) | |
| ≥80 | 1 763 (7.74) | 476 (4.05) | 890 (9.48) | 397 (24.15) | |
| Sex, n (%) | <.001 | ||||
| Male | 9 933 (43.59) | 5 650 (48.09) | 3 818 (40.65) | 465 (28.28) | |
| Female | 12 853 (56.41) | 6 100 (51.91) | 5 574 (59.35) | 1 179 (71.72) | |
| Country, n (%) | <.001 | ||||
| Non-Mediterranean | 15 991 (70.18) | 8 589 (73.10) | 6 464 (68.82) | 938 (57.06) | |
| Mediterranean | 6 795 (29.82) | 3 161 (26.90) | 2 928 (31.18) | 706 (42.94) | |
| Level of education, n (%) | <.001 | ||||
| Low | 8 218 (36.07) | 3 520 (29.96) | 3 645 (38.81) | 1 053 (64.05) | |
| Medium | 9 246 (40.58) | 5 031 (42.82) | 3 768 (40.12) | 447 (27.19) | |
| High | 5 322 (23.36) | 3 199 (27.23) | 1 979 (21.07) | 144 (8.76) | |
| Retirement status, n (%) | <.001 | ||||
| Not retired | 9 956 (43.69) | 5 387 (45.85) | 4 015 (42.75) | 554 (33.70) | |
| Retired | 12 830 (56.31) | 6 363 (54.15) | 5 377 (57.25) | 1 090 (66.30) | |
| Smoking status, n (%) | <.001 | ||||
| Nonsmoker | 15 029 (65.96) | 7 816 (66.52) | 6 061 (64.53) | 1 152 (70.07) | |
| Ex-smoker | 3 635 (15.95) | 1 856 (15.80) | 1 565 (16.66) | 214 (13.02) | |
| Current smoker | 4 122 (18.09) | 2 078 (17.69) | 1 766 (18.80) | 278 (16.91) | |
| Drinking status, n (%) | <.001 | ||||
| Not at all in the last 3 months | 5 819 (25.54) | 2 194 (18.67) | 2 749 (29.27) | 876 (53.28) | |
| Less than once a month | 2 590 (11.37) | 1 244 (10.59) | 1 163 (12.38) | 183 (11.13) | |
| At least once a month | 14 377 (63.10) | 8 312 (70.74) | 5 480 (58.35) | 585 (35.58) | |
| Multimorbiditya, n (%) | <.001 | ||||
| 0 | 6 606 (28.99) | 4 294 (36.54) | 2 184 (23.25) | 128 (7.79) | |
| 1 | 6 683 (29.33) | 3 768 (32.07) | 2 654 (28.26) | 261 (15.88) | |
| 2 | 4 747 (20.83) | 2 279 (19.40) | 2 114 (22.51) | 354 (21.53) | |
| 3 | 2 692 (11.81) | 940 (8.00) | 1 400 (14.91) | 352 (21.41) | |
| ≥4 | 2 058 (9.03) | 469 (3.99) | 1 040 (11.07) | 549 (33.39) | |
| Multimorbidityb, n (%) | <.001 | ||||
| No | 13 289 (58.32) | 8 062 (68.61) | 4 838 (51.51) | 389 (23.66) | |
| Yes | 9 497 (41.68) | 3 688 (31.39) | 4 554 (48.49) | 1 255 (76.34) | |
| Depression, n (%) | <.001 | ||||
| No | 17 086 (74.98) | 10 880 (92.6) | 5 715 (60.85) | 491 (29.87) | |
| Yes | 5 700 (25.02) | 870 (7.4) | 3 677 (39.15) | 1 153 (70.13) | |
Notes: multimorbiditya = multimorbidity was expressed as a continuous variable; multimorbidityb = multimorbidity was represented as a dichotomous variable.
Supplementary Table S4 shows the correlations among frailty, multimorbidity, and depression across the 3 time points. The associations between each variable at the 3 time points were statistically significant, indicating that all variables were stable across the 3 waves. In addition, the associations were similar for all variables between Waves 4, 5, and 6, indicating stable associations.
According to the change assessment results, 25.75% of the older participants experienced increased frailty between Waves 4 and 5, and 28.71% experienced increased frailty between Waves 4 and 6. In 35.59% and 36.91% of older adults, depression increased between Waves 4 and 5 and between Waves 4 and 6, respectively. Multimorbidity increased between Waves 4 and 5 in 23.31% of older adults and between Waves 4 and 6 in 28.36% of older adults (Supplementary Table S5).
