Abstract
Background.
Despite national immunization efforts, including universal childhood hepatitis A (HepA) vaccination recommendations in 2006, hepatitis A virus (HAV)–associated outbreaks have increased in the United States. Unvaccinated or previously uninfected persons are susceptible to HAV infection, yet the susceptibility in the US population is not well known.
Methods.
Using National Health and Nutrition Examination Survey 2007–2016 data, we estimated HAV susceptibility prevalence (total HAV antibody negative) among persons aged ≥2 years. Among US-born adults aged ≥20 years, we examined prevalence, predictors, and age-adjusted trends of HAV susceptibility by sociodemographic characteristics. We assessed HAV susceptibility and self-reported nonvaccination to HepA among risk groups and the “immunization cohort” (those born in or after 2004).
Results.
Among US-born adults aged ≥20 years, HAV susceptibility prevalence was 74.1% (95% confidence interval, 72.9–75.3%) during 2007–2016. Predictors of HAV susceptibility were age group 30–49 years, non-Hispanic white/black, 130% above the poverty level, and no health insurance. Prevalences of HAV susceptibility and nonvaccination to HepA, respectively, were 72.9% and 73.1% among persons who reported injection drug use, 67.5% and 65.2% among men who had sex with men, 55.2% and 75.1% among persons with hepatitis B or hepatitis C, and 22.6% and 25.9% among the immunization cohort. Susceptibility and nonvaccination decreased over time among the immunization cohort but remained stable among risk groups.
Conclusions.
During 2007–2016, approximately three-fourths of US-born adults remained HAV susceptible. Enhanced vaccination efforts are critically needed, particularly targeting adults at highest risk for HAV infection, to mitigate the current outbreaks.
Keywords: hepatitis A virus infection, susceptibility, nonvaccination, risk group
Hepatitis A (HepA) is a vaccine-preventable, acute infectious liver disease caused by hepatitis A virus (HAV) [1]. In the United States, HepA vaccination recommendations were expanded in phases by the Advisory Community on Immunization Practices (ACIP). In 1996, the vaccine was first recommended for children living in areas with the highest disease prevalence and for certain adult risk groups [2]. In 1999, the recommendation was expanded to all children in 17 states that had mean incidence rates consistently higher than the 1987–1997 national average [3]. In 2006, universal vaccination was recommended nationally for children aged 12–23 months [4].
Using National Health and Nutrition Examination Survey (NHANES) data, studies found that overall HAV susceptibility in the United States was 65.1% during 1999–2006, while susceptibility among US-born children aged 6–19 years decreased significantly from 92.0% in 1988–1994 to 79.8% in 1999–2006 [5]. In 2016, national 2-dose HepA vaccination coverage among adolescents aged 13–17 years reached 64.4%, indicating increased HepA vaccination among adolescents, likely due to implementation of the 1996–1999 recommendations and national permissive catch-up vaccination [1, 2, 4, 6]. However, HAV susceptibility has increased among US-born adults aged 20 years or older from 70.5% during 1999–2006 to 75.8% during 2007–2012, with those aged 30–49 years having the highest susceptibility [7]. In 2016, self-reported 2-dose HepA vaccination coverage among adults aged 19 years and older was 9.5% [8]. From 1999 to 2017, HAV-related hospitalization rates among reported surveillance cases significantly increased from 7.3% in 1999 to 67.2% in 2017, underscoring the importance of HAV control and prevention [9–11].
Historically, HAV incidence in the United States has been on a steady downward trend since 1995 [12]. However, HAV infections substantially increased since 2016 due to a quickly growing number of states experiencing person-to-person outbreaks, primarily among drug users and homeless populations [13–15]. During 2016–2018, the Centers for Disease Control and Prevention (CDC) received approximately 15 000 reports of HAV infection, representing a 294% increase compared with 2013–2015 [14]. As of 22 November 2019, a total of 28 466 outbreak-associated HAV infections had been reported from 30 states, with 17 217 (60%) hospitalizations and 288 deaths [15]. As a result of these outbreaks, in 2019, ACIP added persons experiencing homelessness as a risk group recommended for routine HepA vaccination [16].
Persons who report drug use, men who have sex with men (MSM), and persons with hepatitis B or hepatitis C are recommended for vaccination by ACIP due to a higher risk of HAV infection or adverse outcomes if coinfected [2]. Studies indicate that approximately 63% of young persons in San Diego who inject drugs [17] and 40–50% of patients with chronic hepatitis B and hepatitis C among a regional cohort [18, 19] were HAV susceptible. However, national HAV susceptibility among these groups remains ill defined. To help characterize HAV susceptibility trends, it is also essential to understand how HepA vaccination was implemented, particularly among risk groups and the immunization cohort based on the 2006 universal childhood vaccination recommendation. This study aimed to describe prevalence, trends, and predictors of HAV susceptibility in the United States using the most recent decade of data available from NHANES (2007–2016). Hepatitis A virus susceptibility and self-reported nonvaccination among risk groups and the immunization cohort (those aged ≥2 years born in or after 2004) were also assessed.
METHODS
Survey Sample and Design
The NHANES, conducted by the National Center for Health Statistics (NCHS) at the CDC, collects data on the health and nutritional status of the noninstitutionalized civilian US population. NHANES uses a complex, stratified, multistage probability sampling design and collects information from approximately 5000 persons per year through household interviews, physical examination, and testing of biological samples. NHANES is an ongoing survey and releases data in 2-year increments. The average response rate for NHANES interviews during 2007–2016 was 72.5% (78.9% for 2007–2010, 71.8% for 2011–2014, and 61.3% for 2015–2016). More information on the background, survey design, and operation are available on the NHANES website (https://wwwn.cdc.gov/nchs/nhanes). This study used de-identified publicly available data, which do not require institutional review board approval.
Serologic Testing
Serum specimens from persons aged 2 years or older were collected for total HAV antibody (anti-HAV) testing. The laboratory method for detecting total anti-HAV in NHANES 2007–2016 followed the same procedure using a competitive immunoassay technique (Anti-HAV Total; VITROS Immunodiagnostic System [Ortho-Clinical Diagnostics, Inc., Rochester, NY]). Tests considered initially reactive were repeated for confirmation [20]. For this study, we classified indeterminate results (64 of total 39 719 anti-HAV results) as negative. Anti-HAV positivity indicates immunity against HAV acquired either from past infection or vaccination but does not differentiate between the 2. Persons with missing anti-HAV results were excluded for the analysis.
