Abstract
Many multicenter randomized clinical trials in oncology are conducted through the National Clinical Trials Network (NCTN), an organization consisting of 5 cooperative groups. These groups are made up of multidisciplinary investigators who work collaboratively to conduct trials that test novel therapies and establish best practice for cancer care. Unfortunately, disparities in clinical trial leadership are evident. To examine the current state of diversity, equity, and inclusion across the NCTN, an independent NCTN Task Force for Diversity in Gastrointestinal Oncology was established in 2021, the efforts of which serve as the platform for this commentary. The task force sought to assess existing data on demographics and policies across NCTN groups. Differences in infrastructure and policies were identified across groups as well as a general lack of data regarding the composition of group membership and leadership. In the context of growing momentum around diversity, equity, and inclusion in cancer research, the National Cancer Institute established the Equity and Inclusion Program, which is working to establish benchmark data regarding diversity of representation within the NCTN groups. Pending these data, additional efforts are recommended to address diversity within the NCTN, including standardizing membership, leadership, and publication processes; ensuring diversity of representation across scientific and steering committees; and providing mentorship and training opportunities for women and individuals from underrepresented groups. Intentional and focused efforts are necessary to ensure diversity in clinical trial leadership and to encourage design of trials that are inclusive and representative of the broad population of patients with cancer in the United States.
Clinical trials are the cornerstone of cancer research, generating the highest level of evidence on the efficacy of procedural interventions and novel therapeutics to improve outcomes for patients with cancer. Results of clinical trials are disseminated among the oncology community through publications, presentations at conferences, and consensus guidelines; findings can be practice changing. Although the primary goal of a clinical trial is to benefit patients, trial design and conduct play an important role in career development for clinical investigators. A successfully conducted clinical trial may contribute to long-term career success in the form of grant funding and promotion for researchers, especially for those in leadership roles.
Some of the most practice-changing oncology clinical trials are conducted through the National Cancer Institute (NCI) National Clinical Trials Network (NCTN). These trials are opened across more than 2200 academic and community sites across the United States and internationally, establishing the far-reaching influence of NCTN trials and their principal investigators (PIs) (1). In addition, NCTN trials often answer practical, real-world questions that may never be addressed through industry-sponsored trials. The NCTN began as the NCI Clinical Trials Cooperative Group Program more than 50 years ago to provide the administrative infrastructure and regulatory oversight to facilitate the multi-institutional execution of cancer clinical trials (2). The leadership of these cooperative groups lacked diversity and paralleled the demographic of leadership in academic medicine and the oncologic specialties at the time. The process for nominating and choosing the Cooperative Group chairs, roles that helped shape the landscape of cancer clinical research during that era, were not transparent. Moreover, these positions had no term limits, thus restricting the inclusion of women and racially and ethnically underrepresented groups in leadership positions, despite their increasing number in academic medicine and oncology over the past few decades.
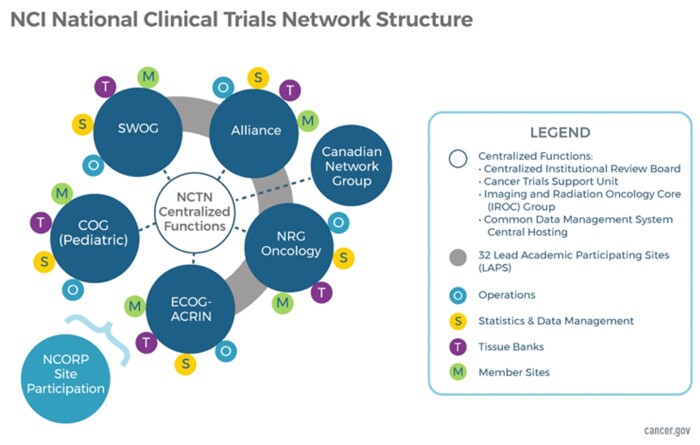
Cooperative groups were ultimately restructured for efficiency in 2014. The NCTN currently comprises 5 adult oncology cooperative groups, including 4 US groups (Alliance for Clinical Trials in Oncology, SWOG, Eastern Cooperative Oncology Group–American College of Radiology Imaging Network (ECOG-ACRIN), and NRG Oncology), 1 Canadian group (Canadian Cancer Trials Group), and the Children’s Oncology Group (Figure 1). Each individual group consists of a community of multidisciplinary investigators, clinicians, and staff and has an independent governance and leadership structure that is not overseen by the NCI.
