Abstract
Background
Lung cancer following lung transplantation (LT) may require thoracic surgery (TS). There is an urgent need for data on surgical feasibility, clinical and surgical characteristics, as well as outcome data.
Methods
We reviewed the medical records of LT patients who had undergone TS at the University Hospital Leipzig between the years 2000 and 2022. Data on medical and surgical history, pulmonary function test, arterial blood gas analysis, six-minute walking distance test, and surgical approach, perioperative management, anesthesiologic, and surgical procedures were analyzed.
Results
Among 248 LT patients, 13 patients (5.2%) developed lung cancer after 4.2 years on average and on 6 of them (46.2%), major TS procedure was performed for the resection of lung cancer. In one patient who underwent TS for a suspicious pulmonary nodule, it turned out to be a parenchymal scar. TS was carried out in 57.1% on the native lung and 42.9% on the transplant lung. Pneumonia and acute renal failure were predominantly observed postoperative complications. We found that the capacity of gas exchange either before or after TS was related to the degree of postoperative complications. The in-hospital survival was 71.4%.
Conclusions
Incidence of lung cancer is increased after LT. Follow-up care allows early diagnosis with a comparably high share of operable tumor stage. Cancer as well as postoperative complications were more likely after single lung transplantation (SLT). Postoperative morbidity and mortality are higher in this scarce group of patients and hence, warrants a centered and experienced interdisciplinary approach.
Keywords: Lung transplantation (LT), lung cancer, thoracic surgery (TS), post-transplant, surgical outcome
Highlight box.
Key findings
• Lung cancer is the prevalent indication for major thoracic surgery (TS) following lung transplantation (LT).
• Oncological intended TS is feasible following LT but carries a higher risk for postoperative complications.
• Bilateral LT (BLT) is associated with a lower risk for lung cancer and postoperative complications when TS is necessary following LT.
What is known and what is new?
• Solid organ transplant recipients have a 3- to 4-fold increased risk to develop a malignancy in years thereafter. The rise of malignancy and infections is certainly predisposed by the chronic immunosuppression following LT. Existing data report that minor TS is feasible and safe following LT.
• We report that oncological intended major TS was feasible but was associated with a higher risk for postoperative complications. Notwithstanding the predicament of organ paucity, our data underline the existing knowledge of the superiority of BLT over single LT.
What is the implication, and what should change now?
• Multidisciplinary team should be aware of the increased risk for pneumonia and renal failure and a centered approach should be preferred whenever possible.
• Selection of donor organs is safe recognizing the small risk of cancer development in transplanted organs even during immunosuppression.
• Balancing the considerable postoperative complications observed (pneumonia, acute renal failure requiring dialysis, postoperative survival) and the potential benefits of TS in the context of lung cancer and breathing efficiency, our data encourage to expand the experience in the field of TS following LT.
Introduction
Lung transplantation (LT) is commonly performed for the indications of end stage lung diseases. Chronic obstructive pulmonary disease (COPD), idiopathic interstitial pneumonia, and cystic fibrosis (CF) cover over 70% of LT indications (1). The type of transplantation principally comprises single lung (SLT), double bilateral lung (BLT), and heart-lung transplantation (HLT). Type of transplantation depends on the organ donor offer, the donor and recipient size matching and other comorbidities. SLT is owed to the paucity of organ donor offer.
Thoracic surgery (TS) embodies the entirety of diagnostic and therapeutic interventions performed by surgeons on the thoracic cavity. Major TS typically includes anatomic resections such as segmentectomies, lobectomies or pneumonectomies. Following LT, certain pathologies will come up that may require surgical intervention on the transplanted or the native lung.
Solid organ transplant recipients have a 3- to 4-fold increased risk to develop a malignancy in years thereafter (2). The rise of malignancy and infections is certainly predisposed by the chronic immunosuppression following LT. In addition, following SLT, hyperinflation of the remaining emphysematous lung can compress the contralateral allograft.
Over the past 30 years, a growing body of literature describing the experience of TS performed on LT patients illustrates the need of data on surgical feasibility, clinical and surgical characteristics [e.g., thoracotomy vs. video-assisted thoracoscopic surgery (VATS)] as well as outcome data. It has already been shown that TS is feasible and useful in the workup of suspicious pathologies of the transplanted or remaining native lung (3,4). Since TS in LT patients is, nevertheless, still a rare procedure, there are predominantly single case reports or, at best, case series found in the literature.
The aim of this study was to critically evaluate our experience with TS in LT patients focusing on oncological-intended TS, the influence of TS on respiratory characteristics and surgery related complications. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1669/rc).
Methods
Study design and subject collection
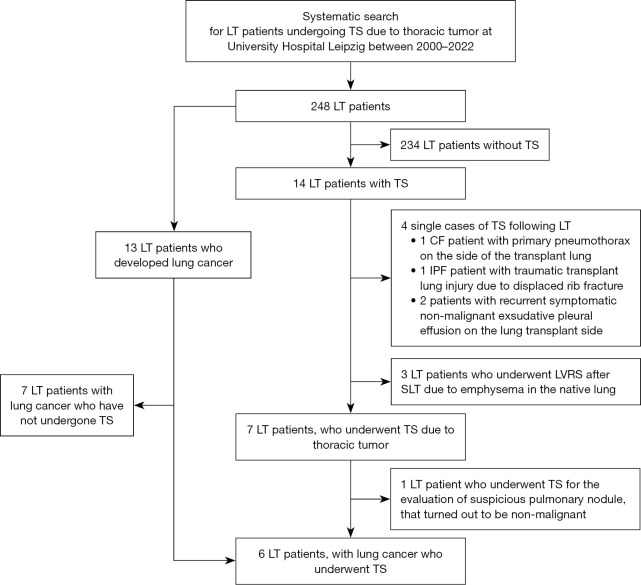
For this retrospective, single-center, observational study, electronic medical records of all LT patients at our center who had undergone TS between the years 2000 and 2022 were reviewed through the hospital’s clinical database (Figure 1). TS was defined as any surgical intervention that opened the thoracic cavity via open thoracotomy or VATS. LT patients were included in the final analysis whose TS procedures were directly related to the evaluation or treatment of thoracic tumor. The median follow-up via in-house outpatient transplant clinic time was 350 days [range, 11 to 2,976 days (approximately 8 years)].
Figure 1.
