Abstract
Introduction
Previous studies in humans and rats suggest that erythritol might positively affect vascular function, xylitol decrease visceral fat mass and both substances improve glycaemic control. The objective of this study was to investigate the impact of a 5-week intake of erythritol and xylitol on vascular function, abdominal fat and blood lipids, glucose tolerance, uric acid, hepatic enzymes, creatinine, gastrointestinal tolerance and dietary patterns in humans with obesity.
Methods
Forty-two participants were randomised to consume either 36 g erythritol, 24 g xylitol, or no substance daily for 5 weeks. Before and after the intervention, arterial stiffness (pulse wave velocity, arteriolar-to-venular diameter ratio), abdominal fat (liver volume, liver fat percentage, visceral and subcutaneous adipose tissue, blood lipids), glucose tolerance (glucose and insulin concentrations), uric acid, hepatic enzymes, creatinine, gastrointestinal tolerance and dietary patterns were assessed. Data were analysed by linear mixed effect model.
Results
The 5-week intake of erythritol and xylitol showed no statistically significant effect on vascular function. Neither the time nor the treatment effects were significantly different for pulse wave velocity (time effect: p=0.079, Cohen’s D (95% CI) −0.14 (−0.54–0.25); treatment effect: p=0.792, Cohen’s D (95% CI) control versus xylitol: −0.11 (–0.61–0.35), control versus erythritol: 0.05 (0.44–0.54), erythritol versus xylitol: 0.07 (–0.41–0.54)). There was no statistically significant effect on abdominal fat, glucose tolerance, uric acid, hepatic enzymes and creatinine. Gastrointestinal tolerance was good except for a few diarrhoea-related symptoms. Participants of all groups reduced their consumption of sweetened beverages and sweets compared with preintervention.
Conclusions
The 5-week intake of erythritol and xylitol showed no statistically significant effects on vascular function, abdominal fat, or glucose tolerance in people with obesity.
Clinical trial registration
Keywords: dietary patterns, metabolic syndrome, nutritional treatment, precision nutrition, weight management
WHAT IS ALREADY KNOWN ON THIS TOPIC
Prior research indicates that among people with diabetes, erythritol consumption improves glycaemic control and vascular function. Diabetic animal models have demonstrated that both polyols enhance blood glucose control, while xylitol decreases visceral fat mass. In humans, both polyols also trigger the release of metabolically advantageous gastrointestinal hormones (incretins).
WHAT THIS STUDY ADDS
This randomised controlled trial in normoglycaemic people with obesity shows no statistically significant effect of a 5-week intake of erythritol and xylitol on vascular function, abdominal fat or glucose tolerance.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The study contributes valuable insights into the metabolic impacts of regular erythritol and xylitol consumption. It reveals that these sugar substitutes do not seem to exhibit adverse effects on vascular function, glycaemic control or fat metabolism and, therefore, hold promise as suitable sugar alternatives for individuals with obesity.
Introduction
Obesity is linked to reduced postprandial incretin secretion1 and increased glucose absorption.2 These characteristics promote hyperglycaemia.3 Additionally, obesity is associated with increased free fatty acid and triglyceride (TG) concentrations.4 5 Hyperglycaemia and hyperlipidaemia combined play a role in the pathogenesis of vascular dysfunction. In human endothelial cells, high glucose concentrations induce apoptosis and overproduction of reactive oxygen species, leading to endothelial dysfunction.6 7 Moreover, in humans, high insulin and TG concentrations have a synergistic association with arterial stiffness.8 Additionally, the retinal venular calibre—a secondary marker of vascular dysfunction—is significantly larger in people with increased fasting glucose levels and glycated haemoglobin (HbA1C).9
Arterial stiffness is an early marker of cardiovascular disease10 and a strong predictor of future cardiovascular events.11 The retinal arteriolar narrowing is associated with hypertension, especially when combined with higher venular diameter.12 13 An increase in the arteriolar-to-venule diameter ratio (AVR) is associated with an increased risk of coronary heart disease and acute myocardial infarction in women.14 Therefore, assessment of the retinal and central blood vessels allows the detection of early changes in vascular function, possibly prior to type 2 diabetes mellitus (T2DM) and its complications.
Given the current obesity epidemic, the WHO recommends reducing sugar intake.15 A possibility to achieve this recommendation is to partially replace sugar with low-calorie sweeteners such as erythritol and xylitol. These sweeteners are interesting for patients with overweight and diabetes due to their low glycemic indexes16 and their ability to induce the secretion of gastrointestinal satiation hormones.17–19 Additionally, erythritol has a protective effect on endothelial cell function, as shown in endothelial cell culture as well as in patients with T2DM, and a 4-week intake reduces central pulse pressure in patients with T2DM.20 21
A recent study suggests a potential link between plasma erythritol levels and cardiovascular events in humans.22 However, erythritol is also produced endogenously from glucose in humans.23 In the studied group, the origin of erythritol is not clear, which makes a causal analysis impossible. Rodent studies hint that sucrose intake may raise internal erythritol production,24 possibly explaining higher erythritol levels.
Xylitol has beneficial effects on visceral fat mass, plasma insulin, glycaemia and lipid concentrations in non-diabetic rats.25 26 In humans, xylitol intake for 18 days tends to decrease cholesterol levels compared with 6-day sucrose intake.27 Finally, both substances show beneficial effects on glycaemic control in both normoglycaemic and diabetic rats.28 29 Therefore, these two substances seem promising in preventing vascular dysfunction and its underlying mechanisms, such as endothelial cell death, hyperlipidaemia and hyperglycaemia.
The objective of this study was to investigate the impact of a 5-week intake of erythritol and xylitol on vascular function (primary objective), abdominal fat and blood lipids, glucose tolerance, uric acid, hepatic enzymes, creatinine, gastrointestinal tolerance and dietary patterns (secondary objectives) in humans with obesity.
