Abstract
Background:
Hand hygiene is a key tool in infection control. While methods of hand washing have been widely researched, there have been fewer studies investigating the effectiveness of available ways to dry hands in public areas.
Aims:
This study compared the efficacy of using paper towels (PT), warm air dryers (WAD) and jet air dryers (JAD) after hand washing in terms of microbiological effectiveness and potential for dispersal of pathogens.
Methods:
Microbial flora on palms and fingertips of 30 subjects were sampled on nutrient agar plates before washing hands and after drying with PTs, WADs and JADs. Total colony forming units (cfus) were recorded. Walls in the vicinity of a PT dispenser, WAD and JAD in female and male washrooms were sampled for the presence of viable microorganisms.
Results:
Mean cfu significantly reduced after drying with PTs (palms t= 2.67, p <0.05; fingertips t=4.44, p<0.01) significantly increased after using WADs (palms t=3.11, p<0.01; fingertips t=2.06, p<0.05), but there was no difference with JAD (palms t= 1.85, p>0.05; fingertips t=0.97, p>0.05). Some dispersal of organisms was detected on the washroom walls, with the least distribution around PT dispensers and unusual opportunistic pathogens isolated from the JAD units.
Discussion:
PTs are more effective at drying hands than WADs and JADs, they are more likely to be used appropriately and lead to minimal dispersal of microorganisms from wet hands.
Keywords: Hand drying, paper towels, warm air dryers, jet air dryers, dispersal
Background
Hand washing and drying practices can be a matter of custom and etiquette (Freeman et al., 2014), but they are also important in personal hygiene and infection control. Following the work of Semmelweis and others in the 19th century (Pittet and Boyce, 2001), good hand hygiene has been promoted to reduce the spread of infectious diseases in the community (Warren-Gash et al., 2013) and nosocomial infections (Loveday et al., 2014; Tacconelli et al., 2014). Evaluating education and training around hand hygiene behaviour is a key area of research for infection control professionals (Chatfield et al., 2017)
Hands are colonised by ‘resident’ organisms and ‘transient’ flora (Allegranzi and Pittet, 2009). The former group are ‘normal’ skin flora, such as coagulase negative Staphylococcus spp. The latter are acquired through direct contact with infected material (faeces, soil or food) or via contaminated fomites; examples include Escherichia coli, norovirus, Salmonella spp. and methicillin resistant Staphylococcus aureus (Pittet and Boyce, 2001). The physical process of washing hands is known to dislodge both types of organism from the skin. While transient flora can be associated with serious diseases, normal flora can also be opportunistic pathogens. Therefore, although infrequent cleaning of hands can be a risk to personal and public health, washing them using poor technique could release organisms from the skin without fully removing them and thus pose a larger hazard. Extensive evaluation of hand washing techniques, including investigations of optimal hand movements and whether to use soap or alcohol based sanitizer (Curtis and Cairncross, 2003; Kac et al., 2005; Foddai et al., 2016) has resulted in clear, well publicised guidelines for thorough washing of hands (WHO, 2009; CDC, 2002).
Evidence suggests that wet or inadequately dried hands can transmit skin organisms (Patrick et al., 1997; Huang et al., 2012; Pitt SJ et al., unpublished data), thus contributing to the spread of potential pathogens. Research into optimal hand drying methods is relatively limited, protocols vary and results are mixed. Most studies have used the technique of ‘seeding’ the subjects’ gloved hands with known concentrations of specific organisms (Ansari et al., 1991; Hanna et al., 1996; Gustafson et al., 2000) or deliberate contamination of food (Snelling et al., 2011). Sampling methods include pressing finger tips and palms onto agar plates before and after washing and drying (Taylor et al., 2000; Redway and Fawdar, 2008), touching tin foil after drying (Snelling et al., 2011) and assessing the microbial count on paper and cloth towels rather than hands (Hanna et al., 1996; Taylor et al., 2000). Despite some studies indicating no difference in outcome according to drying method (Gustafson et al., 2000), results do suggest that paper towels (PTs) are more effective than air dryers (Hanna et al., 1996; Redway and Fawdar, 2008; Huang et al., 2012) and that jet air dryers (JADs) tend to perform better than warm air driers (WADs) (Snelling et al., 2011; Huang et al., 2012). This may be because the physical process of pressing a PT onto the hands is more efficient at removing organisms dislodged by washing (Hanna et al., 1996).
