ABSTRACT
Cardiogenic shock continues to carry a high mortality rate despite contemporary care, with no breakthrough therapies shown to improve survival over the past few decades. It is a time‐sensitive condition that commonly results in cardiovascular complications and multisystem organ failure, necessitating multidisciplinary expertise. Managing patients with cardiogenic shock remains challenging even in well‐resourced settings, and an important subgroup of patients may require cardiac replacement therapy. As a result, the idea of leveraging the collective cognitive and procedural proficiencies of multiple providers in a collaborative, team‐based approach to care (the “shock team”) has been advocated by professional societies and implemented at select high‐volume clinical centers. A slowly maturing evidence base has suggested that cardiogenic shock teams may improve patient outcomes. Although several registries exist that are beginning to inform care, particularly around therapeutic strategies of pharmacologic and mechanical circulatory support, none of these are currently focused on the shock team approach, multispecialty partnership, education, or process improvement. We propose the creation of a Cardiogenic Shock Team Collaborative—akin to the successful Pulmonary Embolism Response Team Consortium—with a goal to promote sharing of care protocols, education of stakeholders, and discovery of how process and performance may influence patient outcomes, quality, resource consumption, and costs of care.
Keywords: cardiogenic shock, education, multidisciplinary teams, process improvement
Subject Categories: Cardiopulmonary Resuscitation and Emergency Cardiac Care, Heart Failure
Nonstandard Abbreviations and Acronyms
- CICU
cardiac intensive care unit
- CS
cardiogenic shock
- PERT
Pulmonary Embolism Response Team
The management of cardiogenic shock (CS) is complicated. Despite innovations in pharmacologic and mechanical circulatory support, no single therapy introduced over the past several decades has resulted in a demonstrable improvement in patient survival. As a result, CS continues to be associated with high morbidity and mortality. 1 , 2 , 3 , 4 More recently, however, it has been proposed that methods for leveraging the collective cognitive and procedural proficiencies of a multidisciplinary team of providers may be helpful; in fact, the concept of a collaborative cardiogenic “shock team” is now endorsed by the American Heart Association and has been successfully implemented at a number of high‐volume clinical centers. 5 , 6 , 7 , 8 At the same time, there is a slowly maturing evidence base that has suggested that CS teams may improve patient outcomes; however, there are currently no established recommendations available to guide the composition, assembly, or employment of such teams. 9 On conducting an informal 2023 survey of critical care cardiology physicians (representing leadership from 25 institutions in the United States) committed to the idea of the shock team, considerable heterogeneity of opinion and practice emerged. Notably, there were numerous differences with regard to staffing, structure, and care processes, along with great variation in team membership, methods of activation, and resource needs. Although several maturing CS registries are beginning to inform care—most with a particular focus on therapeutic strategies for circulatory support—none of these currently focus on the role of the shock team, the potential value of multidisciplinary management, provider education, or process improvement.
It has been nearly a decade since the Pulmonary Embolism Response Team (PERT) Consortium was established to help guide pulmonary embolism (PE) care. The goal of this group was to improve patient outcomes and to validate treatment options, while enhancing education and spearheading innovation. 10 Over time the PERT Consortium has grown substantially in both scale and scope—in addition to its annual scientific meeting, the group has established specialized subcommittees focused on the creation of contemporary PE care protocols and the development of registry and quality databases, all with a goal of cultivating the exchange of ideas, clinical and community instruction, and outcome benchmarking. Analogous to the PERT Consortium, we believe that a CS Team Collaborative is needed to meaningfully influence the future of CS care. The aim of such a collaborative would be to bring together key stakeholders who share a commitment to advancing the implementation and integration of CS teams into clinical practice. This will include advocating for the CS team care model, developing and disseminating team‐based methods and treatment protocols, educating health care professionals and community consumers, and promoting research and innovation. In the sections that follow, we hope to highlight the impact of CS on patients, hospitals, and health care systems; to describe the merits and potential pitfalls of a team‐based approach to care; and to justify the need for a CS Team Collaborative by highlighting goals, deliverables, and keys to its maturation and sustainability.
The Impact of Cardiogenic Shock
Definition, Epidemiology, and Influence of Cardiogenic Shock Care in Today's Cardiac Intensive Care Unit
CS is a state of extreme circulatory failure that results in maladaptive, self‐perpetuating cycles of impaired cardiac output, tissue hypoxemia, peripheral hypoperfusion, and poor vascular reactivity that can lead to irreversible multisystem organ failure (Figure 1). 5 Historically, CS has been defined using central macrovascular parameters of blood pressure, cardiac output/index, intracardiac filling pressures, and ventricular function, in combination with varying assessments of end‐organ perfusion. 11 , 12 Accepted clinical trial definitions usually include 4 elements: (1) hypotension—systolic blood pressure <90 mm Hg for ≥30 minutes or use of mechanical or pharmacological circulatory support, (2) adequacy of preload—pulmonary congestion on chest imaging or pulmonary capillary wedge pressure ≥15 mm Hg, (3) low cardiac output—cardiac index ≤1.8 L/min per m2 without or ≤2.2 L/min per m2 with circulatory support therapies, and (4) hypoperfusion—cold clammy skin, oliguria (eg, urine output <30 mL/h), serum lactate >2.0 mmol/L. The Society of Cardiovascular Angiography and Intervention (SCAI) has proposed a simple, yet holistic assessment of physical examination, biochemical markers, and hemodynamics to classify patients with or at risk for CS into 5 SCAI Shock Stages of escalating severity (A through E), thus providing greater granularity to CS severity. 13 , 14 The society's Shock definition further elaborates important aspects such as normotensive shock, concomitant cardiac arrest, and response to therapeutic interventions in the evaluation of CS, all of which affect clinical outcomes in this population. 15 , 16 , 17
Figure 1. Cycle of clinical decompensation characterizing the patient with cardiogenic shock.
