Abstract
Acute viral infections often induce a transient period of immune deficiency in which the host’s T cells fail to proliferate in response to T-cell mitogens and fail to make an antigen-specific memory recall response. This has been associated with the enhanced sensitivity of these highly activated T cells to undergo apoptosis, or activation-induced cell death (AICD), upon T-cell receptor ligation. Here we show that gamma interferon receptor-deficient (IFN-γ R−/−) mice mount a T-cell response to lymphocytic choriomeningitis virus (LCMV) infection but fail to undergo the transient immune deficiency. Instead, their T cells were hyperproliferative and relatively, but not completely, resistant to AICD. The immune response returned to homeostasis, but with delayed kinetics, in parallel with delayed clearance of the virus. Wild-type mice receiving high doses of disseminating LCMV Clone 13 are known to undergo clonal exhaustion of their virus-specific cytotoxic T lymphocytes (CTL). To determine whether this process was mediated by AICD associated with IFN-γ or with Fas-Fas ligand interactions, LCMV-specific precursor CTL frequencies were examined in LCMV Clone 13-infected IFN-γ R−/− or lpr (Fas-deficient) mice. In both instances, viral persistence was established and CTL precursors were greatly eliminated. This finding indicates that clonal exhaustion of CTL does not require IFN-γ or Fas, even though both molecules influence AICD and the transient immune deficiency seen in the LCMV infection.
Apoptotic regulation of T-cell responses has been implicated in the transient immune deficiencies associated with many viral infections (33, 36), in the silencing of the immune response and its return to homeostasis at the end of infection (32), and in the selective clonal exhaustion of antigen-specific T cells that occurs under conditions of high antigen load during disseminating infections (23). Among the molecules implicated in the regulation of T-cell apoptosis are cell surface receptors such as Fas-Fas ligand (FasL) (4, 6, 15, 37, 38) and tumor necrosis factor-tumor necrosis factor receptor (49), intracellular proteins such as Bcl-2 and its many relatives (35), and caspases, a family of cysteine proteases including interleukin-1 convertase enzyme (ICE) family proteases (21, 27). A number of cytokines have also been proposed as factors inducing apoptosis or conditioning cells to become susceptible to apoptosis (8, 17). One of these, gamma interferon (IFN-γ), is well known for its capacity for immune modulation. IFN-γ can influence the generation of cell-mediated immune responses both by suppressing the growth of Th2 cells and by promoting the differentiation of Th1 cells from either naive or memory CD4+ cells (3, 9). Some evidence implicating IFN-γ as a contributor to T-cell death has been presented. The induction of apoptosis of Th1 cell lines is associated with IFN-γ gene expression and secretion following CD3/T-cell receptor (TCR) mobilization in the absence of accessory cells, and apoptosis can be prevented by the addition of anti-IFN-γ antibodies (11, 18). Recent studies have shown that splenocytes from mice with the inability to respond to IFN-γ exhibit increased levels of proliferation in response to mitogen and alloantigen (5, 48). Immune modulation by IFN-γ via its antiproliferative and proapoptotic actions has been proposed as an additional mechanism that contributes to clonal deletion and the maintenance of self tolerance after antigenic challenge (18), yet its role in the apoptotic processes of T cells induced during viral infection is unknown.
To examine the role of IFN-γ in T-cell apoptosis associated with immune deficiency, immune silencing, and clonal exhaustion in virus infections, we have selected to study the lymphocytic choriomeningitis virus (LCMV) model of infection in mice. Infection of mice with low doses of LCMV strain Armstrong (LCMV-Arm) results in a massive expansion in the number and activity of CD8+ T cells, which are responsible for the clearance of the virus. These mice, like many other virus-infected hosts, develop a period of transient immune deficiency at which time the proliferative responses to common mitogens and recall antigens are depressed (14, 33). This immune deficiency is a consequence of the enhanced susceptibility of the activated T cells to undergo apoptosis upon strong TCR stimulation (33), a phenomenon known as activation-induced cell death (AICD) (39). During this period of immune deficiency, the splenic T-cell number substantially declines in a process we term immune silencing. Immune silencing, which occurs after detectable viral antigen is cleared, is associated with high levels of apoptosis within the T- and B-cell populations of the spleen (32). After the immune silencing process, the mice are left with high levels of virus-specific precursors in their T-cell memory pool. Infection of mice with high doses of a variant of LCMV (Clone 13) that replicates to higher titers and rapidly disseminates results in a transient antiviral cytotoxic T-lymphocyte (CTL) response followed by a clonal exhaustion of the virus-specific CTL response and the development of a persistent infection without CTL memory (23). Because the injection of high levels of T-cell-specific peptides can induce apoptosis and eliminate T-cell responses in vivo (43), a likely mechanism for clonal exhaustion under conditions of this disseminating LCMV infection is AICD (apoptosis) of this antigen activated T-cell population. Thus, regulation of T-cell apoptosis during viral infections can be examined by using LCMV-infected mice for analyses of the susceptibility of activated lymphocytes to undergo apoptosis during the period of immune deficiency, of downregulation of CD8+ cell number, and of clonal exhaustion. If IFN-γ governs efficient downregulation of the CD8+ T-cell response, then one possible outcome of infection of IFN-γ receptor (IFN-γ R)-deficient (IFN-γ R−/−) mice with LCMV-Arm would be failure to reestablish homeostatic numbers of CD8+ T cells and, following infection with LCMV Clone 13, a failure to undergo clonal exhaustion. We report here that mice with a defect in the ability to respond to IFN-γ have T cells that are impaired in the ability to undergo apoptosis in vitro and have no evidence of transient immune deficiency during acute viral infection. These mice are delayed in the process of immune silencing of CD8 T-cell number and activity and clear the infection with delayed kinetics, but the cell numbers ultimately decline, and expected frequencies of CTL precursors (CTLp) are established. The virus-specific T cells from IFN-γ R−/− mice as well as Fas-deficient lpr mice also underwent clonal exhaustion following infection with LCMV Clone 13. These results indicate that IFN-γ is important for the induction of transient immune deficiency but is not required for the immune silencing or clonal exhaustion.