Definitions and Measures
Anti-HAV negativity was defined as HAV susceptible. Since a single dose of HepA vaccine can offer immunity for more than 10 years [21], we were interested in those who never received vaccination. A person who self-reported “no dose” to the question, “Have you ever received hepatitis A vaccine” was defined as nonvaccinated. Persons who refused to answer or answered “don’t know” were excluded from the nonvaccination analysis. Sociodemographic characteristics included sex, age group, race/ethnicity, US-birthplace status, marital status, poverty index, and health insurance coverage. Age was grouped into 2–11, 12–19, 20–29, 30–39, 40–49, 50–59, 60–69, and 70 years or older. Race/ethnicity was categorized as Hispanic (Mexican American or other Hispanic), non-Hispanic white, and non-Hispanic black. Persons who were not classified into one of these groups, including the Asian category that was added in 2011, were classified as “other.” US-birthplace status was categorized as US born and non–US born. Marital status was grouped as never married, married/living with a partner, and separated/widowed/divorced. Poverty index was categorized as less than 130%, 130–350%, and more than 350% using the ratio of family income to poverty guidelines [22]. Health insurance coverage was grouped as either none or any.
Drug use history was identified among adults aged 18–69 years who responded “yes” to any of 2 questions: (1) “Have you ever used cocaine, crack cocaine, heroin, or methamphetamine?” and (2) “Have you ever, even once, used a needle to inject a drug not prescribed by a doctor?” Those who responded “yes” to the first question but “no” to the second question were categorized as having ever used drugs but not injection drug use (IDU); those who responded “yes” to the second question were categorized as IDU. MSM was identified as men aged 18–69 years who responded “yes” to the question “Have you ever had any kind of sex with a man, including oral or anal?” Persons with hepatitis B or hepatitis C were identified as positive for hepatitis B surface antigen, positive for hepatitis C virus RNA, or both. For this study, all of the 3 groups were termed as risk groups. The immunization cohort was defined as those who were born in or after 2004 because the ACIP recommendation to vaccinate all children aged 12–23 months began in 2006. The immunization cohort was limited to those aged 2 years or older due to the data availability. As a supplementary analysis, we examined HAV susceptibility among adults aged 20–59 years with human immunodeficiency virus (HIV) as indicated by positive HIV antibody test results.
Statistical Analysis
We used NHANES survey sampling design and weighting variables to calculate nationally representative estimates and corresponding 95% confidence intervals (CIs) with Stata 14 (StataCorp LP, College Station, TX). Because many immigrants from developing countries may have acquired HAV immunity [23], we first estimated age-specific prevalence of HAV susceptibility stratified by US-birthplace status among persons aged 2 years or older. Then, we focused on US-born adults aged 20 years or older for the rest of this study. We estimated HAV susceptibility by sex, age group, race/ethnicity, marital status, poverty index, health insurance, and survey cycle. We conducted both univariate and multivariate logistic regressions to determine predictors associated with HAV susceptibility. Goodness-of-fit models were assessed by the Hosmer-Lemeshow test. We examined HAV susceptibility trends over 3 periods: 2007–2010, 2011–2014, and 2015–2016. For analyses of risk groups, we grouped the data into 2 periods (2007–2010 and 2011–2016) due to limited sample size. We estimated age-adjusted prevalence by the direct method using the 2000 US Census population and the following age group distribution: 20–29, 30–39, 40–49, 50–59, 60–69, and 70 years or older. We used Stata’s “contrast” command for linear trends and “nlcom” command for relative percentage changes analyses. In parallel to susceptibility analyses, we also assessed predictors and trends of HepA nonvaccination among the general US-born adult population aged 20 years or older as supplementary analyses. For all statistical tests, a significance level of α = .05 was utilized. Data presentation followed NCHS recommendations [24, 25].
RESULTS
Hepatitis A Virus Susceptibility Among Persons Aged 2 Years and Older
Of 47 072 participants aged 2 years or older interviewed in NHANES during 2007–2016, 45 334 (96.3%) were medically examined, of whom HAV susceptibility results were available for 39 719 (87.6% of those examined). During 2007–2016, HAV susceptibility prevalence among persons aged 2 years and older was 59.0% (95% CI, 57.4–60.6%). Susceptibility varied by age group for each 2-year cycle among both US- and non–US-born persons (Figure 1). Among US-born persons aged 2 years or older, susceptibility decreased from approximately 70% in 2007–2010 to 60% in 2011–2016. Substantial decreases in susceptibility occurred from 2007–2008 to 2015–2016 among children aged 2–11 years (57.7% to 13.7%) and aged 12–19 years (66.8% to 28.2%), and to a lesser extent among adults aged 20–29 years (85.9% to 65.2%). Susceptibility among other age groups remained unchanged or increased during 2007–2016. Overall, there was an inverse U-shaped relationship between susceptibility and age groups across years, where those aged 30–59 years had the highest susceptibility (~80%). Among non–US-born persons, susceptibility remained around 20%, and no specific pattern existed by age group over time.
Figure 1.