Bidirectional Association Between Frailty and Multimorbidity
A cross-lagged model of the standardized path estimates of the relationship between frailty and multimorbidity was developed (Figure 1 and Supplementary Table S6). In the overall multigroup path analysis model, the CFI was 0.946, the GFI was 0.977, the IFI was 0.946, the SRMR was 0.054, and the RMSEA was 0.076, indicating an adequate fit for the data. At a given time point, frailty and multimorbidity were positively correlated, but their effects diminished over time (T4: β = 0.259; T5: β = 0.154; T6: β = 0.122, p < .001 for all). It was found that the autoregressive path of frailty and multimorbidity was moderately stable (β = 0.472–0.503 for frailty and β = 0.506–0.582 for multimorbidity, p < .001 for all) at the 3 time points. With all covariates taken into account, the model indicated that greater frailty in Wave 4 predicted greater multimorbidity in Wave 5 (β = 0.107; p < .001) and vice versa (β = 0.159; p < .001). Similarly, a positive relationship existed between Wave 5 frailty and Wave 6 multimorbidity (β = 0.101; p < .001) and vice versa (β = 0.144; p < .001).
Differences in standardized path coefficients were compared to assess the strength of the bidirectional effects between frailty and multimorbidity. The results indicated that the relationship between prior multimorbidity and later frailty was stronger than that between early frailty and subsequent multimorbidity (b1–a1: β = 0.051; b2–a2: β = 0.043; p < .001 for all). Subgroup analyses by sex and age (except for those over 80 years of age) supported the main findings (Supplementary Figures S1 and S2 and Supplementary Table S7).
Bidirectional Association Between Changes in Frailty and Multimorbidity
Figure 2 shows the results of the cross-lagged model standardized estimates of the relationship between changes in frailty and changes in multimorbidity (full results are shown in Supplementary Table S8). This equation fits the data relatively well. Frailty at baseline had a significant positive association with both early and late changes in multimorbidity (β = 0.121 and 0.079, respectively; p < .001 for all). Early frailty change was a significant predictive factor for late multimorbidity change (β = 0.048; p < .001). According to the results, those who had previously been frail were more likely to be multimorbid later in life. Multimorbidity at baseline predicted frailty for early and late changes (β = 0.174 and 0.156, respectively; p < .001 for all). Early multimorbidity was a predictor of later frailty (β = 0.064; p < .001). In other words, older adults with multimorbidity at baseline were at risk of developing frailty over time. In addition, we determined the greater pathway effect by comparing the standardized path coefficients (c1 and c2; d1 and d2; e1 and e2), and the results indicated that the pathway effect of baseline multimorbidity leading to the early change and late change in frailty was stronger than that of the other pathway (all p < .001).
Mediating Effects of Depression on the Bidirectional Association Between Frailty and Multimorbidity
In Figure 3, depression was depicted as a mediator of the cross-lagged association between frailty and multimorbidity (the full list of results can be found in Supplementary Table S9). In this model, the degree of fit of the equation was relatively good. Findings suggested that depression in Wave 5 mediated the association between frailty in Wave 4 and multimorbidity in Wave 6 (f1 * g1: β = 0.004, bootstrap 95% CI: 0.003, 0.006; h1: β = 0.092, bootstrap 95% CI: 0.080, 0.105). However, depression in Wave 5 could not mediate the links between multimorbidity in Wave 4 and frailty in Wave 6 (f2 * g2: β = 0.00062, bootstrap 95% CI: −0.00001, 0.00133). In summary, the above results showed that depression mediates the effects of frailty on subsequent multimorbidity but not vice versa.
Sensitivity Analyses
First, with another frailty measure (ie, frailty index), similar results that frailty and multimorbidity had a bidirectional association and that depression in Wave 5 mediated frailty in Wave 4 and multimorbidity in Wave 6 (Supplementary Tables S10 and S11). Second, with the multiple imputation to fill the missing data on covariates, the results were consistent with the main results (Supplementary Tables S12 and S13). Third, using data from longer time intervals, the results showed that the bidirectional association between frailty and multimorbidity still existed (Supplementary Table S14), and the mediating role of depression in the frailty-to-multimorbidity association was consistent with the main analysis (Supplementary Table S15). Fourth, the bidirectional relationship between frailty and multimorbidity was not affected by limiting the cross-lagged path coefficients to equal (Supplementary Figure S3 and Supplementary Table S16). Fifth, after excluding overlapping variables with frailty from depression, sensitivity analyses revealed that depression mediated the bidirectional association between frailty and multimorbidity (a full list of results can be found in Supplementary Table S17). Sixth, the results of the analyses stratified by disability status were consistent with the results of the main analysis (Supplementary Table S18).