MATERIALS AND METHODS
Mice.
129 SV/EV wild-type control mice and 129 mice homozygous for a targeted mutation disrupting the β subunit of the murine IFN-γ R were originally derived and provided by M. Aguet (13). Mice were housed in a specific pathogen-free environment and were used at 6 to 12 weeks of age. C57BL/6J and B6.MRL-Faslpr (CD95-deficient) mice were purchased from Jackson Laboratory (Bar Harbor, Maine) and placed in conventional housing. Mice were infected either intraperitoneally with 5 × 104 PFU of LCMV-Arm or intravenously with 4 × 107 PFU of LCMV Clone 13 (fourth-passage LCMV Clone 13-Armstrong was obtained from M. B. A. Oldstone, Scripps Clinic and Research Foundation, La Jolla, Calif.).
Proliferation assays.
Spleens were ground between the frosted ends of microscope slides, and the erythrocytes were lysed in 0.84% ammonium chloride. The cells were washed with RPMI 1640 culture medium supplemented with 2 mM l-glutamine, 100 μg of penicillin-streptomycin per ml, 5 × 10−5 M 2-mercaptoethanol (all from Sigma Chemical Co., St. Louis, Mo.), 0.1 μM sodium pyruvate (Gibco, Grand Island, N.Y.), 0.1 mM nonessential amino acids (Gibco), 10 mM HEPES, and 10% fetal calf serum (Sigma). Splenic T cells were enriched by nylon wool purification as previously described (33) and used for the determination of susceptibility to apoptosis. Enriched splenic T cells from infected and uninfected mice were cultured for 36 to 48 h in various culture conditions as previously described (19), with the exception that tissue culture plates were coated overnight with 2 to 5 μg of purified mouse anti-CD3 (clone 145-2C11; Pharmingen). Human recombinant interleukin-2 (IL-2) was from Cetus (Emeryville, Calif.). Half of the culture was used to determine proliferative responses by measuring the incorporation of [3H]thymidine (1 μCi/well; Amersham, Arlington Heights, Ill.) during the final 6 h of incubation.
TUNEL staining.
The remaining half of the culture containing approximately 5 × 105 cells was used to determine the percentage of apoptotic cells by the terminal nucleotidyltransferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL) assay modified for flow cytometry (47). Briefly, the cultured cells were surface labeled with fluorescein isothiocyanate-conjugated anti-mouse CD8a (clone 53-6.7) and phycoerythrin-conjugated anti-mouse CD4 (H129.19) (Pharmingen, San Diego, Calif.) and then fixed with paraformaldehyde. The cells were then permeabilized with 70% cold ethanol, washed, and resuspended in terminal transferase reaction buffer that contained 0.2 M potassium cacodylate, 25 mM Tris-HCl, 0.25 mg of bovine serum albumin per ml, 2.5 mM cobalt chloride, 10 U of TdT, and 0.5 nmol of biotin-16-dUTP (B-dUTP) (all from Boehringer Mannheim, Indianapolis, Ind.) in a total volume of 50 μl. The incorporated B-dUTP was detected by the addition of streptavidin conjugated with Tricolor (Caltag, Burlingame, Calif.). The samples were resuspended in phosphate-buffered saline–2% fetal calf serum for flow cytometric analysis.
Lymphocyte phenotyping.
The percentages of splenic CD3+ and CD8+ T lymphocytes were identified by double surface immunofluorescence staining with anti-mouse CD3 conjugated with phycoerythrin (Gibco) and anti-mouse CD8 conjugated with fluorescein isothiocyanate (Gibco). A total of 106 cells were stained, fixed with 1% paraformaldehyde, and analyzed on either a FACStar Plus or a FACS 440 (both from Becton Dickinson, San Jose, Calif.).
Cytotoxicity assays.
Cell-mediated cytotoxicity was determined by a standard CTL assay (34). Various numbers of effector splenocytes were plated in triplicate to achieve the desired effector-to-target cell ratio. LCMV-infected or uninfected MC57G fibroblasts were used as target cells. Target cells were labeled with 100 μCi of 51Cr (Amersham) and mixed with the effector cells for a 5- to 6-h incubation. Supernatant from each well was harvested and counted. Percent specific lysis was calculated from the formula 100 × [(experimental cpm − spontaneous cpm)/(maximum cpm − spontaneous cpm)]. One lytic unit (LU) was defined as the number of lymphocytes necessary to obtain 30% specific lysis of 104 target cells. Total LU was calculated from the total number of lymphocytes per spleen.
Limiting dilution assay for virus-specific CTLp.