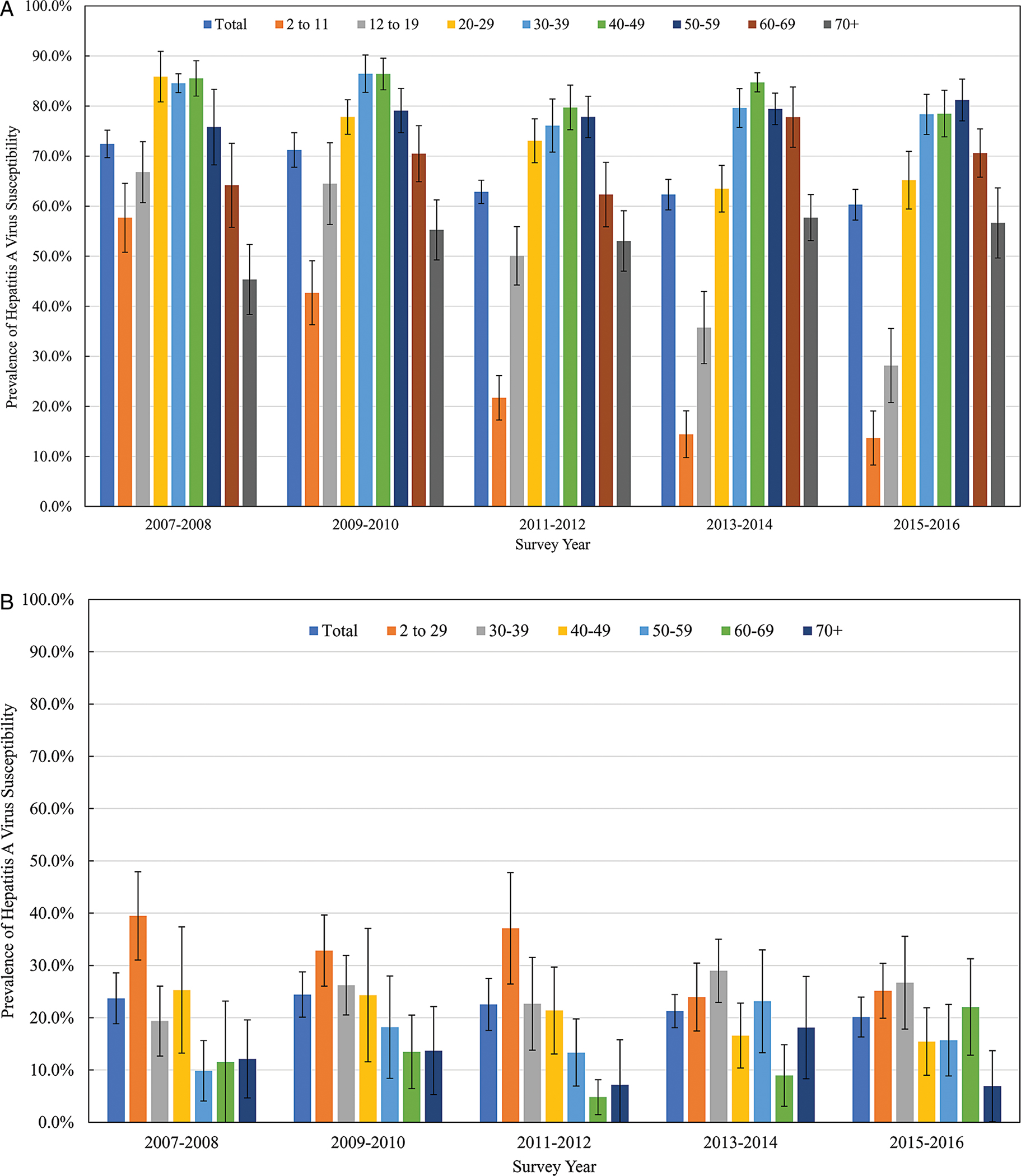
Prevalence of HAV susceptibility by age group and survey years among persons aged ≥2 years in the United States, NHANES 2007–2016. A, US born; B, non–US born. Abbreviations: HAV, hepatitis A virus; NHANES, National Health and Nutrition Examination Survey.
The inverse U-shaped pattern was observed by race/ethnicity groups (Figure 2). Hepatitis A virus susceptibility among Hispanics was significantly lower than non-Hispanic whites and non-Hispanic blacks across all age groups. Non-Hispanic whites and non-Hispanic blacks aged 2–39 years had similar susceptibility. However, non-Hispanic blacks aged 39 years or older had markedly lower susceptibility compared with their non-Hispanic white counterparts.
Figure 2.
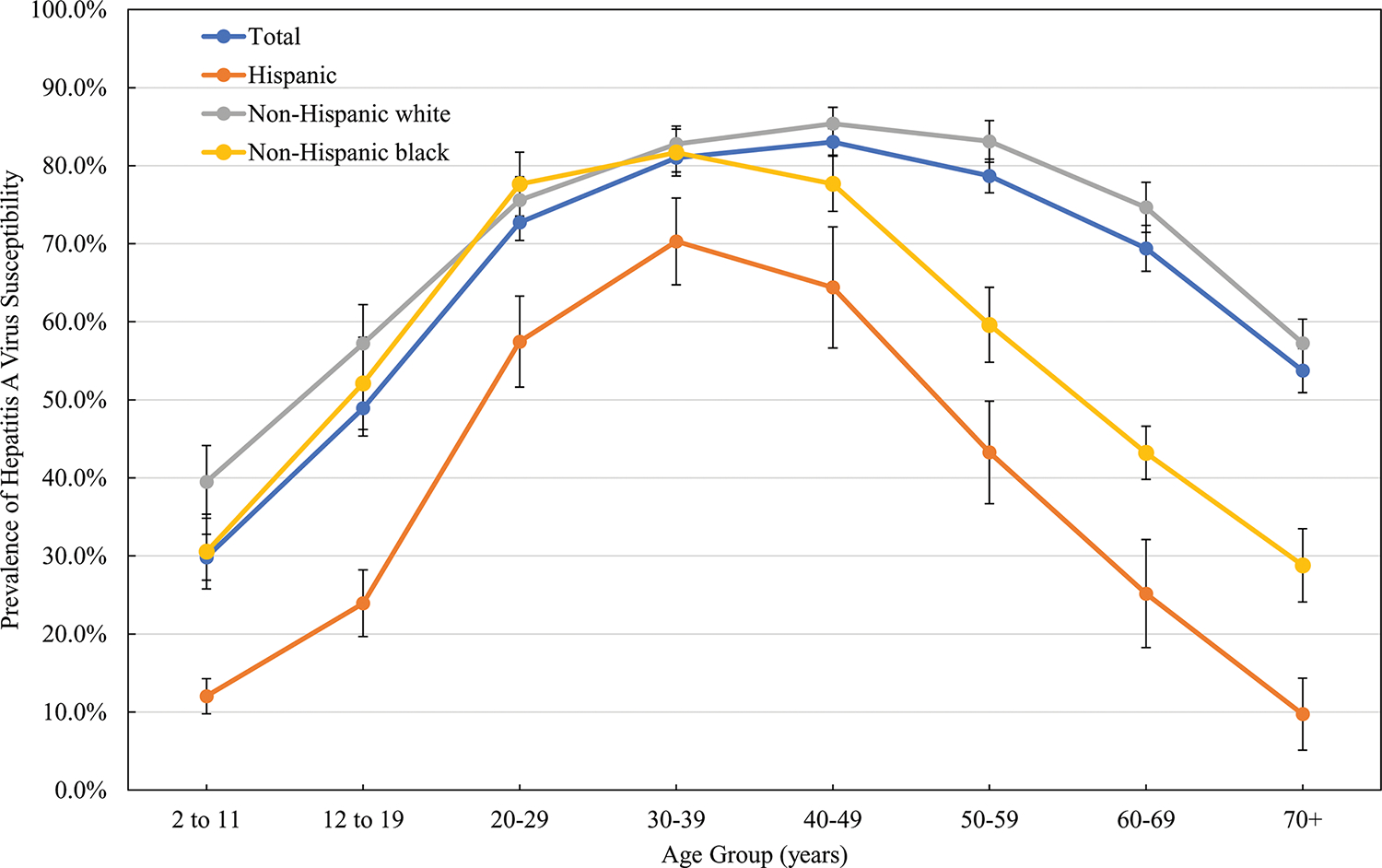
Prevalence of HAV susceptibility by age group and race/ethnicity among US-born persons aged ≥2 years in the United States, NHANES 2007–2016. Abbreviations: HAV, hepatitis A virus; NHANES, National Health and Nutrition Examination Survey.
