Significance
The small molecule AC102 currently undergoing clinical trials for the treatment of sudden sensorineural hearing loss (SSNHL) has been shown to revert hearing loss to near normal levels in a guinea pig model of noise-induced hearing loss. In this model, the compound AC102 exerts the therapeutic effects by concurrently protecting sensory hair cells and neuronal connections within the inner ear which are critical for normal hearing function. Furthermore, in vitro studies indicated that AC102 exerts its function by boosting adenosine triphosphate production and limiting the levels of toxic reactive oxygen species. Thus, AC102 could serve as a potential therapeutic against SSNHL, a serious health condition with no approved drugs.
Keywords: sudden sensorineural hearing loss, hair cell preservation, synaptopathy, hearing recovery, ATP production
Abstract
Although sudden sensorineural hearing loss (SSNHL) is a serious condition, there are currently no approved drugs for its treatment. Nevertheless, there is a growing understanding that the cochlear pathologies that underlie SSNHL include apoptotic death of sensory outer hair cells (OHCs) as well as loss of ribbon synapses connecting sensory inner hair cells (IHCs) and neurites of the auditory nerve, designated synaptopathy. Noise-induced hearing loss (NIHL) is a common subtype of SSNHL and is widely used to model hearing loss preclinically. Here, we demonstrate that a single interventive application of a small pyridoindole molecule (AC102) into the middle ear restored auditory function almost to prenoise levels in a guinea pig model of NIHL. AC102 prevented noise-triggered loss of OHCs and reduced IHC synaptopathy suggesting a role of AC102 in reconnecting auditory neurons to their sensory target cells. Notably, AC102 exerted its therapeutic properties over a wide frequency range. Such strong improvements in hearing have not previously been demonstrated for other therapeutic agents. In vitro experiments of a neuronal damage model revealed that AC102 protected cells from apoptosis and promoted neurite growth. These effects may be explained by increased production of adenosine triphosphate, indicating improved mitochondrial function, and reduced levels of reactive-oxygen species which prevents the apoptotic processes responsible for OHC death. This action profile of AC102 might be causal for the observed hearing recovery in in vivo models.
While sudden sensorineural hearing loss (SSNHL) can result from a variety of identifiable causes (e.g., infectious, autoimmune, vascular, ototoxicity), 90% of cases are idiopathic or unknown (1). Despite an indisputable need for an effective treatment for SSNHL and decades of research, there are currently no approved medications (2, 3). Three elements of the inner ear are key factors for hearing function: 1) The sensory outer hair cells (OHCs) amplifying the sound signal, 2) the sensory inner hair cells (IHCs) responsible for mechanoelectrical transduction, and 3) the neurites of the auditory nerve, which are connected to the IHCs through so-called ribbon synapses, finally transmitting the signals to the auditory cortex of the brain (4). The inner ear is on permanent high activity both in an active and alert state. This high bioenergetic demand makes the cells uniquely vulnerable to stressors such as excessive noise exposure or ototoxic chemicals, putting inner ear cells energetically “on the edge.” Several reports revealed pathological processes induced by noise overstimulation which contribute to inner ear damage. The key pathological process caused by overstimulation is the immediately sharp increase in toxic reactive-oxygen species (ROS) concentration in the cochlea which remains elevated for 7 to 10 d (5, 6). The protracted oxidative stress triggers the release of proapoptotic factors which finally leads to caspase-dependent apoptotic cell death (7). An additional factor implicated in noise trauma is the transient adenosine triphosphate (ATP) depletion in the cochlear perilymph (8). Both factors, the simultaneous increase of ROS and the decrease of ATP suggest that acoustic overexposure causes an acute mitochondrial dysfunction. As a result of this, and through direct mechanical damage, OHCs can undergo cell death, primarily by apoptosis (9–11), which leads to threshold shifts in pure tone audiograms. IHCs are more resistant to cochlear injury, but their ribbon synapses, which connect to the peripheral neurites of the spiral ganglion, can disconnect due to glutamate excitotoxicity after noise exposure, a condition called cochlear synaptopathy (12–14).
A crucial aspect of inner ear diseases is that neither the hair cells (HCs) nor the auditory nerve cells are capable of postembryonic mitosis to produce new cells (15). Therefore, early and effective treatment is required to prevent hearing loss from becoming permanent. Current treatment options are limited to hyperbaric oxygen and glucocorticoids such as dexamethasone or prednisolone, the current standard of care for SSNHL in humans. However, SSNHL treatment with glucocorticoids lacks approval due to unclear efficacy (2, 3, 16, 17). Therefore, the development of drugs to treat inner ear diseases is an enormous medical need that triggered development of several new therapeutic approaches against hearing loss (17).
We previously reported a pyridoindole compound with neuro-restorative potential in an animal model of Parkinson’s disease (18, 19), which prompted us to search for more effective derivatives. This resulted in the identification of 6-fluoro-9-methyl-pyridoindole (AC102), which we subsequently tested in models of neurological disorders. In this study, we set out to investigate the effects of AC102 against hearing loss in vivo and in vitro. Noise-induced hearing loss (NIHL) is a common form of SSNHL in the adult population (20). Therefore, NIHL in guinea pigs (GPs) is a well-accepted model that exhibit the key pathognomonic changes observed in many types of SSNHL including the loss of OHCs and IHC synaptopathy (21). It was selected to examine the protective and restorative potential of AC102 in hearing loss. To this end, we induced NIHL in the GPs and applied AC102 or vehicle into the middle ear after noise trauma and determined the hearing function 14 d after noise exposure. Moreover, we analyzed the number of OHCs and synaptic connections between IHCs and the auditory nerve. To this end, we analyzed the effects of AC102 on neuroprotection, synapse formation, mitochondrial function, free radical accumulation, and apoptosis.
Results
AC102 Restores Hearing in a NIHL Model.
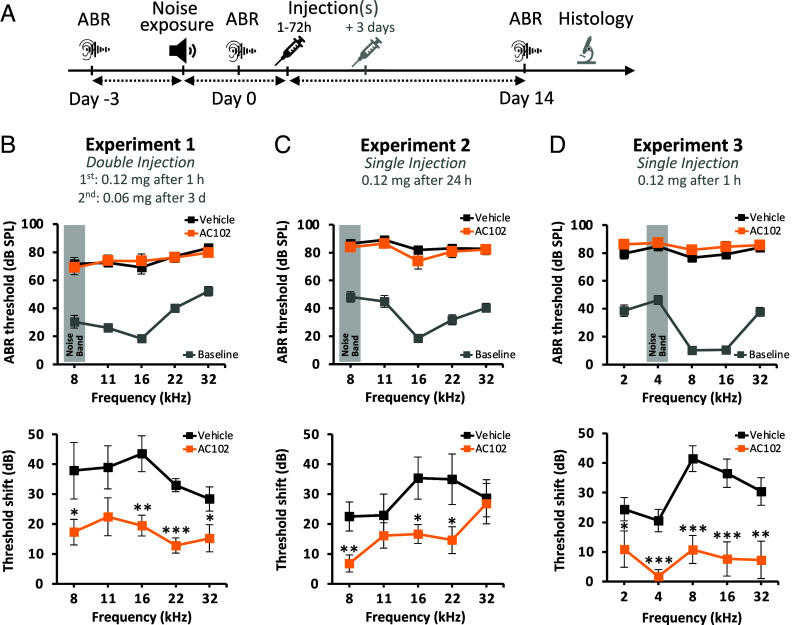
Our main goal was to investigate whether AC102 can reverse or even functionally repair noise-induced inner ear damage in a GP model of NIHL. To this end, we induced hearing loss in GPs by noise exposure and locally applied AC102 or vehicle after defined intervals into the middle ear (Fig. 1A). Auditory brainstem responses (ABRs) were recorded 3 d before noise exposure (D-3) to obtain baseline hearing thresholds and on the day of noise exposure (D0) to confirm the induction of a strong hearing threshold elevation. Finally, ABRs were recorded 14 d after noise trauma (D14) to assess hearing recovery when spontaneous repair has largely plateaued. Permanent threshold shift (PTS) was calculated as the threshold differences between D14 and D-3 for each frequency.
Fig. 1.
The effect of AC102 on hearing in a GP model of NIHL. (A) Schematic overview of the experimental NIHL model in GPs. (B–D, Upper panel) ABR hearing thresholds measured during different experimental settings. Audiograms illustrate the hearing loss induced by noise exposure (AC102: orange, Vehicle: black) compared to mean prenoise hearing thresholds (baseline, gray) of both groups prior to double or single injection of AC102. Generally, noise exposure led to a mean hearing loss of 80 ± 3 dB SPL over the measured frequencies. (B–D, Lower panel) PTS 14 d after noise exposure show that AC102 significantly improves hearing by 13 to 24 dB over the measured frequency range when applied twice and by 13 to 31 dB upon single injection (1 to 24 h) compared to vehicle controls. Data shown as mean ± SEM. Experiment 1: n = 6 (vehicle), n = 7 (AC102); experiment 2: n = 8 (vehicle), n = 7 (AC102); experiment 3: n = 11 (vehicle), n = 9 (AC102). *P < 0.05, **P < 0.01, ***P < 0.001.