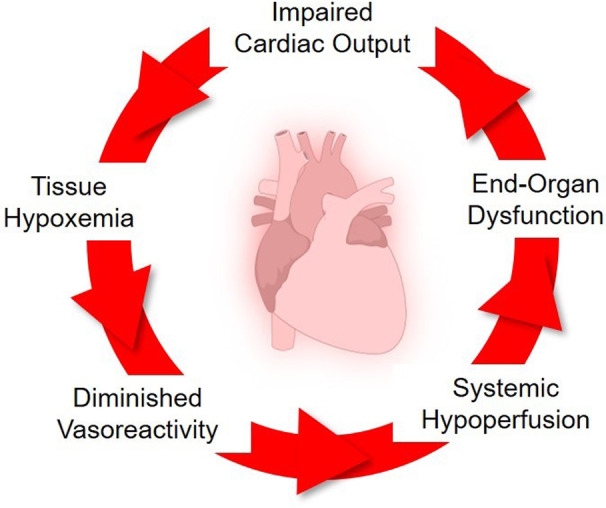
CS is the final common pathway for multiple acute cardiovascular causes including acute myocardial infarction (AMI), acute heart failure (HF), myocarditis, the postcardiotomy state, and others. 1 , 18 Historically, AMI has been the most common cause of CS, with higher rates noted in patients with ST‐segment–elevation MI compared with those with non‐ST‐segment–elevation MI. 19 , 20 In patients with AMI, CS is typically due to primary pump failure of the left ventricle resulting from myocardial necrosis, nonrevascularized ischemia, and myocardial stunning/hibernation. 21 Mechanical complications and electrical instability can also contribute to the development or progression of CS in patients with AMI. 22 , 23
More recently, acute HF appears to be overtaking AMI as the predominant cause for CS in today's cardiac intensive care unit (CICU). 1 , 24 Unlike patients with AMI‐CS who present with loss of compensatory reserve and develop hypotension as a common presenting symptom, hypotension is often the last clinical manifestation of CS in patients with HF. 25 Those with advanced HF typically have low‐normal systolic blood pressure at baseline, higher cardiac filling pressures, and low cardiac output, without compromised end‐organ perfusion. Worsening congestion can then trigger a cycle of worsening hemodynamics and hypoperfusion. 25 The insidious onset of hypoperfusion may lead to delays in detection and an underestimation of the true prevalence of CS in this population. 15
Less common causes of CS include myocarditis, arrhythmias, valvular heart disease, stress cardiomyopathy, and peripartum cardiomyopathy. 26 Although CS is typically defined by elevations in left‐sided filling pressures and a decrease in cardiac output, there is now a greater appreciation for right ventricular CS in conditions including acute PE, right‐sided AMI, pulmonary hypertension, adult congenital heart disease, and acute‐on‐chronic cor pulmonale. 27 Although echocardiography is frequently used for identification of acute right ventricular failure, 28 many other clinical and hemodynamic markers should be considered; among these, a pulmonary artery pulsatility index defined as (systolic pulmonary artery pressure—diastolic pulmonary artery pressure)/right atrial pressure <1.0 has been increasingly used. 29 Finally, there has also been greater recognition of mixed shock—the combination of vasodilatory shock and CS due to concomitant sepsis, systemic inflammation, cardiac arrest, and other factors. 1 , 16 , 17
Although CS can be thought of as a primary cardiovascular condition, the initial hemodynamic insult, if left untreated, can result in progressive metabolic perturbations and subsequent multiorgan failure. 30 In patients with AMI‐CS, recent epidemiologic data have noted an increase in associated noncardiac organ dysfunction including respiratory, renal, hepatic, and neurologic failure. 19 , 20 , 31 , 32 , 33 , 34 Furthermore, contemporary patients with CS are increasingly sicker due to a higher baseline comorbidity burden, the influence of concomitant cardiac arrest, and the greater use of temporary mechanical circulatory support (MCS) options including venoarterial extracorporeal membrane oxygenation. 1 , 35 , 36 , 37 In totality, there has been both an increase in cardiac as well as noncardiac organ support therapies for AMI‐CS, all of which affect health care delivery and staffing in the CICU, requirements for training and proficiency among CICU clinicians, and have led to increasing health care expenditures. 30
Morbidity, Mortality, and Resource Needs of the Patient With Cardiogenic Shock
Patients who develop CS are at high risk of short‐term mortality, with 30% to 50% of patients dying in the hospital or within 30 days. 2 Studies diverge regarding whether the survival of patients with CS is stagnant or slowly improving, but regardless of these prevailing trends, outcomes remain poor overall. 1 , 3 , 4 Persistently high mortality in populations with CS likely reflects the dearth of established therapies that are proven to improve survival in CS when applied broadly. 38 As with mortality, nonfatal major adverse cardiovascular events, sepsis, acute kidney injury, and bleeding are common in patients with CS, particularly among those receiving temporary MCS. Indeed, the ECMO CS (Extracorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock) trial found that more than 60% of all patients with CS had 1 or more serious events. 39 Long ICU and hospital stays are typical, particularly for hospital survivors, and even after discharge patients with CS remain at substantial risk of subsequent cardiovascular events, rehospitalization, and death.
Patients with CS are critically ill and have complex care needs involving the heavy consumption of ICU‐specific therapies. Although resource use varies based upon the severity of CS (being highest among those with refractory, Society for Cardiovascular Angiography and Intervention stage E CS), intensive cardiovascular and noncardiovascular organ support modalities are ubiquitous (Table 1). 40 In major randomized trials of patients with CS, nearly all received parenteral catecholamines and the vast majority were invasively ventilated (Table 2). 41 , 42 , 43 , 44 Noncardiovascular therapies including targeted temperature management, dialysis, or blood transfusion are commonly required, emphasizing the multidisciplinary critical care needs of this population. The dismal prognosis associated with CS in contemporary clinical practice emphasizes the dire need to develop care strategies to improve survival. The failure of randomized clinical trials to demonstrate incremental improvements in patient‐centered outcomes with novel devices and therapeutics in populations with CS is at odds with those studies that have suggested trends toward improving survival with CS over time. If no individual therapy is beneficial when applied in a uniform manner across cohorts with CS, it stands then to reason that individualized application of these therapies could potentially yield benefit. An important approach that can leverage multidisciplinary expertise to match the most appropriate therapeutic strategy to a specific patient would be the shock team and, as detailed in this article, preliminary analyses suggest that implementation of the CS team may improve outcomes.
Table 1.
Cardiovascular and Noncardiovascular Resources Frequently Used in the Management of the Patient With Cardiogenic Shock
| Cardiovascular | Noncardiovascular |
|---|---|
| Inotrope and vasopressor medications | Mechanical ventilation |
| Percutaneous coronary intervention | Renal replacement therapy |
| Antiarrhythmic medications | Targeted temperature management |
| Anticoagulant therapies | Electroencephalography |
| Mechanical circulatory support | Blood transfusion |
| Heart replacement therapy | Antimicrobial therapies |
| Noninvasive cardiac imaging | Nutrition optimization |
Table 2.
Resource Use and Outcomes in Major Cardiogenic Shock Trials With Data Combined Between Study Arms
| SHOCK 21 | IABP SHOCK II 41 | CULPRIT SHOCK 42 | DOREMI 43 | ECMO CS 39 | ECLS SHOCK 44 | |
|---|---|---|---|---|---|---|
| In‐hospital or 30‐d mortality | 51.3% | 40.4% | 47.4% | 39.6% | 48.7% | 48.4% |
| Catecholamines | 99.0% | 90.0% | 89.5% | … | 85.5% | 95.4% |
| Mechanical ventilation | 83.1% | … | 81.0% | … | 72.3% | 86.3% |
| Dialysis | … | … | 14.0% | 19.3% | 6.2% | 11.0% |
| Nonfatal myocardial infarction or stroke | … | 3.3% | 4.2% | 2.1% | … | 4.3% |
| Moderate or severe bleeding | 28.0% | 20.7% | 19.3% | … | 25.6% | 16.5% |
| Cardiac arrest | … | … | … | 8.3% | 12.0% | … |
| Sepsis | 19.0% | … | 18.0% | … | 39.0% | 10.0% |
| Median intensive care unit stay (days) | … | … | 5 | 5 | … | 9 |
Note: Not all variables and outcomes were reported in all studies, and in some cases combination of event counts for different outcomes could have led to overestimation. CULPRIT‐SHOCK indicates Culprit Lesion Only PCI [Percutaneous Coronary Intervention] Versus Multivessel PCI in Cardiogenic Shock; DOREMI, Milrinone as Compared With Dobutamine in the Treatment of Cardiogenic Shock; ECLS‐SHOCK, Extracorporeal Life Support in Cardiogenic Shock; ECMO CS, Extracorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock; IABP‐SHOCK II, Intraaortic Balloon Pump in Cardiogenic Shock II; and SHOCK, Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock.