Air dryers are perceived as more energy efficient than disposable, single-use PTs. Estimates of the relative carbon footprint of each method bear that out (Huang et al., 2012; Joseph et al., 2015). Interestingly, surveys suggest that many people still prefer using PTs in public washrooms as they are considered to be the most ‘hygienic’ drying method (INTERMETRA, 2011; Huang et al., 2012). Also, the noise from air dryers is a notable disadvantage, especially in healthcare situations where people need quiet and rest (Best et al., 2014). Dispersal of organisms from wet hands into the surrounding area is another concern. Studies using deliberate contamination of gloved hands have shown that bacteria (Best et al., 2014), yeasts (Best and Redway, 2015), bacteriophage (Kimmitt and Redway, 2016;) and inert substances (Best and Redway, 2015) are all successfully spread during hand drying. JADs are reported to spread a greater concentration of organisms for longer distances than other methods (Best et al., 2014; Best and Redway, 2016; Kimmitt and Redway, 2015). Previous work has involved recovery of organisms introduced into the experiments by the researchers. However, from an infection control point of view it is more interesting to track what happens to the organisms which people carry on their hands during a normal day.
Therefore the aims of this study were to:
1) Compare the efficacy of three hand drying methods – namely the use of PTs, JADs and WADs - in relation to the microbial flora each individual was carrying before hand washing.
2) Investigate the dispersal of potential pathogens from organisms naturally carried on people’s hands via each type of hand dryer.
Materials and Methods
Hand drying
Thirty subjects who were staff or students in the School of Pharmacy and Biomolecular Sciences (PABS) at the University of Brighton, UK (15 male, 15 female) aged between 18 and 64 were recruited under a protocol which had been approved by the School’s Ethics Committee and included informed consent. Potential participants with obvious skin lesions or conditions causing irritation were excluded from the study. Experiments were carried out over three weeks in three male and three female washrooms at the university, which each contained one of the types of hand dryer. One type of dryer was tested on one day each week and all participants were involved in each set of experiments. The PT dispensers, JADs and WADs used by male and female subjects were of the same type and in adjacent washrooms. All three sets of washrooms were in well used areas of the campus, of similar size and footfall. The drying appliances were checked to be in good working order, but were not cleaned before use. Microbial counts on each subject’s hands were estimated by asking them to place their fingertips onto a standard 90mm Petri dish and their palm onto a contact plate. Both types of plates used Nutrient Agar (NA) (Oxoid, Basingstoke, Hampshire). The dominant hand was sampled before washing. The subject was then required to wash their hands using the WHO recommended protocol (WHO, 2009), which had been demonstrated to them. Hands were dried using either PT, JAD or WAD, until the person felt comfortable. The dominant hand was re-sampled using 90mm and contact plates, as described above.
Plates were incubated at 25° C for 96 hours (to allow for growth of yeasts), after which colony forming unit (cfu) counts were recorded. A value of 900 was arbitrarily assigned to plates where the count was very high, allowing their inclusion in the data analysis. This was chosen to indicate a large cfu, while remaining in the same order of magnitude (100s) as lower counts. The cfu results for male and female subjects were combined and the mean calculated for each type of drying method. Data was normally distributed, so mean cfu counts were compared using paired t–tests in Minitab® v.17. The mean count after washing and then drying was subtracted from the mean count obtained before washing for each method (‘mean cfu difference’).