To test the efficacy of AC102, we performed several independent experiments with different treatment regimens (Fig. 1 B–D). Viewed over all experiments, noise exposure [4 to 8 kHz octave noise band up to 125 dB sound pressure level (SPL) for 30 to 60 min] led to a severe temporary hearing threshold elevation to 80 ± 3 dB SPL over all frequencies. In the first set of experiments we performed double, unilateral injections, either 1 h and 3 d (Fig. 1B) or 72 h and 6 d after noise exposure (SI Appendix, Fig. S1). Animals treated with AC102 1 h and 3 d after trauma, exhibited a PTS of 17 ± 3 dB across all tested frequencies, whereas vehicle-treated GPs showed a PTS of 36 ± 3 dB (Fig. 1B; P = 0.0007). For animals receiving AC102 at 72 h and 6 d after trauma, the mean PTS over all tested frequencies was 17 ± 6 dB in AC102-treated animals versus 24 ± 4 dB in vehicle-treated controls (SI Appendix, Fig. S1B; P = 0.160). In our next experiment, we determined the efficacy of a single injection of AC102 administered 24 h posttrauma (Fig. 1C). This again led to a strong and highly significant reduction in PTS, underscoring the sufficiency of a single injection of AC102 to reach meaningful hearing recovery in this NIHL model. The PTS in vehicle-treated animals was 29 ± 5 dB compared to 16 ± 3 dB in AC102-treated animals (P = 0.020). Next, we evaluated if AC102 could improve hearing after noise exposure even at lower frequencies. Therefore, we shifted the center of the noise band from 8 kHz to 4 kHz. Analogously, we extended the tested frequencies in the ABRs down to 2 kHz. To also achieve a more stable PTS by reducing partial spontaneous recovery in control animals, we increased the noise trauma to 1 h at 125 dB SPL (Fig. 1D). Even in this more pronounced damage model, we observed a strong and highly significant improvement of hearing in animals that received an injection of AC102 1 h post noise exposure compared to vehicle-treated animals. AC102 application 1 h after noise exposure led to a significant recovery of hearing thresholds at all tested frequencies almost to pretrauma levels (PTS of 8 ± 4 dB) while in the vehicle-treated group the mean threshold shift showed only a moderate recovery over the time course of 14 d (PTS of 31 ± 3 dB; P = 0.0001) (Fig. 1D).
AC102 Maintains the OHC Population After Noise Exposure.
Elevated hearing thresholds following noise trauma can be mainly attributed to apoptotic injury of OHCs (10, 22). We thus evaluated the survival of OHCs after AC102 treatment in our NIHL GP model (Fig. 2). Cochleae were harvested on D14 and OHCs quantified along the organ of Corti. After a single injection of AC102 (24 h after noise exposure), treated animals showed a mean OHC loss of less than 7% across all cochlear regions compared to naïve animals not exposed to noise trauma. In contrast, vehicle-treated animals lost on average over 18% of OHCs across all analyzed regions compared to naïve controls (Fig. 2C). Notably, AC102 led to almost 95% survival of OHCs in the most basal regions (P = 0.017), whereas 93.6% of cells were present in the most apical region (P = 0.068). This finding is consistent with functional ABR data (Fig. 1C). Similar results were obtained after the double injection of AC102 at 1 h and 3 d after trauma, where only 6% of OHCs across all frequencies were lost in the AC102-treated group, compared to 21% in vehicle-treated animals (Fig. 2B). Additionally, we investigated whether AC102 prevents cell loss by inhibiting apoptotic cell death in vitro. The known apoptotic inducer staurosporine showed increased caspase 3/7 activities and elevated levels of cleaved caspase 3. Cotreatment with AC102 significantly reduced both the activity and protein levels of active caspase (SI Appendix, Fig. S3).
Fig. 2.
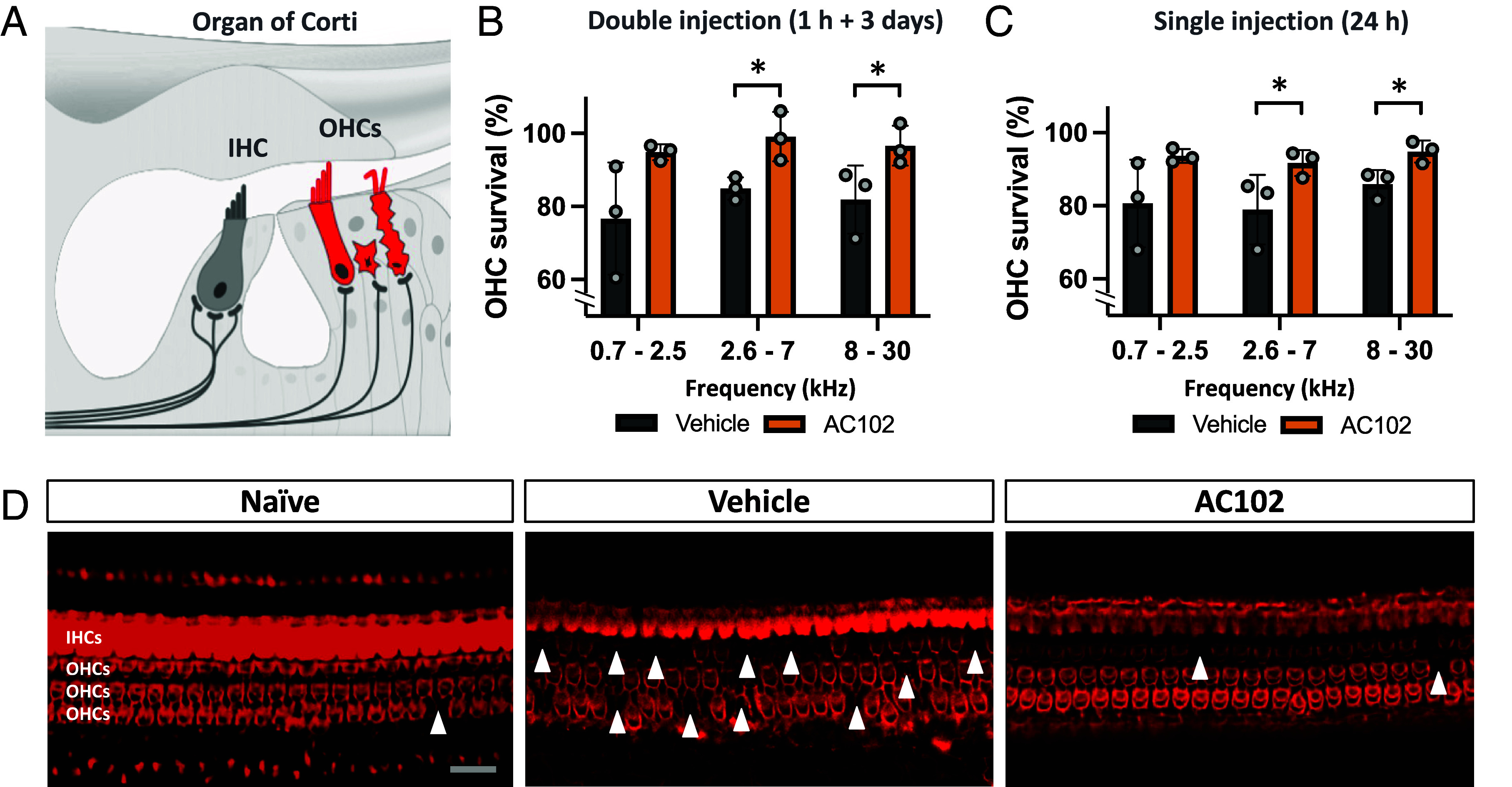
The effect of AC102 on OHC survival after noise exposure in vivo. (A) Schematic of OHCs and IHCs within the cochlea. (B) Quantification of OHCs 14 d after noise exposure revealed that AC102 significantly maintains OHC survival in the mid-basal cochlear region after double injection (1 h and 3 d after noise exposure; Fig. 1B) as well as (C) after single injection (24 h after noise exposure; Fig. 1C). Data shown as mean ± SEM. n = 3 animals per group, frequency range, and experiment. *P < 0.05. (D) Representative confocal images of the middle cochlear turn show the phalloidin stained sensory epithelium of naïve and vehicle- or AC102-treated ears. Arrowheads mark areas of lost OHCs. (Scale bar, 20 µm.)
AC102 Restores IHC Ribbon Synapses After Noise Exposure.