Influence of Cardiogenic Shock Care at the Hospital Level—Resource Needs, Length of Stay, and Costs
The requirements and resources needed for hospitals and health care systems seeking to provide comprehensive care of patients with CS spans multiple domains, including: emergent treatment of the inciting event (eg, primary percutaneous coronary intervention, for ST‐segment–elevation MI), temporary MCS (often necessitating surgical expertise), CICU care, management of noncardiac organ system failures (eg, mechanical ventilation and renal replacement therapy), stepdown level inpatient care, and finally posthospital convalescent care. A variety of providers spanning the spectrum of medical and surgical specialties commonly aid in the management of the patient with complex CS. ICU and hospital lengths of stay vary depending on the cause of CS and comorbidities but are often prolonged for survivors and for those who receive cardiac replacement therapy. For patients discharged alive, median length of stay in the ICU ranges from 5 to 15 days, with hospital stays of 2 to 4 weeks being typical. 45 , 46
Costs of care for CS are in part driven by the number and type of temporary MCS devices used. Several claims and administrative data studies have attempted to address the cost differential between various support modalities, though such investigations are plagued by issues with retrospective data including indication and illness bias (risk‐treatment paradox) and lack of data for many key clinical factors, including the device‐related complications that influence outcomes and costs in CS. A US Medicare study from 2015 to 2017 found that hospital costs for percutaneous ventricular assist device therapy versus venoarterial extracorporeal membrane oxygenation were $188 000 versus $172 000. 46 A propensity‐adjusted study of commercially insured patients with CS from 2015 to 2020 found hospital costs of $101 000 with intra‐aortic balloon pump use versus $161 000 with percutaneous ventricular assist device. 47 One‐year costs of care for survivors were $142 000 for patients with intra‐aortic balloon pump versus $217 000 with a percutaneous ventricular assist device; differences were attributed to device cost as well as the costs of complications including bleeding and renal replacement therapy, which were more common in the latter group. A Finnish single‐center study of consecutive patients receiving venoarterial extracorporeal membrane oxygenation from 2013 to 2017 (78% CS) reported median in‐hospital costs of 130 000€ per patient. 45 Generalizing cost projections is difficult given the heterogeneity in MCS use, but even medically treated patients with CS require substantial resource use. 48 Paradoxically, the more successes an acute, invasive intervention strategy has in reducing acute CS mortality, the more costs may rise disproportionately as patients who are sicker require additional rescue and ongoing hospital care including cardiac replacement therapy.
At least 20% to 30% of patients will require continued home care services or rehabilitation following the index episode of CS, adding to overall costs. 45 Rehospitalization within 60 days and 1 year is common among survivors of CS and can further burden the health system. 49 , 50 An analysis of Medicare patients who survived CS showed that one third were readmitted to a hospital within 60 days. 49 A more recent, large analysis of survivors of AMI‐CS from a Canadian provincial database showed that 42% required increased support from their premorbid baseline, and nearly 50% were rehospitalized within 1 year. 50 Additionally, greater than one‐third of patients under the age of 60 may be unable to work permanently, adding to the overall societal impact and cost. Accordingly, optimization of care during hospitalization is not enough, and patients with CS require treatment within a chronic care paradigm in order to optimize long‐term survival.
System‐Level Impact and Burden of Cardiogenic Shock
The resource intensive nature of CS, with prolonged CICU stays and specialized MCS, has led many health care systems to regionalize care into specialized quaternary care referral centers with the advanced expertise and tools necessary for patient care within the context of a larger network. The ideal characteristics of CS centers remain unclear, but key concepts can be extrapolated from the care of cardiac arrest patients where the American Heart Association has provided class IIA recommendations for transport to specialized cardiac arrest centers when comprehensive postarrest care is not available locally. 51 In CS, a focus on subspecialty care, temporary MCS, CICU and specialized nursing support, and postdischarge rehabilitation therapies would be analogous to these cardiac arrest centers. Additionally, patients with CS may be bridged to heart transplant or durable MCS, both of which have dramatic implications for length of stay, health care costs, complexity of care, and outcomes, and all of which underscore the importance of an integrated, multidisciplinary team‐based approach to care.
The Merits and Risks of Team‐Based Care
The Promise of Team‐Based Care in Cardiogenic Shock
In view of the intractably high morbidity and mortality associated with CS, many experts have advocated for collaborative care models, including the employment of multidisciplinary CS teams at dedicated CS centers. 5 Multiple examples of this type of disease‐specific, team‐based care exist in cardiovascular medicine, including the use of Heart Teams for structural and advanced coronary artery disease—a concept embraced by cardiovascular professional societies for more than a decade. 52 , 53 In the case of CS, several unique considerations make a team‐focused approach particularly attractive, including the time‐sensitive nature of CS care, the multiple stakeholders involved in the management of patients with CS, the potential need for cardiac replacement therapies, and the substantial variation in practice patterns within and across health care institutions and systems in contemporary clinical practice.
The quintessential syndrome of cardiac critical illness, CS is characterized by hemodynamic impairment leading to systemic hypoperfusion that, if sustained, can lead to extensive multiorgan dysfunction and a worse prognosis. 33 Prompt recognition and early intervention in CS may therefore be important for disrupting the deadly spiral that underpins CS mortality. A team‐based approach, in which all relevant providers are notified about a CS case simultaneously by a single phone call or page, rather than through dyssynchronous and often protracted individual consultation, has the potential to expedite clinical decision‐making and reduce delays in care.
Given the complex mix of medical, catheter‐based, and surgical therapies used to treat CS, management of patients with CS is inherently multidisciplinary. In many cases, subspecialists in critical care cardiology, advanced HF and transplant cardiology, interventional cardiology, and cardiac surgery directly contribute to the care of patients, each offering a unique perspective and distinctive skill set (Figure 2). Bringing together each of these stakeholders into formalized teams with a clear organizational structure thus holds the promise of building a collaborative mentality and streamlining care delivery to those with CS.
Figure 2. Key members of an institution's cardiogenic shock team and critical partnerships necessary for establishing and supporting a cardiogenic shock team collaborative.
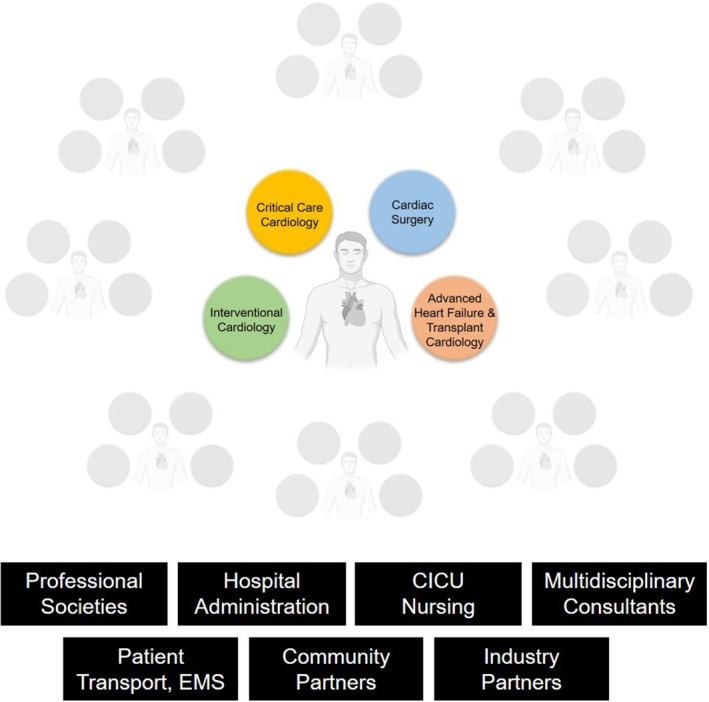
CICU indicates cardiac intensive care unit; and EMS, emergency medical services.
Finally, given the well‐documented variation in CS management, team‐based approaches may allow for standardization of best practices by drawing on the experience of providers from different backgrounds. Indeed, observational analyses support a possible volume‐outcome relationship in CS, and by creating teams of multiple highly capable physicians, the collective experience (or “collective volume”) of the care team is amplified. 54 , 55 Methods to facilitate management led by cardiovascular experts and organized within cardiac‐specific subspecialty intensive care settings embedded within high‐volume centers should be prioritized as these are known to improve outcomes and resource allocation. 56 , 57 Moreover, team‐based care often leads to other process improvements, such as team meetings focused on continuous case review and quality benchmarking, which support broader programmatic and institutional growth and improvement in CS care.
