Abstract
Patient: Female, Newborn-year-old
Final Diagnosis: Fetal ileal atresia at 22 weeks gestation
Symptoms: None
Clinical Procedure: —
Specialty: Obstetrics and Gynecology • Pediatrics and Neonatology
Objective:
Congenital defects/diseases
Background:
Maldevelopment of the fetal bowel can result in the rare condition of intestinal atresia, which results in congenital bowel obstruction. This report describes a case of prenatal diagnosis of fetal ileal atresia at 22 weeks’ gestation.
Case Report:
Here, we present a 24-year old woman who was 22 weeks into her first pregnancy when she underwent routine fetal ultrasound. She was diagnosed with gestational diabetes mellitus. Her body mass index was normal and she had normal weight gain. The ultrasonographic examination performed revealed a hyperechoic bowel and a small dilatation of the bowel. The couple was counselled for possible intestinal atresia and its postnatal implications. At 33 weeks of gestation, polyhydramnios appeared, and the intestinal distension was much more pronounced, with hyperechoic debris in the intestinal lumen (succus-entericus). After birth, surgery was performed and we concluded the patient had type II atresia, which was surgically treated.
Conclusions:
This report has highlighted the importance of antenatal ultrasound in detecting fetal abnormalities, and has shown that rare conditions such as intestinal atresia can be accurately diagnosed and successfully managed. Surgical correction, if implemented promptly after stabilizing the general condition, can have a relatively good prognosis. Coexisting fetal ileal atresia and gestational diabetes mellitus are rare occurrences, which can make each condition even more difficult to treat.
Keywords: Intestinal Atresia, Jejunal Atresia, Prenatal Diagnosis, Gestational Diabetes, Congenital Abnormalities, Neonatology
Introduction
Intestinal atresia is a congenital malformation in which a part of the small intestine or the entire segment is absent, resulting in an anatomical discontinuity of the digestive tract. The remedy in these situations requires prompt major surgical interventions with the aim of restoring the continuity of the digestive tract as quickly as possible. The incidence of this malformation is between 1 in 5000 and 1 in 14 000 live births, of which approximately 20% are associated with chromosomal anomalies. About 33% of the affected children are born prematurely, and intestinal atresia has no gender predominance as it is equally common in male and female children [1]. Familial cases of intestinal atresia have been reported, but the majority of intestinal atresia occurs sporadically. Prenatal diagnosis using ultrasound imaging techniques can be used to identify intestinal obstruction, but only 29% to 50% of cases receive a positive diagnosis prenatally. Prenatal diagnosis relies on the demonstration of dilated loops of the fetal bowel and the presence of polyhydramnios at the end of the second or more frequently during the third trimester of pregnancy. Data from 18 European registries document the importance of ultrasound examinations [2]. However, these signs are not specific and can be found in other congenital anomalies of the gastrointestinal tract, such as meconium ileus, colonic atresia, Hirschsprung’s disease, and imperforate anus. Associated conditions are cystic fibrosis, intestine malrotation, and gastroschisis, in about 10% of the cases [3].
Among the mechanisms most frequently found to underlie the occurrence of intestinal atresia are intrauterine ischemic vascular injuries at the level of the branches of the mesenteric artery, especially at the level of the middle intestine [3]. Others are: Tandler’s theory (which states that intestinal atresia is due to the progressive stoppage of the development of the intestine during the gestational period of 5–8 weeks), and conditions that lead to insufficient blood supply to the small intestine (strangulation, intestinal volvulus, intussusception, hernia) [4]. Damage to the mesenteric vessels seems to be more common in the case of intestinal atresias without familial aggregation, while in the case of familial aggregation with multiple associated lesions, the most frequently involved mechanism is documented to be the malformations [5].