Discussion
The present study first demonstrated a positive bidirectional association between frailty and multimorbidity among older Europeans. Prior frailty positively predicted subsequent multimorbidity, and prior multimorbidity positively predicted subsequent frailty, with the latter having a greater effect. Additionally, frailty at baseline and early change in frailty were significant predictors of late change in multimorbidity, and vice versa. Our study revealed that frailty may exacerbate multimorbidity, creating a vicious cycle that leads to frailty over time. Further, depression was investigated as a mediating factor in this association; the results suggested that the effect of frailty on multimorbidity is partially mediated by depression. These findings provide new insights into the direction and mechanisms underlying the association between frailty and multimorbidity.
Most previous studies have revealed a potential bidirectional relationship between frailty and multimorbidity (14). A retrospective cohort study conducted at the Singapore General Hospital showed that the risk of developing frailty increases with the number of comorbidities (39). Similarly, Guaraldi et al. have demonstrated that frailty at baseline predicts multimorbidity events in a 10-year prospective clinical cohort (11). However, these studies have only explored the association between frailty and multimorbidity in a single direction and have not simultaneously explored bidirectional association. Thus, they may be limited in clarifying the temporal relationship. By contrast, our study analyzed the interrelationship between frailty and multimorbidity with cross-lagged panel models using a nationally representative longitudinal data set, revealing a positive bidirectional association between them and finding that the effect of prior multimorbidity on subsequent frailty is greater than the effect of prior frailty on subsequent multimorbidity. Therefore, preventing multimorbidity among older adults can help control frailty; conversely, the assessment of frail people can help in identifying high-risk groups and maximizing health benefits for these groups.
There are several explanations for the bidirectional correlation between frailty and multimorbidity. On the one hand, most frail patients have chronic inflammation, which is a major risk factor for a variety of chronic diseases (40). It has been shown that high levels of interleukin-6 at baseline can predict the accelerated longitudinal accumulation of multiple chronic diseases in older adults (41). On the other hand, when older adults have multiple chronic diseases simultaneously and do not receive timely and effective treatment, the evolution of their condition may accelerate. Their physical condition can change rapidly from healthy to frail within a short period, thereby increasing their risk of developing new diseases (13). Another study by Chang et al. on a population of women showed that the cumulative effects of certain inflammatory diseases may increase the risk of frailty (42). Specifically, when frail older adults suffer from multiple chronic diseases, the accumulation of inflammatory diseases may prolong the pro-inflammatory state, which may elevate cortisol levels and reduce muscle mass, physiological reserve, and immune capacity, thus creating a vicious cycle (42,43).
Notably, our study also revealed that depression partially mediated the association between prior frailty and subsequent multimorbidity. A better understanding of these factors may have important implications for improving the management and prevention of multimorbidity, especially in frail populations. The first half of the longitudinal mediating relationship found in our study was supported by a longitudinal study showing that worsening baseline frailty was associated with subsequent severe depression (44). There are several possible explanations for this observation. Frail older adults may experience disability or functional dependence due to pain, activity limitations, poor endurance, and other factors that lead to depression (45). Overlapping mechanisms of frailty and depression, such as chronic inflammation, mitochondrial dysfunction, and oxidative stress, may also partially explain this relationship (16). One prospective study concluded that depression at baseline increases the risk of future multimorbidity, which is consistent with the second half of our longitudinal mediation relationship (17). Inflammatory biomarkers such as C-reactive protein and interleukin-6 may act as an intermediate bridge between depression and multimorbidity (18,19). Admittedly, we explored only 1 possible mechanism for the interrelationship between multimorbidity and frailty and found the mediating effect of depression. Although the effect was statistically significant, this may play only a small role in the contribution of frailty to multimorbidity, and we cannot be sure whether the effect over time is caused primarily by depression, or other mechanisms. Further research is needed to address this question.