Challenges Associated With Team‐Based Care in Cardiogenic Shock
Despite the many potential benefits of CS teams, the collaboration required for successful implementation of team‐based CS care poses several challenges (Table 3), including those related to defining a successful organizational structure, alleviating burdens of communication and inefficiency, ensuring equity of workload and reimbursement, promoting team member engagement, and minimizing physicians' sense of loss of autonomy and responsibility. Defining successful organization structure for a CS team requires addressing concerns of leadership, authority, responsibility, and ownership. The actualization of such a partnership requires buy‐in from numerous parties to a shared vision. From a CS team's inception, member alignment and agreement are imperative but are often exacting to achieve.
Table 3.
Potential Challenges With Team‐Based Care in Cardiogenic Shock
| Defining organizational structure |
| Limiting burden of excessive communication |
| Promoting and maintaining team member engagement |
| Minimizing loss of autonomy or authority |
| Maintenance of consistency |
| Reimbursement for member time and participation |
| Aligning necessary skill sets with multiple personnel |
| Maintaining stakeholder satisfaction |
An ideal process lacks variability—it is consistent across time without heterogeneity in its protocols. For a CS team, this requires the ability to provide a reliable, reproducible, and consistent response regardless of time of day or day of the week. CS team membership, therefore, requires commitment from multiple members of multiple specialties, each with varying capabilities and capacity. For service lines with variable overnight coverage or only a few individuals capable of (or interested in) participating as part of a CS team, consistency of coverage or engagement may challenge the process. Additionally, reimbursement for time and participation must be addressed by each institution. Time spent on a multidisciplinary call may not be reimbursed for all providers. This frequently requires team members to participate out of their own interest or benevolence, which may not be ideal for long‐term sustainability. Between specialties, competing demands for time exist, leading to the potential for variable engagement among team members and even liability concerns.
Depending upon the member composition, CS team‐based care may also risk alienating subspecialty colleagues with considerable experience and expertise in managing these patients. Care must be taken to identify appropriate stakeholders, provider skill sets, and individuals committed to the team mission. Furthermore, undermining the revered doctor–patient relationship, a valuable cornerstone of medicine, as well as loss of physician autonomy and ownership in decision‐making, oppose traditional paradigms of medical practice. Such team‐based approaches may have the unintended consequence of decreasing physician engagement or satisfaction with potential erosion of a sense of personal responsibility.
Finally, team‐based care may also present challenges for efficiency and communication. Multidisciplinary care increases the number of participants in any given decision, and facilitating communication between numerous individuals may be cumbersome. Although recent data suggest delays in care are not a byproduct of larger team‐based decisions, this remains a concern at many institutions and among individual providers. 6
Unique Challenges at the Unit, Hospital, and System Level
Each unit, hospital, and health care system, with its unique size, structure, culture, and experience, may face distinct challenges when it comes to implementing a CS team. At the unit level, for example, individual physicians may feel that their expertise and autonomy are being threatened when they are obligated to call other providers, particularly because they may be accustomed to making decisions independently. Therefore, for unit leaders, attention to interpersonal issues may be especially important for gaining support for CS teams.
At the hospital level, financial considerations are more likely to challenge the successful implementation of CS teams. Because programmatic support from hospital administrators often requires a clear financial incentive, hospitals may be uninterested in providing resources to support these programs. Without institutional support, the development and maintenance of formalized CS teams often falls entirely on individuals, which may be impossibly burdensome for even the most motivated physician.
At the system level, coordinating shock care across regional networks with “spoke” and “hub” hospitals requires buy‐in from an even broader set of stakeholders, including allied health personnel (eg, emergency medical systems). 7 Lessons from other models of regionalized systems of care (eg, ST‐segment–elevation MI) may provide a helpful template for overcoming such challenges. We speculate that an increased degree of cross‐collaboration between centers within a care network could have far‐reaching implications for improving regional care beyond the CS population. With that being said, concerns from smaller, transferring institutions must also be recognized and addressed; among these, the fear of losing patients with acute cardiovascular conditions to receiving centers and the fear of being considered or perceived as less proficient in shock care must both be recognized and assuaged in order to ensure effective and durable collaboration.
Cardiogenic Shock Teams and Outcomes—Lessons Learned from Successful Programs
The development of CS team‐based approaches at several vanguard institutions has been highly illustrative for the field. Here we examine lessons learned that were considered critical to the implementation, sustained growth, and success of 3 such programs at Inova Heart and Vascular, the University of Utah, and the University of Ottawa (Figure 3).
Figure 3. Important lessons learned from 3 vanguard cardiogenic shock team‐based programs—Inova Heart and Vascular, University of Utah, and University of Ottawa.

CVICU indicates cardiovascular intensive care unit; and MCS, mechanical circulatory support.
Inova Heart and Vascular
In July 2016, leaders at the Inova Schar Heart and Vascular Institute assembled a multidisciplinary task force composed of stakeholders from the specialties of advanced HF and transplant services, cardiovascular critical care (including cardiologists and intensivists), interventional cardiology, and cardiac surgery to review the state of CS care at the institution. Members of this team performed a comprehensive analysis of the cardiovascular service line's infrastructure, to include the historical volumes of patients admitted to the health system with a primary diagnosis of CS, mechanisms in place to facilitate transfer of patients with CS in the community to the Level 1 (highest tier) shock center, the spectrum of available therapies, and historical clinical outcomes. Their findings were consistent with contemporary clinical trial and registry data, with a survival rate to 30 days postdischarge of <50%. 8 , 21 , 41 , 55 During this analysis, the team identified 4 important themes with accompanying opportunities for improvement: (1) the care model was fractured, as there was not a standardized multidisciplinary evaluation process for patients with a suspected or confirmed diagnosis of CS; (2) patients with CS were often diagnosed late in the natural course of the disease state, when they were too ill or frail to derive any meaningful benefit from goal‐directed or salvage therapies 58 ; (3) there was inconsistent access to care, in which patients with CS at the Level 2 or 3 shock centers in the community were unable to be transferred in an expedited fashion to the Level 1 center for comprehensive, multiorgan system care 59 ; and (4) there were significant variations in practice patterns among providers and hospitals, in part due to the absence of adequately powered clinical trials from which to inform guidelines and management.
In response to these identified gaps in care delivery, the CS taskforce developed a diagnostic and therapeutic algorithm to help standardize care. The algorithm was predicated on 5 foundational principles of organizational structure and systems of care delivery: (1) early identification of shock; (2) comprehensive, invasive hemodynamic assessment to appropriately phenotype the disease with respect to congestive profiles; (3) minimizing the dose and duration of vasopressors, given their inherent cardiotoxic profiles; (4) when appropriate, early, selective, and hemodynamically tailored MCS with associated best practices around vascular access and closure; and (5) multidisciplinary and longitudinal 24/7 care in an American Heart Association Level 1 CICU with the full gamut of complex services—to include complex coronary revascularization, advanced cardiac replacement therapies, and dedicated, high‐intensity staffing. 5 A 1‐call “shock line” was also developed so that when presented with a case of suspected CS, there was timely assembly of the on‐call physicians from the various specialties comprising the “shock team” to then facilitate multidisciplinary consultation and clinical decision‐making, including initial guidance to the referring physician. All cases were reviewed and discussed at biweekly quality improvement meetings. This initiative was associated with a significant improvement in short‐term outcomes, with 30‐day survival rates >70% in all patients with CS within 18 months of implementation of this new care model. Inova further developed a validated, multimodality risk score incorporating baseline demographics and hemometabolic variables at dynamic time points to help quantify risk and inform clinical decision‐making. 8 Notably, hemodynamic parameters including cardiac power output and pulmonary artery pulsatility index at 24 hours were most prognostic of short‐term mortality, suggesting that the clinical trajectory in the first day was critical to a patient's survival.
