Abstract
The role of serum response factor (Srf), a central mediator of actin dynamics and mechanical signaling, in cell identity regulation is debated to be either a stabilizer or a destabilizer. We investigated the role of Srf in cell fate stability using mouse pluripotent stem cells. Despite the fact that serum-containing cultures yield heterogeneous gene expression, deletion of Srf in mouse pluripotent stem cells leads to further exacerbated cell state heterogeneity. The exaggerated heterogeneity is detectible not only as increased lineage priming but also as the developmentally earlier 2C-like cell state. Thus, pluripotent cells explore more variety of cellular states in both directions of development surrounding naïve pluripotency, a behavior that is constrained by Srf. These results support that Srf functions as a cell state stabilizer, providing rationale for its functional modulation in cell fate intervention and engineering.
Keywords: Srf, Pluripotent stem cells, Cell identity, Heterogeneity
Graphical Abstract
Graphical Abstract.

Significance Statement.
By analyzing gene expression heterogeneity, we found that Srf confers cell state stability in mouse pluripotent stem cells. These results clarify Srf’s role in cell identity regulation and illustrate an instance how divergent cell states surrounding naïve pluripotency could emerge and co-exist in the same culture. Even though mouse pluripotent stem cells can undergo unlimited propagation, the reduced presence of the main cell identity (naïve pluripotency) and prevalence of alternative cell states (2C-like and linage positive cells) suggest semblance to cell identity degeneration in other biology, such as aging.
Introduction
Cell state stability is required for effective execution of specific biochemical and biophysical functions, loss of which underlies degeneration.1-4 On the other hand, noisy cell states contribute to plasticity, enabling the emergence of new cell identity5 required for development and homeostasis throughout life. Consistent with this notion, cell-to-cell heterogeneity is known to impart development robustness6 and culture conditions that reduce pluripotency gene expression noise also compromises multipotency.7 Pathological cell states, such as malignancy, could be viewed as cells in protracted state excursions or cells entrapped in a proliferative state.8 Organismal well-being rests on a delicate balance between cell state plasticity and stability. An example where this balance is compromised is aging: Increased cellular heterogeneity is one of the hallmarks of aging,9,10 coupled with the loss of the dominant cell identity.11 Understanding the molecular regulators of cell fate stability could offer potential therapeutic interventions for degenerative diseases. The ubiquitously expressed transcription factor, Srf, has been implicated in regulating cell fate stability. Specifically, decreased Srf activity has been seen in multiple aging systems12 and that instigators of Srf signaling has recently been shown to rejuvenate oligodendrogenesis in aged mice.13
Srf is a ubiquitously expressed transcription factor (TF), responding to many extracellular signals to control the expression of 2 large categories of genes. One is components of the actomyosin cytoskeleton, in complex with the myocardin family of transcriptional coactivators, MKL1/2. These genes are involved in adhesion and motility14,15 and underlies cell size and morphology. The other major group of Srf target genes is the “immediate-early” genes, such as Jun, Fos, and Egr1, whose expression is rapidly activated by mitogenic stimuli.16,17 Strikingly, cell size/morphology and immediate early gene expression are the top features highly predictive of gene expression heterogeneity.18 Furthermore, Srf is a major mediator of circadian oscillation.19,20 Therefore, it is conceivable that the cell-to-cell variability in gene expression is significantly contributed by Srf, whose loss-of-function could confer a less variable gene expression state. However, global Srf knockout is embryonic lethal with defective gastrulation17,21; Cre-mediated cell type-specific deletion often results in compensatory cells escaping Cre-mediated conditional Srf allele excision,22-25 confounding the assessment of cellular heterogeneity. Unlike somatic cells, Srf appears dispensable for pluripotent stem cells for both human26 and mouse.17,27 We previously reported the derivation of Srf null iPSCs,28 providing the cellular system to examine the role of Srf in controlling cell state heterogeneity in pluripotent stem cells.
Mouse pluripotent stem cells can interconvert between several related states.29 For example, they can be maintained in the naïve pluripotent state (ICM-like) or primed pluripotent state (epiblast-like) depending on culture conditions,30,31 with the latter being a developmentally more advanced stage than the naïve state. Signaling pathways, such as the MAPK cascade and retinoic acid signaling, induces the exit from naïve pluripotency and committing to specific lineages. Preceding the pluripotent ICM cells, the zygote and 2-cell (2C) stage blastomeres are totipotent.32 The 2C-like cells can arise spontaneously in mESC cultures at low frequency (~0.5%).32 Similar to 2C-stage embryos, 2C-like cells reactivate 2C-specific transcripts including Zscan4, Eif1a-like, Gm6763, major satellites (MajSat) repeats, and murine endogenous retrovirus with leucine tRNA primer (MuERV-L, also known as MERVL and Erv4).32-34 As each cell state is associated with different developmental potential, the molecular control of the interconversion between these cell states have been intensely studied. For example, depletion of the p150 or the p60 subunits of chromatin assembly factor-1 (Caf-1), or expression of Dux in mESCs promotes the emergence of 2C-like-cells.35-37 Taken together, exiting naïve pluripotency to enter the 2C-like state or lineage priming is developmentally divergent and is under distinct regulatory mechanisms. Here, we report the surprising finding that these divergent cell states could both arise following the loss of a single gene, Srf.