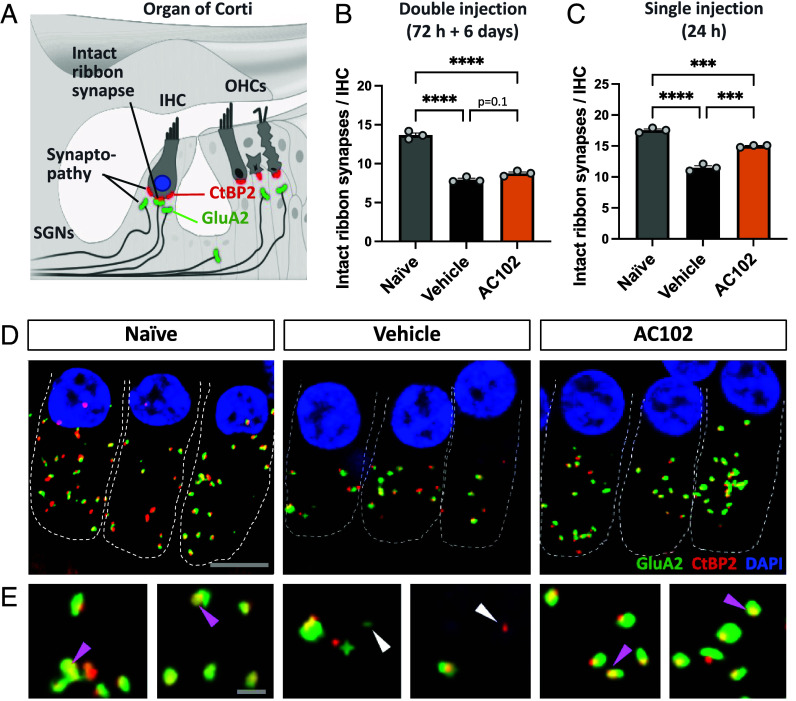
Cochlear synaptopathy, the loss of ribbon synapses and disconnection between IHCs and spiral ganglion neurites (SGNs), is a prominent feature in NIHL (23, 24). To evaluate the potency of AC102 to stop or reverse NIHL-induced synaptopathy, the number of ribbon synapses was quantified in confocal microscopy imaging (Fig. 3). We used organ of Corti tissues from the studies investigating the efficacy of AC102 after double (72 h and 6 d after noise exposure, SI Appendix, Fig. S1) and single injection (24 h after noise exposure, experiment 2, Fig. 1C). Our analysis showed a significant loss of paired ribbon synapses of 38%, averaged over both experiments, in vehicle-treated controls compared to naïve animals 14 d after noise trauma. In contrast, delayed, double application of AC102 (72 h and 6 d after noise exposure, Fig. 3B and SI Appendix, Fig. S1) led to a slightly higher number of intact, connected synapses per IHC (64%) compared to the vehicle group. This effect highly significantly increased (86%) when AC102 was applied once (24 h after noise exposure, Figs. 1C and 3C).
Fig. 3.
Effect of AC102 on synaptopathy after noise exposure in vivo. (A) Schematic overview of staining paradigm: pre- and postsynaptic proteins were stained by CtBP2 (red) and GluA2 (green), respectively. Colocalization labeled intact ribbon synapses, whereas individual staining labeled synaptopathy. SGNs = spiral ganglion neurites. (B) Quantification of pre- and postsynaptic pairs of CtBP2- and GluA2-labeled puncta 14 d after noise exposure at the 16 kHz region revealed that a delayed double injection of AC102 (72 h and 6 d after noise exposure; SI Appendix, Fig. S1) does not affect synaptopathy. (C) In contrast, a highly significant reduction of synaptopathy is observed when AC102 is locally applied as single injection (24 h after noise exposure; Fig. 1C). Data shown as mean ± SEM. n = 3 per group and experiment. ***P ≤ 0.001, ****P ≤ 0.0001. (D) Representative confocal images of IHCs and their ribbon synapses in naïve, vehicle and AC102-treated animals from experiment shown in C. (Scale bar, 10 µm.) (E) High magnification images of ribbon synapses from D. Purple arrows denote paired, and white arrows denote unpaired receptor patches. (Scale bar, 2 µm.)
Thus, AC102 reduces cochlear synaptopathy in a NIHL model in vivo. These effects were more pronounced when AC102 was applied closer to the time of injury.
AC102 Protects Cells from Rotenone- and Ethanol-Mediated Damage.
To analyze mechanisms underlying the phenotypes observed in our in vivo studies, we employed the mouse hippocampal neuron-derived immortalized cell line HT22. While direct conclusions about mechanisms of the inner ear cannot be drawn from this model, it allows us to assess biological pathways of neuronal cell function, providing insights into potential mechanisms of action of AC102 relevant to cochlear function. Our in vivo data showed that AC102 prevented HC loss after noise exposure in GPs within 14 d (Fig. 2). To substantiate these protective properties of AC102, we employed an in vitro model of acute cell damage using the mitochondrial toxin rotenone as an inducer. Rotenone primarily works by inhibiting the function of complex-I of the mitochondrial electron transport chain (25). We observed that treatment of HT22 cells with rotenone alone caused 70% damaged cells (P = 0.009) while AC102 showed a concentration-dependent significant protection against cell damage starting from cotreatment of 10 µM AC102 (P = 0.002) (Fig. 4 A and C).
Fig. 4.
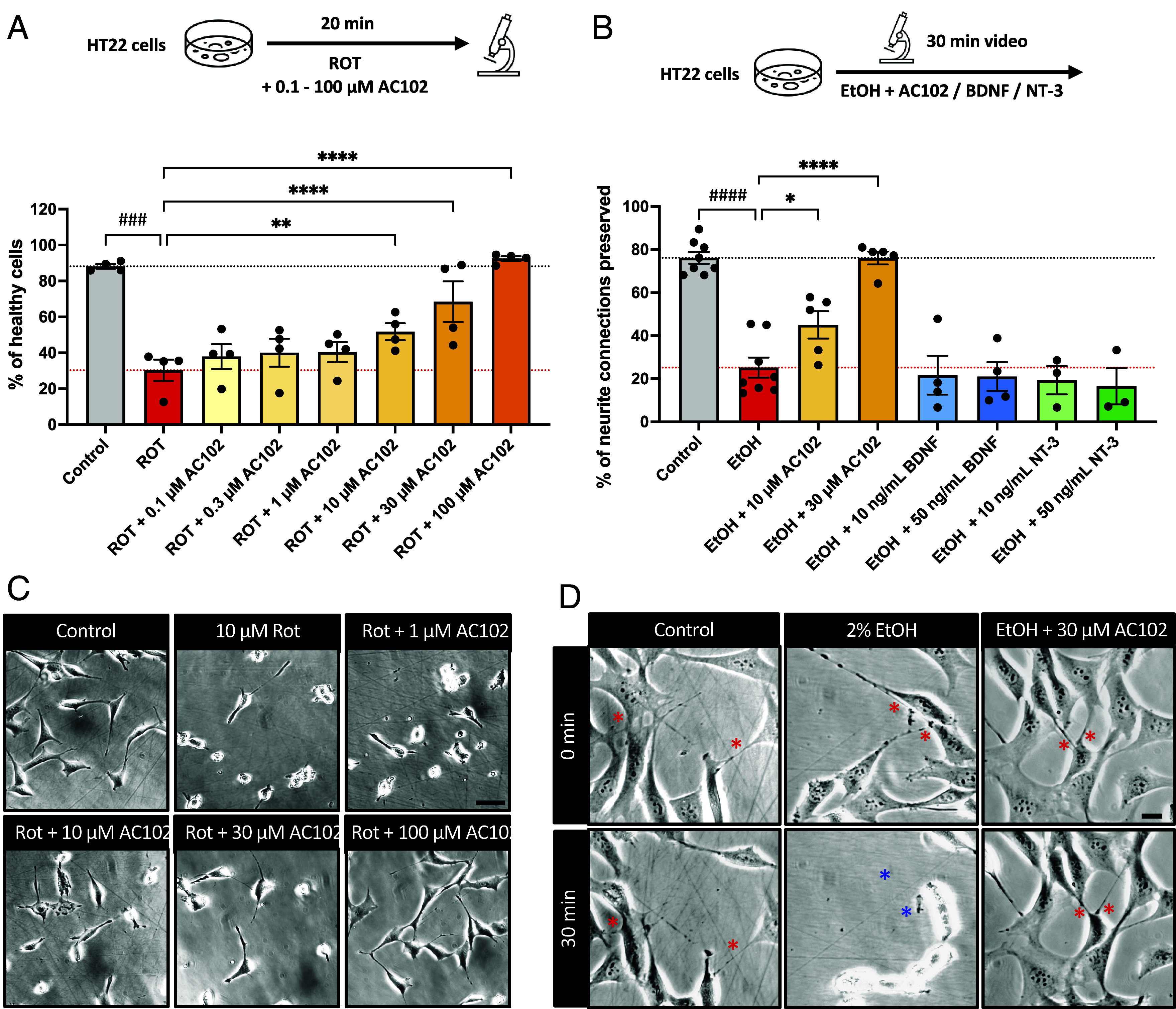
Protective effects of AC102 on cell damage and neurite connections. (A) Quantification of healthy cells in the presence of rotenone (ROT) alone or cotreatment of AC102. Shown are mean ± SEM of the percentage of cells that showed healthy morphology. n = 4 biological replicates (B) Quantification of preserved neurite connections in the presence of 2% ethanol (EtOH) alone or cotreatment of AC102. Shown are mean ± SEM of the percentage of neurite connections that were preserved throughout a 30 min observation period. n = 8 (for Control and EtOH), n = 5 (EtOH + AC102), n = 4 (EtOH + BDNF), n = 3 (EtOH + NT-3) biological replicates. ###P ≤ 0.001; ####P ≤ 0.0001 (one-sided paired t test); *P ≤ 0.05, **P ≤ 0.01, ****P ≤ 0.0001 (repeated measures one-way ANOVA and Fisher’s LSD test). (C and D) Representative images for A and B, respectively. Red asterisks indicate neurite connections and blue asterisks indicate lost connections. (Scale bar, 20 µm.)
