Abstract
Human pluripotent stem cells (hPSCs) are inherently sensitive cells. Single-cell dissociation and the establishment of clonal cell lines have been long-standing challenges. This inefficiency of cell cloning represents a major obstacle for the standardization and streamlining of human-induced pluripotent stem cell (hiPSC) gene editing in basic and translation research. Here, we describe a chemically defined protocol for robust single-cell cloning using microfluidics-based cell sorting in combination with the CEPT small molecule cocktail. This advanced strategy promotes the viability and cell fitness of self-renewing stem cells. The use of low-pressure microfluidic cell dispensing ensures gentle and rapid dispensing of single cells into 96- and 384-well plates, while the fast-acting CEPT cocktail minimizes cellular stress and maintains cell structure and function immediately after cell dissociation. The protocol also facilitates clone-picking and produces genetically stable clonal cell lines from hPSCs in a safe and cost-efficient fashion. Depending on the proliferation rate of the clone derived from a single cell, this protocol can be completed in 7 to 14 days and requires experience with aseptic cell culture techniques. Altogether, the relative ease, scalability, and robustness of this workflow should boost gene editing in hPSCs and leverage a wide range of applications, including cell line development (e.g., reporter and isogenic cell lines), disease modeling, and applications in regenerative medicine.
Introduction
Since the derivation of the first human embryonic stem cell (hESC) lines and the introduction of iPSC technology, significant progress has been made in characterizing pluripotent cells and defining in vitro conditions that maintain their capacity for long-term expansion and functional differentiation1–4. hPSCs are known to be sensitive cells and that might be one of the reasons why the first hESC lines were established 17 years after the isolation of mouse ESCs5,6. Consequently, special cell culture conditions, media formulations, coating substrates, growth factors, and differentiation-inducing reagents were tested and established specifically for human cells over the past two decades7–9. While hESCs were initially cultured on mouse embryonic fibroblasts (MEFs) in the presence of fetal bovine serum (FBS), the advent of hiPSCs technology and their promise for regenerative medicine and personalized cellular therapies has made the use of chemically defined media more attractive. For instance, an important advance for reproducible culture of hPSCs is the development of fully-defined Essential 8 (E8) Medium and the use of recombinant proteins such as vitronectin (VTN-N) and laminin 521 (LN-521) as coating substrates10,11. Moreover, hPSCs require sufficient concentrations of fibroblast growth factor 2 (FGF2) for proliferation and to maintain their undifferentiated state. Since FGF2 can quickly degrade in cell culture12, the availability of beads and special formulations that ensure a more constant release of growth factors over longer periods of time has improved stem cell experiments. For instance, commercially available StemFlex Medium or mTeSR Plus with stabilized FGF2 allow a more flexible feeding schedule, including weekend-free maintenance.
Despite these advances, culturing hPSCs remains challenging and is further complicated by cell-line-to-cell-line variability and other factors that are difficult to control (e.g., investigator bias). Typically, a significant fraction of hPSCs are lost during each routine cell passaging step, and some cell lines require the use of higher cell densities to ensure long-term expansion13,14. Alternatively, cell viability can be improved by inhibiting the ROCK pathway, which otherwise will become hyperactive during passaging or thawing of cryopreserved cells and lead to apoptosis and anoikis due to uncontrolled cell contractions15–17. The introduction of the ROCK pathway inhibitor Y-27632 by Sasai and colleagues15 was a critical discovery for the stem cell field and is still the most widely used method to improve cell survival. However, even in the presence of Y-27632 or subsequently reported ROCK pathway inhibitors (e.g., blebbistatin, pinacidil, thiazovivin)17–19, cellular stress and cell death remain major hurdles. The sensitivity of hPSCs is particularly evident when cells are passaged at low cell densities or at the most extreme condition possible, which is plating one-cell-per-well to establish clonal cell lines20.
Single-cell cloning for establishment of next-generation hPSC lines
Single-cell cloning is a mainstay of stem cell biology and represents the most rigorous assay to investigate self-renewal and differentiation, which are the key defining properties of stem cells irrespective of developmental potential and tissue of origin23. The dynamic nature of cells during the reprogramming process complicates the isolation of true clones and establishment of monoclonal hPSC lines that originate from a single cell24. Single-cell cloning is an advanced and urgently needed cell culture method for probing “stemness” and establishment of next-generation clonal cell lines with improved quality, genomic stability, and homogeneity25. Establishing robust protocols that ensure efficient and safe single-cell cloning of hPSCs is therefore of great relevance for basic research as well as for manufacturing cellular products for clinical applications (e.g., fate mapping experiments, cell differentiation, characterization of cell type identities, disease modeling).
Single-cell cloning of hPSCs for gene editing
hiPSCs represent a powerful platform technology for the study of human biology, genetics, and disease mechanisms4. Being able to genetically modify patient-derived iPSCs by introducing or correcting mutations has enormous potential for biomedical research. The CRISPR/Cas9 system is currently the method of choice for gene editing due to its efficiency and simplicity as compared to previous efforts using transcription activator-like effector nucleases (TALENs) and zinc finger nucleases (ZNFs)26,27. Delivery of Cas9 endonuclease protein together with sequence-specific single guide RNA (sgRNA) precisely targets DNA double-strand breaks. However, clonal isolation of correctly edited cells and establishment of genetically modified cell lines in a safe and efficient fashion remain formidable challenges for the stem cell field.
Among these challenges are low cloning efficiency, uncontrolled cellular stress when cells are cultured in isolation, risk of inducing genetic mutations, drug selection, and time-consuming follow-up experiments to identify the correctly edited cell that needs to be recovered and established as a self-renewing cell line28–30. Moreover, some experimental needs may require not only monoallelic but also biallelic introduction and this may further prolong the timeline of producing gene-edited cell lines. To address the challenges of gene editing of sensitive hPSCs, some authors suggest strategies such as optimization of cell culture conditions20, inhibition of p5331, overexpression of BCL-XL32, or simultaneous reprogramming and gene editing of human fibroblasts25.
Cytoprotection and superior cell survival by using the CEPT cocktail
We recently reported the development of a four-part small molecule cocktail, which we named CEPT (chroman 1, emricasan, polyamines, trans-ISRIB), to improve cell survival of hPSCs21. The CEPT cocktail was developed by using a high-throughput luminescent cell viability assay (CellTiter-Glo® from Promega) to screen diverse chemical libraries with nearly 16,000 small molecule compounds, followed by iterative combination screening at various cell densities, establishment of full dose-response curves, and follow-up experiments to better understand the underlying cellular stress mechanisms. Therefore, this study not only established a new chemical platform for efficient and safe culture and utilization of hPSCs but also revealed that cellular stress and DNA double-strand breaks are more common, even during routine cell passaging, than previously anticipated. Importantly, cellular stress and the impairment of cell structure and function were prominent during the first minutes to several hours after cell dissociation, which can go unnoticed when cells are measured at 24 h post-passage (Figs. 1, 2).
Fig. 1: Live-cell imaging of acutely dissociated hPSCs.
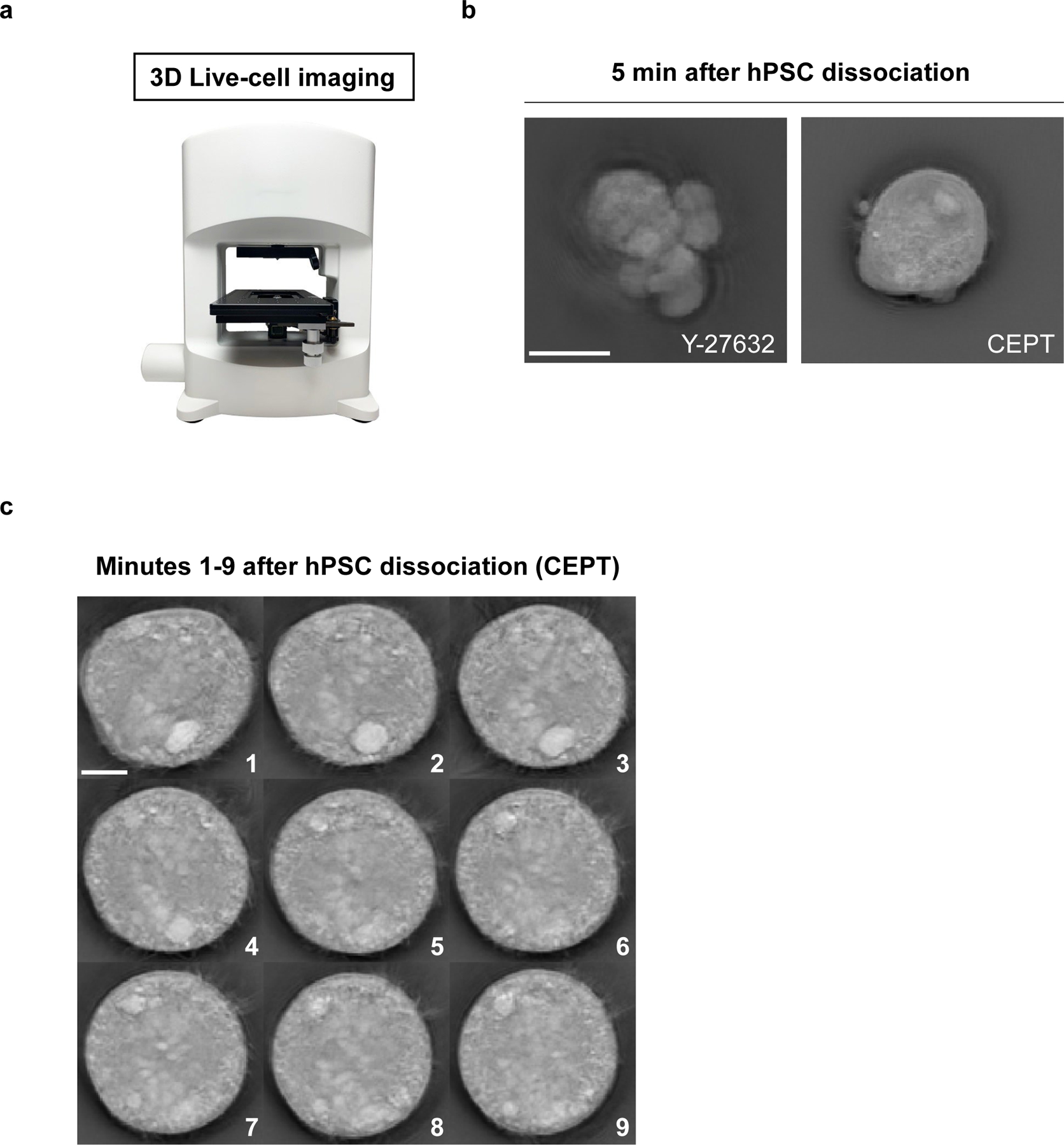
a, Nanolive (3D Cell Explorer) microscope enabling label-free cell imaging. b, Fast-acting CEPT is cytoprotective and maintains cell morphology of WA09 dissociated cells, whereas ongoing cell contractions lead to abnormal cells in the presence of Y-27632. Representative image was taken 5 minutes after cell dissociation. c, Monitoring the morphology of dissociated hPSCs and treatment with CEPT. Images were taken every minute for the first 9 minutes after cell dissociation and plating into the well. Scale bars, 10 µm (b) and 5 µm (c).
Fig. 2: Cell morphology and cell adhesion profile of dissociated hPSCs after treatment with Y-27632, CloneR, or CEPT.