The Inova team was among the first in the nation to demonstrate the merits of standardized care for CS across multiple hospitals and associated health systems. The team built upon early success to engage in systematic outreach efforts with 34 partnering Level 2 and 3 shock centers, spanning a geographic area of nearly 6000 square miles in Maryland, Virginia, West Virginia, and the District of Columbia. 60 , 61 In addition to disseminating their treatment algorithm and facilitating access to their shock team, physicians and advanced practice providers from Inova participated in focused educational sessions with clinical and administrative leaders at outside hospitals, reviewing contemporary evidence in CS management, including early diagnosis, timely invasive hemodynamic assessment, indications for MCS, best practices around vascular access and critical care. Providers from the Level 2 and 3 centers were invited to the biweekly shock meetings and bidirectional feedback was provided for quality improvement. Using this approach, the Inova experience demonstrated that the implementation of a dedicated and regionalized shock network based upon standardized protocols, interhospital collaboration, and early transfer could be associated with similar short‐term outcomes (including in‐hospital mortality, 30‐day mortality, major bleeding complications, stroke, and 30‐day major adverse cardiovascular and cerebrovascular events) for all patients, independent of whether they initially presented to a “hub” or “spoke” shock care center. 7
The University of Utah
The University of Utah (UU) Shock Team was established in April 2015, aiming to manage patients with CS using a multidisciplinary approach. The team included the following specialties: advanced HF cardiology, cardiothoracic surgery, interventional cardiology, cardiovascular ICU medicine and nursing. The team at UU opted to develop a simple, pragmatic algorithm for team activation, with a focus on ease of implementation. As a result, there are currently no hemodynamic or other clinical criteria required to justify the team's engagement. The rationale for this simplified approach was to encourage a low threshold for activation and hence more widespread use of the shock team. This has proven over the years to be effective and has resulted in relatively low rates of “false alarms.” However, after feedback from key stakeholders, suggesting that more specific criteria for team activation should be considered, reexamination of this algorithm is currently in process.
After the shock team is activated through the institution's paging system, a discussion regarding patient management is initiated between all involved parties. The on‐call shock team members are not required to be immediately present in the hospital during weekends or after hours. Once a patient has been vetted and a diagnosis of CS has been corroborated, empiric medical therapy is initiated and right heart catheterization is performed. The escalation to short‐term MCS is considered by the shock team based upon specific, predetermined criteria. In general, the philosophy the UU shock team has been to use the shock team to facilitate early identification and triage of patients with CS and leave a majority of the other decisions to the discretion of the primary cardiovascular ICU team; review of internal data did not suggest that this degree of primary team autonomy resulted in either delays in care or conflicts between health care professionals.
The HF cardiology service operates as the hub responsible for coordinating care; this seemed prudent given that the institution's HF specialists have a well‐established culture of collaboration with the different specialties involved in CS management. The importance of including and prioritizing cardiovascular ICU nursing in the central structure of the shock team was one of the lessons learned at UU. Initially this group was not included structurally on the shock team, but a change was quickly implemented thereafter and led to enhanced cohesiveness and improved communication.
Because UU is the only academic medical center in the Mountain West and cares for individuals across nearly 10% of the geographic continental United States, physical transfer of patients with CS can be unwieldy. Given the tremendous expansion of telehealth platforms, frequently leveraged during the COVID19 pandemic, the idea of a virtual collaborative shock team composed of clinicians across institutions was examined. After careful vetting, the team at the UU is currently preparing for its implementation. Continuous refinement of processes, along with ongoing research and quality improvement initiatives have been keys to the growth and sustainability of the shock team platform at UU.
The University of Ottawa
The first lesson learned at the University of Ottawa was the importance of early engagement of major stakeholders, primarily critical care, interventional cardiology, cardiac surgery, and hospital administration. Institutional resources were needed, specifically the support of clinical services and access to MCS devices. Moreover, the shock team model was, to varying extents, a paradigm shift that included adoption of a dedicated mobile team to care for patients who were already being managed by another clinical team (often critical care). Demonstrating the incremental value of a separate shock team for these patients was imperative. Validating the merits of overlapping subspecialty skill sets and considering competing interests and renumeration considerations that could generate tension among team members was a priority. The early engagement of all stakeholders, with clear delineation of individual roles and the identification of specialty leads and champions was critical for acceptance, adoption, and successful clinical implementation.
A second valuable lesson was recognizing the need to adapt published management algorithms to local systems and resources. Unique institutional considerations impact the feasibility of implementing a universal model across all health care facilities. The University of Ottawa adopted elements of the shock team model vital to patient outcomes including multidisciplinary involvement to deliver multifaceted care, a coordinating physician to facilitate rapid patient triage, a 1‐call system to streamline team activation, methods for efficient virtual communication, and the use of invasive hemodynamics to guide management. Although early delivery of temporary MCS was advocated, the overall use of specific devices commonly employed in shock management algorithms was generally lower due to limited access to interventions heavily dependent upon hospital funds.
The University of Ottawa is a Level 1 shock center with a catchment of more than 30 hospitals (mostly Level 3 institutions) and is the only facility with cardiac catheterization laboratories and capability for percutaneous coronary intervention, cardiac surgery and durable, end‐stage HF therapies; therefore, the institution has a high volume and diverse patient population with CS. As a consequence, activation of the shock team in all patients with CS is impractical and risks overwhelming institutional resources. Because management protocols cannot encompass all possible patient permutations and clinical scenarios, the University of Ottawa emphasizes the value of multispecialty expertise and collective input to improve decision‐making.
A final important lesson has been the concept of “building gradually and reviewing frequently.” The program began with a limited number of highly committed shock team members and adhered to relatively narrow patient selection criteria. Over time, these procedures and approaches have been refined in parallel with the growth of team experience, expansion in the number of members, addition of new expertise, and in response to changes in patient referral patterns and the acquisition of new knowledge. This experience emphasizes the numerous similarities and occasional differences faced by institutions from different countries and regions.
The PE Response Team and PERT Consortium—A Model for Partnership and Discovery
History of PERT and the PERT Consortium
PE is the third leading cause of cardiovascular mortality, posing a significant burden to the US health care system. In contrast to stroke and AMI, the relative paucity of data informing optimal PE treatment strategies has complicated approaches to management. 62 The armamentarium for PE care has greatly expanded beyond systemic anticoagulation alone, particularly highlighted by the rapid evolution in interventional approaches. However, as data are largely limited to observational studies and underpowered trials exploring surrogate outcomes, the optimal management of patients with intermediate‐ and high‐risk PE is still unclear and increasingly heterogeneous. 63 Accordingly, a multidisciplinary, team‐based approach that includes several specialties has been recommended to improve clinical decision‐making. 64
Modeled after other successful rapid‐response systems, the first PERT was formed in 2012 at the Massachusetts General Hospital. Inspired by their efforts, there are now >150 PERT centers internationally. 10 , 62 Analogous to the Heart Team concept, PERTs capitalize on the expertise of several experts convened for immediate and real‐time consultation, tasked with reaching a consensus on individualized care for complex patients. 10 , 65 Cross‐specialty collaboration serves to challenge the “silo mentality,” dismantle biases, leverage appropriate treatment options, and obviate the need for serial consultation. 66 PERT programs also help with risk stratification, trainee and staff education, and the creation of longitudinal care pathways for survivors of PE. 63 , 66
The composition of the original PERT included cardiology, cardiothoracic surgery, echocardiography, emergency medicine, hematology, pulmonary/critical care, vascular medicine, and interventional cardiology. 10 Although the most optimal structure is still debatable, most centers use 3 to 5 specialists on their team, with critical care physicians (or intensivists) almost always present. 63 , 67 Interventional radiology, cardiac surgery, and cardiology have historically been included in at least 85% of PERT programs, depending upon center expertise and practice patterns. 68 Beyond the assembly of key stakeholders, PERT implementation and integration requires careful attention to design, mechanisms for efficient consultation, quality improvement, and iterative assessment. 63 , 65
The Massachusetts General Hospital PERT established a 24‐hour emergency activation line, promoted with an educational campaign consisting of posters and seminars. 63 Although most commonly initiated in the emergency department, PERT activations could occur from any internal site or outside facility. Intermediate‐ and high‐risk PEs have been considered indications for PERT activation at all participating institutions, whereas low‐risk PE or clinically unclear PE cases may or may not be included (although at times PERT is also called upon for diagnostic assistance). 67 , 69 Upon activation, most teams convene in their entirety; a tiered approach whereby an individual evaluates a patient before group assembly is less frequently practiced. 69 Infrastructural malleability allowing for the inclusion of non‐traditional team members (eg, obstetrics for the pregnant patient) is viewed as crucial for PERT success.
