Cancer health disparities are measurable remediable, but persistent, differences in cancer-related quality of life or mortality across groups of people. Health disparities are rooted in historical injustices, including systemic racism (1), which, in turn, create inequities in risk exposures and access to and quality of health services (2-4).
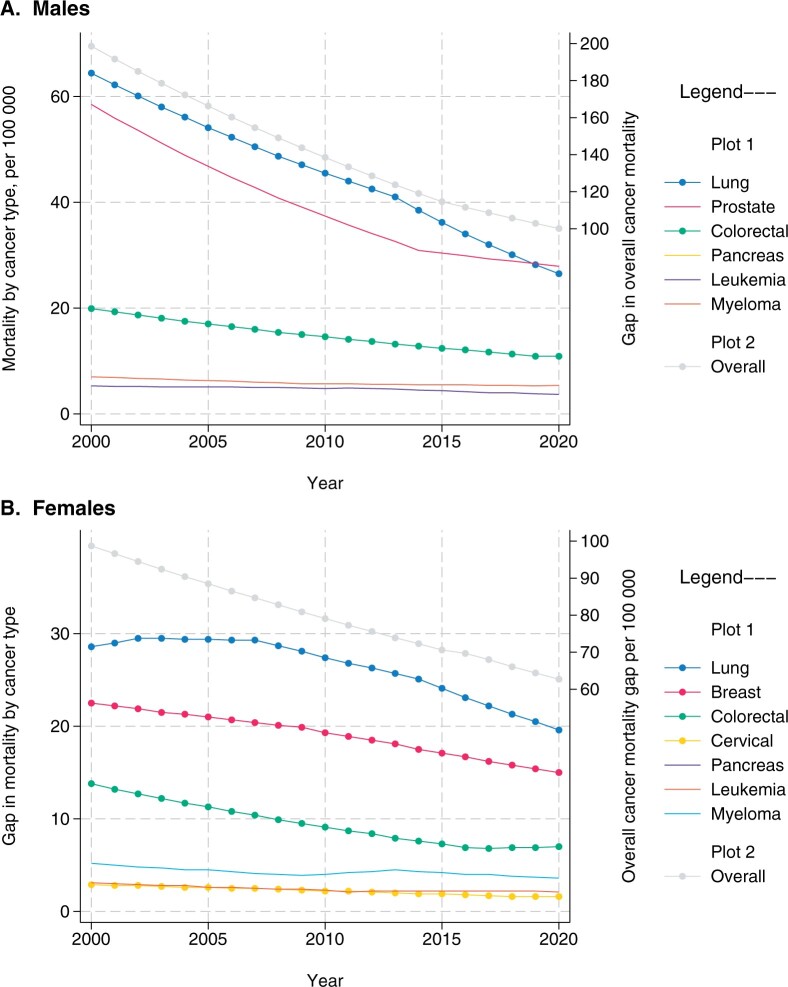
In this issue of the Journal, Cancer Intervention and Surveillance Modeling Network (CISNET) researchers collaborated on population simulation models to evaluate how interventions across the cancer care continuum contribute to mortality disparities between Black people and the overall US population (Figure 1). Across racial and ethnic groups currently defined in Surveillance, Epidemiology, and End Results Program data, Black people have the highest cancer mortality rate and the Asian and Pacific Islander group the lowest (5). Encouragingly, the overall US cancer mortality rate declined by 33% from 1991 to 2020 (4,5), with improvements across all racial and ethnic groups along with the narrowing of the gap across groups, overall and among cancers with a Grade A or B screening recommendation from the US Preventive Services Task Force (USPSTF) (Figure 1) (5). However, the progress is heterogenous across cancer sites; mortality disparities are largest for lung and smallest for cervical cancer (5). Nonetheless, these trends are promising and point to opportunities to optimize care along the cancer care continuum (Figure 2). Further, evidence on eliminating disparities between Black and White people in both incidence and mortality for colorectal cancer using sustained, coordinated efforts across the cancer care continuum is a strong catalyst for future progress (6).
