Abstract
Obesity, cardiometabolic disease, cognitive decline, and osteoporosis are symptoms of postmenopause, which can be modeled using 4-vinylcyclohexene diepoxide (VCD)–treated mice to induce ovarian failure and estrogen deficiency combined with high-fat diet (HFD) feeding. The trend of replacing saturated fatty acids (SFAs), for example coconut oil, with seed oils that are high in polyunsaturated fatty acids, specifically linoleic acid (LA), may induce inflammation and gut dysbiosis, and worsen symptoms of estrogen deficiency. To investigate this hypothesis, vehicle (Veh)- or VCD-treated C57BL/6J mice were fed a HFD (45% kcal fat) with a high LA:SFA ratio (22.5%: 8%), referred to as the 22.5% LA diet, or a HFD with a low LA:SFA ratio (1%: 31%), referred to as 1% LA diet, for a period of 23 to 25 weeks. Compared with VCD-treated mice fed the 22.5% LA diet, VCD-treated mice fed the 1% LA diet showed lower weight gain and improved glucose tolerance. However, VCD-treated mice fed the 1% LA diet had higher blood pressure and showed evidence of spatial cognitive impairment. Mice fed the 1% LA or 22.5% LA diets showed gut microbial taxa changes that have been associated with a mix of both beneficial and unfavorable cognitive and metabolic phenotypes. Overall, these data suggest that consuming different types of dietary fat from a variety of sources, without overemphasis on any particular type, is the optimal approach for promoting metabolic health regardless of estrogen status.
Keywords: dietary fatty acids, ovary-intact postmenopause, energy homeostasis, blood pressure, cognition, gut microbiota
The average age of natural menopause globally is 48.8 years and 51 years in the United States (1). With increasing longevity, at least one-third of a woman's lifespan is spent in menopause and postmenopause when ovaries no longer produce 17β-estradiol (E2) (2). Symptoms of E2-deficiency are experienced by 75% of women (1) and include vasomotor disturbances, alteration of gut microbiota, and chronic diseases such as obesity, cardiovascular disease (CVD), osteoporosis, cognitive decline, and gastrointestinal disorders (3-5). Hormone replacement therapy (HRT) has been the standard of care for menopausal and postmenopausal women. HRT use within the first 5 years of postmenopause is considered beneficial to alleviate vasomotor symptoms and bone loss, but HRT is not recommended for chronic disease prevention (6). HRT use in postmenopausal women over 65 years or use for more than 5 years increases the risk of breast cancer, coronary heart disease, stroke, thromboembolic events, and cholecystitis (7). Due to these potential harms, postmenopausal women need other options to reduce chronic disease risk resulting from E2 decline.
Compared with premenopausal women, postmenopausal women have approximately 5-fold higher risk of abdominal obesity (8). Postmenopausal women with both increased abdominal fat deposition and high insulin resistance had increased blood pressure, hyperglycemia, and hypertriglyceridemia (9). One in 3 postmenopausal women are affected by low-trauma fracture due to osteoporosis, leading to excess morbidity, mortality, and diminished life quality (4). Poor memory is a common symptom during menopausal transition and early postmenopause (10). Independent of age, women in the late stage of postmenopause performed worse in tests of executive function, including planning and mental flexibility, than subjects in the early stage of postmenopause (11), suggesting that hormonal changes were responsible. Dietary modulation of the gut microbiota may contribute to improved health outcomes in postmenopause. Studies in obese postmenopausal women identified gut bacterial species that were positively and negatively correlated with metabolic risk markers such as insulin resistance, obesity, osteoporosis, and memory deficits (5). Probiotics such as Lactobacillus reuteri and L. rhamnosus GG were shown to decrease gut permeability and protect estrogen-deficient mice and postmenopausal women from bone loss (12-14). Gut bacteria play a role in control of systemic estrogens (14). Bacteria that harbor β-glucuronidases with specificity for estrogen can deconjugate biologically inactive estrogen metabolites allowing reabsorption back into enterohepatic circulation (15, 16).
Dietary fatty acids (FAs) are not only high-energy nutrients, but also versatile molecules involved in numerous metabolic pathways. For example, different species of FAs have a variety of effects on energy homeostasis, insulin resistance, and FA oxidation (17-20.) In the US diet, the daily consumption of the most abundant polyunsaturated FA, linoleic acid (LA, C18:2), has remarkably increased to approximately 6% of total dietary energy due to a 20-fold increase in the availability of vegetable oil (21, 22). Concerns about the benefits of high levels of dietary LA have been raised resulting from the correlation with increased prevalence of obesity (23). Studies provide evidence that reducing dietary LA alleviated lipopolysaccharide-induced neuroinflammation in rats (24). Coconut oil is composed of 93% saturated fatty acids (SFAs) and has a unique profile of 10% to 15% caprylic (C8:0), 10% to 15% capric (C10:0) acid, and 45% to 50% lauric acid (C12:0) (25). Defined as medium-chain SFAs, these coconut oil SFAs are more readily absorbed in the proximal small intestine and transported to the liver for conversion into energy rather than fat deposition (26). Coconut oil SFAs have been associated with improved gut microbial profiles and intestinal health in animal models (26). Conflicting conclusions have been presented of whether coconut oil raises the cholesterol level and consequently increases risk of CVD (25, 27). Coconut oil supplementation may enhance memory partially through increased cholinergic activity and reduced oxidative stress (28).
Unlike women, aged mice do not develop a dramatic decline in E2 levels, therefore ovariectomized (OVX) mice are widely used to model E2 deficiency; however, in removing the ovaries other ovarian hormones are also lost (29). 4-Vinylcyclohexene diepoxide (VCD) is an occupational toxin that selectively destroys primary and primordial ovarian follicles while leaving the ovary intact. VCD treatment results in gradual loss of ovarian cycling and closely resembles the natural perimenopause to menopause transition, which is characterized by the gradual increase in the level of follicle stimulating hormone and decrease in androgen and E2 production (29). As in postmenopausal women, the ovaries of VCD-treated rodents continue producing hormones such as androstenedione (30). In this study, we used vehicle (Veh)- and VCD-treated mice to compare the differential effects of HFD enriched with coconut oil SFA or omega-6 LA on metabolic, bone, and cognitive phenotypes and biomarkers in association with the gut microbial changes.
Materials and Methods
Animals and Treatment
Animal studies were conducted according to protocols approved by Rutgers University Institutional Animal Care and Use Committee. After 1 week of acclimation in the animal facility, female wild-type C57BL/6J mice (n = 44) aged 4 weeks (Jackson Laboratory, Bar Harbor, ME) were weighed and injected intraperitoneally (IP) with sesame oil as Veh or VCD (130 mg/kg body weight, 5 days/week) for 3 weeks. Estrous disruption, defined as 10 days of consecutive diestrus, was assessed by vaginal cytology performed 2, 4, and 6 months after VCD/Veh injections. Mice were group housed (5 mice/cage) under controlled conditions (24 ± 1 °C, 12 hours light/dark cycle) with access to food ad libitum (Lab Diet 5V75) until estrous cycle disruption was confirmed. Once confirmed, mice were pair-housed and assigned to 4 diet subgroups. Mice were euthanized between weeks 23 and 25 postintervention, age 53-55 weeks.
Diet
High-fat diets (HFDs, 45% kcal fat) used in this study were purchased from Research Diets (New Brunswick, NJ) and included (1) a HFD rich in coconut oil SFA (1% LA, 31% SFA), referred to as the 1% LA diet, and (2) a HFD rich in omega-6 PUFA (22.5% LA, 8% SFA), referred to as the 22.5% LA diet. Nutritional composition of experimental diets is elsewhere (Table 1 (31)). Veh- and VCD-treated mice were fed the 1% LA diet or 22.5% LA diet after confirmation of estrous cycle disruption. The 4 experimental groups were Veh-1% (n = 10), Veh-22.5% (n = 10), VCD-1% (n = 12), and VCD-22.5% (n = 12).
Behavioral Test
After 10 weeks of dietary intervention and before metabolic phenotyping, to assess the effects of different dietary FAs and E2 deficiency on spatial working and recognition memory, all mice were randomly assigned into 4 subgroups for behavioral tests that were conducted under red lighting during the night cycle. Three behavioral tests took place in the order of Y-maze, spatial object recognition (SOR) and novel object recognition (NOR) as detailed previously (32, 33). All tests were performed by the same investigator and recorded by a camera suspended above the testing arena (ANY-maze, Version 6, Stoelting, USA). A visual cue was hung on the room wall for all 3 tests and another visual cue was located on the inside wall of the open field box for the SOR and NOR tests. Visual cues were not moved throughout all the tests. An opaque cage was used as a holding cage to keep mice away from visual cues during intertrial delay. The entire interior surface of testing arenas and the holding cage were sanitized by MB10 between each testing trial to minimize olfactory cues.
Y-maze testing procedure
The Y-shaped maze had 3 opaque plastic arms (7 w × 35 l × 15 h cm) at a 120° angle from each other. One arm was designated the starting arm and the other 2 arms were defined as the known or novel arm for each test which was counterbalanced between mice. For the exposure trial, 1 randomly chosen mouse was placed at the end of the starting arm, facing toward the center of the maze, with the recording starting simultaneously when the mouse was released. After 5 minutes of undisturbed exploration of the starting arm and known arm, the mouse was put into the holding cage for a 5-minute intertrial delay. Then the divider was removed from the novel arm and the mouse was returned to the Y-maze to freely explore all 3 arms for 5 minutes. With completion of the test session, the mouse was returned to its home cage. An entry was counted when 4 legs or 90% of the body crossed the boundary of an arm. Number, duration, and percentage of entries in each arm was recorded and analyzed.
SOR procedure
For 3 consecutive habituation days, mice were individually placed in the empty opaque open field (40 × 40 × 40 cm, open top), and allowed to freely explore for 5 minutes before being returned to the home cage. On the test day (ie, the fourth day of SOR test), 2 identical objects (50-mL conical tubes) were presented in 2 of 4 parallel quadrants at designated locations 7 cm from the corner walls of the open field. The mouse was placed in the bottom left corner of the open field while being simultaneously recorded. After 5 minutes of free interaction with the objects as the exposure trial, the mouse was put in the holding cage for a 5-minute intertrial delay. One of the objects was displaced, at random, to its diagonal position. For the test trial, the mouse was reintroduced to the open field for exploration with the new object configuration and recorded for 5 minutes. After the test session, the mouse was returned to the home cage. Bouts of exploration number into each object area within 2 cm of the objects, duration, percentage of exploration number, and time with each object were recorded and analyzed.
NOR procedure
After the SOR test, mice had 5 consecutive days of habituation during which individual mice were allowed to explore the empty open field for 5 minutes. On the sixth day, each mouse experienced a 5-minute exposure trial where 2 new identical objects (either 2 250-mL glass flasks or 2 cast iron brackets) were again presented in 2 of the 4 parallel quadrants of the open filed, followed by a 5-minute intertrial delay. One of the familiar objects was replaced, at random, with a novel object while the second familiar item stayed in the same location as it had been during the exposure trial. The mouse was reintroduced into the open field and recorded exploring for 5 minutes as the test trial. Bouts of exploration number, duration, and percentage of exploration number and time were recorded and analyzed.
Hemodynamic Measurements
A noninvasive tail-cuff CODA instrument (Kent Scientific, Torrington, CT, USA) was utilized to assess whether different dietary FAs affected hemodynamic measurements, including systolic, diastolic, and mean blood pressures, and heart rate. Mice were acclimated to the mouse restrainer and measuring method for 20 to 30 minutes a day for 4 consecutive days. On the test day (day 5), each run consisted of 10 cycles for acclimation followed by 20 cycles for data collection. Measurements were determined by averaging more than 3 acceptable values for each run (34).
Metabolic Phenotyping
Body composition
Mice were pair-housed with free access to food and water. Body weight and food intake were monitored weekly for the first 14 weeks of dietary intervention and at weeks 20 to 22 and weeks 23 to 25. Body composition was measured using an EcoMRI 3-in-1 Body Composition Analyzer at baseline and at weeks 20 to 22 of dietary intervention (at age of 50-52 weeks).