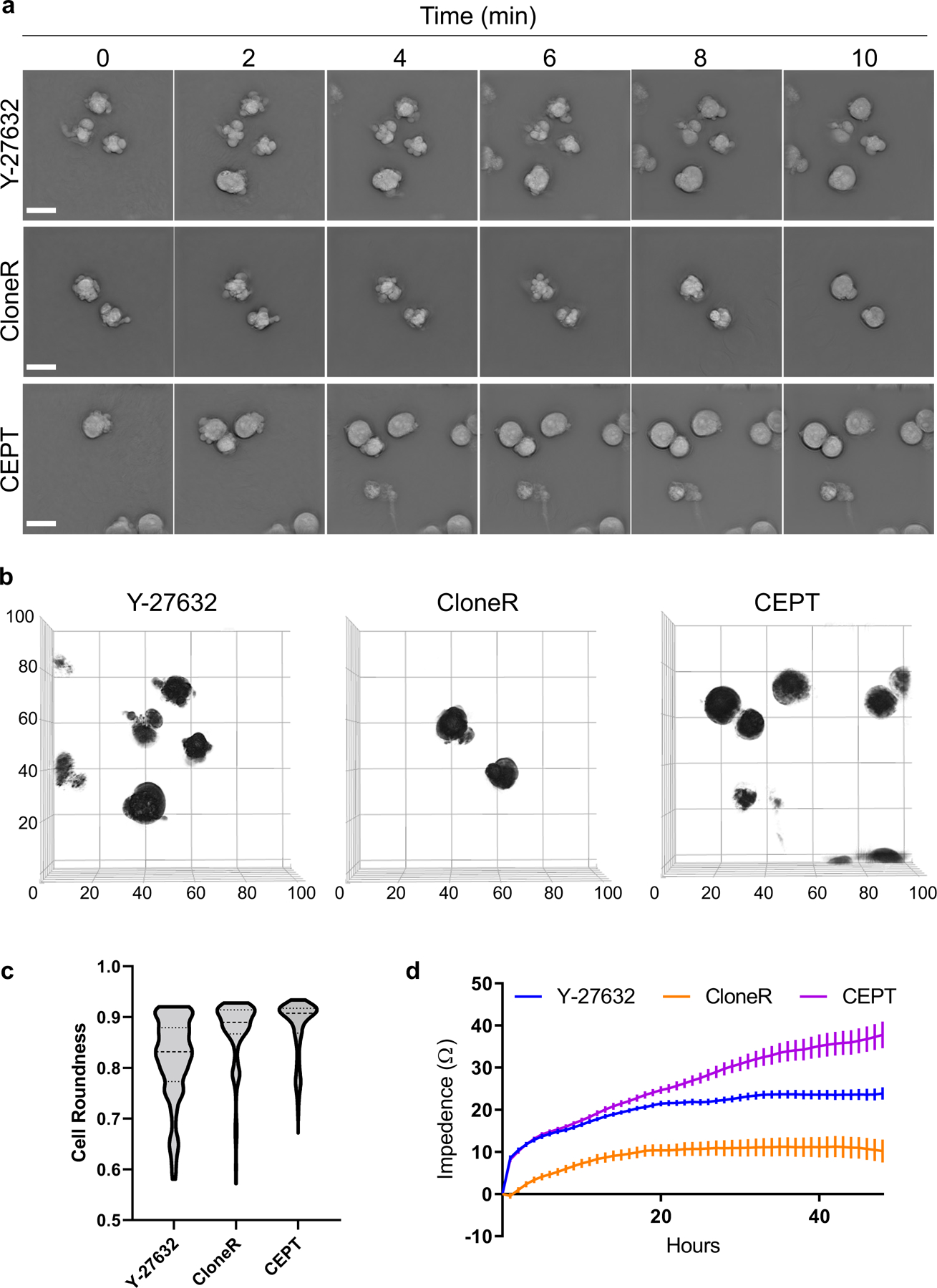
a, Label-free live-cell imaging (Nanolive) showing the first 10 min after plating dissociated cells (WA09). Abnormal phenotypes are caused by cell contractions and membrane blebbing in the presence of Y-27632 (10 µM) and CloneR (1X), whereas treatment with fast-acting CEPT mitigates cell stress and ensures circularity of cell bodies. b, Analysis of membrane blebbing and cell roundness of single cells at 10 min after cell dissociation and treatment with Y-27632 (10 µM), CloneR (1X), or CEPT. Images represent 3D renditions of 60–70 z-planes stacked together. A customized fully automated digital image analysis algorithm segmented each cell as an individual object and measured the shape. Cell roundness is quantified per cell as deviation from perfect mathematical roundness. Six fields of view were analyzed for each condition. Note that CloneR has less cells (black circles) in the field of view because the addition of CloneR (1:10 dilution as recommended by the manufacturer) changes the density of the medium and the cells do not settle to the bottom of the plate as fast as they do with Y-27632 and CEPT. c, Violin plots of live-cell images (3D Cell Explorer) showing distribution of cell roundness and membrane blebbing at 10 min post-treatment with Y-27632, CloneR, or CEPT. Data are from n ≥ 230 for each condition. d, Cell adhesion time-course measured by impedance analysis of cells passaged with Y-27632 (10 µM), CloneR (1X), or CEPT. Data are mean ± s.e.m.; n = 16 wells for each group. Scale bars, 20 µm (a and b); x and y-axes in µm.
While the CEPT cocktail prevented cellular stress and DNA damage during cell passaging, none of the currently used reagents such as Y-27632, RevitaCell, CloneR, and SMC4 could provide similar cytoprotective effects21. When compared to Y-27632 (at the standard dose of 10 µM) and the commercially available reagent developed for single-cell cloning CloneR (at the recommended dose of 1X), CEPT more rapidly reduced cell blebbing and maintained cell roundness and circularity. Of note, these differences were already detectable within the first 10 min after plating dissociated cells (Figs. 1a-c and 2a-c). Furthermore, CEPT promoted cell adhesion to the coating substrate more efficiently as measured by impedance analysis21 (Fig. 2d). Altogether, our previous report21 and the new data presented here establish that CEPT improves routine cell passaging, embryoid body (EB) differentiation, organoid formation, cryopreservation and thawing of hPSCs, single-cell cloning, gene editing, and robotic cell culture22.
Comparison with other methods
Because single-cell cloning of hPSCs has been notoriously difficult, improving the efficiency and safety of this procedure is of great relevance. Having developed the CEPT cocktail, we recently demonstrated its usefulness with independent single-cell cloning strategies (Fig. 3a)21. First, using FACS-based cell sorting instruments (FACSAria lll from BD Biosciences) showed that CEPT increased cloning efficiency when compared to Y-27632 (10 µM). Second, using an image-based single-cell printing method (Cytena SCP from Molecular Devices), CEPT again had a higher cloning efficiency than Y-27632 (10 µM) (Fig. 3a). Although image-based cell dispensing increases confidence that a single cell is strictly dispensed into each well, carrying out the experimental procedure itself turned out to be time-consuming and labor-intensive as silicone-glass cartridges are susceptible to breakage, prolonging instrument setup for up to 1 h, and dispensing single cells into each well of a 96-well plate takes over 5 min per plate.
Fig. 3: CEPT improves single-cell cloning efficiency and maintains pluripotency.
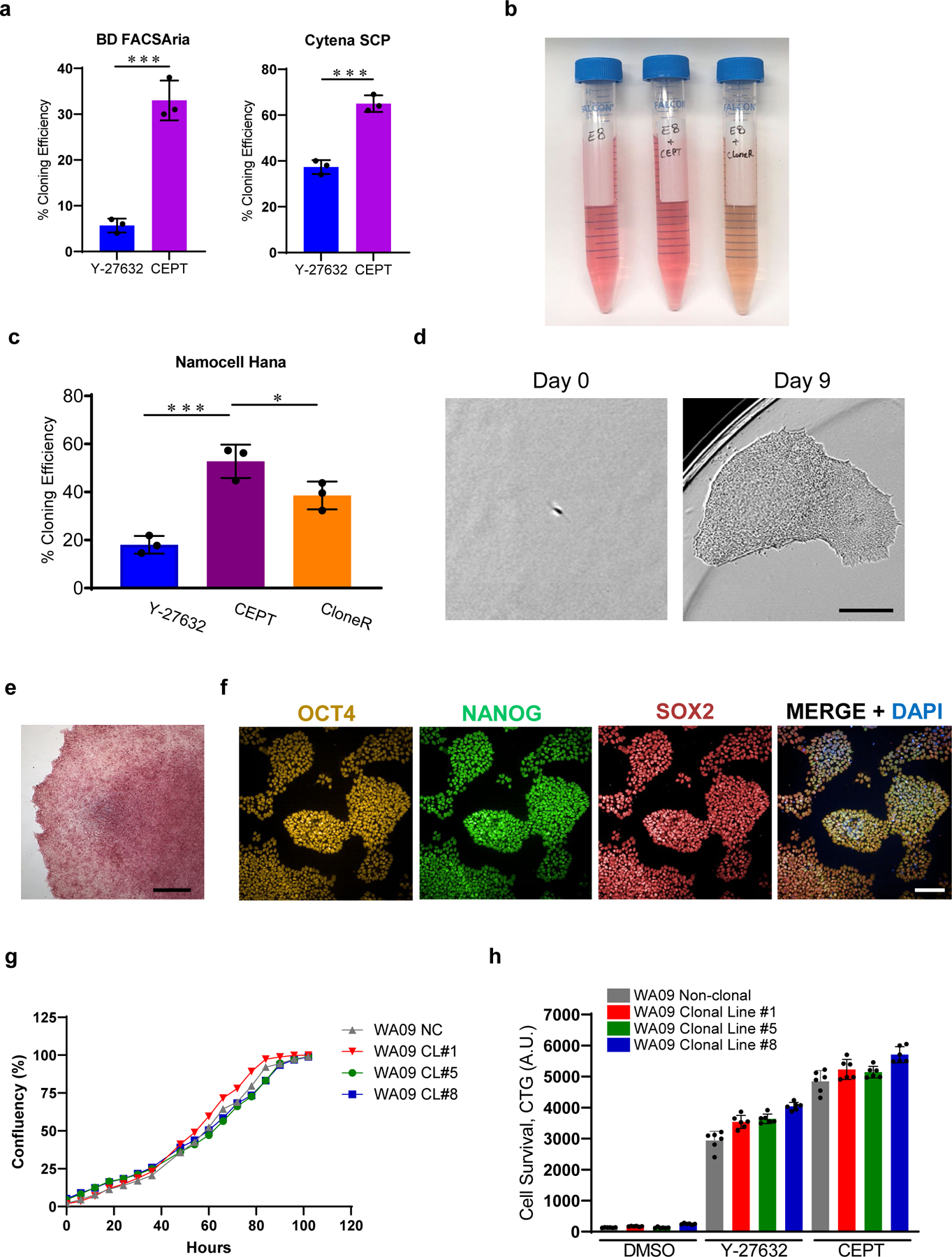
a, Single-cell cloning efficiency in the presence of Y-27632 (10 µM) and CEPT as tested on two different platforms including single-cell printing with Cytena (Molecular Devices), and cell sorting with FACSAria (Becton Dickinson). Figure adapted with permission from ref. 21. b, E8 Medium after supplementation with CEPT or CloneR. Note that addition of CloneR (1:10 dilution according to manufacturer) changes the color of cell culture medium. c, Quantification of single-cell cloning efficiency (WA09) using microfluidics-based cell sorting (Namocell Hana) after treatment with Y-27632 (10 µM), CloneR (1X), or CEPT. d, Phase-contrast images showing a single cell (WA09) that survived dissociation and generated a clonal colony in 9 days. e, Representative image of a clonal colony (WA09) expressing pluripotency-associated marker alkaline phosphatase. f, Representative immunocytochemical images showing that clonal cell lines maintain pluripotency and express OCT4, NANOG and SOX2 (clonal cell line #1 from WA09). g, Image-based analysis comparing cell growth rate of non-clonal parental line (NC) and derived clonal cell lines 1, 5, and 8 (CL #1, 5 and 8). h, Luminescent cell viability assay showing that the sensitivity to enzymatic cell dissociation is comparable in non-clonal parental line and derived clonal cell lines 1, 5, and 8. Scale bars, 300 µm (d), 400 µm (e), and 100 µm (f). Data are mean ± s.d.; n = 3 96-well plates for each group (a, c), *P ≤0.05, **P ≤0.01, ***P ≤0.001, two-way ANOVA and Student’s t-test, respectively. Data are mean ± s.d.; n ≥ 3 (g, h).
We further tested a microfluidic-based cell sorting instrument (Hana from Namocell) in combination with the CEPT cocktail (Fig. 3c). This provided overall superior results compared to FACS regarding fast setup (≤3 min vs >15 min) and gentle cell dispensing (≤2 psi vs >2–5 psi) into 96-well plates in 1–2 min per plate (and 6 min for a 384-well plate). Such low-pressure cell dispensing can minimize mechanical stress and cell damage and is typically not achieved by traditional FACS instruments. Also, CEPT again showed a higher cloning efficiency than Y-27632 (10 µM) and CloneR (1X) using the microfluidic-based system (Fig. 3c), without changing the properties of the culture medium as is observed with CloneR (Fig. 3b). In addition, clonal cell lines derived using the microfluidic-based cell sorting instrument in conjunction with the CEPT cocktail expressed pluripotency-associated markers and showed normal cell growth (Fig. 3e-h). Based on these findings and the comparisons from our earlier study21, the protocol described here will focus on user-friendly microfluidic cell sorting in combination with the CEPT cocktail for stress-free and robust single-cell cloning in high-throughput fashion.