Methods
The study was conducted as a randomised, controlled trial and performed in accordance with the current version (V.2013) of the Declaration of Helsinki on medical research involving human subjects (https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/).
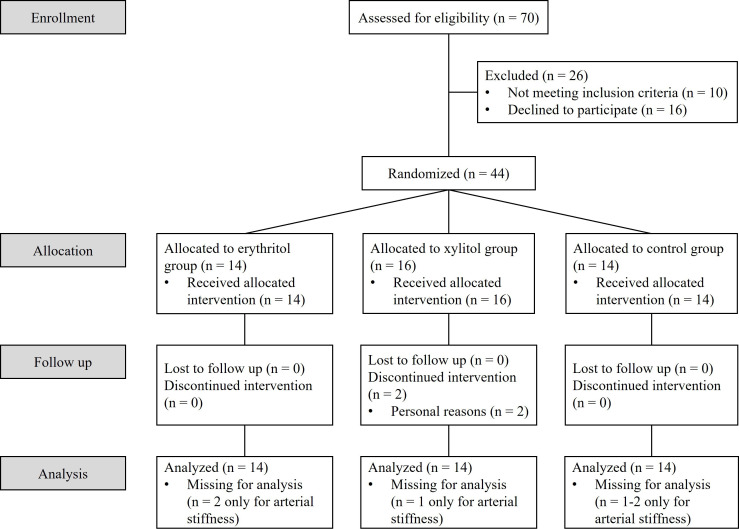
A total of 44 normoglycaemic participants with obesity were recruited via advertisement between November 2016 and January 2022. Exclusion criteria included any prior medical conditions, any surgery with major changes to the gastrointestinal tract, regular medications use, pregnancy or consumption of substances in abuse. Participants did not have any dietary restrictions or regular consumption of erythritol or xylitol. Two participants dropped out for personal reasons and were replaced, resulting in 42 participants who completed the study (see participants’ flowchart in figure 1). The baseline characteristics for each group are presented in table 1.
Figure 1.
Participants flowchart.
Table 1.
Baseline characteristics (mean±SD) for each group
| Parameter | Erythritol group | Xylitol group | Control group | P values |
| Sex | n=14 (8♂; 6♀) | n=14 (9♂; 5♀) | n=14 (7♂; 7♀) | 0.583* |
| Age (years) | 31.3±8.8 | 30.4±10.6 | 30.6±10.1 | 0.972† |
| BMI (kg/m2) | 33.9±3.7 | 34.8±2.8 | 34.9±3.7 | 0.709† |
| Systolic blood pressure (mm Hg) | 131.9±12.9 | 129.4±7.3 | 126.4±17.2 | 0.552† |
| Diastolic blood pressure (mm Hg) | 85.9±10.9 | 85.6±10.2 | 78.7±8.6 | 0.107† |
| Pulse rate (1 /min) | 72.9±11.2 | 73.9±11.2 | 75.3±11.2 | 0.862† |
♀: females, ♂: males.
*Chi-square test.
†Analysis of variance.
BMI, body mass index.
The participants were randomly assigned—by a third person, using a computer-based randomisation system—to consume either 12 g of erythritol, or 8 g of xylitol dissolved in water three times a day (together with the main meals) for 5 weeks or to the control group (no substance) in a 1:1:1 ratio.
The first week of intervention was an adaptation period: one portion per day for 2 days, then two portions per day for 3 days, finally three portions per day for two last days. Then, participants went on with three daily portions for the remaining 4 weeks. Participants in the control group did not consume any substances. In the intervention groups, the trial was double-blind, meaning that the study participants and the study personnel were blinded concerning the type of substance consumed.
Erythritol and xylitol were purchased from Mithana GmbH (Zimmerwald, Switzerland). The duration of intervention and the dosage of erythritol were based on a previous pilot study of Flint et al,21 which showed reduced central pulse pressure and improved endothelial function in patients with T2DM after a 4-week intake of 36 g/day of erythritol. This quantity of erythritol is a feasible dosage to replace the daily added sugar intake in Switzerland and represents real-life conditions. Xylitol was given in an equisweet dosage to erythritol.
Before and after the intervention period, participants were invited to three study visits to assess arterial stiffness and retinal vessels diameters, abdominal fat quantification (including subcutaneous, visceral and hepatic distribution), and glycaemic control, blood lipids, uric acid, hepatic enzymes and creatinine. In addition, gastrointestinal tolerance and dietary patterns were assessed before and during the second and fourth week of intervention. Further information on the methodology is found in online supplemental appendix S1.
bmjnph-2023-000764supp001.pdf (74KB, pdf)
Statistical analysis
This study is a pilot trial. Therefore, a minimum number of 14 participants per group was chosen for reasons of comparability and practicability. Imputation of isolated missing values, which constituted less than 0.5% of the data set, except for glucose (3.3%), was performed using the median value corresponding to the respective treatment group. Therefore, the imputation did not alter the distribution of the values for the parameter in question.
For longitudinal parameters, a linear mixed effect model with subsequent Šidak test was applied using the time (pre- or post-intervention) as a within-subject factor and the treatment (erythritol, xylitol, control) as a fixed between-subject factor. Non-longitudinal parameters were analysed by general linear modelling. SPSS for Windows software, V.25.0 was used (IBM, Armonk, New York). Values are reported as means±SD and displayed in figures as means±SE of the mean (SEM) or median and IQR for boxplots. Differences were considered to be statistically significant when p<0.05. For the primary endpoint, effect sizes were calculated as Cohen’s D with their corresponding 95% CIs in Python (V.3.11) using the modules Statsmodels (V.0.13.5)30 and Scipy (V.1.10.1).
Results
Vascular function: arterial stiffness, retinal vessel diameters
No statistically significant effect of erythritol or xylitol intake on vascular function was found. For arterial stiffness: Neither the time (preintervention or postintervention) nor the treatment (erythritol, xylitol, control) effects were significantly different for the left brachial pulse wave velocity (LB-PWV).