HT22 cells produce neurites that form connections with other cells. These neurite connections mimic the in vivo neuronal connections which are critical for the function of neuronal networks. As neuronal connections between IHCs and SGNs are important for auditory function (26) we asked whether AC102 could protect neuronal connections. We developed a damage model of neuronal connection loss using 2% ethanol as a stressor. Treatment of HT22 cells with 2% ethanol caused significant loss of neuronal connections within 30 min. Only 25% (P < 0.0001) of connections were preserved (Fig. 4B). Interestingly, cotreatment of cells with 10 or 30 µM of AC102 showed 45% (P < 0.0001) and 76% (P = 0.022) preservation of neurite connection, respectively, which was almost identical to the 76% preservation seen after 30 min in control cells without ethanol damage (Fig. 4 B and D). The neurotrophins brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) have been shown to induce neurite outgrowth (27, 28), and are the two mainly expressed neurotrophins in the cochlea. Therefore, we also conducted these experiments in the presence of BDNF or NT-3. In contrast to AC102, these neurotrophins did not provide protection of neurite connections (SI Appendix, Fig. S2C).
AC102 Stimulates Neurite Outgrowth.
The data above suggest that AC102 may either prevent synaptopathy or restore ribbon synapse connections between IHCs and neurites of the spiral ganglion after noise exposure (Fig. 3). While prevention of synaptopathy may occur through neuroprotective mechanisms as shown in Fig. 4, the repair of synapses would require neurite regrowth. We therefore evaluated whether AC102 can stimulate neurite outgrowth in vitro. Exposure of HT22 cells to varying concentrations of AC102 induced a significant increase in neurite outgrowth per cell, compared to control cells treated with vehicle alone (Fig. 5A). This effect was concentration-dependent, starting in the nanomolar (nM) range and plateauing in the micromolar (µM) range. An increase in neurite outgrowth was first observed at 3 nM AC102 (10% more neurite outgrowth), which became significant at 100 nM AC102 (25% increase; P = 0.003) and plateaued at 10 µM AC102 (30% increase, P = 0.0006). We also quantified the neurite growth–enhancing effects of AC102 on HT22 cells by immunostaining with ß-III tubulin. We observed a similar significant effect on neurite outgrowth in the presence of AC102 at higher concentrations (SI Appendix, Fig. S2 A and B).
Fig. 5.
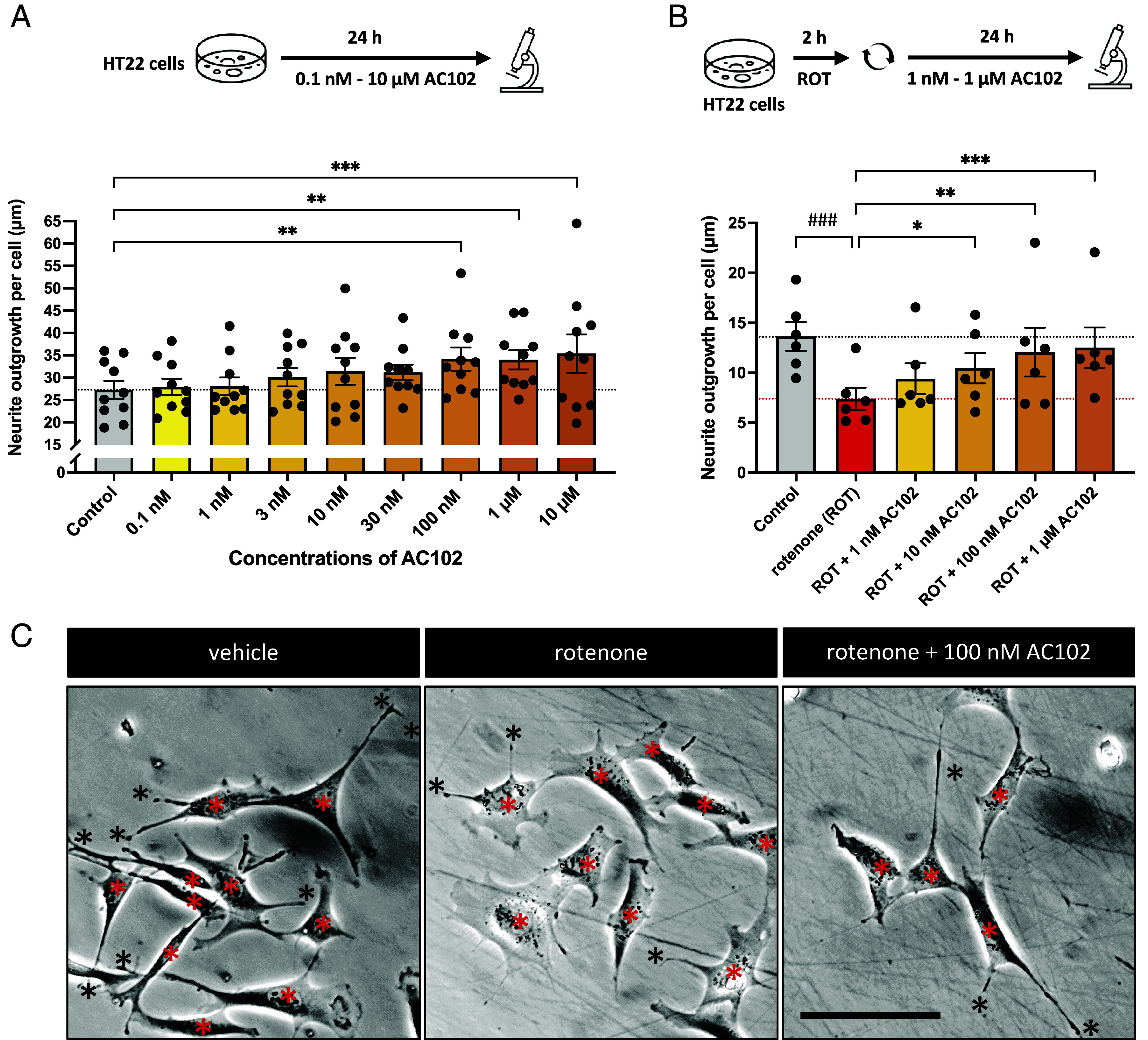
Neuroregenerative properties of AC102. (A) Effects of varying concentrations of AC102 on neurite outgrowth in HT22 cells. Shown are mean ± SEM (n = 10). Mixed effects analysis and Fisher’s LSD test for comparison of AC102-treated groups against vehicle (**P ≤ 0.01, ***P ≤ 0.001). (B) Regenerative effect of AC102 of neurite outgrowth in a rotenone damage model, in which different concentrations of AC102 were applied to cells for 24 h after a 2 h damage with rotenone. Shown are mean ± SEM (n = 6). One-sided paired t test for damage model (###P ≤ 0.001). Repeated measures one-way ANOVA and Fisher’s LSD test for comparison of AC102-treated groups against damage alone (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001). (C) Representative images from selected groups of b. Cells and neurites are marked with red and black asterisks, respectively. (Scale bar, 100 µm.)
Because of the known effect of neurotrophins on neurite outgrowth (27), we repeated the experiment, comparing the neurite growth–enhancing effect of selected concentrations of AC102 to that of different concentrations of BDNF or NT-3. At all concentrations tested, the effect of neurotrophins was less pronounced than that of AC102 (SI Appendix, Fig. S2C).
Next, we analyzed the neurite growth–enhancing effects of AC102 in an injury model to evaluate whether AC102 could elicit regenerative effects on neurites after cell damage. We adapted a rotenone damage model (29) for HT22 cells and optimized timing and concentration until we reached a neurite outgrowth reduction of around 50% with minimal cell death. This was achieved with a 2 h treatment with 10 µM rotenone. After the rotenone injury, we replaced the media and observed neurite outgrowth for an additional 24 h (Fig. 5 B and C) in the presence of varying concentrations of AC102. Rotenone-treated cells showed a 46% decrease in neurite outgrowth compared to vehicle-treated cells (P = 0.001). Treatment with AC102 after the damage restored neurite outgrowth in a concentration-dependent manner. AC102 concentrations as low as 10 nM reduced the rotenone-induced decrease of neurite outgrowth to 23% vs. 46%, and 1 µM AC102 reduced it to 8.4% vs. 46% (P = 0.027 and P = 0.0006, respectively).
These data provide clear evidence of the neurite growth–enhancing properties of AC102 in healthy (Fig. 5A) as well as damaged cells (Fig. 5B), even at low nM concentrations.