Results
Srf Null iPSCs Display Heightened Cell State Heterogeneity
Srf null (SrfΔ/Δ) iPSCs were generated by reprogramming myeloid progenitors of adult reprogrammable mouse, followed by Cre-mediated excision of a conditional Srf allele, as we described before.28 Consistent with the previous reports that Srf null mESCs remain pluripotent, these Srf null iPSCs can be propagated in mESC culture conditions, display reduced cortical actins, and maintain highly accessible chromatin.28 To explore whether and how Srf controls cell state heterogeneity, Srf null (KO) or wild type (WT) iPSCs cultured in serum-containing medium were subject to single-cell RNA sequencing (scRNA-seq). After quality filtering and normalization, the transcriptomes of 857 WT and 960 Srf KO cells were obtained and analyzed. The mean and median numbers of detected genes per cell were 3915 and 4135 for KO and 4114 and 4275 for WT, respectively. Using the 3000 most variable genes, Manifold Approximation and Projection (UMAP) dimensional reduction identified 2 major cell clusters (Fig. 1A): A large cluster encompasses most cells in both genotypes and a smaller cluster limited to 11.7% Srf KO cells (Fig. 1A). Srf mRNA is not detected in KO cells, confirming the inactivated status of the gene (Fig. 1B). Even in the WT population, only ~20% (163 cells) displayed detectible Srf expression, indicating that WT iPSCs naturally display a spectrum of Srf levels (Fig. 1B). In addition to Srf itself, the expression of an Srf target gene Actb mirrors that of Srf, consistent with its greatly reduced transcriptional activity. In contrast, the expression of core pluripotency genes including Pou5f1 (Oct4) and Nanog are similar in both genotypes (Fig. 1B), confirming the pluripotent nature of both WT and KO cells.
Figure 1.

Loss of Srf increases transcriptional heterogeneity of mouse iPSCs. (A) Projection of all cells onto 2 principal dimensions using UMAP based on the 3000 most variable genes. Genotypes are distinguished by color. (B) Violin plots of select genes in Srf KO and WT iPSCs. Lower expression in Srf and Actb confirms the null status of Srf in KO cells. (C) Violin plots depicting the mean gene expression levels in Srf KO and WT iPSCs. Median and 95%CI of mean gene expression levels for the 5943 genes in the Robust gene set (Supplementary Table S2) are shown. Wilcoxon rank test, P = .097. (D) Dot plot depicting the mean gene expression of 5943 genes in Srf KO vs. WT iPSCs. (E) Violin plots depicting the coefficient of variation (σ2/μ2, CV2) of gene expression in Srf KO and WT iPSCs. Wilcoxon rank test, P < 2.22e−16. (F) Binned gene expression CV2 across the all gene expression averages for Srf KO and WT iPSCs. Wilcoxon rank test, P < 1e−5 for all bins. (G) Mean expression vs. CV2 for all 5943 genes. (H) Distribution of the Fano factor (σ2/μ) of all 5943 genes in Srf KO vs. WT iPSCs. Overlay of density contours reveals how center of mass (red-dashed line) lies above the diagonal line, as shown for mean values in D and the Black-dashed lines.
We next assessed the transcriptome-wide cell-to-cell variation in gene expression levels (ie, intercellular gene expression heterogeneity) in Srf KO and WT iPSCs. While the mean-expression levels for most genes exhibit minimal changes between KO and WT iPSCs (Fig. 1C, 1D), Srf KO iPSCs displayed higher cell-to-cell variability in transcript levels (ie, transcript noise) for virtually all genes across the genome, as analyzed by CV2 (σ2/µ2) versus mean (Fig. 1E-1G). Higher CV is seen in KO cells independent of the gene expression levels (Fig. 1G). In addition, we quantified transcript noise using the Fano factor (σ2/μ). Despite mean-expression levels exhibiting minimal changes (Fig. 1C, 1D), the Fano factor is higher for >90% of genes in Srf KO iPSCs (Fig. 1H). These results indicate that Srf KO iPSCs display a global increase in transcript noise with little change in mean levels for the great majority of the transcriptome. Importantly, the overall noisy cell state is not contributed by the small population unique to the KO cells, as most KO cells display higher CV2 and Fano factor (Fig. 1E-1H). Thus, pluripotent stem cells exist in a state of heightened heterogeneity when Srf is inactivated.
Srf KO iPSCs Display Increased Expression of Differentiation Markers
To examine the cellular consequence of this gene expression heterogeneity, we sought to examine the potential emergence of cell states deviating or departing from the naïve pluripotent cell state. While scRNA-seq data resolve cell state heterogeneity, the data are often sparse and may not be sufficient to capture the expression of genes that are present in low levels or transiently. Therefore, we performed bulk RNA-seq of parallel Srf KO and WT iPSCs. We identified 816 upregulated and 247 downregulated differentially expressed genes (DEGs) (Fig. 2A; Supplementary Table S1). Gene set enrichment analysis (GSEA) indicated that epithelial-to-mesenchymal transition (EMT), inflammatory, and interferon pathways were upregulated in Srf KO iPSCs compared with WT controls (Fig. 2B). Many Srf target genes including Actb, Actg1, Fos, and Egr1 were substantially downregulated, corroborating Srf loss of function (Fig. 2C; Supplementary Table S1). The reduced immediate early genes in Srf KO iPSCs further confirm the similar observation made with Srf null ESCs.17
Figure 2.