The effect size (Cohen’s D (95% CI)) for the time effect was −0.14 (−0.54–0.25), and the effect sizes (Cohen’s D (95% CIs)) for the treatment effects were control versus xylitol: −0.11 (−0.61–0.35), control versus erythritol: 0.05 (0.44–0.54) and erythritol versus xylitol: 0.07 (−0.41–0.54).
For retinal vessel diameters: neither the time nor the treatment effects were significantly different for the AVR. The effect size (Cohen’s D (95% CI)) for the time effect was −0.14 (-0.034–0.01), and the effect sizes (Cohen’s D (95% CIs)) for the treatment effects were control versus xylitol: −0.23 (−0.61–0.26), control versus erythritol: 0.13 (−0.11–0.16) and erythritol versus xylitol: 0.12 (0.10–0.14). The vascular parameters are reported in table 2.
Table 2.
Arterial stiffness and retinal vessel diameters (mean±SD) for each group before and after intervention
| Parameter | Time point | Erythritol group | Xylitol group | Control group | Time effect (P value) |
Treatment effect (P value) |
| LB-PWV(m/s) | Preintervention | (n=12) 6.0±0.9 |
(n=13) 6.1±0.9 |
(n=13) 5.9±0.9 |
0.079 | 0.792 |
| Postintervention | (n=12) 5.8±0.6 |
(n=13) 6.0±0.9 |
(n=12) 5.9±0.9 |
|||
| AVR (n=14) | Preintervention | 0.8±0.0 | 0.8±0.1 | 0.8±0.1 | 0.900 | 0.698 |
| Postintervention | 0.8±0.0 | 0.8±0.1 | 0.8±0.1 |
Linear mixed effect model with subsequent Šidak test.
AVR, arteriolar-to-venular diameter ratio; LB-PWV, left-brachial pulse wave velocity.
Abdominal fat: quantification and distribution, blood lipids
No statistically significant effect of erythritol or xylitol intake on abdominal fat and blood lipids was found. Abdominal fat: neither the time nor the treatment effects were significantly different for the liver volume, the liver fat percentage, the visceral adipose tissue and the subcutaneous adipose tissue volumes.
Blood lipids: neither the time nor the treatment effects were significantly different for TGs, total cholesterol levels and high-density lipoprotein cholesterol. There was a significant time, but no treatment effect for the low-density lipoprotein (LDL) cholesterol. In all treatment groups, the LDL cholesterol levels were significantly decreased after the intervention compared with before. The respective parameters are reported in table 3.
Table 3.
Abdominal fat and blood lipids parameters (mean±SD) for each group before and after intervention
| Parameter | Time point | Erythritol group (n=14) | Xylitol group (n=14) |
Control group (n=14) |
Time effect (P value) |
Treatment effect (P value) |
| Liver volume (L) | Preintervention | 1.70±0.40 | 1.81±0.47 | 1.72±0.30 | 0.307 | 0.564 |
| Postintervention | 1.70±0.47 | 1.89±0.52 | 1.70±0.32 | |||
| Liver fat percentage (%) | Preintervention | 9.5±7.8 | 7.3±6.6 | 6.1±7.3 | 0.436 | 0.892 |
| Postintervention | 8.7±7.6 | 7.3±7.5 | 6.0±6.5 | |||
| VAT volume (L) | Preintervention | 4.64±2.62 | 3.98±1.85 | 4.21±1.53 | 0.216 | 0.583 |
| Postintervention | 5.11±3.00 | 4.39±2.03 | 4.32±1.72 | |||
| SAT volume (L) | Preintervention | 13.06±3.73 | 12.84±2.71 | 13.27±4.32 | 0.300 | 0.995 |
| Postintervention | 12.96±4.22 | 13.07±2.71 | 13.48±4.57 | |||
| Triglycerides (mmol/L) | Preintervention | 1.9±1.3 | 1.4±0.7 | 2.2±2.1 | 0.158 | 0.837 |
| Postintervention | 1.6±1.0 | 1.7±1.0 | 1.2±0.5 | |||
| Total cholesterol (mmol/L) |
Preintervention | 5.4±1.4 | 4.8±0.9 | 4.7±1.0 | 0.489 | 0.365 |
| Postintervention | 5.0±1.1 | 4.8±1.1 | 4.6±0.9 | |||
| HDL-cholesterol (mmol/L) |
Preintervention | 1.8±1.8 | 1.4±0.3 | 1.2±0.3 | 0.240 | 0.364 |
| Postintervention | 1.3±0.4 | 1.3±0.2 | 1.2±0.3 | |||
| LDL-cholesterol (mmol/L) |
Preintervention | 3.0±0.9 | 3.0±0.5 | 3.1±0.7 | 0.038* | 0.943 |
| Postintervention | 2.9±0.7 | 2.9±0.6 | 2.9±0.9 |
Linear mixed effect model with subsequent Šidak test.
*Significant with p<0.05.