Figure 1.
Absolute cancer mortality rate difference across racial and ethnic groups in the United States over time, Surveillance, Epidemiology, and End Results (SEER) (2000-2020). A) Males. B) Females. Based on SEER program data. The lines depict the difference between the rate in the racial/ethnic group with the lowest (best) and the group with the highest (worst) mortality rates (per 100 000) for each cancer type or overall rate in each year.
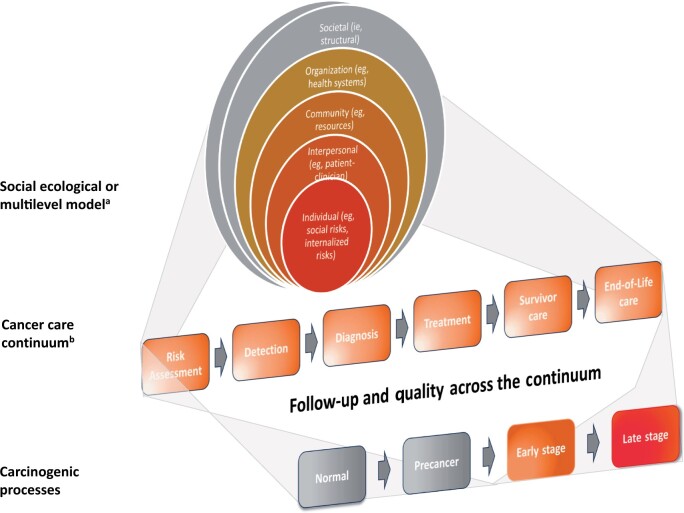
Figure 2.
Depiction of the cancer care continuum and interrelationship to the carcinogenic process and the social-ecological model. aThe levels of the social-ecological or multilevel model as shown encompass factors at societal (policies, laws, health-care policies); organization (location, capabilities, barriers, methods of service delivery such as direct outreach, tracking and monitoring systems, language assistance); community (resources, availability of services), interpersonal (family, social networks, patient–clinician or provider relationships, stigma); and individual (biological vulnerability, comorbid conditions, environmental, social, cultural, values, and preference) levels. bThe cancer care continuum as shown is a simplication and varies across cancer types and the care pathway used. It also involves interfaces and transitions across care steps or care settings.
Cancer health equity and frameworks for modeling cancer health disparities
The modeling studies in this issue were guided by an antiracism framework with the goal of identifying strategies to advance health equity among Black people and beyond. Health equity is the “absence of unfair, avoidable or remediable differences [in health-promoting opportunities and health outcomes] among groups of people.” (7) It means that everyone has a fair opportunity to receive the best quality of cancer preventive and treatment services possible, and no one is disadvantaged by social, economic and structural barriers. Equity connotes cancer health (across the care continuum) that is not undermined by racism and other “isms” or social and/or societal injustices. It presumes evidence-based health services are delivered equally for everyone while consistently identifying and addressing adverse drivers of health so that people are not returned to conditions that made them sick(er) (8). This framing reflects the complexity and multidimensionality of cancer health disparities and can serve as the undergirding for modeling studies. It builds on the interconnectedness among the cancer care continuum, the social-ecological model, and carcinogenic processes (Figure 2). Doing so positions the CISNET model as an indispensable resource for advancing the National Cancer Plan and the Cancer Moonshot goal of reducing “the cancer death rate by half within 25 years.”
To assess cancer health disparities, the analyses in this issue of the Journal used hypothesized pathways that may plausibly manifest as differences, according to race, in cancer stage, biomarkers, or aggressiveness. Such assumptions are plausible given racism’s potential direct and indirect effects on biological mechanisms across the continuum of carcinogenesis. The effects of racism across the cancer care continuum were conceptualized using a framework that mirrors the SEM or multilevel model. The SEM considers factors at societal, organization, community, interpersonal, and individual levels (see Figure 2) (9). Thus, racism could be also decomposed into individual (internalized), interpersonal, institutional, community (eg, redlining and targeted marketing practices), and societal (structural) levels.