Flow chart of systematic search for patients who underwent TS following LT. LT, lung transplantation; TS, thoracic surgery; CF, cystic fibrosis; IPF idiopathic pulmonary fibrosis; LVRS, lung volume reduction surgery; SLT, single LT.
To compare the outcome of LT patients who underwent TS for lung cancer with lung cancer patients without LT, survival indices were collected from a matched retrospective cohort stemming from the same TS department which performed TS for lung cancer in LT patients. The matching comprised tumor stage and tumor histology. Survival was defined as the time between diagnosis of lung cancer and death.
Clinical assessment
We collected patient’s data on medical and surgical history including the time between LT and TS, the comorbidities, and present medication (e.g., immunosuppressive therapy). LT patients were regularly followed up through the LT outpatient clinic—usually every 3 months. LT patients underwent pulmonary function tests (PFT) including spirometry and body plethysmography, arterial blood gas (ABG) analysis, and exercise capacity tests such as six-minute walking distance (6MWD) test at every follow up visit; in particular before and after TS. The last measurement of PFT, ABG and 6MWD before TS and the first after TS were included in the analysis. The range and severity of comorbidities were classified and quantified according to the age-modified Charlson comorbidity index (5). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the local ethics committee at the Medical Faculty, University of Leipzig (IRB00001750, AZ 264/19-ek and AZ 259/18-ek). All participants consented to the usage of their personal data for research purposes by signing the hospital’s admission agreement.
Surgical procedures
TS was performed in the operating room under general anesthesia and comprised thoracotomy, according to clinical indication based on multidisciplinary team discussion (6). Data on preoperative management, anesthesiologic and surgical procedures including operative time and surgical techniques, and postoperative management were collected. All anatomic resections to resect lung cancer comprised systematic mediastinal lymphadenectomy for the establishment of a complete pathological TNM. Particular attention was paid to postoperative characteristics including duration of invasive ventilation, time of chest tube removal, surgical intensive care unit (SICU) and hospital length of stay as well as complications. Postoperative complications were assessed according to the Clavien-Dindo classification (7).
Pathological and oncologic assessment
Thoracic specimens collected by means of TS comprising the lung, pleura, pleural effusion, or lymph nodes underwent routine procedures of pathologic analysis. The local department of pathology performed histopathologic evaluation of the surgical specimen collected during TS. In case of confirmed thoracic tumor diagnosis, tumor cells were characterized, and tumor stage was established according to the 5th edition of the World Health Organization classification (8), the 8th edition of TNM classification of malignant tumors (9).
We added to the result section survival rates from our local lung cancer center stemming from an in-hospital lung cancer registry. We extracted lung cancer patient’s demographics, pathologic tumor stage, histology, and survival (1-year survival rate and median overall survival).
Statistical analysis
Data were compiled and arranged according to the clinical groups. Descriptive statistics were used to describe characteristics and outcome parameters of LT patients. Continuous variables are expressed as mean or median with range (minimum to maximum), as indicated. Differences between clinical data that derived from two time points of one LT patient were assessed by paired t-test or Wilcoxon matched pairs signed rank test, depending on data distribution. Categorical variables are shown as counts and proportions (i.e., percentages). Statistically significant difference was accepted at a level of a two-sided P<0.05. Data management including compilation, analysis, and preparation of figures was conducted using the software package GraphPad Prism (v9.0.2 for macOS, La Jolla, CA, USA).
Results
Clinical characteristics of LT patients who underwent TS
Between the years 2000 and 2022, 248 patients (93 women, 37.5%) underwent LT, of whom 166 patients (66.9%) received BLT, 74 patients (29.8%) SLT, and 8 patients (3.2%) HLT. Among them, 14 (5.6%) patients (3 women, 21.4%) underwent TS for different indications (Figure 1): 7 (2.8%) LT patients (1 woman, 14.3%) received TS for the evaluation or treatment of lung cancer, while in 6 of them lung cancer diagnosis was confirmed. In 7 other cases, TS was unrelated to the evaluation of thoracic tumor disease: spontaneous pneumothorax on the side of the transplant lung in 1 patient with CF, traumatic transplant lung injury due to displaced rib fracture (n=1), recurrent symptomatic exudative pleural effusion on the side of the transplant lung (n=2), as well as lung volume reduction surgery (LVRS) due to progressive emphysema of the native lung that compressed the allograft (n=3).
Among the 248 LT patients, 13 LT patients (5.2%) have developed lung cancer after 4.2 years on average (range, 0.4 to 8.3 years), during the observation period. TS was performed in curative intent in 46.2% (6/13) of all lung cancer patients. In the remaining 7 LT patients, lung cancer was already advanced or metastasized qualifying primarily for systemic therapy.
Table 1 and Figure 2 summarize the cohort’s clinical characteristics. Only 14.3% of LT patients who underwent TS was female (1/7) whereas almost two thirds was female in the entire LT cohort (155/248, 62.5%). Five (71.4%) patients received SLT and 2 (28.6%) BLT. LT procedures in patients with COPD (3/7, 42.9%) included 1 SLT and 2 BLT. All patients with idiopathic pulmonary fibrosis (IPF) received SLT (4/7, 57.1%). In all SLT cases who underwent TS for the evaluation of lung cancer, surgical procedure was performed on the native lung.
Table 1. Clinical characteristics of LT patients who underwent TS for lung cancer resection.
| Characteristic | Value |
|---|---|
| Participants | 7 (100.0) |
| Sex, male/female | 6 (85.7)/1 (14.3) |
| Body mass index, kg/m2 | 23.3 (19.7; 30.3) |
| Indication for LT | |
| COPD | 3 (42.9) |
| SLT/BLT | 1 (33.3)/2 (66.6) |
| IPF | 4 (57.1) |
| SLT/BLT | 4 (100.0)/0 |
| Transplant procedure | |
| SLT | 5 (71.4) |
| BLT | 2 (28.6) |
| Age at LT, years | 56.6 (46.0; 66.0) |
| Age at TS, years | 64.0 (54.0; 67.0) |
| Time from LT to TS (overall), years | 5.7 (1.5; 9.3) |
| Site of TS | |
| Native lung | 4 (57.1) |
| Transplant lung | 3 (42.9) |
| Smoking status prior to LT | |
| Never smoker | 1 (14.3) |
| Former smoker | 6 (85.7) |
| Pack years | 20 (5; 45) |
| Immunosuppressive therapy | |
| Prednisolone + MMF | |
| + tacrolimus | 4 (57.1) |
| + ciclosporin | 3 (42.9) |
| Comorbidities | |
| Charlson comorbidity index | 10 (7; 14) |
| CLAD prior to TS | 5 (71.4) |
| Arterial hypertension | 5 (71.4) |
| CAD | 2 (28.6) |
| Chronic renal failure | 3 (42.9) |
| Hyperlipoproteinemia | 5 (71.4) |
| Diabetes mellitus | 3 (42.9) |
Data are shown as number with percentage or median (minimum; maximum). LT, lung transplantation; TS, thoracic surgery following transplantation; COPD, chronic obstructive pulmonary disease; SLT, single LT; BLT, double LT; IPF, idiopathic pulmonary fibrosis; MMF, mycophenolate; CLAD, chronic lung allograft dysfunction; CAD, coronary artery disease.