Hepatitis A Virus Susceptibility Among US-Born Adults Aged 20 Years or Older
Among US-born adults aged 20 years or older, HAV susceptibility was 70% or more by most sociodemographic characteristics and survey years examined (Table 1). Susceptibility was higher among US-born adults aged 30–39 years (81.0%; 95% CI, 79.2–82.8%), 40–49 years (83.0%; 95% CI, 81.3–84.7%), 50–59 years (78.7%; 95% CI, 76.5–80.8%), those without health insurance (78.3%; 95% CI, 76.2–80.4%), those who were 350% above the poverty level (77.7%; 95% CI, 75.9–79.5%), and non-Hispanic whites (77.2%; 95% CI, 75.8–78.6%).
Table 1.
Prevalence and Predictors of Hepatitis A Virus Susceptibility Among US-Born Adults Aged 20 Years or Older in the United States, NHANES 2007–2016
| Characteristic | n/N | Univariate Logistic Regression |
Multivariate Logistic Regressiona |
||||
|---|---|---|---|---|---|---|---|
| Crude % (95% CI) | Crude OR (95% CI) | P | Adjusted % (95% CI) | Adjusted OR (95% CI) | P | ||
|
| |||||||
| Total | 12 451/18 486 | 74.1 (72.9–75.3) | ... | ... | ... | ||
| Sex | |||||||
| Male | 6021/8 930 | 74.6 (73.2–76.1) | ... | 72.6 (71.2–74.1) | ... | ||
| Female | 6430/9556 | 73.7 (72.2–75.1) | 0.95 (.88–1.03) | .229 | 73.1 (71.6–74.6) | 1.03 (.93–1.14) | .596 |
| Age group (years) | |||||||
| 20–29 | 2331/3261 | 72.7 (70.4–75.0) | 73.7 (71.4–76.0) | ||||
| 30–39 | 2466/3035 | 81.0 (79.2–82.8) | 1.60 (1.40–1.83) | <.001 | 80.1 (78.1–82.1) | 1.46 (1.26–1.70) | <.001 |
| 40–49 | 2330/2886 | 83.0 (81.3–84.7) | 1.84 (1.57–2.15) | <.001 | 80.8 (79.0–82.7) | 1.54 (1.30–1.82) | <.001 |
| 50–59 | 2007/2854 | 78.7 (76.5–80.8) | 1.38 (1.17–1.64) | <.001 | 75.6 (73.3–78.0) | 1.12 (.92–1.35) | .257 |
| 60–69 | 1665/2965 | 69.4 (66.5–72.4) | 0.85 (.71–1.02) | <.001 | 66.7 (63.6–69.7) | 0.70 (.57–.86) | <.001 |
| ≥70 | 1652/3485 | 53.7 (50.9–56.6) | 0.44 (.37–.51) | <.001 | 51.4 (48.7–54.0) | 0.36 (.30–.43) | <.001 |
| Race/ethnicity | |||||||
| Hispanic | 1162/2423 | 54.7 (50.6–58.7) | ... | 53.2 (48.8–576) | ... | ||
| Non-Hispanic white | 7857/10 573 | 77.2 (75.8–78.6) | 2.80 (2.33–3.38) | <.001 | 778 (76.5–79.1) | 3.30 (2.65–4.11) | <.001 |
| Non-Hispanic black | 2922/4687 | 66.9 (64.5–69.2) | 1.67 (1.38–2.03) | <.001 | 66.3 (63.9–68.6) | 1.79 (1.43–2.23) | <.001 |
| Marital status | |||||||
| Never married | 2670/3742 | 73.0 (70.8–75.1) | ... | NC | NC | ||
| Married/with a partner | 7182/10 326 | 76.3 (74.8–778) | 1.19 (1.04–1.36) | .012 | ... | ... | |
| Separated/widowed/divorced | 2598/4414 | 68.4 (66.5–70.4) | 0.80 (.70–.92) | .002 | ... | ... | |
| Poverty indexb | |||||||
| <130% | 3345/5187 | 69.3 (671–71.4) | .... | 69.4 (674–71.5) | ... | ||
| 130–350% | 4200/6386 | 72.5 (70.9–74.1) | 1.17 (1.05–1.31) | .007 | 73.0 (71.5–74.5) | 1.21 (1.08–1.35) | .001 |
| >350% | 4083/5622 | 777 (75.9–79.5) | 1.55 (1.35–1.77) | <.001 | 74.9 (73.0–76.7) | 1.34 (1.16–1.55) | <.001 |
| Health insurance | |||||||
| Any | 10 069/15 294 | 73.5 (72.1–74.8) | ... | 71.9 (70.6–73.2) | ... | ||
| None | 2376/3179 | 78.3 (76.2–80.4) | 1.30 (1.15–1.48) | <.001 | 772 (75.1–79.4) | 1.36 (1.18–1.57) | <.001 |
| Survey year | |||||||
| 2007–2008 | 2631/4004 | 75.7 (72.5–79.0) | ... | 74.0 (70.8–773) | ... | ||
| 2009–2010 | 2778/3914 | 773 (74.8–79.7) | 1.09 (.87–1.36) | .455 | 75.9 (73.6–78.1) | 1.11 (.88–1.40) | .363 |
| 2011–2012 | 2264/3415 | 71.6 (69.2–74.0) | 0.81 (.65–1.00) | .046 | 69.8 (672–72.5) | 0.80 (.63–1.01) | .057 |
| 2013–2014 | 2636/3829 | 74.1 (72.0–76.3) | 0.92 (.74–1.13) | .414 | 73.2 (70.9–75.6) | 0.96 (.76–1.20) | .704 |
| 2015–2016 | 2142/3324 | 72.3 (69.5–75.1) | 0.84 (.67–1.05) | .115 | 71.8 (69.0–74.6) | 0.89 (.69–1.13) | .332 |
All estimates are weighted except for n/N.