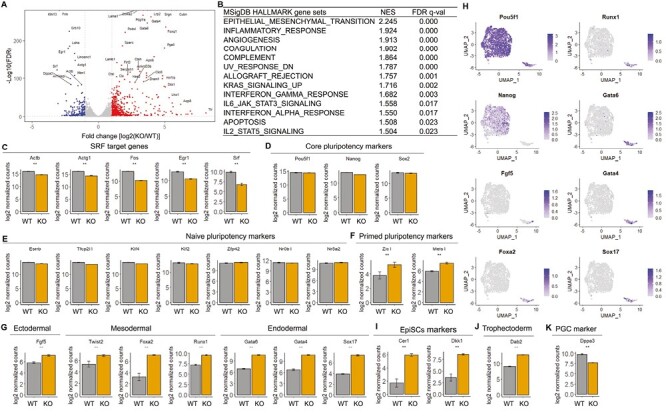
Srf KO iPSCs display increased expression of differentiation markers. (A) Volcano plot of differentially expressed genes (DEGs), defined as FDR-adjusted P-value ≤ .05 and a log2 fold change of at least 1. The red and blue dots represent upregulated and downregulated DEGs in Srf KO iPSCs compared with WT controls. Shown are the top 40 most differentially expressed genes. (B) Upregulated genes in Srf KO iPSCs are enriched in multiple pathways, as detected by gene set enrichment analysis (GSEA) on MSigDB HALLMARK gene sets. (C) Expression of known Srf target genes in bulk RNA-seq of Srf KO and WT iPSCs. Lower expression of these genes confirm the reduced Srf function in the KO cells. (D) Expression of core pluripotency genes. (E) Expression of naïve pluripotency marker genes. (F) Expression of primed pluripotency marker genes. (G) Expression of lineage-specific genes, including ectodermal (Fgf5), mesodermal (Twist2, Foxa2, and Runx1), and endodermal (Gata6, Gata4, and Sox17) markers. (H) Expression of lineage genes on scRNA-seq UMAP. (I) Expression of epiblast stem cells (EpiSCs) marker genes. (J) Expression of trophectoderm marker genes. (K) Expression of primordial germ cell (PGC) marker genes. *|log2FC| > 1 and FDR < .05; **|log2FC| > 1 and FDR < .01.
Consistent with the scRNA-seq results, core pluripotency-associated genes such as Pou5f1 (Oct4), Nanog, and Sox2 were unaffected (Fig. 2D). Expression of additional naïve pluripotency state genes including Esrrb, Tfcp2l1, Klf4, Klf2, Zfp42, Nr0b1, and Nr5a2 were also unaffected (Fig. 2E). These results further support that Srf is dispensable for naïve pluripotency. However, primed pluripotency genes such as Zic1 and Meis1 were significantly upregulated in Srf KO iPSCs (Fig. 2F).
To determine whether Srf KO iPSCs deviated further from pluripotency, we examined their expression of lineage-specifying transcription factors. Srf KO iPSCs displayed upregulation of markers of all 3 germ layers. These include the ectoderm lineage marker Fgf5; mesoderm lineage markers Twist2, Foxa2, and Runx1; endoderm lineage markers Gata6, Gata4, and Sox17 (Fig. 2G). Moreover, many of these lineage-specific genes were expressed in the small cluster specific to Srf KO in the scRNA-seq data (Fig. 2H). Finally, epiblast stem cell (EpiSC) marker genes Cer1 and Dkk1 (Fig. 2I) and the trophectoderm marker gene Dab2 (Fig. 2J) were significantly upregulated in Srf KO iPSCs. Taken together, when Srf activity is low or absent, pluripotent stem cells more frequently explore a differentiated/committed state. The only exception appears to be the primordial germ cell (PGC) lineage, whose marker gene Dppa3 (Stella) was downregulated in Srf KO iPSCs (Fig. 2K), suggesting Srf’s role in supporting germ cell potential.
Srf Null iPSCs Display Increased Expression of Marker Genes for 2C-like State
On the surface, the results above could be seen as Srf KO iPSCs are prone to differentiate, which is surprising given that Srf null embryos fail to specify germ layers.21 An alternative interpretation is that Srf KO iPSCs display reduced cell state stability. In this scenario, one would expect the emergence of cell states that are developmentally earlier than naïve pluripotency. One well-known cell state that pluripotent cells fluctuate in and out is the 2C-like cell state, which precedes the ICM state developmentally. Therefore, we compared our data to 2C-like cells isolated from ESCs (MERVL+/Zscan4+, Zscan4+)38-40 by GSEA. The top of the Srf KO transcriptome was significantly enriched in 2C-specific genes (2C-up) both in Zscan4+ and MERVL+/Zscan4+ 2C-like ESCs (Fig. 3A, 3B). Srf inactivation resulted in upregulation of many more 2C-specific genes including Zscan4 genes (Zscan4a, Zscan4b, Zscan4c, Zscan4d, and Zscan4e) and Eif1a-like genes (Gm5662, Gm2022, Gm4027, BB287469, Gm2016, Gm21319, and Gm8300), Dux, Zfp352, Usp17lb, Tcstv1, Tcstv3, major satellites (MajSat), LINE1, and MERVL (Fig. 3C). These results indicate that deletion of Srf promotes the emergence of 2C-like cells.