AC102 Reduces the Levels of ROS.
Thus far our data suggests a protective and neurite growth–enhancing role of AC102. Next, we evaluated potential molecular mechanisms underlying the protective effects of AC102. As increased ROS is a major cause for OHC death after noise exposure, mainly affecting mitochondria, we sought out to understand whether AC102 has any ROS-reducing effect. For determining this, we treated HT22 cells with the ROS inducer antimycin A alone or in combination with different concentrations of AC102 for 20 min. Treatment of cells with 100 µM of antimycin A significantly increased the levels of ROS while cotreatment of AC102 with antimycin A significantly reduced the ROS levels at 30 and 100 µM AC102 by 41 % (P < 0.0001) and 83 % (P < 0.0001), respectively (Fig. 6A). These data indicate that protective effects of AC102 may be mediated by its ROS-reducing properties.
Fig. 6.
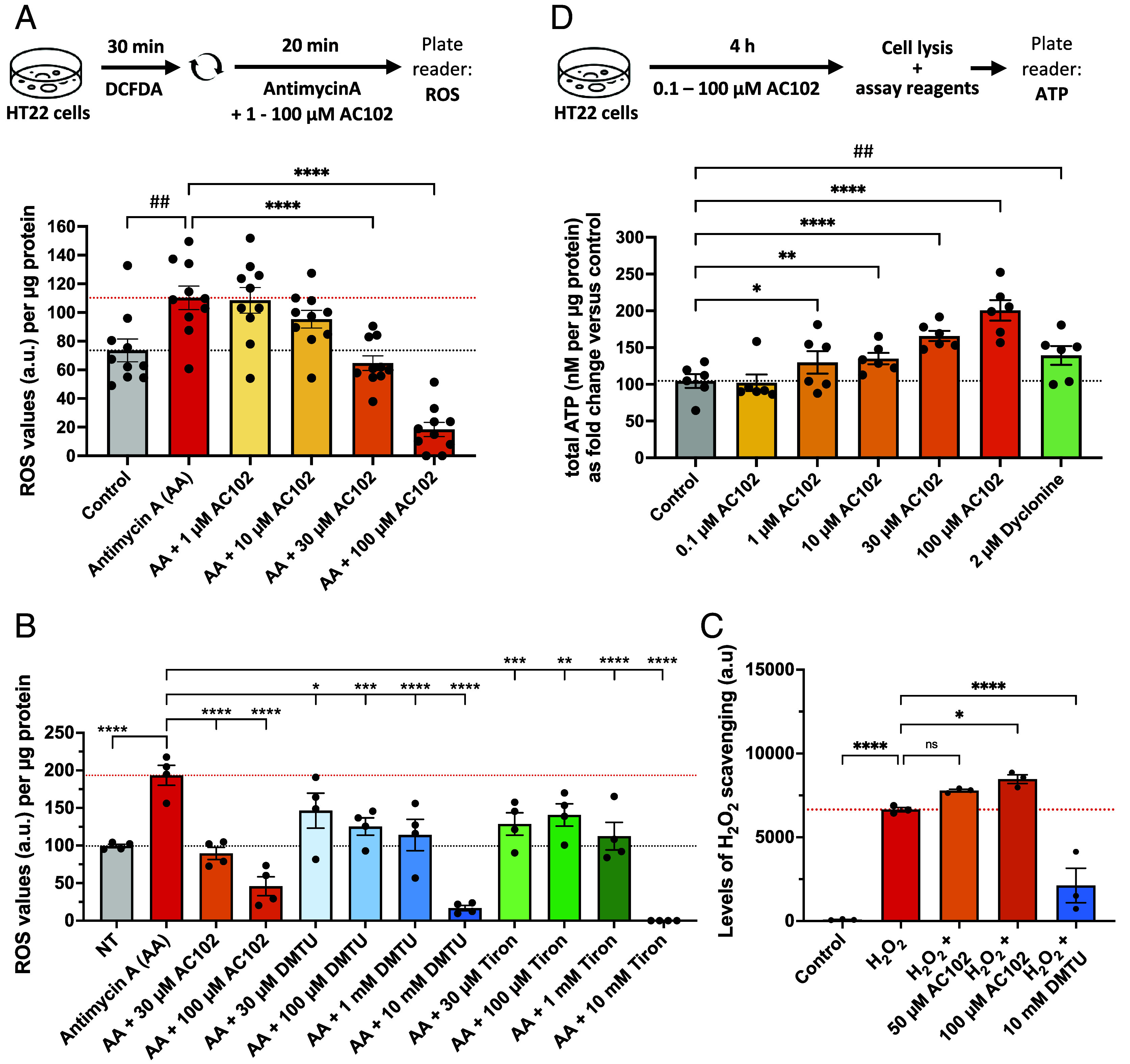
AC102 reduces ROS levels and elevates ATP production. (A) Quantification of DCFDA fluorescence in the presence of ROS inducer antimycin A alone or cotreatment with varying concentrations of AC102 (n = 10). (B) Quantification of DCFDA fluorescence in the presence of ROS inducer antimycin A alone (red) or cotreatment with selected concentrations of AC102 (orange) or cotreatment with varying concentrations of established ROS scavengers (blue, green) (n = 4). (C) Quantification of H2O2 scavenging in cell-free assay in the presence of AC102 or DMTU (n = 3). (D) Total ATP production of HT22 cells, treated with varying concentrations of AC102 or Dyclonine as positive control (n = 6). Shown are mean ± SEM. One-sided paired t test (##P ≤ 0.01). Repeated measures one-way ANOVA and Fisher’s LSD test for comparison of AC102-treated groups against vehicle (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001).
To compare the ROS-reducing properties of AC102 to those of established ROS scavengers, we repeated the experiment including cotreatments with N,N′-dimethylthiourea (DMTU), a scavenger of mainly H2O2 and Tiron, a scavenger of mainly O2−. Applied in typical concentrations of 10 mM, both of these scavengers greatly reduced ROS (reduction of 91% and 100%, respectively, with each P < 0.0001). At a concentration of 100 µM AC102, reduced ROS by 76%, DMTU by 35%, and Tiron by 27% (Fig. 6B). Moreover, to understand whether AC102 has a direct scavenging role on ROS we coincubated H2O2 in a cell-free environment with AC102 or DMTU. We noticed DMTU was able to significantly reduce H2O2 levels (68%) but not AC102. This suggests that ROS reducing properties of AC102 could be at the cellular level (Fig. 6C).
AC102 Increases ATP Production.
Neurite outgrowth is an energy-demanding process (30) and evidence suggests that noise-induced death of OHCs is at least partly related to their vulnerability due to their high energy demand (31). We therefore investigated in vitro whether AC102 acts beneficially on cellular ATP production. Indeed, AC102 promoted a significant increase in ATP production compared to vehicle-treated cells (Fig. 6D). An increase in ATP production was observed with as low as 1 µM AC102 (24% increase; P = 0.027). This effect was concentration-dependent, and the ATP increase reached 92% at 100 µM AC102 (P < 0.0001), which was even higher than the positive control of dyclonine (33% increase; P = 0.003), a known inducer of ATP production (32).
Discussion
To date, the vast majority of studies aiming at restoration of hearing in SNHL have targeted either regeneration of HCs through genetic manipulation (33–35), or prevention of OHC loss through antiapoptotic (36), antioxidative (37), or anti-inflammatory agents such as glucocorticoids (38, 39). None of these approaches, aiming at OHC protection, have demonstrated effects on synaptopathy. This may explain why many promising preclinical results (40, 41) failed in the transition into the clinic and warrant for multimodal approaches.
In the current study, we present functional in vivo and histological evidence of significant recovery of hearing in a GP model of NIHL. We report that both immediate (1 h) and delayed application of AC102 after acoustic trauma (24 or 72 h) showed improvement in functional hearing, while the decrease of the effect over time supports the concept of a window of opportunity for successful treatment. Similar results were obtained both after single (1 h) and double application (1 h and 3 d) of AC102. Therefore, a single dose of AC102 with a remarkably low effective amount (0.12 mg AC102 per GP) was as effective as repeated application. Hearing thresholds normalized to almost pretrauma levels, achieving a mean improvement up to 31 dB at 8 kHz (being within the optimal hearing range of GPs) compared to the vehicle control and highlighting AC102’s therapeutic potential against acute hearing loss.
In our study, we demonstrated that AC102 can mitigate noise-induced loss of HCs. Cytocochleograms obtained after double (1 h + 3 d) and single (24 h) application of AC102 show a similar effect on OHC survival. The mean survival rate increased by a maximum of 16% compared to the vehicle group and resulted in almost complete preservation of the sensory epithelium. These data are consistent with functional hearing measurements, confirming that there exists a window of opportunity during which AC102 can confer benefits and that single dosing with AC102 is sufficient to maintain auditory HC viability after noise exposure.