Abbreviations: CI, confidence interval; HAV, hepatitis A virus; NC, not included; NHANES, National Health and Nutrition Examination Survey; n/N, number of negative anti-HAV results over total number of tested samples.
Model was adjusted by sex, age group, race/ethnicity, poverty index, health insurance, and survey years. Goodness-of-model fit was assessed by the Hosmer-Lemeshow test.
Poverty index level is based on the Supplemental Nutrition Assistance Program (SNAP) eligibility.
Multivariate models (Table 1) showed that US-born adults aged 30–39 years (adjusted odds ratio [AOR], 1.46; 95% CI, 1.26–1.70) and 40–49 years (AOR, 1.54; 95% CI, 1.30–1.82) were more susceptible than adults aged 20–29 years. Non-Hispanic whites (AOR, 3.30; 95% CI, 2.65–4.11) and non-Hispanic blacks (AOR, 1.79; 95% CI, 1.43–2.23) had higher susceptibility than Hispanics. The AOR of susceptibility increased with increasing poverty index. Lack of health insurance was a significant predictor of susceptibility (AOR, 1.36; 95% CI, 1.18–1.57).
Overall, age-adjusted HAV susceptibility decreased (P = .021 for linear trend test) during 2007–2016 (Table 2). By characteristics, linear decreases were observed among females (P = .029), those who were 350% above the poverty level (P = .002), and those with health insurance (P = .041). However, susceptibility remained stable among non-Hispanic whites, non-Hispanic blacks, those who were 350% under the poverty level, and those without health insurance.
Table 2.
Age-adjusted Prevalence of Hepatitis A Virus Susceptibility by Selected Characteristics Among US-Born Adults Aged 20 Years or Older in the United States, NHANES 2007–2016
| 2007–2010 |
2011–2014 |
2015–2016 |
||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | n/N | % (95% CI) | n/N | % (95% CI) | n/N | % (95% CI) | P a | Relative % Change (95% CI)b |
|
| ||||||||
| Total | 5409/7918 | 772 (75.2–79.3) | 4900/7244 | 73.5 (71.8–75.1) | 2142/3324 | 72.8 (70.0–75.7) | .021* | −5.7 (−9.9 to −1.6)* |
| Sex | ||||||||
| Male | 2609/3814 | 76.9 (74.8–79.1) | 2388/3513 | 74.3 (72.3–76.3) | 1024/1603 | 72.9 (68.8–770) | .061 | −5.2 (−10.7 to .3) |
| Female | 2800/4104 | 775 (75.2–79.9) | 2512/3731 | 72.8 (70.5–75.2) | 1118/1721 | 72.8 (70.1–75.5) | .029* | −6.1 (−10.3 to −1.9)* |
| Race/ethnicity | ||||||||
| Hispanic | 522/1051 | 49.9 (43.6–56.2) | 375/752 | 51.5 (46.4–56.5) | 265/620 | 475 (38.0–570) | .282 | −4.8 (−25.8 to 16.1) |
| Non-Hispanic white | 3713/4961 | 80.8 (78.5–83.1) | 2964/4012 | 76.0 (74.0–78.0) | 1180/1600 | 75.7 (71.9–79.4) | .056 | −6.4 (−11.3 to −1.4) |
| Non-Hispanic black | 1061/1735 | 64.9 (61.5–68.2) | 1292/2058 | 66.4 (63.2–69.6) | 569/894 | 66.6 (61.3–71.9) | .540 | 2.7 (−6.4 to 11.8) |
| Poverty indexc | ||||||||
| <130% | 1338/2037 | 69.8 (66.6–73.0) | 1524/2320 | 69.0 (66.3–71.7) | 483/830 | 71.4 (65.6–773) | .446 | 2.4 (−6.6 to 11.3) |
| 130–350% | 1846/2806 | 76.4 (73.6–79.1) | 1516/2306 | 73.4 (71.0–75.7) | 838/1274 | 74.0 (71.5–76.6) | .255 | −3.0 (−75 to 1.5) |
| >350% | 1875/2512 | 80.5 (78.4–82.7) | 1553/2167 | 75.0 (71.9–78.2) | 655/943 | 72.9 (68.8–770) | .002* | −9.5 (−14.7 to −4.3)* |
| Health insurance | ||||||||
| Any | 4296/6455 | 775 (75.3–79.7) | 3923/5926 | 73.0 (71.2–74.8) | 1850/2913 | 72.4 (69.5–75.4) | .041* | −6.6 (−10.9 to −2.2)* |
| None | 1111/1459 | 76.5 (71.8–81.2) | 975/1311 | 74.4 (68.8–80.1) | 290/409 | 72.4 (65.2–79.6) | .163 | −5.4 (−15.7 to 4.9) |
All estimates are weighted except for n/N.
Abbreviations: CI, confidence interval; HAV, hepatitis A virus; NHANES, National Health and Nutrition Examination Survey; n/N, number of negative anti-HAV results over total number of tested samples.