HDL, high-density lipoprotein; LDL, low-density lipoprotein; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Glucose tolerance
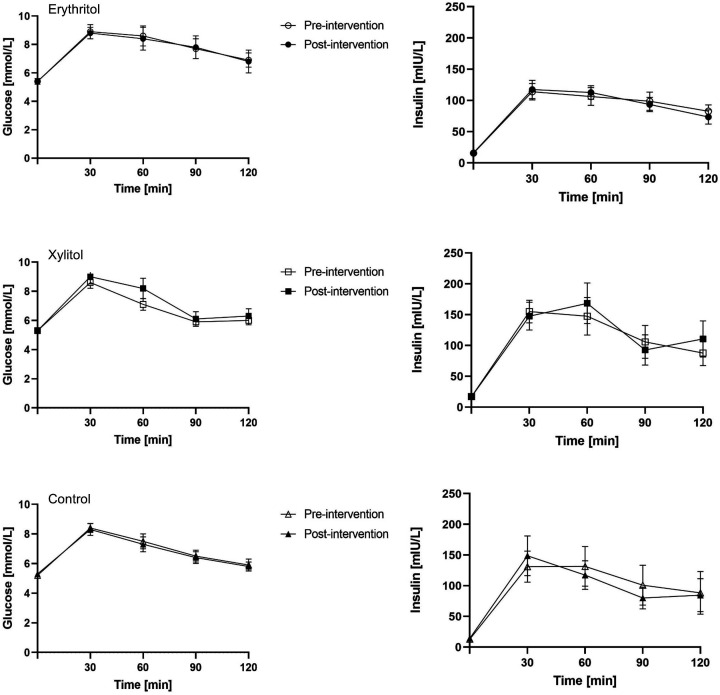
No statistically significant effect of erythritol or xylitol intake on glucose tolerance was found. Neither the time nor the treatment effects were significantly different for the glucose and insulin concentrations during oral glucose tolerance test (see figure 2), and for the areas under the glucose and insulin concentration curves at 120 min (glucose: p=0.482 and p=0.412, respectively; insulin: p=0.902 and p=0.583, respectively).
Figure 2.
Glucose and insulin concentrations during glucose tolerance test for each group before and after intervention (mean±SEM).
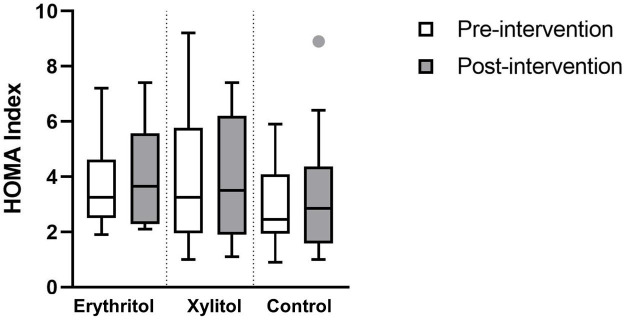
No statistically significant effect of erythritol or xylitol intake on the Homeostatic Model Assessment index = (fasting glucose × fasting insulin)/22.5 was found. Neither the time nor the treatment effects were significantly different (p=0.339, p=0.780, respectively, see figure 3).
Figure 3.
HOMA Index for each group before and after intervention (median and IQR). HOMA, Homeostatic Model Assessment.
Uric acid, hepatic enzymes and creatinine
No statistically significant effect of erythritol or xylitol intake on uric acid, hepatic enzymes and creatinine was found. Neither the time nor the treatment effects were significantly different for uric acid, aspartate aminotransferase, alanine aminotransferase and creatinine. The respective parameters are reported in table 4.
Table 4.
Uric acid, hepatic enzymes and creatinine (mean±SD) for each group before and after intervention
| Parameter | Time point | Erythritol group (n=14) | Xylitol group (n=14) |
Control group (n=14) |
Time effect (P value) |
Treatment effect (P value) |
| Uric acid(mmol/L) | Preintervention | 350.5±84.6 | 391.2±108.3 | 342.1±70.5 | 0.704 | 0.330 |
| Postintervention | 342.9±77.4 | 380.6±92.4 | 351.9±60.2 | |||
| ASAT(U/L) | Preintervention | 22.0±9.7 | 25.9±14.5 | 23.1±10.5 | 0.436 | 0.876 |
| Postintervention | 25.6±11.7 | 25.7±13.0 | 22.3±7.3 | |||
| ALAT(U/L) | Preintervention | 38.4±19.4 | 33.6±22.6 | 31.1±23.5 | 0.800 | 0.518 |
| Postintervention | 40.5±26.9 | 32.2±17.9 | 28.6±16.1 | |||
| Creatinine (mmol/L) |
Preintervention | 69.3±21.8 | 72.9±13.0 | 70.8±12.9 | 0.611 | 0.491 |
| Postintervention | 69.3±13.0 | 70.1±14.5 | 70.7±13.8 |
Linear mixed effect model with subsequent Šidak test.
ALAT, alanine aminotransferase; ASAT, aspartate aminotransferase.
Gastrointestinal tolerance and dietary patterns
Gastrointestinal Symptoms Rating Scale (GSRS)-Question 15 (sensation of not completely emptying the bowels) was removed from the analysis due to many missing values. Overall, the gastrointestinal tolerance was good. No statistically significant effect of erythritol or xylitol intake on abdominal pain, indigestion or constipation was found. Neither the time nor the treatment effects were significantly different for the GSRS questions regarding those symptoms. There was a significant treatment effect with regard to the experience of reflux (question 2: ‘Have you been bothered by heartburn during the past week (meaning retrosternal discomfort or unpleasant burning sensation in the chest)?’) and loose stools (question 12: ‘Have you been bothered by loose stools during the past week?’). In the control group, participants experienced significantly more reflux compared with the erythritol group, and in the xylitol group, participants have been significantly more bothered by loose stools compared with the erythritol group. In addition, there was a significant time effect with regard to sensations of an urgent need for bowel movement (question 14: ‘Have you been bothered by an urgent need to have a bowel movement during the past week?’). In all treatment groups, the sensations of urgent need for bowel movement were significantly increased after the second week compared with preintervention. The mean scores of the different gastrointestinal symptoms are displayed in table 5.
Table 5.