Data limitations in modeling cancer health disparities
The types of studies presented in this issue of the Journal seek to be more than just an exercise in simulations but used available data on Black people on outcomes across the cancer care continuum. The models reported in this issue of the Journal relied on national registries, trials, and large observation studies for inputs. However, national registries have varying levels of completeness and disaggregation according to sex, race, and ethnicity (which may be imputed) and do not have data on precancerous conditions like adenomatous polyps or high-grade cervical neoplasia. Also, Black and other marginalized populations are typically underrepresented in clinical trials because of noninclusive research practices and practices of labeling many in underresourced communities as “hard-to-reach” (10). Despite well-documented disparities (5), data are often sparse or lacking on disease outcomes related to educational attainment, income, insurance status, sexual and gender minority, and geography making it difficult to evaluate intersections of social identities that are critical for a fuller understanding of cancer health disparities.
The cancer care continuum as an anchor for modeling cancer health disparities
The cancer care continuum (9) is foundational to the National Cancer Plan (11) and the USPSTF’s analytic framework (12) and is essential for modeling cancer health disparities. For instance, risk assessment (Figure 2) enables risk reduction (such as smoking cessation), screening, or interception. It helps guide people to the right care, including timely diagnostic services for people with cancer-related symptoms. A consequence of systemic racism, including residential segregation, is the clustering of cancer risk from environmental exposures and restricted access to healthful resources among people in groups that are socially and economically disadvantaged. At the same time, biological consequences of chronic stress and factors related to access, delivery, and quality of health services may exacerbate primary risk factors (12). The lack of inclusive research practices contributes to underrepresentation in the evidence base and creates potential biases in risk assessment tools used in clinical decision making. For instance, despite higher smoking prevalence and a higher lung cancer death rate, disproportionately fewer Black than White males (15.1% vs 19.7%) and females (7.6% vs 14.9%) may be eligible for lung cancer screening even with revised eligibility criteria (13). This is likely compounded by potential differences in the completeness and accuracy of information (eg, smoking history) in the electronic health record.
Delivery of evidence-based preventive and therapeutic interventions is critical for optimizing cancer health (10,12). People in groups that are socially or economically disadvantaged have lower rates of cancer screening and follow-up and lower quality of care (14). The CISNET models use observed data from large screening studies to measure screening test delivery among Black people. The results suggest differences in the effects of screening delivery on mortality disparities across cancer sites based on whether screening can have a preventive effect through removal of precancerous lesions (eg, cervical and colorectal cancers) and based on receipt of effective therapy (eg, lung cancer). Those findings are consistent with the pattern of narrowing disparities in mortality over time for cancers with guideline-recommended screening strategies but should be interpreted with caution given limited data. Also, data are limited on downstream care after screening, and there is substantial heterogeneity in health experiences among people of African ancestry. Additionally, the analysis did not consider community context or influences of concurrent social identities.
Few studies focus on the transitions across the care continuum, including the quality of an examination or timeliness in follow-up testing for an abnormal screening result (15). In one health-care system research network, Black females were less likely to receive follow-up for abnormal mammograms (16), but data are limited from other settings. There are substantial variations in timeliness of follow-up of a positive fecal test (17). As noted by the USPSTF, “positive results on … screening tests require follow-up … for the screening benefits to be achieved.” (18) In this issue of the Journal, the CISNET cervical cancer modeling reports that 12.7% of the modeled mortality disparities between Black women and the overall population were from delays in follow-up, reinforcing the need for accurate data across the care continuum to inform future analyses.