Overview of the Procedure
The protocol presented here (Figs. 4 and 5) provides an advanced, rapid, and practical approach to perform single-cell cloning and overcomes current challenges in hPSC technology. The first section of this protocol describes how to thaw and maintain hPSC cell lines in E8 Medium supplemented with CEPT (Steps 1–19). The second section describes single-cell dissociation of non-clonal cell lines and the procedure used to generate hPSC clonal cell lines derived from a single hPSC (Steps 20–66). hPSC clonal cell lines generated using this protocol exhibit critical quality attributes of hPSCs that can assessed using various assays (Box 2 – 4) and differentiation protocols (Box 5).
Fig. 4: Scalable single-cell cloning workflow for hPSCs.
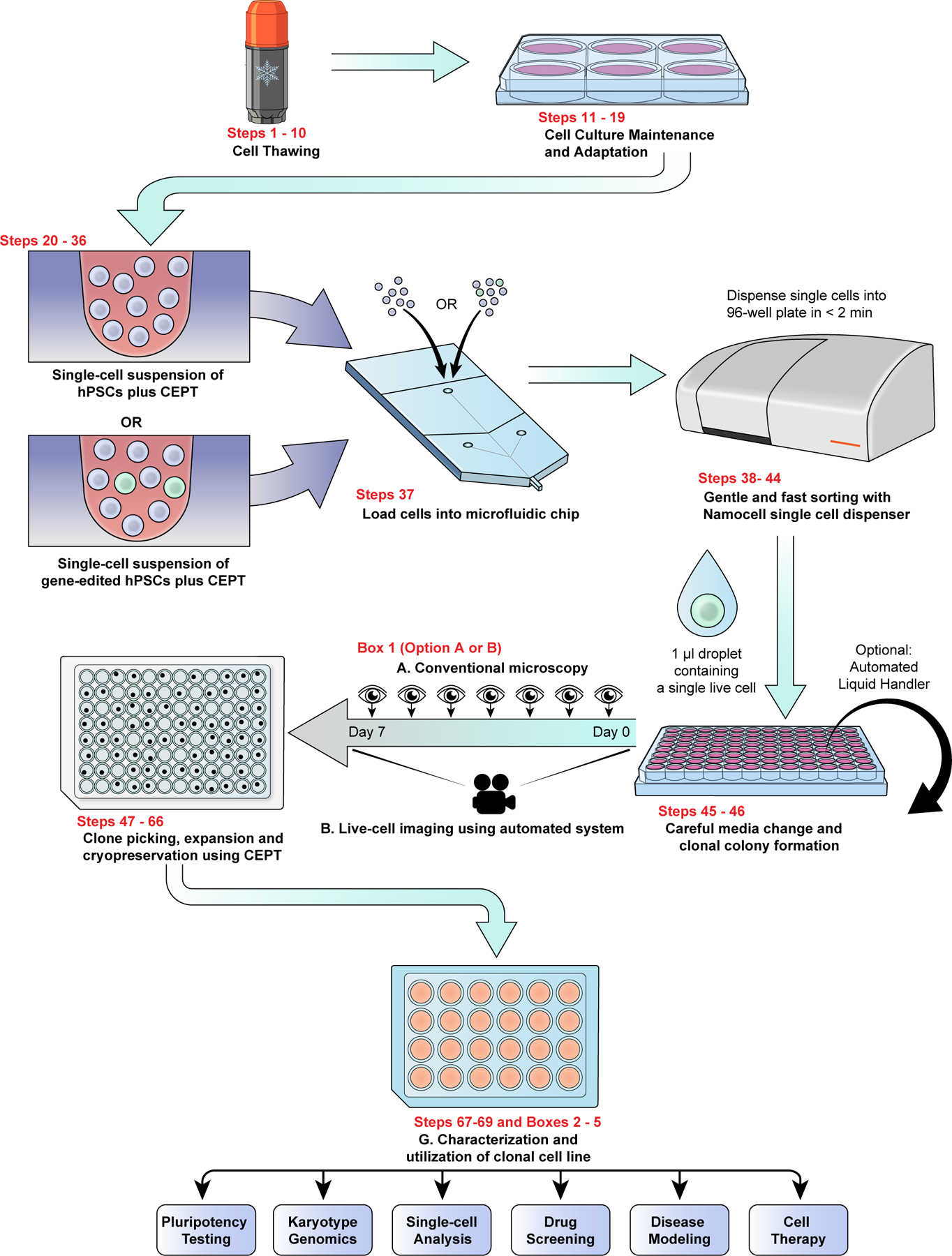
Combined use of the CEPT cocktail and microfluidic cell-dispensing ensures that single-cell cloning is safe, efficient, and practical. Overview of workflow depicting the various steps to perform streamlined single-cell cloning in high-throughput fashion followed by recommended cell characterization and indication of potential downstream applications.
Fig. 5. Live-cell staining and microfluidic single-cell dispensing of hPSCs.
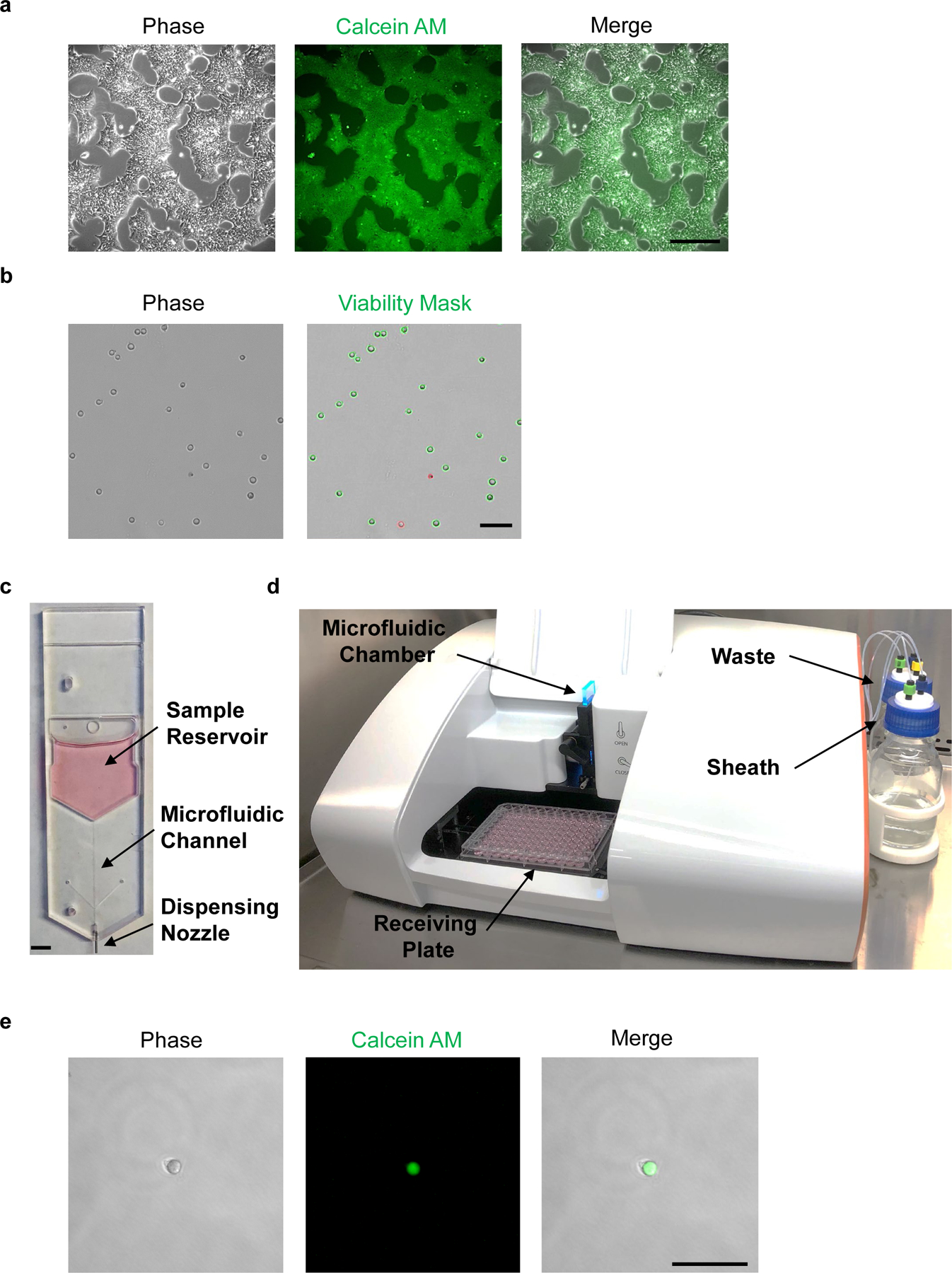
a, Phase-contrast image of hPSCs before Calcein AM staining (left panel). Fluorescence microscopic analysis of hPSCs at 1 h post-staining with Calcein AM (center and right panels). b, Cell suspension of dissociated hPSCs should avoid clumps and yield low frequency of doublets (left panel). Trypan blue exclusion test can be used to measure cell viability; live and dead cells are shown with green or red outline, respectively (right panel). c, Disposable microfluidic cell cartridge depicting sample reservoir, microfluidic channel, and dispensing nozzle. d, Hana (Namocell) single-cell dispenser with microfluidic chamber, receiving plate, waste and sheath bottles. e, Fluorescence microscopic image of a single calcein-labeled hPSC after microfluidic dispensing in a 1 μl droplet. Scale bars, 400 µm (a), 200 µm (b and e) and 5 mm (c).
Box 2. Alkaline Phosphatase Staining.
Additional material required
StemAb Alkaline Phosphatase Staining Kit II (Stemgent, cat. no. 00–0055)
Reagent setup
DPBST
Dilute 5 μL of Tween 20 into 10 mL of DPBS for a final concentration of 0.05%. Mix well until Tween 20 is completely dissolved and store at room temperature up to 1 year.
AP Substrate Solution
Mix Solution A with Solution B at a 1:1 ration in a conical tube. Incubate for 2 min before adding Solution C so that the final ratio of Solutions A:B:C is 1:1:1. Use the AP solution within 30 min of preparation.
Procedure
Timing 3 days
AP Staining
Seed clonal cell lines (step 60) on rhLaminin-521 or VTN-N coated 96-well plates at density of 100,000 cells per mL in E8 medium supplemented with CEPT. Use 64 µL of medium per well.
Maintain culture with daily media changes for 3 days.
Aspirate cell culture medium and wash cells with DPBST.
-
Add Fix Solution (from the Alkaline Phosphatase Staining Kit) and incubate at RT for 2–5 min. Use 80 µL of Fix Solution per well of a 96-well plate.
CRITICAL STEP: Optimal fixation is between 2–5 min. Over fixation will decrease AP activity and staining.
Aspirate Fix Solution and wash cell twice with DPBST. Do not allow wells to dry.
Aspirate DPBST and add AP Substrate Solution. Use 80 µL of Substrate Solution per well of a 96-well plate, respectively. Do not allow wells to dry.
Incubate cells in the dark at RT for 20 min.
Stop reaction by aspirating AP Substrate Solution. Wash cells twice with DPBS.
Add enough DPBS to each well to cover the cells and prevent drying.
Confirm wells with AP positive clones by eye or using a widefield microscope. (Fig. 3e). The presence of AP will result in red or purple staining, while the absence will result in no stain.
Store plates at 4 °C for ≥ 2 weeks.
Box 4. QC clonal cell lines using TaqMan hPSC Scorecard.
Additional material required
6-well ultralow attachment (ULA) plates (Corning, cat. no. 3471)
Essential 6 Medium (Thermo Fisher Scientific, cat. no. A1516401)
RNeasy Plus Mini Kit (Qiagen, cat. no. 74134)
TaqMan hPSC Scorecard Kit (Thermo Fisher Scientific, cat. no. A15871)
Additional equipment required
Compatible RT-PCR instrument
Procedure
Preparation of embryoid bodies (EBs) for Scorecard analysis
Timing 7 d
-
1
Day 0: Aspirate medium from clonal cell line (step 60), gently wash with DPBS. Add 1 mL pre-warmed Accutase per well of a 6-well plate. Incubate plate at 37 °C, 5% CO2 for 10 min.
-
2
Gently detach cells by washing bottom of the plate with 1 mL DPBS and transfer into a 50 mL conical tube.
-
3
Dilute cell suspension with an additional 8 mL of DPBS for a total volume of 10 mL.
-
4
Centrifuge tube with cells (100 g, 1 min, RT), aspirate supernatant, and resuspend pellet with 2 mL of E6 Medium supplemented with CEPT. Using a 1 mL pipette equipped with a narrow-bore tip, gently triturate cell suspension up and down to break up any cell clumps.