Acoustic trauma can lead to ROS formation, glutamate-induced oxidative stress, and activation of caspase-3, triggering a sequence of events within the cochlea that can culminate in apoptosis and regulated necrosis of auditory HCs (42, 43). In the context of hearing loss, a transient ATP depletion has been shown in the cochlear perilymph following acoustic trauma (8) and it has been proposed that transient ATP depletion weakens the ability of the cochlea to recover from acoustic trauma (31). Both factors suggest that acoustic trauma causes an acute mitochondrial dysfunction. Additionally, a maintenance of ATP homeostasis and reduction of ROS levels both have been implicated in reducing OHCs loss and improvement of hearing function (44). Antioxidants and free radical scavengers that can neutralize ROS and downstream effects of oxidative stress have been shown to protect HCs and auditory function in NIHL and drug-induced hearing loss (45, 46). Our in vitro data show that AC102 is able to reduce antimycin A-induced ROS formation. Compared to established ROS scavengers, its effect at the tested concentrations of 30 and 100 µM is stronger than those of ROS scavengers DMTU and Tiron. Importantly, our cell-free assay data further suggest that AC102 lacks direct scavenging action but instead prevents mitochondrial ROS formation. In vitro, AC102 reduced staurosporine-induced caspase 3/7 activity and expression of active caspase 3 [SI Appendix, Fig. S3; (47)]. In summary, these protective mechanisms of AC102 may contribute to increased survival of OHCs.
While OHCs are vulnerable to the apoptotic and necrotic processes initiated by noise exposure, in vivo NIHL models consistently demonstrate that the ribbon synapses connecting the IHC to the SGN are the cochlear structures most vulnerable to noise trauma, due, at least in part, to their susceptibility to glutamate excitotoxicity (48). Rapid (within 24 h) cochlear synaptopathy which may only partially reverse over the following weeks, is a prominent feature in GP models of NIHL (23, 24). In agreement with these studies, we observed that noise exposure led to substantially fewer intact synapses per IHC at 14 d post trauma than the IHC of naïve controls. A highly significant increase in the restoration of synapses was observed following application of AC102 shortly (24 h) post acoustic trauma.
Anatomical measurements of cochlear ribbon synapses are based on confocal microscopy with antibodies labeling the presynaptic ribbons (CtBP2) and postsynaptic glutamate receptors (GluA2) (49). Morphological analysis of the synaptic complex and neurites provides evidence for the potential of synaptic and neurite plasticity following noise exposure, namely re-extension and synaptogenesis even in the fully mature GP ear (23). AC102 may reactivate repair mechanisms following noise exposure to increase synaptic recovery and regeneration.
In vitro, a rapid (within 24 h) concentration-dependent stimulation of neurite outgrowth was observed when AC102 was applied after 2 h of neuronal injury induced by rotenone. Importantly, the effects of AC102 on neurite outgrowth are mirrored in a second in vivo model of otic injury. A single application of AC102 led to near-complete preservation of both IHC synapses and auditory nerve fibers (ANFs) throughout the cochlea in a model of electrode insertion trauma (47). Taken together, these in vivo and in vitro data suggest a restorative and/or protective effect of AC102 on neuronal processes. In the literature, rescue of the synaptopathic phenotype has, so far, only been demonstrated by treatment with neurotrophins (50). However, while neurotrophins exert potent neuroregenerative effects, there is no evidence to date that neurotrophins prevent apoptosis of OHCs.
Postmortem studies of human temporal bones have found that subjects with a history of noise exposure exhibit loss of ANFs and that this loss is predictive of poor word discrimination (51). Moreover, an age-related loss of cochlear synapses has been reported in humans with no history of otological disease (51, 52).This underscores the importance of IHC ribbon synapses in speech perception, not just in individuals with normal audiograms (i.e. hidden hearing loss) but also in patients with measurable hearing impairments. This highlights the need to develop therapies that address not only damage to the HCs themselves but also cochlear synaptopathy and ANF degeneration. Neuroregeneration is energy demanding and neurite outgrowth has been linked to neuronal energy levels in vitro (30) and in vivo (53). Therefore, the observed stimulation of ATP production might contribute to the detected recovery of the ribbon synapses.
In conclusion, the data presented here demonstrate that AC102 may be a suitable therapeutic for the treatment of hearing loss associated with damage to OHCs, cochlear synaptopathy, and increased hearing thresholds: pathologies that underlie many forms of SSNHL (Fig. 7). These data show comprehensively the bimodal effect of a single compound on both OHCs survival and IHC synaptopathy, leading to an effective improvement of hearing function. Clinical data from a Phase I study in healthy volunteers have shown that AC102 is safe and well tolerated. Currently, AC102 is under evaluation in comparison to oral steroids in a Phase II clinical trial for the treatment of idiopathic SSNHL (ClinicalTrials.gov, NCT05776459).
Fig. 7.
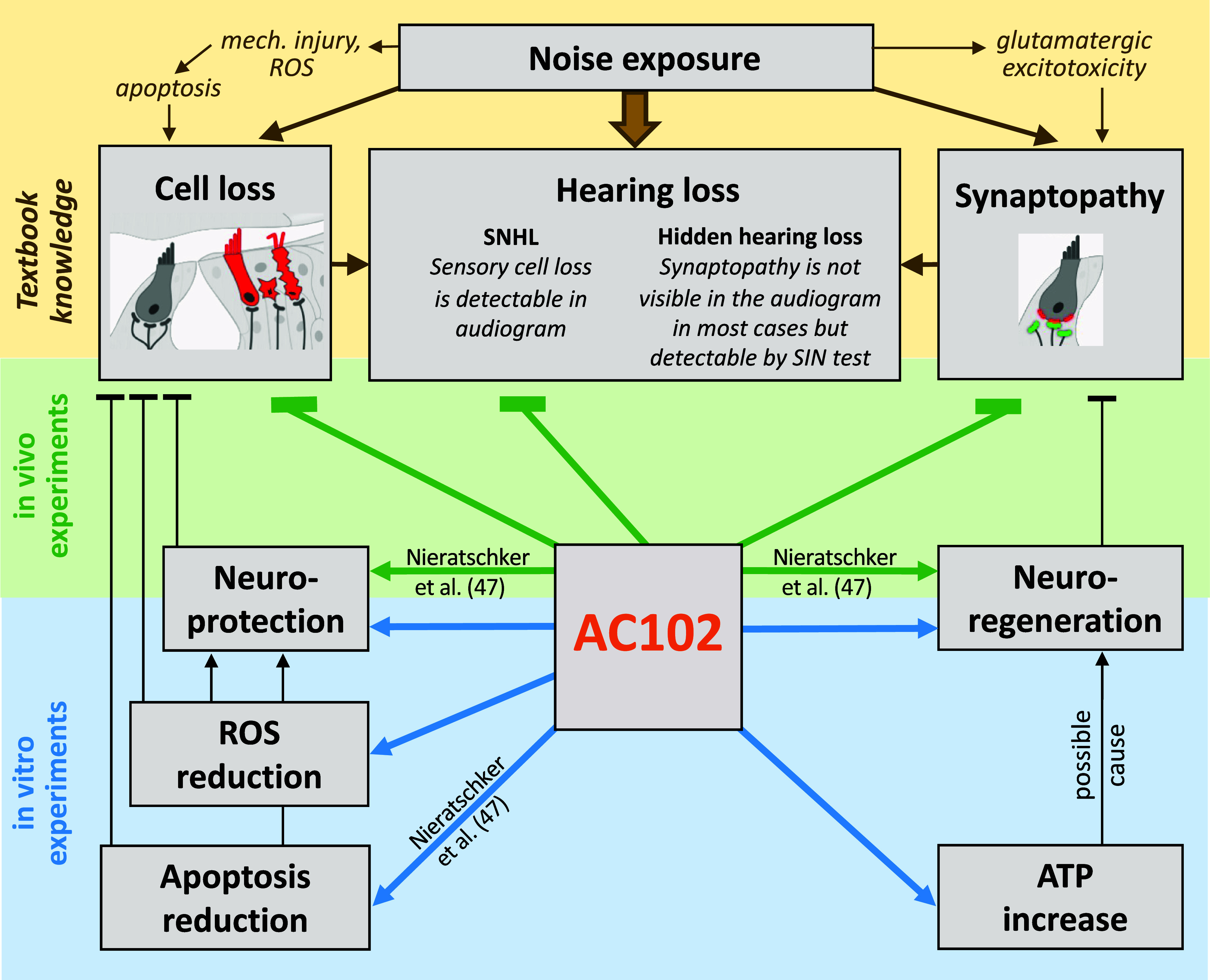
Graphical overview of experimental findings. Hearing loss, as a direct consequence of noise trauma is caused by loss of OHCs as well as synaptopathy. Our in vivo studies show compelling evidence that AC102 can prevent OHC loss, reverse synaptopathy, and subsequently restore hearing after acoustic exposure. Our in vitro experiments demonstrate neuroprotective and neuroregenerative properties of AC102 and revealed a reduction of ROS and increase of ATP as potential molecular mechanism that could explain the observed in vivo effects. SIN = Speech-in-Noise.
Materials and Methods
An extended description of the materials and methods used in the present manuscript is provided as SI Appendix, Materials and Methods.