Gastrointestinal symptoms scores (mean±SD) for each group before and after intervention
| Parameter | Time point | Erythritol group (n=14) | Xylitol group (n=14) | Control group (n=14) | Time effect (P value) |
Treatment effect (P value) |
| Q1: Abdominal pain (pain or discomfort in the upper abdomen) | Preintervention | 1.2±0.4 | 1.4±0.9 | 1.1±0.4 | 0.108 | 0.390 |
| Week 2 of intervention | 1.6±1.6 | 1.6±1.1 | 1.8±0.9 | |||
| Week 4 of intervention | 1.3±0.8 | 1.6±1.0 | 1.9±1.4 | |||
| Q2: Reflux (heart burn) | Preintervention | 1.1±0.4 | 1.7±1.3 | 1.4±0.6 | 0.560 | 0.015* (C vs E) |
| Week 2 of intervention | 1.3±0.5 | 1.5±0.9 | 1.3±0.6 | |||
| Week 4 of intervention | 1.1±0.5 | 1.6±1.0 | 1.8±1.2 | |||
| Q3: Reflux (acid reflux) | Preintervention | 1.1±0.4 | 1.4±1.1 | 1.4±0.8 | 0.872 | 0.531 |
| Week 2 of intervention | 1.4±0.7 | 1.3±0.6 | 1.2±0.4 | |||
| Week 4 of intervention | 1.3±0.8 | 1.2±0.6 | 1.6±0.9 | |||
| Q4: Abdominal pain (hunger pains) | Preintervention | 1.9±0.9 | 2.7±1.4 | 2.2±1.5 | 0.813 | 0.141 |
| Week 2 of intervention | 2.1±1.4 | 2.7±1.4 | 2.6±1.5 | |||
| Week 4 of intervention | 1.8±1.1 | 2.6±1.3 | 2.2±1.0 | |||
| Q5: Abdominal pain (nausea) | Preintervention | 1.0±0.0 | 1.4±0.8 | 1.3±0.7 | 0.261 | 0.480 |
| Week 2 of intervention | 1.6±1.4 | 1.5±1.2 | 1.2±0.4 | |||
| Week 4 of intervention | 1.3±0.8 | 1.7±1.3 | 1.6±1.0 | |||
| Q6: Indigestion (rumbling in the stomach) | Preintervention | 1.8±1.1 | 2.2±1.1 | 2.0±1.6 | 0.170 | 0.113 |
| Week 2 of intervention | 1.7±0.9 | 2.5±1.3 | 2.6±1.7 | |||
| Week 4 of intervention | 1.4±0.6 | 2.6±1.8 | 1.8±1.3 | |||
| Q7: Indigestion (bloating) | Preintervention | 2.5±1.8 | 2.4±1.5 | 2.1±1.4 | 0.807 | 0.442 |
| Week 2 of intervention | 2.1±1.9 | 2.8±1.9 | 2.2±1.4 | |||
| Week 4 of intervention | 1.7±1.1 | 2.7±1.8 | 2.4±1.9 | |||
| Q8: Indigestion (burping) | Preintervention | 1.6±0.8 | 2.1±1.3 | 1.9±1.2 | 0.436 | 0.079 |
| Week 2 of intervention | 1.5±0.9 | 1.9±1.3 | 2.0±1.3 | |||
| Week 4 of intervention | 1.3±0.6 | 2.1±1.4 | 2.5±2.0 | |||
| Q9: Indigestion (passing gas/flatus) | Preintervention | 2.4±1.4 | 2.9±1.4 | 2.5±1.1 | 0.935 | 0.482 |
| Week 2 of intervention | 2.6±1.7 | 2.9±1.7 | 2.4±1.5 | |||
| Week 4 of intervention | 2.2±1.2 | 2.9±1.9 | 2.8±1.9 | |||
| Q10: Constipation (reduced emptying) | Preintervention | 1.0±0.0 | 1.5±1.3 | 2.1±1.4 | 0.701 | 0.159 |
| Week 2 of intervention | 1.6±1.3 | 1.8±1.5 | 1.6±0.9 | |||
| Week 4 of intervention | 1.1±0.5 | 2.0±1.9 | 1.5±1.2 | |||
| Q11: Diarrhoea (frequent emptying) | Preintervention | 1.4±0.7 | 1.6±0.9 | 1.9±1.0 | 0.212 | 0.236 |
| Week 2 of intervention | 1.9±1.2 | 2.4±1.7 | 1.2±0.6 | |||
| Week 4 of intervention | 1.7±0.8 | 2.1±1.6 | 2.3±1.3 | |||
| Q12: Diarrhoea (loose stools) | Preintervention | 1.4±0.9 | 1.6±0.9 | 2.1±1.2 | 0.297 | 0.022* (E vs X) |
| Week 2 of intervention | 1.4±0.6 | 2.9±2.1 | 1.5±0.8 | |||
| Week 4 of intervention | 1.5±0.8 | 2.5±2.0 | 2.4±1.8 | |||
| Q13: Constipation (hard stools) | Preintervention | 1.2±0.8 | 1.7±1.4 | 1.9±1.3 | 0.563 | 0.630 |
| Week 2 of intervention | 1.9±1.4 | 1.4±0.9 | 1.9±1.3 | |||
| Week 4 of intervention | 1.4±0.6 | 1.9±1.2 | 1.4±0.8 | |||
| Q14: Diarrhoea (urgent need of bowel movement) | Preintervention | 1.4±0.5 | 1.1±0.5 | 1.4±0.6 | 0.006** (pre vs W2) |
0.262 |
| Week 2 of intervention | 1.6±0.9 | 2.7±1.9 | 1.5±0.8 | |||
| Week 4 of intervention | 1.6±1.0 | 2.4±2.2 | 2.1±1.8 | |||
| Q15: Diarrhoea (incomplete emptying) | Removed from the analysis due to many missing values | |||||
Linear mixed effect model with subsequent Šidak test.
**Significant with p<0.01; *significant with p<0.05.
C, control group; E, erythritol group; W2, week 2; X, xylitol group.