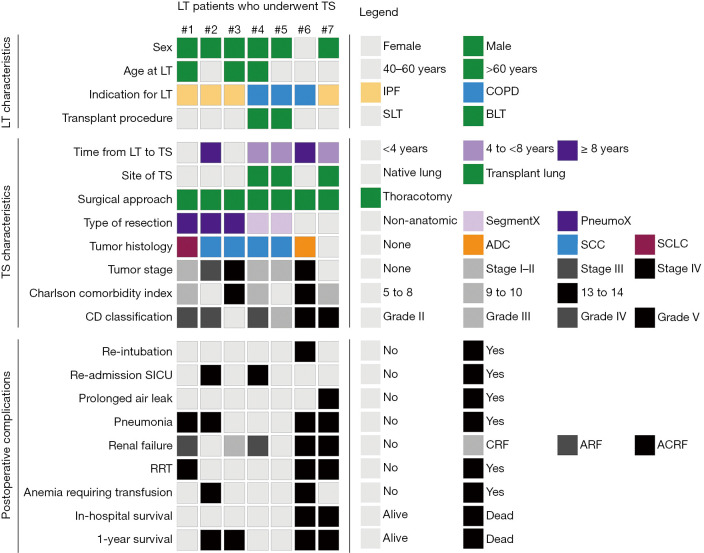
Figure 2.
Schematic overview of clinical and surgical characteristics, postoperative complications, and outcome parameters. Each column represents one LT patient who underwent TS: patients #1 to #7 were evaluated for lung cancer. Each row represents a certain characteristic. The legend of the right side of the panel defines the meaning of color and shading regarding the squares on left side. #, LT patients identifier; LT, lung transplantation; TS, thoracic surgery; IPF, idiopathic pulmonary fibrosis; COPD, chronic obstructive pulmonary disease; SLT, single LT; BLT, double LT; SegmentX, segmentectomy; PneumoX, pneumonectomy; ADC, adenocarcinoma; SCC, squamous cell carcinoma; SCLC, small cell lung cancer; CD, Clavien-Dindo; SICU, surgical intensive care unit; CRF, chronic renal failure without acute deterioration; ARF, acute renal failure developed from normal renal function; ACRF, acute on chronic renal failure; RRT, renal replacement therapy (dialysis).
The median age at the time of LT and TS was 56.6 and 64.0 years, respectively, leading to a median time difference of 5.7 years (range, 1.5 to 9.3 years).
Immunosuppressive therapy following LT consisted of prednisolone and mycophenolate, together with tacrolimus (57.1%), or with ciclosporin (42.9%). For the perioperative management of TS, mycophenolate was discontinued until wound healing was completed. Prednisolone and tacrolimus or ciclosporine were continued perioperatively. Following the diagnosis of lung cancer in LT patient #2, the inhibitor of mechanistic target of rapamycin (mTOR), everolimus, was added to the immunosuppressive therapy. For the other 5 LT patients, postoperative immunosuppression remained unchanged compared to the preoperative immunosuppression (Table 1).
In 5/7 LT patients, bronchiolitis obliterans syndrome (BOS) was diagnosed prior to TS (Table 1). In Table S1, we assigned BOS to the respective LT patient and listed the established treatment regimens (e.g., azithromycin thrice a week, pulse corticosteroid therapy, or extracorporeal photopheresis). Patient #2 suffered from BOS grade 3, for which he was treated with azithromycin thrice a week, pulse corticosteroid therapy, and regular extracorporeal photopheresis. He deceased within the first year following TS for lung cancer.
Surgical characteristics of LT patients
Regarding the 240 patients who underwent BLT or SLT, oncological intended TS was performed in 1.2% (2/166) and 5.4% (4/74) of BLT or SLT patients, respectively.
Surgical characteristics including the distribution of surgical indications and procedures among LT patients are summarized in Tables 2,3 and in Figure 2. All surgical indications were approved by the institutional multidisciplinary thoracic tumor board and with informed consent of all patients. The surgical approach and the type of resection critically depended on the surgical indication.
Table 2. Procedures of TS in LT patients.
| Characteristic | Total | Site of TS | |
|---|---|---|---|
| Native lung | Transplant lung | ||
| Indication for LT | |||
| COPD | 3 (42.9) | 1 | 2 |
| IPF | 4 (57.1) | 3 | 1 |
| Surgical approach | |||
| Thoracotomy | 7 (100.0) | 4 | 3 |
| Time from LT to TS, years | 5.7 (4.6; 7.0) | 5.7 (1.5; 9.3) | 5.7 (4.6; 7.0) |
| Type of resection | |||
| Anatomic | 5 (71.4) | 3 | 2 |
| Segmentectomy | 2 (40.0) | 0 | 2 |
| Lobectomy | 0 (0.0) | 0 | 0 |
| Pneumonectomy | 3 (60.0) | 3 | 0 |
| Non-anatomic | 2 (28.6) | 1 | 1 |
Data are shown as number, number with percentage, or median (minimum; maximum). Percentages may not add up to 100% due to rounding. TS, thoracic surgery; LT, lung transplantation; COPD, chronic obstructive pulmonary disease; IPF, idiopathic pulmonary fibrosis.