P value for overall linear trend tests. *P < .05.
Relative percentage changes between 2007–2010 and 2015–2016. *P < .05.
Poverty index level is based on the Supplemental Nutrition Assistance Program (SNAP) eligibility.
Hepatitis A Virus Susceptibility Among Risk Groups and Immunization Cohort
Among US-born risk groups aged 20 years or older, HAV susceptibility was 72.9% (95% CI, 67.2–78.7%) among persons who reported IDU, 67.5% (95% CI, 60.7–74.3%) among MSM, and 55.2% (95% CI, 46.7–63.6%) among persons with hepatitis B or hepatitis C during 2007–2016 (Table 3). Susceptibility remained unchanged among risk groups from 2007–2010 to 2011–2016. Among the immunization cohort, susceptibility was 22.6% (95% CI, 20.1–25.2%) during 2007–2016. Susceptibility substantially decreased by 53.8% (95% CI, 45.0–62.6%) among the immunization cohort from 39.6% in 2007–2010 to 18.3% in 2011–2016.
Table 3.
Prevalence of Hepatitis A Virus Susceptibility Among Risk Groups and Immunization Cohort in the United States, NHANES 2007–2016
| 2007–2016 |
2007–2010 |
2011–2016 |
Relative % Change (95% CI)a | ||||
|---|---|---|---|---|---|---|---|
| n/N | % (95% CI) | n/N | % (95% CI) | n/N | % (95% CI) | ||
|
| |||||||
| Persons with hepatitis B or hepatitis Cb | 146/294 | 55.2 (46.7–63.6) | 51/105 | 56.1 (46.8–65.4) | 95/189 | 63.8 (55.6–71.9) | 13.7 (−9.3 to 36.7) |
| Men who had any sex with menc | 205/317 | 675 (60.7–74.3) | 43/62 | 68.2 (56.1–80.3) | 162/255 | 68.2 (59.7–76.7) | −0.1 (−20.5 to 20.3) |
| Persons who reported drug used | |||||||
| Having used, not IDU | 1840/2452 | 78.6 (76.2–81.1) | 790/997 | 81.6 (78.3–85.0) | 1050/1455 | 75.7 (72.3–79.1) | −7.3 (−12.8 to −1.8)* |
| IDU | 245/378 | 72.9 (672–78.7) | 106/141 | 75.0 (678–82.1) | 139/237 | 70.9 (62.0–79.8) | −5.4 (−19.9 to 9.1) |
| Immunization birth cohorte | 1331/6622 | 22.6 (20.1–25.2) | 499/1455 | 39.6 (34.9–44.3) | 832/5167 | 18.3 (15.4–21.2) | −53.8 (−62.6 to −45)* |
Prevalence estimates among risk groups were age-adjusted using the US 2000 standard population. Risk groups only included US-born adults aged ≥20 years. All estimates are weighted except for n/N.
Abbreviations: CI, confidence interval; HAV, hepatitis A virus; IDU, injection drug use; NHANES, National Health and Nutrition Examination Survey; n/N, number of negative anti-HAV results over total number of tested samples.
Relative percentage changes from 2007–2010 to 2011–2016.
P < .05.
Those who tested positive for hepatitis B surface antigen or hepatitis C RNA.
Males 20–69 years old only. Data are not available in NHANES 2007–2008.
Adults 20–69 years old only. Included cocaine, crack cocaine, heroin, or methamphetamine. Excluded marijuana.
Those born after 2004 and aged ≥2 years during 2007–2016. Since 2006, the Advisory Community on Immunization Practice (ACIP) recommends all children aged 12–23 months to have hepatitis A routine vaccination.
Among adults aged 20–59 years with HIV, HAV susceptibility was 39.2% (95% CI, 24.8–53.6%) during 2007–2016 (Supplementary Figure 1). Susceptibility among US-born adults with HIV during 2007–2016 was not estimated due to limited sample size.
Self-reported HepA Nonvaccination Among Risk Groups and Immunization Cohort
Among US-born risk groups aged 20 years or older during 2007–2016, self-reported HepA nonvaccination prevalence was 75.1% (95% CI, 68.9–81.2%) among persons with hepatitis B or hepatitis C, 73.1% (95% CI, 67.2–79.0%) among persons who reported IDU, and 65.2% (95% CI, 58.4–72.1%) among MSM (Table 4). Nonvaccination remained unchanged among persons who reported IDU and MSM from 2007–2010 to 2011–2016. Among persons with hepatitis B or hepatitis C, nonvaccination marginally decreased by 18.4% (95% CI, 1.4–35.4%). Among the immunization cohort, nonvaccination prevalence was 25.9% (95% CI, 24.0–27.8%) and decreased by 32.4% (95% CI, 22.4–42.4%) from 34.6% in 2007–2010 to 23.4% in 2011–2016.
Table 4.
Prevalence of Nonvaccination to Hepatitis A Among Risk Groups and Immunization Cohort in the United States, NHANES 2007–2016
| 2007–2016 |
2007–2010 |
2011–2016 |
Relative % Change (95% CI)a | ||||
|---|---|---|---|---|---|---|---|
| n/N | % (95% CI) | n/N | % (95% CI) | n/N | % (95% CI) | ||
|
| |||||||
| Persons with hepatitis B or hepatitis Cb | 232/315 | 75.1 (68.9–81.2) | 107/144 | 78.3 (70.9–85.6) | 125/171 | 63.8 (51.5–76.2) | −18.4 (−35.4 to −1.4)* |
| Men who had any sex with menc | 183/289 | 65.2 (58.4–72.1) | 37/062 | 52.4 (38.1–66.8) | 146/227 | 64.0 (56.2–71.7) | 21.9 (−12.2 to 56) |
| Persons who reported drug used | |||||||
| Having used, not IDU | 1556/2197 | 71.7 (69.0–74.3) | 682/910 | 75.2 (72.1–78.3) | 874/1287 | 65.2 (61.2–69.2) | −13.3 (−19.5 to −71)* |
| IDU | 276/390 | 73.1 (672–79.0) | 121/163 | 75.1 (678–82.4) | 155/227 | 674 (58.6–76.1) | −10.3 (−24.4 to 3.8) |
| Immunization birth cohorte | 1884/8560 | 25.9 (24.0–278) | 608/2013 | 34.6 (30.4–38.8) | 1276/6547 | 23.4 (21.2–25.6) | −32.4 (−42.4 to −22.4)* |
All estimates are weighted except for n/N.
