Video
Superinfected intragastric balloon removal.
Intragastric balloon placement is becoming increasingly popular worldwide. This is a generally safe procedure, but adverse events do exist. The incidence of infection of the intragastric balloon is rare and cited less than 1% in the available literature.1, 2, 3, 4 Here, we present a case of a 26-year-old woman with a medical history of obesity (body mass index = 30 kg/m2) who underwent intragastric balloon placement abroad for weight loss (Video 1, available online at www.videogie.org). The balloon was filled with 600 mL of fluid during the procedure. This procedure was uncomplicated, and she later returned to the United States.
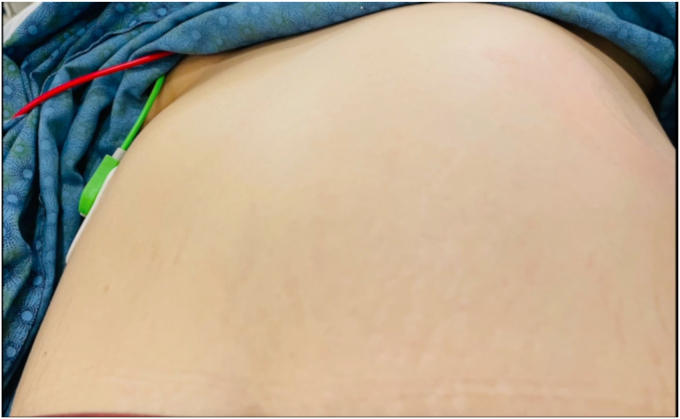
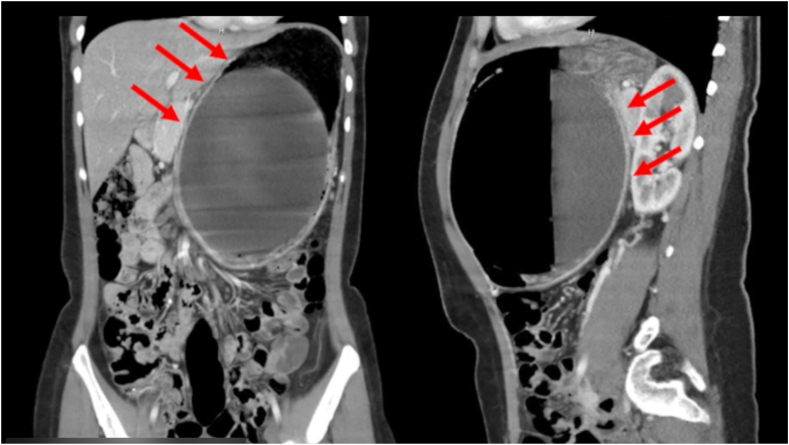
One month following placement, the patient experienced nausea, vomiting, abdominal distension, abdominal pain, and back pain. She presented to the emergency department for evaluation. She was afebrile and hemodynamically stable and had no chills at the time. Physical examination revealed asymmetry of her abdominal wall (Fig. 1). A palpable mass was appreciated over her left upper quadrant. She had a normal white blood cell count of 4700/μL and hypokalemia level of 3.3 mmol/L. The other laboratory values, including a venous lactate, were normal. CT of the abdomen revealed a markedly distended intragastric balloon noted to measure 17 × 15.4 cm with an air-fluid level with concomitant extensive dilation of the gastric lumen and marked thinning of the anterior stomach wall and adjacent linear pockets of air (Fig. 2). Given these findings, there was concern for impending perforation, and an emergent upper endoscopy was pursued to remove the intragastric balloon.
Figure 1.
Asymmetry of the patient’s abdominal wall as noted on physical examination.
Figure 2.
Markedly distended intragastric balloon noted to measure 17 15.4 cm with an air-fluid level with concomitant extensive dilation of the gastric lumen and marked thinning of the anterior stomach wall (red arrows) and adjacent linear pockets of air as seen on CT of the abdomen.