Figure 3.

Srf KO iPSCs display increased expression of marker genes for 2C-like state. (A) GSEA of the 229 upregulated genes in the Zscan4+ 2C-like cells.40 The x-axis shows the log2(fold change)-ranked Srf KO transcriptome. The enrichment score increases when a gene in the Srf KO transcriptome is also present in the 2C-like gene set, and a Black vertical bar is drawn at bottom; the enrichment score decreases when a gene is absent in the 2C-like gene set. The P-value was empirically determined on the basis of 1000 permutations of ranked gene lists. (B) GSEA of the 310 upregulated genes in the MERVL+/Zscan4+ 2C-like cells.40 (C) Expression of a panel of 2C-like genes between Srf KO and WT iPSCs. * |log2FC| > 1 and FDR < .05; **|log2FC| > 1 and FDR < .01. (D) GSEA of the top 500 most upregulated genes in p150 knockdown (KD) mESCs. (E) GSEA of the top 500 most upregulated genes in p60 KD mESCs.
In addition to natural fluctuation, 2C-like cells can be induced following specific genetic perturbation. Ishiuchi et al35 reported that depletion of either the p150 or the p60 subunits of chromatin assembly factor-1 (Caf-1) in ES cells increases the population of 2C-like cells. Therefore, we compared our Srf KO transcriptome with that of p150 or p60 knockdown (KD). Strikingly, we observed significant overlap between the transcriptomes following Srf loss of function with those following p150 or p60 KD (Supplementary Fig. S1A, S1B; P <2.2e−16 and P = 4.3e−16 for p150 and p60 RNAi, respectively, by 2-sided Fisher’s exact test). Moreover, GSEA indicates that the most upregulated genes in Srf KO iPSCs are enriched in the most upregulated genes in p150 or p60 KD (Fig. 3D, 3E). These results demonstrate that Srf KO iPSCs and Caf-1 KD cells share substantial overlapping gene-expression patterns. This overlap occurs without significant changes in the expression of Caf-1 RNA or protein levels (Supplementary Table S1 and Fig. S2), suggesting that Caf1 itself is not directly regulated by Srf.
We next explored mechanistically how such a phenomenon could occur. In human pluripotent stem cells, reduced SRF expression leads to DNA replication stress and chromosomal abnormalities, especially after prolonged culture.26 Therefore, we stained the cells for γH2AX, a marker for DNA damage.41 The KO cells displayed elevated levels of γH2AX (Fig. 4A, 4B) in the absence of any other genomic insults. Therefore, the co-existence of 2C-like cells, pluripotent stem cells and lineage marker positive cells in the Srf KO iPSCs is associated with increased baseline DNA damage. Taken together, Srf loss of function in pluripotent stem cells increases the likelihood of otherwise rare or transient cell states, including the 2C-like cells and various lineage marker positive cells (Fig. 4C). These results collectively support that Srf functions as a cell fate stabilizer. In its absence, gene expression becomes noisier and divergent cell fates arises more frequently.
Figure 4.

Srf KO iPSCs display elevated markers for DNA damage, and working model of Srf conferring cell state stability in pluripotent stem cells. (A) Representative confocal fluorescence images of iPSC cultures stained with γH2AX antibody. (B) Quantification of γH2AX fluorescence intensity as in A. n = 80 each, Wilcoxon test, P = .005. (C) Pluripotency state, at least when cultured in serum containing medium, is heterogeneous containing a spectrum of cell states arising spontaneously (blue line, WT). The spread of these cell states is constrained by Srf (yellow line, KO). Deleting Srf allows more cells to appear in the tail regions in both directions of development.
Discussion
Our results here support that mouse pluripotent stem cells, at least in serum containing culture conditions supporting naïve pluripotency, could depart from this main state and enter other cell states in both developmental directions (Fig. 4C). This is consistent with the notion that cell state variability is integral to pluripotency.42 The cell state excursion is constrained at least in part by Srf. The apparent preservation of pluripotency genes with simultaneously increased differentiation markers as well as 2C-like genes are rooted in the increased cell state heterogeneity. Specifically, when Srf is inactivated, more cells depart from the pluripotent state to explore alternative states (Fig. 4C, indicated by the lower but widening peak). However, these exploratory cells are always fewer than the main population. On the bulk expression level, a small decrease in the percentage of pluripotent cells will likely be difficult to detect; in contrast, an increase in lineage positive or 2C-like state should be easier to detect as their baseline expression is low. We note that while Srf is required to constrain cell state variability in pluripotent stem cells, Srf gain of function also antagonizes pluripotency,27,43 suggesting that a narrow or optimal range of Srf activity might be required to strike the balance between cell state plasticity and stability.