Indirect calorimetry
Indirect calorimetry and locomotor activities measurement (72-hour run including 24-hour acclimation and 48-hour data collection) were conducted during weeks 20 to 22 of dietary intervention using the Oxymax Comprehensive Lab Animal Monitoring System (Oxymax/CLAMS, Columbus Instruments, Columbus, OH). As previously described, volume of oxygen consumption and carbon dioxide production were measured and used for calculation of respiratory exchange ratio (RER) and energy expenditure (EE) (35). Physical activity was detected by wheel running and total and ambulatory infrared beam interruption (36). The Oxymax/CLAMS system was configured with triple axis (X-, Y-, and Z-) detection of animal movement, determined as number of times the infrared photocell beams are interrupted in each of the 3 planes. X_total, Y_total, and Z_total indicate the total number of beam interruptions in each plane whereas X_ambulatory and Y_ambulatory indicate when the mouse traverses the cage. Subtraction of ambulatory counts from the total counts indicates the number of beam interruptions that are due to repetitive movements such as grooming and scratching.
Feeding behavior
Biological Data Acquisition (BioDAQ, Research Diets, New Brunswick, NJ) chambers were used for monitoring of feeding behaviors after assessment of indirect calorimetry as previously described (37). Mouse food pellets were loaded in a touch-sensitive hopper and food consumption was obtained by reduction of food weight in the hopper. When the mouse interacted with the hopper and removed any of its diet, the system denoted each reduction as a “bout.” When intervals between each bout registered as greater than 300 seconds apart, the food consumed was determined to be a “meal.” Meal size, frequency, and duration were recorded for 72 hours (ie, 3 days) after an initial 96 hours of habituation. Food intake at corresponding hours for the 3 days of monitoring were averaged and graphed as hourly food intake from 0 to 23 hours and summed over the 72-hour period for total food intake.
Oral glucose tolerance test
The oral glucose tolerance test (OGTT) was performed on all mice during week 19 of dietary intervention, as previously described (35). Mice were fasted for 5 hours with ad libitum access to water in a clean cage (9 Am to 2 Pm) and perorally gavaged with glucose (2 g/kg body weight) immediately after baseline measurement. Blood glucose measurements were collected from tail pricks at baseline (t = 0 minutes), and then 15, 30, 60, 90, and 120 minutes after glucose challenge. After 120 minutes, all mice were returned to their home cages.
Insulin tolerance test
During weeks 20 to 21 of dietary intervention, an insulin tolerance test (ITT) was performed as previously described (38). Mice were fasted for 4 hours (9 Am to 1 Pm) with ad libitum access to water. Fasted mice were intraperitoneally injected with 0.75 units/kg insulin in 0.9% saline solution (Humulin R; Lilly, Indianapolis, IN, USA). Blood samples were collected from tail pricks before injection (t = 0 minutes) and then 15, 30, 60, 90, and 120 minutes after injection.
Tissue Collection
Four days after the final physiological test (at 53-55 weeks of age), mice were euthanized after sedation with ketamine (100 µL of 100 mg/mL) after 2 hours of fasting. Tissues including liver, intestinal segments, and gonadal white adipose tissue (GWAT) were harvested, snap frozen in liquid nitrogen, and stored at −80 °C freezer for future analysis. The brain was removed from the skull and the basal hypothalamus was cut using a brain slicer (Ted Pella, Inc., Redding, CA, USA) into 1-mm-thick coronal rostral and caudal blocks corresponding to plates 42 to 47 and plates 48 to 53, respectively (39). The basal hypothalamus blocks were transferred to RNAlater (Life Technologies, Inc., Grand Island, NE, USA) and stored overnight at 4 °C. The rostral and caudal parts of the arcuate nucleus were dissected from slices using a dissecting microscope. Dissected arcuate nucleus tissue was stored at −80 °C until gene expression analyses. Ovaries were collected, fixed in formalin, embedded in paraffin, sectioned (5 µm each) into 20 to 25 sections, and stained with hematoxylin and eosin staining for microscopic imaging and follicle counting. Follicles were counted every fifth section and classified as primordial, primary, secondary, or other, which includes postsecondary, preantral, and antral (40, 41). Mouse carcasses were stored frozen at −80 °C until bone analyses.
17β-Estradiol Enzyme-Linked Immunosorbent Assay
Serum E2 was measured in VCD-treated (n = 20) and Veh-treated (n = 6) mice using a 17β-estradiol enzyme-linked immunosorbent assay kit (Abcam cat# ab108667, RRID:AB_2927550) according to the manufacturer's instructions. Color intensity was measured at 450 nm using a ClarioStar multimode plate reader.
Bone Densitometry
Areal bone mineral density (aBMD) and bone mineral content (BMC) of whole body and individual bone samples including femur, tibia, humerus, and lumbar spine (L1-L5) was measured using dual-energy X-ray absorptiometry (GE-Lunar PIXImus mouse densitometer software version 2.10.41) as detailed previously (42).
Hepatic, Gonadal White Adipose Tissue (GWAT), and Basal Hypothalamic Tissue Quantitative Polymerase Chain Reaction
Total hepatic and GWAT RNA from tissues were extracted using NucleoSpin RNA extraction Kits (Macherey-Nagel, Inc.) according to the manufacturer's protocol followed by quantification and qualification using Nanodrop (Thermo Fisher Scientific Inc.) as previously described (39). Basal hypothalamic tissue was extracted using Ambion Micro-RNA kits (see other Roepke papers for explanations). cDNA was synthesized from 500 ng of total RNA and reverse transcription quantitative polymerase chain reaction (RT-qPCR) was performed on a CFX-Connect Real-time PCR instrument (BioRad, Inc.) using either PowerSYBR Green master mix (Life Technologies) or Sso Advanced SYBR Green (BioRad, Inc.). Primers (Table 2 (31)) were designed to span exon–exon junctions using Clone Manager 5 software (Sci Ed Software, Cary, NC, USA) and were synthesized by Life Technologies, Inc. The conditions were as follows: at 95 °C for 10 minutes (PowerSYBR) or 3 minutes (Sso Advanced) followed by 45 cycles (PowerSYBR) or 40 cycles (Sso Advanced) of 94 °C for 10 seconds (denaturation), 60 °C for 45 seconds (annealing), and completed with a dissociation step for melting point analysis with 60 cycles of 95 °C for 10 seconds, 65 °C to 95 °C (in increments of 0.5 °C) for 5 seconds, and 95 °C for 5 seconds. The Cq geomean of reference genes, Hprt, Actb, and Gapdh, were used to calculate fold change and the relative gene expression data were analyzed using the ΔΔCT method.
Ileum Tissue qPCR
RNA from ileum tissues (30-35 mg) was extracted following the manufacturer's protocol using the Qiagen Universal RNA extraction kit. RNA concentrations were quantified by Nanodrop (Thermo Fisher Scientific Inc.). cDNA was synthesized from 5 µg of RNA and RT-qPCR was performed (with technical duplicates) on a thermocycler (Quantstudio 3, Thermo Fisher Scientific Inc) using Taqman Fast Universal PCR master mix using conditions: 20 seconds at 95 °C followed by 40 cycles of 95 °C for 1 second (denaturation) and 60 °C for 20 seconds (annealing and extension). TaqMan assay primers (Life Technologies) used are summarized elsewhere (Table 3 (31)). The housekeeping gene (Hmbs) was used to calculate gene expression using the ΔΔCT method.
Histology and Immunohistochemistry
The distal colonic segment together with a whole fecal pellet were harvested: the first 4 to 6 cm from the pyloric sphincter was collected as colon. Tissue was fixed overnight at 4 °C in 3% paraformaldehyde and 2% sucrose then embedded in paraffin. For histological analysis, 5-μm intestinal tissue sections were stained with hematoxylin and eosin (H&E) (Histopathology Core Facility, New Brunswick, NJ). Goblet cells along the crypts and surface of the epithelium were counted from images (5.5×) of H&E stains. Goblet cell parameters for each group were determined using an arbitrary (450 × 450 pixels) square region of interest as previously described (43, 44). Specifically, binary color threshold and particle size range were determined and kept constant. The numbers of goblet cells within the region of interest were recorded. Total length and average width of mucosa analyzed in each region of interest were recorded. ImageJ software was used for data acquisition (https://imagej.nih.gov/ij). For histomorphometry all tissues sections were blindly scored by a board-certified pathologist.
For immunohistochemical studies, intestinal sections were deparaffinized, rehydrated, and subsequently blocked with 10%, 50%, or 100% normal goat serum at room temperature for 2 hours. The tissue sections were then incubated overnight at 4 °C with primary rabbit affinity purified polyclonal antibodies against structural components of the intestine as described below, or with rabbit IgG (RRID:AB_1940281, ProSci Inc., Poway, CA) as a control. Colon segments were stained for cyclooxygenase-2 (Cox-2, 1:200, RRID:AB_2085144, Abcam, Cambridge, MA), mucin-2 (Muc-2, 1:2000, RRID:AB_2888616, Abcam), inducible nitric oxide synthase (1:500, RRID:AB_2927640, Abcam) expression. Tissue sections were then incubated for 30 minutes with a biotinylated goat antirabbit secondary antibody (Vector Labs, Burlingame, CA). Antibody binding was visualized using a DAB Peroxidase Substrate Kit (RRID:AB_916366, Vector Labs). Tissue sections were photographed using the VS120-S5 System (Olympus, Center Valley, PA). For histomorphometry all tissues sections were blindly scored by a board-certified pathologist. Slide staining was reported using a dual number system (#×#). The first number was the intensity of the stain, and the second number was the amount of stain present in the specimen. The intensity was graded 1 to 4 and the amount indicated as follows: 1, <10%; 2, 11% to 40%; 3, 41% to 60%; and 4, >60%. The total score was obtained by multiplying the 2 numbers.
Gut Microbiota Analysis
Genomic DNA (gDNA) was extracted from fecal samples using DNeasy PowerSoil Pro Kit (QIAGEN). Library preparation and paired-end sequencing of 16S rRNA V3-V4 amplicons (2 × 250 bp configuration) was performed by Azenta LifeSciences (Piscataway, NJ) using an Illumina MiSeq instrument. Paired-end sequences were imported into QIIME2-2021.8 (45), demultiplexed, and quality filtered using the q2-demux plugin. Demultiplexed sequences were denoised, decluttered, and merged using q2-dada2 (46) to generate amplicon sequence variants (ASVs). Taxonomy was assigned to ASVs using the q2-feature-classifier (47) and classify-sklearn Naïve Bayes taxonomy classifier trained on the Silva 138 SSU full-length reference sequences. Beta diversity metrics analysis of variance was determined using ADONIS and 10 000X permutation analysis in the vegan package within R Studio v.4.1.2 (R Studio Software, Boston, MA, USA). Differences of gut bacteria at the phylum and genus levels were determined by nonparametric Kruskal–Wallis test followed by 2-stage step-up method of Benjamini, Krieger, and Yekutieli with false discovery rate adjusted P value, q < .05.
Statistical Analysis
Data were analyzed with a 2-way analysis of variance (ANOVA) with treatment (Veh or VCD) and diet (1% LA or 22.5% LA) as factors followed with the Holm–Sidak multiple comparison using GraphPad Prism 9.4 (GraphPad Software, La Jolla, CA, USA) and expressed as mean ± SD. Values of P < .05 were considered to be statistically significant.
Results
Coconut Oil SFA Decreased Weight Gain and Food Consumption
Twenty-four weeks after VCD treatment, cessation of the estrus cycle was confirmed by vaginal cytology and defined as 10 days of consecutive diestrus. Furthermore, histological analysis of ovaries collected at the end of the experiment showed follicles and corpora lutea were absent in VCD-treated mice (Fig. 1A and 1B) while Veh-treated groups had all 4 types of follicles present as an indicator of their regular ovulation (Fig. 1A and 1B). Compared with Veh-treated mice, VCD-treated mice had significantly lower serum E2 levels (Fig. 1A (31)). After postmenopause confirmation, at age 30 weeks, Veh- or VCD-treated mice were fed 22.5% LA diet or 1% LA for a total of 4 intervention groups: Veh-22.5% LA, Veh-1% LA, VCD-22.5% LA, VCD-1% LA.