The assay used the procedure of Moskophidis et al. (24), modified as noted elsewhere (26, 40). Briefly, dilutions of splenocytes from infected mice were stimulated with irradiated LCMV-infected syngeneic peritoneal exudate cells and with syngeneic splenocytes as a source of feeder cells. The cultures were maintained in AIM-V medium (Gibco) supplemented with a 16% culture supernatant from a gibbon lymphoma tumor cell line, MLA.144 (American Type Culture Collection, Rockville, Md.), as a source of IL-2 (31). The cultures were restimulated on day 4 with irradiated virus-infected PECs. The level of cytolytic activity was measured on day 8, at which time the individual wells were split two ways and incubated 8 h with a 51Cr-labeled target cell line KO (a murine H-2b cell line from simian virus 40-transformed kidney cells from S. Tevethia, Pennsylvania State University, Hershey), either infected with LCMV or uninfected. Positive wells were identified as wells that exceeded the mean chromium release from wells without effector cells by 3 standard deviations. Wells containing cells that lysed uninfected syngeneic targets were eliminated from the calculations. The precursor frequency was determined by chi-square analysis based on maximum likelihood (45) by a computer program provided by R. Miller (University of Michigan, Ann Arbor).
Virus titer assay.
Blood or spleen homogenates from infected mice were titrated for virus by plaque formation on Vero (American Type Culture Collection) cell monolayers.
RESULTS
CD8+ T-cell silencing and LCMV clearance are delayed in IFN-γ R−/− mice.
Mice infected with LCMV have a major expansion of antiviral CD8+ T cells that are responsible for clearing the infection. Changes in total splenic T-cell number and in CD8+ cell number, and the virus-specific CTL responses from IFN-γ R−/− mice and the 129 SV/EV controls, are shown in Table 1. In the 129 SV/EV mice, both splenic T-cell numbers and CD8+ cell numbers doubled over the first 10 days of infection, with the exception of experiment 4, in which the CD8+ cell number doubled but the overall T-cell expansion was modest. In these mice, peak viral titers were detected 4 days postinoculation (p.i.), and no virus-positive mice were detected after day 8 of infection (Table 2) (25). In contrast to the efficient clearance of the virus in the 129 SV/EV mice, there was prolonged viral replication in the IFN-γ R−/− mice (Table 2). Clearance of virus from the spleens of the IFN-γ R−/− mice occurred between 10 and 15 days p.i., and significantly higher titers of virus were found 8 days p.i. (P = 0.0003) in IFN-γ R−/− mice compared to the wild-type controls. The trend of increased viral titers in IFN-γ R−/− mice was also present at 5 (P = 0.03) and 6 (P = 0.04) days p.i. Associated with the delayed clearance of virus was a three- to fivefold expansion in the number of splenic CD8 cells that was increased 13 to 15 days p.i. (Table 1).
TABLE 1.
Total splenic CD3+ and CD8+ lymphocyte number and splenic CTL activity in 129SV/EV and IFN-γ R−/− mice infected with LCMV
| Expt | Mouse strain | na | No. of splenocytes/spleen
|
Splenic CTL activity
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 p.i.
|
Days 7–8 p.i.
|
Days 10–11 p.i.
|
Days 13–15 p.i.
|
Day 0 p.i.
|
Days 7–8 p.i.
|
Days 10–11 p.i.
|
Days 13–15 p.i.
|
|||||||||||
| CD3+ (107) | CD3+ CD8+ (107) | CD3+ (107) | CD3+ CD8+ (107) | CD3+ (107) | CD3+ CD8+ (107) | CD3+ (107) | CD3+ CD8+ (107) | % Specific lysisc | Total LUd | % Specific lysis | Total LU | % Specific lysis | Total LU | % Specific lysis | Total LU | |||
| 1 | 129 | 2 | 5.8 | 0.6 | 9.5 | 0.6 | 14.3 | 2.8 | 8.3 | 1.0 | 7 | <1 | 38 | 108 | 64 | 551 | 30 | 35 |
| IFN-γ R−/− | 2 | 6.2 | 0.5 | 10.4 | 1.2 | 11.3 | 2.4 | 15.3 | 2.0 | 6 | <1 | 60 | 256 | 69 | 339 | 58 | 341 | |
| 2 | 129 | 2 | 3.9 | 0.6 | 4.7 | 0.8 | 2.3 | 1.9 | 3.6 | 1.5 | −2 | <1 | 28 | 111 | 39 | 199 | 21 | 75 |
| IFN-γ R−/− | 2 | 3.2 | 0.7 | 4.6 | 1.1 | 3.4 | 1.9 | 4.9 | 2.0 | −3 | <1 | 29 | 171 | 43 | 222 | 27 | 149 | |
| 3 | 129 | 4 | NDb | ND | 5.7 | 2.0 | ND | ND | 4.0 | 1.8 | ND | ND | 17 | 73 | ND | ND | 18 | 78 |
| IFN-γ R−/− | 3 | ND | ND | 2.5 | 1.2 | ND | ND | 3.9 | 3.0 | ND | ND | 13 | 75 | ND | ND | 30 | 396 | |
| 4 | 129 | 2 | 2.9 | 0.6 | ND | ND | 3.5 | 2.4 | 2.5 | 1.6 | −1 | <1 | ND | ND | 37 | 173 | 24 | 48 |
| IFN-γ R−/− | 2 | 2.1 | 1.5 | ND | ND | 2.9 | 1.4 | 4.7 | 3.6 | −2 | <1 | ND | ND | 39 | 196 | 34 | 233 | |
| 5 | 129 | 2 | ND | ND | 1.7 | 1.3 | ND | ND | ND | ND | ND | ND | 50 | 175 | ND | ND | ND | ND |
| IFN-γ R−/− | 2 | ND | ND | 1.7 | 0.9 | 1.5 | 1.1 | 4.3 | 3.0 | ND | ND | 31 | 111 | 36 | 155 | 35 | 181 | |
Number of mice per experiment. The results for the 129 mice are from pooled spleens. The results for the IFN-γ R−/− mice are averages of individual mice.
ND, not done.
At effector-to-target cell ratio of 100:1 (see Materials and Methods).
See Materials and Methods.
TABLE 2.