In the CISNET models, the quality of therapy explained up to three-quarters of the mortality disparities in cancer sites with established effective treatment. Like other parts of the continuum, the delivery of cancer treatment is complex and has well-documented variations that disproportionately affect people from groups that are socially disadvantaged (10). Although guideline-concordance is often used as a summative measure of treatment quality, the accuracy and completeness of treatment information is limited and often sparse in Black populations.
It should be recognized that the cancer care continuum as depicted in Figure 2 is a convenient simplification. It should be adapted for individual cancers and care pathways. Expanded elements such as screening process steps described by Selby et al. (19) and Doubeni et al. (20) should be incorporated as appropriate.
Implications for future modeling
Modeling has been used extensively to inform screening recommendations (18), screening delivery (21), and coverage decisions (22) and to evaluate gaps in care (eg, timeliness of follow-up) (23), capacity and quality (24), and service disruptions (eg, COVID-19 pandemic) (25). Models use multiple birth cohorts across calendar periods and can capture changes over time in demographics, risk factors (eg, smoking), and therapies. Models can specify population-specific biological pathways where plausible, such as location of colorectal cancers or molecular subtypes of breast cancer. The routine inclusion of life expectancy and deaths averted in CISNET models enables applications to disparities. However, many considerations are needed as researchers apply health equity frameworks.
The extension of modeling to racism is a critical frontier. However, to meaningfully inform policy decision making, the studies need to integrate racism’s multilevel structure as well as the direct and collateral costs of inaction. It requires data and/or methods beyond what is currently presented.
As noted above, there is a paucity of nationally representative data for evaluating the cancer care continuum across multiple population groups. There is limited evidence on interventions with demonstrated reductions in health disparities as well as the time horizon for realizing improvements across cancers, contexts, and points of intervention. A particular area of concern is the potential for biases from using information from flawed data systems. As pointed out in the paper by Trentham-Dietz and colleagues in this issue of the Journal, electronic health records data contain limited information on people with restricted access to health care.
In the CISNET models, race is used as a proxy measure in portraying events along the cancer control continuum because of data limitations. However, this approach could serve to encode historical biases into the modeling and perpetuate disparities in survival and life expectancy. However, alternatives are limited and the perfect can become the enemy of good as long as interpretations are qualified.
One size does not fit all, particularly in the era of precision health care. Thus, race-specific clinical guidelines has been an area of ongoing debate. For instance, the USPSTF considered lowering age to initiate colorectal cancer screening only in Black people but instead expanded the age for everyone based on CISNET modeling and empirical evidence, albeit limited, showing lack of differential effectiveness according to race (18). There are many other arguments against race-specific recommendations, including assigning biological meaning to race rather than using risk factors or underlying drivers of inequities as the basis for risk assessment. For instance, biases in current lung screening eligibility likely stem from limited understanding of the relation of smoking intensity and duration with risk. Race-based recommendations also ignore social groupings other than race that experience similar levels of risk or disparities. As illustrated in the body of CISNET research in this issue of the Journal, a major driver of disparities is the knowledge-translation, implementation, or discovery-delivery disconnect (2,10). Thus, expanding screening eligibility criteria does not improve disparities in the absence of effective strategies to improve delivery of evidence-based health services in Black or other marginalized populations.
There are several indirect ways to mitigate the role of systemic racism in disparities across the continuum of cancer care. Evidence suggests that interventions may not be effective or provide long-term benefit if used in isolation. Thus, rather than output single interventions, modeling could provide the needed components of an intervention strategy for specified contexts. Because benefits of disease prevention and treatment are predicated on effectiveness, disparities may be more pronounced in clinical settings with high potential for improved health outcomes. Such assumptions could be evaluated along with potential mitigations. In that vein, there are current opportunities to model the impact of the recent Centers for Medicare and Medicaid Services coverage decision that “non-invasive, stool-based test and the follow-on colonoscopy are both part of a continuum of a complete CRC [colorectal cancer] screening” as well as phased implementation of waivers of coinsurance for polypectomy, biopsy, pathology, and anesthesia fees (26,27).