Figure 1.
Follicle counting, body weight, food intake, body composition, and feeding behavior. (A) Representative images of H&E-stained ovary sections. Data for B-K are presented as mean ± SD and significant differences were determined by a 2-way ANOVA followed by Holm–Sidak post hoc analysis. # denotes significant difference between VCD-1% and VCD-22.5% LA diet groups. & denotes significant difference between Veh-1% and Veh-22.5% groups. % sign denotes significant difference between Veh-1% vs VCD-1% group. @ denotes significant differences between Veh-22.5% and VCD-22.5% groups. Differences between individual groups are indicated as *P < .05 and **P < .01. (B) Counts of primordial, primary, secondary, and other (preantral and antral) follicles in Veh- and VCD-treated mice. Data represent counts from 5 or 6 sections per ovary from 4 mice per group. (C) Cumulative weight gain for week 0 to 14 of dietary intervention (n = 10 for Veh-treated groups and n = 12 for VCD-treated groups). Significant ANOVA results were obtained for time, F(13, 560) = 92.93, P < .0001 and treatment × diet interaction, F(3, 560) = 30.49, P < .0001. (D) Weight gain from baseline after 20 to 22 weeks and 23 to 25 weeks on diets. Significant ANOVA results were obtained for week 20 to 22, diet F (1, 36) = 10.91 P = .0022 and for week 23 to 25, diet F(1,35) = 8.872, P = .0052). (E) Percent fat mass measured during week 20 to 22 of dietary intervention. (F) Percent lean mass measured during week 20 to 22 of dietary intervention with significant difference for diet: F(1,36) = 12.66, P = .0011. For D-F, n = 10 for Veh-treated groups and n = 11 for VCD-treated groups. (G) Cumulative food intake for week 0 to 14 of dietary intervention (n = 10 for Veh-treated groups and n = 12 for VCD-treated groups). Significant results were obtained for time, F(13, 252) = 942.9, P < .0001 and treatment × diet interaction, F(3, 252) = 50.72, P < .0001. Feeding behavior monitored by Biodaq for (H) number of meals (I) average meal size (significance was obtained for diet, F(1,36) = 19.69, P < .0001) (J) total food intake for 72 hours (significance was obtained for diet, F(1,37) = 7.340, P = .0102). (K) 24-hours feeding pattern (significance detected for time, F(23 867) = 46.59, P < .0001 and treatment × diet interaction, F(3867) = 5.037, P = .0018). For H-K, sample sizes were 10 or 11 for each group.
All groups of mice showed similar body weight gain for the first 9 weeks of intervention (Fig. 1C). Between 10 and 14 weeks of dietary intervention, VCD-treated mice fed the 22.5% LA diet gained more weight than VCD-treated mice fed the 1% LA diet (Fig. 1C). During weeks 20 to 22 and 23 to 25 of dietary intervention, Veh-treated mice fed the 22.5% LA diet gained more weight than Veh-treated mice fed the 1% LA diet (Fig. 1D). Compared with VCD-treated mice fed the 1% LA diet, VCD-treated mice fed the 22.5% LA diet gained more body weight during weeks 20 to 22 of dietary intervention and showed a trend of increased weight gain during weeks of 23 to 25 of dietary intervention (Fig. 1D). Compared with Veh-treated mice fed the 1% LA diet, Veh-treated mice fed the 22.5% LA diet had higher final body weights after 23 to 25 weeks of dietary intervention; however, there were no significant differences in the final body weights of VCD-treated mice (Fig. S1B (31)). After 20 to 22 weeks on diet, there were no differences in the percent fat mass due to treatment or diet (Fig. 1E). Regardless of estrus cycle, mice fed the 22.5% LA diet had lower percent lean mass than those fed 1% LA diet in both Veh- and VCD-treated groups (Fig. 1F), suggesting FAs from coconut oil have a beneficial effect on preservation of skeletal muscle mass (48). Consistent with changes in weight gain, the VCD-treated mice fed the 22.5% LA diet had more cumulative food intake than the VCD-treated mice fed the 1% LA diet between weeks 10 and 14 of dietary intervention (Fig. 1G). Compared with Veh-treated mice fed the 1% LA diet, the Veh-treated mice fed the 22.5% LA diet had more cumulative food intake from weeks 11 to 14 of dietary intervention (Fig. 1G).
During weeks 20 to 23 of diet intervention, feeding behavior was assessed with a BioDAQ instrument for analysis of meal frequency, meal size, hourly food intake, and total food intake. During the 72 hours of data collection, the number of meals was similar among the 4 groups (Fig. 1H). Compared with Veh-treated mice fed the 1% LA diet, Veh-treated mice fed the 22.5% LA diet consumed more per meal (Fig. 1I) and showed higher total food intake during 72 hours of monitoring (Fig. 1J). Compared with VCD-treated mice fed the 1% LA diet, mice the fed 22.5% LA diet consumed more food per meal (Fig. 1I), but their total food intake was similar (Fig. 1J), possibly because the number of meals consumed was lower on average, although not significantly (Fig. 1H). Hourly food intake was similar among the 4 groups at most time points (Fig. 1K) with transient differences at night: 01:00, 02:00, and 23:00 hours. Veh-treated mice fed the 22.5% LA diet displayed a spike in food consumption at 01:00, 02:00, and 23:00 hours compared with the Veh-treated mice fed the 1% LA diet (Fig. 1K). At the 1-hour time point, increased food intake was observed in VCD-treated mice fed the 1% LA diet compared with Veh-treated mice fed the 1% LA diet (Fig. 1K). At the 2-hour time point, there was lower food consumption in the VCD-treated mice fed the 22.5% diet than Veh-treated mice fed the 22.5% LA diet (Fig. 1K).
Dietary Fatty Acid Composition Did not Alter Glucose or Insulin Tolerance
To assess the impact of different dietary FAs on glucose homeostasis in estrogen-sufficient and estrogen-deficient stages, glucose and insulin tolerance tests were performed. Compared with VCD-treated mice fed the 22.5% LA diet, VCD-treated mice fed the 1% LA diet had lower blood glucose at the 60- and 90-minute time points (Fig. 2B). The main diet effect for blood glucose area under the curve (AUC) was F(1, 40) = 4.736, P = .0355; however, the post hoc comparison between VCD-treated mice fed the 22.5% LA diet and VCD-treated mice fed the 1% LA did not reach significance (Fig. 2B; AUC, P = .071). Compared with Veh-treated mice fed the 1% LA diet, Veh-treated mice fed the 22.5% LA diet had higher blood glucose levels 15 minutes after insulin injection (Fig. 2C). There was a significant main effect of interaction between treatment × diet (F(1, 38) = 4.275, P = .0455); however, dietary FAs did not affect insulin tolerance (Fig. 2D). Compared with the Veh-treated mice fed the 22.5% LA diet, VCD-treated mice fed the 22.5% LA diet had lower blood glucose AUC (Fig. 2D), suggesting improved insulin sensitivity in VCD-treated mice, although the distribution of data points indicates high individual variability.
Figure 2.
Oral glucose tolerance test (OGTT) and insulin tolerance test (ITT). (A) Blood glucose curve obtained for OGTT and (B) area under the blood glucose curve (AUC). (C) Blood glucose curve obtained for ITT and (D) AUC. Data are presented as mean ± SD. Sample sizes were 10 for Veh-treated groups and 12 for VCD-treated groups. Data were analyzed by a 2-way ANOVA followed by Holm–Sidak multiple comparison (*P < .05) between and within Veh or VCD treatment. Significantly different results of ANOVA tests were obtained (A) at 60 minutes, diet: F(1, 40) = 4.105, P = .0495, at 90 minutes, F(1, 40) = 6.878, P = .0123; (B) diet: F(1, 40) = 4.736, P = .0355; (C) at 15 minutes, treatment × diet: F(1, 38) = 7.549, P = .0091, at 30 minutes, treatment × diet: F(1, 38) = 4.231, P = .0466; (D) treatment × diet: F(1, 38) = 4.275, P = .0455. # denotes significant difference between VCD-1% and VCD-22.5% groups. & denotes significant difference between Veh-1% and Veh-22.5% groups.
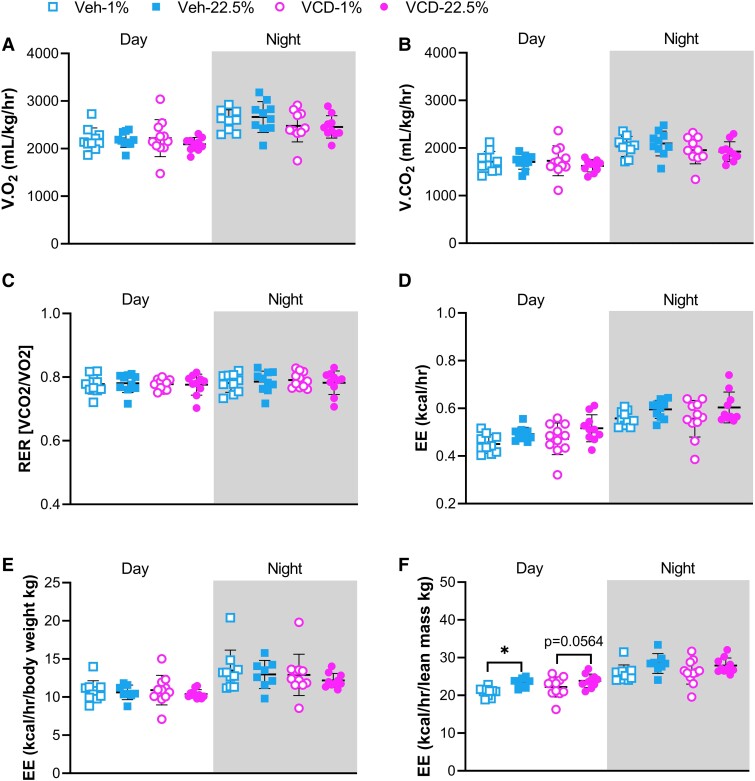
High LA Diet Increased Energy Expenditure in Veh-treated Mice and VCD-treated Mice Displayed Less Locomotor Activity
The Oxymax/CLAMS system was used to measure metabolic parameters over a 48-hour period, including volume of oxygen consumed, volume of carbon dioxide produced, RER, EE measured as heat generation, and locomotor activity. The 4 groups exhibited similar levels of oxygen consumption and carbon dioxide production during the day and night phases (Fig. 3A and 3B). RER was close to 0.7 in all groups, indicating that FAs were utilized as primary substrate for oxidative metabolism (Fig. 3C). EE and EE normalized to body weight were similar among all groups (Fig. 3D and 3E); however, when normalized to lean mass, Veh-treated mice fed the 22.5% LA diet showed increased EE, and VCD-treated mice fed the 22.5% diet showed a trend of higher EE during daytime (Fig. 3F). Difference in activity due to FA type was only observed in total Y-axis activity where Veh-treated mice fed the 22.5% LA diet interrupted the infrared radiation beam less frequently than Veh-treated mice fed the 1% LA diet (Fig. 4D). During the night phase, spontaneous activity as measured by beam interruption of X-, Y-, and Z-axes together with wheel running was suppressed due to VCD-induced estrogen deficiency (Fig. 4A-4F). VCD-treated mice fed the 22.5% LA diet had lower activity than Veh-treated mice fed the 22.5% LA diet in all measurements (Fig. 4A-4F). Compared with Veh-treated mice fed the 1% LA diet, VCD-treated mice fed the 1% LA diet showed less activity in X- and Y-axis movements during nighttime and in total Y-axis activity during daytime (Fig. 4B-4E).
Figure 3.