Hand dryer environment
The area around the PT dispenser, WAD and JAD in male and female washrooms (six separate facilities) in university buildings were sampled at a different time to the hand drying studies. Experiments were done at approximately the same time of day (between 11am and 12pm) on each occasion. Routine cleaning of the facilities took place before 8am daily. A 30cm square card template was prepared and laminated and 1cm2 holes were cut out at 10cm intervals (Figure 1). The card was placed on the washroom wall and a cotton swab moistened in phosphate buffered saline (PBS) was used to sample the wall through each of the holes. Sampling was carried out at two positions with respect to the each of the dryers. One set of swabs were collected by placing the template directly underneath the unit and the other set were taken to the right hand side of it, at a position where splashing could occur. Each dryer was also swabbed in specific places – the knob used to turn the towel on the PT dispensers, the sides and underside of the WADs and the trough at the bottom of the area that hands are held within the JAD. Each swab was placed into 1mL PBS and vortexed for 10 seconds; 100µL of suspension was plated out onto NA, Cystine-Lactose-Electrolyte Deficient agar (CLED) and Blood Agar (BA) (Oxoid, Basingstoke, Hampshire) and incubated at 37° C for 48 hours. The cfu counts from NA plates were recorded and mean counts from the male and female washrooms for each type of dryer were recorded. Isolates from the CLED and BA plates were used to aid species identification.
Figure 1.

Sampling template placed on washroom wall to the right hand side of paper towel dispenser.
Template was 30 cm2 ; thus sampling positions were at 10cm intervals.
Results
Comparison of hand drying methods
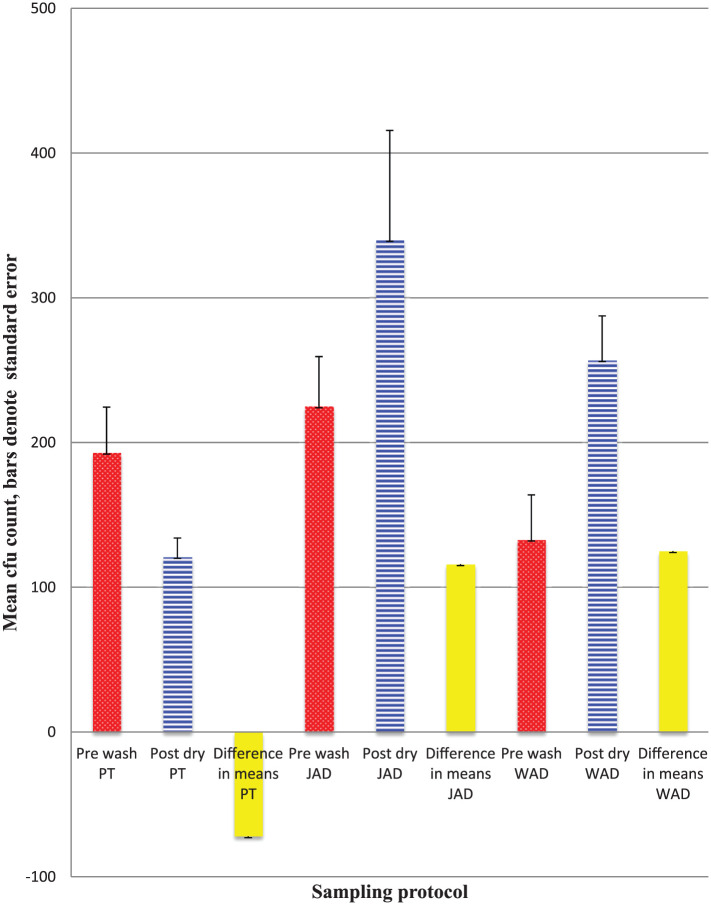
Figure 2 shows the mean cfu counts on subjects’ fingertips ( Figure 2a) and palms (Figure 2b) before washing and after drying. There were statistically significant differences between counts for both fingertips and palms after using PTs (decrease) and WADs ( increase), but not JADs. Ninety morphologically different isolates were selected for further identification through standard methods. The majority (60) were found to be coagulase negative Staphylococcus spp., with some Staph.aureus, bacilli and yeasts.
Figure 2a.
Mean cfu counts on fingertips pre washing and post drying by three hand drying methods, n=30.
Statistical test of differences in means: PT t= 2.67, p <0.05; JAD t= 1.85, p>0.05; WAD t=3.11, p<0.01.
Hand dryer environment
The mean cfu count obtained from male and female washroom walls at each sampling point on the template underneath and to the right hand side of each dryer is shown in Table 1. It shows that bacteria were found in most 1cm2 areas sampled, although there was no apparent pattern. Overall, fewer organisms were recovered underneath and to the right of the PT dispenser, while the highest counts were found underneath the WAD. Notable counts were also observed at the point 20cm to the right of the JAD in a vertical line down the wall (grid positions 7, 8, 9 - see Figure 1 and Table 1).