Splenic LCMV titers in 129 SV/EV and IFN-γ R−/− micea
| Days p.i. | Titer (log10 PFU/spleen ± SD)b
|
|
|---|---|---|
| 129 SV/EV | IFN-γ R−/− | |
| 3 | 3.7 ± 1.3 (5c) | 5.1 ± 0.2 (2) |
| 4 | 5.2 ± 1.1 (6) | 4.9 ± 0.6 (3) |
| 5 | 4.1 ± 1.2 (6) | 5.4 ± 0.2 (3) |
| 6 | 2.7 ± 0.7 (6) | 3.4 ± 0.2 (4) |
| 8 | <2.4 (8) | 3.4 ± 0.4d (6) |
| 10 | <2.0 (4) | <2.4 (4) |
| 13 | <2.0 (7) | <1.0 (7) |
Age-matched IFN-γ R−/− mice and wild-type control (129 SV/EV) mice were infected i.p. with 5 × 104 PFU of LCMV.
Spleen homogenates were titrated on Vero cells for 4 days in an agarose overlay assay. Plaques were detected on day 5 by a neutral red overlay.
Number of mice per time point. Individual spleens were titrated.
Statistically significant increase (P < 0.05).
Primary CTL activity was measured against syngeneic LCMV-infected target cells. As expected, the LCMV-specific cytotoxic activity peaked in the control mice 10 to 11 days p.i. and then rapidly declined (Table 1). Some of the IFN-γ R−/− mice also had peak levels of activity days 10 to 11 p.i., but a notable finding was the levels of lytic activity present on days 13 to 15 p.i. This activity was even more pronounced when the overall cellularity of the spleen was considered (total LU). In each experiment, there was an increase in the total LU present in the spleens from the IFN-γ R−/− mice compared to the wild-type controls 13 to 15 days p.i. Even though the total LU was high 13 to 15 days p.i. in the IFN-γ R−/− mice, the level dropped dramatically 17 days p.i. (total LU = 30) and returned to normal (total LU < 1) 20 days p.i. These results indicate that the LCMV-infected IFN-γ R−/− mice have elevated levels of virus replication, a delayed rate of virus clearance, and a much delayed rate of immune silencing (both in cell number and in activity) compared to the 129 SV/EV mice.
Splenic T cells from IFN-γ R−/− mice infected with LCMV are hyperproliferative and fail to develop transient immune deficiency.
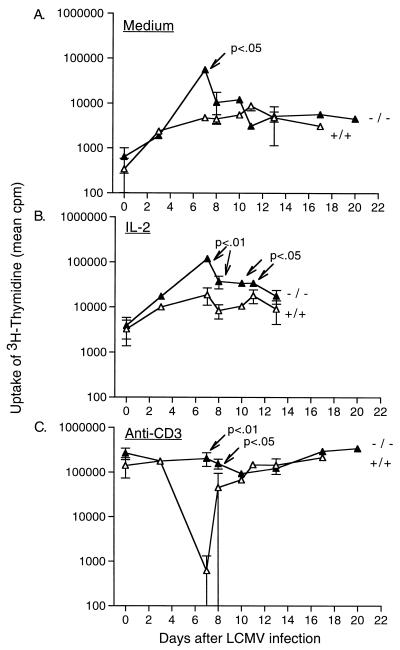
Immune deficiency and the susceptibility to AICD of T cells from LCMV-Arm-infected mice was determined by the combined methods of measurement of lymphocyte proliferation in response to stimulation by immobilized anti-CD3 and detection of apoptotic cells in these cultures by flow cytometry. As shown in Fig. 1C, contrasting with the transient 100-fold reduction in [3H]thymidine uptake observed in the 129 SV/EV littermates on days 7 to 8 p.i., mice with an inability to respond to IFN-γ had a slight (1.7-fold) reduction in the proliferative response compared to the response on day 0 (P = 0.03). Even at a comparable time point where there was minimal virus replication in the IFN-γ R−/− mice, and when the T-cell population was expanding and expressing activation markers (days 10 to 14), there was no major impairment in the ability of splenocytes to proliferate to anti-CD3: the average inhibition of the proliferative response 10 to 14 days p.i. was from 2.1- to 2.8-fold less than the proliferative response on day 0. In addition, the proliferative response of T cells to IL-2 in the absence of receptor stimulation was significantly elevated in IFN-γ R−/− mice on days 7 to 10 p.i.: Fig. 1B shows that it was nearly 10-fold higher than in control infected mice (P values range from <0.01 to <0.05). As IFN-γ can mediate both antiproliferative and proapoptotic effects, the T-cell proliferative results from IFN-γ R−/− mice could have reflected either increased proliferation or decreased levels of apoptosis in the cultures.
FIG. 1.
Proliferative responses of splenic T cells from wild-type 129 SV/EV (+/+) and IFN-γ R−/− (−/−) mice after 36 to 48 h of culture in medium alone (A) or supplemented with IL-2 (B) or anti-CD3 (C). The mean counts per minute of [3H]thymidine incorporation are ± 1 standard deviation plotted. The results are averaged from the values for individual mice, with a minimum of two mice tested per time point. The Student t test was used to generate P values for data from days 7 to 13 p.i.
Splenic CD8+ T cells from LCMV-infected IFN-γ R−/− mice are resistant to AICD during the period of immune silencing.