Indirect calorimetry. (A) volume of oxygen consumed, VO2, (B) volume of carbon dioxide produced, VCO2, (C) respiratory exchange ratio, RER, (D) energy expenditure, EE, (E) EE normalized to body weight (kg), and (F) EE normalized to lean mass (kg). Data are presented as mean ± SD. Sample sizes were 10 for Veh-treated groups and 12 for VCD-treated groups. Data were analyzed by a 2-way ANOVA followed by Holm–Sidak multiple comparisons (*P < .05) between and within Veh or VCD treatment for daytime or nighttime. Significantly different ANOVA results are (D) daytime, diet: F(1, 37) = 7.404, P = .0099; nighttime, diet: F(1, 37) = 5.874, P = .0204; (F) daytime, diet: F(1, 35) = 11.53, P = .0017; nighttime, diet: F(1, 35) = 6.526, P = .0151.
Figure 4.
Locomotor activity. (A) Wheel running, (B) X-plane total activity, (C) X-plane ambulatory activity, (D) Y-plane total activity, (E) Y-plane ambulatory activity, (F) Z-plane total activity. Data are presented as mean ± SD. Sample sizes were 10 for Veh-treated groups and 12 for VCD-treated groups. Significant differences were determined by 2-way ANOVA followed by Holm–Sidak multiple comparison (*P < .05, **P < .01, ***P < .001) between and within treatment for daytime or nighttime. Significantly different results of ANOVA tests were (A) for nighttime, treatment: F(1,34) = 12.68, P = .0011; (B) for nighttime, treatment: F(1,36) = 15.626, P = .0004; (C) for nighttime, treatment: F(1,36) = 15.93, P = .0003; (D) for daytime, treatment: F(1,36) = 5.443, P = .0254; diet: F(1,36) = 5.136, P = .0295. For nighttime, treatment: F(1,36) = 24.41, P < .0001. (E) For daytime, treatment: F(1,37) = 7.318, P = .0103. For nighttime, treatment: F(1,35) = 16.83, P = .0002. (F) For daytime, treatment: F(1,34) = 4.176, P = .0488. For nighttime, treatment: F(1,35) = 11.38, P = .0018.
LA Reduced Blood Pressure in VCD-Treatment Mice
During weeks 16 and 17 of dietary intervention, systolic, diastolic, and mean blood pressures and heart rate were measured using a CODA system. VCD-treated mice fed the 22.5% LA diet had lower systolic and mean blood pressure than VCD-treated mice fed the 1% LA diet (Fig. 5A and 5C). Dietary FAs had the main effects on diastolic blood pressure (F(1,39) = 4.105, P = .0496) and heart rate (F(1,39) = 6.105, P = .0181); however post hoc analysis did not reach significance (Fig. 5B and 5D). Veh or VCD treatment showed no impact on hemodynamics. There were no diet-induced differences on blood pressure or heart rate among Veh-treated mice (Fig. 5). Overall, these results suggest that PUFAs such as LA exert a hypotensive effect in E2 deficiency.
Figure 5.
Blood pressure. (A) Systolic blood pressure. (B) Diastolic blood pressure. (C) Mean blood pressure. (D) Heart rate. Data are presented as mean ± SD. Sample sizes were 9 or 10 for Veh-treated groups and 12 for VCD-treated groups. Differences were determined by 2-way ANOVA followed with Holm–Sidak post hoc comparison (* P < .05) between and within treatment groups. Significantly different results of ANOVA test were (A) diet: F(1,39) = 5.096, P = .0297; (B) diet: F(1,39) = 4.105, P = .0496; (C) diet: F(1,39) = 4.597, P = .00383; (D) diet: F(1,39) = 6.105, P = .0181.
VCD-Treated Mice fed the Coconut Oil SFA-Rich Diet Showed Evidence of Reduced Cognitive Function
During weeks 9 to 12 of dietary intervention, mice underwent behavioral tests to assess the differential effects of dietary FAs, with and without E2 deficiency, on cognitive function. In the Y-maze spatial recognition test, the main effects caused by VCD treatment were observed in both entries (Fig. 6A) and time in the unknown arm (Fig. 6B), indicating impaired spatial recognition due to E2 deficiency. There was a main effect of treatment on the percentage of entries into the unknown arm and a moderate effect of treatment on the percentage of time spent in the unknown arm (Fig. 6C and 6D). In particular, VCD-treated mice fed the 1% LA diet entered fewer times (Fig. 6A) and spent less time (Fig. 6B) than Veh-treated mice fed the 1% LA diet. There were no differences between Veh- and VCD-treated mice fed the 22.5% LA diet (Fig. 6A-6D), suggesting that LA rather than coconut oil SFA may protect cognitive function in the E2-deficient state.
Figure 6.
Behavioral tests including Y-maze (A,B), spatial object recognition test (SOR, E-H), and novel object recognition (NOR, I-L). (A) Unknown arm entries. (B) Unknown arm time. (C) Percent entries into the unknown arm. (D) Percent time in the unknown arm. (E) Head entries in the area of displaced object. (F) Head time in the area of displaced object. (G) Percent of head entries in the area of displaced object. (H) Percent of head time in the area of displaced object. (I) Head entries in the area of novel object. (J) Head time in the area of novel object. (K) Percent of head entries in the area of novel object. (L) Percent of head time in the area of displaced object. Veh-treated groups, n = 10 and VCD-treated groups, n = 12. Differences were determined by 2-way ANOVA followed with Holm–Sidak post hoc comparison (*P < .05) between and within treatment groups. Significant ANOVA results are shown under graph.
For the SOR test, the interaction between treatment and diet exhibited significant impact on the percentage of exploration bouts of the displaced object (Fig. 6G); however, there were no main effects of treatment × diet on exploration bouts, time, or percentage of time in the exploration of a displaced object (Fig. 6E, 6F, and 6H). VCD-treated mice fed the 1% LA diet showed decreased exploration of the displaced object compared with Veh-treated mice fed the 1% LA diet, suggesting E2 deficiency combined with coconut oil SFA induced impaired cognition (Fig. 6G). Spatial memory was not altered by individual factors (Fig. 6E-6H). For the NOR test, the 4 groups showed similar tendency and time spent in exploring the novel object (Fig. 6I-6L).
E2 Deficiency Induced Bone Loss Assessed by Dual-Energy X-ray Absorptiometry
Bones were evaluated for BMC and BMD after 23 to 25 weeks of dietary intervention, when mice were 53-55 weeks old. There was a significant main effect of treatment on BMC of humerus and spine (Table 1). VCD-induced E2 deficiency significantly reduced BMD of individual bone samples, including femur, tibia, and spine, compared with Veh-treated E2-sufficient mice (Table 1). The BMD of spine in VCD-treated mice was significantly lower than Veh-treated counterparts fed the same diet (Table 1). A trend of reduced BMD was also detected in humerus bone samples of VCD-treated mice (P = .085) (Table 1). No significant main effects of diet or interaction (treatment × diet) were found on BMD and BMC of individual bones or whole body (Table 1).
Table 1.
Bone parameters
| Measurements | Groups | P value | |||||
|---|---|---|---|---|---|---|---|
| Veh-1% | Veh-22.5% | VCD-1% | VCD-22.5% | Treatment | Diet | Interaction (treatment × diet) |
|
| Whole body BMD (g/cm3) | 0.0570 ± 0.0022 | 0.0562 ± 0.0015 | 0.0561 ± 0.0037 | 0.0555 ± 0.0006 | .290 | .304 | .891 |
| Femur BMD (g/cm3) | 0.0654 ± 0.0021 | 0.0657 ± 0.0027 | 0.0630 ± 0.0032 | 0.0630 ± 0.0038 | .006 | .595 | .815 |
| Tibia BMD (g/cm3) | 0.0511 ± 0.0022 | 0.0512 ± 0.0014 | 0.0498 ± 0.0018 | 0.0495 ± 0.0018 | .021 | .882 | .882 |
| Humerus BMD (g/cm3) | 0.0497 ± 0.0021 | 0.0504 ± 0.0016 | 0.0492 ± 0.0017 | 0.0487 ± 0.0022 | .085 | .892 | .320 |
| Spine BMD (g/cm3) | 0.0603 ± 0.0012* | 0.0595 ± 0.0026* | 0.0564 ± 0.0009 | 0.0556 ± 0.0020 | <.0001 | .195 | .941 |
| Whole body BMC (g) | 0.6772 ± 0.0866 | 0.5923 ± 0.0642 | 0.6595 ± 0.1195 | 0.6442 ± 0.1332 | .625 | .126 | .308 |
| Femur BMC (g) | 0.0310 ± 0.0017 | 0.0316 ± 0.0025 | 0.0302 ± 0.0020 | 0.0304 ± 0.0022 | .123 | .552 | .750 |
| Tibia BMC (g) | 0.0254 ± 0.0014 | 0.0255 ± 0.0012 | 0.0247 ± 0.0020 | 0.0252 ± 0.0013 | .301 | .560 | .709 |
| Humerus BMC (g) | 0.0145 ± 0.0007 | 0.0151 ± 0.0007 | 0.0142 ± 0.0007 | 0.0143 ± 0.0011 | .035 | .194 | .337 |
| Spine BMC (g) | 0.0487 ± 0.0076 | 0.0490 ± 0.0045 | 0.0455 ± 0.0031 | 0.0448 ± 0.0030 | .019 | .887 | .733 |
Values are means ± SD. Sample sizes: n = 10 for Veh-treated groups, n = 11 for VCD-treated groups. Data were analyzed by 2-way ANOVA followed by Holm–Sidak multiple comparison between and within treatments, P < .05.
Abbreviations: BMD, bone mineral density; BMC, bone mineral content.
*Denotes significant difference between Veh- and VCD-treated mice fed the same diet.
Neuropeptide and Hormone Receptor Genes Were Regulated by Dietary FAs and Treatment
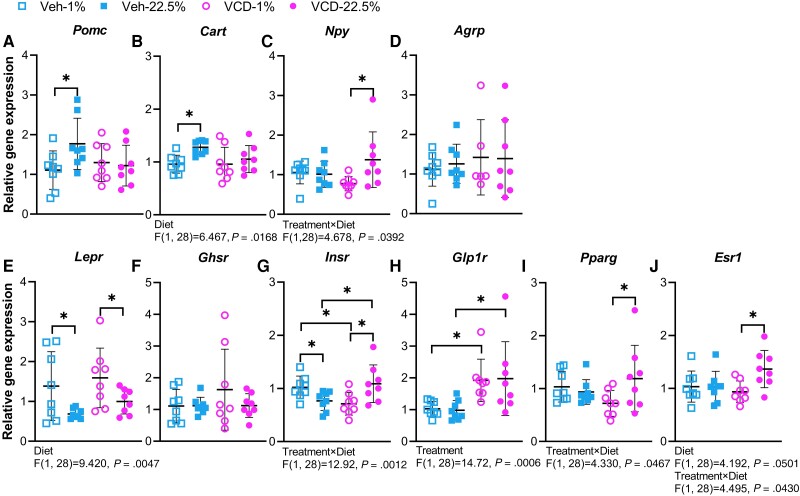
Gene expression levels of 4 neuropeptides expressed in the arcuate nucleus were analyzed to assess effects of diet and/or treatment on markers of feeding and energy homeostasis. Activation of proopiomelanocortin (POMC), encoded by Pomc, and cocaine-and-amphetamine-regulated transcript (CART), encoded by Cart, is linked to suppression of food intake and increased energy expenditure (49). Veh-treated mice fed the 22.5% LA diet showed increased Pomc mRNA levels compared with Veh-treated mice fed the 1% LA diet (Fig. 7A). Cart gene expression was influenced by diet and Veh-treated mice fed the 22.5% LA diet had higher Cart mRNA levels than Veh-treated mice fed the 1% LA diet (Fig. 7B). Increased Pomc and Cart in Veh-treated mice fed the 22.5% LA diet was inconsistent with their higher food intake (Fig. 1G and 1J). Activation of neuropeptide Y (NPY) and agouti-related peptide (AgRP) is related to increased appetite and weight gain (38). There was a main effect of interaction between treatment and diet on the mRNA level of Npy (Fig. 7C). VCD-treated mice fed the 22.5% LA diet had higher Npy mRNA levels than VCD-treated mice fed the 1% LA (Fig. 7C), which was consistent with the increased food intake of VCD-treated mice fed the 22.5% LA diet (Fig. 1G). There was no main effect of diet or treatment on the gene expression of Agrp (Fig. 7D).