Table 3. Surgical characteristics and complications.
| Characteristic | Value |
|---|---|
| Subjects evaluated or treated for lung cancer | 7 (100.0) |
| Operative time, min | 187 (59; 246) |
| Anatomic resection | 208 (149; 246) |
| Non-anatomic resection | 75 (59; 90) |
| Postoperative characteristics | |
| Duration of invasive ventilation, days | 0 (0; 3) |
| Time to chest tube removal, days | 5 (2; 7) |
| On site of native lung | 3 (2; 6) |
| On site of transplant lung | 6 (5; 7) |
| SICU length of stay, days | 4 (0; 30) |
| Hospital length of staya, days | 19 (10; 43) |
| Complications | |
| Re-intubation | 1 (14.3) |
| Re-admission to SICU | 2 (28.6) |
| SICU re-admission length of stay, days | 3 (1; 5) |
| Pneumonia | 4 (57.1) |
| Prolonged air leak (>7 days) | 1 (14.3) |
| Acute renal failure | 4 (57.1) |
| Requiring RRT | 3 (75.0) |
| Anaemia requiring transfusion | 2 (28.6) |
| Urinary tract infection | 2 (28.6) |
| CDI | 1 (14.3) |
| VTE or PE | 0 |
| Wound infection | 0 |
| Pleural empyema | 0 |
| Revision surgery | 0 |
| Clavien-Dindo classification | |
| Grade I | 0 |
| Grade II | 1 (14.3) |
| Grade III | 1 (14.3) |
| Grade IV | 3 (42.9) |
| Grade V | 2 (28.6) |
| In-hospital survival | 5 (71.4) |
| 1-year survival | 3 (42.9) |
Data are shown as number with percentage or median (minimum; maximum). a, defined as time from surgery to discharge. SICU, surgical intensive care unit; RRT, renal replacement therapy (dialysis); CDI, Clostridium difficile infection with pseudomembranous colitis; VTE, venous thromboembolism; PE, pulmonary embolism.
Overall, thoracotomy was performed in all cases, while in this series VATS was never applied. Lung resection was performed in all cases, of which 71.4% (5/7) were anatomic and 28.6% (2/7) non-anatomic resections. Pneumonectomy was required in 42.9% (3/7) of resections and was exclusively performed on the native lung following SLT.
Pathologic characteristics of LT patients
Table 4 and Figure 2 summarize the oncologic characteristics of the 7 LT patients who underwent TS for the diagnostic or therapy of lung cancer. Among them, lung cancer diagnosis was confirmed in 85.7% (6/7) of the cases. In case #7 (Figure 2), a suspicious solitary pulmonary nodule located in the transplant lung showed increased fluorodeoxyglucose uptake by means of positron emission tomography (PET) suggesting malignancy. The histologic results of computed tomography (CT)-guided biopsy during the preoperative work-up remained inconclusive. Due to high pretest-probability for malignancy, multidisciplinary team argues in support of surgical resection. Postoperative histopathological examination revealed a parenchymal scar tissue most probably due to preceding pulmonary embolism. Unfortunately, this patient died in the aftermath of surgery due to pneumonia and renal failure.
Table 4. Pathologic characteristics of LT patients following TS.
| Characteristic | Total | SCC | ADC | SCLC | Scar tissuea |
|---|---|---|---|---|---|
| Subjects | 7 (100.0) | 4 (57.1) | 1 (14.3) | 1 (14.3) | 1 (14.3) |
| Site of TS | |||||
| Native | 4 (57.1) | 2 (IPF) | 1 (COPD) | 1 (IPF) | 0 |
| Transplant | 3 (42.9) | 2 (COPD) | 0 | 0 | 1 (IPF) |
| Type of resection | |||||
| Pneumonectomy | 3 (42.9) | 2 (IPF) | 0 | 1 | 0 |
| Lobectomy | 0 | 0 | 0 | 0 | 0 |
| Segmentectomy | 2 (28.6) | 2 (COPD) | 0 | 0 | 0 |
| Non-anatomic | 2 (28.6) | 0 | 1 | 0 | 1 |
| Pathologic TNM stage | |||||
| IA | 2 (28.6) | 1 | 0 | 1 | 0 |
| IIB | 1 (14.3) | 1 | 0 | 0 | 0 |
| IIIB | 1 (14.3) | 1 | 0 | 0 | 0 |
| IVA | 2 (28.6) | 1 | 1 | 0 | 0 |
| Survival | |||||
| In-hospital | 5 (71.4) | 4 | 0 | 1 | 0 |
| 1-year | 3 (42.9) | 2 | 1 | 0 | 0 |
All lung resections to evaluate lung cancer were performed using thoracotomy. Data are shown as numbers with percentages. Lung diseases for which LT was performed are shown in parenthesis. Percentages may not add up to 100% due to rounding. a, pulmonary infarction probably due to pulmonary embolism. LT, lung transplantation; TS, thoracic surgery; SCC, squamous cell cancer; ADC, adenocarcinoma; SCLC, small cell lung cancer; IPF, idiopathic pulmonary fibrosis; COPD, chronic obstructive pulmonary disease.
Pulmonary squamous cell carcinoma (SCC) was the most prevalent histologic type in this series, in whom we found lung cancer (4/6, 67%). Two of them (#2 and #3) were diagnosed in the native lung of IPF patients at pathologic stage IIIB (pT3pN2) and IVA [pT4pN1pM1 (pulmonary lymphangitic carcinomatosis, PLE)], respectively, and required pneumonectomy. The 2 cases of SCC (#4 and #5) occurring in the transplant lung were found at pathologic stage IA3, which was removed by lingula resection, and at stage IIB (pT3pN0), which required an S1 segmentectomy plus S2 wedge resection. Pulmonary adenocarcinoma (ADC) was found only in the native lung of a COPD patient (#6). Pulmonary small cell lung cancer (SCLC) was found in the native lung of an IPF patient (#1) He received neoadjuvant platinum-based chemotherapy prior to TS.
Regarding the remaining native lung as the origin of lung cancer, two cases of SCC and one case of SCLC developed in the IPF lung. All three were curatively resected through pneumonectomy. SCC located in the transplant lung of two patients who were transplanted for COPD underwent segmentectomy. The final pathologic stage ranged between IA2 and IVA. Of note, 3 of 6 lung cancer patients following LT showed postoperatively an unforeseen locally advanced or metastasized pathologic tumor stage (IIIB to IVA). Those three cases require further consideration:
Male patient #2 (55-year-old, former occasional smoker) developed a SCC in the right upper lobe of the remaining native lung 9.3 years after SLT due to IPF. Staging included PET/CT (cT3cN0cM0, stage IIB). Everolimus was added to immunosuppressive therapy. The fibrotic lung parenchyma of the tumor bearing native lung did only contribute 5% of lung perfusion (quantitative ventilation-perfusion scintigraphy). Pneumonectomy was recommended. Pathologic tumor stage revealed an unforeseen mediastinal lymph node involvement leading to an advanced stage IIIB (pT3pN2cM0). The patient was postoperatively unfit for adjuvant systemic therapy (chemotherapy), which was critically discussed in the context of immunosuppresion following LT. Patient died at home of unknown reasons 146 days after TS.