Figure 1.
NCI National Clinical Trials Network (NCTN) structure. COG = Children’s Oncology Group; ECOG-ACRIN = Eastern Cooperative Oncology Group–American College of Radiology Imaging Network; NCI = National Cancer Institute; NCORP = National Community Oncology Research Program.
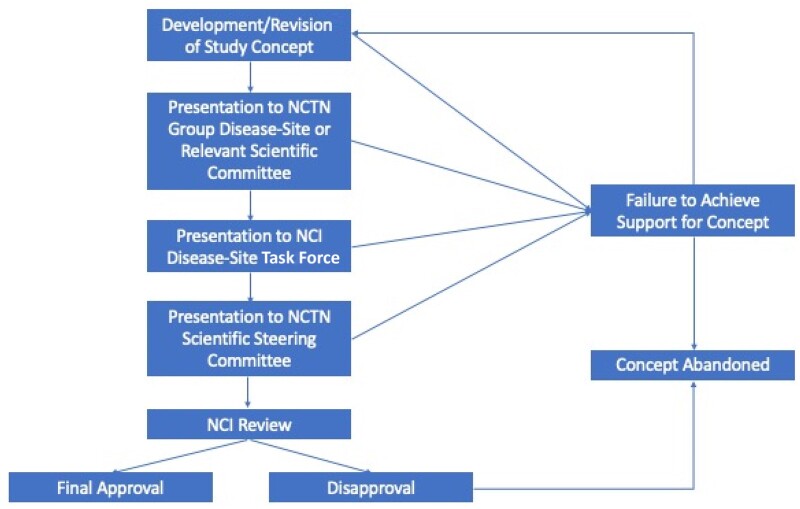
The development of a clinical trial in the NCTN is a multistep process that typically begins with presentation of a study concept to an NCTN group disease-site or nondisease scientific committee that is then revised in an iterative process until there is strong committee support for the concept within the NCTN group. The study concept is then presented to the disease-specific NCI Task Force and, if supported, to the NCTN Scientific Steering Committee, followed by the NCI for review and final approval (Figure 2). Although the process is designed to ensure broad support and feasibility for clinical trials, trial conceptualization and activation may take several years. The system’s inherent complexity and ambiguity make navigating the process quite onerous, particularly for early-career investigators who lack adequate mentorship, making it even more challenging for women and underrepresented investigators.
Figure 2.
Process of clinical trial development within the NCTN groups. NCI = National Cancer Institute; NCTN = National Clinical Trials Network.
JNCI and JNCI: Cancer Spectrum editors, with support from the publisher, Oxford University Press, recently announced a diversity, equity, and inclusion (DEI) initiative to increase diversity and inclusion of underrepresented groups in cancer research in terms of the conduct of that research (clinicians, researchers, and patients) and its publication, including both authorship and peer review (3). In parallel with this initiative, we drafted this viewpoint to outline some of the most important DEI issues facing the cancer clinical trial workforce and to summarize the efforts of our recently developed investigator-initiated task force to promote diversity within the NCTN. Herein, we discuss the existing landscape of DEI among NCTN investigators and leaders, review value added by diversity of the clinical research workforce, and outline specific opportunities to advance DEI within the NCTN infrastructure.
To ensure clarity of language regarding DEI within this discussion, we have defined diversity as individual representation within an organization from different demographic groups, such as race, gender, ethnicity, sexual orientation, age, religion, and nationality (4). For the purpose of this viewpoint, our discussion speaks to the construct of gender or gender identity (ie, men/women) rather than biological sex (ie, male/female). Underrepresented groups include racial or ethnic populations historically minoritized in medicine, oncology, and clinical research. The term equity represents fair treatment of all individuals, such that each is provided with equal access to opportunities. Finally, inclusion illustrates the concept that individuals feel accepted for their unique identities and ideas and feel part of the organizational system (4). Within this viewpoint, we will primarily focus on the concept of diversity but will include recommendations to address intersectional issues of DEI within the clinical trial leadership workforce.