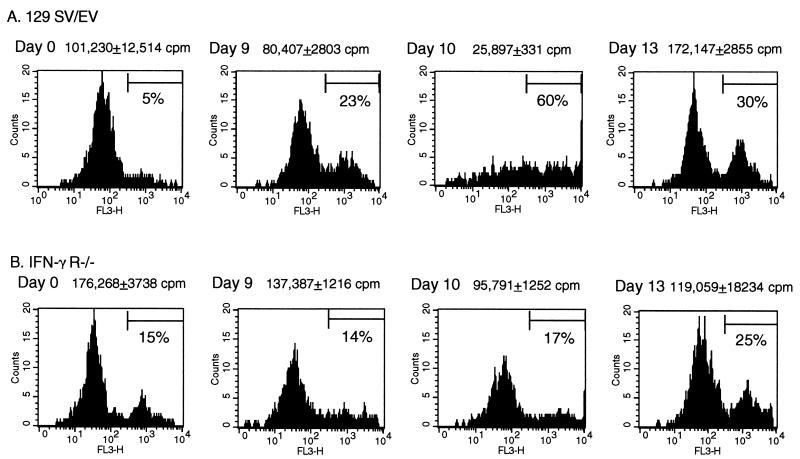
To address the question of whether the cells from the IFN-γ R−/− mice were impaired in the ability to undergo apoptosis, we used the TUNEL flow assay to specifically measure the level of apoptotic CD8+ cells in the anti-CD3-stimulated cultures. Representative histograms from individual experiments have been compiled to show the overall trend of susceptibility of CD8+ cells to apoptosis at different time points during LCMV infection. T cells from uninfected (day 0) 129 SV/EV mice or IFN-γ R−/− mice proliferated after incubation with anti-CD3 (Fig. 1C), and the CD8+ cell population was 4 to 11% apoptotic (Fig. 2). Nine days after LCMV infection of the 129 SV/EV mice, the numbers of apoptotic cells in culture rose to 25% following anti-CD3 stimulation. This susceptibility to anti-CD3-mediated cell death was greatest on day 10 of infection, when 60% of the CD8 cells were apoptotic after 48 h in culture. Day 10 corresponded to the peak of splenic CD8+ cell expansion in the 129 SV/EV mice (Table 1). By 13 days p.i., the proportion of the CD8 cells that were driven into death fell to 30% as the splenic CD8 number declined. In contrast to the 129 SV/EV mice, the appearance of CD8+ cells susceptible to apoptosis was delayed in the IFN-γ R−/− mice, consistent with the delay in CD8+ cell expansion and viral clearance (Tables 1 and 2). Nine to 10 days p.i., at the cusp of CD8+ cell expansion and the start of viral clearance, CD8+ cells from the IFN-γ R−/− mice remained resistant to anti-CD3-mediated death (Fig. 2B). At this time point, only 15 to 16% of the CD8 cells were apoptotic. The majority of the IFN-γ R−/− mice cleared LCMV by 13 days p.i., by which time there was a three- to fivefold expansion in the number of CD8 cells, similar to the conditions observed on day 10 in the 129 SV/EV mice, at which point the CD8 cells from 129 SV/EV mice showed maximum susceptibility to anti-CD3-mediated cell death. On day 13, the CD8+ cells from IFN-γ R−/− mice were more susceptible to anti-CD3-mediated cell death (25% of the cells were apoptotic after culture), but there were many more viable cells present than at the day 10 time point in wild-type mice, when one considers the 100-fold difference in proliferation (Fig. 1C). Thus, CD8+ cells from LCMV-infected IFN-γ R−/− mice are relatively resistant to apoptosis in addition to being hyperproliferative. This finding suggests that IFN-γ is a factor required for efficient induction of AICD and that it is important for the transient immune deficiency during virus infections.
FIG. 2.
Detection of DNA fragmentation associated with CD8+ apoptotic cells following stimulation for 36 to 48 h in the presence of anti-CD3. Apoptotic CD8+ cells were detected by the TUNEL assay followed by the addition of streptavidin-Tricolor. The percent apoptotic cells was determined from the cell numbers present in the M1 region. The profiles are from individual mice and are representative of at least two mice tested per time point.
T cells from IFN-γ R−/− mice and Fas-deficient mice undergo clonal exhaustion in persistent viral infection.
Infection of mice with a high dose of a disseminating strain of virus, LCMV Clone 13, results in elimination of virus specific CTL and leaves the host in a the state of viral persistence (23). In that immunological setting, both high levels of circulating antigen and virus-specific activated T cells would be present in the same environment, the conditions necessary for induction of AICD. To test the hypothesis that clonal exhaustion may occur by AICD, we infected wild-type, Fas-deficient (lpr), or IFN-γ R−/− mice with LCMV Clone 13 and measured CTLp frequencies 6 weeks p.i. When infected with low doses of LCMV-Arm, all of the mice were capable of developing normal memory CTL responses. From 200 to over 4,700 CTLp per 50,000 CD8+ cells were detected in both wild-type and mutant mice 6 weeks p.i. with LCMV-Arm (Table 3). The values for the LCMV-Arm-infected 129 SV/EV and IFN-γ R−/− mice used in these experiments were comparable to CTLp frequencies reported elsewhere by our group (129 SV/EV, 1,785 and 3,333 CTLp/50,000 CD8+ cells; IFN-γ R−/−, 1,282 and 4,545 CTLp/50,000 CD8+ cells). These values were within the normal range for mice immune to LCMV-Arm (41, 42). However, when infected with LCMV Clone 13, all mice became persistently infected after intravenous inoculation. The titer of LCMV Clone 13 from the blood of infected C57BL/6 mice was 3.7 ± 0.7 log PFU/ml (n = 11), the titer for lpr mice was 5.2 ± 0.3 PFU/ml (n = 7), the titer for 129 SV/EV mice was 2.3 ± 0.3 PFU/ml (n = 3), and the titer for IFN-γ R−/− mice was 3.5 ± 0.1 PFU/ml (n = 4). Persistent infection resulted in a pronounced 2- to 3-log10 reduction in the number of CTLp present 6 weeks after infection of both lpr and IFN-γ R−/− mice and their wild-type controls. Thus, mice with selective deficiencies in the ability to undergo AICD are clearly capable of undergoing clonal exhaustion of virus-specific CTL in the presence of circulating viral antigen.