Figure 7.
(A-J) Arcuate gene expression of neuropeptides and hormone receptors. Data are presented as mean ± SD. Sample sizes were n = 8 per group. Differences determined by 2-way ANOVA followed with Holm–Sidak post hoc comparison (*P < .05) between and within treatment groups. Significant ANOVA results are shown under graph.
The gene expression levels of 6 hormone receptors from the arcuate nucleus tissue were analyzed. Leptin predominantly secreted by adipose tissue binds to hypothalamic leptin receptor (LEPR), encoded by Lepr, and signals satiety and energy sufficiency, leading to reduced food intake and weight gain. Compared with Veh- and VCD-treated mice fed the 1% LA diet, Veh- and VCD-treated mice fed the 22.5% LA diet had lower Lepr gene expression (Fig. 7E), which was consistent with increased food intake and weight gain in mice fed the 22.5% LA diet (Fig. 1). Growth hormone secretagogue receptor (GHSR), encoded by Ghsr, is bound by ghrelin, a hormone secreted predominantly by the stomach in response to hunger. Augmentation of NPY/AgRP gene expression is potently induced by ghrelin activation of GHSR. However, the gene expression level of Ghsr was not affected by diet or treatment (Fig. 7F). Insulin transports across the blood–brain barrier and interacts with hypothalamic insulin receptor (INSR), encoded by Insr, to regulate energy homeostasis. A main effect of diet and treatment interaction was observed in the gene expression level of Insr. Within Veh-treated groups, mice fed the 22.5% LA diet had lower expression level of Insr than those fed 1% LA diet (Fig. 7G). Within VCD-treated groups, mice fed the 22.5% LA diet showed a higher expression level of Insr than those fed the 1% LA diet (Fig. 7G). Compared with Veh-treated mice fed the 22.5% LA diet, VCD-treated mice fed the 22.5% LA diet had higher Insr mRNA levels (Fig. 7G). Glucagon-like peptide 1 (GLP1) binds to the receptor GLP1R, encoded by Glp1r, to stimulate insulin secretion. There was a main effect of treatment on the mRNA level of Glp1r, and VCD-treated mice showed upregulated Glp1r mRNA level compared with Veh-treated counterparts on the same diet (Fig. 7H). The gene expression level of Pparg, encoding peroxisome proliferator-activated receptor gamma (PPARγ), was increased in VCD-treated mice fed the 22.5% LA diet compared with VCD-treated mice fed the 1% LA diet, which may trigger the upregulation of NPY/AgRP to induce food intake (50) (Fig. 7I). Estrogen receptor alpha, encoded by Esr1, is the most extensively studied estrogen receptors for mediating metabolism. There were main effects of diet and treatment × diet on the gene expression level of Esr1, and VCD-treated mice fed the 22.5% LA diet showed increased Esr1 mRNA levels compared with VCD-treated mice fed the 1% LA diet (Fig. 7J). Although serum E2 was not measured, it is possible that the increase in Esr1 expression may be due to lower levels of E2 circulating in mice fed the 22.5% LA diet.
Dietary FAs and/or VCD Treatment Altered Markers of Fat and Glucose Metabolism in Liver and GWAT
Gene expression levels were assessed to investigate the effects of coconut oil SFA and LA on hepatic genes involved in FAs, triglycerides, and glucose metabolism. Forkhead box O1 (FOXO1), a key transcription factor in insulin-dependent pathways, activates glucose 6-phosphatase (G6Pase), and phosphoenolpyruvate carboxykinase (PEPCK) to promote hepatic gluconeogenesis (51). Compared with Veh-treated mice fed the 22.5% LA diet, mRNA levels of Foxo1 were reduced in the VCD-treated mice fed the 22.5% LA diet while in Veh-treated groups mice fed the 22.5% LA diet had higher mRNA levels of Foxo1 compared with mice fed the 1% LA diet (Fig. 8A). Compared with Veh-treated mice fed the 1% LA diet, VCD-treated mice fed the 1% LA diet had lower expression of G6pc (Fig. 8A) There were no differences in the gene expression levels of Pepck and Insr (Fig. 8A). Hepatic lipogenesis is regulated by various enzymes, including acetyl-CoA carboxylase (ACC), ATP citrate lyase (ACLY), and diglyceride acyltransferase (DGAT2) (52). Compared with VCD-treated mice fed the 1% LA diet, VCD-treated mice fed the 22.5% LA diet had suppressed mRNA level of Acly, Acc1, and Acc2, encoding ACLY, ACC1, and ACC2, respectively, suggesting repressed hepatic lipogenesis and activated FA β-oxidation (Fig. 8A). Compared with Veh-treated mice fed the 1% LA diet, Veh-treated mice fed the 22.5% LA diet also showed decreased Acc2 mRNA levels (Fig. 8A). No differences were shown in the gene expression levels of Fasn and Dgat as well as 2 FA transport proteins, FATP2 and FATP5, encoded by Fatp2 and Fatp5 (Fig. 8A).
Figure 8.
Liver and GWAT qPCR. Gene expression of markers of lipid and glucose metabolism pathways in (A) liver samples and (B) gonadal white adipose tissue samples. Data are presented as mean ± SD. Sample sizes were n = 8 per group. Differences were determined by 2-way ANOVA followed with Holm–Sidak post hoc comparison (*P < .05) between and within treatment groups. Significant ANOVA results are shown under graphs.
Markers for FA metabolism were assessed in GWAT. There was main effect of diet on the mRNA levels of Ppara and Pparg, affecting fat metabolism, deposition, and inflammation; however, differences between intervention groups were not detected (Fig. 8B). The gene expression level of lpl, encoding lipoprotein lipase (LPL), was suppressed in the VCD-treated mice compared with Veh-treated counterparts fed the same diet, suggesting more fat deposition in E2-deficient mice regardless dietary FA type consumed (Fig. 8B). There was no effect of diet or treatment on mRNA level of Lepr (Fig. 8B).
High LA Diet Induced Colonic Inflammation
H&E staining revealed that colon morphology was similar between diet groups (Fig. 9A). Goblet cells were quantified in AB-PAS–stained tissues. VCD-treated mice fed the 1% LA diet had more colonic goblet cells than Veh-treated mice fed the 1% LA diet, while VCD-treated mice fed the 22.5% LA diet had fewer goblet cells than Veh-treated mice fed the 22.5% LA diet (Fig. 9A). Immunohistochemistry of colon tissues were performed to assess markers of inflammation and mucin levels. In Veh- or VCD-treated groups, mice fed the 22.5% LA diet had higher Cox-2 expression than those fed 1% LA diet (Fig. 9B). Expression levels of Cox-2 in the VCD-treated mice fed the 22.5% LA diet was increased compared with the Veh-treated mice fed the 22.5% LA diet (Fig. 9B). These data suggest that the LA is proinflammatory in the intestine. There were no differences in inducible nitric oxide synthase or Muc-2 among all the 4 groups (Fig. 9B).
Figure 9.
Colonic histology and ileal qPCR. (A) H&E staining of colon tissues to visualize morphology and AB-PAS staining to detect mucin-producing goblet cells. (B) Detection of colonic COX-2, iNOS, and MUC-2 by immunohistochemistry. (C) Gene expression of inflammatory, tight junction proteins, and glucose metabolism markers in ileum. For A,B, sample sizes were 6 for each group; for C, n = 8-9 for Veh-treated groups and n = 11 for VCD-treated groups. Data are presented as mean ± SD. Differences were determined by 2-way ANOVA followed with Holm–Sidak post hoc comparison (*P < .05) between and within treatment groups. Significant ANOVA results are shown under graphs.
Relative mRNA levels of genes related to inflammation, gut barrier integrity, and glucose metabolism were assessed in ileum samples. No changes were observed in the gene expression levels of inflammatory markers such as Il6, Tnf, and Nos2 (Fig. 9C). Within each treatment, mice fed the 22.5% LA diet had higher gene expression level of Tjp1, encoding tight junction protein 1 (ZO-1), compared with those fed 1% LA diet, which may be augmented to protect inflammation-induced leaky gut (Fig. 9C). Gcg encodes preproglucagon, which is cleaved by PC1/3 protease (Pcsk1) to yield the incretin peptide Glp1 and Glp2, which regulates gut barrier and intestinal hexose transport (53). Pcsk1 expression was similar among the 4 groups; however, compared with Veh-treated mice on the same diet, VCD-treated mice fed the 1% LA diet had lower Gcg mRNA and VCD-treated 22.5% LA diet had a higher level of Gcg mRNA (Fig. 9C), suggesting the 22.5% LA diet may induce more GLP-1 and GLP-2 production. Upregulated gene expression levels of Slc5a1 and Slc2a2, encoding carbohydrate transporters SGLT1 and GLUT2 respectively, was observed in the Veh-treated mice fed the 22.5% LA diet compared with the Veh-treated mice fed the 1% LA diet, suggesting elevated carbohydrate absorption (Fig. 9C). There was no difference in the mRNA level of Fiaf, encoding fasting-induced adipose factor (FIAF), suggesting that circulating LPL was not affected (Fig. 9C).
Gut Microbiota Profiling Was Altered by FAs and E2 Deficiency
16S rRNA V3-V4 amplicon sequencing was performed to assess the impact of different dietary FAs on fecal microbial communities of estrogen-sufficient and estrogen-deficient mice. Faith's phylogenetic diversity index indicated that all groups had similar ASVs. There were no differences in richness and evenness among 4 groups (Fig. 2A (31)). To assess the dissimilarity of the gut microbial community among samples, 4 metrics of beta diversity analysis were performed. Both Bray–Curtis, based on ASV abundance, and Jaccard, based on ASV presence, indicated that dietary intervention and treatment had effects on separation of gut microbiota along PC1 and PC2. Taking phylogenetic distance between ASVs and their presence/absence into consideration, unweighted unifrac showed 4 groups separated from each other along PC1 and PC2. However, weighted unifrac, accounting for abundance of taxa and phylogenetic distance, showed that gut microbial communities were separated due to treatment and diet × treatment interaction (Fig. 2B (31)).
At phylum level, Veh-treated mice fed the 1% LA diet had lowest relative abundance of Firmicutes compared with the other 3 groups while all groups had similar relative abundance of Bacteroidetes (Fig. 10A). VCD-treated mice fed the 1% LA diet had a higher ratio of Firmicutes to Bacteroidetes (F/B) compared with Veh-treated mice (Fig. 10A). Increased F/B ratio has been widely examined in human and mouse gut microbiota associated to gut dysbiosis and metabolic disorders (54). Veh-treated mice fed the 1% LA diet had the highest relative abundance of Verrucomicrobiota and Akkermansia (Fig. 10A and B). Mice fed the 22.5% LA diet had lower relative abundance of Verrucomicrobiota regardless of E2 status (Fig. 10A). The higher relative abundance of Akkermansia in the Veh-treated mice fed the 1% LA diet (Fig. 10B) was associated with the reduced weight gain observed in this group (Fig. 1C) and consistent with studies correlating Akkermansia abundance to metabolic health (55, 56). Parabacteroides and Rikenella were increased in VCD-treated mice fed the 22.5% LA diet compared with VCD-treated mice fed the 1% LA diet (Fig. 10B). Parabacteroides was reported to catabolize branched-chain amino acids and protect against formation of atherosclerosis lesions in ApoE−/− mice (57). Rikenella was positively correlated with HDL levels and negatively correlated with serum triglyceride, total cholesterol, and low-density lipoprotein levels in HFD-fed rats (58). Eubacterium coprostanoligenes was increased in Veh-treated mice fed the 1% LA diet and showed a trending increase in VCD-treated mice fed the 1% LA diet (Fig. 10B). Eubacterium sp. are butyrate producers and have been associated with gut barrier integrity, reduced metabolic endotoxemia, and lower systemic inflammation which contributes to atherosclerosis (59) suggesting coconut oil SFAs promote abundance of beneficial bacteria. Postmenopausal women reporting more than 2 subjective memory complaints had higher relative abundance of family Desulfovibrionaceae than women reporting 1 or no complaints (60). Compared with VCD-treated mice fed the 1% LA diet, there was a decrease in the relative abundance of Desulfovibrionaceae ASV-2 in VCD-treated mice fed the 22.5% LA diet (Fig. 10B), suggesting LA consumption can reduce bacterial taxa associated with postmenopausal cognitive decline. Compared with VCD-treated mice fed the 1% LA diet, VCD-treated mice fed the 22.5% LA had higher relative abundance of Butyricimonas and reduced relative abundance of Blautia (Fig. 10B). As butyrate-producing bacteria, Butyricimonas has been linked to improvement of postmenopausal osteoporosis and insulin resistance (61, 62). Relative abundance of Blautia is inversely associated with visceral fat area (63, 64), consistent with more weight gain in the VCD-treated mice fed the 22.5% LA diet (Fig. 1C and 1D). Gut microbial β-glucuronidase deconjugate estrogens, allowing reabsorption of biologically active forms of estrogens back to circulation (15, 16). Eubacterium, Blautia, and Parabacteroides, which harbor the gus gene encoding β-glucuronidase (16, 65), were differentially altered by dietary FAs; however, further study is needed to evaluate the specificity of their β-glucuronidase enzymes for glucuronidated estrogens.