Male patient #3 (67-year-old, former smoker, 45 pack years) developed a centrally located SCC in the left main bronchus of the remaining native lung 1.5 years after SLT due to IPF. Preoperative workup including PET/CT measured a clinical stage IIB (cT2bcN0cM0) and multidisciplinary team discussion suggested pneumonectomy as procedure of choice due to tumor’s size and location. Histopathologic examination reported a 5.5 cm large tumor mass that showed local invasion of the mediastinum, the great venous vessels and the left atrial wall resulting in a R1 situation. In the end, a single metastasis of the visceral pleura was identified leading to an unforeseen pathologic stage IVA (PLE, pT4pN1pM1a). After sequential chemoradiotherapy, the patients remained free of relapse until death 10 months (293 days) after TS due to septic multiorgan failure with cytomegalovirus and aspergillus pneumonia.
Female patient #6 (54-year-old, former smoker, 30 pack years) presented with unclear exudative hemorrhagic pleural effusion around the native lung 8.3 years after SLT due to COPD. Open-surgical evaluation showed altered visceral pleural tissue without evidence of intrapulmonary tumor. Histopathologic analysis of a wedge resection revealed poorly differentiated pulmonary ADC with pleural metastasis. She died of multiorgan failure due to pneumonia 11 days after TS.
Influence of TS on respiratory characteristics in LT patients
In LT patients undergoing lung resection for lung cancer (n=7), TS led to a reduction of the forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), residual volume (RV), and oxygenation index [arterial oxygen partial pressure (PaO2)/inspiratory oxygen fraction (FiO2)] and an increase of the arterial carbon dioxide tension (PaCO2). However, diffusion capacity for carbon monoxide (DLCO) and arterial oxygen saturation (SaO2) remained unchanged following TS (Figure 3, Table S2).
Figure 3.
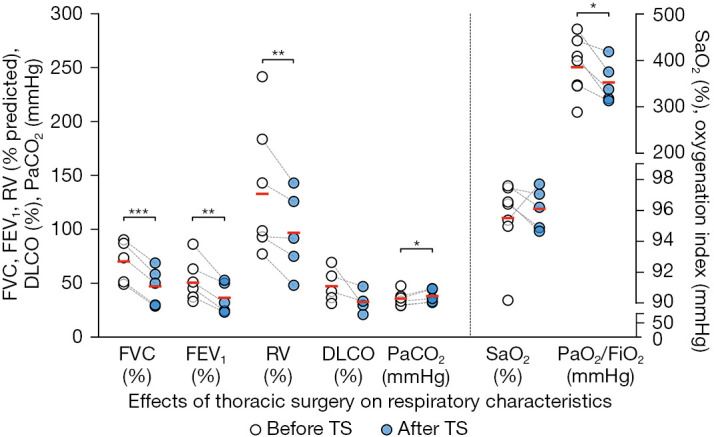
Effects of thoracic surgery on respiratory characteristics. Before-after plot of LT patients who underwent subsequent TS for suspicious tumor showing the respiratory characteristics before and after TS of each patient by means of dashed lines. The red horizontal line indicates the mean. The vertical dashed line divides the graph into two groups of plots according to the axis they referring to. PaO2/FiO2 signifies oxygenation index. Statistical analyses were performed using paired t-test or Wilcoxon matched pairs signed rank test in function of distribution (*, P<0.05; **, P<0.01; ***, P<0.001). Statistically not significantly different comparisons are not labelled. FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; RV, residual volume; DLCO, diffusion capacity for carbon monoxide after single breath; PaCO2, arterial carbon dioxide tension; SaO2, arterial oxygen saturation; PaO2, arterial oxygen partial pressure; FiO2, inspiratory oxygen fraction; LT, lung transplantation; TS, thoracic surgery.
The median preoperative, postoperative, and perioperative times between PFT, ABG and 6MWD testing and TS were 25, 17, or 33 days, respectively. LT patients were regularly seen in the outpatient clinic. We further investigated the course of PFT of each study participant. Individual respiratory characteristics of 4 LT patients following TS are shown in Table S1. For patients #6 and #7, no postoperative PFT was available since they were in no condition for PFT and died during the hospital stay following TS. Patient #2 underwent PFT once after TS but has not been able to show up for further clinical follow up visits in the outpatient clinic.
For the data available, all five patients underwent PFT within the first 3 months following TS (0.3 to 2.3 months). The last PFT was performed for two individuals within the first postoperative year (11.0 and 7.3 months) and for another two individuals more than 5 years after TS (61.9 and 73.1 months). PFT parameters of the short-term group (#1 and 3) who underwent pneumonectomy showed the following development: FVC: −0.19 and −0.06 L; FEV1: −0.16 and +0.30 L; total lung capacity (TLC): +1.3% and −4.1%; RV: −9.6% and +16.1%; and DLCO/VA (alveolar volume): −15.6%. For those LT patients following pneumonectomy, dynamic and static lung volumes showed rather decreased relative values.
PFT parameters of the long-term group (#4 and #5) who underwent segmentectomy showed the following development: FVC: +0.61 and −0.26 L; FEV1: +0.56 and −0.38 L; TLC: −7.9% and −21.0%; RV: −17.3% and −36.0%; and DLCO/VA: +14.8% and +19.5%. For those LT patients following segmentectomy, static lung volumes and CO diffusion seemed to improve (normalized RV, TLC, DLCO/VA).
Postoperative characteristics, complications, and survival
Postoperative characteristics and surgery-related complications following TS in LT patients are shown in Table 3, Figures 2,4.
Figure 4.
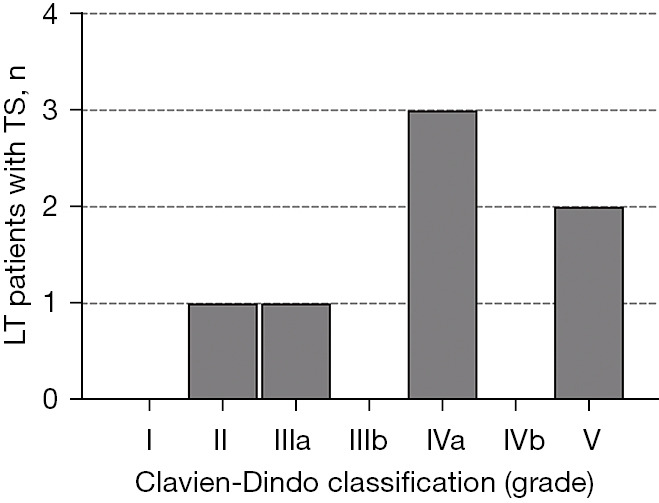
Postoperative complications in relation to respiratory characteristics. Histogram of complications following TS according to the Clavien-Dindo classification, of which one who died did not have lung cancer. LT, lung transplantation; TS, thoracic surgery.