Abbreviations: CI, confidence interval; HAV, hepatitis A virus; IDU, injection drug use; NHANES, National Health and Nutrition Examination Survey; n/N, number of negative anti-HAV results over total number of tested samples.
Prevalence estimates among risk groups were age-adjusted using the US 2000 standard population. Risk groups only included US-born adults aged ≥20 years.
Relative percentage changes between 2007–2010 and 2011–2016.
P < .05.
Those who tested positive for hepatitis B surface antigen or hepatitis C RNA.
Males 20–69 years old only. Data are not available in NHANES 2007–2008.
Adults 20–69 years old only. Included cocaine, crack cocaine, heroin, or methamphetamine. Excluded marijuana.
Those born after 2004 and aged ≥2 years during 2007–2016. Since 2006, the Advisory Community on Immunization Practice (ACIP) recommends all children aged 12–23 months to have hepatitis A routine vaccination.
HepA nonvaccination prevalence among the general US-born adults aged 20 years or older was 72.9% (95% CI, 71.7–74.1%) during 2007–2016 and decreased over time by most characteristics examined (except those without health insurance) (Supplementary Tables 1 and 2).
DISCUSSION
Using nationally representative data, we found that HAV susceptibility among US-born adults aged 20 years or older was approximately 74.1% during 2007–2016. Adults aged 30–59 years, non-Hispanic whites, those without health insurance, and risk groups were most susceptible to HAV. This high susceptibility persisted over time, indicating that vaccination efforts, which have increased among children, have not increased for adults, including those at risk. Adults may also have less chance of acquiring HAV immunity through exposure to asymptomatic infected children as occurred in the prevaccination era. A better understanding of HAV susceptibility overall and among subgroups may help identify vaccination gaps and improve efforts in HAV control and prevention.
Compared with previous NHANES studies, overall HAV susceptibility among persons aged 2 years or older decreased from 65.1% during 1999–2006 [5] to 59.0% during 2007–2016. Among US-born adults aged 20 years or older, HAV susceptibility slightly declined from 75.8% during 1999–2012 [7] to 72.8% during 2015–2016. These declines were likely attributable to childhood immunization efforts and death of older cohorts. In support of this, a substantial decrease in HAV susceptibility was observed among the immunization cohort during 2007–2016.
We found an inverse U-shaped curve of age-specific HAV susceptibility prevalence among US-born person aged 2 years or older, where those aged 30–59 years were most susceptible during 2007–2016. The older age groups (≥60 years) had relatively lower susceptibility, which may be partially due to the acquired immunity from past infection. Because of the aging of the immunization cohort and death among older cohorts, we expect that the inverse U-shaped curve may shift toward older age groups over time, meaning that HAV-susceptible persons would more likely be older in future years. This shift could result in more aging-related complications including hospitalization and premature mortality [9, 26]. Hepatitis A virus susceptibility also varied by race/ethnicity and socioeconomic status. Larger proportions of non-Hispanic whites, those with higher household income, and those without health insurance were HAV susceptible.
Although the ACIP has recommended routine vaccination for risk groups such as persons with IDU and MSM since 1996, this study showed that HAV susceptibility among these groups was as high as 70%. Consistently, we found that approximately 70% of at-risk adults remained unvaccinated against HAV. Lack of vaccination in these groups made it possible for outbreaks to spread nationwide and are challenging to control. These findings underscore the urgency of improving HepA vaccination coverage among at-risk adults. This would need joint efforts from public and private sectors on better understanding the barriers and facilitators of HepA vaccination among risk groups; application of standards for adult immunization practice to avoid missed opportunities of vaccination [27]; implementation of evidence-based strategies such as increasing vaccination demand, enhancing vaccination access, and provider- or system-based interventions [28]; and utilization of targeted approaches such as enhancing resources to access hard-to-reach populations (eg, the homeless) and integrating vaccination with other programs/settings for at-risk adults.
This study has several limitations. First, NHANES data are representative of the noninstitutionalized US civilian population. Our estimates on HAV susceptibility and nonvaccination may have been underestimated because some high-risk groups such as persons experiencing homelessness, prisoners, and military personnel were not included [29]. Although there was a decline in response rate for NHANES 2015–2016 from previous cycles, it could be compensated for by sampling weights and no evidence of substantial nonresponse bias has been found [30]. Second, MSM and illicit drug use were based on self-reported questions, which may be subject to reporting bias. We had limited sample sizes for risk groups, which required combining more years of data. Third, we considered persons with hepatitis B or hepatitis C as a group with adverse outcomes of HAV infection [18]. However, because at the time of this analysis ACIP only recommended chronic liver disease as a comorbidity indication for immunization [4], those with viral hepatitis coinfections who had not yet developed chronic liver disease may not have received the vaccination. Fourth, the immunization cohort did not include children aged younger than 2 years due to data unavailability. Children who were immunized before 2006 were also excluded. Finally, HAV susceptibility was solely based on laboratory testing but did not account for the likelihood of recent exposure due to risk behaviors. Risk groups could be more vulnerable to HAV infection even though the susceptibility prevalence is lower than in other populations.
In summary, although HepA vaccination coverage among children has improved, approximately three-fourths of US-born adults aged 20 years or older were HAV susceptible during 2007–2016. Susceptibility and nonvaccination among risk groups remained high and stable over time. Given the recent outbreaks that have been prolonged and challenging to control, these findings underscore that more immunization efforts are critically needed among adults at risk of HAV infection to mitigate the current outbreaks.
Supplementary Material
Acknowledgments.
The authors acknowledge Dr Eyasu H. Teshale and Dr Neil Gupta, the Centers for Disease Control and Prevention, for their suggestions.
Financial support.
All authors are US federal employees of the Centers for Disease Control and Prevention and have no outside funding to declare.