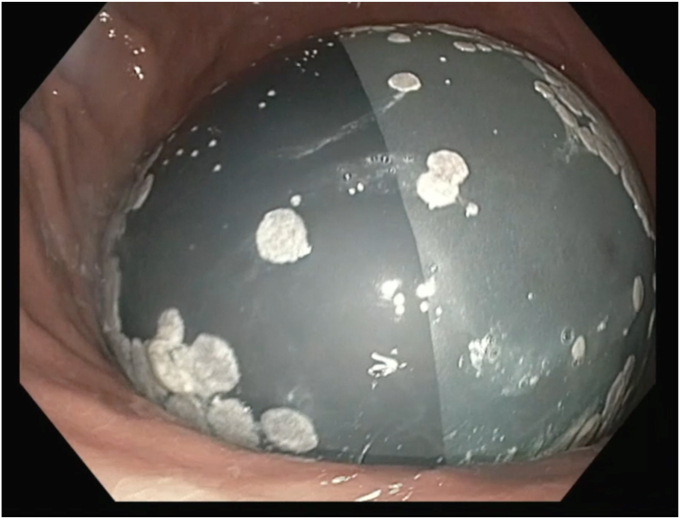
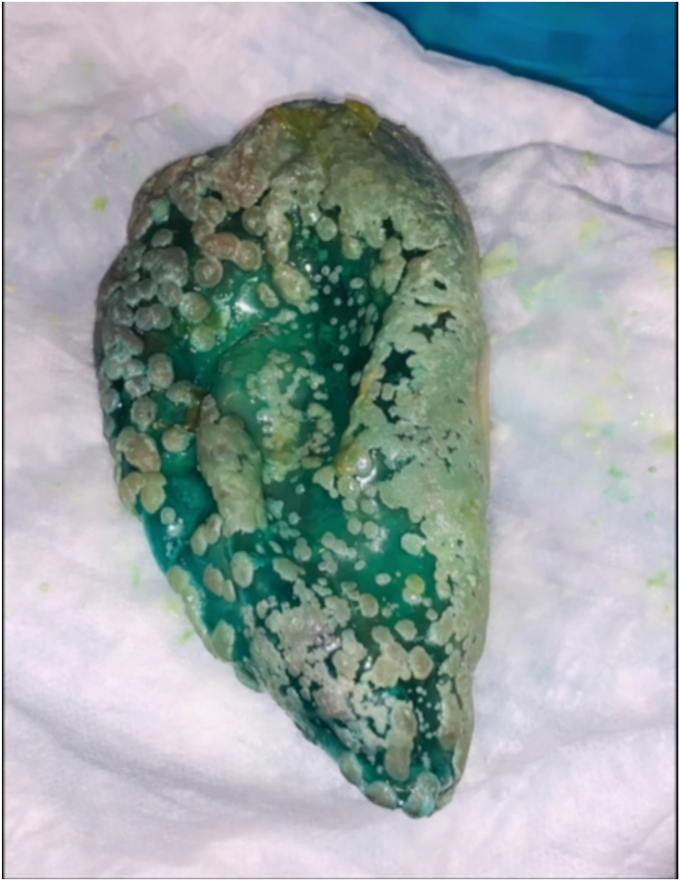
The patient was intubated for airway protection at the time of the procedure. During the endoscopy, the balloon was noted to be hyperinflated. White plaques were visualized throughout the surface of the balloon, suggestive of bacterial or fungal colonization. A distinct air-fluid level was also seen within the balloon, suggestive of hyperinflation (Fig. 3). A puncture device was used to spear the balloon, to ultimately drain the fluid. Care was taken to advance the device at a perpendicular angle to the balloon to secure access, and not beyond the back wall of the balloon where it could also puncture the gastric wall. Suction was applied to the puncture catheter, to allow drainage of fluid. However, given the infected, hyperinflated balloon, it is assumed that the integrity of the balloon wall was compromised, leading to leaking of fluid around the puncture site. Thus, the excess fluid was suctioned through the endoscope, and the fluid in the balloon was safely evacuated (Fig. 4). Given that the procedure was performed with the patient under general anesthesia in the semi-upright position, the contents of the fluid were immediately suctioned, and the gastric mucosal integrity was not significantly impaired. The risk of both aspiration pneumonitis and infection, including bacterial translocation, was deemed to be low. Close examination of the stomach revealed a single, localized erosion (Fig. 5). Plaques from the balloon were swabbed and sent to microbiology for culture.