We encountered several noteworthy issues during sensitivity analyses. First, sensitivity analyses of the mediating effects showed that depression partially mediated the effect of multimorbidity on frailty when the components of depression that overlapped with frailty were removed. Analysis of the results revealed that these factors possibly hindered the effect of multimorbidity on depression; further studies are needed to explore this relationship. Second, a subgroup analysis showed that in the age group of 80 years and older, the effect of frailty on multimorbidity was greater than the effect of multimorbidity on frailty in the first stage (Waves 4–5), whereas it was the opposite in the second stage (Waves 5–6). Previously, a study showed that frailty trajectories in older adults follow a U-shaped curve, with accelerated growth in frailty disappearing as age continues to increase after reaching a certain age (33). This may partially explain our results.
Strengths and Limitations
The current study had several strengths. First, our study is the first to use cross-lagged models that could analyze the correlation, stability, and bidirectional relationship of the entire theoretical model in a single analysis to examine the bidirectional associations between frailty and multimorbidity at 3 time points and the association between frailty and multimorbidity dynamics in older Europeans. Second, it has been suggested that there may be differences in the proportion of multimorbidity and frailty in different age groups (14); therefore, we further investigated this association in different age groups. Third, most previous studies have defined multimorbidity as 2 or more diseases; however, this strict cutoff is likely influenced by a ceiling effect that does not account for the large difference in disease burden between those with 2 or more diseases and those with 5 or more diseases (14). Our study used a continuous measure of multimorbidity to compensate for the shortcomings of previous studies.
It is important to note that despite the strengths of this study, some limitations exist. First, the chronic disease history was based on self-reported, which may not be as reliable as medical records or clinical assessments. However, previous studies have shown that the use of self-reports to identify multimorbidity is a common and feasible way in epidemiological studies (46). Second, regarding chronic diseases, only the number of diseases was considered, not the patterns of multimorbidity. Future research should explore longitudinal associations between multimorbidity patterns and frailty. Third, we must acknowledge several limitations inherent in cross-lagged models. The cross-lagged panel model lacks a more specific assessment of within-person effects, and future research is needed to explore between-person effects in within-person change using some alternative models (ie, random intercepts cross-lagged panel model) (47). In addition, the cross-lagged panel model may suffer from omitted variable bias, resulting in biased estimates of relationships between variables. Finally, future studies should consider the potential ethnic or cultural differences in the relationship between multimorbidity, frailty, and depression.
Conclusion
This longitudinal study showed a positive bidirectional association between frailty and multimorbidity and revealed changes in multimorbidity and frailty. We found that multimorbidity had a greater effect on frailty and that depression may lead to multimorbidity in frail people later in life. Early monitoring of frailty and depression as well as attention to the mental health of frail older adults may be beneficial in preventing or slowing the progression of multimorbidity. Further research is needed to reveal the potential pathways underlying the association between multimorbidity and frailty to interrupt the possible vicious cycle.
Supplementary Material
Acknowledgments
We thank the German Ministry of Education and Research, the Max Planck Society for the Advancement of Science, and the U.S. National Institute on Aging (U01_AG09740-13S2, P01_AG005842, P01_AG08291, P30_AG12815, R21_AG025169, Y1-AG-4553-01, IAG_BSR06-11, OGHA_04-064, HHSN271201300071C) and various national funding sources is gratefully acknowledged (see www.share-project.org). We would like to thank Editage (www.editage.cn) for English language editing.
Contributor Information
Zhaolong Feng, Department of Epidemiology and Biostatistics, School of Public Health, Medical College of Soochow University, Suzhou, China.
Ze Ma, Department of Epidemiology and Biostatistics, School of Public Health, Medical College of Soochow University, Suzhou, China.
Wei Hu, Department of Epidemiology and Biostatistics, School of Public Health, Medical College of Soochow University, Suzhou, China.
Qida He, Department of Epidemiology and Biostatistics, School of Public Health, Medical College of Soochow University, Suzhou, China.
Tongxing Li, Department of Epidemiology and Biostatistics, School of Public Health, Medical College of Soochow University, Suzhou, China.
Jiadong Chu, Department of Epidemiology and Biostatistics, School of Public Health, Medical College of Soochow University, Suzhou, China.
Xuanli Chen, Department of Epidemiology and Biostatistics, School of Public Health, Medical College of Soochow University, Suzhou, China.