Figure 10.
Gut microbial community response to treatment and dietary fatty acids. Relative abundance of amplicon sequence variants (ASVs) identified in fecal gut microbiota samples collected after 23 to 25 weeks of dietary intervention (n = 10 for Veh-treated groups and n = 11 for VCD-treated groups) classified at the (A) phyla and (B) genera level. Different letters (a, b, c) indicate significant difference as determined by the Kruskal–Wallis test followed by 2-stage step-up method of Benjamini, Krieger, and Yekutieli with false discovery rate adjusted P value, q < .05.
Discussion
In this study, we showed that E2-deficient mice fed a HFD enriched in PUFAs, primarily LA, or SFAs in the form of coconut oil can have both beneficial and negative effects on metabolic phenotypes, cognitive behavior, related biomarkers, and gut microbial community profiles. Compared with VCD-treated mice fed the 22.5% LA diet, VCD-treated mice fed the 1% LA diet rich in coconut oil SFAs had less weight gain (Fig. 1C and 1D), improved glucose tolerance (Fig. 2A and 2B), and increased relative abundance of bacterial taxa (E. coprostanoligenes and Blautia) associated with improved energy homeostasis (Fig. 10B), but also negative phenotypes such as elevated blood pressure (Fig. 5A-5C) and a trending increase in heart rate (Fig. 5D). In contrast, VCD-treated mice fed the 22.5% LA diet had increased relative abundance of bacterial taxa (Parabacteroides, Rikenella, Butyricimonas) (Fig. 10B) had improved cardiovascular phenotypes (Fig. 5A-5C) and decreased abundance of Desulfovibrionaceace ASV-2 (Fig. 10B), which is associated with impaired working memory (Fig. 6A, B, and 6G).
Excessive LA intake has been linked to the development of obesity (39, 66). After 8 weeks of dietary intervention on the 1% LA and 22.5% LA diets, there were no differences in final body weight in Veh- or E2-treated ovariectomized mice (39). In this study, VCD-treated mice fed the 22.5% LA showed more weight gain from weeks 10 to 14 of dietary intervention, and this obesogenic trend continued during weeks 23 to 25 of dietary intervention (Fig. 1C and D). Due to the presence of E2, weight gain in Veh-treated groups was delayed and the cumulative weight gain of mice fed the HFD rich in LA exceeded that of mice fed the HFD rich in coconut oil SFAs at weeks 20 to 22 on the diets (Fig. 1C and D). Overall, these data indicate that in E2-sufficient and -deficient states, long-term feeding of a HFD rich in LA is more obesogenic than a diet rich in coconut oil SFAs. Studies in postmenopausal women have indicated that their daily lipid intake was higher than recommended (67, 68), which leads to higher risk of cardiometabolic disease. Interestingly, a low-fat diet was associated with weight gain in postmenopausal women while a reduced carbohydrate diet was inversely associated with weight gain (69). These reports indicate that both amount of fat and diet quality are important determinants of metabolic health outcomes.
Normalization of EE to body weight or lean mass yields different results (70). During daytime, EE normalized to lean mass in Veh-treated mice fed the 22.5% LA diet was higher than Veh-treated mice fed the 1% LA diet; however, EE was similar in both groups when normalized to body weight (Fig. 3E). Body weight includes highly variable fat mass, which may distort the estimate of EE. Resting energy expenditure is the largest component of EE, and lean mass acts as the main determinant of resting energy expenditure. The HFD rich in 22.5% LA appeared to increase resting energy expenditure and the effect was most obvious in E2 sufficient state (Fig. 3F).
Compared with Veh-treated OVX mice fed a normal fat diet for 8 weeks, OVX mice fed the 22.5% LA diet had impaired glucose clearance after intraperitoneal glucose challenge or intraperitoneal insulin challenge (39). The blood glucose AUC of OVX mice fed the 1% LA diet was similar to Veh-treated OVX mice fed a normal fat diet, suggesting that SFAs from coconut oil can benefit glucose metabolism in the context of E2 deficiency (39). However, there were no differences shown in blood glucose clearance in the comparison of mice fed the 1% LA diet and 22.5% LA diet (30). In this study, where dietary intervention lasted 23 to 25 weeks, VCD-treated mice fed the 22.5% LA diet showed a trend of impaired glucose disposal compared with VCD-treated mice fed the 1% LA diet (Fig. 2B). These data suggest that prolonged consumption of a high LA diet can augment glucose intolerance. Upregulated mRNA level of insulin receptor in hypothalamus in VCD-treated mice fed the 22.5% LA diet compared with the counterparts fed 1% LA diet (Fig. 7B) suggests development of insulin resistance; however, measuring plasma insulin after feeding and fasting are needed to fully evaluate insulin resistance.
It is common that obese mice have higher circulating leptin (71, 72). Leptin acts on the arcuate nucleus by stimulating anorexigenic POMC-containing neurons and suppressing orexigenic AgRP/NPY-containing neurons to regulate appetite and energy homeostasis (73). Previously, Veh-treated OVX mice fed the 22.5% LA diet had higher leptin than their counterparts fed the 1% LA diet (39). Reduced mRNA levels of hypothalamic leptin receptor due to the 22.5% LA diet in both Veh- and VCD-treated mice suggest leptin resistance (Fig. 7E), resulting in increased food intake (Fig. 1G and 1J). The unexpected increase in POMC/CART tone in Veh-treated mice fed the 22.5% LA diet may result from the effect of estrogen on energy homeostasis. It has been shown that estrogen can regulate appetite and weight gain via increasing excitatory inputs on POMC neurons, which is effective in leptin-deficient and leptin receptor–deficient mice (74). The interaction between estrogen and leptin signaling should be addressed in future work.
Decline in circulating estrogen is directly related to changes in memory performance. In our study, VCD-induced E2 deficiency had negative effects on spatial memory and recognition memory as assessed by Y-maze and SOR tests (Fig. 6A-6H), which are dependent on hippocampal integrity. Interestingly, we did not observe any changes in the NOR test (Fig. 6I-6L). Most studies have indicated that novel object preference is spared in rodents with hippocampal lesions (75). Evidence of less intention to explore the unknown arm in Y-maze or displaced object in SOR was found in the VCD-treated mice fed the 1% LA diet rich in coconut oil (Fig. 6A-6H). Excessive LA intake increases brain tissue inflammation, while virgin coconut oil (1.42 mL/kg/day) was shown to reduce neuronal inflammation and oxidative damage while improving working memory in aged cycling and noncycling rats (76). It is possible that our long-term feeding of HFD rich in coconut oil masked the protective effects of the medium chain SFAs in coconut oil.
Hypertension is a major risk factor for CVD and the prevalence is higher in women after menopause; however, whether the absence of E2 and the change of E2/androgen ratio promotes hypertensin is controversial (77). In our VCD model, blood pressure was not affected by E2 status (Fig. 5). Epidemiological studies showed that replacement of SFA with LA reduces low-density lipoprotein and higher plasma LA levels was associated with lower total cholesterol (78-80). A diet rich in extra virgin coconut oil increased high-density lipoprotein in aged coronary artery disease patients (81). Elevated blood pressure in VCD-treated mice fed the 1% LA diet rich in coconut oil (Fig. 5) suggests that long-term excessive consumption of SFAs from coconut oil may raise low-density lipoprotein. The optimal ratio of coconut oil and LA for cardiovascular and cardiometabolic health needs more evidence from preclinical and clinical studies.
Estrogen deficiency accelerates aging-related bone loss in women. In mice aged 8.5 months, spinal and femoral microarchitecture showed more severe deterioration in OVX mice than VCD-treated mice, suggesting that skeletal changes in the VCD model occur slowly with lower magnitude, which better mimics natural postmenopausal osteoporosis (30). VCD-induced ovarian failure led to a pronounced reduction in BMD of femur, tibia, and spine that was unaltered by FAs (Table 1). In contrast, the HFD rich in coconut oil diet had no effect on BMD although coconut oil has been reported to prevent osteoporosis and lipid peroxidation in OVX rats (82, 83).
Our findings raise questions about the interaction of the gut microbiota, dietary FAs, and sex steroid hormones. In women aged 25-65, serum E2 was negatively related to Slackia and Butyricimonas (84). Another comparison between premenopausal and postmenopausal women found that serum E2 was positively correlated with Gammaproteobacteria and Myxococcales and negatively correlated with Prevotellaceae (85). In our study, the VCD-treated mice fed the 1% LA diet had lowest relative abundance of Butyricimonus; however, we did not observe alteration of the other genera (Fig. 10B). Genera that can produce β-glucuronidase were modulated by FAs (Fig. 10B); however, we do not know if their β-glucuronidase product has specificity for estrogens. This study indicates links between the gut microbiota, blood pressure, and spatial memory, but further research is needed to understand how dietary modulation of the microbiota reduce risks of CVD and dementia in postmenopausal women.
The VCD model has been used to study the effects of ovarian estrogen decline on bone loss (30, 86, 87), hypertension (88), and cognitive decline (89). Compared with Veh-treated mice, VCD-treated mice developed glucose intolerance 26 weeks after VCD treatment; however, VCD-treated mice fed a HFD showed glucose intolerance by 16 weeks after VCD treatment (90-92.) Future studies comparing normal fat diet vs HFD in VCD-treated mice would be useful to model ovary-intact postmenopause under different dietary patterns. Metabolic syndrome–related disorders such as insulin resistance, glucose intolerance, hypertension, and overweight/obesity often already exist in premenopausal women and may be exacerbated by E2 decline in menopause. A model of pre-existing diet-induced metabolic syndrome followed by VCD-induced E2 deficiency would be difficult to implement as cycling female mice fed a HFD are resilient to symptoms of metabolic syndrome (93). Early studies indicated that VCD can specifically target the ovaries to induce premature ovarian failure (29, 92) while more recent reports indicated nonspecific toxicity to liver and kidney in rats (94), although differences in toxicity may be due to species-specific effects. We did not observe obvious morphological abnormalities at necropsy, but it is possible that VCD treatments may have induced unmeasured/undetected toxicity to tissues other than the ovaries.
In a previous study, OVX mice were fed 1% LA diet, 22.5% LA diet, or normal fat diet (39). While a limitation of this study is the absence of Veh- and VCD-treated mice fed a normal fat diet, taken together, our results indicate that in the VCD menopause mouse model, coconut oil SFAs alleviate weight gain and improve glucose tolerance, whereas LA can benefit blood pressure and cognitive function. Furthermore, correlations between gut bacteria and dietary FAs suggests novel FA-induced mechanisms for improving health outcomes in postmenopausal state.
Acknowledgment
The authors would like to thank Dr. Stanley Lightfoot for blind scoring of histomorphometry of colon tissues sections and Captain Ronald P. Tango for his technical expertise and for assistance in crafting last minute fixes for broken behavior apparatus pieces.