High blood sugar levels during pregnancy correlate very well with the occurrence of embryopathy responsible for the production of congenital malformations [6]. Gestational diabetes mellitus (GDM) is characterized by high blood sugar levels during the second or third trimester of pregnancy [7,8]. Some congenital malformations are more frequently found to be related to the existence of diabetes mellitus (DM) (Table 1) [9,10], and the concomitant association of several malformations is associated with pre-existing DM [9]. Among these, duodenal atresia is the most commonly found gastrointestinal malformation linked to GDM.
Table 1.
Malformations associated with diabetes mellitus during pregnancy. (* more frequent in gestational diabetes mellitus).
|
The link between GDM and congenital malformations of the digestive tract is still uncertain. However, there are studies that support this idea, even circulating the hypothesis that severe forms of GDM increase the risk of these malformations by up to threefold (in contrast to moderate GDM, which does not bring additional risks) [11]. Pre-pregnancy DM is associated with an increased risk of congenital malformations [9], but further studies are required on larger cohorts to support this hypothesis.
Infection with COVID-19 during pregnancy can have a double impact. COVID-19 during pregnancy can have immediate but also subsequent implications through the appearance of post-COVID syndrome, with the possibility of affecting neonatal systems directly or indirectly through the activation of pro-inflammatory mechanisms with increased reactivity over time, or the induction of hypercoagulability states [12,13]. Congenital damage to the fetus can be secondary to intrauterine vascular injuries at the level of the placental circulation, and among the most frequent manifestations are intestinal atresia and limb anomalies; however, the picture is not yet very clear and evidence is still evolving [14–17].
The postnatal diagnosis can be suggested by the presence in the newborn of a significant abdominal distension which will also be highlighted in plain abdominal radiography as the presence of air in the proximal intestine and the absence of gas downstream of it [18]. The use of barium enemas can highlight the anatomical structures located downstream of the malformation [19]. Also, prolonged vomiting syndromes, most often with a bilious appearance in the immediate post-natal period (the first 24–48 hours of life) can suggest the existence of this malformation [18,20]. The absence of intestinal transit in the first days of life can be another alarm sign that must prompt the neonatologist to rule out the existence of intestinal atresia [21]. The most frequently used classification of intestinal atresia is that of Grosfeld et al [22]. Based on this classification, they described the survival rate for each type, varying between 29% for type IV intestinal atresias and 85% for type I intestinal atresias [22].
The therapeutic approach to these patients is always surgical, using multiple anastomosis techniques, with the attempt to restore the continuity of the digestive tube as quickly as possible and with the smallest possible amount of resected intestine, obtaining the lowest complication rate at the anastomosis level, as well as preventing the occurrence of short bowel syndrome [23]. The primary resection of the dilated proximal segment and the creation of a definitive anastomosis between the remaining intestinal segments is the idea behind the techniques [24,25]. One of the simplest techniques with a good survival rate is the end-to-side oblique anastomotic technique [23]. The use of transient ileostomies followed by subsequent intestinal reconstruction is among other surgical techniques with increased utility especially in the case of the association of meconium peritonitis [26]. Poor prognosis for the survival of newborn surgical patients is associated with prematurity (regardless of age or birth weight) as well as the presence of other congenital anomalies. However, prognosis is also associated with the experience of the surgical center where the intervention is performed, as dexterity plays a pivotal role, and poor prognosis is commonly associated with high chances of contracting postoperative sepsis [23,24]. The subsequent quality of life of the newborn is profoundly influenced by the function of the postsurgically recovered bowel [23].
This report describes a case of prenatal diagnosis of fetal ileal atresia at 22 weeks’ gestation.
Case Report
A 24-year old woman who was 22 weeks into her first pregnancy underwent routine fetal ultrasound. The evolution of the pregnancy up to the age of 22 weeks was normal, without the recording of any special events from a clinical point of view. At the 22nd week of pregnancy, she underwent routine scheduled obstetric evaluation, during which investigations revealed elevated blood sugar levels and she was diagnosed as having GDM. She had a normal body mass index (BMI) before pregnancy and a weight gain within normal limits during pregnancy. The ultrasonographic examination revealed morphological aspects within normal limits except for the following 2 findings: a hyperechoic bowel and a small dilatation of the bowel (Figure 1).