Table 1.
Mean cfu counts ‡ on washroom walls underneath and to the right hand side of PT dispensers, WAD and JAD dryers.
| Mean | Colony | Forming | Units | |||
|---|---|---|---|---|---|---|
| Template position | Underneath PT dispenser | Underneath JAD | Underneath WAD | Right hand side of PT dispenser | Right hand side of JAD | Right hand side of WAD |
| 1 | 2 | 3 | 7 | 5 | 1.5 | 6 |
| 2 | 0.5 | 0 | 4.5 | 2.5 | 0.5 | 6 |
| 3 | 7.5 | 3.5 | 10 | 1.5 | 10 | 8 |
| 4 | 0.5 | 1.5 | 4.5 | 1 | 2 | 2 |
| 5 | 1.5 | 0.5 | 10.5 | 0 | 0 | 5 |
| 6 | 2 | 3.5 | 9.5 | 2 | 1 | 5 |
| 7 | 0.5 | 2 | 19 | 0.5 | 10 | 2 |
| 8 | 2 | 2.5 | 12 | 0.5 | 13 | 1 |
| 9 | 2 | 4.5 | 21 | 3.5 | 11.5 | 3 |
| 10 | 2.5 | 6.5 | 10 | 8.5 | 5 | 15 |
| 11 | 1 | 2.5 | 1.5 | 1 | 0 | 1 |
| 12 | 1 | 2 | 1.5 | 1 | 1.5 | 2 |
The majority of isolates were Stapylococcus spp. or environmental Bacillus spp.; a small number of filamentous fungi and Candida spp. were also grown.
Sampling of the knobs of the PT dispenser, the sides of the WAD and the trough of the JAD yielded high bacterial counts (‘too many to count’) in all cases. Most isolates were either Staphylococcus spp. (Staph. epidermidis or Staph. aureus) or non-pathogenic Bacillus spp. Organisms recovered from the trough in the JADs also included Staph.haemolyticus from the dryer in the female washroom and Pantoea agglomerans (Figure 3) from the one in the male facility.
Figure 3.
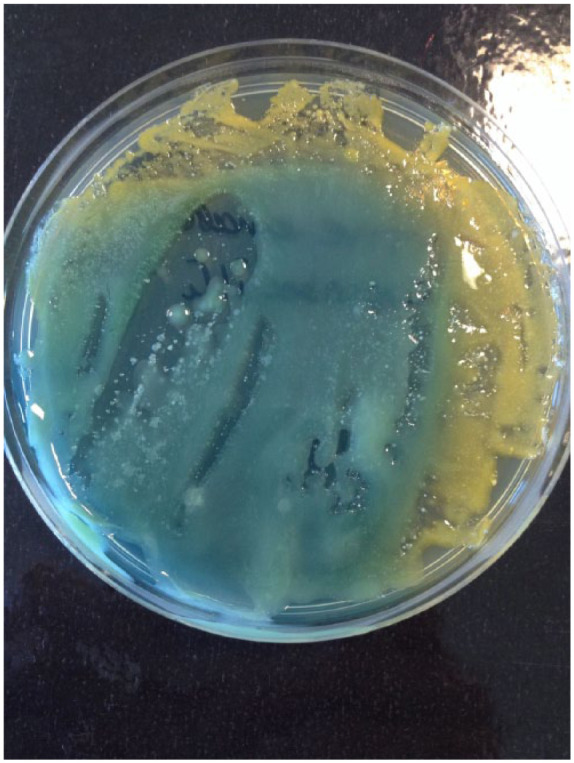
Pantoea agglomerans (isolated from a JAD trough) on CLED.