TABLE 3.
LCMV-specific CD8+ CTLp frequencies in mice infected with either LCMC-Arm or LCMV Clone 13
| Mouse strain | Expt | LCMV-Arm
|
LCMV Clone 13
|
||
|---|---|---|---|---|---|
| CTLp/50,000 CD8+ cells | 95% confidence interval | CTLp/50,000 CD8+ cells | 95% confidence interval | ||
| C57BL/6 | 1 | 262 | 331–192 | <3 | |
| 2 | 1,250 | 1,724–793 | <3 | ||
| 3 | 2,173 | 3,125–1,282 | <2 | ||
| 4 | 355 | 467–244 | <2 | ||
| 5 | 575 | 735–423 | 23 | 34–12 | |
| 6 | 982 | 1,372–590 | 11 | 13–9 | |
| 7 | 20 | 29–10 | |||
| Avg | 933 | 9 | |||
| B6.MRL-Faslpr | 1 | 1,613 | 2,000–1,250 | <2 | |
| 2 | 1,190 | 1,563–820 | <2 | ||
| 3 | 459 | 568–320 | 100 | 135–66 | |
| 4 | <2 | ||||
| 5 | 29 | 40–18 | |||
| 6 | 20 | 26–15 | |||
| 7 | <1 | ||||
| 8 | 14 | 18–10 | |||
| Avg | 1,087 | 21 | |||
| 129 SV/EV | 1 | 1,667 | 2,083–1,190 | 34 | 45–23 |
| 2 | 204 | 277–132 | |||
| Avg | 935 | 34 | |||
| IFN-γ R−/− | 1 | >1,163 | <1.5 | ||
| 2 | >4,762 | 9 | 15–3 | ||
| 3 | 6 | 11–1 | |||
| 4 | 10 | 17–2 | |||
| Avg | >2,962 | 6.6 | |||
DISCUSSION
Highly activated cycling T cells are prone to undergo apoptosis if stimulated strongly through the TCR in a process termed AICD (39). In vivo, AICD has been demonstrated in a murine CD4+ transgenic T-cell system to require Fas-FasL interactions (43), and IFN-γ has been shown to upregulate Fas expression on T cells (28–30). AICD is a mechanism for the transient immune deficiency observed during the height of an acute viral infection, at which time T cells fail to proliferate in the presence of mitogen or recall antigens (12, 22, 32, 33). The model of LCMV infection allows investigation of the mechanism(s) for induction of apoptosis in the settings of transient immune deficiency, immune silencing, and clonal exhaustion. We report here that mice with a defect in the ability to respond to IFN-γ had no evidence of transient immune deficiency during acute LCMV infection, as measured by proliferative responses, but were delayed in the process of viral clearance and immune downregulation. T cells from these mice exhibited impaired ability to undergo apoptosis in vitro, but immune silencing eventually occurred at low doses of virus, and clonal exhaustion occurred at high doses. Together with our previous studies with lpr mice (19, 32), this finding indicates that neither Fas nor IFN-γ is necessary for immune silencing or clonal exhaustion of CD8+ T cells, although these molecules affect the susceptibility of T cells to AICD.
AICD has also been postulated to play a role in immune silencing and return to homeostasis (10, 20, 44, 46). In the setting of low-dose LCMV-Arm infection, this seems unlikely, as viral antigen is cleared prior to the onset of immune silencing (16), and mice with deficiencies in molecules known to regulate AICD, such as Fas and FasL, undergo normal immune silencing (19, 32). There is also evidence that CD8+ T cells do not require a second encounter with antigen to undergo apoptosis (2). AICD is thought to depend on reexposure of activated T cells to TCR ligation, but Alexander-Miller et al. suggest that the commitment to apoptosis has already occurred during the initial antigenic encounter, indicating that in their system cycling CTL do not require a second encounter with antigen to induce apoptosis (2). This finding suggests that AICD may not be the major mechanism for establishment of CD8+ T-cell homeostasis after resolution of viral infection.
The levels of antigen present at the end of the immune response have been proposed to affect the outcome of TCR ligation of activated cells: AICD is the result when large quantities of antigen are present (1). However, when concentrations of antigen are low, as in the setting of LCMV-Arm infection, activated T cells may be prone to death resulting from deprivation of growth factors such as IL-2 and stromal cell factor(s) which are postulated to promote survival of T cells by maintaining high levels of Bcl-xL and intracellular glutathione, an antioxidant (1), in a process independent of Fas ligation. We have previously shown that mice with enforced expression of Bcl-2, which can protect lymphocytes from cytokine withdrawal-induced apoptosis, clear LCMV normally, have a normal reduction in T-cell number, and exhibit increased numbers of apoptotic cells in the spleens of mice recovering from infection (32). This finding suggests that cytokine deprivation alone is not sufficient to drive cells into apoptosis, although the special effects of Bcl-xL were not investigated (32). Also, the frequencies and specificities of LCMV-specific CTLp are only marginally affected by the decline in T-cell number during immune silencing (42), arguing against receptor-dependent apoptosis of those T cells and consistent with stromal cell-mediated rescue of activated cells independent of antigen.