The PERT Consortium, an international nonprofit organization, was established in 2015—an aspirational goal of the Massachusetts General Hospital program to standardize PE management. Initially introduced at an inaugural meeting of >40 North American centers, the consortium now includes global membership from Europe, Asia, South America, and Australia. 69 In an effort to promote the PERT model, educate the public and health care professionals, and drive research initiatives, the consortium publishes position statements, collaborates on clinical trials, and hosts podcasts, webinars, and meetings. 65 Committees including Governance, Research, Education, Clinical Practice and Protocols, Development, and Communication serve as foundational elements for the group's mission. 69 , 70
Current PERT Consortium initiatives include the creation of PE Centers of Excellence, interhospital transfer projects to facilitate patient access to care regardless of geography, and an educational bootcamp for physicians‐in‐training. 70 The consortium also houses a quality database of >6800 patients from >30 institutions, and the embedded Pulmonary Embolism Research Collaborative is developing the foundation for the evaluation, treatment, and assessment of patients with PE in the domains of clinical care, quality assurance, and investigation. 70 In collaboration with industry partners, the PERT Consortium is involved in several ongoing, multicenter randomized clinical trials aimed at evaluating the impact of various reperfusion therapies on patient outcomes and designed to provide the first Level 1 evidence for many contemporary therapies. 70
Little more than a decade since its inception, the rapid and broad adoption of the PERT model has simultaneously fortified and been influenced by the evolution of advanced PE therapeutics. As the field matures, the PERT Consortium offers guidance to individual institutions while supporting research, promoting education, and benchmarking care quality across the world, and may serve as an exploitable model to accomplish the same in the team‐based management of CS.
Drawing Inspiration From the PERT Experience
Launching PERT at the first institution required navigating challenges and forging partnerships across service lines and divisions; extrapolating the PERT model to multiple institutions added another layer of analogous obstacles to consider. 10 , 66 Some of the tactics that promoted the success of the PERT concept have parallels at both local and national levels, and such themes and lessons can inform and bolster the approach to a nascent Cardiogenic Shock Team Collaborative.
Clear vision and mission—PERT devoted itself to a single clinical entity and focused on a clear bottom line to “advance PE care” and “improve patient survival and outcomes.” PE is an underserved public health issue as the most common cardiovascular disease after MI and stroke. PERT also inhabited the entire universe of PE care including diagnosis, prognosis, and treatment and from acute evaluations in the emergency and critical care settings to interventional and pharmacologic therapy to transitions of care including follow‐up clinics and prevention.
Shared goals and purpose—PERT defined specific, actionable goals that allowed multiple stakeholders to contribute. PERT sought to expand its model, contribute to the scientific literature on PE, assist with funding, and educate the general public. This mission purposefully parallels the “triple threat” in academic medicine: clinical, educational, and research excellence.
Passionate, committed leadership—visionary leadership is required to affect visionary change. PERT leaders manifested a commitment founded on the pure aim of addressing PE but were tireless in activity, giving voice to a previously unorganized group of like‐minded professionals.
“Big tent” approach—PERT not only allowed but actively welcomed all entities and persons interested in contributing to their vision. From within health care, all phases of PE support, all specialties, all role groups, and all levels of expertise were welcomed. PERT programs proliferated across the United States and globally with hundreds of organizations adopting the concept.
Building partnerships and collaborations—PERT forged partnerships among hospitals, but importantly with industry and government, including the US Food and Drug Administration.
Embedded educational objectives—PERT endeavored to use education in the form of a continuing medical education conference and patient materials from the very outset. Additionally, PERT used multimodal channels including innovative social media and asynchronous learning for delivery of content on a regular basis.
Promotion of mentorship—The collaborative nature of the PERT organization facilitated mentorship outside of traditional specialty siloes and across institutions.
Contribution to scholarship—PERT created a forum for PE scholarship to complement existing channels and sought to proactively drive research agendas.
“Buzz”—PERT successfully undertook marketing, social media, and custom‐branded materials to build identify and cultivate interest.
Applying these lessons, the PERT Consortium and its member institutions have enjoyed several successes along multiple dimensions of its mission (Table 4). 71 , 72 , 73 , 74 , 75 , 76 , 77 PE and CS have parallels as acute cardiovascular conditions, with high morbidity and mortality, and without an obvious evidence‐based best practice strategy, and thus for which multidisciplinary care, empiric interventional treatment, and networks of hub‐and‐spoke teams became a foundation of care delivery.
Table 4.
Successful Initiatives From the PERT Consortium
| Clinical |
|---|
| PERT Consensus Clinical Practice Guidelines for Diagnosis and Treatment 71 |
| PERT may reduce length of stay, possible mortality impact 72 |
| Interhospital Transfer Guide (2020) 68 |
| PE “Center of Excellence” certification, forthcoming |
| Research |
| National PERT Database, >12 000 patient entries as of 2023 |
| PE Research Consortium to define research goals and priorities |
| Collaboration with industry, recognizing evolving interventional paradigms for PE |
| Leadership in clinical trials: HI‐PEITHO, 73 STORM‐PE, 74 APEX‐AV, 75 PEERLESS, 76 PE‐TRACT 77 |
| Abstracts and poster sessions at PERT Symposium |
| Educational and Outreach |
| Annual National PERT Symposium, launched 2015 |
| Monthly digest of papers on PE published by PERT members |
| Webinars with clinical discussion, generally monthly |
| PERT Bootcamp |
| Efforts to recruit and mentor women clinicians |
| Patient and community engagement |
APEX‐AV indicates Evaluating the Safety and Efficacy of the AlphaVac Multipurpose Mechanical Aspiration (MMA) F1885 PE for Treatment of Acute Pulmonary Embolism; HI‐PEITHO, Ultrasound‐Facilitated, Catheter‐Directed, Thrombolysis in Intermediate‐High Risk Pulmonary Embolism; PE, pulmonary embolism; PERT, Pulmonary Embolism Response Team; PE‐TRACT, Pulmonary Embolism‐Thrombus Removal With Catheter‐Directed Therapy; and STORM‐PE, Prospective, Multicenter, Randomized Controlled Trial Evaluating Anticoagulation Alone Vs Anticoagulation Plus Mechanical Aspiration With the Indigo Aspiration System for the Treatment of Intermediate High Risk Acute Pulmonary Embolism.