Discussion
This study has shown that PTs are a more effective method of removing microorganisms from hands after washing than either WADs or JADs. This is consistent with the findings of some previous studies (Hanna et al., 1996; Redway and Fawdar, 2008, Huang et al., 2012), although others have concluded that air drying is better (Ansari et al., 1991) or that there is no observable difference (Gustafson et al., 2000; Taylor et al., 2000). Fewer viable microorganisms were recovered from the walls around the PT dispensers than near the other two types of dryer (Table 1), suggesting that these are better from a public health perspective. While the contamination of the vicinity of various drying units has been investigated in controlled environments (Margas et al., 2013; Best et al., 2014; Best and Redway, 2015; Kimmitt and Redway, 2016), random sampling of public washroom walls has not been fully investigated.
Counts of microorganisms were significantly reduced on both fingertips and palms after washing according to WHO guidelines (WHO, 2009) and then drying with PTs, while cfu increased when WADs were used (Figures 2a and 2b). Other authors have reported similar findings (Hanna et al., 1996; Redway and Fawdar, 2008; Huang et al., 2012). Since wet hands are associated with transmission of infectious agents (Patrick et al., 1997, Taylor et al., 2000), these results suggest that using paper towels is appropriate in healthcare settings. Friction involved in the action of using the towels possibly contributes to removal of organisms (Huang et al., 2012). The raised cfu counts recorded after washing and drying with WAD are not unprecedented (e.g. Snelling et al., 2011; Redway and Fawdar, 2008), but since the amount of time the subjects held their hands under the dryer was not strictly monitored in this study, it may simply have occurred because hands were still slightly wet. The more surprising finding was that the cfu count slightly decreased on fingertips, but slightly increased on palms after using the JAD, although differences between readings before washing and after drying were not statistically significant (Figures 2a and 2b). Most studies report a modest reduction in counts after using JAD (Redway and Fawdar, 2008, Taylor et al., 2000; Snelling et al., 2011), albeit usually less marked than after using PTs. Although comparisons between JADs and WADs have suggested that the former are a better hand drying method between the two (e.g. Snelling et al., 2011), the results presented here do not conclusively support that. This could be due to the decision to allow subjects to dry their hands as they usually would, rather than prescribe drying times. The absence of coliforms among the isolates suggests that the WHO hand washing guidelines (WHO, 2009) are effective for removing such transient flora and that they are rinsed away into the sink. However, this would need to be further investigated in experiments using suitable selective media.
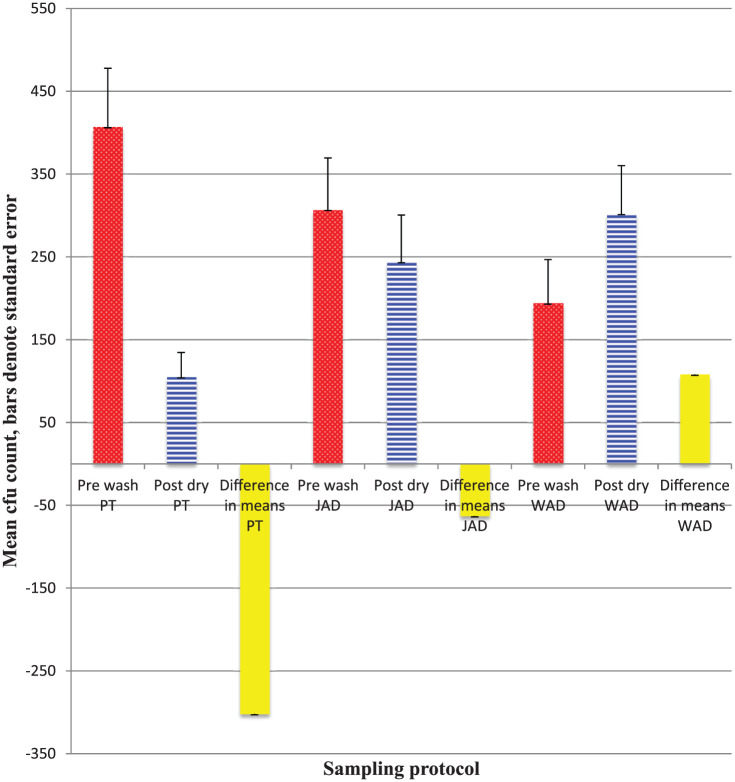
Figure 2b.
Mean cfu counts on palms pre washing and post drying by three hand drying methods, n=30.