The analyses in this issue of the Journal focused predominantly on comparing Black people to the overall population. Although comparisons are typically made to the largest population group (ie, White people) in most studies, an ideal state is not often known. Counterfactual assumptions could be made to specify the best life expectancy across all groups as the expected outcome and thus use the best performing group as the comparator (Figure 1).
Cancer health disparities result from complex social and structural inequities across cancer care and disease continua through multifactorial, multidimensional influences with changes over time that may be difficult to characterize accurately. The analyses in this issue of the Journal, which are based on an antiracism framework, represent a great starting point for CISNET to inform how interventions may decrease cancer health disparities across major cancer sites. Models can more fully inform decisions on mitigating disparities that would advance the National Cancer Plan and Cancer Moonshot goals by using strong conceptual frameworks, filling existing data gaps, and incorporating new advances in cancer control. To gain a more comprehensive understanding, future endeavors could be expanded to other social groups, including groups defined by intersections of multiple social, stigmatized, or marginalized identities.
Contributor Information
Chyke A Doubeni, Department of Family and Community Medicine, The Ohio State University College of Medicine, Ohio State University Comprehensive Cancer Center, Wexner Medical Center, Columbus, OH, USA.
Zinzi D Bailey, Department of Epidemiology and Community Health, University of Minnesota, Minneapolis, MN, USA.
Robert A Winn, Massey Comprehensive Cancer Center, Virginia Commonwealth University, Richmond, VA, USA.
Data Availability
No primary data were generated or analyzed for this paper. Information on racial/ethnic differences in incidence and mortality are publically available from https://seer.cancer.gov/statistics-network/explorer/.
Author contributions
Chyke A Doubeni, MD (Conceptualization; Writing—original draft; Writing—review & editing) Zinzi Bailey, ScD (Conceptualization; Project administration), and Robert Winn, MD (Conceptualization; Project administration)
Funding
This paper was supported in part by funding from the National Cancer Institute of the National Institutes of Health (NIH) under award numbers R01CA213645, R61CA278594, R01CA278052, and R01CA269832.
Conflicts of interest
The funding source had no role in this paper. Dr Doubeni authors topics on UpToDate and the content is solely the responsibility of the authors and does not necessarily represent the views and policies of the NIH or UpToDate.
References
- 1. Bailey ZD, Krieger N, Agenor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: Evidence and interventions. Lancet . 2017;389(10077):1453-1463. [DOI] [PubMed] [Google Scholar]
- 2. Doubeni CA, Selby K, Gupta S. Framework and strategies to eliminate disparities in colorectal cancer screening outcomes. Annu Rev Med . 2021;72:383-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158(2):354-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48. [DOI] [PubMed] [Google Scholar]
- 5. Surveillance Research Program, National Cancer Institute. SEERExplorer: an interactive website for SEER cancer statistics. 2023 Apr 19. [updated: 2023 Jun 8; cited 2023 Aug 18]. Data source(s): U.S. Mortality Data (1969-2020). National Center for Health Statistics, CDC. https://seer.cancer.gov/statistics-network/explorer/. Accessed: August 18, 2023.
- 6. Doubeni CA, Corley DA, Zhao W, Lau Y, Jensen CD, Levin TR. Association between improved colorectal screening and racial disparities. N Engl J Med . 2022;386(8):796-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization. Health Equity. 2015. https://www.who.int/health-topics/health-equity. Accessed August 16, 2023.
- 8. McKenna C. Michael Marmot: the health of nations. BJPsych Bull . 2023;47(1):56-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taplin SH, Rodgers AB. Toward improving the quality of cancer care: addressing the interfaces of primary and oncology-related subspecialty care. J Natl Cancer Inst Monogr. 2010;2010(40):3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doubeni CA, Simon M, Krist AH. Addressing systemic racism through clinical preventive service recommendations from the US Preventive Services Task Force. JAMA. 2021;325(7):627-628. [DOI] [PubMed] [Google Scholar]
- 11. National Cancer Institute. National Cancer Plan. 2023. https://nationalcancerplan.cancer.gov/national-cancer-plan.pdf. Accessed August 18, 2023.