The median time from surgery to chest tube removal was 5 days (range, 2 to 7 days) and the median length of SICU stay was 4 days (range, 0 to 30 days) leading to a median length of hospital stay of 19 days (range, 10 to 43 days). TS was carried out on the native or transplant lung in 4 and 3 cases, respectively. Prolonged air leak was observed in 14% (1/7) of TS. Re-intubation was required in 1 case (14%) and re-admission to SICU in 2 cases (30%). No revision surgery was required.
The Clavien-Dindo classification categorizes the postoperative complications (Table 3, Figures 2,4). The most prevalent and relevant clinical postoperative complications were pneumonia and acute renal failure (4, 57.1%, respectively). In 3 of 4 cases (75%), pneumonia occurred in the native lung. Renal replacement therapy (dialysis) was required in 75% (3/4) of the LT patients following an acute onset of renal failure, which started from a chronic renal failure in two cases and from a normal renal function in one case. Moreover, two patients showed a chronically reduced glomerular filtration rate without deterioration following the TS (reviewed in Figure 2). There were no reports on venous thromboembolism, pulmonary embolism, wound infections, or pleural empyema.
To estimate the effect of transplantation on TS in patients who were surgically treated for lung cancer, data on survival were compared to a matched retrospective lung cancer cohort stemming from the same thoracic surgery department. Of 2,856 lung cancer patients, 83 lung cancer patients matched to tumor stage (IA, IIB, IIIB and IVA) and histopathology (SCLC, SCC, ADC) of those lung cancer patients who had underwent prior LT (Table S3). The 1-year survival rate of LT patients who were diagnosed with lung cancer was 50% (3/6) with a median overall survival of 17.6 months, while the 1-year survival rate in the match register cohort was 53% (44/83) with a median overall survival of 13.9 months. Taken together, given the limitation of the small sample size, 1-year survival rate (50% vs. 53%) and median overall survival (17.6 vs. 13.9 months) between LT cohort with lung cancer and the historical lung cancer cohort were comparable.
Discussion
We aimed to report on our single-center experience of oncological intended TS in the unique cohort of LT patients over a period of 20 years. Among 248 LT patients, we found lung cancer in 13 LT patients (5.2%). TS procedure was performed in 46.2% (6/13) of all lung cancer patients. Among all TS procedures, TS for the evaluation or treatment of lung cancer made up the largest part (7/14). Our results indicate that the diffusion capacity (DLCO) and SaO2 remained unchanged following TS, whereas static and dynamic lung volumes (FEV1, FVC, RV) as well as the oxygenation index decreased. Acute renal failure (newly onset or acute on chronic), which led in 75% into renal replacement therapy (dialysis), and postoperative pneumonia (57%) were the most prevalent and relevant complications following TS in LT patients.
Studies to TS in LT are scarce and of heterogenic designs. In one retrospective cohort of 340 heart transplantation or HLT patients, 21 cardiothoracic procedures were carried out (6.2%), of which 6 were TS (10): TS for lung cancer represented half of the TS indications (n=3), while the other were pneumonia (n=2) and lung torsion (n=1). In another heterogenic study, 10% (45/442) of LT or HLT patients underwent TS, of which 80% were open lung biopsies and only 20% VATS (11), not representing the questions of major thoracic surgery in LT.
In our present study, 7 LT patients underwent TS for the evaluation or treatment of lung cancer, of which 1 case turned out not to be lung cancer, leading to an overall proportion of 2.4% (6/248) of all LT patients at our center. We found a higher proportion of TS procedures for lung cancer following SLT (5.4%) than for lung cancer following BLT (1.8%). In line with this observation, results from a retrospective analysis and a recent review suggested that the prevalence of lung cancer found in the native lung following SLT is considerably higher (approximately 2.8%; range, 0.4% to 8.9%) than the prevalence of lung cancer found in the transplant lung (0.3% to 0.4%) (12,13). These findings were also found in a study from Pennsylvania analyzing a cohort of 905 patients (14). In the total LT population at our center, we found lung cancer in 13 LT patients, while 6 of them underwent TS (46.2%). The one-year survival rate of patients who developed lung cancer following LT, as reported in this study, was comparable to that of lung cancer patients without prior LT, as reported by the National Cancer Institute (42.9% vs. 44.2%) (15). Current state of literature indicates that LT prior to lung cancer diagnosis affects overall survival in comparison to lung cancer patients without prior LT (16). But in general, the prognosis of LT patients who were found to have lung cancer tends to be rather poor ranging from 50% of LT patients who survived at 1 year to 25% at 5 years from time of lung cancer diagnosis (12).
Even though TS is described in LT patients, TS was performed on the transplant lung in 3 (42.9%) patients: TS revealed pulmonary SCC in two cases and one suspect nodule, which turned out to be scar tissue (probably due to an unrecognized pulmonary embolism turned into an organized infarct). In 4 of 7 cases presented in this study, the thoracic cavity had never been surgically approached before. A surgically unapproached thorax may not involve the same intraoperative conditions, such as pleural adhesions compared to a re-opened thorax. In these cases, operative approach may have been minimally invasive instead of thoracotomy as well.
Reason for LT was equally distributed in our cohort of oncological intended TS following LT (3/6 COPD, 3/6 IPF). This is contradictory to the results of Fernández et al., who reported lung cancer following LT only in COPD patients in a cohort of 161 LT patients (17). There is solid evidence that interstitial lung diseases, such as IPF, increase the risk for the development of lung cancer (18): For instance, the 10-year cumulative incidence of lung cancer in a cohort of 103 IPF patients was 55% (19), while in another cohort the risk for lung cancer was increased 7-fold in IPF patients compared to those without IPF (20).