Statistical test of differences in means: PT t=4.44, p <0.01; JAD t= 0.97, p>0.05; WAD t=2.06, p<0.05.
Microbial contamination found on and around the air dryers, PT dispensers and washroom walls (Table 1) confirms that wet hands can spread organisms efficiently (Patrick et al., 1997). Viable organisms were found to have been distributed across the washroom walls unevenly (Table 1). Although this was semi-quantitative sampling, some patterns emerged. The cfu counts were lowest in the vicinity of the PT dispenser, which is consistent with previous reports from more systematic experiments (Best et al., 2014; Best and Redway, 2015; Kimmitt and Redway, 2016). Bacterial count was a slightly raised to the right hand side of the dispenser at template positions 9 and 10 (30cm diagonally and directly away from the side of the dispenser respectively). Best and Redway (2015) also noted slightly raised microbial counts on agar plates placed at heights between 0.6 and 1.2 m from the floor underneath a PT dispenser; this would be at about waist height for most people and could be consistent with flicking off water from wet hands when taking a towel. The comparatively high counts obtained from sampling underneath the WAD unit shows that water reaches the wall and suggests that organisms from people’s wet hands can remain viable there. This is similar to patterns detected by other authors recovering ‘seeded’ organisms from around WADs (Best and Redway, 2015; Kimmitt and Redway, 2016). In this study, there was a clear configuration of spread to the right of the JAD, with raised cfu readings at template positions 7, 8 and 9 which are in a vertical line 20cm from the side of the unit. Best et al. (2014) used lactobacilli and sampled the air to the sides of the same three types of hand drying units used in this study. They found that at 1m away, the mean cfu beside the JAD was 40 times higher than that in the area around the PT dispenser (Best et al., 2014). Previous studies have used paint, lemon juice or suspensions containing known organisms and measured their dispersal during hand drying. They all indicated that microorganisms could present a hazard during the drying process, particularly near JADs (Best et al., 2014; Best and Redway, 2015; Kimmitt and Redway, 2016). In this study it was found that normal skin flora (Staphylococcus spp.) and environmental organisms, probably coming from the water (Bacillus spp.) remained viable on walls surrounding the three types of dryer. Despite the use of CLED and BA, no faecal coliforms or respiratory pathogens were found, which again points to the efficacy of the WHO washing method (WHO, 2009). It should be noted that the walls surrounding the dryers were mostly tiled, so this finding may be attributable to lack of viability of human pathogens on that type of surface. However, higher counts were obtained from the drying units themselves, although the isolates were mostly Staphylococcus spp. and Bacillus spp. For the knob on the side of the PT dispenser this is not surprising, since people would touch that with wet hands. It is likely that the underside of the WAD unit is contaminated by air pushed upwards when hands are moved around in it. The sampling of the trough of the jet air dryer again yielded high counts and also led to isolation of two interesting bacteria. Staph.haemolyticus is an emerging opportunistic pathogen in humans, which is known to form biofilms (Barros et al., 2015). Pantoea agglomerans (Figure 3) is a plant pathogen, also commonly found in food and human faeces. It is a rare, but serious cause of disease in immunocompromised patients (Walterson and Stavrinides, 2015). Redway and Fawdar (2008) isolated Escherichia coli, Klebsiella spp. and Pseudomonas aeruginosa from a public JAD, but this does not appear to have been followed up by other authors. The isolation of faecal organisms and potential opportunistic pathogens from the JAD troughs does indicate that they provide a moist but protected environment for bacterial growth. These results suggest that in a healthcare environment, JADs should only be installed in sites where their cleanliness can be guaranteed.
Although the data from the cfu counts from subjects’ palms and fingertips before and after washing was sufficient to process and showed clear trends, it could have been strengthened by repeating each type of drying with individuals (e.g. each person using each type of dryer three times). Since people were asked to commit to three days of experiments, it was felt that 15 males and 15 females was a manageable number to follow up, but the results could have been enhanced by having a larger number of participants. Use of CLED or MacConkey to specifically look for coliforms may also have enhanced results. The sampling of the walls included CLED and BA, which should have increased the chances of isolating any coliforms and other potential pathogens from people’s wet hands. The choice of mid-morning for the experiments was on the expectation that the areas were cleaned early in the morning and there would have been significant traffic of people on arrival in the building and during mid-morning breaks. However, this was not verified and a mid–afternoon sampling might have revealed different results. This could be explored by swabbing the walls and drying units repeatedly and at regular intervals over the course of a working day to ascertain the optimal sampling time.