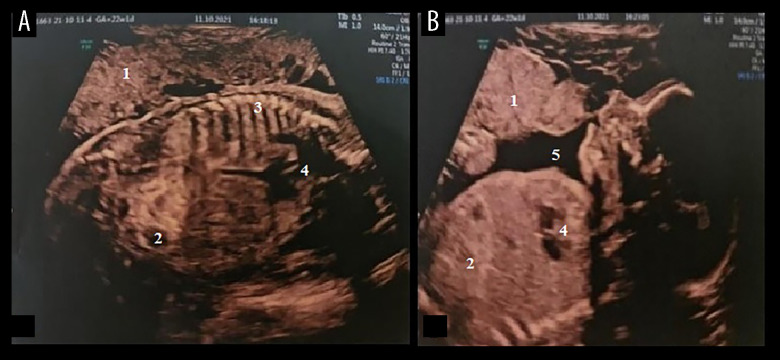
Figure 1.
(A, B) Transabdominal ultrasound of the fetus at 22 weeks, showing a hyperechoic bowel and dilated loop. 1, Placenta; 2, dilated intestinal loops; 3, vertebral column; 4, heart; 5, amniotic fluid.
Because, at that moment, we suspected intestinal atresia, the parents were counselled in detail about the diagnosis of fetal intestinal atresia, its possible association with other abnormalities, and information on postnatal management and prognosis. To assess the risk of the coexistence of other congenital malformations, the parents were advised to carry out a genetic test from the amniotic fluid. The procedure was performed the following day at 22 weeks and 4 days and the results of this test (karyotype, array-based comparative genomic hybridization) were normal (46,XX). The parents were also tested for cystic fibrosis and this was found to be negative. One week later (week 23 of gestation), we reevaluated the fetus, and we detected highly dilated bowel loops compared with the previous examination (Figure 2).
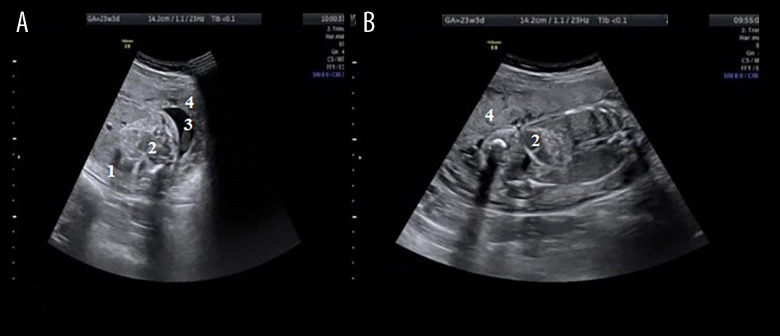
Figure 2.
(A) Transabdominal ultrasound of the fetus, aged 23-weeks and 3-days, showing a hyperechoic bowel. (B) Dilated loop. 1, section through the abdomen; 2, dilated intestinal loops; 3, amniotic fluid; 4, placenta.
This rapid evolution of intestinal distension highlights the diagnosis of bowel atresia. Subsequently, the patient was examined weekly by ultrasound (Figure 3) with the aim of adopting the best surgical solution immediately postpartum.
Figure 3.
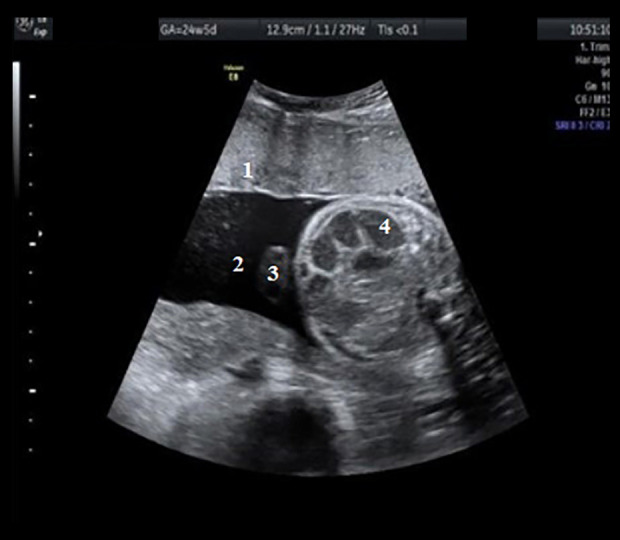
Transabdominal ultrasound of the fetus, aged 24 weeks and 4 days, showing a dilated loop with diameter >7 mm. Hyperechoic dilated intestinal loops are visible. 1, placenta; 2, amniotic fluid; 3, umbilical cord with 2 arteries and a vein; 4, dilated intestinal loops.