Sustainability and Growth of PERT
The ability of the PERT Consortium to attract, retain, and rapidly increase membership is remarkable given the ever‐growing demands on time and financial resources of health care providers and organizations. Brent Keeling, past president of the PERT Consortium, shared insights into important strategies and decisions made by leaders that were important steps toward gaining early traction and facilitating subsequent growth.
At its inception, the PERT Consortium was a coalition of the willing—a grassroots effort that brought together individuals and institutions interested in PE. In the early days, PERT members joined to explore and discuss the current state of knowledge and strategies for improving care for a deadly disease that was not the primary focus of any existing organization. Maintaining engagement required more than simply common interest, and necessitated offering unique benefits from belonging to the PERT Consortium. At the time, there were no high‐quality data characterizing acute PE. The formation of a one‐of‐a‐kind national PE data repository created immediate value for members, offering access to data for research and for institutions to use in order to assess performance and organize quality improvement efforts—collecting and maintaining the highest quality data about acute PE for these reasons remains a foundational principle of the group upon which growth and sustainability rest.
Engagement, collaboration, and inclusivity with all stakeholders are the common themes at the core of PERTs and the evolution of the consortium's membership. The new PERT model of multidisciplinary team‐based decision‐making shifted the burden of managing complex, high‐risk patients away from individual providers, leading to increases in the number of PE clinical experts, PE researchers, and institution leaders focused on PE quality—all of whom found a professional home within the PERT Consortium. Mid‐ and late‐career professionals joined early. Opportunities to engage early‐career members and trainees were created by offering valuable research and leadership roles, including opportunities within trainee councils and invited roles for fellows, residents, and even high school students at the annual PERT symposium. Social media‐savvy members were leveraged to recruit their colleagues to join. The PERT Consortium has partnered effectively with other professional societies such as the National Thrombosis Forum and works collaboratively with international organizations such as the EXPERT‐PE group in Europe. There is also engagement at the institutional level with membership in the PERT Consortium and recognition through the “center of excellence” program, which identifies high‐performing sites as offering best quality PE care. From its inception, patients have also been welcomed into the PERT Consortium, with prominent roles in the national meeting and campaigns such as the “PE Looks Like Me” effort that seeks to bridge the gap between patients and care providers to spread awareness about the illness.
Building financial strength is necessary for the growth of any organization, and consistently adequate funding is often difficult to obtain. The PERT Consortium has addressed this challenge with a strategy of cultivating a myriad of diverse funding sources. As with other similar organizations, industry collaboration is key. Additional funding comes from a variety of sources including events such as the annual PERT gala, membership dues, “center of excellence” fees, and philanthropy.
The Cardiogenic Shock Team Collaborative—Mission, Goals, and Deliverables
We have outlined the many challenges associated with building and growing shock teams and have described several institutional success stories, albeit with important lessons learned along the way. Additionally, the PERT Consortium serves as a noble model for the creation of a similar program focused on the single clinical entity of CS. In order to improve outcomes for these patients within the complex and varied contemporary clinical landscape, we believe that it will be important to put together a shock team collaborative to identify and disseminate best practice principles for multidisciplinary care.
Toward a Mission Statement
As described in this article, the management of the patient with CS is complex and frequently requires multidisciplinary collaboration. 5 Care heterogeneity exists within and across health care institutions and systems. Such heterogeneity threatens to stymie collaborative scientific endeavors and potentially undermine patient outcomes. Challenges in CS care include but are not limited to (1) the ability to identify patients early; (2) the institution of timely, life‐saving interventions; (3) the transfer of patients to centers with advanced resources; (4) an understanding of the utility of temporary MCS devices; (5) the vetting of indications for escalation or deescalation of support; and (6) the capacity to define optimal methods to facilitate transition from temporary to more durable heart‐replacement strategies. Few would argue that CS care is easy, and complicated problems often require coordinated decision‐making. We believe that the employment of a CS team can lead to better outcomes and more thoughtful resource use. Although there exist several high‐quality CS registries and collaboratives that have been pivotal toward our understanding of the contemporary landscape, none have specifically focused on the shock team, multidisciplinary involvement, or process improvement.
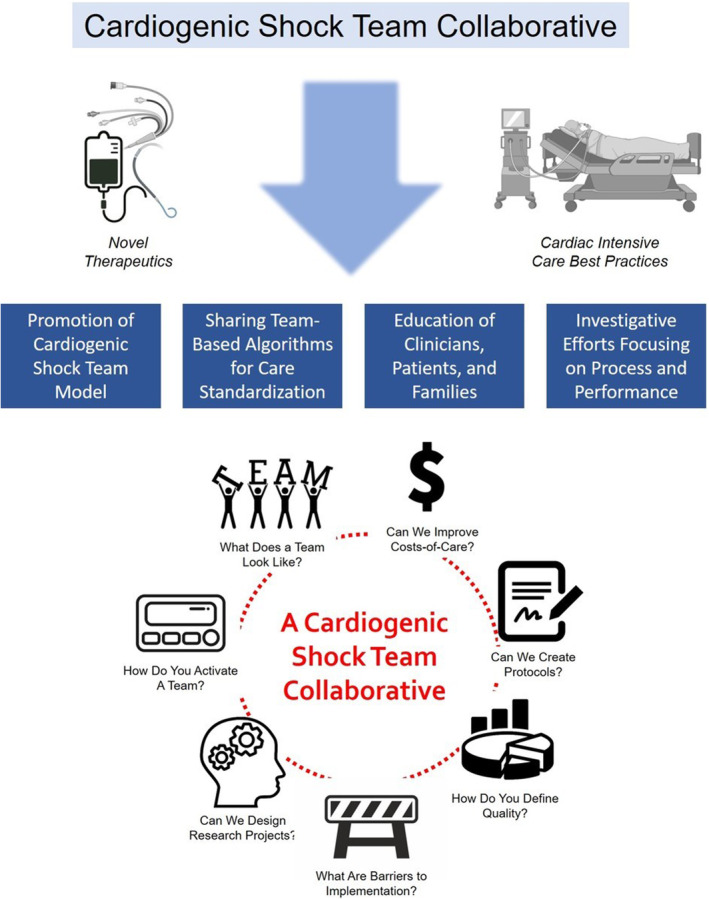
The mission of the CS Team Collaborative will be to improve the management and outcomes of patients with CS through innovative, efficient, multidisciplinary, and reliable team‐based models that leverage novel therapeutics and state‐of‐the‐art cardiovascular critical care principles. The goals of this alliance will include (1) the promotion of the CS team model within health care institutions across the United States, (2) the sharing of team‐based algorithms and the identification of opportunities for care standardization, (3) the education of health care professionals as well as the general public about CS, and (4) the systematic exploration of how process and performance affects patients, care quality, resource allocation, and costs (Figure 4).
Figure 4. Mission, goals, and potential deliverables of a proposed cardiogenic shock team collaborative.
Resource Needs and Partnerships
CS teams serve as the infrastructure for a system of care aimed at early identification of patients at risk for destabilization and effective communication between invested members. Building a CS team collaborative necessitates partnerships between multiple physician specialists, including providers from community to quaternary care facilities. The scope of this collaborative should not be limited to physicians alone; instead, a comprehensive CS team collaborative should also include input from members of patient transport teams, nonphysician practitioners, nursing, hospital administration, bed flow management personnel, and others. In order to foster knowledge exchange and promote a global perspective on the transformation of CS management, the collaborative should likewise seek the inclusion of international medical experts. Engagement and input from relative stakeholders will be critical. By working together on recommendations with a common goal to improve patient outcomes, this model can fortify the mutually beneficial relationships and establish requisite trust to build highly functional teams. The collaborative should also seek to include representation from CS data repositories, including the Cardiogenic Shock Working Group, the American Heart Association Cardiogenic Shock Registry, Extracorporeal Life Support Organization, Society of Thoracic Surgery Intermacs, and the Critical Care Cardiology Trials Network, to inform recommendations and to identify areas for organized research efforts, and even industry‐based registries necessary to evaluate real‐world practices and complication rates. These partnerships can serve as the foundation for systematic expansion of the current evidence‐base pertaining to CS management.