- 12. Davidson KW, Mangione CM, Barry MJ, et al. ; U. S. Preventive Services Task Force. Actions to transform US Preventive Services Task Force methods to mitigate systemic racism in clinical preventive services. JAMA. 2021;326(23):2405-2411. [DOI] [PubMed] [Google Scholar]
- 13. Pinsky PF, Lau YK, Doubeni CA. Potential disparities by sex and race or ethnicity in lung cancer screening eligibility rates. Chest. 2021;160(1):341-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gupta S, Sussman DA, Doubeni CA, et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Inst . 2014;106(4):dju032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doubeni CA, Gabler NB, Wheeler CM, et al. Timely follow-up of positive cancer screening results: a systematic review and recommendations from the PROSPR Consortium. CA Cancer J Clin . 2018;68(3):199-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McCarthy AM, Kim JJ, Beaber EF, et al. Follow-up of abnormal breast and colorectal cancer screening by race/ethnicity. Am J Prev Med. 2016;51(4):507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chubak J, Garcia MP, Burnett-Hartman AN, et al. ; PROSPR Consortium. Time to colonoscopy after positive fecal blood test in four U.S. health care systems. Cancer Epidemiol Biomarkers Prev . 2016;25(2):344-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Davidson KW, Barry MJ, Mangione CM, et al. ; U. S. Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. [DOI] [PubMed] [Google Scholar]
- 19. Selby K, Jensen CD, Levin TR, et al. Program components and results from an organized colorectal cancer screening program using annual fecal immunochemical testing. Clin Gastroenterol Hepatol . 2022;20(1):145-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doubeni CA, Fedewa SA, Levin TR, et al. Modifiable failures in the colorectal cancer screening process and their association with risk of death. Gastroenterology. 2019;156(1):63-74.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meester RG, Doubeni CA, Zauber AG, et al. Public health impact of achieving 80% colorectal cancer screening rates in the United States by 2018. Cancer. 2015;121(13):2281-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peterse EFP, Meester RGS, Gini A, et al. Value of waiving coinsurance for colorectal cancer screening in medicare beneficiaries. Health Aff (Millwood). 2017;36(12):2151-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meester RG, Zauber AG, Doubeni CA, et al. Consequences of increasing time to colonoscopy examination after positive result from fecal colorectal cancer screening test. Clin Gastroenterol Hepatol . 2016;14(10):1445-1451.e1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meester RGS, Doubeni CA, Zauber AG, van Ballegooijen M, Corley DA, Lansdorp-Vogelaar I. Impact of adenoma detection on the benefit of faecal testing vs. colonoscopy for colorectal cancer. Int J Cancer . 2017;141(11):2359-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sharpless NE. COVID-19 and cancer. Science . 2020;368(6497):1290. [DOI] [PubMed] [Google Scholar]
- 26. Centers for Medicare and Medicaid Services. Removal of a National Coverage Determination & Expansion of Coverage of Colorectal Cancer Screening. 2022. https://www.cms.gov/files/document/mm13017-removal-national-coverage-determination-expansion-coverage-colorectal-cancer-screening.pdf. Accessed August 22, 2023.
- 27. Centers for Medicare and Medicaid Services. Colorectal Cancer Screening Tests: Changes to Coinsurance for Related Procedures. 2022. https://www.cms.gov/files/document/mm12656-changes-beneficiary-coinsurance-additional-procedures-furnished-during-same-clinical.pdf. Accessed August 22, 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No primary data were generated or analyzed for this paper. Information on racial/ethnic differences in incidence and mortality are publically available from https://seer.cancer.gov/statistics-network/explorer/.