At 33 weeks of gestation, polyhydramnios appeared, and the intestinal distension was much more pronounced with hyper-echoic debris in the intestinal lumen (succus-entericus). Also, intestinal peristalsis was much increased (Figure 4).
Figure 4.
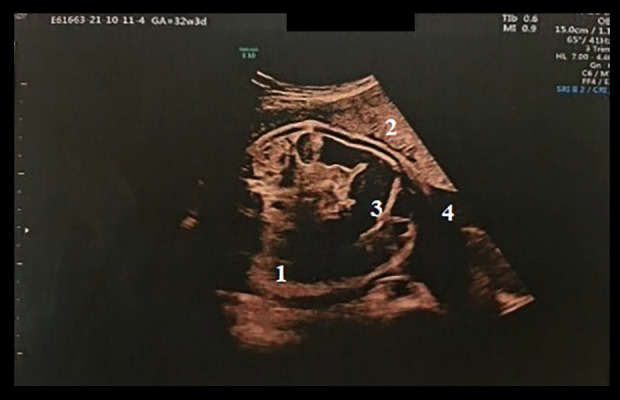
Transabdominal ultrasound of the fetus, aged 33 weeks, showing intestinal distension with hyperechoic debris in the intestinal lumen (succus-entericus) with continuous peristalsis, by real-time imaging examination. 1, section through the abdomen; 2, placenta; 3, dilated intestinal loop; 4, amniotic fluid.
The patient delivered a female neonate of 3460 g, with an Apgar Index Score of 8 points, by natural birth. Morphopathological examination of the placenta did not reveal any vascular changes such as malperfusion, hematomas or infarction.
After birth, the neonate was examined by a neonatologist who observed pronounced abdominal distention. Biliary vomiting appeared in the first 2 days of life. Biliary vomiting in the newborn is considered secondary to a mechanical obstruction until proven otherwise, and emergency surgical evaluation is required. A nasogastric tube was placed for suction of the gastric secretions, and fluid and electrolyte imbalance correction was started. Parenteral nutrition was started the same day. Neonatal jaundice appeared shortly after birth, and laboratory tests revealed hyperbilirubinemia. The jaundice was treated with the help of phototherapy. Blood sugar levels were 50 mg/dL (normal value: 70–100 mg/dL).
The newborn underwent radiographic evaluation that showed large, dilated loops in the upper abdomen with no distal gas. The baby was referred to the pediatric surgeon and underwent surgery for ileal atresia. The intraoperative findings of bowel atresia were as follows: The atretic portion of the small bowel (10 cm) was resected with termino-terminal anastomosis for type II atresia (Figure 5). Dilated intestinal loops could be seen, and the rest of the loops distal to the obstruction were collapsed because the air did not enter at that level. The length of the intestine was normal. The proximal blind pouch was dilated and cyanotic.