The results presented here show that while hand washing is important in reducing transmission of potentially pathogenic microorganisms, it is also vital that hands are dried properly. This study supports the idea put forward by previous authors that PTs are the best hand drying method. The reasons for this are because they provide the most effective means to dry hands in microbiological terms and drying is likely to be carried out properly without need for special instructions. In addition, PT dispenser units minimise the spread of organisms from wet hands during the drying process. However, using hand towels can be seen as wasteful (even when the paper is recycled) and JADs in particular have been shown to have a lower carbon footprint. Paper dispensers must be replenished regularly, which requires ordering of towels and employing a person to do it. This could be perceived as costly and there is a risk of some time periods when no towels are available. An air dryer should not need daily checks, so may be preferred in a healthcare setting. However, in a busy environment, professionals and patients may not take sufficient time to dry their hands adequately which may contribute to the spread of infection. Therefore the conclusion of these findings are that PTs are preferable in public health terms.
Acknowledgments
Thanks are due to Mrs C Dedi and Dr A Gunn for helpful comments on drafts of this article and also to the two anonymous reviewers for useful feedback on an earlier version of the manuscript.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Peer review statement: Not commissioned; blind peer-reviewed.
ORCID iD: Sarah J Pitt  https://orcid.org/0000-0002-9215-6517
https://orcid.org/0000-0002-9215-6517
References
- Allegranzi B, Pittet D. (2009) Role of hand hygiene in healthcare-associated infection prevention. Journal of Hospital Infection 73: 305–315. [DOI] [PubMed] [Google Scholar]
- Ansari SA, Springthorpe VS, Sattar SA, Tostowaryk W, Wells GA. (1991). Comparison of cloth, paper, and warm air drying in eliminating viruses and bacteria from washed hands. American Journal of Infection Control 19: 243–249. [DOI] [PubMed] [Google Scholar]
- Barros EM, Lemos M, Souto-Padrón T, Giambiagi-deMarval M. (2015) Phenotypic and genotypic characterization of biofilm formation in Staphylococcus haemolyticus. Current Microbiology 70: 829–834. [DOI] [PubMed] [Google Scholar]
- Best EL, Redway K. (2015) Comparison of different hand-drying methods: the potential for airborne microbe dispersal and contamination. Journal of Hospital Infection 89: 15–217. [DOI] [PubMed] [Google Scholar]
- Best EL, Parnell P, Wilcox MH. (2014). Microbiological comparison of hand-drying methods: the potential for contamination of the environment, user, and bystander. Journal of Hospital Infection 88: 99–206. [DOI] [PubMed] [Google Scholar]
- CDC (2002) Centers for Disease Prevention and Control. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Morbidity and Mortality Weekly Report (No. RR-16) 51: 1-48. [PubMed] [Google Scholar]
- Chatfield SL, DeBois K, Nolan R, Crawford H, Hallam JS. (2017) Hand hygiene among healthcare workers: A qualitative meta summary using the GRADE-CERQual process. Journal of Infection Prevention 18: 104–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis V, Cairncross S. (2003) Effect of washing hands with soap on diarrhoea risk in the community: a systematic review. The Lancet Infectious Diseases 3: 275–281. [DOI] [PubMed] [Google Scholar]
- Foddai AC, Grant IR, Dean M. (2016) Efficacy of instant hand sanitizers against foodborne pathogens compared with hand washing with soap and water in food preparation settings: A systematic review. Journal of Food Protection 79: 1040–1054. [DOI] [PubMed] [Google Scholar]
- Freeman MC, Stocks ME, Cumming O, Jeandron A, Higgins J, Wolf J, et al. (2014) Systematic review: hygiene and health: systematic review of handwashing practices worldwide and update of health effects. Tropical Medicine and International Health 19: 906–916. [DOI] [PubMed] [Google Scholar]