Disparities in oncology and clinical trials leadership
Over the past few decades, gender diversity among oncology clinicians and researchers has increased, with women now comprising 37% of medical oncologists, 31% of radiation oncologists, and 39% of surgical oncologists (5). These levels are still not commensurate with current medical school composition, however, in which more than 50% of students are women (6). Racial disparities persist, as well; only 2.3% of practicing oncologists self-identify as Black or African American, and 5.8% identify as Hispanic, illustrating underrepresentation compared with current medical student demographics (8.2% and 10.5%, respectively) and the general US population (13.6% and 18.9%, respectively) (6-9). The underrepresentation of women and individuals from minoritized racial and ethnic groups is even more apparent in faculty leadership positions and NCI-designated cancer center leadership (5,10,11).
These gender disparities extend to leadership of oncology clinical trials. In an evaluation of phase III randomized controlled trials in oncology published between 2003 and 2018, only 25.9% of NCTN group trials (48/185 trials) were led by female corresponding authors (12). Even lower rates of female corresponding authors were identified among gastrointestinal (GI) (7.9%), genitourinary (7.2%), and hematologic cancer trials (9.3%) (12). Perhaps most concerning, no female corresponding authors were identified for published NCTN surgical trials (12). Individuals from racial and ethnic minoritized groups are also underrepresented among clinical trial investigators. A recent study found that non-White investigators conduct and initiate fewer clinical trials than their White counterparts, likely because of limited clinical research infrastructure and support (13).
When examining oncology articles published in high-impact medical journals from 2002 to 2018, less than one-third of authors were identified as women (14). Senior authors were less likely to be women, regardless of journal type, year, or primary article type. Further, women were less likely to be authors of clinical trials at each authorship position than authors at that respective position for observational studies in oncology (14). Similar trends have been observed when examining authorship by race: the proportion of Black and Hispanic first and senior authors in high-impact journals, specifically the Journal of the American Medical Association and the New England Journal of Medicine, has changed very little over the past 3 decades (15). For example, the percentage of Black authors in the New England Journal of Medicine has increased only 0.5% per year since 2000. The authors suggest that it would take more than 140 years for the proportion of Black senior authors to match the proportion of Black individuals in the United States (15).
The case for diversity in clinical trial leadership
Despite these disappointing statistics, the benefits of investigator diversity in scientific research and clinical trials are abundant (16). First, gender diversity in leadership appears to promote a more equitable and supportive environment. When performing speaker introductions at the 2017 and 2018 annual American Society for Clinical Oncology meetings, men were less likely to introduce women speakers with their professional title, whereas no gender differences were observed when women performed the introductions (17). Although implicit bias has been demonstrated in professional speaker introductions, it likely extends across other arenas of clinical and academic oncology, as well. Communication styles differ by gender, race and ethnicity, and other elements of our lived experiences. Active listening, interrupting, and delivery of criticism can affect individuals’ willingness to participate in discussions and influence group decision making. To promote effective and inclusive communication, cultural competency training is often required in many industries, including medicine. Research suggests that teams with more women achieve greater equity in participation and that women more accurately recognize the expertise of their team members than men (18,19).
Second, a more diverse team of scientists leads to more innovative, inclusive, and impactful research. A cross-sectional study analyzing dissertation data from nearly all US PhD recipients found that demographically underrepresented students—specifically, women and non-White scholars—innovate—or introduce novel conceptual scientific links—at higher rates than majority students (20). In another study, a positive association was identified between women’s authorship and a research study’s likelihood of conducting gender- and sex-based analysis (21). Several studies of published scientific and medical manuscripts have demonstrated that publications by mixed-gender teams and ethnically diverse teams are substantially more novel and impactful than publications of same-gender or less ethnically diverse teams of equivalent size (22,23). Although just a few examples, the scientific advantages of culturally and gender-diverse teams are evident.