For dietary pattern, there was a significant time, and a significant treatment effect regarding the consumption of dairy products. In all treatment groups, the consumption of dairy products was significantly reduced after the fourth week compared with preintervention (p=0.027). Additionally, in the erythritol group, participants consumed significantly more dairy products compared with the xylitol group (p=0.028). Otherwise, there was only a significant time, but no treatment effect on the consumption of beverages with added sugar (preintervention vs week 2, p=0.024, and preintervention vs week 4, p=0.048), sugar-sweetened beverages (preintervention vs week 4, p=0.025) and sweets (preintervention vs week 2, p=0.001, and preintervention vs week 4, p=0.022). In all treatment groups, the consumption of beverages with added sugar, sugar-sweetened beverages and sweets was significantly reduced compared with preintervention.
Discussion
In this randomised controlled trial, we examined the effect of a 5-week intake of erythritol and xylitol on vascular function, abdominal fat and glucose tolerance in humans with obesity. Additionally, we examined blood lipids, uric acid, hepatic enzymes and creatinine, assessed gastrointestinal symptoms and evaluated changes in dietary patterns.
Flint et al 21 found a significant decrease in central pulse pressure and a trend for reduced PWV after a 4-week erythritol intake in patients with T2DM.21 Our study found no statistically significant effect of erythritol and xylitol intake on vascular function (PWV and retinal vessel diameters) in normoglycaemic participants with obesity. This discrepancy may be due to differences in the study populations, as participants with T2DM typically have higher PWV compared with healthy individuals.31 Participants in our study were, considering a clinically healthy upper limit for PWV of 10 m/s,32 33 already in the normal range before the intervention. However, even if erythritol and xylitol consumption did not improve vascular function in our trial, the fact that their ingestion showed no statistically significant effect concerning vascular function in our population argues for their use as a sugar substitutes, as hyperglycaemia associated with sugar intake is known to impact vascular function.34 Of note, a recent cohort study by Witkowski et al 22 showed a possible correlation between erythritol blood levels and risk of cardiovascular events in humans. Given that erythritol is also endogenously synthesised in humans via the pentose-phosphate pathway from glucose,23 determining the source of erythritol in this particular group remains uncertain, making it impossible to establish a causal relationship. Interestingly, studies on rodents suggest that sucrose intake can stimulate the endogenous production of erythritol.24 Consequently, the observed high plasma erythritol levels could potentially be attributed to heightened sugar consumption.
Amo et al 25 found that in rats fed a high-fat diet and receiving xylitol during 8 weeks, visceral fat mass was significantly lower compared with the control group.25 In our trial, the 5-week intake of erythritol and xylitol showed no statistically significant effect on abdominal fat mass and its distribution. Of note, participants were instructed to consume their habitual diet, and, therefore, did not profit from the possible beneficial effect of xylitol found when combined with a high-fat diet. Our results concerning blood lipids are in line with a human study looking at an intake of high doses (up to 100 g/day, during 18 days) of xylitol in healthy volunteers, which found no changes in TG levels and a trend in reduction of cholesterol levels.27 Therefore, erythritol and xylitol seem superior compared with sucrose, which is known to increase blood lipids and promote liver fat accumulation.35 36
Huttunen et al 37 found no effect of chronic xylitol intake (30 g/day) for 2 years on fasting insulin or glucose concentrations in healthy volunteers.37 In line, we also found no statistically significant effect of a 5-week erythritol or xylitol intake on glucose tolerance. However, in another study assessing the effect of 20 g/day erythritol during 2 weeks on glucose tolerance in patients with T2DM, Ishikawa et al 38 found a trend for decreased fasting blood glucose and decreased HbA1C.38 Here again, the difference in study populations might explain the discrepancy. In conclusion, we show that erythritol and xylitol do not lead to statistically significant changes in glucose tolerance, which make them promising sugar alternatives, especially in patients at risk for T2DM.
We have previously found that acute ingestion of 35 g xylitol led to an increase in uric acid, while there was no effect after 50 g erythritol.18 19 An increase in uric acid was also found in an acute study in healthy volunteers given 35 g xylitol during physical exercise.39 Förster et al 27 reported that plasma uric acid was unchanged in healthy volunteers after 18 days of up to 100 g/day xylitol consumption. Here, we did not find any statistically significant elevation in uric acid in either group. We conclude that an increase in uric acid can be observed when xylitol is given acutely in healthy volunteers, but not after a 5-week exposure in volunteers with obesity but without T2DM.
Gastrointestinal tolerance in our trial was good except for a few diarrhoea-related symptoms at the beginning of the intervention, especially in the xylitol group. This is in line with other studies, showing that the acute consumption of xylitol might cause some gastrointestinal inconvenience,19 39 and that subjects over time adapt to chronic intake.40
There were some modifications in dietary patterns during the intervention. Participants of all treatment groups reduced their consumption of dairy products, sweetened beverages and sweets compared with preintervention. However, as these changes also occurred in participants of the control group, we rather interpret them as a ‘study effect’ than any intervention effect.
It is necessary to acknowledge some limitations of this study. First, as this is a pilot study, we cannot exclude that the sample size has been too small to detect significant changes. Second, the duration of intake was only 5 weeks. Therefore, no conclusions can be drawn for longer periods. Third, as no placebo substance is available, which would be metabolically inert and sweet in taste, the study was not placebo-controlled. Therefore, participants in the control group were not blinded. Fourth, no biomarker of intake was assessed, therefore compliance to the study intervention could not be objectively measured. Fifth, the participants consumed 36 g/day, or 24 g/day of erythritol or xylitol, respectively. Therefore, we cannot exclude that the use of higher amounts of erythritol and xylitol would have induced an effect on the parameters studied. However, higher dosages might cause more severe gastrointestinal symptoms, leading to poorer treatment adherence, and might not represent real-life settings.
In conclusion, we showed that the 5-week intake of erythritol and xylitol in people with obesity had no statistically significant effects on vascular function, abdominal fat and blood lipids, glucose tolerance, uric acid, hepatic enzymes and creatinine and was well tolerated except loose stools in the xylitol group. These results are relevant given the current recommendation to reduce sugar consumption, as the dosages and intake time points correspond to everyday-life sugar consumption. The study adds important information to the knowledge about erythritol and xylitol, showing that they are promising sugar alternatives, especially for people with obesity and, therefore, at risk of hypertension and cardiovascular diseases, hepatic steatosis and type 2 diabetes.