Qiang Han, Department of Epidemiology and Biostatistics, School of Public Health, Medical College of Soochow University, Suzhou, China.
Na Sun, Department of Epidemiology and Biostatistics, School of Public Health, Medical College of Soochow University, Suzhou, China.
Yueping Shen, Department of Epidemiology and Biostatistics, School of Public Health, Medical College of Soochow University, Suzhou, China.
Funding
This work was supported by the National Natural Science Foundation of China (project number 81973143), the Liyang Chronic Disease Risk Factor Monitoring Cohort Study (project number P113911618), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Data Availability
Data are available upon request from the SHARE website (see http://www.share-project.org/data-access/user-registration.html). Our study uses data from SHARE Wave 4 (doi:10.6103/SHARE.w4.710), 5 (doi:10.6103/SHARE.w5.710), and 6 (doi:10.6103/SHARE.w6.710).
Author Contributions
Study design: Z.F., W.H., and Y.S. Data collection and management: Z.F., Z.M., W.H., Q.H., T.L., J.C., X.C., Q.H., N.S., and Y.S. Data analyses: Z.F., Z.M., and W.H. All authors were involved in the manuscript preparation. The author(s) read and approved the final manuscript. All of the authors have participated actively in this study, and agree to the content of the manuscript and they’re being listed as an author on the paper.
Ethics Approval and Consent to Participate
The SHARE project received ethical approval from the Ethics Committee of the Max Planck Society for the Advancement of Science, and all participants provided informed consent at recruitment. All methods were performed in accordance with the relevant guidelines and regulations.
References
- 1. Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10(4):430–439. doi: 10.1016/j.arr.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 2. Vetrano DL, Calderón-Larrañaga A, Marengoni A, et al. An international perspective on chronic multimorbidity: approaching the elephant in the room. J Gerontol A Biol Sci Med Sci. 2018;73(10):1350–1356. doi: 10.1093/gerona/glx178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Qiao Y, Liu S, Li G, et al. Longitudinal follow-up studies on the bidirectional association between ADL/IADL disability and multimorbidity: results from two national sample cohorts of middle-aged and elderly adults. Gerontology. 2021;67(5):563–571. doi: 10.1159/000513930 [DOI] [PubMed] [Google Scholar]
- 4. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–263. doi: 10.1093/gerona/59.3.m255 [DOI] [PubMed] [Google Scholar]
- 5. Skou ST, Mair FS, Fortin M, et al. Multimorbidity. Nat Rev Dis Primers. 2022;8(1):48. doi: 10.1038/s41572-022-00376-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shi SM, Olivieri-Mui B, McCarthy EP, Kim DH. Changes in a frailty index and association with mortality. J Am Geriatr Soc. 2021;69(4):1057–1062. doi: 10.1111/jgs.17002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tardini F, Pinciroli R, Berra L. The intensive care unit: how to make this unfriendly environment geriatric-friendly. Eur J Surg Oncol. 2020;46(3):379–382. doi: 10.1016/j.ejso.2019.12.022 [DOI] [PubMed] [Google Scholar]
- 8. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet 2019;394(10206):1365–1375. doi: 10.1016/S0140-6736(19)31786-6 [DOI] [PubMed] [Google Scholar]
- 9. Alvarado BE, Zunzunegui MV, Beland F, Bamvita JM. Life course social and health conditions linked to frailty in Latin American older men and women. J Gerontol A Biol Sci Med Sci. 2008;63(12):1399–1406. doi: 10.1093/gerona/63.12.1399 [DOI] [PubMed] [Google Scholar]
- 10. Curcio CL, Henao GM, Gomez F. Frailty among rural elderly adults. BMC Geriatr. 2014;14:2. doi: 10.1186/1471-2318-14-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guaraldi G, Brothers TD, Zona S, et al. A frailty index predicts survival and incident multimorbidity independent of markers of HIV disease severity. AIDS. 2015;29(13):1633–1641. doi: 10.1097/QAD.0000000000000753 [DOI] [PubMed] [Google Scholar]