-
5
Add 3 mL of E6 Medium supplemented with CEPT for a final cell suspension volume of 5 mL.
-
6
Strain cell suspension through a 15 μm cell strainer to remove residual cell clumps.
-
7
Take a 10 μL aliquot of cell suspension and mix with a 10 μL aliquot of trypan blue.
-
8
Verify single-cell suspension and count cells.
-
9
Remove 500,000 cells to use as undifferentiated control, transfer into a 1.5 mL Eppendorf tube, centrifuge at 200 g for 2 min, aspirate supernatant, PAUSEPOINT Store cell pellet at −20 °C until use.
-
10
Resuspend the remaining cells at a density of 1 million cells per mL in E6 Medium supplemented with CEPT and plate 2 mL of cell suspension into one well of a 6-well ULA plate.
-
11
Day 1–6.: Replace the medium with fresh, pre-warmed (37 °C) E6 Medium without CEPT. To replace medium, gently transfer EB suspension to a 15 mL conical tube, centrifuge at 200g for 1 min, gently aspirate spent medium, add fresh pre-warmed E6 Medium and return EB suspension to ULA plate. Repeat every other day thereafter.
-
12
Day 7: Harvest EBs by gently transferring the EB suspension to a 15 mL conical tube, centrifuge at 200g for 1 min, gently aspirate spent medium, resuspend EBs with DPBS using a wide bore tip and transfer into a 1.5 mL Eppendorf tube. Centrifuge at 200 g for 2 min, aspirate supernatant.
PAUSE POINT: If there is not enough time to extract RNA cell pellet can be store at −20 °C until use.
-
13
Extract RNA using the RNeasy Plus Mini Kit as suggested by manufacturer.
-
14
QC RNA and analyze samples using the TaqMan hPSC Scorecard Kit as suggested by manufacturer.
Box 5. QC clonal cell lines using directed differentiation.
Additional material required
Essential 6 Medium (Thermo Fisher Scientific, cat. no. A1516401)
DMEM/F12 (Thermo Fisher Scientific, cat. no. 1132033)
LDN-193189 (R&D Systems, cat. no. 6053)
A83–01 (Tocris, cat. no. 2939)
STEMdiff Mesoderm Induction Medium (STEMCELL Technologies, cat. no. 05220)
STEMdiff Definitive Endoderm Kit TeSR-E8 Optimized (STEMCELL Technologies, cat. no. 05115)
RIPA Lysis and Extraction Buffer (Thermo Fisher Scientific, cat. no. 89900)
Halt Protease and Phosphatase Inhibitor Cocktail (100X) (Thermo Fisher Scientific, cat. no. 78446)
BCA Protein Assay Kit (Thermo Fisher Scientific, cat. no. 23225)
Antibodies (See Table 1)
Additional equipment required
Sonicator (optional): e.g., Sonicator (QSonica)
Automated Western Blot Platform or Western Blot Equipment (optional): e.g., WES (ProteinSimple), Western blotting system (BioRad)
Reagent setup
LDN-193189 (10,000X) 10 mM stock solution
Dissolve 10 mg powder in 2.09 mL DMSO. Aliquot and store at −80 °C for up to 2 years.
A83–01 (10,000X) 20 mM stock solution
Dissolve 10 mg powder in 1.19 ml DMSO. Aliquot and store at −20 °C for ≥ 2 years.
Ectoderm differentiation medium
Supplement E6 Medium with LDN-193189 and A83–01 stock solutions to a final concentration of 100 nM and 2 µM, respectively. Prepare enough medium for the entire differentiation process and store at 4 °C for up to 10 days.
STEMdiff Mesoderm Induction Medium
Thaw medium at 4 °C overnight. Once thawed, use immediately or store at 4 °C for up to 1 month.
STEMdiff Definitive Endoderm Basal Medium
Thaw STEMdiff Definitive Endoderm Basal Medium at RT or overnight at 2 – 8 °C and mix thoroughly. Once thawed store at 2 – 8 °C for up to 2 months.
TeSR-E8 Pre-Differentiation Medium
Add cold (2 – 8 °C) STEMdiff Definitive Endoderm TeSR E8 Supplement to cold (2 – 8 °C) E8 Medium at a 1:20 dilution. TeSR-E8 Pre-Differentiation Medium can be stored at (2 – 8 °C) for up to 2 weeks.
Medium 1
Thaw STEMdiff Definitive Endoderm Supplement MR and CJ on ice and dilute MR and CJ supplements 1:100 in cold (2 – 8 °C) STEMdiff Endoderm Basal Medium. Use prepared Medium 1 immediately.
Medium 2
Thaw STEMdiff Definitive Endoderm Supplement CJ on ice and dilute 1:100 in cold (2 – 8 °C) STEMdiff Endoderm Basal Medium. Medium 2 can be stored at 2 – 8 °C for up to 1 week.
RIPA Lysis and Extraction Buffer
Add protease and phosphatase inhibitor cocktail to RIPA lysis buffer at a final concentration of 1X, keep on ice and use immediately
Procedure
Directed differentiation of clonal cell lines into ectoderm
Timing 8 d
Day 0: Dissociate clonal cell lines (step 64) using pre-warmed Accutase, as described in steps 26–29. Resuspend cells in E8 medium supplemented with CEPT, and plate at a density of 50,000 cells/cm2 onto VTN-N-coated 6-well plates (see Reagent Setup). Use 2 mL of E8 medium supplemented with CEPT per well of a 6-well plate.
Day 1–6. Every day, replace the medium with fresh, pre-warmed (37 °C) Ectoderm differentiation medium. Use 2 mL of Ectoderm differentiation medium per well of a 6-well plate.
- Day 7. Characterize cells. Either:
- Aspirate medium, rinse with DPBS. Add 1 mL of DPBS and harvest ectoderm cells by scraping cells down. Transfer cell suspension into a 1.5 mL Eppendorf tube, centrifuge (200 g, 2 min, RT) aspirate supernatant, and store cell pellet at - 20 °C until required for immunoblot analysis (see below).
- Fix cells and analyze by immunocytochemistry for expression of ectoderm marker PAX6 (See Box 3).
Directed differentiation of clonal cell lines into mesoderm
Timing 6 d
Day 0. Dissociate clonal cell lines (step 64) using pre-warmed Accutase, as described in steps 26–29. Resuspend cells in E8 medium supplemented with CEPT, and plate at a density of 50,000 cells/cm2 onto VTN-N-coated 6-well plates (see Reagent Setup). Use 2 mL of E8 medium supplemented with CEPT per well of a 6-well plate.
Day 1–4. Every day, replace the medium with fresh, pre-warmed (37 °C) STEMdiff mesoderm induction medium. Use 3 mL of mesoderm induction medium per well of a 6-well plate.
- Day 5. Characterize cells. Either:
- Aspirate medium, rinse wash with DPBS. Add 1 mL of DPBS and harvest mesoderm cells by scraping cells down. Transfer cell suspension into a 1.5 mL Eppendorf tube, centrifuge at 200 g for 2 min, aspirate supernatant, and store cell pellet at −20 °C until required for immunoblot analysis (see below).
- Fix cells and analyze by immunocytochemistry for expression of mesoderm marker Brachyury (See Box 3).
Directed differentiation of clonal cell lines into endoderm
Timing 7 d
Day 0. Dissociate clonal cell lines (step 64) using pre-warmed Accutase, as described in steps 26–29. Resuspend cells in E8 medium supplemented with CEPT, and plate at a density of 150,000 cells/cm2 onto VTN-N-coated 6-well plates. Use 2 mL of E8 medium supplemented with CEPT per well of a 6-well plate.
Every day, replace the medium with fresh E8 medium without CEPT until cells reach 50–60% confluency.
After reaching 50–60% confluency, switch to TeSR-E8 Pre-Differentiation medium for 24 h or until cells reach 70% confluency.
After reaching 70% confluency in TeSR-E8 Pre-Differentiation medium, remove medium, gently rinse with DPBS and dissociate cells using EDTA diluted to 0.5 mM in Ca2+ and Mg2+-free PBS for 10–15 min at 37 °C. Gently was cells down, transfer into a 15 mL conical tube, centrifuge at 200 g for 2 min, aspirate supernatant and resuspend in TeSR-E8 Pre-Differentiation medium supplemented with CEPT.
Plate cells at a density of 210,000 cells/cm2 onto VTN-N-coated 6-well plates (See Reagent Setup). Use 2 mL of TeSR-E8 Pre-Differentiation medium supplemented with CEPT per well of a 6-well plate.
Day 1. After 24 h, gently rinse cells with 1 mL of DMEM/F12 and replace medium with 2 mL of pre-warmed (37 °C) Medium 1.
Day 2–4. The next day and everyday thereafter, replace the medium with pre-warmed Medium 2.
- Day 5. Characterize cells. Either:
- Aspirate medium, rinse wash with DPBS. Add 1 mL of DPBS and harvest endoderm cells by scraping cells down. Transfer cell suspension into a 1.5 mL Eppendorf tube, centrifuge at 200 g for 2 min, aspirate supernatant, and store cell pellet at −20 °C until required for immunoblot analysis (see below).
- Fix cells and analyze by immunocytochemistry for expression of endoderm marker SOX17 (See Box 3).
Immunoblot analysis
Timing 1 d
Thaw cell pellets and resuspend in 200 µL of RIPA buffer with protease and phosphatase inhibitors.
Sonicate on ice for three 10 sec pulses at 40% output.
Quantify protein concentration for all cell lysates using the BCA Protein Assay Kit.
Analyze samples for expression of pluripotent and/or lineage specific markers using an automated or traditional western blot system. See Table 1 for suggested antibodies.
Applications and limitations
Optimal physico-chemical conditions are necessary for efficient and safe single-cell cloning. This is now possible by using fast and gentle microfluidic cell dispensing together with the CEPT cocktail. Clonal cell lines from hPSCs lines can be generated in a streamlined fashion. Typically, clones with significant colony size emerge within 7–10 days of plating single cells (Fig. 3d) and can be picked and expanded as a stably growing cell line in the presence of CEPT, applied for 24 h at each passage. Cloning efficiencies are consistently higher in comparison to the widely used ROCK inhibitor Y-27632 (10 µM) and CloneR (1X) (Fig. 3a,c). By using the CEPT cocktail, we previously validated this protocol with multiple cell lines (WA01, WA07, WA09, LiPSC-GR1.1 and GM25256) and observed cloning efficiencies up to 80% for robust cell lines21.
However, we also noted cell-line-to-cell-line variability regarding clonogenicity, suggesting that some cell lines are inherently more sensitive to single-cell dissociation and cloning experiments. In such cases, cloning efficiencies were as low as 20% but still higher in the presence of CEPT when compared Y-27632 (10 µM) and CloneR (1X). Low cloning efficiency in such cell lines might be attributed to slower proliferation rate, enhanced or hyperactive stress response, and other unknown mechanisms that warrant future investigation. Lastly, it is worth emphasizing that the use of chemically-defined conditions, as exemplified here, should enable the seamless transition to current good manufacturing practice (cGMP) applications.
Experimental design
Before using this protocol, it is recommended that the cell line of interest should be adapted to routine cell passaging with the CEPT cocktail21. Irrespective of enzymatic or enzyme-free cell passaging, the cell line should be exposed to the CEPT cocktail for 24 h at each passage for a minimum of 7 passages to improve the overall quality of the cell line. Stress-free passaging ensures robust cell growth, increased cell numbers at each passage, and makes culturing and differentiating hPSCs more predictable. In addition, to increased experimental reproducibility, it is recommended to use chemically defined media (e.g., E8 Medium, mTeSR), while MEF feeders, and less defined media should be avoided.
rhLaminin-521 or VTN-N coating can be used for regular maintenance of hPSCs in E8 Medium. However, given the lean nature of E8 Medium, we find that adaptation of hPSC cultures to StemFlex Medium 24 h prior to single-cell dissociation and dispensing of single cells into StemFlex Medium supplemented with CEPT further improves cloning efficiency. Although CEPT also increases survival of single-cells in E8 Medium, due to the lean nature of E8 Medium it is not recommended for initial establishment of single-cell cultures. StemFlex Medium supplemented with CEPT allows for superior clonal expansion when compared to E8 Medium. Similarly, when compared to VTN-N, rhLaminin-521 coating better supports single-cell cloning. Therefore, we recommend the combination of CEPT, StemFlex and rhLaminin-521 for optimal single-cell cloning experiments.