Our data show a higher probability for lung cancer development in SLT than BLT as well as a higher complication rate for TS following SLT. It seems reasonable to infer that the persistence of the native lung in SLT patients still pose a variety of risk factors itself potentially leading to a worse outcome of SLT patients: i.e., development of malignant thoracic tumors (12) and infections (21). Following SLT, it was generally reported that postoperative complications in the native lung occurred in 50% of SLT patients leading to a mortality of 25% (21): of those complications, invasive aspergillosis (16%), bacterial pneumonia (5%), and hemothorax (5%) were found early after SLT (within 6 weeks from transplant), whereas recurrent pneumothorax (21%), progressive hyperinflation of the emphysematous native lung with functional deterioration (11%), and pulmonary nocardiosis (5%) came under late complications. More precisely, bacterial or fungal native airway colonization [e.g., mycobacteria species (spp.), pseudomonas spp., aspergillus spp.] acquired over the time of chronic lung disease, which may, together with the immunosuppressive therapy, be more likely paralleled by postoperative complications following TS such as pneumonia, respiratory failure, sepsis, or organ rejection (22). To this end, immunosuppression in SLT patients increases the risk of such postoperative complications.
It is remarkable, that the development of cancer is rather unlikely in transplanted organs (2/166 patients that received BLT, 1.2%) in comparison to native lung following LT (4/74 patients that received SLT, 5.4%). It is plausible that the native lung remains pre-injured and hence has a higher risk of complications as described above. Yet beyond that, one can subsume that selection of donor organs seems to be trustworthy and safe facing that immunosuppression would lead to progression of unrecognized precancerous lesions.
Postoperative complications are categorized by the Clavien-Dindo classification (7) and were reported low grade (grade II and III) in 50% of all TS cases. We found that postoperative pneumonia (50%) and acute renal failure (40%) were the most prevalent complications contributing to a median length of SICU stay of 2 days (range, 0 to 30 days). Complications in general following TS of LT patients were reported to occur in 6% (3) to 12% (23).
Prolonged air leak (>7 days) occurred in 14% (1/7) of LT patients, whereas others observed a rate of 16.7% (4/24) (24) or even of 6% (3/48) (3) following minor surgical procedures, such as open lung biopsy. Moreover, the median time to chest tube removal was 5 days and the length of stay in hospital averaged 19 days. Depending on the indication for TS in LT patients (e.g., lung cancer, LVRS, pulmonary infections, allograft rejection), the pooled median length of hospital stay for LT patients undergoing open lung biopsy is reportedly 15 days (n=58) (24,25) and 14 days for LT patients undergoing LVRS (n=25) (26-28).
In the oncological intended TS cohort, postoperative pneumonia developed in 57% (4/7), of which 75% (3/4) were found in SLT patients, mainly in IPF patients (75%). According to the literature, less than half of patients who were found to have a malignancy following LT have undergone surgery (29), which is why data on postoperative outcome in lung cancer following LT is rather scarce. In here, two LT patients undergoing TS for lung cancer died during the hospital stay and two more within the first postoperative year. The latter two death cases were unrelated to the TS but were presumably due to relapse or progression of lung cancer disease. Compared to a historical lung cancer cohort, we found comparable survival rate between lung cancer patients following LT and those without LT. Adjuvant systemic therapy following lung cancer surgery has to be discussed individually in the context of immunosuppression and LT patient’s eligibility.
TS in LT patients involves postoperative complications including redo thoracotomy and its attendant problems such as bleeding adhesions, prolonged air leak, and prolonged intensive care unit stay (30). However, appropriate preoperative multidisciplinary team discussion as foreseen for every lung cancer patients (6), strict selection of patients eligible for TS following LT, and structured postoperative care may contribute to acceptable or improved survival rates.
This observational study aimed to provide an overview of a relevant surgical indication for and complications of oncological intended TS in LT patients. Nevertheless, it discloses certain limitations: The strength of evidence is hampered by the limited sample size, which only allows for cautious generation of hypotheses, given the retrospective nature of this study.
Conclusions
We present descriptive results on TS in LT patients covering two decades of a single-center experience. Lung cancer was the most prevalent indication for major TS (7/14, 50%). Balancing the considerable postoperative complications observed (pneumonia, acute renal failure requiring dialysis, postoperative survival) and the potential benefits of TS in the context of lung cancer and breathing efficiency, our data encourage to expand the experience in the field of TS following LT. Oncological intended TS was feasible following LT but carries a higher risk for postoperative complications. BLT was associated with a lower risk for lung cancer and postoperative complications when TS was necessary following LT. Notwithstanding the predicament of organ paucity, our data underline the existing knowledge of the superiority of BLT over SLT. Facing the low share of cancer developed in transplanted organs, one can subsume that selection of donor organs was safe.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This publication was funded by the Open Access Publishing Fund of Leipzig University and supported by the German Research Foundation within the program Open Access Publication Funding.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the local ethics committee at the Medical Faculty, University of Leipzig (IRB00001750, AZ 264/19-ek and AZ 259/18-ek). All participants consented to the usage of their personal data for research purposes by signing the hospital’s admission agreement.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1669/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1669/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1669/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1669/coif). A.F. was supported by the postdoctoral fellowship “MetaRot program” from the Federal Ministry of Education and Research (BMBF), Germany (FKZ 01EO1501, IFB Adiposity Diseases), a research grant from the “Mitteldeutsche Gesellschaft für Pneumologie (MDGP) e.V.” (2018-MDGP-PA-002), a junior research grant from the Medical Faculty, University of Leipzig (934100-012), a graduate fellowship of the “Novartis-Stiftung für therapeutische Forschung” (Novartis Foundation), and the “LuCaPET” project (ERAPerMed_324), which was funded with tax funds on the basis of the budget passed by the Saxon State Parliament (Germany) under the frame of ERA PerMed (Horizon 2020). A.F. reports that this publication was funded by the Open Access Publishing Fund of Leipzig University and supported by the German Research Foundation within the program Open Access Publication Funding. The other authors have no conflicts of interest to declare.