In the clinical trial setting specifically, studies have demonstrated that a more diverse research staff may improve clinical trial participation by individuals from minoritized or underrepresented racial and ethnic groups. In 1 survey, physicians from underrepresented racial and ethnic groups who have participated in clinical trial research reported that they perceive their race as important to overcoming patients’ reluctant to participate in clinical research (13). Similarly, in a study of focus groups to explore perceptions of clinical trial participation among Black women, Black investigator representation on the research team was identified as a positive motivator to participation (24). This relationship appears to translate to other racial and ethnic groups, as well. Focus groups conducted with Native American individuals identified that study leadership by Native American community members would promote participation in clinical trial programs around smoking cessation (25). In addition to increasing accrual of diverse populations, enhancing diversity among the leaders and committees responsible for clinical trial development may similarly promote design of trials that are more inclusive and generalizable or trials that study differential response to interventions across unique populations.
Focusing on solutions
To examine the current state of DEI across the NCTN group leadership and among clinical trial investigators, we established an independent NCTN Task Force for Diversity in GI Oncology in 2021. Our task force consists of a complement of medical oncology, radiation oncology, and surgical oncology early-career and mid- to senior faculty members with diverse racial, ethnic, and gender-identity backgrounds from each of the 5 NCTN groups to promote information sharing and collaboration across groups. We defined our task force mission to include an assessment of the historical and current trends of leadership within the NCTN and identification of opportunities to advance and promote diversity within the organization. Although our group initially intended to address issues within GI oncology clinical trials, we have since expanded our initiatives more broadly, given the need for these efforts across all disease sites in the NCTN.
We first sought to understand the existing landscape of diversity among NCTN groups by reaching out to leaders in each group to request data on demographics and policies. Through these efforts, we identified that most NCTN groups historically have not and presently do not routinely collect or track demographic data for their members or leaders. Further, the organizational leadership infrastructure differs across NCTN groups, with tremendous variation in delegation of opportunities, pathways toward leadership, and tenure periods for committee chairs. Regarding leadership of clinical trials and publications, none of the groups routinely tracks demographic records of PIs or authorship of primary or secondary manuscripts. Each group has a unique policy regarding authorship; many give PIs priority authorship for all secondary manuscripts. In addition, many groups require authorship inclusion and senior author designation to committee chairs and other group leaders. These requirements can be structural impediments to the advancement of more junior investigators who have performed the work of conducting the trial while perpetuating success for a small subset of individuals. In general, we identified a consistent lack of transparency in the specific parameters for membership, leadership, authorship, and advancement within the NCTN groups. When processes lack transparency, equity becomes difficult—if not impossible—to measure.
In response to building momentum around the importance of DEI in clinical medicine, the NCI established the NCI Equity and Inclusion Program (EIP) in 2021, which is overseen and supported by the NCI Equity Council (26). This program is designed to enhance research on cancer health disparities, ensure diversity of thought and background in the cancer research workforce, promote an inclusive and equitable community at the NCI, and systematically track and evaluate equity activities. Shortly following the creation of the program, the NCI put out a request for information titled “Seeking Stakeholder Input on Enhancing Diversity and Inclusion in the Cancer Research Workforce” (Notice No. NOT-CA-21-067). Our recently formed task force was poised to respond and described both the challenges caused by gender disparities in clinical trial leadership as well as potential solutions. Our response was one of the driving forces behind the NCI’s decision to form a subcommittee of the EIP. The EIP subcommittee conducted a survey of the NCTN leadership in 2023 in an effort to benchmark diversity. This survey will be the first of its kind. Although we had initially intended to collect these data as a primary objective in our task force, we are encouraged that the NCI is now leading this important centralized effort. We anticipate that this will be an excellent first step toward addressing diversity across the NCI, which should include additional, focused efforts specific to the NCTN.