Single-cell sorting with traditional FACS sorters can result in mechanical cell damage associated with shear forces depending on the sorting pressure and nozzle size that decrease viability and reduce single-cell cloning efficiency even in the presence of CEPT or Y-27632. The present protocol describes efficient, scalable single-cell cloning using Namocell’s Hana microfluidic platform that minimizes mechanical cell damage by using low dispensing pressure (≤ 2 psi) in combination with the cytoprotective CEPT cocktail (Fig. 4). However, this protocol can be easily adopted by laboratories using other platforms and FACS-sorting (Fig. 3a) and improvement in cloning efficiency should be detectable after using the CEPT cocktail irrespective of the cell sorting method.
Furthermore, to ensure that only viable single cells are dispensed into each well, we recommend staining hPSC cultures with Calcein AM, 1 to 2 hours prior to single cell dissociation (Fig. 5). The Calcein AM concentration recommended in this protocol is compatible with many cell sorting platforms, as it allows for gating of viable Calcein AM positive cells without any cytotoxic effects. To avoid mechanical stress that occurs during media change, single cells growing in a single well can be exposed to StemFlex and CEPT for 72 h followed by a half medium change. This approach avoids physical stress within the first three days.
The choice of plate format for plating single cells depends on user preference, however, 96-well plates are easier to handle and media change, while 384-well plates allow for higher throughput. Imaging within 2 hours after single cell plating and every 6–12 hours thereafter is critical to document and ensure that clones are derived from single cells. However, some automated imaging systems (e.g., Incucyte from Sartorius) generate heat during the imaging process that result in high temperature fluctuations when imaging an entire 96- or 384-well plate. Stem cells plated as single cells are very sensitive to temperature fluctuations and may die during repeated imaging. To reduce these temperature fluctuations, reduce the imaging frequency and/or scan entire plates in sectioned intervals.
Pluripotency after single cell cloning is first confirmed via live cell TRA-1–60 staining (Step 47). After this, quality control of derived clonal cell lines can include karyotype analysis, whole-exome sequencing, and various standardized assays to measure pluripotent differentiation potential (e.g., embryoid bodies and directed differentiation in monolayer cultures) (Fig. 6). Here we used karyotype and whole exome sequencing analysis to demonstrate that clonal cell lines derived by this method are karyotypically normal and remain free of widespread genetic abnormalities or cancer-causing mutations. Furthermore, we suggest a colorimetric assay to measure the presence of pluripotency marker, alkaline phosphatase (Box 2), immunocytochemistry to establish expression of OCT4, SOX2 and Nanog (Box 3), the TaqMan hPSC Scorecard for verification of multi-lineage differentiation potential (Box 4) and directed multi-lineage differentiation protocols (Box 5) for additional quality control analysis of derived clonal cell lines.
Fig. 6: Karyotype analysis, whole-exome sequencing, and multi-lineage differentiation of clonal cell lines.
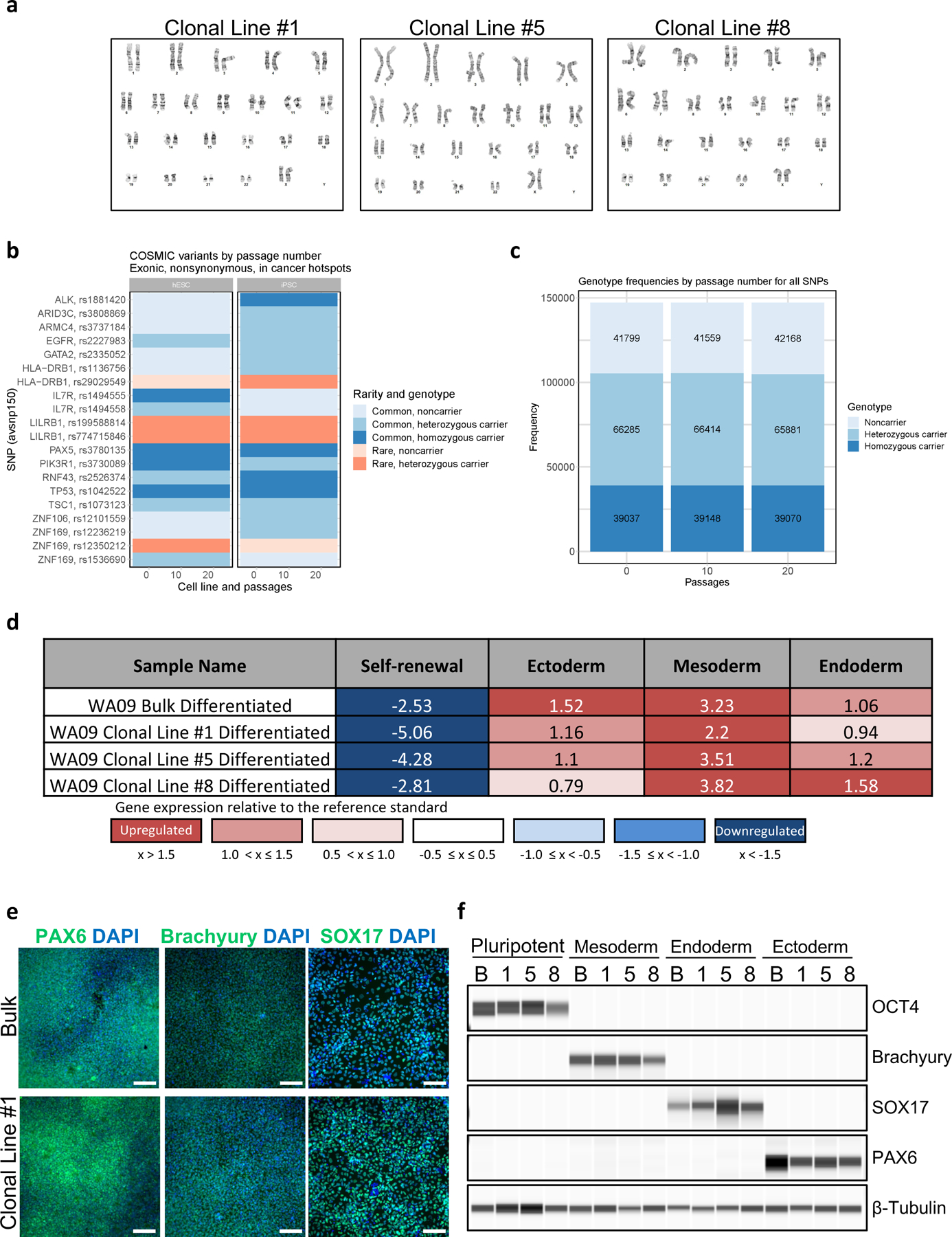
a, Three representative examples of clonal cell lines (WA09) that maintained normal karyotypes after 32 passages. Single-cell cloning, clone-picking, cell line establishment, cell expansion, and routine passaging were performed by using the CEPT cocktail. b, WES variant, genotype, and CNV analysis show genetic stability of WA09 and LiPSC-GR1.1 passaged with CEPT for 20 passages. COSMIC variants by passage number in cancer hotspots show no change in rarity or genotype over passaging. c, Genotype frequencies in WA09 cells by passage number for all SNPs are proportionally constant. Figures b and c were adapted with permission from ref. 21. d, EB differentiation potential of non-clonal parental line (WA09) and derived clonal cell lines measured by using Scorecard analysis. e, f, Immunocytochemistry and immunoblot analysis of non-clonal parental (N) and derived clonal cell lines (1, 5, and 8) after directed differentiation into ectoderm, mesoderm, and endoderm. Scale bar, 200 µm.
Box 3. Immunocytochemical analysis of pluripotency-associated markers in clonal cell lines.
Additional material required
Paraformaldehyde (Electron Microscopy Sciences, cat. no. 15710)
CAUTION: Paraformaldehyde can be toxic upon ingestion, inhalation or contact with skin. Handle using appropriate safety precautions.
Triton X-100 (ThermoFisher Scientific, cat. no. 85111)
Donkey Serum (Sigma, cat. no. D9663)
Antibodies (see Table 1)
Hoechst 33342 (ThermoFisher Scientific, cat. no. H3570)
Reagent setup
4% (wt/vol) Paraformaldehyde
Dilute 16% (wt/vol) PFA to 4% (wt/vol) PFA using DPBS. Use only freshly prepared 4% (wt/vol) PFA.
Donkey Serum
Thaw donkey serum at 4 °C overnight. Mix thawed donkey serum, aliquot and store at - 20 °C until use.
Permeabilization and Blocking Solution
Prepare a DPBS solution containing 5% (vol/vol) donkey serum and 0.1% (vol/vol) Triton X-100. Filter using Bottle-top Vacuum Filter System. Use within 1 month. Store at 4 °C.
Procedure
Timing 6 d
Preparation for Immunocytochemistry
-
1
Day 0: Seed clonal cell lines (step 60) on rhLaminin-521 or VTN-N coated 6-well plates at density of 100,000 cells per mL in E8 medium supplemented with CEPT. Use 2 mL of medium per well of a 6-well plate.
-
2
Day 1–2: Aspirate medium and replace with E8 media without CEPT. Use 2 mL of medium per well of a 6-well plate.
Fixing cells
-
3
Day 3: Remove medium from the well, and gently wash the cells with DPBS.
-
4
Remove the PBS and fix the cells using 4% (wt/vol) PFA solution for 10 min at RT.
-
5
After 10 min, remove the PFA and gently wash the cells three times with DPBS.
PAUSE POINT: If there is not enough time to stain the cells, the plates can be sealed using Parafilm to avoid desiccation and stored at 4 °C until use.
Immunocytochemistry
-
6
Remove DPBS from the wells, and permeabilize the cells with permeabilization and blocking solution for 1 h at RT.
-
7
Meanwhile dilute the primary antibodies in the permeabilization and blocking solution. Information on suggested antibody dilution is indicated in Table 1.
-
8
After 1 h, remove the permeabilization and blocking solution and add enough diluted primary antibody to cover the cells. Incubate at 4 °C overnight with gentle shaking.
-
9
Remove antibody solution and wash three times using DPBS.
-
10
Dilute the secondary antibodies in the permeabilization and blocking solution.
-
11
Information on suggested antibody dilution is indicated in Table 1.
-
12
After washing three times, add enough secondary antibody solution to cover the cells. Incubate at RT with gentle shaking for 2 hours in the dark.
-
13
Dilute Hoechst to 1 µg/mL using DPBS.
-
14
After 2 h, remove secondary antibody solution and add enough prepared Hoechst solution to cover the cells. Incubate at RT with gentle shaking for 10 min in the dark.
-
15
After 10 min, wash three times using DPBS.
-
16
Seal plates using Parafilm to avoid desiccation, wrap plates with foil to prevent photobleaching and stored at 4 °C until imaged with fluorescence microscope.
MATERIALS
Biological materials
Various NIH-approved hESC and hiPSC lines. Cell lines were tested and maintained under mycoplasma-free conditions.
WA01 (WAe001-A) (https://scicrunch.org/resolver/RRID:CVCL_9771)
WA07 (WAe007-A) (https://scicrunch.org/resolver/CVCL_9772)
WA09 (WAe009-A) (https://scicrunch.org/resolver/CVCL_9773)
LiPSC-GR1.1 (RUCDRi002-A) (https://scicrunch.org/resolver/CVCL_RH35)
JHU0781 (https://scicrunch.org/resolver/CVCL_JT73)
GM25256 (UCSFi001-A) (https://scicrunch.org/resolver/CVCL_Y803)
CAUTION: Before working with human cell lines, ensure that all national regulations are followed, and that required institutional ethics and review board approval is obtained and adhered to. NIH-supported research with human pluripotent stem cells is conducted under the terms of the NIH Guidelines for Human Stem Cell Research. Embryonic stem cells used in this study were eligible for use in NIH funded research and listed on the NIH Human Embryonic Stem Cell Registry.
CAUTION: The cell lines used in your research should be analyzed regularly to ensure authenticity and that they are not infected with mycoplasma.
Reagents
-
Essential 8 (E8) Medium (Thermo Fisher, cat. no. A1517001)
CAUTION: Thaw E8 supplement for 1–2 h at RT or overnight at 2–8°C. Do not thaw supplement at 37°C.
-
StemFlex Medium (Thermo Fisher, cat. no. A3349401)
CAUTION: Thaw StemFlex supplement for 1–2 h at RT or overnight at 2–8°C. Do not thaw supplement at 37°C.
Recombinant Human Laminin 521 (rhLaminin-521) (Thermo Fisher, cat. no. A29249)
Vitronectin (VTN-N) Recombinant Human Protein, Truncated (Thermo Fisher, cat. no. A31804)
DPBS with Calcium and Magnesium (Thermo Fisher, cat. no. 14040133)
DPBS without Calcium and Magnesium (Thermo Fisher, cat. no. 14190144) CRITICAL: Unless otherwise specified DPBS refers to DPBS without Calcium and Magnesium.