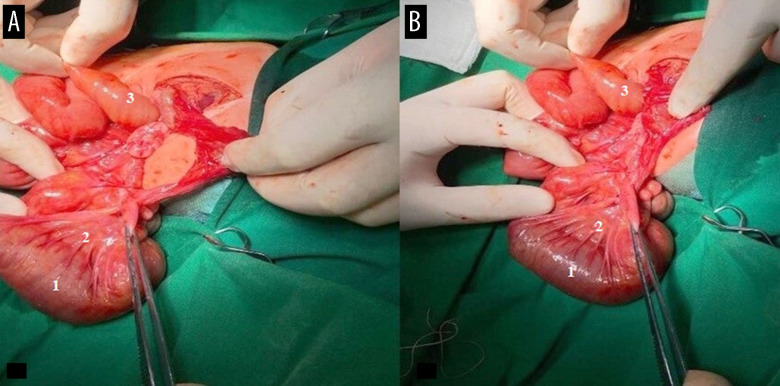
Figure 5.
(A, B) Resection of the entire section of the small bowel in the newborn, aged 5 days. 1, ileum; 2, mesenteries; 3, jejunum.
Postoperative evolution was favorable, without any complications. After 7 days of surgical intervention, the nasogastric tube was removed and enteral feeds were initiated. The child was discharged 14 days after the surgery with a good general status.
Discussion
The importance of early diagnosis in congenital malformations is the key to a better prognosis, as seen in the case presented here. The lack of appropriate monitoring of fertile women through screening programs is frequently associated with the impossibility of accurately establishing the form of DM discovered during pregnancy [6]. Moreover, the increasingly frequent presence of obesity among women of childbearing age further complicates diagnosis because obesity is associated with both type 2 DM and GDM [6].
Our patient did not have pathological changes in body mass before or during pregnancy, and she had no personal or family history of DM (neither the mother, nor the child’s father, had a family history of DM). Also, her obstetric history included a previous pregnancy that culminated at term with the natural birth of a normal live fetus. During the detailed anamnesis performed on the pregnant woman, no malformative risk elements could be detected either through accidental or voluntary exposure to prohibited substances. The possibility of symptomatic or asymptomatic infection with COVID-19 in the periconceptual period or during the course of pregnancy was also excluded, both in the case of the mother and the extended family members.
The relatively small incidence of this pathology may be underestimated due to reporting flaws. Also, due to the limited number of cases, the development of easily accessible and specialized medical teams in the management of these cases is very difficult because any intervention requires a practical learning curve [27,28], which is associated with a high rate of morbidity and mortality among these newborns [18]. In our hospital, this was 1 case reported in almost 250 births, the average number of births in our hospital each month.
The frequent association of intestinal atresia with cystic fibrosis required the evaluation of the parents for the existence of the carrier allele of the Cystic Fibrosis Transmembrane Gene mutation, as soon as changes suggestive of congenital damage of the digestive tract were detected by ultrasound. However, the testing turned out to be negative.
The appearance of polyhydramnios in the 33rd week of gestation can erroneously classify the malformation as duodenal atresia because it occurs frequently in the natural course of this malformation. This increased the degree of complexity in the present case.
A retrospective study published in September 2023 by Sümeyra Gürel et al analyzed a group of 242 pregnant women with idiopathic polyhydramnios, who had been registered over a 5-year period. They found an incidence of 2% of cases showing gastrointestinal malformations, the incidence of these cases being directly dependent on the presence of severe polyhydramnios [OR 3.2 (95% CI 1.9–5.5)] [29]. The association between gestational diabetes and gastrointestinal malformations is not yet unequivocally proven. However, there are complex malformation issues that have been associated with GDM, including digestive tube malformations. One of these pathologies is caudal regression syndrome, in which variable degrees of sacral and coccygeal vertebral agenesis is associated with gastrointestinal, genitourinary, and cardiovascular malformations [30]. The presence of this syndrome was excluded in the case of our patient because there were no coexisting malformations at the level of the spine.
The presence of pre-pregnancy DM exerts an important impact on the malformation risk of the fetus. A Canadian study carried out over a period of 11 years indicated an increase in malformations in general in these patients, but also a very important increase in the population-attributable risk of registered gastrointestinal defects (0.8% in the general population, ranging between 0.7 and 2.6%; 95% CI) in the case of patients with pre-pregnancy DM [31]. The involvement of pre-pregnancy DM in our case is firmly excluded because the patient did not present with elevated blood glucose values up to this point, as well as by the fact that there was no recorded diabetic heredity in this family.