Wild-type mice receiving high doses of disseminating LCMV Clone 13 are known to undergo clonal exhaustion of their virus-specific CTL. The mechanism driving this elimination of antigen-specific T cells remains unclear. Clonal exhaustion may occur during generalized infections with noncytopathic viruses, when an excess of antigen on localized and peripheral antigen-presenting cells may induce all the matured effector T cells to die off within a few days. This repeated exposure to antigen would drive them into apoptosis, resulting in a deletion of this specificity from the repertoire (7, 50). Attempts to prevent clonal exhaustion by infusions of IL-1 or IL-2 were unsuccessful, indicating that interleukins do not seem to be the limiting factor in the exhaustion of CTLs (23). We report here that viral persistence was established and CTLp were greatly eliminated following LCMV Clone 13 infection of IFN-γ R−/− and lpr (Fas-deficient) mice, suggesting that clonal exhaustion of CTL also does not require IFN-γ or Fas, even though both molecules influence AICD and the transient immune deficiency seen in the LCMV infection.
ACKNOWLEDGMENTS
This work was supported by USPHS NIH research grant AI 17672 and training grant AI 07272.
We thank T. Krumpoch and B. Fournier for flow cytometry and R. Budd for the terminal transferase protocol.
REFERENCES
- 1.Akbar A N, Salmon M. Cellular environments and apoptosis: tissue microenvironments control activated T-cell death. Immunol Today. 1997;18:72–76. doi: 10.1016/s0167-5699(97)01003-7. [DOI] [PubMed] [Google Scholar]
- 2.Alexander-Miller M A, Leggatt G R, Sarin A, Berzofsky J A. Role of antigen, CD8, and cytotoxic T lymphocyte (CTL) avidity in high dose antigen induction of apoptosis of effector CTL. J Exp Med. 1996;184:485–492. doi: 10.1084/jem.184.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley L M, Dalton D K, Croft M. A direct role for IFN-γ in regulation of Th1 cell development. J Immunol. 1996;157:1350–1358. [PubMed] [Google Scholar]
- 4.Brunner T, Mogil R J, LaFace D, Yoo N J, Mahboubi A, Echeverri F, Martin S J, Force W R, Lynch D H, Ware C F, Green D R. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 5.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–1745. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 6.Dhein J, Walczak H, Baumler C, Debatin K-M, Krammer P H. Autocrine T-cell suicide mediated by APO-1 (Fas/CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 7.Doherty P C. Immune exhaustion: driving virus-specific CD8+ T cells to death. Trends Microbiol. 1993;1:207–209. doi: 10.1016/0966-842x(93)90133-c. [DOI] [PubMed] [Google Scholar]
- 8.Duke R C, Cohen J J. IL-2 addiction: withdrawal of growth factor activates a suicide program in dependent T cells. Lymphokine Res. 1986;5:289–299. [PubMed] [Google Scholar]
- 9.Gajewski T F, Fitch F W. Anti-proliferative effect of IFN-γ in immune regulation: IFN-γ inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988;140:4245–4252. [PubMed] [Google Scholar]
- 10.Glickstein L J, Huber B T. Karoushi—death by overwork in the immune system. J Immunol. 1995;155:522–524. [PubMed] [Google Scholar]
- 11.Groux H, Monte D, Plouvier B, Capron A, Ameisen J-C. CD3-mediated apoptosis of human medullary thymocytes and activated peripheral T cells: respective roles of interleukin-1, interleukin-2, interferon-γ and accessory cells. Eur J Immunol. 1993;23:1623–1629. doi: 10.1002/eji.1830230734. [DOI] [PubMed] [Google Scholar]
- 12.Groux H, Torpier G, Monte D, Mouton Y, Capron A, Ameisen J C. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med. 1992;175:331–340. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-γ receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs R P, Cole G A. Lymphocytic choriomeningitis virus-induced immunosuppression: a virus-induced macrophage defect. J Immunol. 1976;117:1004–1009. [PubMed] [Google Scholar]
- 15.Ju S T, Panka D J, Cui H, Ettinger R, El-Khatib M, Sherr D H, Stanger B Z, Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 16.Lau L, Jamieson B D, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–652. [PubMed] [Google Scholar]
- 17.Lenardo M J. Interleukin-2 programs mouse αβ T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Janeway C J. Interferon gamma plays a critical role in induced cell death of effector T cell: a possible third mechanism of self-tolerance. J Exp Med. 1990;172:1735–1739. doi: 10.1084/jem.172.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohman B L, Razvi E S, Welsh R M. T-lymphocyte downregulation after acute viral infection is not dependent on CD95 (Fas) receptor-ligand interactions. J Virol. 1996;70:8199–8203. doi: 10.1128/jvi.70.11.8199-8203.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch D H, Ramsdell F, Alderson M R. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;16:569–574. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 21.Martin S J, Green D R. Protease activation during apoptosis: death by a thousand cuts? Cell. 1995;82:349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- 22.Meyaard L, Otto S A, Jonker R R, Mijnster M J, Keet R P M, Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992;257:217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 23.Moskophidis D, Lechner F, Pircher H, Zinkernagel R M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 24.Moskophidis D, Assmann-Wicher U, Simon M M, Lehmann-Grube F. The immune response of the mouse to lymphocytic choriomeningitis virus. V. High numbers of cytolytic T lymphocytes are generated in the spleen during acute infection. Eur J Immunol. 1987;17:937–942. doi: 10.1002/eji.1830170707. [DOI] [PubMed] [Google Scholar]