-
Accutase (Thermo Fisher, cat. no. 00–4555-56)
CRITICAL: Thaw frozen Accutase for 1–2 h at room temperature (RT; 19–22°C) or overnight at 2–8°C. Do not thaw at 37°C. Thawed Accutase can be stored at 2–8°C for up to 2 months.
UltraPure 0.5M EDTA (Thermo Fisher, cat. no. 15575020)
pluriStrainer 15 μm (pluriSelect, cat. no. 43–50015-03)
Calcein AM (Thermo Fisher, cat. no. C3099)
Trypan Blue Solution 0.4% (Thermo Fisher, cat. no. 15250061)
-
Chroman 1 (MedChem Express, cat. no. HY-15392)
CRITICAL: Since we established Chroman 1 as a potent and selective ROCK-pathway inhibitor, we do not recommend using an alternative type of ROCK inhibitor.
Emricasan (SelleckChem, cat. no. S7775)
Polyamine Supplement (Sigma-Aldrich, cat. no. P8483)
Trans-ISRIB (R&D Systems, cat. no. 5284)
Tween 20 (Sigma-Aldrich, cat. no. P1379)
TRA-1–60 Alexa Fluor 488 Conjugate Kit (Thermo Fisher Scientific, cat. no. A25618)
FluoroBrite DMEM (Thermo Fisher Scientific, cat. no. A1896701)
Bottle-top Vacuum Filter System (Sigma-Aldrich, cat. no. CLS431096)
-
Dimethylsulfoxide (DMSO) (ATCC, cat. no. 4-X)
CAUTION: Wear protective gloves and protective eyewear.
Wide-bore pipette tips (Rainin, cat. no. 30389218)
Narrow-bore pipette tips (Rainin, cat. no. 30389232)
CoolCell Freezing Containers (Biocision, cat. no. BCS-170)
Cryovials (Brooks Life Sciences, cat. no. 65–7661)
Cell culture plates 6, 12, 24, 48, 96-well plates (Corning, cat. no. 3506, 3512, 3524, 3548, 3596)
Cell culture plate 384-well (Greiner, cat. no. 781091)
Equipment
Cell culture incubator: e.g., Thermo Scientific Heracell VIOS 160i
Benchtop centrifuge: e.g., Eppendorf Centrifuge 5810R
BSL2 biosafety cabinet: e.g., NuAire 6’ Class II Type A2
Fluorescence cell counter: e.g., Nexcelom Bioscience Cellometer Auto 2000
Fluorescence cell counter chambers: e.g., Nexcelom Bioscience Cellometer Disposable Counting Chambers
37°C bead bath: e.g., Fisherbrand Isotemp bath with Lab Armor beads
Cell Thawing System: e.g., BioCision ThawSTAR Automated Cell Thawing System
Thermomixer: e.g., Eppendorf Thermomixer C
Fluorescence microscope: e.g., Leica DMi8 microscope
Single-cell sorter and dispenser: e.g., Hana (Namocell), SCP (Cytena), FACSAria lll (BD Biosciences)
Time-lapse imaging systems (optional): e.g., Incucyte ZOOM system (Sartorius), 3D Cell Explorer (Nanolive)
Automated liquid handler (optional): e.g., Biomek i7 Automated Workstation (Beckman Coulter), VIAFLO 96/384 (Integra)
Reagent setup
Coating cell culture plates with recombinant human laminin-521 (rhLaminin-521)
-
1
Slowly thaw one vial of rhLaminin-521 on ice or at 2–8 °C.
-
2
Once thawed, dilute 1:40 in DPBS containing calcium and magnesium and gently mix by pipetting up and down. Dilution will result in a working concentration of 2.5 μg/mL.
CRITICAL STEP: Ensure DPBS containing calcium and magnesium is used when coating plates with LN521.
-
3
Coat plate using a final coating concentration of 0.5 μg/cm2 (volumes for different culture plates are given in the table below). Sensitive cell lines may benefit from a higher concentration such as 1 µg/cm2.
| Culture Plate (Surface Area) | rhLaminin-521 working solution (2.5 μg/ml) to use per well |
|---|---|
| 6-well plate (9.6 cm2) | 2 mL |
| 12-well plate (3.8 cm2) | 0.8 mL |
| 24-well plate (1.9 cm2) | 0.4 mL |
| 48-well plate (1 cm2) | 0.2 mL |
| 96-well plate (0.32 cm2) | 0.064 mL |
| 384-well plate (0.056 cm) | 0.010 mL |
-
4
Incubate at 37 °C and 5% CO2 for 2 h to overnight. Once coated, the plate can be stored at 2–4 °C, sealed with parafilm, for up to one week.
-
5
Prior to use, pre-warm plate at 37 °C and 5% CO2 for at least 30 min.
Coating cell culture plates with recombinant human vitronectin (VTN-N)
-
1
Thaw one vial of VTN-N at room temperature.
-
2
Once thawed, dilute 1:100 in DPBS without calcium and magnesium and gently mix by pipetting up and down. Dilution will result in a working concentration of 5 μg/mL.
CRITICAL STEP: Ensure DPBS without calcium and magnesium is used when coating plates with VTN-N.
-
3
Coat plates using a final coating concentration of 0.5 μg/cm2 (volumes for different culture plates are given in the table below). Sensitive cell lines may benefit from a higher concentration such as 1 µg/cm2.
| Culture Plate (Surface Area) | VTN-N working solution (5 μg/ml) to use per well |
|---|---|
| 6-well plate (9.6 cm2) | 1 mL |
| 12-well plate (3.8 cm2) | 0.4 mL |
| 24-well plate (1.9 cm2) | 0.2 mL |
| 48-well plate (1 cm2) | 0.1 mL |
| 96-well plate (0.32 cm2) | 0.032 mL |
| 384-well plate (0.056 cm) | 0.005 mL |
-
4
Incubate at 37 °C and 5% CO2 for 1 h to overnight. Once coated, the plate can be stored at 2–4 °C, sealed with parafilm, for up to one week.
-
5
Prior to use, pre-warm plate at 37 °C and 5% CO2 for at least 30 min.
E8 Medium
Thaw E8 supplement for 1–2 h at RT or overnight at 2–8°C. Do not thaw supplement at 37°C. Once thawed, add supplement to basal medium according to manufacturer’s instructions. Complete E8 Medium with supplements can be stored at 2–8°C for up to 2 weeks.
StemFlex Medium
Thaw StemFlex supplement for 1–2 h at RT or overnight at 2–8°C. Do not thaw supplement at 37°C. Once thawed, add supplement to basal medium according to manufacturer’s instructions. Complete StemFlex medium with supplements can be stored at 2–8°C for up to 2 weeks.
Chroman 1 (10,000X), 0.5 mM stock solution
Add 22.91 mL of DMSO to 5 mg of chroman 1. Store at 4 °C for up to a month or 20 °C for up to 1 year.
Emricasan (10,000X), 50 mM stock solution
Add 175.6 µL of DMSO to 5 mg of emricasan. Vortex until completely dissolved. Store at 4 °C for up to a month or 20 °C for up to 1 year.
Trans-ISRIB (10,000X), 7 mM stock solution
Add 3.165 mL of DMSO to 10 mg of trans-ISRIB. Gently warm at 37–70 °C and vortex until completely dissolved. Store at 4 °C for up to a month or 20 °C for up to 1 year. If precipitates form, warm at 67 °C on a thermomixer to dissolve precipitate prior to use.
E8 Medium supplemented with CEPT
Add chroman 1, emricasan, and trans-ISRIB at 1:10,000 to E8 Medium. Add polyamine supplement at 1:1,000 to E8 Medium. Final concentrations; chroman 1 (50 nM), emricasan (5 μM), polyamine supplement (1X) and trans-ISRIB (0.7 μM). Sterile filter the prepared medium using a bottle-top vacuum filtration system. Prepared medium can be stored at 4 °C for up to 2 weeks.
CRITICAL: The CEPT cocktail provides comprehensive cytoprotection from various forms of cellular stress. It is recommended that all components of the CEPT cocktail are used for optimal outcome.
StemFlex Medium supplemented with CEPT
Add chroman 1, emricasan, and trans-ISRIB at 1:10,000 to StemFlex Medium. Add polyamine supplement at 1:1,000 to StemFlex Medium. Final concentrations; chroman 1 (50 nM), emricasan (5 μM), polyamine supplement (1X) and trans-ISRIB (0.7 μM). Sterile filter the prepared medium using a bottle-top vacuum filtration system. Prepared medium can be stored at 4 °C for up to 2 weeks.
CRITICAL: The CEPT cocktail provides comprehensive cytoprotection from various forms of cellular stress. It is recommended that all components of the CEPT cocktail are used for optimal outcome.
0.5 mM EDTA
Dilute UltraPure 0.5 M EDTA to 0.5 mM in DPBS. Store at RT for up to 1 month.
E8 Medium Freezing Solution
Add DMSO in E8 Medium at a final concentration of 10% (vol/vol). Then supplement with CEPT to get the following final concentrations: chroman 1 (50 nM), 22mricasan (5 μM), polyamine supplement (1X), and trans-ISRIB (0.7 μM). Sterile-filter E8 Medium Freezing Solution using a bottle-top vacuum filtration system.
Procedure
Thawing hPSCs
Timing 12.5 h
-
1
Coat a cell culture plate with rhLaminin-521 or VTN-N as described in the Reagent Setup section. Our laboratory generally maintains cell cultures on VTN-N coated 6-well plates with 2 mL of cell culture medium per well.
-
2
Once coated plates are ready, place cryovial in an automated cell thawing system until thawed. Alternatively, place the cryovial in a 37 °C bead bath until thawed.
-
3
Using a 1 mL pipette equipped with a wide-bore tip, gently transfer cells into a 50 mL conical tube.
-
4
Add 1 mL of pre-warmed (37 °C) E8 Medium supplemented with CEPT dropwise at a rate of 1 drop every 2 seconds. Then slowly and dropwise add an additional 3 mL of pre-warmed (37 °C) E8 Medium supplemented with CEPT at a rate of 2 drops every second for a total volume of 5 mL.
CRITICAL STEP: Dropwise addition of cell culture media minimizes osmotic shock on the thawed cells.
-
5
Centrifuge tube with cells (200 g, 3 min, RT) aspirate supernatant, and resuspend pellet with 2 mL of pre-warmed (37 °C) E8 Medium supplemented with CEPT.
-
6
Take a 10 μL aliquot of cell suspension and mix with a 10 μL aliquot of trypan blue.
-
7
Count cells using an automated cell counter or haemocytometer and resuspend in pre-warmed (37 °C) E8 Medium supplemented with CEPT at a density of 150,000 cells/mL.
TROUBLESHOOTING
-
8
Remove coating solution, from rhLaminin-521 or VTN-N coated plates then plate 210 μL of cell suspension (150,000 cells/mL) per cm2 of plate surface area for a final plating density of 30,000 cells/cm2 in E8 Medium supplemented with CEPT.
-
9
Gently rock plate to evenly distribute cells and place in a cell culture incubator at 37 °C, 5% CO2 for at least 12h.
-
10
10. 12–24 h post-plating, aspirate medium and replace with fresh E8 Medium without CEPT, pre-warmed at 37°C. Use 210 µL of media per cm2 of plate surface area.
CRITICAL STEP: Work quickly when replacing the rhLaminin-521 or VTN-N coating solution to avoid drying of the coated surface, otherwise, the performance of rhLaminin-521 or VTN-N will be compromised.
Routine maintenance of hPSCs
Timing 3–4 days
-
11
Replace medium daily with fresh E8 Medium without CEPT until cells reach 60–80% confluency at which point cells are ready to be passaged (Fig. 5a). Cells should be passaged at a 1:6 ratio every 3–4 days for routine maintenance. Use 210 µL of media per cm2 of plate surface area.
-
12
Before passaging, coat a cell culture plate with rhLaminin-521 or VTN-N as described in the Reagent Setup section.
-
13
To passage cells, first aspirate the spent E8 Medium. Wash the cells once with DPBS.
-
14
Aspirate DPBS and add 0.5 mM EDTA solution to the cells and incubate for 4 to 5 min at 37 °C for partial dissociation of the colonies. Use 100 µL of 0.5 mM EDTA solution per cm2 of plate surface area.
-
15
Aspirate the EDTA solution completely and use E8 Medium supplemented with CEPT to wash off and break up the colonies into small clumps. Use a 1 mL pipette equipped with a wide-bore tip or a serological pipette to break up colonies into smaller clumps (ideally clumps of 5 to 10 cells).
-
16
Transfer the clump suspension to the prepared rhLaminin-521 or VTN-N-coated plate.