Abbreviations
- aBMD
areal bone mineral density
- ACC
acetyl-CoA carboxylase
- ACLY
ATP citrate lyase
- AgRP
agouti-related peptide
- ANOVA
analysis of variance
- ASV
amplicon sequence variant
- AUC
area under the curve
- BMC
bone mineral content
- CART
cocaine-and-amphetamine-regulated transcript
- Cox-2
cyclooxygenase-2
- CVD
cardiovascular disease
- DGAT2
diglyceride acyltransferase
- E2
17β-estradiol
- EE
energy expenditure
- FA
fatty acid
- FOXO1
Forkhead box O1
- G6Pase
glucose 6-phosphatase
- GHSR
Growth hormone secretagogue receptor
- GLP
Glucagon-like peptide
- GWAT
gonadal white adipose tissue
- H&E
hematoxylin and eosin
- HFD
high-fat diet
- HRT
hormone replacement therapy
- INSR
insulin receptor
- ITT
Insulin tolerance test
- LA
linoleic acid
- LEPR
leptin receptor
- LPL
lipoprotein lipase
- Muc-2
mucin-2
- NOR
novel object recognition
- NPY
neuropeptide Y
- OGTT
oral glucose tolerance test
- OVX
ovariectomized
- PEPCK
phosphoenolpyruvate carboxykinase
- POMC
proopiomelanocortin
- PPARγ
peroxisome proliferator-activated receptor gamma
- PUFA
polyunsaturated fatty acid
- RER
respiratory exchange ratio
- RT-qPCR
reverse transcription quantitative polymerase chain reaction
- SFA
saturated fatty acid
- SOR
spatial object recognition
- VCD
4-vinylcyclohexene diepoxide
- Veh
vehicle
Contributor Information
Ke Sui, Department of Food Science, NJ Institute for Food Nutrition and Health (Rutgers Center for Lipid Research and Center for Nutrition Microbiome and Health), Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA.
Ali Yasrebi, Department of Animal Sciences, Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA; NJ Institute for Food Nutrition and Health (Rutgers Center for Lipid Research, Center for Human Nutrition, Exercise and Metabolism Center, and Center for Nutrition Microbiome and Health), Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA.
Candace R Longoria, Department of Kinesiology and Applied Physiology, Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA; NJ Institute for Food Nutrition and Health (Rutgers Center for Lipid Research, Center for Human Nutrition, Exercise and Metabolism Center, and Center for Nutrition Microbiome and Health), Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA.
Avery T MacDonell, Department of Food Science, NJ Institute for Food Nutrition and Health (Rutgers Center for Lipid Research and Center for Nutrition Microbiome and Health), Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA.
Zehra H Jaffri, Department of Food Science, NJ Institute for Food Nutrition and Health (Rutgers Center for Lipid Research and Center for Nutrition Microbiome and Health), Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA.
Savannah A Martinez, Department of Food Science, NJ Institute for Food Nutrition and Health (Rutgers Center for Lipid Research and Center for Nutrition Microbiome and Health), Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA.
Samuel E Fisher, Department of Animal Sciences, Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA.
Natasha Malonza, Department of Kinesiology and Applied Physiology, Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA; NJ Institute for Food Nutrition and Health (Rutgers Center for Lipid Research, Center for Human Nutrition, Exercise and Metabolism Center, and Center for Nutrition Microbiome and Health), Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA.
Katie Jung, Department of Nutritional Sciences, Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA.
Kevin M Tveter, Department of Food Science, NJ Institute for Food Nutrition and Health (Rutgers Center for Lipid Research and Center for Nutrition Microbiome and Health), Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA.
Kimberly R Wiersielis, Department of Animal Sciences, Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA; NJ Institute for Food Nutrition and Health (Rutgers Center for Lipid Research, Center for Human Nutrition, Exercise and Metabolism Center, and Center for Nutrition Microbiome and Health), Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA.
Mehmet Uzumcu, Department of Animal Sciences, Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA.
Sue A Shapses, Department of Nutritional Sciences, Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA; NJ Institute for Food Nutrition and Health (Rutgers Center for Lipid Research, Center for Human Nutrition, Exercise and Metabolism Center, and Center for Nutrition Microbiome and Health), Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA.
Sara C Campbell, Department of Kinesiology and Applied Physiology, Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA; NJ Institute for Food Nutrition and Health (Rutgers Center for Lipid Research, Center for Human Nutrition, Exercise and Metabolism Center, and Center for Nutrition Microbiome and Health), Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA.
Troy A Roepke, Department of Animal Sciences, Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA; NJ Institute for Food Nutrition and Health (Rutgers Center for Lipid Research, Center for Human Nutrition, Exercise and Metabolism Center, and Center for Nutrition Microbiome and Health), Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA.
Diana E Roopchand, Department of Food Science, NJ Institute for Food Nutrition and Health (Rutgers Center for Lipid Research and Center for Nutrition Microbiome and Health), Rutgers, The State University of New Jersey, New Brunswick, NJ 08901, USA.
Funding
This work was supported by a seed grant from the NJ Institute for Food Nutrition and Health, Rutgers University.
Author Contributions
D.E.R., T.A.R., and S.C.C. conceived the study, designed the experiments and provided oversight to the project. K.S. and A.Y. performed animal experiments. A.T.M. and K.S. analyzed follicle counting under the guidance from M.U. A.T.M., Z.H.J., S.A.M., and S.E.F. performed qPCR. C.R.L. and N.M. performed intestinal histology. K.J. performed bone dissections and DEXA scans. S.A.S. provided guidance on bone-related endpoints. K.S. performed behavioral tests under guidance from K.R.W. K.S. and K.M.T. performed gut microbial analysis. K.S., A.Y., C.L., N.M., and K.M.T. performed statistical analysis, and prepared final figures. K.S. drafted manuscript. D.E.R. edited and wrote the final manuscript. All authors read and approved final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data Availability
16S amplicon sequences for fecal microbiota analyses were submitted to Sequence Read Archive (SRA) and can be retrieved with accession number PRJNA887004.
Ethics Statement
The animal study was reviewed and approved by Rutgers institutional animal care and use committee.
References
- 1. Davis SR, Baber RJ. Treating menopause—MHT and beyond. Nat Rev Endocrinol. 2022;18(8):490-502. [DOI] [PubMed] [Google Scholar]
- 2. Lobo RA, Gompel A. Management of menopause: a view towards prevention. Lancet Diabetes Endocrinol. 2022;10(6):457-470. [DOI] [PubMed] [Google Scholar]
- 3. Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34(3):309–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Premaor MO, Pilbrow L, Tonkin C, Parker RA, Compston J. Obesity and fractures in postmenopausal women. J Bone Miner Res. 2010;25(2):292–297. [DOI] [PubMed] [Google Scholar]
- 5. Brahe L, Le Chatelier E, Prifti E, et al. Specific gut microbiota features and metabolic markers in postmenopausal women with obesity. Nutr Diabetes. 2015;5(6):e159–e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flores VA, Pal L, Manson JE. Hormone therapy in menopause: concepts, controversies, and approach to treatment. Endocr Rev. 2021;42(6):720–752. [DOI] [PubMed] [Google Scholar]
- 7. Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US preventive services task force recommendation statement. JAMA. 2017;318(22):2224–2233. [DOI] [PubMed] [Google Scholar]
- 8. Donato GB, Fuchs SC, Oppermann K, Bastos C, Spritzer PM. Association between menopause status and central adiposity measured at different cutoffs of waist circumference and waist-to-hip ratio. Menopause. 2006;13(2):280–285. [DOI] [PubMed] [Google Scholar]
- 9. Piché M-È, Weisnagel SJ, Corneau L, Nadeau A, Bergeron J, Lemieux S. Contribution of abdominal visceral obesity and insulin resistance to the cardiovascular risk profile of postmenopausal women. Diabetes. 2005;54(3):770–777. [DOI] [PubMed] [Google Scholar]
- 10. Gholizadeh S, Sadatmahalleh SJ, Ziaei S. The association between estradiol levels and cognitive function in postmenopausal women. Int J Reprod Biomed. 2018;16(7):455. [PMC free article] [PubMed] [Google Scholar]
- 11. Elsabagh S, Hartley DE, File SE. Cognitive function in late versus early postmenopausal stage. Maturitas. 2007;56(1):84–93. [DOI] [PubMed] [Google Scholar]
- 12. Li J-Y, Chassaing B, Tyagi AM, et al. Sex steroid deficiency–associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016;126(6):2049–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Britton RA, Irwin R, Quach D, et al. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol. 2014;229(11):1822–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu J, Cao G, Yuan S, Luo C, Yu J, Cai M. Probiotic supplements and bone health in postmenopausal women: a meta-analysis of randomised controlled trials. BMJ Open. 2021;11(3):e041393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sui Y, Wu J, Chen J. The role of gut microbial β-glucuronidase in estrogen reactivation and breast cancer. Front Cell Dev Biol. 2021;9:631552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ervin SM, Li H, Lim L, et al. Gut microbial β-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J Biol Chem. 2019;294(49):18586–18599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakamura MT, Yudell BE, Loor JJ. Regulation of energy metabolism by long-chain fatty acids. Prog Lipid Res. 2014;53:124–144. [DOI] [PubMed] [Google Scholar]
- 18. Wallace TC. Health effects of coconut oil—A narrative review of current evidence. J Am Coll Nutr. 2019;38(2):97–107. [DOI] [PubMed] [Google Scholar]
- 19. Martins AR, Crisma AR, Masi LN, et al. Attenuation of obesity and insulin resistance by fish oil supplementation is associated with improved skeletal muscle mitochondrial function in mice fed a high-fat diet. J Nutr Biochem. 2018;55:76–88. [DOI] [PubMed] [Google Scholar]
- 20. Buckley JD, Howe PR. Long-chain omega-3 polyunsaturated fatty acids may be beneficial for reducing obesity—a review. Nutrients. 2010;2(12):1212–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93(5):950–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wright J, Kennedy-Stephenson J, Wang C, McDowell M, Johnson C. Trends in intake of energy and macronutrients-United States, 1971-2000. JAMA. 2004;291(10):1193–1193. [Google Scholar]
- 23. Naughton SS, Mathai ML, Hryciw DH, McAinch AJ. Linoleic acid and the pathogenesis of obesity. Prostaglandins Other Lipid Mediat. 2016;125:90–99. [DOI] [PubMed] [Google Scholar]
- 24. Taha AY, Blanchard HC, Cheon Y, et al. Dietary linoleic acid lowering reduces lipopolysaccharide-induced increase in brain arachidonic acid metabolism. Mol Neurobiol. 2017;54(6):4303–4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lima RdS, Block JM. Coconut oil: what do we really know about it so far? Food Qual Saf. 2020;68(7):67-72. [Google Scholar]
- 26. DeLany JP, Windhauser MM, Champagne CM, Bray GA. Differential oxidation of individual dietary fatty acids in humans. Am J Clin Nutr. 2000;72(4):905–911. [DOI] [PubMed] [Google Scholar]
- 27. Eyres L, Eyres MF, Chisholm A, Brown RC. Coconut oil consumption and cardiovascular risk factors in humans. Nutr Rev. 2016;74(4):267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rahim NS, Lim SM, Mani V, Abdul Majeed AB, Ramasamy K. Enhanced memory in Wistar rats by virgin coconut oil is associated with increased antioxidative, cholinergic activities and reduced oxidative stress. Pharm Biol. 2017;55(1):825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mayer LP, Devine PJ, Dyer CA, Hoyer PB. The follicle-deplete mouse ovary produces androgen. Biol Reprod. 2004;71(1):130–138. [DOI] [PubMed] [Google Scholar]
- 30. Wright LE, Christian PJ, Rivera Z, et al. Comparison of skeletal effects of ovariectomy versus chemically induced ovarian failure in mice. J Bone Miner Res. 2008;23(8):1296–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sui K, Yasrebi A, Longoria CR, et al. Supplementary materials for: Coconut oil saturated fatty acids improved energy homeostasis but not blood pressure or cognition in VCD-treated female mice. 2022. doi: 10.5281/zenodo.7473294. Zenodo. Date of deposit 17 December 2022. [DOI]
- 32. Wang JH, Ma YY, van den Buuse M. Improved spatial recognition memory in mice lacking adenosine A2A receptors. Exp Neurol. 2006;199(2):438–445. [DOI] [PubMed] [Google Scholar]