A role of Srf in stabilizing the naïve pluripotent cell state extends our previous work in identifying the actin-MKL1/SRF pathway in stabilizing somatic cell fate.28 In the case of somatic cell reprogramming driven by the Yamanaka factors, the activity of actin-MKL1/SRF pathway needs to be sufficiently weakened to allow the entry into pluripotency. Together, our results lend further support that Srf is part of the actin-MKL1/SRF pathway contributing to cell fate stability. With Srf appreciated as a cell fate stabilizer, its function in somatic and pluripotent stem cells can be understood in one unified model (Fig. 4C). These insights contrast that of a recent study suggesting Srf destabilizes cell identity.44 Our results offer a molecular explanation why certain small molecules with diverse targets or mechanisms, such as ROCK inhibitors and Arp2/3 inhibitors, are potent cell fate modulators in the derivation and maintenance of pluripotent stem cells.28,45,46 On the other hand, once the desired cell fate is attained and needs to be propagated, further attenuation of Srf activity could be counterproductive.
Our results unveiling elevated baseline DNA damage in Srf KO iPSCs (Fig. 4A, 4B) are consistent with a recent report identifying DNA repair pathway in regulating gene expression noise and cell fate plasticity.5 These results further agree with our GSEA results showing that Srf KO iPSCs have upregulated interferon response and inflammatory genes (Fig. 2B). Elevated DNA damage triggering interferon and inflammatory response is often features of the inflammaging process.47 Therefore, even though mouse pluripotent stem cells are not thought to undergo aging, the reduced main cell identity (ie naïve pluripotency) and increased alternative cell states (2C-like and linage-positive cells), when Srf is inactivated, are reminiscent of cell identity degeneration in aging.9-11
Given that Srf loss of function has been reported in several human diseases via alternative splicing or caspase-mediated cleavage yielding dominant negative form,48-51 our results provide potential insights into additional cell state abnormality beyond pluripotency.
Methods
Bulk RNA Sequencing (RNA-Seq) and Data Analysis
Mouse iPSCs with SrfΔ/Δ (KO) and Srff/f (WT) were generated and cultured in serum containing medium as reported earlier.28 Total RNA was extracted using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.), from 3 replicates of each Srf genotype. The quality and integrity of the RNA samples were examined as previously.28 RNA-seq was performed with Illumina Hiseq 2500 platform that included quality control, library preparation, fragmentation, and PCR enrichment of target RNA according to standardized procedures. One hundred and fifty base pairs paired-end raw reads were initially processed to obtain clean reads by removing adaptor sequences, low-quality sequences and empty reads. After quality control, the clean reads were mapped to mouse genome (mm10) using STAT. Genes expression level was quantitated with TEtranscripts. Differentially expressed genes (DEGs) were identified with DESeq2. An absolute log2 fold change >1 and the adjusted P-value (FDR) significance score <.05 were used as thresholds to identify DEGs. Gene set enrichment analysis (GSEA) was performed using GSEA software (https://www.gsea-msigdb.org/gsea/index.jsp) with default parameters.
Single-Cell RNA-Seq Library Preparation, Sequencing, and Data Analysis
10× Genomics Chromium platform was used to capture and barcode cells to generate single-cell Gel beads-in-emulsion (GEMs) by following the manufacturer’s protocol. The resulting libraries were sequenced on an Illumina NovaSeq 6000 System. Sequences from scRNA-seq were processed with Cellranger (v.2.0.0) software (10× genomics). In short, demultiplexing, UMI (unique molecular identifier) collapsing, and alignment to the mouse (Mus musculus) reference genome (version: mm10) were performed. Only confidently mapped, non-PCR duplicates with valid barcodes and unique molecular identifiers were used to generate the gene-barcode matrix that contained 954 cells for WT and 1073 cells for Srf KO iPSCs. Further analysis—including data cleaning, normalization, scaling quality, and filtering—was performed using Seurat v3.1.1.52 To exclude genes that might be random noise, we filtered genes whose expression was detected in fewer than 10 cells. To exclude poor quality cells that might result from multiplets or other technical noise, we filtered cells that were considered outliers (>3rd quartile + 1.5× interquartile range or < 1st quartile − 1.5× interquartile range) based on the number of expressed genes detected, the sum of UMI counts and the proportion of mitochondrial genes. In addition, we limited the proportion of mitochondrial genes to a maximum of 0.1 to further remove potential poor-quality data from broken cells. After removing unwanted cells from the dataset, we normalized the data by the total expression, multiplied by a scale factor of 10,000 and log-transformed the result. To reduce noise that may be introduced by considering all the genes, we used “FindVariableFeatures” function to select ~3000 highly variable genes (HVGs) that contribute greatly to cell-to-cell variation. Then, the expression of the HVGs was used as the feature set for linear dimensional reduction of the data through principal component analysis (PCA) using Seurat’s RunPCA function. Uniform Manifold Approximation and Projection (UMAP) dimensional reduction was performed on the scaled matrix (with most variable genes only) using the first 30 components of principal component analysis (PCA) to obtain a 2-dimensional representation of the cell states.
Protein Analysis by Western Blotting and Immunofluorescence Staining
Cell lysates of Srf KO and WT iPSCs were harvested by directly lysing the cells with 2× sample buffer (Bio-Rad). Proteins were separated by SDS-PAGE, transferred onto nitrocellulose membranes (Bio-Rad). The membranes were blocked with 5% nonfat dry milk in TBS-Tween (TBST) for 1 hour, incubated with primary antibodies overnight at 4 °C, followed by incubation with horseradish-peroxidase-conjugated secondary antibodies for 1 hour, and illuminated by enhanced chemiluminescence (ECL). Primary antibodies used include β-tubulin (1:5000, Abcam Ab6046) and Caf1 (p60) (1:500, Santa Cruz, #sc-393662).