Conclusions and recommendations
Women and individuals from minoritized racial and ethnic groups remain underrepresented in NCTN clinical trial leadership, although the full extent of these disparities remains difficult to capture because of the absence of available data. While we await results of the NCI EIP survey, our task force has focused our efforts on identifying discrete and actionable efforts to address diversity within the NCTN (Table 1). First, we believe that it is critical that we begin collecting information about the demographics of our membership, PIs, committees and steering committees, committee chairs, and group leaders. This information should include demographics on sex and gender identity, race and ethnicity, faculty rank, and institution, among others. Although the NCTN groups are independent entities, the process for membership and leadership—including term limits—should be streamlined and standardized across all NCTN groups. Opportunities for early-career faculty to gain leadership experience can be created within smaller subcommittees, which can facilitate the development of a more diverse complement of future leaders. Efforts focused on ensuring DEI should be embedded within all NCTN groups, such as the recently created Vice Chair for Diversity, Equity, Inclusion, and Professional Integrity position within the SWOG Cancer Research Network and the creation of the ECOG-ACRIN Task Force on Advancement of Women. We must ensure that our task forces and steering committees consist of diverse individuals who can contribute a broad range of perspectives and expertise to the clinical trial development process.
Table 1.
Opportunities to address workforce disparities within the National Clinical Trials Network
| Type of intervention | Anticipated outcome |
|---|---|
| Membership | |
| Collect and publish annual demographic data on NCTN group committee membership. | Ensure accountability to address current gaps in gender and racial workforce representation within the NCTN. |
| Publish standardized criteria for NCTN group and committee membership eligibility. | Enhance diversity across NCTN group membership and committees to better represent the spectrum of perspectives and priorities within the broader oncology community. |
| Streamline membership application processes. | Encourage and facilitate membership application and selection from a more diverse population of investigators through a standardized, transparent process. |
| Promote diverse representation among NCTN task forces and steering committees. | Design clinical trials that are considerate and inclusive of racial, ethnic, gender, and other underrepresented minority populations. |
| Leadership | |
| Collect annual demographic data on NCTN principal investigators, committee chairs, task force members, steering committee members, and group leaders. | Ensure accountability to address existing disparities in leadership across the NCTN groups and NCI leadership. |
| Standardize NCTN committee and task force leadership term limits. | Create leadership opportunities for faculty to promote and maintain an engaged clinical trial workforce and program. |
| Establish a more diverse cadre of experienced, senior leaders to serve as mentors and role models for new and underrepresented investigators. | |
| Create a designated leadership position (ie, vice chair or equivalent) responsible for DEI within each NCTN group. | Communicate clear prioritization of DEI within the NCTN groups, and establish specific, intentional programmatic efforts to enhance DEI. |
| Education | |
| Create a framework to promote respectful communication. | Offer structured recommendations to NCTN leadership and members to ensure that communication is respectful and professional, that all individuals are addressed equitably by professional titles, and that every member feels encouraged and empowered to participate in discussions and decisions. |
| Implement implicit bias training for NCTN leadership. | Ensure that NCTN leadership, including committee chairs and task force members, are aware of their own potential implicit bias to minimize the influence of bias during committee discussions and decisions. |
| Develop specific training opportunities or funding awards for women and underrepresented junior investigators interested in clinical trial design and leadership. | Build a more diverse workforce by specifically providing clinical trial education and training to underrepresented individuals and those from less resource-rich institutions. |
| Establish a formal mentorship program for junior faculty. | Encourage and support early-career investigators from diverse backgrounds and institutions to engage in the NCTN. |
| Establish orientation materials for new members. | Educate new members on the NCTN group processes, which will foster transparency and equity. |
| Publications | |
| Review and revise publication policies for primary and secondary manuscripts. | Ensure that authorship is equitable and accurately represents the effort of participating investigators. |
| Standardize authorship contribution criteria across NCTN groups. | |
| Rotate authorship and presentations for secondary manuscripts to provide opportunities for junior investigators. | Increase engagement of new and junior investigators by ensuring fair and equitable credit for time and effort. |
DEI = diversity, equity, and inclusion; NCI = National Cancer Institute; NCTN = National Clinical Trials Network.
Strategies to promote inclusivity, such as a best-practice guidelines to promote professional and respectful communication and implicit bias training, should be developed. To promote equity, dedicated mentorship and training opportunities should be offered to women and underrepresented individuals interested in clinical trial design and leadership through the NCTN. Further, we must carefully evaluate and revise existing publication policies to represent the efforts of underrepresented and early-career investigators more equitably. Solutions could include rotating authorship for secondary manuscripts, allowing junior members of the research team to present study findings at national and regional meetings, and standardizing authorship roles and contribution requirements across the NCTN. Finally, retention of midcareer and established professionals, including women and individuals from underrepresented backgrounds, should be prioritized through intentional strategies such as committee leadership opportunities, dedicated support for mentorship of junior faculty, and involvement in steering committees or task forces.