- Gustafson DR, Vetter EA, Larson DR, Ilstrup DM, Maker MD, Thompson RL, Cockerill FR, III. (2000) Effects of 4 hand-drying methods for removing bacteria from washed hands: a randomized trial. Mayo Clinic Proceedings 75: 705–708. [DOI] [PubMed] [Google Scholar]
- Hanna PJ, Richardson BJ, Marshall M. (1996) A comparison of the cleaning efficiency of three common hand drying methods. Applied Occuputional and Environmental Hygiene 11: 37–43. [Google Scholar]
- Huang C, Ma W, Stack S. (2012) The hygienic efficacy of different hand-drying methods: a review of the evidence. Mayo Clinic Proceedings 87: 791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INTERMETRA B and Market Research Group. (2011) Study of the consumers’ attitudes to different handdrying systems for European Tissue Symposium June 2008. Brussels: European Tissue Symposium. [Google Scholar]
- Joseph T, Baah K, Jahanfar A, Dubey B. (2015). A comparative life cycle assessment of conventional hand dryer and roll paper towel as hand drying methods. Science of the Total Environment 515: 109–117. [DOI] [PubMed] [Google Scholar]
- Kac G, Podglajen I, Gueneret M, Vaupre S, Bissery A, Meyer G. (2005) Microbiological evaluation of two hand hygiene procedures achieved by healthcare workers during routine patient care: a randomized study. Journal of Hospital Infection 60: 32–39. [DOI] [PubMed] [Google Scholar]
- Kimmitt PT, Redway KF. (2016) Evaluation of the potential for virus dispersal during hand drying: a comparison of three methods. Journal of Applied Microbiology 120: 478–486. [DOI] [PubMed] [Google Scholar]
- Loveday HP, Wilson J, Pratt RJ, Golsorkhi M, Tingle A, Bak A, et al. (2014). epic3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. Journal of Hospital Infection 86: S1-S70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margas E, Maguire E, Berland CR, Welander F, Holah JT. (2013) Assessment of the environmental microbiological cross contamination following hand drying with paper hand towels or an air blade dryer. Journal of Applied Microbiology 115: 572–582. [DOI] [PubMed] [Google Scholar]
- Patrick DR, Findon G, Miller TE. (1997) Residual moisture determines the level of touch-contact-associated bacterial transfer following hand washing. Epidemiology and Infection 119: 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet D, Boyce JM. (2001) Hand hygiene and patient care: pursuing the Semmelweis legacy. The Lancet Infectious Diseases 1: 9–20.11871420 [Google Scholar]
- Redway K, Fawdar S. (2008) A comparative study of three different hand drying methods: paper towel, warm air dryer, jet air dryer. Brussels: European Tissue Symposium. [Google Scholar]
- Snelling AM, Saville T, Stevens D, Beggs CB. (2011) Comparative evaluation of the hygienic efficacy of an ultra-rapid hand dryer vs conventional warm air hand dryers. Journal of Applied Microbiology 110: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacconelli E, Cataldo MA, Dancer SJ, Angelis G, Falcone M, Frank U, et al. (2014) ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clinical Microbiology and Infection 20(s1): 1–55. [DOI] [PubMed] [Google Scholar]
- Taylor JH, Brown KL, Toivenen J, Holah JT. (2000) A microbiological evaluation of warm air hand driers with respect to hand hygiene and the washroom environment. Journal of Applied Microbiology 89: 910–919. [DOI] [PubMed] [Google Scholar]
- Walterson AM, Stavrinides J. (2015) Pantoea: insights into a highly versatile and diverse genus within the Enterobacteriaceae. FEMS Microbiology Reviews 39: 968–984. [DOI] [PubMed] [Google Scholar]
- Warren-Gash C, Fragaszy E, Hayward AC. (2013) Hand hygiene to reduce community transmission of influenza and acute respiratory tract infection: a systematic review. Influenza and Other Respiratory Viruses 7: 738–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2009) World Health Organisation. Guidelines on Hand Hygiene in Healthcare. Geneva: World Health Organisation. [Google Scholar]