- 12. Huang ST, Tange C, Otsuka R, et al. Subtypes of physical frailty and their long-term outcomes: a longitudinal cohort study. J Cachexia Sarcopenia Muscle 2020;11(5):1223–1231. doi: 10.1002/jcsm.12577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zheng Z, Guan S, Ding H, et al. Prevalence and incidence of frailty in community-dwelling older people: Beijing Longitudinal Study of Aging II. J Am Geriatr Soc. 2016;64(6):1281–1286. doi: 10.1111/jgs.14135 [DOI] [PubMed] [Google Scholar]
- 14. Vetrano DL, Palmer K, Marengoni A, et al. ; Joint Action ADVANTAGE WP4 Group. Frailty and multimorbidity: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci. 2019;74(5):659–666. doi: 10.1093/gerona/gly110 [DOI] [PubMed] [Google Scholar]
- 15. Mezuk B, Edwards L, Lohman M, Choi M, Lapane K. Depression and frailty in later life: a synthetic review. Int J Geriatr Psychiatry. 2012;27(9):879–892. doi: 10.1002/gps.2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown PJ, Rutherford BR, Yaffe K, et al. The depressed frail phenotype: the clinical manifestation of increased biological aging. Am J Geriatr Psychiatry. 2016;24(11):1084–1094. doi: 10.1016/j.jagp.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qiao Y, Liu S, Zhang Y, Wu Y, Shen Y, Ke C. Bidirectional association between depression and multimorbidity in middle-aged and elderly Chinese adults: a longitudinal cohort study. Aging Ment Health. 2022;26(4):784–790. doi: 10.1080/13607863.2021.1877609 [DOI] [PubMed] [Google Scholar]
- 18. Lindqvist D, Dhabhar FS, James SJ, et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology. 2017;76:197–205. doi: 10.1016/j.psyneuen.2016.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b [DOI] [PubMed] [Google Scholar]
- 20. Hajek A, Konig HH. Asymmetric effects of obesity on loneliness among older Germans. Longitudinal findings from the Survey of Health, Ageing and Retirement in Europe. Aging Ment Health. 2021;25(12):2293–2297. doi: 10.1080/13607863.2020.1822285 [DOI] [PubMed] [Google Scholar]
- 21. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 22. Jarach CM, Tettamanti M, Nobili A, D’Avanzo B. Social isolation and loneliness as related to progression and reversion of frailty in the Survey of Health Aging Retirement in Europe (SHARE). Age Ageing. 2021;50(1):258–262. doi: 10.1093/ageing/afaa168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Santos-Eggimann B, Cuenoud P, Spagnoli J, Junod J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci. 2009;64(6):675–681. doi: 10.1093/gerona/glp012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim KI, Lee JH, Kim CH. Impaired health-related quality of life in elderly women is associated with multimorbidity: results from the Korean National Health and Nutrition Examination Survey. Gend Med. 2012;9(5):309–318. doi: 10.1016/j.genm.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 25. Salive ME. Multimorbidity in older adults. Epidemiol Rev. 2013;35:75–83. doi: 10.1093/epirev/mxs009 [DOI] [PubMed] [Google Scholar]
- 26. Marques A, Gaspar de Matos M, Bordado J, Gouveia ER, Peralta M, Gomez-Baya D. Different levels of physical activity and depression symptoms among older adults from 18 countries: a population-based study from the Survey of Health, Ageing and Retirement in Europe (SHARE). Eur J Sport Sci 2021;21(6):887–894. doi: 10.1080/17461391.2020.1795273 [DOI] [PubMed] [Google Scholar]
- 27. Prince MJ, Reischies F, Beekman AT, et al. Development of the EURO-D scale—a European, Union initiative to compare symptoms of depression in 14 European centres. Br J Psychiatry. 1999;174:330–338. doi: 10.1192/bjp.174.4.330 [DOI] [PubMed] [Google Scholar]
- 28. Foverskov E, Glymour MM, Mortensen EL, Holm A, Lange T, Lund R. Education and cognitive aging: accounting for selection and confounding in linkage of data from the Danish Registry and Survey of Health, Ageing and Retirement in Europe. Am J Epidemiol. 2018;187(11):2423–2430. doi: 10.1093/aje/kwy162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peleg S, Nudelman G. Associations between self-rated health and depressive symptoms among older adults: does age matter? Soc Sci Med. 2021;280:114024. doi: 10.1016/j.socscimed.2021.114024 [DOI] [PubMed] [Google Scholar]
- 30. Vitman Schorr A, Khalaila R. Aging in place and quality of life among the elderly in Europe: a moderated mediation model. Arch Gerontol Geriatr. 2018;77:196–204. doi: 10.1016/j.archger.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 31. Berry D, Willoughby MT. On the practical interpretability of cross-lagged panel models: rethinking a developmental workhorse. Child Dev. 2017;88(4):1186–1206. doi: 10.1111/cdev.12660 [DOI] [PubMed] [Google Scholar]