Acknowledgments
We would like to thank V. Rahmel, C. Cudré-Mauroux, K. Roser (students), A. Etter-Atlass, D. Brosi, J. Brosi and S. Gagliardo (study nurses), Dr. L. Streese (sport scientist) and C. Hauser (PhD Student) for their help in the current study.
Footnotes
ACM-G and BKW contributed equally.
Contributors: OB, AS-T, HH, CB, ACM-G and BKW designed the research. VB, FT and PM conducted the research. JD performed the statistical analysis. VB wrote the paper. ACM-G and BKW are responsible for the overall content as guarantors. All authors have read and approved the final manuscript.
Funding: This work was supported by: 'Freiwillige Akademische Gesellschaft' and 'Stiftung zur Förderung der gastroenterologischen und allgemeinen klinischen Forschung sowie der medizinischen Bildauswertung'. Grant receiver: ACM-G.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data described in the manuscript will be made available upon request to the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The protocol was approved by the Ethikkommission Nordwest- und Zentralschweiz: 2016-00781. Participants gave informed consent to participate in the study before taking part.
References
- 1. Meyer-Gerspach AC, Wölnerhanssen B, Beglinger B, et al. Gastric and intestinal satiation in obese and normal weight healthy people. Physiol Behav 2014;129:265–71. 10.1016/j.physbeh.2014.02.043 [DOI] [PubMed] [Google Scholar]
- 2. Nguyen NQ, Debreceni TL, Bambrick JE, et al. Accelerated intestinal glucose absorption in morbidly obese humans: relationship to glucose transporters, Incretin hormones, and Glycemia. J Clin Endocrinol Metab 2015;100:968–76. 10.1210/jc.2014-3144 [DOI] [PubMed] [Google Scholar]
- 3. Seimon RV, Brennan IM, Russo A, et al. Gastric emptying, mouth-to-cecum transit, and glycemic, insulin, Incretin, and energy intake responses to a mixed-nutrient liquid in lean, overweight, and obese males. Am J Physiol Endocrinol Metab 2013;304:E294–300. 10.1152/ajpendo.00533.2012 [DOI] [PubMed] [Google Scholar]
- 4. Mavrelis PG, Ammon HV, Gleysteen JJ, et al. Hepatic free fatty acids in alcoholic liver disease and morbid obesity. Hepatology 1983;3:226–31. 10.1002/hep.1840030215 [DOI] [PubMed] [Google Scholar]
- 5. Lee Y, Hirose H, Zhou Y-T, et al. Increased lipogenic capacity of the islets of obese rats: a role in the pathogenesis of NIDDM. Diabetes 1997;46:408–13. 10.2337/diab.46.3.408 [DOI] [PubMed] [Google Scholar]
- 6. Risso A, Mercuri F, Quagliaro L, et al. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab 2001;281:E924–30. 10.1152/ajpendo.2001.281.5.E924 [DOI] [PubMed] [Google Scholar]
- 7. Tsuneki H, Sekizaki N, Suzuki T, et al. Coenzyme Q10 prevents high glucose-induced oxidative stress in human umbilical vein endothelial cells. Eur J Pharmacol 2007;566:1–10. 10.1016/j.ejphar.2007.03.006 [DOI] [PubMed] [Google Scholar]
- 8. Salomaa V, Riley W, Kark JD, et al. Non-insulin-dependent diabetes mellitus and fasting glucose and insulin concentrations are associated with arterial stiffness indexes. Circulation 1995;91:1432–43. 10.1161/01.cir.91.5.1432 [DOI] [PubMed] [Google Scholar]
- 9. Nguyen TT, Wang JJ, Sharrett AR, et al. Relationship of retinal vascular caliber with diabetes and retinopathy: the multi-ethnic study of atherosclerosis (MESA). Diabetes Care 2008;31:544–9. 10.2337/dc07-1528 [DOI] [PubMed] [Google Scholar]
- 10. Franklin SS. Beyond blood pressure: arterial stiffness as a new biomarker of cardiovascular disease. J Am Soc Hypertens 2008;2:140–51. 10.1016/j.jash.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 11. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010;55:1318–27. 10.1016/j.jacc.2009.10.061 [DOI] [PubMed] [Google Scholar]
- 12. Ikram MK, Witteman JCM, Vingerling JR, et al. Retinal vessel diameters and risk of hypertension the Rotterdam study. Hypertension 2006;47:189–94. 10.1161/01.HYP.0000199104.61945.33 [DOI] [PubMed] [Google Scholar]
- 13. Streese L, Lona G, Wagner J, et al. Normative data and standard operating procedures for static and dynamic retinal vessel analysis as biomarker for cardiovascular risk. Sci Rep 2021;11:14136. 10.1038/s41598-021-93617-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar narrowing and risk of coronary heart disease in men and women the atherosclerosis risk in communities study. JAMA 2002;287:1153–9. 10.1001/jama.287.9.1153 [DOI] [PubMed] [Google Scholar]
- 15. WHO . Guideline: sugars intake for adults and children. Geneva: World Health Organization, 2015. [PubMed] [Google Scholar]
- 16. Livesey G. Health potential of polyols as sugar replacers, with emphasis on low glycaemic properties. Nutr Res Rev 2003;16:163–91. 10.1079/NRR200371 [DOI] [PubMed] [Google Scholar]
- 17. Wölnerhanssen BK, Cajacob L, Keller N, et al. Gut hormone secretion, gastric emptying, and glycemic responses to erythritol and xylitol in lean and obese subjects. Am J Physiol Endocrinol Metab 2016;310:E1053–61. 10.1152/ajpendo.00037.2016 [DOI] [PubMed] [Google Scholar]