-
17
Twelve to twenty-four hours post-passaging aspirate medium and replace with fresh E8 Medium without CEPT for at least 2 days before subsequent passages or preparation for single-cell dissociation. Use 210 µL of medium per cm2 of plate surface area.
TROUBLESHOOTING
-
18
Maintain cell culture by repeating steps 11 – 17, until ready to prepare cells for single-cell dissociation.
Preparation of hPSCs for Single-Cell Dissociation
Timing 1–2 days
-
19
Twenty-four to forty-eight hours prior to single cell dissociation aspirate E8 Medium and replace with StemFlex Medium. Use 210 µL of media per cm2 of plate growth surface area.
Calcein AM staining of hPSCs
Timing ≤ 2 h
-
20
Prepare a 2x working solution of Calcein AM Staining Medium by diluting Calcein AM to 1:25,000 (40 nM) in StemFlex Medium without CEPT.
-
21
One to two hours before single-cell dissociation, add Calcein AM Staining Medium at a 1:1 ratio to the existing cell culture medium and gently shake the plate to mix. The final dilution of Calcein AM is 1:50,000 (20 nM).
CRITICAL STEP: Dilution of Calcein AM at 1:50,000 (20 nM) stains hPSCs cultures within 1–2 h in the absence of cytotoxicity.
-
22
Incubate in a cell culture incubator at 37°C, 5% CO2 for 1 h. Use a fluorescence microscope to confirm that Calcein AM fluorescence has labelled the vast majority of cells. If the signal is weak, incubate for an additional hour.
TROUBLESHOOTING
-
23
Once cell labeling with Calcein AM is confirmed, proceed to single-cell dissociation (Fig. 5a).
Single-cell dissociation of hPSCs
Timing ≤ 10 min
-
24
Pre-warm Accutase to room temperature for 30 min.
-
25
Aspirate medium then gently wash cells with DPBS.
-
26
Add 100 μL of pre-warmed (37 °C) Accutase per cm2 of plate surface area; incubate plate at 37 °C, 5% CO2 for 10 min. Most cells will detach after the 10 min incubation.
-
27
Gently collect cell suspension and recover additional cells by washing bottom of the plate with 100 µL of DPBS per cm2 of plate surface area and combine into a 50 mL conical tube.
-
28
Add an additional 800 µL of DPBS per cm2 surface area of source plate to further dilute the cell suspension.
-
29
Centrifuge tube with cell suspension (100 g, 1 min, RT), aspirate supernatant, and resuspend pellet with 200 µL of StemFlex Medium supplemented with CEPT per cm2 surface area of source plate. Using a 1 mL pipette equipped with a narrow-bore tip, gently triturate cell suspension up and down to break up any cell clumps.
CRITICAL STEP: Centrifugation at 100 g for 1 min reduces cell aggregation and helps maintain a single-cell suspension. With higher centrifugation speeds and times cells tend to re-aggregate or clump.
-
30
Add 300 µL of StemFlex Medium supplemented with CEPT per cm2 surface area of source plate.
-
31
Strain cell suspension through a 15 μm cell strainer to remove residual cell clumps.
-
32
Take a 10 µL aliquot of cell suspension and mix with a 10 µL of trypan blue.
-
33
Verify single-cell suspension and count cells (Fig. 5b).
TROUBLESHOOTING
Dispensing single cells into 96- or 384-well plates
Timing 2–3 min
-
34
Prepare a rhLaminin-521 coated 96- or 384-well plate as described in Reagent Setup.
CRITICAL STEP: For optimal cloning efficiency, rhLaminin-521 coating is recommended at this step. VTN-N coating can also be used, but cloning efficiency may be reduced when compared to rhLaminin-521.
-
35
Aspirate the coating solution using a multi-channel aspirator and immediately add StemFlex Medium supplemented with CEPT. Add 100 μL or 50 μL per well of a 96- or 384-well plate, respectively. Do not allow the plate to dry.
-
36
Dilute cells in StemFlex Medium supplemented with CEPT to a concentration of 5,000 cells/mL.
-
37
Load 750 μL of cell suspension into Hana (Namocell) microfluidic chip sample reservoir (Fig. 5c).
-
38
Load microfluidic chip onto the cell dispenser (Fig. 5d) and run analysis function on the Hana (Namocell) Single Cell Dispenser System to ensure a flow rate of 1.6–2.0 cells/s.
CRITICAL STEP: Flow rates ≥2.0 cells/s will result in a higher rate of droplets with more than one cell per droplet. Flow rates ≤1.0 cells/s will result in longer dispense times per plate. For a 96-well plate the ideal dispense time per plate is 2–3 min.
-
39
In the Hana (Namocell) software, set trigger to FITC and set gates to SSCH: 400, SSCL: 25, FSCH: 450, FSCL: 50, FITCH: 1000, FITCL: 25, PEH: 4000 and PEL: 0.
-
40
Load a 96- or 384-well plate with the lid onto the plate stage of the Hana (Namocell) Single Cell Dispenser. In the Hana (Namocell) software select 10 wells and dispense onto the clean 96- or 384-well plate lid.
-
41
Inspect the droplets dispensed onto the clean lid under a fluorescence microscope and confirm that only a single Calcein AM positive cell is present in each droplet (Fig. 5e).
TROUBLESHOOTING
-
42
In the Hana (Namocell) software select recipient wells and dispense single cells into rhLaminin-521 or VTN-N coated 96- or 384-well plates with StemFlex Medium supplemented with CEPT. Ensure plate lid is removed from plate at this step.
-
43
Immediately after cell dispensing, replace plate lid, and centrifuge plate (100 g, 1 min, RT) to accelerate settlement of cells to bottom of cell culture plate.
-
44
Incubate in a cell culture incubator at 37 °C, 5% CO2.
Maintaining single-cell cultures in 96- or 384-well plates
Timing 7–14 days
CRITICAL: Automated liquid-handling workstations are recommended as they will facilitate and increase throughput when handling multiple plates.
CRITICAL: Imaging within 2 hours after plating cells and every 6–12 hours thereafter is critical to document and ensure clones are derived from a single cell.
-
45
Day 0–3: On each of the first 3 days (recovery period), add 50 or 100 µL per well of StemFlex Medium without CEPT to 96- or 384-well plates, respectively. No media removal is required during the recovery period. Image cells as described in Box 1.
CRITICAL STEP: Addition of 50–100 µL per well of StemFlex Medium without CEPT during the first 3 days is used to feed cells and dilute CEPT. Prolonged exposure to CEPT during the recovery period does not affect pluripotency (Figs. 3e, f and 6).
-
46
Day 4–14: Replace half of the medium volume in each well with StemFlex Medium without CEPT. Daily half-medium changes will gradually dilute and remove CEPT through day 14.
CRITICAL STEP: When performing media changes, ensure that cell cultures are not disturbed by avoiding contact with the bottom of the well. Gently aspirate and dispense media.
Box 1. Imaging single-cell cultures in 96- or 384-well plates.
Timing ≤ 14 days
Image the single cell culture either using a manual (option A) or automated imaging platform (option B).
A. Manual live-cell imaging at different timepoints
For microscopes equipped with a temperature, humidity, and CO2 controller, set to 37 °C, 95% humidity, and 5% CO2. Alternatively, work quickly to minimize the time cells are out of the cell culture incubator. A widefield microscope equipped with a 4X objective and camera is sufficient for documentation of single-cell derived cultures.
Place the cell culture plate on the microscope stage.
Using the 4X objective, focus on the bottom of the well, scan the entire well until a single cell is found and capture an image within 2 hours after plating. If possible, save coordinates for future imaging. Calcein AM staining can help visualize cells immediately after plating when using a fluorescence microscope.
Repeat manual imaging every 6–12 h for up to 14 days to monitor cell survival and clone formation (Fig. 3d).
Maintain media addition and half media change schedule as described in steps 45 and 46.
B. Automated, long-term time-lapse imaging
-
1
Prepare an automated time-lapse imaging system equipped with a temperature, humidity, and CO2 controller to maintain cells at 37 °C, 95% humidity, and 5% CO2.
-
2
Immediately after plating, place the cell culture plate in the automated imaging system.
-
3
Using a 4X objective, focus on the bottom of the well and capture images of the entire surface area of each well within 2 hours after plating.
CRITICAL STEP: Scanning at 4X allows for enough resolution to capture a single cell while reducing the scanning time.
-
4
Repeat imaging every 6 h for up to 14 days to monitor cell survival, clone formation and generate time course data (Fig. 3d).
-
5
Maintain media addition and half media change schedule as described in steps 45 and 46.
Validation of pluripotency after single-cell cloning
Timing ≤ 30 min
Single-cell clone establishment
-
47
To validate pluripotency after single-cell cloning using the TRA-1–60 Alexa Fluor-488 conjugate kit for Live Cell Imaging, add TRA-1–60 mouse anti-human, Alexa Fluor-488 conjugated monoclonal antibody directly into the cell culture medium of cells to be stained at a dilution of 1:50. Gently shake the plate to mix.
-
48
Incubate at 37 °C, 5% CO2 for 15–30 min.
-
49
Remove the staining solution and gently wash cells twice with FluoroBrite DMEM. Use 100 µL of FluoroBrite DMEM per cm2 of plate surface area.
-
50
Immediately image cells with a fluorescence microscope to identify pluripotent clones expressing cell surface pluripotency marker TRA-1–60.
Expansion of single-cell clones
Timing ≥ 3 days
-
51
Coat a 24-well plate with rhLaminin-521 as described in Reagent Setup section. Remove the coating solution and replace with StemFlex Medium supplemented with CEPT. Use 400 μL of StemFlex Media supplemented with CEPT per well of 24-well plate.
-
52
Prepare 0.5 mM EDTA Solution by diluting 0.5 M EDTA at 1:1000 in DPBS.
-
53
Aspirate cell culture medium from wells with TRA-1–60 positive clone from step 51 and rinse once with DPBS.
-
54
Replace cell culture media with 0.5 mM EDTA Solution and incubate at 37 °C, 5% CO2 for 5 min. Use 80 μL or 15 µL of 0.5 mM EDTA solution per well of a 96- or 384-well plates, respectively.
-
55
Aspirate EDTA Solution and gently detach cells by washing bottom of plate with StemFlex Medium supplemented with CEPT. Gently pipette up and down 5 times to dissociate cell clumps. Use 80 μL or 15 µL of StemFlex Medium Supplemented with CEPT per well of a 96- or 384-well plates, respectively.
-
56
Transfer entire volume of cell suspension from one well of the 96-well plate into an independent well of the rhLaminin-521-coated 24-well plate containing 400 µL of StemFlex Medium supplemented with CEPT. Pick as many single-cell clones as desired by repeating steps 54 – 57 for each clone.
-
57
Twelve to twenty-four hours post plating, aspirate medium and replace StemFlex with 400 µL of fresh E8 Medium without CEPT.
-
58
Change medium daily with fresh E8 Medium without CEPT until cells reach 60 – 80% confluency.
-
59
To further expand cell cultures, passage cells at a 1:6 ratio every 3–4 days as described in steps 11–17. Cells may also be transferred to 12- or 6-well plates to facilitate further expansion. Use E8 Medium supplemented with CEPT at every passage during the first 12–24 h post-passage, followed by daily media changes using E8 Medium without CEPT.
Cryopreservation of hPSCs
Timing 1 day
CRITICAL: The cryopreservation of stocks derived from expanded clones is recommended prior to their analysis, as some assays may take several days to complete.
-
60
Aspirate cell culture medium and rinse once with DPBS.
-
61
Add 0.5 mM EDTA Solution to cell culture and incubate at 37 °C, 5% CO2 for 5 min.
-
62
Aspirate EDTA Solution and gently detach cells by washing bottom of plate with 500 μL of E8 Medium Freezing Solution. Gently pipette up and down 5 times to dissociate cell clumps.
-
63
Take a 10 μL aliquot of cell suspension and mix with a 10 μL aliquot of trypan blue.
-
64
Count cells and resuspend in E8 Medium Freezing Solution at a density of 1–5 × 106 cells/mL.
-
65
Transfer 1 mL aliquots into 1.5 mL cryovials.
-
66
Transfer vials into a CoolCell freezing container and place at −80 °C for 24 h, then transfer vials to liquid nitrogen for long-term storage.
Analysis of clonal cell lines
CRITICAL: Clonal cell lines can be assayed through some or all the following approaches, according to the user’s preference.
Timing ≥ 30 min
- 67
-
68
Analyze clones for the pluripotency markers by staining for alkaline phosphatase (Box 2) and/or for the expression of typical pluripotency-associated markers such as, OCT4, NANOG, and SOX2 (Box 3) (Fig. 3e,f).