References
- 1.Perch M, Hayes D, Jr, Cherikh WS, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-ninth adult lung transplantation report-2022; focus on lung transplant recipients with chronic obstructive pulmonary disease. J Heart Lung Transplant 2022;41:1335-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Raemdonck D, Vos R, Yserbyt J, et al. Lung cancer: a rare indication for, but frequent complication after lung transplantation. J Thorac Dis 2016;8:S915-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weill D, McGiffin DC, Zorn GL, Jr, et al. The utility of open lung biopsy following lung transplantation. J Heart Lung Transplant 2000;19:852-7. [DOI] [PubMed] [Google Scholar]
- 4.Decaluwe H, Van Raemdonck D, Verleden G, et al. Thoracoscopic lobectomy after bilateral lung transplantation. Interact Cardiovasc Thorac Surg 2014;19:515-7. 10.1093/icvts/ivu144 [DOI] [PubMed] [Google Scholar]
- 5.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 6.Hardavella G, Frille A, Theochari C, et al. Multidisciplinary care models for patients with lung cancer. Breathe (Sheff) 2020;16:200076. 10.1183/20734735.0076-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery 1992;111:518-26. [PubMed] [Google Scholar]
- 8.WHO Classification of Tumours Editorial Board. Thoracic tumours. 5th edition ed. WHO classification of tumours series, vol 5. Geneva (Switzerland): International Agency for Research on Cancer. WHO Press; 2021. [Google Scholar]
- 9.Brierley JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. Eighth edition. John Wiley & Sons; 2017. [Google Scholar]
- 10.Rothenburger M, Hülsken G, Stypmann J, et al. Cardiothoracic surgery after heart and heart-lung transplantation. Thorac Cardiovasc Surg 2005;53:85-92. 10.1055/s-2004-830472 [DOI] [PubMed] [Google Scholar]
- 11.Burdett CL, Critchley RJ, Black F, et al. Invasive biopsy is effective and useful after lung transplant. J Heart Lung Transplant 2010;29:759-63. 10.1016/j.healun.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 12.Dickson RP, Davis RD, Rea JB, et al. High frequency of bronchogenic carcinoma after single-lung transplantation. J Heart Lung Transplant 2006;25:1297-301. 10.1016/j.healun.2006.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olland AB, Falcoz PE, Santelmo N, et al. Primary lung cancer in lung transplant recipients. Ann Thorac Surg 2014;98:362-71. 10.1016/j.athoracsur.2014.04.014 [DOI] [PubMed] [Google Scholar]
- 14.Lashari BH, Vender RJ, Fleitas-Sosa DC, et al. Lung cancer in recipients after lung transplant: single-centre experience and literature review. BMJ Open Respir Res 2022;9:e001194. 10.1136/bmjresp-2021-001194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Cancer Institute: Surveillance, Epidemiology, and End Results (SEER) Program. Lung and Bronchus, Recent Trends in SEER Relative Survival Rates, 2000-2018. Accessed August 23, 2021. 2021. Available online: https://seer.cancer.gov/explorer/application.html?site=47&data_type=4&graph_type=2&compareBy=survival_interval&chk_survival_interval_1=1&sex=1&race=1&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&advopt_display=1
- 16.Sigel K, Veluswamy R, Krauskopf K, et al. Lung Cancer Prognosis in Elderly Solid Organ Transplant Recipients. Transplantation 2015;99:2181-9. 10.1097/TP.0000000000000715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernández AM, Poveda DS, Ruiz E, et al. Incidence of Carcinoma in the Native Lung After Single Lung Transplantation. Transplant Proc 2022;54:57-8. 10.1016/j.transproceed.2021.10.009 [DOI] [PubMed] [Google Scholar]
- 18.Alberg AJ, Brock MV, Ford JG, et al. Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e1S-e29S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozawa Y, Suda T, Naito T, et al. Cumulative incidence of and predictive factors for lung cancer in IPF. Respirology 2009;14:723-8. 10.1111/j.1440-1843.2009.01547.x [DOI] [PubMed] [Google Scholar]
- 20.Hubbard R, Venn A, Lewis S, et al. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med 2000;161:5-8. 10.1164/ajrccm.161.1.9906062 [DOI] [PubMed] [Google Scholar]
- 21.Venuta F, Boehler A, Rendina EA, et al. Complications in the native lung after single lung transplantation. Eur J Cardiothorac Surg 1999;16:54-8. 10.1016/s1010-7940(99)00141-4 [DOI] [PubMed] [Google Scholar]
- 22.Schulman LL, Htun T, Staniloae C, et al. Pulmonary nodules and masses after lung and heart-lung transplantation. J Thorac Imaging 2000;15:173-9. 10.1097/00005382-200007000-00004 [DOI] [PubMed] [Google Scholar]
- 23.Chaparro C, Maurer JR, Chamberlain DW, et al. Role of open lung biopsy for diagnosis in lung transplant recipients: ten-year experience. Ann Thorac Surg 1995;59:928-32. 10.1016/0003-4975(95)00015-d [DOI] [PubMed] [Google Scholar]
- 24.Bertolotti A, Defranchi S, Vigliano C, et al. Surgical lung biopsy in transplant patients with diffuse lung disease: how much worse when the lung is the graft? Ann Thorac Surg 2013;96:279-85. 10.1016/j.athoracsur.2013.02.037 [DOI] [PubMed] [Google Scholar]
- 25.Defranchi S, Bertolotti AM, Vigliano CA, et al. Open lung biopsy for diffuse disease in patients with and without previously transplanted solid organs. Ann Thorac Surg 2010;90:965-71; discussion 971-2. 10.1016/j.athoracsur.2010.05.053 [DOI] [PubMed] [Google Scholar]
- 26.Wilson H, Carby M, Beddow E. Lung volume reduction surgery for native lung hyperinflation following single-lung transplantation for emphysema: which patients? Eur J Cardiothorac Surg 2012;42:410-3. 10.1093/ejcts/ezs086 [DOI] [PubMed] [Google Scholar]
- 27.Reece TB, Mitchell JD, Zamora MR, et al. Native lung volume reduction surgery relieves functional graft compression after single-lung transplantation for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg 2008;135:931-7. 10.1016/j.jtcvs.2007.10.069 [DOI] [PubMed] [Google Scholar]
- 28.Schulman LL, O’Hair DP, Cantu E, et al. Salvage by volume reduction of chronic allograft rejection in emphysema. J Heart Lung Transplant 1999;18:107-12. 10.1016/s1053-2498(98)00021-7 [DOI] [PubMed] [Google Scholar]
- 29.Robbins HY, Arcasoy SM. Malignancies following lung transplantation. Clin Chest Med 2011;32:343-55. 10.1016/j.ccm.2011.02.011 [DOI] [PubMed] [Google Scholar]
- 30.Souilamas R, Saueressig M, Boussaud V, et al. Pulmonary resection after lung transplantation in cystic fibrosis patients. Asian Cardiovasc Thorac Ann 2011;19:202-6. 10.1177/0218492311409242 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as