Footnotes
Potential conflicts of interests. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Disclaimer. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1.Cuthbert JA. Hepatitis A: old and new. Clin Microbiol Rev 2001; 14:38–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Prevention of hepatitis A through active or passive immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1996; 45:1–30. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Prevention of hepatitis A through active or passive immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1999; 48:1–37. [PubMed] [Google Scholar]
- 4.Advisory Committee on Immunization Practices; Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55:1–23. [PubMed] [Google Scholar]
- 5.Klevens RM, Kruszon-Moran D, Wasley A, et al. Seroprevalence of hepatitis A virus antibodies in the U.S.: results from the National Health and Nutrition Examination Survey. Public Health Rep 2011; 126:522–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson NP, Yankey D, Singleton JA, Elam-Evans LD. Hepatitis A vaccination coverage among adolescents (13–17 years) in the United States, 2008–2016. Vaccine 2018; 36:1650–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klevens RM, Denniston MM, Jiles-Chapman RB, Murphy TV. Decreasing immunity to hepatitis A virus infection among US adults: findings from the National Health and Nutrition Examination Survey (NHANES), 1999–2012. Vaccine 2015; 33:6192–8. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Vaccination coverage among adults in the United States, National Health Interview Survey, 2016. Available at: https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/NHIS-2016.html. Accessed 9 September 2019.
- 9.Ly KN, Klevens RM. Trends in disease and complications of hepatitis A virus infection in the United States, 1999–2011: a new concern for adults. J Infect Dis 2015; 212:176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy TV, Denniston MM, Hill HA, et al. Progress toward eliminating hepatitis A disease in the United States. MMWR Suppl 2016; 65:29–41. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Surveillance for viral hepatitis—United States, 2017. Available at: https://www.cdc.gov/hepatitis/statistics/2017surveillance/index.htm. Accessed 13 September 2019.
- 12.Wasley A, Samandari T, Bell BP. Incidence of hepatitis A in the United States in the era of vaccination. JAMA 2005; 294:194–201. [DOI] [PubMed] [Google Scholar]
- 13.Foster M, Ramachandran S, Myatt K, et al. Hepatitis A virus outbreaks associated with drug use and homelessness—California, Kentucky, Michigan, and Utah, 2017. MMWR Morb Mortal Wkly Rep 2018; 67:1208–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster MA, Hofmeister MG, Kupronis BA, et al. Increase in hepatitis A virus infections—United States, 2013–2018. MMWR Morb Mortal Wkly Rep 2019; 68:413–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Widespread outbreaks of hepatitis A across the United States. Available at: https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed 22 Novermber 2019.
- 16.Doshani M, Weng M, Moore KL, Romero JR, Nelson NP. Recommendations of the Advisory Committee on Immunization Practices for use of hepatitis A vaccine for persons experiencing homelessness. MMWR Morb Mortal Wkly Rep 2019; 68:153–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collier MG, Drobeniuc J, Cuevas-Mota J, Garfein RS, Kamili S, Teshale EH. Hepatitis A and B among young persons who inject drugs—vaccination, past, and present infection. Vaccine 2015; 33:2808–12. [DOI] [PubMed] [Google Scholar]
- 18.Moorman AC, Xing J, Nelson NP; Chronic Hepatitis Cohort Study (CHeCS) Investigators. Need for increasing hepatitis A virus vaccination among patients infected with hepatitis B virus and hepatitis C virus. Gastroenterology 2018; 154:2015–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henkle E, Lu M, Rupp LB, et al. ; Chronic Hepatitis Cohort Study (CHeCS) Investigators. Hepatitis A and B immunity and vaccination in chronic hepatitis B and C patients in a large United States cohort. Clin Infect Dis 2015; 60:514–22. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Laboratory procedure mannual for hepatitis A antibody. Available at: https://wwwn.cdc.gov/nchs/data/nhanes/2007-2008/labmethods/hepa_e_hepatitis-a-antibody_met.pdf. Accessed 7 November 2019.
- 21.Ott JJ, Wiersma ST. Single-dose administration of inactivated hepatitis A vaccination in the context of hepatitis A vaccine recommendations. Int J Infect Dis 2013; 17:e939–44. [DOI] [PubMed] [Google Scholar]
- 22.Jun S, Cowan AE, Tooze JA, et al. Dietary supplement use among U.S. children by family income, food security level, and nutrition assistance program participation status in 2011(−)2014. Nutrients 2018; 10:1212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ooi WW, Gallagher A, Chen LH. Immunity to hepatitis A and hepatitis B in Indian and Chinese immigrants seen in a travel clinic in Massachusetts, United States. J Travel Med 2006; 13:212–8. [DOI] [PubMed] [Google Scholar]
- 24.Parker JD, Talih M, Malec DJ, et al. National Center for Health Statistics data presentation standards for proportions. Vital Health Stat 2 2017; 175:1–22. [PubMed] [Google Scholar]
- 25.Ingram DD, Malec DJ, Makuc DM, et al. National Center for Health Statistics guidelines for analysis of trends. Vital Health Stat 2 2018; 179:1–17. [PubMed] [Google Scholar]
- 26.Collier MG, Tong X, Xu F. Hepatitis A hospitalizations in the United States, 2002–2011. Hepatology 2015; 61:481–5. [DOI] [PubMed] [Google Scholar]
- 27.National Vaccine Advisory Committee. Recommendations from the National Vaccine Advisory Committee: standards for adult immunization practice. Public Health Rep 2014; 129:115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willis BC, Ndiaye SM, Hopkins DP, Shefer A; Task Force on Community Preventive Services. Improving influenza, pneumococcal polysaccharide, and hepatitis B vaccination coverage among adults aged <65 years at high risk: a report on recommendations of the Task Force on Community Preventive Services. MMWR Recomm Rep 2005; 54:1–11. [PubMed] [Google Scholar]
- 29.Hofmeister MG, Rosenthal EM, Barker LK, et al. Estimating prevalence of hepatitis C virus infection in the United States, 2013–2016. Hepatology 2019; 69:1020–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: impact of declining response rates on nonresponse bias, executive summary. Available at: https://wwwn.cdc.gov/nchs/data/nhanes/2011-2012/analyticguidelines/nonresponse_executive_summary_13_16.pdf. Accessed 13 November 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