Srf KO and WT iPSCs were cultured in µ-Slide 8-well chambers (ibidi GmbH, Martinsried, Germany) for immunofluorescence. The cells were washed twice with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde (PFA) for 10 minutes. After washing twice with PBS, cells were permeabilized with 0.25% Triton X-100 in PBS for 10 minutes and followed by 2 washes in PBS. Cells were then blocked with 1% bovine serum albumin (BSA), 5% goat serum in PBST for 1 hour at room temperature, and then incubated with the γH2AX antibodies (1:300, Millipore, #05-636) overnight at 4 °C, followed by 2 washes with PBS. The cells were subsequently incubated with the secondary antibody (Alexa Fluor 488, 1:500, Invitrogen, #A27034) for 1 hour at RT. DNA wascounterstained with DAPI. Images were acquired using the Leica SP5 or Stellaris (Leica Microsystems, Germany) confocal microscope platform. To measure the intensity of γH2AX in nuclei, polylines were drawn along the edge of the nuclei based on DAPI label, and the mean pixel values of Alexa Fluor 488 labeled γH2AX were measured using LAS AF software (Leica Microsystems, Germany). Eighty nuclei were measured for each condition. Statistical assessment was performed with Wilcoxon test, and plots were generated using Graphpad Prism9 software.
Supplementary Material
Contributor Information
Jian Zhang, Department of Cell Biology, Yale University, New Haven, CT, USA; Yale Stem Cell Center, Yale University, New Haven, CT, USA.
Qiao Wu, Department of Cell Biology, Yale University, New Haven, CT, USA; Yale Stem Cell Center, Yale University, New Haven, CT, USA.
Xiao Hu, Department of Cell Biology, Yale University, New Haven, CT, USA; Yale Stem Cell Center, Yale University, New Haven, CT, USA.
Yadong Wang, Yale Stem Cell Center, Yale University, New Haven, CT, USA; Department of Genetics, Yale University, New Haven, CT, USA.
Jun Lu, Yale Stem Cell Center, Yale University, New Haven, CT, USA; Department of Genetics, Yale University, New Haven, CT, USA.
Raja Chakraborty, Department of Medicine, Section of Cardiovascular Medicine, Yale University, New Haven, CT, USA.
Kathleen A Martin, Department of Medicine, Section of Cardiovascular Medicine, Yale University, New Haven, CT, USA.
Shangqin Guo, Department of Cell Biology, Yale University, New Haven, CT, USA; Yale Stem Cell Center, Yale University, New Haven, CT, USA.
Funding
Research reported in this publication was supported in part by DP2GM123507 (S.G.) and the Kutnick Family Foundation.
Conflict of Interest
The authors indicated no financial relationships.
Author Contributions
J.Z. performed all bioinformatics analysis, produced figures and tables, and wrote the manuscript. Q.W. performed cell culture and related protein and RNA analyses, produced figures, and co-wrote the manuscript. X.H. performed some cell culture experiments and collected samples. Y.W. and J.L. performed some of the RNA analysis. R.C. and K.M. provided control samples for some of the RNA analysis. S.G. supervised the project, designed experiments, and wrote the manuscript. All the authors reviewed the manuscript.
Data Availability
The data underlying this article are available in GEO under accession code GSE210074. All data analyzed during this study are included in the manuscript and supporting files. Source data files are provided for all figures and figure supplements.
References
- 1. Angelidis I, Simon LM, Fernandez IE, et al. An atlas of the aging lung mapped by single cell transcriptomics and deep tissue proteomics. Nat Commun. 2019;10(1):963. 10.1038/s41467-019-08831-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bahar R, Hartmann CH, Rodriguez KA, et al. Increased cell-to-cell variation in gene expression in ageing mouse heart. Nature. 2006;441(7096):1011-1014. 10.1038/nature04844 [DOI] [PubMed] [Google Scholar]
- 3. Herndon LA, Schmeissner PJ, Dudaronek JM, et al. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419(6909):808-814. 10.1038/nature01135 [DOI] [PubMed] [Google Scholar]
- 4. Martinez-Jimenez CP, Eling N, Chen HC, et al. Aging increases cell-to-cell transcriptional variability upon immune stimulation. Science. 2017;355(6332):1433-1436. 10.1126/science.aah4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Desai RV, Chen X, Martin B, et al. A DNA repair pathway can regulate transcriptional noise to promote cell fate transitions. Science. 2021;373(6557):abc6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Torres-Padilla ME, Chambers I. Transcription factor heterogeneity in pluripotent stem cells: a stochastic advantage. Development. 2014;141(11):2173-2181. 10.1242/dev.102624 [DOI] [PubMed] [Google Scholar]