-
69
Assay for pluripotency and cell differentiation potential by generating embryoid bodies and analyzing using hPSC Scorecard analysis (Box 4) or differentiating cells into ectoderm, mesoderm or endoderm and immunoblotting with antibodies against cell type-specific markers (Box 5) (Fig. 6d-f).
TIMING
Steps 1–10, thawing and plating cells: 12.5 h
Steps 11–19, routine maintenance of hPSCs and adaptation: 3–4 days
Steps 20–23, Calcein AM staining: ≤ 2 h
Steps 24–33, single-cell dissociation: ≤ 10 min
Steps 34–44, singe-cell dispensing: 2–3 min per plate
Steps 45–46, maintaining single-cell cultures: 7–14 days
Steps 47–50, validation of pluripotency: ≤ 30 min
Steps 51–59, expansion of single cell clones: ≥ 3 days
Steps 60–66, cryopreservation of hPSCs: 1 day
Steps 67–69, analysis of clonal cell lines: ≥ 30 min
Box 1, imaging single-cell clones: ≤ 14 days
Box 2, alkaline phosphatase staining: 3 days
Box 3, immunocytochemical analysis pluripotency-associated markers in clonal cell lines: 6 days
Box 4, QC clonal cell lines using TaqMan hPSC Scorecard: 7 days
Box 5, QC clonal cell lines using directed differentiation: 6–9 days
Anticipated results
Single cells can be observed after cell attachment within 10–15 min post-plating and the first indication for successful clone formation is typically detectable after 2–3 days, depending on the sensitivity and proliferation rate of the parental cell line. Based on our experience, robust single-cell colonies can be established in 1–2 weeks from most cell lines. Supplementation of StemFlex Medium with CEPT provides comprehensive cytoprotection and increases single-cell cloning efficiencies up to 6-fold when compared to Y-27632 and up to 1.5-fold when compared to CloneR in the cell lines tested (Fig. 3c).
Single-cell clones established using CEPT remain undifferentiated as demonstrated by expression of alkaline phosphatase, OCT4, NANOG, and SOX2 (Fig. 3d-f) and show similar proliferation rates and display expected sensitivity to single-cell dissociation when compared to the parental cell line (Fig. 3g, h). Furthermore, clonal cell lines maintain normal karyotypes, exhibit pluripotent differentiation potential, and WES analysis can be used to demonstrate absence of chromosomal abnormalities, p53 mutations, and changes at cancer hotspots (Fig. 6a-f).
Limitations
Over the last several years we generated single-cell clones from various hESC and hiPSC lines and in all instances the protocol described here increased cloning efficiency. Indeed, clonogenicity was not only higher in comparison to the widely used ROCK inhibitor Y-27632, but also more efficient when compared to the commercially available CloneR reagent. We observed cloning efficiencies up to 80% for more robust cell lines. However, sensitive cell lines such as WA01 still had cloning efficiencies as low as 20%, although significantly improved when compared to Y-27632 (10 µM)21. We speculate that lower cloning efficiency in these cell lines could be attributed to slower proliferation rates, variability in activated cell signaling pathways that cause an exaggerated stress response during cell dissociation, and other variables33.
Troubleshooting
Troubleshooting advice can be found in Table 2.
Table 2.
Troubleshooting table
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 7 | Poor cell survival after thawing | Cell thawing process was too slow and/or cells were not plated in media supplemented with CEPT | Ensure cells are thawed within 5 min and plate in media supplemented with CEPT for maximum cell survival |
| 17 | Poor cell survival after passaging | Passaging induces several cell stress mechanisms that may reduce cell survival upon passaging | Use CEPT consistently at each passage to reduce cell stress and increase quality of cell line |
| 22 | Cells are not Calcein AM positive after 2h | Calcein AM concentration may be too low | Increase Calcein AM concentration to 1:25,000 (40 nM) and repeat steps 19–21 |
| 33 | Cell suspension has aggregates or clumps after straining | Cell culture was overgrown or too confluent (> 80%) at the time of dissociation. | Only use cell cultures that are ≤ 80% confluent |
| Remove cell clumps by centrifugation at 50 g for 30 seconds. Without disturbing cell pellet, take cell suspension supernatant from top. Supernatant will have fewer aggregates or clumps. | |||
| 41 | Dispensed droplets have multiple cells | Cell suspension is too dense (> 5,000 cells/mL) or flow rate is too high (≥ 2 cells/s) | Dilute cells suspension until flow rate is ≤ 2 cells/s |
| 47 | Low single-cell cloning efficiency | Cells were not adapted to StemFlex media prior to single-cell dissociation | Ensure cell culture is adapted to StemFlex media for at least 24 h prior to single-cell dissociation |
| Cells were aspirated during media changes | Aspirate media from side of well and avoid contact with the bottom of the well | ||
| CEPT was not added to StemFlex media | Ensure StemFlex single-cell plating media is supplemented with CEPT | ||
| 47 | No colonies | High temperatures generated during imaging killed cells | Reduce imaging intervals |
| Use alternative imaging platforms that do not generate too much heat during the imaging process |
Supplementary Material
Table 1.
Antibodies used for clonal cell line characterization
| Antibody | Manufacturer | Cat. No. | Dilution | RRID |
|---|---|---|---|---|
| Anti-OCT4 | Santa Cruz Biotechnology | SC-5279 | ICC:1:200, WB:1:50 | AB_628051 |
| Anti-SOX2 | R&D Systems | AF2018 | ICC:1:100, WB:1:50 | AB_355110 |
| Anti-NANOG | Cell Signaling Technology | 4903S | ICC:1:200, WB:1:50 | AB_10559205 |
| Anti-beta Tubulin | Novus | NB600–936 | WB:1:5000 | AB_10000656 |
| Anti-SOX17 | Cell Signaling Technology | 81778 | ICC:1:1000, WB:1:50 | AB_2650582 |
| Anti-PAX6 | Biolegend | 901301 | ICC:1:200, WB:1:50 | AB_2565003 |
| Anti-Brachyury | Cell Signaling Technology | 81694 | ICC:1:1000, WB:1:50 | AB_2799983 |
| Donkey anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 568 | Thermo Fisher Scientific | A10037 | ICC:1:1000 | AB_2534013 |
| Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Thermo Fisher Scientific | A21206 | ICC:1:1000 | AB_2535792 |
| Donkey anti-Goat IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 647 | Thermo Fisher Scientific | A32849 | ICC:1:1000 | AB_2762840 |
ACKNOWLEDGEMENTS
We are grateful for the support from the Regenerative Medicine Program (RMP) of the NIH Common Fund, NIH HEAL Initiative, and in part by the intramural research program of the National Center for Advancing Translational Sciences (NCATS), NIH. The funders had no role in study design, data collection and data analysis, decision to publish, or preparation of the manuscript. We are also thankful to the NIH Medical Arts Design Section for preparing Figure 4.
Footnotes
EDITORIAL SUMMARY:
This protocol combines microfluidic cell sorting with the CEPT small molecule cocktail to minimize cellular stress, and enable the robust single-cell cloning of human pluripotent stem cells in a high-throughput fashion.
PROPOSED TWEET: Efficient and safe single-cell cloning of human pluripotent stem cells by combining microfluidics and the CEPT cocktail
PROPOSED TEASER: Single cell cloning of hPSCs with the CEPT cocktail
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Ethics declarations
Competing interests
I.S., Y.C., and A.S. are co-inventors on a U.S. Department of Health and Human Services patent application covering the CEPT cocktail and its use.
Supplementary Information
Source Data Fig. 2
Cell roundness and impedance data
Source Data Fig. 3
% Cloning efficiency, growth rate confluency and cell survival data
Source Data Fig. 6
Unprocessed blots
Data availability statement
The main data discussed in this protocol were generated as part of the studies published in the supporting primary research papers in refs. 21 and 22. Whole-exome files have been deposited to the Sequence Read Archive under BioProject PRJNA552890. Source data are provided with this paper.
REFERENCES
- 1.Thomson JA et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998). [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K et al. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 131, 861–872 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Shi Y, Inoue H, Wu JC & Yamanaka S Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug Discov. 16, 115–130 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma A, Sances S, Workman MJ & Svendsen CN Multi-lineage Human iPSC-Derived Platforms for Disease Modeling and Drug Discovery. Cell Stem Cell 26, 309–329 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans MJ & Kaufman MH Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156 (1981). [DOI] [PubMed] [Google Scholar]
- 6.Pal R A small-molecule cocktail that beats cellular stress. Nat. Methods 18, 457–458 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Xu RH et al. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat. Methods 2, 185–190 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Ludwig TE et al. Feeder-independent culture of human embryonic stem cells. Nat. Methods 3, 637–646 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Ludwig TE et al. Derivation of human embryonic stem cells in defined conditions. Nat. Biotechnol. 24, 185–187 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Chen G et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods 8, 424–429 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodin S et al. Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in defined and xeno-free environment. Nat. Commun. 5, 1–13 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Levenstein ME et al. Basic Fibroblast Growth Factor Support of Human Embryonic Stem Cell Self-Renewal. Stem Cells 24, 568–574 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garitaonandia I et al. Increased risk of genetic and epigenetic instability in human embryonic stem cells associated with specific culture conditions. PLoS One 10, 1–25 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbaric I et al. Time-lapse analysis of human embryonic stem cells reveals multiple bottlenecks restricting colony formation and their relief upon culture adaptation. Stem Cell Reports 3, 142–155 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe K et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 25, 681–686 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Ohgushi M et al. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell 7, 225–239 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Chen G, Hou Z, Gulbranson DR & Thomson JA Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Cell Stem Cell 7, 240–248 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbaric I et al. Pinacidil enhances survival of cryopreserved human embryonic stem cells. Cryobiology 63, 298–305 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Xu Y et al. Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc. Natl. Acad. Sci. U. S. A. 107, 8129–8134 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen YH & Pruett-Miller SM Improving single-cell cloning workflow for gene editing in human pluripotent stem cells. Stem Cell Res. 31, 186–192 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Chen Y et al. A versatile polypharmacology platform promotes cytoprotection and viability of human pluripotent and differentiated cells. Nature Methods 18, 528–541 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tristan CA et al. Robotic high-throughput biomanufacturing and functional differentiation of human pluripotent stem cells Stem Cell Reports. 16, 1–17 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singec I et al. Defining the actual sensitivity and specificity of the neurosphere assay in stem cell biology. Nat. Methods 3, 801–806 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Paull D et al. Automated, high-throughput derivation, characterization and differentiation of induced pluripotent stem cells. Nat. Methods 12, 885–892 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Howden SE, Thomson JA & Little MH Simultaneous reprogramming and gene editing of human fibroblasts. Nat. Protoc. 13, 875–898 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doudna JA The promise and challenge of therapeutic genome editing. Nature 578, 229–236 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yumlu S et al. Gene editing and clonal isolation of human induced pluripotent stem cells using CRISPR/Cas9. Methods 121–122, 29–44 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Merkle FT et al. Human pluripotent stem cells recurrently acquire and expand dominant negative P53 mutations. Nature 545, 229–233 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts B et al. Systematic gene tagging using CRISPR/Cas9 in human stem cells to illuminate cell organization. Mol. Biol. Cell 28, 2854–2874 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrews PW et al. Assessing the Safety of Human Pluripotent Stem Cells and Their Derivatives for Clinical Applications. in Stem Cell Reports vol. 9 1–4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ihry RJ et al. P53 inhibits CRISPR-Cas9 engineering in human pluripotent stem cells. Nat. Med. 24, 939–946 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Li XL et al. Highly efficient genome editing via CRISPR–Cas9 in human pluripotent stem cells is achieved by transient BCL-XL overexpression. Nucleic Acids Res. 46, 10195–10215 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ortmann D & Vallier L Variability of human pluripotent stem cell lines. Curr. Opin. Genet. Dev. 46, 179–185 (2017). [DOI] [PubMed] [Google Scholar]
Key references using this protocol:
- Chen Y, Tristan CA, et al. A versatile polypharmacology platform promotes cytoprotection and viability of human pluripotent and differentiated cells. Nat Methods 18, 528–541 (2021). 10.1038/s41592-021-01126-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristan CA et al. Robotic high-throughput biomanufacturing and functional differentiation of human pluripotent stem cells Stem Cell Reports. 16, 1–17 (2021). 10.1016/j.stemcr.2021.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The main data discussed in this protocol were generated as part of the studies published in the supporting primary research papers in refs. 21 and 22. Whole-exome files have been deposited to the Sequence Read Archive under BioProject PRJNA552890. Source data are provided with this paper.