Animal Model of NIHL, Drug Administration, and Hearing Assessment.
All animal experiments were approved by the Animal Use and Care Committee of the Senate of Berlin (reference no. G0199/15, A0187/17) and were conducted in accordance with the regulations based on the EU-directive 2010/63/EU. Fully anesthetized male Dunkin Hartley albino GPs (Envigo, 450 to 1,400 g) were exposed to a single continuous noise band of 1 octave centered at 4 kHz (125 dB SPL, 1 h) or at 8 kHz (120 dB SPL, 30 min) inside a sound-proof chamber (e.g., refs. 54 and 55). In total, 57 animals were used for the in vivo experiments [experiment 1: n = 6 (vehicle), n = 7 (AC102); experiment 2: n = 8 (vehicle), n = 7 (AC102); experiment 3: n = 11 (vehicle), n = 9 (AC102)]. Furthermore, three naïve animals were included as untreated controls for each experiment.
The intratympanic application of AC102 was performed unilaterally as a sterile suspension of 10 µL (0.12 mg) in a poloxamer-based hydrogel (e.g., refs. 56 and 57) at defined time points after the noise trauma. For double injections, a second dose of 5 µL (0.06 mg) was administered 3 d after the initial dose. The concentration of 0.12 mg AC102 was selected based on a previously performed in vivo dose–response study.
Auditory function was measured by recording ABRs 3 d before, on the day of, and 14 d after noise exposure. The TDT System 3 (Tucker-Davis-Technologies, Alachua, FL) was used for presentation of stimuli and recording of ABRs. Anesthetized animals were presented sinus tones with frequencies between 2 and 32 kHz for a duration of 10 ms in 5 dB steps from 0 to 90 dB SPL. ABR analysis was performed under fully blinded conditions using the TDT BioSigRZ software.
Immunohistochemistry, Image Acquisition, and Histological assessment.
Cochleae were dissected, fixated, and stained with primary antibodies against presynaptic C-terminal-binding protein 2 (anti-CtBP2, #612044, BD, Franklin Lakes, NJ) and postsynaptic glutamate ionotropic receptor AMPA type subunit 2 (anti-GluA2, #MAB397, Millipore, Burlington, MA). After washing, samples were stained with appropriate secondary antibodies and a marker against F-actin (phalloidin 405, #A30104, Thermo Fisher Scientific, Waltham, MA) to visualize HCs. After washing the sensory epithelium was dissected out from the cochlear tissue, cut into sections and flat mounted in a tonotopic manner on slides.
Ribbon synapses and OHCs were imaged with 40× and 20× oil immersion lenses, respectively, at an SP5 confocal microscope (Leica, Wetzlar, Germany). Phalloidin-stained OHCs were counted manually with ImageJ (version 1.53t, NIH) and represented per µm length of the sensory epithelium. OHCs were considered lost when both stereocilia bundles and cuticular plates were absent. For the scoring of ribbon synapses, each punctum with colocalized CtBP2 and GluA2 signal was scored as an intact, connected synapse and presented as synapses per IHC (58, 59). A minimum of four z-stacks were analyzed per cochlea and synapses from at least ten IHCs per z-stack quantified.
Cell Damage, Neurite Connection, and Neurite Outgrowth Assays.
For the cell damage assay, mouse hippocampal neuron-derived immortalized neuronal cells (HT22; SCC129, Sigma-Aldrich, St. Louis, MO) were treated with 10 µM rotenone (R8875-1G, Merck, Darmstadt, Germany) alone or cotreated with varying concentrations of AC102 for 20 min. After imaging, damaged cells (identified by round morphology) and healthy cells (rather oval shaped or looking like typical neuronal cells, often with neurites) were quantified using ImageJ software.
For the neurite connection assay, time-lapse videos were acquired for 30 min and videos were analyzed using ImageJ. Number of neurite connections (neurites touching a cell or another neurite) per cell were quantified at time “0” and “30” min, and the number of connections lost between the time points was reported as percentage of original connections.
For the neurite outgrowth assay, HT22 cells were treated with appropriate concentrations of either AC102, or one of the neurotrophins, BDNF (Biochrom AG, Berlin, Germany), or NT-3 (Peprotech, Cranbury, NJ). For neurite outgrowth after rotenone damage, cells were treated with 10 µM rotenone for 2 h after which rotenone was exchanged for fresh media containing vehicle or varying concentrations of AC102. After 24 h treatment, imaging was performed, neurites and cells were quantified by ImageJ and represented as total neurite length per cell.
ROS and ATP Assays.
For ROS measurement, HT22 cells were treated with carboxy-H2-dichlorodihydrofluorescein diacetate (DCFDA) reagent (C400, Thermo Fisher Scientific, Waltham, MA) in Hank’s balanced salt (HBSS) medium (14175-095, Gibco, Thermo Fisher Scientific, Waltham, MA) for 30 min, washed and treated with 100 µM of ROS inducer antimycin A (J63522, Sigma-Aldrich, St. Louis, MO) alone or with different concentrations of AC102 or DMTU (D188700, Sigma-Aldrich, St. Louis, MO) or Tiron (A0447328, Thermo Fisher Scientific, Waltham, MA) for 20 min in DMEM. After the incubation, DCFDA fluorescence (Ex: 485 nm; Em: 535 nm) was measured at ClarioStar cell analyzer (BMG Labtech, Ortenberg, Germany) and blanks from cell-free wells were subtracted. For cell-free ROS assays, 1 µM H2O2 was incubated with AC102 or with DMTU for 2 h and levels of H2O2 was determined by luminescence-based assay (ROS-Glo, G8820, Promega, Madison, WI) and normalized to protein levels. For ATP measurement, HT22 cells were treated with varying concentrations of AC102 for 4 h. Total ATP production was measured with the Luminescence ATP Detection Assay Kit (Cayman Chemical, 700410) according to the manufacturer’s instructions. All data were normalized against the protein levels determined by the bicinchoninic acid (BCA) assay (23223, Pierce, Thermo Fisher Scientific, Waltham, MA) under the same experimental conditions.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank Dr. Jörn Niessing for help with graphical illustrations and Dr. Christoph Enzensperger for the synthesis of AC102.
Author contributions
H.R., S.B., B.B., R.W., T.Z., S.K.P., J.H.-R., and R.S. designed research; S.B., B.B., R.W., M.K., T.Z., F.T., B.Ü., and N.E. performed research; S.B., B.B., R.W., M.K., T.Z., F.T., B.Ü., N.E., M.N., and C.A. analyzed data; H.R., J.H.-R. and R.S. interpreted data; S.K.P. interpreted results; J.H.-R. designed methods; and H.R., S.B., B.B., R.W., M.K., and R.S. wrote the paper.
Competing interests
H.R., S.B., B.B., R.W., M.K., B.Ü., N.E., and R.S. are current employees and T.Z. and F.T. are former employees of AudioCure Pharma GmbH, which is developing treatments for sudden sensorineural hearing loss. H.R. and R.S. own shares of AudioCure Pharma GmbH. H.R., T.Z., and R.S. are named on patents from AudioCure Pharma GmbH, covering AC102 for use in otic diseases. J.H.-R. has an ongoing paid collaboration with AudioCure Pharma GmbH. C.A. is currently holding a grant from the Christian Doppler Research Association (CD Laboratory for Inner Ear Research) and is receiving funding from MED-EL Corporation, Innsbruck, Austria. S.K.P. is a scientific board member and was previously a paid consultant of AudioCure Pharma GmbH. M.N. and C.A. are and were (Principal) Investigators in previous and ongoing clinical studies of AudioCure Pharma GmbH. S.K.P. is a coordinating investigator of one of their ongoing clinical studies.