In 2010, Oddsberg et al published a study carried out between January 1, 1982 and December 31, 2007, that reached the conclusion that the presence of DM is associated with an increased risk of esophageal atresia, suggesting that these 2 pathologies could be interconnected. (They recorded a 70% higher risk of esophageal atresia in mothers with DM) [32].
A 2001 study in Sweden compared malformation risk between children born to mothers with pre-pregnancy DM and those with GDM. They found that, in general, there was a higher incidence of malformations in children born to mothers with pre-pregnancy DM, but for digestive tube malformations specifically, a higher incidence was observed among mothers with GDM [33]. Other cases presented by Toni et al and Vigrone et al highlight the importance of early diagnosis in such anomalies, specifying the role of ultrasound examinations which can prove highly beneficial [34,35].
The hyperbilirubinemia detected in our newborn is part of a physio-pathological cascade that is a common event in these patients. In our case, the ileal obstruction increased the enterohepatic circulation of bilirubin, leading to pronounced jaundice. Perforation can occur rapidly in the evolution of patients with intestinal atresia, but in our case, there was no perforation as the surgical intervention was done shortly after birth. Gastrointestinal atresia is also associated with an increased rate of asphyxia and antenatal death even in the presence of a normal karyotype and the absence of congenital malformation associations. The mechanism involved appears to be overstimulation of the fetal vagal nervous system by compression exerted by distension of the upstream digestive tract, or due to an injury, resulting in bradycardia or asystole [36]. If, in terms of the benefits of antenatal versus postnatal diagnosis of intestinal atresia, the balance of benefits seems to be tilted towards antenatal diagnosis in newborns, this may appear different from the obstetric point of view [37]. Polyhydramnios during pregnancy increases the need for cesarean birth [38]. Also, the antenatal diagnosis of alimentary canal atresia has been associated with preterm birth without influencing the rate of hospitalization of the baby [37].
Conclusions
This report has highlighted the importance of antenatal ultra-sound in detecting fetal abnormalities, and has shown that rare conditions such as intestinal atresia can be accurately diagnosed and successfully managed. Carrying out studies on the subsequent outcomes of mothers and their children who have undergone interventions to correct intestinal atresia can contribute to the adoption of new medical management guidelines for these patients.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Institution Where the Patient Was Treated
University Emergency Hospital “Odobescu”, Timişoara, Romania; “Louis Turcanu” Pediatric Emergency Children Hospital, Timişoara, Romania.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Adams SD, Stanton MP. Malrotation and intestinal atresias. Early Hum Dev. 2014;90(12):921–25. doi: 10.1016/j.earlhumdev.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Haeusler MC, Berghold A, Stoll C, et al. Prenatal ultrasonographic detection of gastrointestinal obstruction: Results from 18 European congenital anomaly registries. Prenat Diagn. 2002;22(7):616–23. doi: 10.1002/pd.341. [DOI] [PubMed] [Google Scholar]
- 3.Osuchukwu OO, Rentea RM. Ileal atresia. StatPearls; 2023. [cited 2023 July 1st]; Available from: https://www.ncbi.nlm.nih.gov/books/NBK557400/ [PubMed] [Google Scholar]
- 4.Louw JH. Congenital intestinal atresia and stenosis in the newborn. Observations on its pathogenesis and treatment. Ann R Coll Surg Engl. 1959;25(4):209–34. [PMC free article] [PubMed] [Google Scholar]