In conclusion, addressing DEI in membership and leadership across the NCTN groups will lead to more creative, innovative, and inclusive clinical trials. Ultimately, we advocate that an equitable workplace and equitable patient care are 2 sides of the same coin. To promote diversity of ideas and clinical trial leadership that is more reflective of our members, we must invite women and investigators from a variety of ethnic, racial, cultural, and gender-identity backgrounds to the table. We—and our patients—deserve it.
Acknowledgements
None.
Role of the funder: Not applicable.
Contributor Information
Rebecca A Snyder, Departments of Surgical Oncology and Health Services Research, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Barbara Burtness, Department of Internal Medicine, Section of Medical Oncology, and Yale Cancer Center, Yale School of Medicine, New Haven, CT, USA.
May Cho, Department of Medicine, Division of Hematology/Oncology, University of California, Irvine, Irvine, CA, USA.
Jaydira Del Rivero, Developmental Therapeutics Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Deborah B Doroshow, Department of Medicine, Division of Hematology and Oncology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Kathryn E Hitchcock, Department of Radiation Oncology, University of Florida, Gainesville, FL, USA.
Aparna Kalyan, Department of Medicine, Division of Hematology & Oncology and Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, IL, USA.
Christina A Kim, Department of Internal Medicine, Section of Medical Oncology and Hematology, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, MB, Canada.
Jelena Lukovic, Radiation Medicine Program, Princess Margaret Cancer Centre, University Health Network and Department of Radiation Oncology, University of Toronto, ON, Canada.
Aparna R Parikh, Massachusetts General Cancer Center, Division of Hematology/Oncology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Nina N Sanford, Department of Radiation Oncology, The University of Texas Southwestern Medical Center, Dallas, TX, USA.
Bhuminder Singh, Department of Medicine, Division of Gastroenterology, Hepatology, and Nutrition, Vanderbilt University Medical Center, Nashville, TN, USA.
Chan Shen, Departments of Surgery and Public Health Sciences, Penn State College of Medicine, Hershey, PA, USA.
Rachna T Shroff, Department of Medicine, Division of Hematology/Oncology, University of Arizona Cancer Center, Tucson, AZ, USA.
Namrata Vijayvergia, Department of Hematology/Oncology, Fox Chase Cancer Center, Philadelphia, PA, USA.
Karyn A Goodman, Department of Radiation Oncology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Pamela L Kunz, Department of Internal Medicine, Section of Medical Oncology, and Yale Cancer Center, Yale School of Medicine, New Haven, CT, USA.
Data availability
No new data were generated or analyzed in this manuscript.
Author contributions
Rebecca A. Snyder, MD, MPH (Conceptualization; Formal analysis; Investigation; Project administration; Writing—original draft; Writing—review & editing), Namrata Vijayvergia, MD (Conceptualization; Investigation; Writing—review & editing), Rachna T. Shroff, MD (Conceptualization; Investigation; Writing—review & editing), Chan Shen, PhD (Conceptualization; Investigation; Writing—review & editing), Bhuminder Singh, PhD (Conceptualization; Investigation; Writing—review & editing), Nina N. Sanford, MD (Conceptualization; Investigation; Writing—review & editing), Aparna R. Parikh, MD (Conceptualization; Validation; Writing—review & editing), Karyn A. Goodman, MD, MS (Conceptualization; Investigation; Supervision; Writing—review & editing), Jelena Lukovic, MD, MPH (Conceptualization; Investigation; Writing—review & editing), Aparna Kalyan, MD (Conceptualization; Investigation; Writing—review & editing), Kathryn E. Hitchcock, MD, PhD (Conceptualization; Investigation; Writing—review & editing), Deborah B. Doroshow, MD, PhD (Conceptualization; Investigation; Writing—review & editing), Jaydira Del Rivero, MD (Conceptualization; Investigation; Writing—review & editing), May Cho, MD (Conceptualization; Investigation; Writing—review & editing), Barbara Burtness, MD (Conceptualization; Investigation; Writing—review & editing), Christina A. Kim, MD (Conceptualization; Investigation; Writing—review & editing), Pamela L. Kunz, MD (Conceptualization; Investigation; Supervision; Writing—review & editing).