- 18. Wölnerhanssen BK, Drewe J, Verbeure W, et al. Gastric emptying of solutions containing the natural sweetener erythritol and effects on gut hormone secretion in humans: a pilot dose-ranging study. Diabetes Obes Metab 2021;23:1311–21. 10.1111/dom.14342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meyer-Gerspach AC, Drewe J, Verbeure W, et al. Effect of the natural sweetener xylitol on gut hormone secretion and gastric emptying in humans: a pilot dose-ranging study. Nutrients 2021;13:174. 10.3390/nu13010174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boesten D, Berger A, de Cock P, et al. Multi-targeted mechanisms underlying the endothelial protective effects of the diabetic-safe sweetener erythritol. PLoS One 2013;8:e65741. 10.1371/journal.pone.0065741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flint N, Hamburg NM, Holbrook M, et al. Effects of erythritol on endothelial function in patients with type 2 diabetes mellitus: a pilot study. Acta Diabetol 2014;51:513–6. 10.1007/s00592-013-0534-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Witkowski M, Nemet I, Alamri H, et al. The artificial sweetener erythritol and cardiovascular event risk. Nat Med 2023;29:710–8. 10.1038/s41591-023-02223-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hootman KC, Trezzi J-P, Kraemer L, et al. Erythritol is a pentose-phosphate pathway metabolite and associated with adiposity gain in young adults. Proc Natl Acad Sci U S A 2017;114:E4233–40. 10.1073/pnas.1620079114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ortiz SR, Field MS. Sucrose intake elevates erythritol in plasma and urine in male mice. J Nutr 2023;153:1889–902. 10.1016/j.tjnut.2023.05.022 [DOI] [PubMed] [Google Scholar]
- 25. Amo K, Arai H, Uebanso T, et al. Effects of xylitol on metabolic parameters and visceral fat accumulation. J Clin Biochem Nutr 2011;49:1–7. 10.3164/jcbn.10-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kishore P, Kehlenbrink S, Hu M, et al. Xylitol prevents NEFA-induced insulin resistance in rats. Diabetologia 2012;55:1808–12. 10.1007/s00125-012-2527-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Förster H, Quadbeck R, Gottstein U. Metabolic tolerance to high doses of oral xylitol in human volunteers not previously adapted to xylitol. Int J Vitam Nutr Res Suppl 1982;22:67–88. [PubMed] [Google Scholar]
- 28. Islam MS. Effects of xylitol as a sugar substitute on diabetes-related parameters in nondiabetic rats. J Med Food 2011;14:505–11. 10.1089/jmf.2010.0015 [DOI] [PubMed] [Google Scholar]
- 29. Msomi NZ, Erukainure OL, Salau VF, et al. Comparative effects of xylitol and erythritol on modulating blood glucose; inducing insulin secretion; reducing dyslipidemia and redox imbalance in a type 2 diabetes rat model. Food Science and Human Wellness 2023;12:2052–60. 10.1016/j.fshw.2023.03.023 [DOI] [Google Scholar]
- 30. Seabold S, Perktold J. Statsmodels: econometric and statistical modeling with python. Python in Science Conference; Austin, Texas.2010. 10.25080/Majora-92bf1922-011 [DOI] [Google Scholar]
- 31. Prenner SB, Chirinos JA. Arterial stiffness in diabetes mellitus. Atherosclerosis 2015;238:370–9. 10.1016/j.atherosclerosis.2014.12.023 [DOI] [PubMed] [Google Scholar]
- 32. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013;31:1281–357. 10.1097/01.hjh.0000431740.32696.cc [DOI] [PubMed] [Google Scholar]
- 33. Koivistoinen T, Kööbi T, Jula A, et al. Pulse wave velocity reference values in healthy adults aged 26-75 years. Clin Physiol Funct Imaging 2007;27:191–6. 10.1111/j.1475-097X.2007.00734.x [DOI] [PubMed] [Google Scholar]
- 34. Kobayashi R, Sakazaki M, Nagai Y, et al. Effects of different types of carbohydrates on arterial stiffness: a comparison of Isomaltulose and Sucrose. Nutrients 2021;13:4493. 10.3390/nu13124493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Geidl-Flueck B, Hochuli M, Németh Á, et al. Fructose- and sucrose- but not glucose-sweetened beverages promote hepatic de novo lipogenesis: a randomized controlled trial. J Hepatol 2021;75:46–54. 10.1016/j.jhep.2021.02.027 [DOI] [PubMed] [Google Scholar]
- 36. Marckmann P, Raben A, Astrup A. Ad libitum intake of low-fat diets rich in either starchy foods or sucrose: effects on blood lipids, factor VII coagulant activity, and fibrinogen. Metabolism 2000;49:731–5. 10.1053/meta.2000.6237 [DOI] [PubMed] [Google Scholar]
- 37. Huttunen JK, Mäkinen KK, Scheinin A. Turku sugar studies XI: effects of sucrose, fructose and xylitol diets on glucose, lipid and Urate metabolism. Acta Odontol Scand 1976;34:345–51. 10.3109/00016357609004646 [DOI] [PubMed] [Google Scholar]
- 38. Ishikawa M, Miyashita M, Kawashima Y, et al. Effects of oral administration of erythritol on patients with diabetes. Regul Toxicol Pharmacol 1996;24:S303–8. 10.1006/rtph.1996.0112 [DOI] [PubMed] [Google Scholar]
- 39. Wołyniec W, Szwarc A, Kasprowicz K, et al. Impact of hydration with beverages containing free sugars or xylitol on metabolic and acute kidney injury markers after physical exercise. Front Physiol 2022;13:841056. 10.3389/fphys.2022.841056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scheinin A, Mäkinen KK. Turku sugar studies: an overview. Acta Odontol Scand 1976;34:405–8. 10.3109/00016357609004651 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjnph-2023-000764supp001.pdf (74KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data described in the manuscript will be made available upon request to the corresponding author.