- 5.Puri P, Fujimoto T. New observations on the pathogenesis of multiple intestinal atresias. J Pediatr Surg. 1988;23(3):221–25. doi: 10.1016/s0022-3468(88)80726-7. [DOI] [PubMed] [Google Scholar]
- 6.Correa A, Gilboa SM, Besser LM, et al. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008;199(3):237.e1–9. doi: 10.1016/j.ajog.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.International Diabetes Federation Management of gestational diabetes in the community. [cited 2023 29 June] Available from: https://idf.org/about-diabetes/gestational-diabetes/
- 8.No Authors Listed Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 9.Aberg A, Westbom L, Källén B. Congenital malformations among infants whose mothers had gestational diabetes or preexisting diabetes. Early Hum Dev. 2001;61(2):85–95. doi: 10.1016/s0378-3782(00)00125-0. [DOI] [PubMed] [Google Scholar]
- 10.Tinker SC, Gilboa SM, Moore CA, et al. Specific birth defects in pregnancies of women with diabetes: National Birth Defects Prevention Study, 1997–2011. Am J Obstet Gynecol. 2020;222(2):176.e1–e11. doi: 10.1016/j.ajog.2019.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheffield JS, Butler-Koster EL, Casey BM, et al. Maternal diabetes mellitus and infant malformations. Obstet Gynecol. 2002;100(5 Pt 1):925–30. doi: 10.1016/s0029-7844(02)02242-1. [DOI] [PubMed] [Google Scholar]
- 12.Mocanu V, Bhagwani D, Sharma A, et al. COVID-19 and the human eye: Conjunctivitis, a lone COVID-19 finding – a case-control study. Med Princ Pract. 2022;31(1):66–73. doi: 10.1159/000521808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosca CI, Branea HS, Sharma A, et al. Rhythm disturbances in post-acute COVID-19 syndrome in young men without pre-existing known cardiovascular disease; A case series. Biomedicines. 2023;11(4):1146. doi: 10.3390/biomedicines11041146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reppucci ML, Kaizer AM, Prendergast C, et al. Incidence of congenital complications related to COVID-19 infection during pregnancy. J Neonatal Perinatal Med. 2023;16(2):227–34. doi: 10.3233/NPM-221122. [DOI] [PubMed] [Google Scholar]
- 15.Brandibur TE, Manea AM, Sharma A, et al. Macronutrients management for growth in neonates with congenital gastrointestinal malformation. Med Sci Monit. 2022;28:e938106. doi: 10.12659/MSM.938106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alhumaid S, Al Alawi Z, Alnaim AA, et al. Intussusception and COVID-19 in children: A systematic review and meta-analysis. Children. 2022;9(11):1745. doi: 10.3390/children9111745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calisti A, Olivieri C, Coletta R, et al. Jejunoileal atresia: Factors affecting the outcome and long-term sequelae. J Clin Neonatol. 2012;1(1):38–41. doi: 10.4103/2249-4847.92237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akinmoladun JA, Lawal TA, Hafiz A. Late third trimester ultrasound diagnosis of duodenal atresia – the importance of detailed prenatal ultrasound screening. Ann Ib Postgrad Med. 2019;17(1):71–74. [PMC free article] [PubMed] [Google Scholar]
- 19.Jagatiani N, Ariwala N, Shan J. Antenatal diagnosis of duodenal atresia. Gujarat Med J. 2009;64(2):77–78. [Google Scholar]
- 20.Nelson LH, Clark CE, Fishburne JI, et al. Value of serial sonography in the in utero detection of duodenal atresia. Obstet Gynecol. 1982;59(5):657–60. [PubMed] [Google Scholar]
- 21.Cunha SBd, Rasteiro C, Silva M, et al. Cystic fibrosis and jejunoileal atresia: A clinical case. Clin Case Rep. 2022;10(8):e05869. doi: 10.1002/ccr3.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricardo Riego de Dios EMC. Imaging in ileal atresia. MedScape. 2019. [updated 2019; cited 2023 august]; Available from: https://emedicine.medscape.com/article/409746-overview.