Funding
Not applicable.
Conflicts of interest
D.B.D.: consultant, AstraZeneca, Mirati, Summit Therapeutics, G1 Therapeutics, Sonata Therapeutics
K.A.G.: consultant/Advisory Board, RenovoRX, NCI, Philips Healthcare; Data Safety Monitoring Committee (DSMC), Viewray
C.A.K.: research funding, Celgene Inc; educational honorarium, Amgen
P.L.K.: research, Novartis (Advanced Accelerator Applications); steering committees, Novartis (Advanced Accelerator Applications), Rayze Bio; advisory boards, Amgen, Genentech, Crinetics, Natera, HutchMed, Ipsen, Isotopen Technologien München AG (ITM)
K.E.H.: research funding, Merck, Naveris; travel to speak at conference, MVision, Philips, NRG Oncology; medical writing, Medscape
R.T.S.: research funding, Bayer, BMS, Bristol-Meyers Squibb, Exelixis Pharmaceuticals, IMV Inc, LOXO, Novocure, NUCANA, Pieris, QED Therapeutics, Rafael Pharmaceuticals, Seagen, Taiho; advisory boards: AstraZeneca, Boehringer Ingelheim Pharma, Clovis, Genentech, Incyte, Merck, QED Therapeutics, Servier, Taiho, Zymeworks Biopharm; consulting, SYROS
References
- 1. Unger JM, LeBlanc M, George S, et al. Population, clinical, and scientific impact of National Cancer Institute’s National Clinical Trials Network treatment studies. J Clin Oncol. 2023;41(11):2020-2028. doi: 10.1200/jco.22.01826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keating P, Cambrosio A. Cancer on Trial: Oncology as a New Style of Practice. Chicago: University of Chicago Press; 2012. [Google Scholar]
- 3. Ganz PA, Chen RC, Boehm AL. Addressing diversity, equity, and inclusion at the JNCI journals. JNCI Cancer Spectr. 2022;6(5):pkac046. doi: 10.1093/jncics/pkac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moore KJ, Xiong S, Bhattacharya M, Bustamante G, Calvert C. Beyond diversity: focusing on and enhancing inclusion in the society for epidemiologic research. Am J Epidemiol. 2020;189(10):1042-1046. doi: 10.1093/aje/kwaa111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chowdhary M, Chowdhary A, Royce TJ, et al. Women’s representation in leadership positions in academic medical oncology, radiation oncology, and surgical oncology programs. JAMA Netw Open. 2020;3(3):e200708. doi: 10.1001/jamanetworkopen.2020.0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. 2022 FACTS: applicants and matriculants data. Association of American Medical Colleges. https://www.aamc.org/data-reports/students-residents/interactive-data/2022-facts-applicants-and-matriculants-data. Accessed January 13, 2023.
- 7. American Society of Clinical Oncology. The state of cancer care in America, 2017: a report by the American Society of Clinical Oncology. J Oncol Pract. 2017;13(4):e353-e394. doi: 10.1200/jop.2016.020743. [DOI] [PubMed] [Google Scholar]
- 8. QuickFacts: United States. Population estimates, July 1, 2022, (V2022): population and housing unit estimates. US Census Bureau. https://www.census.gov/quickfacts/fact/table/US/PST045221. Accessed January 13, 2023.