- 7. Choi J, Huebner AJ, Clement K, et al. Prolonged Mek1/2 suppression impairs the developmental potential of embryonic stem cells. Nature. 2017;548(7666):219-223. 10.1038/nature23274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen X, Burkhardt DB, Hartman AA, et al. MLL-AF9 initiates transformation from fast-proliferating myeloid progenitors. Nat Commun. 2019;10(1):5767. 10.1038/s41467-019-13666-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheung P, Vallania F, Warsinske HC, et al. Single-cell chromatin modification profiling reveals increased epigenetic variations with aging. Cell. 2018;173(6):1385-1397.e14. 10.1016/j.cell.2018.03.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mahmoudi S, Mancini E, Xu L, et al. Heterogeneity in old fibroblasts is linked to variability in reprogramming and wound healing. Nature. 2019;574(7779):553-558. 10.1038/s41586-019-1658-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Izgi H, Han D, Isildak U, et al. Inter-tissue convergence of gene expression during ageing suggests age-related loss of tissue and cellular identity. Elife. 2022;11:e68048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lahoute C, Sotiropoulos A, Favier M, et al. Premature aging in skeletal muscle lacking serum response factor. PLoS One. 2008;3(12):e3910. 10.1371/journal.pone.0003910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iram T, Kern F, Kaur A, et al. Young CSF restores oligodendrogenesis and memory in aged mice via Fgf17. Nature. 2022;605(7910):509-515. 10.1038/s41586-022-04722-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Connelly JT, Gautrot JE, Trappmann B, et al. Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat Cell Biol. 2010;12(7):711-718. 10.1038/ncb2074 [DOI] [PubMed] [Google Scholar]
- 15. Medjkane S, Perez-Sanchez C, Gaggioli C, Sahai E, Treisman R. Myocardin-related transcription factors and SRF are required for cytoskeletal dynamics and experimental metastasis. Nat Cell Biol. 2009;11(3):257-268. 10.1038/ncb1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Posern G, Treisman R. Actin’ together: serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol. 2006;16(11):588-596. 10.1016/j.tcb.2006.09.008 [DOI] [PubMed] [Google Scholar]
- 17. Schratt G, Weinhold B, Lundberg AS, et al. Serum response factor is required for immediate-early gene activation yet is dispensable for proliferation of embryonic stem cells. Mol Cell Biol. 2001;21(8):2933-2943. 10.1128/MCB.21.8.2933-2943.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Battich N, Stoeger T, Pelkmans L. Control of transcript variability in single mammalian cells. Cell. 2015;163(7):1596-1610. 10.1016/j.cell.2015.11.018 [DOI] [PubMed] [Google Scholar]
- 19. Esnault C, Stewart A, Gualdrini F, East P, Horswell S, Matthews N, et al. Rho-actin signaling to the MRTF coactivators dominates the immediate transcriptional response to serum in fibroblasts. Genes Dev. 2014;28(9):943-958. 10.1101/gad.239327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gerber A, Esnault C, Aubert G, et al. Blood-borne circadian signal stimulates daily oscillations in actin dynamics and SRF activity. Cell. 2013;152(3):492-503. 10.1016/j.cell.2012.12.027 [DOI] [PubMed] [Google Scholar]
- 21. Arsenian S, Weinhold B, Oelgeschlager M, Ruther U, Nordheim A. Serum response factor is essential for mesoderm formation during mouse embryogenesis. EMBO J. 1998;17(21):6289-6299. 10.1093/emboj/17.21.6289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li S, Czubryt MP, McAnally J, et al. Requirement for serum response factor for skeletal muscle growth and maturation revealed by tissue-specific gene deletion in mice. Proc Natl Acad Sci USA. 2005;102(4):1082-1087. 10.1073/pnas.0409103102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ragu C, Elain G, Mylonas E, et al. The transcription factor Srf regulates hematopoietic stem cell adhesion. Blood. 2010;116(22):4464-4473. 10.1182/blood-2009-11-251587 [DOI] [PubMed] [Google Scholar]
- 24. Costello P, Sargent M, Maurice D, et al. MRTF-SRF signaling is required for seeding of HSC/Ps in bone marrow during development. Blood. 2015;125(8):1244-1255. 10.1182/blood-2014-08-595603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miano JM, Ramanan N, Georger MA, et al. Restricted inactivation of serum response factor to the cardiovascular system. Proc Natl Acad Sci USA. 2004;101(49):17132-17137. 10.1073/pnas.0406041101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lamm N, Ben-David U, Golan-Lev T, et al. Genomic instability in human pluripotent stem cells arises from replicative stress and chromosome condensation defects. Cell Stem Cell. 2016;18(2):253-261. 10.1016/j.stem.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 27. Weinhold B, Schratt G, Arsenian S, et al. Srf(-/-) ES cells display non-cell-autonomous impairment in mesodermal differentiation. EMBO J. 2000;19(21):5835-5844. 10.1093/emboj/19.21.5835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu X, Liu ZZ, Chen X, et al. MKL1-actin pathway restricts chromatin accessibility and prevents mature pluripotency activation. Nat Commun. 2019;10(1):1695. 10.1038/s41467-019-09636-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kolodziejczyk AA, Kim JK, Tsang JC, et al. Single cell RNA-sequencing of pluripotent states unlocks modular transcriptional variation. Cell Stem Cell. 2015;17(4):471-485. 10.1016/j.stem.2015.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4(6):487-492. 10.1016/j.stem.2009.05.015 [DOI] [PubMed] [Google Scholar]