- 23.Joda AE, Abdullah AF. Outcomes of end-to-side oblique anastomosis as a surgical technique for jejuno-ileal atresia. Updates Surg. 2019;71(3):587–93. doi: 10.1007/s13304-019-00666-9. [DOI] [PubMed] [Google Scholar]
- 24.Kumaran N, Shankar KR, Lloyd DA, Losty PD. Trends in the management and outcome of jejuno-ileal atresia. Eur J Pediatr Surg. 2002;12(3):163–67. doi: 10.1055/s-2002-32726. [DOI] [PubMed] [Google Scholar]
- 25.Gupta S, Gupta R, Ghosh S, et al. Intestinal atresia: Experience at a busy center of North-West India. J Neonatal Surg. 2016;5(4):51. doi: 10.21699/jns.v5i4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.del Ciampo IR, Oliveira TQ, del Ciampo LA, et al. [Early manifestations of cystic fibrosis in a premature patient with complex meconium ileus at birth] Rev Paul Pediatr. 2015;33(2):241–45. doi: 10.1016/j.rpped.2014.12.004. [in Portuguese] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chirdan LB, Uba AF, Pam SD. Intestinal atresia: Management problems in a developing country. Pediatr Surg Int. 2004;20(11–12):834–37. doi: 10.1007/s00383-004-1152-4. [DOI] [PubMed] [Google Scholar]
- 28.Gupta R, Soni V, Valse PD, et al. Neonatal intestinal obstruction associated with situs inversus totalis: Two case reports and a review of the literature. J Med Case Rep. 2017;11(1):264. doi: 10.1186/s13256-017-1423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gürel S, Ayhan I, Uygur L, et al. What to expect after birth in idiopathic polyhydramnios? An analysis of postnatal diagnoses and their relationship to the polyhydramnios degree. Arch Gynecol Obstet. 2023 doi: 10.1007/s00404-023-07216-0. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Tilfine C, Laamrani FZ, Dafiri R. [Caudal regression syndrome: About a case] Pan Afr Med J. 2018;30:219. doi: 10.11604/pamj.2018.30.219.8691. [in French] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu S, Rouleau J, León JA, et al. Impact of pre-pregnancy diabetes mellitus on congenital anomalies, Canada, 2002–2012. Health Promot Chronic Dis Prev Can. 2015;35(5):79–84. doi: 10.24095/hpcdp.35.5.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oddsberg J, Lu Y, Lagergren J. Maternal diabetes and risk of esophageal atresia. J Pediatr Surg. 2010;45(10):2004–8. doi: 10.1016/j.jpedsurg.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Åberg A, Westbom L, Källén B. Congenital malformations among infants whose mothers had gestational diabetes or preexisting diabetes. Early Human Dev. 2001;61(2):85–95. doi: 10.1016/s0378-3782(00)00125-0. [DOI] [PubMed] [Google Scholar]
- 34.Tonni G, Grisolia G, Granese R, et al. Prenatal diagnosis of gastric and small bowel atresia: a case series and review of the literature. J Matern Fetal Neonatal Med. 2016;29(17):2753–61. doi: 10.3109/14767058.2015.1107902. [DOI] [PubMed] [Google Scholar]
- 35.Virgone C, D’Antonio F, Khalil A, et al. Accuracy of prenatal ultrasound in detecting jejunal and ileal atresia: Systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;45(5):523–29. doi: 10.1002/uog.14651. [DOI] [PubMed] [Google Scholar]
- 36.Brantberg A, Blaas HG, Salvesen KA, et al. Fetal duodenal obstructions: Increased risk of prenatal sudden death. Ultrasound Obstet Gynecol. 2002;20(5):439–46. doi: 10.1046/j.1469-0705.2002.00831.x. [DOI] [PubMed] [Google Scholar]
- 37.Simeonova-Brachot II, Larroque M, Pierre F, et al. What are the top benefits of a prenatal diagnosis of fetal esophageal or intestinal atresia? J Clin Gynecol Obstet. 2023;12(1):1–7. [Google Scholar]
- 38.Luo QQ, Zou L, Gao H, et al. Idiopathic polyhydramnios at term and pregnancy outcomes: A multicenter observational study. J Matern Fetal Neonatal Med. 2017;30(14):1755–59. doi: 10.1080/14767058.2016.1224835. [DOI] [PubMed] [Google Scholar]