- 9. Winkfield KM, Levit LA, Tibbits M, et al. Addressing equity, diversity, and inclusion of Black physicians in the oncology workforce. JCO Oncol Pract. 2021;17(5):224-226. doi: 10.1200/op.21.00079. [DOI] [PubMed] [Google Scholar]
- 10. Lerman C, Hughes-Halbert C, Falcone M, et al. Leadership diversity and development in the nation’s cancer centers. J Natl Cancer Inst. 2022;114(9):1214-1221. doi: 10.1093/jnci/djac121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morgan A, Shah K, Tran K, Chino F. Racial, ethnic, and gender representation in leadership positions at National Cancer Institute-designated cancer centers. JAMA Netw Open. 2021;4(6):e2112807. doi: 10.1001/jamanetworkopen.2021.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ludmir EB, Mainwaring W, Miller AB, et al. Women’s representation among lead investigators of clinical trials in oncology. JAMA Oncol. 2019;5(10):1501-1502. doi: 10.1001/jamaoncol.2019.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Getz K, Faden L. Racial disparities among clinical research investigators. Am J Ther. 2008;15(1):3-11. doi: 10.1097/MJT.0b013e31815fa75a. [DOI] [PubMed] [Google Scholar]
- 14. Yalamanchali A, Zhang ES, Jagsi R. Trends in female authorship in major journals of 3 oncology disciplines, 2002-2018. JAMA Netw Open. 2021;4(4):e212252. doi: 10.1001/jamanetworkopen.2021.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abdalla M, Abdalla M, Abdalla S, Saad M, Jones DS, Podolsky SH. The under-representation and stagnation of female, Black, and Hispanic authorship in the Journal of the American Medical Association and the New England Journal of Medicine. J Racial Ethn Health Disparities. 2023;10(2):920-929. doi: 10.1007/s40615-022-01280-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nielsen MW, Alegria S, Börjeson L, et al. Gender diversity leads to better science. Proc Natl Acad Sci U S A. 2017;114(8):1740-1742. doi: 10.1073/pnas.1700616114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duma N, Durani U, Woods CB, et al. Evaluating unconscious bias: speaker introductions at an international oncology conference. J Clin Oncol. 2019;37(36):3538-3545. doi: 10.1200/jco.19.01608. [DOI] [PubMed] [Google Scholar]
- 18. Joshi A. By whom and when is women’s expertise recognized? The interactive effects of gender and education in science and engineering teams. Administr Sci Qu. 2014;59(2):202-239. doi: 10.1177/0001839214528331. [DOI] [Google Scholar]
- 19. Williams Woolley A, Chabris CF, Pentland A, Hashmi N, Malone TW. Evidence for a collective intelligence factor in the performance of human groups. Science. 2010;330(6004):686-688. doi: 10.1126/science.1193147. [DOI] [PubMed] [Google Scholar]
- 20. Hofstra B, Kulkarni VV, Munoz-Najar Galvez S, He B, Jurafsky D, McFarland DA. The diversity-innovation paradox in science. Proc Natl Acad Sci U S A. 2020;117(17):9284-9291. doi: 10.1073/pnas.1915378117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nielsen MW, Andersen JP, Schiebinger L, Schneider JW. One and a half million medical papers reveal a link between author gender and attention to gender and sex analysis. Nat Hum Behav. 2017;1(11):791-796. doi: 10.1038/s41562-017-0235-x. [DOI] [PubMed] [Google Scholar]
- 22. Yang Y, Tian TY, Woodruff TK, Jones BF, Uzzi B. Gender-diverse teams produce more novel and higher-impact scientific ideas. Proc Natl Acad Sci U S A. 2022;119(36):e2200841119. doi: 10.1073/pnas.2200841119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. AlShebli BK, Rahwan T, Woon WL. The preeminence of ethnic diversity in scientific collaboration. Nat Commun. 2018;9(1):5163. doi: 10.1038/s41467-018-07634-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith YR, Johnson AM, Newman LA, Greene A, Johnson TRB, Rogers JL. Perceptions of clinical research participation among African American women. J Women's Health (Larchmt). 2007;16(3):423-428. doi: 10.1089/jwh.2006.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fu SS, Rhodes KL, Robert C, Widome R, Forster JL, Joseph AM. Designing and evaluating culturally specific smoking cessation interventions for American Indian communities. Nicotine Tob Res. 2014;16(1):42-49. doi: 10.1093/ntr/ntt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.About the NCI Equity and Inclusion Program. National Cancer Institute. https://www.cancer.gov/research/key-initiatives/nci-equity-inclusion-program/about. Updated June 14, 2021. Accessed January 3, 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in this manuscript.