Figure 3.
Endoscopic examination demonstrating a distinct air-fluid level within the balloon suggestive of hyperinflation.
Figure 4.
Colonized intragastric balloon after removal.
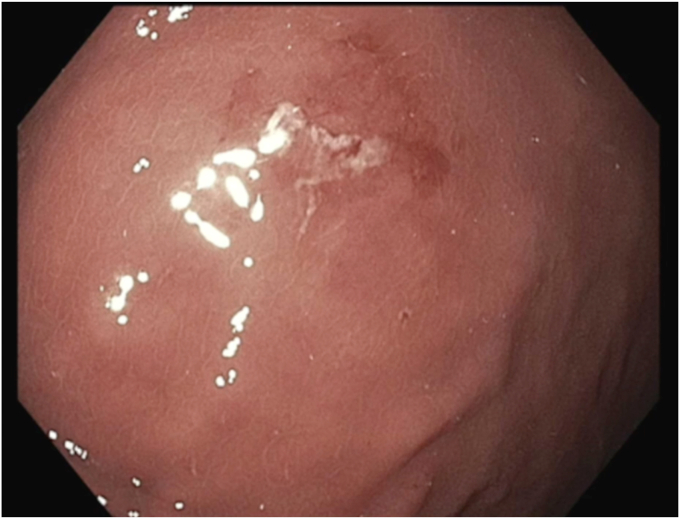
Figure 5.
Single erosion found in gastric lumen after removal of the intragastric balloon.
The patient felt remarkably improved following balloon removal and continued to feel well for at least 4 weeks after the procedure when she was lost to follow-up. Culture results ultimately revealed a polymicrobial infection including Candida tropicalis, Streptococcus anginosus, Serratia marcescens, and Klebsiella pneumoniae. The infectious disease team was consulted, and they recommended a short course of fluconazole and amoxicillin/clavulanate.
Infection is a rare adverse events of intragastric balloon placement, occurring in less than 1% of patients.1, 2, 3, 4 Delayed gastric emptying leading to gastric stasis has been proposed as a potential risk factor for infection, but more data are needed.5 Appropriate cleaning and storage of endoscopes and use of recommended personal protective equipment may be important to minimize this risk, but additional research is needed.
Disclosure
The authors disclosed no financial relationships relevant to this publication.
Supplementary data
Superinfected intragastric balloon removal.
References
- 1.Abu Dayyeh B.K., Kumar N., Edmundowicz S.A., et al. ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest Endosc. 2015;82 doi: 10.1016/j.gie.2015.03.1964. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan S., Edmundowicz S.A., Thompson C.C. Endoscopic bariatric and metabolic therapies: new and emerging technologies. Gastroenterology. 2017;152:1791–1801. doi: 10.1053/j.gastro.2017.01.044. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan S., Moore R.L., Kroh M. Endoscopic bariatric therapy: a guide to the intragastric balloon. Am J Gastroenterol. 2020;115:629. doi: 10.14309/ajg.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 4.Moore R.L., Eaton L., Ellner J. Safety and effectiveness of an intragastric balloon as an adjunct to weight reduction in a post-marketing clinical setting. Obes Surg. 2020;30:4267–4274. doi: 10.1007/s11695-020-04798-5. [DOI] [PubMed] [Google Scholar]
- 5.Coskun H., Bozkurt S. A case of asymptomatic fungal and bacterial colonization of an intragastric balloon. World J Gastroenterol. 2009;15:5751–5753. doi: 10.3748/wjg.15.5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Superinfected intragastric balloon removal.
Superinfected intragastric balloon removal.