Footnotes
This article is a PNAS Direct Submission. A.G.C. is a guest editor invited by the Editorial Board.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Chau J. K., Lin J. R., Atashband S., Irvine R. A., Westerberg B. D., Systematic review of the evidence for the etiology of adult sudden sensorineural hearing loss. Laryngoscope 120, 1011–1021 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Marx M., et al. , International consensus (ICON) on treatment of sudden sensorineural hearing loss. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 135, S23–S28 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Chandrasekhar S. S., et al. , Clinical practice guideline: Sudden hearing loss (update) executive summary. Otolaryngol. Head Neck Surg. 161, 195–210 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Dallos P., Cheatham M. A., Cochlear hair cell function reflected in intracellular recordings in vivo. Soc. Gen. Physiol. Ser. 47, 371–393 (1992). [PubMed] [Google Scholar]
- 5.Shi X., Nuttall A. L., Upregulated iNOS and oxidative damage to the cochlear stria vascularis due to noise stress. Brain Res. 967, 1–10 (2003). [DOI] [PubMed] [Google Scholar]
- 6.Yamashita D., Jiang H. Y., Schacht J., Miller J. M., Delayed production of free radicals following noise exposure. Brain Res. 1019, 201–209 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Yamashita D., Miller J. M., Jiang H. Y., Minami S. B., Schacht J., AIF and EndoG in noise-induced hearing loss. Neuroreport 15, 2719–2722 (2004). [PubMed] [Google Scholar]
- 8.Chen F. Q., Zheng H. W., Hill K., Sha S. H., Traumatic noise activates Rho-family GTPases through transient cellular energy depletion. J. Neurosci. 32, 12421–12430 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei-Ju H., Xiao-Rui S., Nuttall A., Noise exposure induced cochlear hair cell death pathways in guinea pig. J. Otol. 5, 51–56 (2010). [Google Scholar]
- 10.Wong A. C., Ryan A. F., Mechanisms of sensorineural cell damage, death and survival in the cochlea. Front. Aging Neurosci. 7, 58 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng H. W., Chen J., Sha S. H., Receptor-interacting protein kinases modulate noise-induced sensory hair cell death. Cell Death Dis. 5, e1262 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen J. B., Lysaght A. C., Liberman M. C., Qvortrup K., Stankovic K. M., Immediate and delayed cochlear neuropathy after noise exposure in pubescent mice. PLoS ONE 10, e0125160 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberman M. C., Kujawa S. G., Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear. Res. 349, 138–147 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu N., Rutherford M. A., Green S. H., Protection of cochlear synapses from noise-induced excitotoxic trauma by blockade of Ca(2+)-permeable AMPA receptors Proc. Natl. Acad. Sci. U.S.A. 117, 3828–3838 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan M., Agerman K., Ernfors P., Canlon B., Complementary roles of neurotrophin 3 and a N-methyl-D-aspartate antagonist in the protection of noise and aminoglycoside-induced ototoxicity. Proc. Natl. Acad. Sci. U.S.A. 97, 7597–7602 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei B. P., Stathopoulos D., O’Leary S., Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database Syst. Rev. 2013, CD003998 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schilder A. G. M., et al. , Early phase trials of novel hearing therapeutics: Avenues and opportunities. Hear. Res. 380, 175–186 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Wernicke C., et al. , 9-methyl-β-carboline has restorative effects in an animal model of Parkinson’s disease. Pharmacol. Rep. 62, 35–53 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Frenzel M., Rommelspacher H., Sugawa M. D., Dencher N. A., Ageing alters the supramolecular architecture of OxPhos complexes in rat brain cortex. Exp. Gerontol. 45, 563–572 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Cunningham L. L., Tucci D. L., Hearing loss in adults. N. Engl. J. Med. 377, 2465–2473 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naert G., Pasdelou M. P., Le Prell C. G., Use of the guinea pig in studies on the development and prevention of acquired sensorineural hearing loss, with an emphasis on noise. J. Acoust. Soc. Am. 146, 3743 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibata S. B., Raphael Y., Future approaches for inner ear protection and repair. J. Commun. Disord. 43, 295–310 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hickman T. T., Hashimoto K., Liberman L. D., Liberman M. C., Cochlear synaptic degeneration and regeneration after noise: Effects of age and neuronal subgroup. Front. Cell Neurosci. 15, 684706 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song F., Gan B., Wang N., Wang Z., Xu A. T., Hidden hearing loss is associated with loss of ribbon synapses of cochlea inner hair cells. Biosci. Rep. 41, BSR20201637 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li N., et al. , Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 278, 8516–8525 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Sun S., et al. , Hair cell mechanotransduction regulates spontaneous activity and spiral ganglion subtype specification in the auditory system. Cell 174, 1247–1263.e1215 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwasaki K., Isaacs K. R., Jacobowitz D. M., Brain-derived neurotrophic factor stimulates neurite outgrowth in a calretinin-enriched neuronal culture system. Int. J. Dev. Neurosci. 16, 135–145 (1998). [DOI] [PubMed] [Google Scholar]
- 28.Huang E. J., Reichardt L. F., Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677–736 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krug A. K., et al. , Evaluation of a human neurite growth assay as specific screen for developmental neurotoxicants. Arch. Toxicol. 87, 2215–2231 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Trigo D., Goncalves M. B., Corcoran J. P. T., The regulation of mitochondrial dynamics in neurite outgrowth by retinoic acid receptor beta signaling. FASEB J. 33, 7225–7235 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mao H., Chen Y., Noise-induced hearing loss: Updates on molecular targets and potential interventions. Neural Plast. 2021, 4784385 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varkuti B. H., et al. , High-throughput small molecule screen identifies modulators of mitochondrial function in neurons. iScience 23, 100931 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Izumikawa M., et al. , Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat. Med. 11, 271–276 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Kraft S., Hsu C., Brough D. E., Staecker H., Atoh1 induces auditory hair cell recovery in mice after ototoxic injury. Laryngoscope 123, 992–999 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng F., Zuo J., Cochlear hair cell regeneration after noise-induced hearing loss: Does regeneration follow development? Hear. Res. 349, 182–196 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eshraghi A. A., et al. , Preclinical and clinical otoprotective applications of cell-penetrating peptide D-JNKI-1 (AM-111). Hear. Res. 368, 86–91 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Kil J., Pierce C., Tran H., Gu R., Lynch E. D., Ebselen treatment reduces noise induced hearing loss via the mimicry and induction of glutathione peroxidase. Hear. Res. 226, 44–51 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Trune D. R., Canlon B., Corticosteroid therapy for hearing and balance disorders. Anat. Rec. (Hoboken) 295, 1928–1943 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plontke S. K., et al. , Efficacy and safety of systemic, high-dose glucocorticoid therapy for idiopathic sudden sensorineural hearing loss: Study protocol for a three-armed, randomized, triple-blind, multicenter trial (HODOKORT). HNO 70, 30–44 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paciello F., et al. , Pioglitazone represents an effective therapeutic target in preventing oxidative/inflammatory cochlear damage induced by noise exposure. Front. Pharmacol. 9, 1103 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coleman J. K., Littlesunday C., Jackson R., Meyer T., AM-111 protects against permanent hearing loss from impulse noise trauma. Hear. Res. 226, 70–78 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Dinh C. T., Goncalves S., Bas E., Van De Water T. R., Zine A., Molecular regulation of auditory hair cell death and approaches to protect sensory receptor cells and/or stimulate repair following acoustic trauma. Front. Cell Neurosci. 9, 96 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicotera T. M., Hu B. H., Henderson D., The caspase pathway in noise-induced apoptosis of the chinchilla cochlea. J. Assoc. Res. Otolaryngol. 4, 466–477 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minami S. B., Yamashita D., Ogawa K., Schacht J., Miller J. M., Creatine and tempol attenuate noise-induced hearing loss. Brain Res. 1148, 83–89 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Prell C. G., Yamashita D., Minami S. B., Yamasoba T., Miller J. M., Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hear. Res. 226, 22–43 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pak J. H., Kim Y., Yi J., Chung J. W., Antioxidant therapy against oxidative damage of the inner ear: Protection and preconditioning. Antioxidants (Basel) 9, 1076 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nieratschker M., et al. , A novel pyridoindole improves the recovery of residual hearing following cochlear implantation after a single preoperative application. bioXriv [Preprint] (2024). 10.1101/2024.02.14.580226 (Accessed 20 February 2024). [DOI]
- 48.Kujawa S. G., Liberman M. C., Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear. Res. 330, 191–199 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kujawa S. G., Liberman M. C., Adding insult to injury: Cochlear nerve degeneration after "temporary" noise-induced hearing loss. J. Neurosci. 29, 14077–14085 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki J., Corfas G., Liberman M. C., Round-window delivery of neurotrophin 3 regenerates cochlear synapses after acoustic overexposure. Sci. Rep. 6, 24907 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu P. Z., O’Malley J. T., de Gruttola V., Liberman M. C., Primary neural degeneration in noise-exposed human cochleas: Correlations with outer hair cell loss and word-discrimination scores. J. Neurosci. 41, 4439–4447 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Viana L. M., et al. , Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue. Hear. Res. 327, 78–88 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou B., et al. , Facilitation of axon regeneration by enhancing mitochondrial transport and rescuing energy deficits. J. Cell Biol. 214, 103–119 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J., et al. , Inhibition of the c-Jun N-terminal kinase-mediated mitochondrial cell death pathway restores auditory function in sound-exposed animals. Mol. Pharmacol. 71, 654–666 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Wang J., et al. , Riluzole rescues cochlear sensory cells from acoustic trauma in the guinea-pig. Neuroscience 111, 635–648 (2002). [DOI] [PubMed] [Google Scholar]
- 56.Harrop-Jones A., et al. , The sustained-exposure dexamethasone formulation OTO-104 offers effective protection against noise-induced hearing loss. Audiol. Neurootol. 21, 12–21 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Salt A. N., Hartsock J., Plontke S., LeBel C., Piu F., Distribution of dexamethasone and preservation of inner ear function following intratympanic delivery of a gel-based formulation. Audiol. Neurootol. 16, 323–335 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin H. W., Furman A. C., Kujawa S. G., Liberman M. C., Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. J. Assoc. Res. Otolaryngol. 12, 605–616 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi L., et al. , Ribbon synapse plasticity in the cochleae of Guinea pigs after noise-induced silent damage. PLoS ONE 8, e81566 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.