- 33. Wiersielis KR, Ceretti A, Hall A, et al. Sex differences in corticotropin releasing factor regulation of medial septum-mediated memory formation. Neurobiol Stress. 2019;10:100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bello NT, Walters AL, Verpeut JL, Cunha PP. High-fat diet-induced alterations in the feeding suppression of low-dose nisoxetine, a selective norepinephrine reuptake inhibitor. J Obes. 2013;2013:457047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mezhibovsky E, Knowles KA, He Q, et al. Grape polyphenols attenuate diet-induced obesity and hepatic steatosis in mice in association with reduced butyrate and increased markers of intestinal carbohydrate oxidation. Front Nutr. 2021;8:675267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grobe JL. Comprehensive assessments of energy balance in mice. The Renin-Angiotensin-Aldosterone System. Springer; 2017;1614:123–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vail GM, Walley SN, Yasrebi A, Maeng A, Conde KM, Roepke TA. The interactions of diet-induced obesity and organophosphate flame retardant exposure on energy homeostasis in adult male and female mice. J Toxicol Environ Health A. 2020;83(11-12):438–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yasrebi A, Rivera JA, Krumm EA, Yang JA, Roepke TA. Activation of estrogen response element–independent ER α signaling protects female mice from diet-induced obesity. Endocrinology. 2017;158(2):319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mamounis KJ, Hernandez MR, Margolies N, Yasrebi A, Roepke TA. Interaction of 17β-estradiol and dietary fatty acids on energy and glucose homeostasis in female mice. Nutr Neurosci. 2018;21(10):715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. Reproduction. 1968;17(3):555–557. [DOI] [PubMed] [Google Scholar]
- 41. Myers M, Britt KL, Wreford NGM, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004;127(5):569–580. [DOI] [PubMed] [Google Scholar]
- 42. Wang Y, Dellatore P, Douard V, et al. High fat diet enriched with saturated, but not monounsaturated fatty acids adversely affects femur, and both diets increase calcium absorption in older female mice. Nutr Res. 2016;36(7):742–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thiagarajah JR, Yildiz H, Carlson T, et al. Altered goblet cell differentiation and surface mucus properties in Hirschsprung disease. PLoS One. 2014;9(6):e99944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wisniewski P, Wahler G, Gardner C, Lightfoot S, Joseph L, Campbell S. Voluntary wheel running reduces colon inflammation in female but not male mice fed a high-fat diet. Comp Exerc Physiol. 2019;15(1):35–47. [Google Scholar]
- 45. Bolyen E, Rideout JR, Dillon MR, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bokulich NA, Kaehler BD, Rideout JR, et al. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2's q2-feature-classifier plugin. Microbiome. 2018;6(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang L, Luo L, Zhao W, et al. Lauric acid accelerates glycolytic muscle fiber formation through TLR4 signaling. J Agric Food Chem. 2018;66(25):6308–6316. [DOI] [PubMed] [Google Scholar]
- 49. Hill JW. Gene expression and the control of food intake by hypothalamic POMC/CART neurons. Open Neuroendocrinology Journal. 2010;3:21–27. [PMC free article] [PubMed] [Google Scholar]
- 50. Garretson JT, Teubner BJ, Grove KL, Vazdarjanova A, Ryu V, Bartness TJ. Peroxisome proliferator-activated receptor γ controls ingestive behavior, agouti-related protein, and neuropeptide Y mRNA in the arcuate hypothalamus. J Neurosci. 2015;35(11):4571–4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ge W, Zhao Y, Yang Y, et al. An insulin-independent mechanism for transcriptional regulation of Foxo1 in type 2 diabetic mice. J Biol Chem. 2021;297(1):100846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhao G, Cardenas H, Matei D. Ovarian cancer—why lipids matter. Cancers (Basel). 2019;11(12):1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schiellerup SP, Skov-Jeppesen K, Windeløv JA, et al. Gut hormones and their effect on bone metabolism. Potential drug therapies in future osteoporosis treatment. Front Endocrinol. 2019;10:75, 1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Castaner O, Goday A, Park Y-M, et al. The gut microbiome profile in obesity: a systematic review. Int J Endocrinol. 2018;2018:4095789. doi: 10.1155/2018/4095789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Roopchand DE, Carmody RN, Kuhn P, et al. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet–induced metabolic syndrome. Diabetes. 2015;64(8):2847–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cani PD, de Vos WM. Next-generation beneficial microbes: the case of Akkermansia muciniphila. Front Microbiol. 2017;8:1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Qiao S, Liu C, Sun L, et al. Gut Parabacteroides merdae protects against cardiovascular damage by increasing commensal bacteria-driven branched-chain amino acid catabolism. Nat Metab 2021;4(10):1271–1286. [DOI] [PubMed] [Google Scholar]
- 58. Wan X, Li T, Liu D, et al. Effect of marine microalga Chlorella pyrenoidosa ethanol extract on lipid metabolism and gut microbiota composition in high-fat diet-fed rats. Mar Drugs. 2018;16(12):498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mukherjee A, Lordan C, Ross RP, Cotter PD. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes. 2020;12(1):1802866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wu F, Davey S, Clendenen TV, et al. Gut Microbiota and subjective memory complaints in older women. J Alzheimers Dis 2022;88(1):251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang S, Wang S, Wang X, et al. Effects of icariin on modulating gut Microbiota and regulating metabolite alterations to prevent bone loss in ovariectomized rat model. Front Endocrinol (Lausanne). 2022;13:874849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Moreno-Indias I, Sánchez-Alcoholado L, García-Fuentes E, Cardona F, Queipo-Ortuño MI, Tinahones FJ. Insulin resistance is associated with specific gut microbiota in appendix samples from morbidly obese patients. Am J Transl Res. 2016;8(12):5672. [PMC free article] [PubMed] [Google Scholar]
- 63. Ozato N, Saito S, Yamaguchi T, et al. Blautia genus associated with visceral fat accumulation in adults 20–76 years of age. NPJ Biofilms Microbiomes. 2019; 5(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liu X, Mao B, Gu J, et al. Blautia—a new functional genus with potential probiotic properties? Gut microbes. 2021;13(1):1875796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Creekmore BC, Gray JH, Walton WG, et al. Mouse gut microbiome-encoded β-glucuronidases identified using metagenome analysis guided by protein structure. MSystems. 2019;4(4):e00452-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Alvheim AR, Malde MK, Osei-Hyiaman D, et al. Dietary linoleic acid elevates endogenous 2-AG and anandamide and induces obesity. Obesity. 2012;20(10):1984–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tardivo AP, Nahas-Neto J, Nahas EA, Maesta N, Rodrigues MA, Orsatti FL. Associations between healthy eating patterns and indicators of metabolic risk in postmenopausal women. Nutr J. 2010;9(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dde A V, Vde M F, Ramos EG, et al. Association between quality of the diet and cardiometabolic risk factors in postmenopausal women. Nutr J. 2014;13(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ford C, Chang S, Vitolins MZ, et al. Evaluation of diet pattern and weight gain in postmenopausal women enrolled in the Women's Health Initiative Observational Study. Br J Nutr. 2017;117(8):1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Butler AA, Kozak LP. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes. 2010;59(2):323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Banks WA, DiPalma CR, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity. Peptides. 1999;20(11):1341–1345. [DOI] [PubMed] [Google Scholar]
- 72. Zhang Y, Scarpace PJ. The role of leptin in leptin resistance and obesity. Physiol Behav. 2006;88(3):249–256. [DOI] [PubMed] [Google Scholar]
- 73. Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8(5):571–578. [DOI] [PubMed] [Google Scholar]
- 74. Gao Q, Mezei G, Nie Y, et al. Anorectic estrogen mimics leptin's Effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007;13(1):89–94. [DOI] [PubMed] [Google Scholar]
- 75. Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci USA. 2004;101(40):14515–14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Young L, Smith B, Webber-Waugh A, Thaxter K. Virgin coconut oil improved discriminative learning and working memory in aging cycling and non-cycling female Sprague-Dawley rats supporting its beneficial effect in retarding age-related cognitive decline. Adv Aging Res. 2021;10(5):97–112. [Google Scholar]
- 77. Lima R, Wofford M, Reckelhoff JF. Hypertension in postmenopausal women. Curr Hypertens Rep. 2012;14(3):254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010;7(3):e1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mensink RP. Effects of Saturated Fatty Acids on Serum Lipids and Lipoproteins: A Systematic Review and Regression Analysis. World Health Organization; 2016.
- 80. Marangoni F, Agostoni C, Borghi C, et al. Dietary linoleic acid and human health: focus on cardiovascular and cardiometabolic effects. Atherosclerosis. 2020;292:90–98. [DOI] [PubMed] [Google Scholar]
- 81. Cardoso DA, Moreira AS, de Oliveira GM, Luiz RR, Rosa G. A coconut extra virgin oil-rich diet increases HDL cholesterol and decreases waist circumference and body mass in coronary artery disease patients. Nutr Hosp. 2015;32(5):2144–2152. [DOI] [PubMed] [Google Scholar]
- 82. Abujazia MA, Muhammad N, Shuid AN, Soelaiman IN. The effects of virgin coconut oil on bone oxidative status in ovariectomised rat. Evid Based Complement Alternat Med. 2012;2012:525079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hayatullina Z, Muhammad N, Mohamed N, Soelaiman I-N. Virgin coconut oil supplementation prevents bone loss in osteoporosis rat model. Evid Based Complement Alternat Med. 2012;2012:237236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shin J-H, Park Y-H, Sim M, Kim S-A, Joung H, Shin D-M. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res Microbiol. 2019;170(4-5):192–201. [DOI] [PubMed] [Google Scholar]
- 85. Santos-Marcos JA, Rangel-Zuñiga OA, Jimenez-Lucena R, et al. Influence of gender and menopausal status on gut microbiota. Maturitas. 2018;116:43–53. [DOI] [PubMed] [Google Scholar]
- 86. Cao LB, Leung CK, Law PW, et al. Systemic changes in a mouse model of VCD-induced premature ovarian failure. Life Sci. 2020;262:118543. doi: 10.1016/j.lfs.2020.118543. [DOI] [PubMed] [Google Scholar]
- 87. Kalam A, Talegaonkar S, Vohora D. Effects of raloxifene against letrozole-induced bone loss in chemically-induced model of menopause in mice. Mol Cell Endocrinol. 2017;440:34–43. [DOI] [PubMed] [Google Scholar]
- 88. Pollow DP J, Romero-Aleshire MJ, Sanchez JN, Konhilas JP, Brooks HL. ANG II-induced hypertension in the VCD mouse model of menopause is prevented by estrogen replacement during perimenopause. Am J Physiol Regul Integr Comp Physiol. 2015;309(12):R1546–R1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Han G, Choi J, Cha SY, et al. Effects of radix polygalae on cognitive decline and depression in estradiol depletion mouse model of menopause. Curr Issues Mol Biol. 2021;43(3):1669–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Romero-Aleshire MJ, Diamond-Stanic MK, Hasty AH, Hoyer PB, Brooks HL. Loss of ovarian function in the VCD mouse-model of menopause leads to insulin resistance and a rapid progression into the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R587–R592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Konhilas JP, Sanchez JN, Regan JA, et al. Using 4-vinylcyclohexene diepoxide as a model of menopause for cardiovascular disease. Am J Physiol Heart Circ Physiol. 2020;318(6):H1461–H1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Brooks HL, Pollow DP, Hoyer PB. The VCD mouse model of menopause and perimenopause for the study of sex differences in cardiovascular disease and the metabolic syndrome. Physiology (Bethesda). 2016;31(4):250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pettersson US, Waldén TB, Carlsson PO, Jansson L, Phillipson M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS One. 2012;7(9):e46057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ahmadian S, Sheshpari S, Mahdipour M, et al. Toxic effects of VCD on kidneys and liver tissues: a histopathological and biochemical study. BMC Res Notes. 2019;12(1):446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
16S amplicon sequences for fecal microbiota analyses were submitted to Sequence Read Archive (SRA) and can be retrieved with accession number PRJNA887004.