- 32. Best JR, Liu-Ambrose T, Boudreau RM, et al. ; Health, Aging and Body Composition Study. An evaluation of the longitudinal, bidirectional associations between gait speed and cognition in older women and men. J Gerontol A Biol Sci Med Sci. 2016;71(12):1616–1623. doi: 10.1093/gerona/glw066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sha S, Pan Y, Xu Y, Chen L. Associations between loneliness and frailty among older adults: evidence from the China Health and Retirement Longitudinal Study. BMC Geriatr. 2022;22(1):537. doi: 10.1186/s12877-022-03044-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bentley JP, Brown CJ, McGwin G Jr., Sawyer P, Allman RM, Roth DL. Functional status, life-space mobility, and quality of life: a longitudinal mediation analysis. Qual Life Res. 2013;22(7):1621–1632. doi: 10.1007/s11136-012-0315-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mackinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: distribution of the product and resampling methods. Multivariate Behav Res 2004;39(1):99. doi: 10.1207/s15327906mbr3901_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107(2):238–246. doi: 10.1037/0033-2909.107.2.238 [DOI] [PubMed] [Google Scholar]
- 37. Vermeulen J, Neyens JC, van Rossum E, Spreeuwenberg MD, de Witte LP. Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: a systematic review. BMC Geriatr. 2011;11:33. doi: 10.1186/1471-2318-11-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peng S, Wang S, Feng XL. Multimorbidity, depressive symptoms and disability in activities of daily living amongst middle-aged and older Chinese: evidence from the China Health and Retirement Longitudinal Study. J Affect Disord. 2021;295:703–710. doi: 10.1016/j.jad.2021.08.072 [DOI] [PubMed] [Google Scholar]
- 39. Au Yong PSA, Sim EYL, Ho CYX, et al. Association of multimorbidity with frailty in older adults for elective non-cardiac surgery. Cureus 2021;13(5):e15033. doi: 10.7759/cureus.15033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–522. doi: 10.1038/s41569-018-0064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fabbri E, An Y, Zoli M, et al. Aging and the burden of multimorbidity: associations with inflammatory and anabolic hormonal biomarkers. J Gerontol A Biol Sci Med Sci. 2015;70(1):63–70. doi: 10.1093/gerona/glu127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chang SS, Weiss CO, Xue QL, Fried LP. Patterns of comorbid inflammatory diseases in frail older women: the Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2010;65(4):407–413. doi: 10.1093/gerona/glp181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Castle SC, Uyemura K, Rafi A, Akande O, Makinodan T. Comorbidity is a better predictor of impaired immunity than chronological age in older adults. J Am Geriatr Soc. 2005;53(9):1565–1569. doi: 10.1111/j.1532-5415.2005.53512.x [DOI] [PubMed] [Google Scholar]
- 44. Liu H, Li D, Zhao X, Fang B, Zhang Q, Li T. Longitudinal impact of frailty states and sleep duration on subsequent depressive symptoms of older adults. J Am Geriatr Soc. 2021;69(4):1003–1011. doi: 10.1111/jgs.16999 [DOI] [PubMed] [Google Scholar]
- 45. Woods NF, LaCroix AZ, Gray SL, et al. ; Women’s Health Initiative. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005;53(8):1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x [DOI] [PubMed] [Google Scholar]
- 46. Horton M, Rudick RA, Hara-Cleaver C, Marrie RA. Validation of a self-report comorbidity questionnaire for multiple sclerosis. Neuroepidemiology. 2010;35(2):83–90. doi: 10.1159/000311013 [DOI] [PubMed] [Google Scholar]
- 47. Liu X, Zhang L, Wu G, Yang R, Liang Y. The longitudinal relationship between sleep problems and school burnout in adolescents: a cross-lagged panel analysis. J Adolesc. 2021;88:14–24. doi: 10.1016/j.adolescence.2021.02.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request from the SHARE website (see http://www.share-project.org/data-access/user-registration.html). Our study uses data from SHARE Wave 4 (doi:10.6103/SHARE.w4.710), 5 (doi:10.6103/SHARE.w5.710), and 6 (doi:10.6103/SHARE.w6.710).