- 31. Ying QL, Wray J, Nichols J, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453(7194):519-523. 10.1038/nature06968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Macfarlan TS, Gifford WD, Driscoll S, et al. Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature. 2012;487(7405):57-63. 10.1038/nature11244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Falco G, Lee SL, Stanghellini I, et al. Zscan4: a novel gene expressed exclusively in late 2-cell embryos and embryonic stem cells. Dev Biol. 2007;307(2):539-550. 10.1016/j.ydbio.2007.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zalzman M, Falco G, Sharova LV, et al. Zscan4 regulates telomere elongation and genomic stability in ES cells. Nature. 2010;464(7290):858-863. 10.1038/nature08882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ishiuchi T, Enriquez-Gasca R, Mizutani E, et al. Early embryonic-like cells are induced by downregulating replication-dependent chromatin assembly. Nat Struct Mol Biol. 2015;22(9):662-671. 10.1038/nsmb.3066 [DOI] [PubMed] [Google Scholar]
- 36. Hendrickson PG, Dorais JA, Grow EJ, et al. Conserved roles of mouse DUX and human DUX4 in activating cleavage-stage genes and MERVL/HERVL retrotransposons. Nat Genet. 2017;49(6):925-934. 10.1038/ng.3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Whiddon JL, Langford AT, Wong CJ, Zhong JW, Tapscott SJ. Conservation and innovation in the DUX4-family gene network. Nat Genet. 2017;49(6):935-940. 10.1038/ng.3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Akiyama T, Xin L, Oda M, et al. Transient bursts of Zscan4 expression are accompanied by the rapid derepression of heterochromatin in mouse embryonic stem cells. DNA Res. 2015;22(5):307-318. 10.1093/dnares/dsv013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eckersley-Maslin MA, Svensson V, Krueger C, Stubbs TM, Giehr P, Krueger F, et al. MERVL/Zscan4 network activation results in transient genome-wide DNA demethylation of mESCs. Cell Rep. 2016;17:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang Y, Huang Y, Dong Y, et al. Unique patterns of H3K4me3 and H3K27me3 in 2-cell-like embryonic stem cells. Stem Cell Rep. 2021;16(3):458-469. 10.1016/j.stemcr.2021.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bonner WM, Redon CE, Dickey JS, et al. GammaH2AX and cancer. Nat Rev Cancer. 2008;8(12):957-967. 10.1038/nrc2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Silva J, Smith A. Capturing pluripotency. Cell. 2008;132(4):532-536. 10.1016/j.cell.2008.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schratt G, Philippar U, Berger J, et al. Serum response factor is crucial for actin cytoskeletal organization and focal adhesion assembly in embryonic stem cells. J Cell Biol. 2002;156(4):737-750. 10.1083/jcb.200106008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ikeda T, Hikichi T, Miura H, et al. Srf destabilizes cellular identity by suppressing cell-type-specific gene expression programs. Nat Commun. 2018;9(1):1387. 10.1038/s41467-018-03748-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maldonado M, Luu RJ, Ramos ME, Nam J. ROCK inhibitor primes human induced pluripotent stem cells to selectively differentiate towards mesendodermal lineage via epithelial-mesenchymal transition-like modulation. Stem Cell Res. 2016;17(2):222-227. 10.1016/j.scr.2016.07.009 [DOI] [PubMed] [Google Scholar]
- 46. Rajan A, Tien AC, Haueter CM, Schulze KL, Bellen HJ. The Arp2/3 complex and WASp are required for apical trafficking of Delta into microvilli during cell fate specification of sensory organ precursors. Nat Cell Biol. 2009;11(7):815-824. 10.1038/ncb1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhao Y, Simon M, Seluanov A, Gorbunova V. DNA damage and repair in age-related inflammation. Nat Rev Immunol. 2023;23(2):75-89. 10.1038/s41577-022-00751-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chang J, Wei L, Otani T, et al. Inhibitory cardiac transcription factor, SRF-N, is generated by caspase 3 cleavage in human heart failure and attenuated by ventricular unloading. Circulation. 2003;108(4):407-413. 10.1161/01.CIR.0000084502.02147.83 [DOI] [PubMed] [Google Scholar]
- 49. Davis FJ, Gupta M, Pogwizd SM, et al. Increased expression of alternatively spliced dominant-negative isoform of SRF in human failing hearts. Am J Physiol Heart Circ Physiol. 2002;282(4):H1521-H1533. 10.1152/ajpheart.00844.2001 [DOI] [PubMed] [Google Scholar]
- 50. Patten LC, Belaguli NS, Baek MJ, et al. Serum response factor is alternatively spliced in human colon cancer. J Surg Res. 2004;121(1):92-100. 10.1016/j.jss.2004.02.031 [DOI] [PubMed] [Google Scholar]
- 51. Zhang X, Azhar G, Huang C, et al. Alternative splicing and nonsense-mediated mRNA decay regulate gene expression of serum response factor. Gene. 2007;400(1-2):131-139. 10.1016/j.gene.2007.06.008 [DOI] [PubMed] [Google Scholar]
- 52. Macosko EZ, Basu A, Satija R, et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015;161(5):1202-1214. 10.1016/j.cell.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in GEO under accession code GSE210074. All data analyzed during this study are included in the manuscript and supporting files. Source data files are provided for all figures and figure supplements.