- 25.Muller U, Steinhoff U, Reis L F L, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 26.Nahill S R, Welsh R M. High frequency of cross-reactive cytotoxic T lymphocytes elicited during the virus induced polyclonal cytotoxic T lymphocyte response. J Exp Med. 1993;177:317–327. doi: 10.1084/jem.177.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholson D W, Ali A, Thornberry N A, Vaillancourt J P, Ding C K, Gallant M, Gareau Y, Griffin P R, Labelle M, Lazebnik Y A, Munday N A, Raju S M, Smulson M E, Yamin T-T, Yu V L, Miller D K. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 28.Novelli F, Bernabei P, Ozmen L, Rigamonti L, Allione A, Pestka S, Garotta G, Forni G. Switching on the proliferation or apoptosis of activated human T lymphocytes by IFN-γ is correlated with the differential expression of the α- and β-chains of its receptor. J Immunol. 1996;157:1935–1943. [PubMed] [Google Scholar]
- 29.Novelli F, D’Elios M M, Bernabei P, Ozmen L, Rigamonti L, Almerigogna F, Forni G, Del Prete G. Expression and role in apoptosis of the α- and β-chains of the IFN-γ receptor on human Th1 and Th2 clones. J Immunol. 1997;159:206–213. [PubMed] [Google Scholar]
- 30.Oyaizu N, McCloskey T W, Than S, Hu R, Kalyanaraman V S, Pahwa S. Cross-linking of CD4 molecules upregulates Fas antigen expression in lymphocytes by inducing interferon-γ and tumor necrosis factor-α secretion. Blood. 1994;84:2622–2631. [PubMed] [Google Scholar]
- 31.Rabin H, Hopkins III R F, Ruscetti F W, Neubauer R H, Brown R L, G K T. Spontaneous release of a factor with properties of T cell growth factor from a continuous line of primate tumor T cells. J Immunol. 1981;127:1852–1856. [PubMed] [Google Scholar]
- 32.Razvi E S, Jiang Z, Woda B A, Welsh R M. Lymphocyte apoptosis during the silencing of the immune response to acute viral infections in normal, lpr, and Bcl-2 transgenic mice. Am J Pathol. 1995;147:79–91. [PMC free article] [PubMed] [Google Scholar]
- 33.Razvi E S, Welsh R M. Programmed cell death of T lymphocytes during acute viral infection: a mechanism for virus-induced immune deficiency. J Virol. 1993;67:5754–5765. doi: 10.1128/jvi.67.10.5754-5765.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Razvi E S, Welsh R M, McFarland H I. In vivo state of antiviral CTL precursors: characterization of a cycling cell population containing CTL precursors in immune mice. J Immunol. 1995;154:620–632. [PubMed] [Google Scholar]
- 35.Reed J C. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 36.Rouse B T, Horohov D W. Immunosuppression in viral infections. Rev Infect Dis. 1986;8:850–873. doi: 10.1093/clinids/8.6.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell J H, Rush B, Weaver C, Wang R. Mature T cells of autoimmune lpr/lpr mice have a defect in antigen-stimulated suicide. Proc Natl Acad Sci USA. 1993;90:4409–4413. doi: 10.1073/pnas.90.10.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russell J H, Wang R. Autoimmune gld mutation uncouples suicide and cytokine/proliferation pathways in activated, mature T cells. Eur J Immunol. 1993;23:2379–2382. doi: 10.1002/eji.1830230951. [DOI] [PubMed] [Google Scholar]
- 39.Russell J H, White C L, Loh D Y, Meleedy-Rey P. Receptor-stimulated death pathway is opened by antigen in mature T cells. Proc Natl Acad Sci USA. 1991;88:2151–2155. doi: 10.1073/pnas.88.6.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selin L K, Nahill S R, Welsh R M. Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J Exp Med. 1994;179:1933–1943. doi: 10.1084/jem.179.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selin, L. K., S. M. Varga, I. C. Wong, and R. M. Welsh. Protective heterologous anti-viral immunity and enhanced immunopathogenesis mediated by memory T cell populations. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 42.Selin L K, Vergilis K, Welsh R M, Nahill S R. Reduction of otherwise remarkably stable virus-specific cytotoxic T lymphocyte memory by heterologous viral infections. J Exp Med. 1996;183:2489–2499. doi: 10.1084/jem.183.6.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singer G G, Abbas A K. The Fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994;1:365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 44.Strasser A. Apoptosis: death of a T cell. Nature. 1995;373:385–386. doi: 10.1038/373385a0. [DOI] [PubMed] [Google Scholar]
- 45.Taswell C. Limiting dilution analysis for the determination of immunocompetent cell frequencies. I. Data Analysis. J Immunol. 1981;126:1614–1619. [PubMed] [Google Scholar]
- 46.van Parijs L, Abbas A K. Role of Fas-mediated cell death in the regulation of immune responses. Curr Opin Immunol. 1996;1996:355–361. doi: 10.1016/s0952-7915(96)80125-7. [DOI] [PubMed] [Google Scholar]
- 47.Vincent M S, Roessner K, Lynch D, Wilson D, Cooper S M, Tschopp J, Sigal L H, Budd R C. Apoptosis of Fashigh CD4+ synovial T cells by Borrelia-reactive Fas-ligandhigh γδ T cells in Lyme arthritis. J Exp Med. 1996;184:2109–2117. doi: 10.1084/jem.184.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao Y, Tarkowski A. Impact of interferon-γ receptor deficiency on experimental Staphylococcus aureus septicemia and arthritis. J Immunol. 1995;155:5736–5742. [PubMed] [Google Scholar]
- 49.Zheng L, Fisher G, Miller R E, Peschon J, Lynch D H, Lenardo M J. Induction of apoptosis in mature T cells by tumor necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 50.Zinkernagel R M. Immunology taught by viruses. Science. 1996;271:173–178. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]