To bring together this extensive network of leaders in CS care, with the objective of developing necessary clinical, educational, and research initiatives, there will be a need for substantial resources. Much like with the PERT Consortium, financial sponsorship in the form of grants or industry funding, endorsement from a broad set of professional organizations, and a platform for dissemination of collaborative outcomes will be imperative. This platform may include various elements such as publications, a dedicated website, educational materials, and interdisciplinary conferences designed for a broad audience. Furthermore, the resources allocated for the collaborative should be designed for long‐term sustainability, including iterative processes for the inclusion of data and feedback from self‐instituted quality assessment measures. A quality assurance and review process should be integrated into the planning phase to ensure that the collaborative's mission and objectives are being successfully addressed. Finally, the successful execution of a project of this magnitude requires robust administrative support, technical assistance, and committed project management time from leadership.
Initial Deliverables—Research, Quality Improvement, and Cost‐Containment
Although a number of institutions have introduced and refined their employment of CS teams, there remains a notable absence of comprehensive data and consensus necessary to determine optimal team composition, appropriate activation processes, and the specific roles of clinician members. Additionally, although there are observational data to suggest that CS teams likely enhance patient outcomes, concrete evidence for this is lacking, as is a clear understanding of the financial implications for patients and institutions.
Collaborative initiatives, such as the one proposed, are particularly vital for situations where multiple practice patterns exist in the absence of clear guidance to address superiority. We hope that this collaborative can serve as a platform for amassing data to help our community better understand the impact of CS teams on important outcomes and that these data will inform future investigation. Not only that, but we believe this endeavor can function as a multi‐institutional and even multinational forum to facilitate real‐world discussions on the challenges and successes of CS teams and provide a platform for thoughtful analyses, derivation of prudent quality metrices, and institutional self‐reflection. These collaborations enable the sharing of experiences and therefore can foster mutual learning opportunities and a deeper, shared understanding of a complex subject matter—something vital to our work in CS (Figure 4).
Conclusions and Future Directions
Despite notable advances in pharmacologic and mechanical therapies for CS, the burden of morbidity and mortality remains unacceptably high. 5 Recognizing the inherent complexity of these patients, their often extensive multiorgan involvement, and the imperative need for earlier disease recognition and timely intervention, novel approaches must be considered and vetted as quickly as possible. Emerging data suggest that the involvement of a multidisciplinary CS team may improve outcomes. 6 , 7 , 61 Consequently, professional societies have expressed support for these dedicated programs.
Although a growing number of health care institutions have embraced the concept of the CS team approach to patient care, many challenges exist to their creation and implementation. Even where CS teams have been embedded within health care systems, substantial heterogeneity exists in regard to composition and care processes. This variability can be attributed, in part, to a lack of consensus within the cardiology and critical care communities as to the optimal model and role for CS team‐based management; hence, there are few data to inform guidelines and limited guidance from which to inform standards of care.
In light of these challenges, we have proposed the establishment of a Cardiogenic Shock Team Collaborative comprising key stakeholders in the field. The primary objective of this consortium will be to address and mitigate the inter‐institutional disparities in care seen with early team‐based methods. Our approach will involve thoughtful dialogue, rigorous research, and the structured examination of various multidisciplinary care models to determine optimal composition and operational strategies—akin to the successful development and maturation of the PERT Consortium model nearly a decade ago. From this, we aspire to cultivate a comprehensive CS team registry to aggregate and generate evidence to inform future practice. An inaugural meeting, perhaps embedded within an existing shock conference, will look to serve as an opportunity to unite key partners, facilitate discussion on the current landscape, identify gaps in knowledge and research priorities, and exchange insights on site‐based successes and challenges that can be disseminated to the community at large. Through an iterative process, we believe that the CS Shock Team Collaborative will help to foster a community focused on practical guidance, the generation of novel data, and the development of innovation for a complex pathology and clinical condition that continues to kill our patients at an alarming rate.
Sources of Funding
None.
Disclosures
Dr. Berg has received institutional grants to Brigham & Women’s Hospital from AstraZeneca and Pfizer; honoraria from the Medical Education Speakers Network and USV Private Limited; consulting feeds from AstraZeneca, MobilityBio, Pfizer, and Youngene Therapeutics; and serves on clinical endpoint committees for studies sponsored by Beckman Coulter, Kowa Pharmaceuticals, and Tosoh Biosciences. Dr. Drakos serves as a consultant for Abbott Corporation and receives research support from Novartis.
The American Heart Association celebrates its 100th anniversary in 2024. This article is part of a series across the entire AHA Journal portfolio written by international thought leaders on the past, present, and future of cardiovascular and cerebrovascular research and care. To explore the full Centennial Collection, visit https://www.ahajournals.org/centennial.
This article was sent to Sula Mazimba, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 16.
References
- 1. Jentzer JC, Ahmed AM, Vallabhajosyula S, Burstein B, Tabi M, Barsness GW, Murphy JG, Best PJ, Bell MR. Shock in the cardiac intensive care unit: changes in epidemiology and prognosis over time. Am Heart J. 2021;232:94–104. doi: 10.1016/j.ahj.2020.10.054 [DOI] [PubMed] [Google Scholar]
- 2. Tyler JM, Brown C, Jentzer JC, Baran DA, van Diepen S, Kapur NK, Garberich RF, Garcia S, Sharkey SW, Henry TD. Variability in reporting of key outcome predictors in acute myocardial infarction cardiogenic shock trials. Catheter Cardiovasc Interv. 2022;99:19–26. doi: 10.1002/ccd.29710 [DOI] [PubMed] [Google Scholar]
- 3. Ghajar A, Ordonez CP, Philips B, Pinzon PQ, Fleming LM, Motiwala SR, Sriwattanakomen R, Ho JE, Grandin EW, Sabe M, et al. Cardiogenic shock related cardiovascular mortality trends in US population: heart failure vs acute myocardial infarction as contributing causes. Int J Cardiol. 2022;367:45–48. doi: 10.1016/j.ijcard.2022.08.043 [DOI] [PubMed] [Google Scholar]
- 4. Osman M, Syed M, Patibandla S, Sulaiman S, Kheiri B, Shah MK, Bianco C, Balla S, Patel B. Fifteen‐year trends in incidence of cardiogenic shock hospitalization and in‐hospital mortality in the United States. J Am Heart Assoc. 2021;10:e021061. doi: 10.1161/JAHA.121.021061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–e268. doi: 10.1161/CIR.0000000000000525 [DOI] [PubMed] [Google Scholar]
- 6. Taleb I, Koliopoulou AG, Tandar A, McKellar SH, Tonna JE, Nativi‐Nicolau J, Villela MA, Welt F, Stehlik J, Gilbert EM, et al. Shock team approach in refractory cardiogenic shock requiring short‐term mechanical circulatory support. Circulation. 2019;140:98–100. doi: 10.1161/CIRCULATIONAHA.119.040654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tehrani BN, Sherwood MW, Rosner C, Truesdell AG, Lee SB, Damluji AA, Desai M, Desai S, Epps KC, Flanagan MC, et al. A standardized and regionalized network of care for cardiogenic shock. JACC:Heart Fail. 2022;10:768–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tehrani BN, Truesdell AG, Sherwood MW, Desai S, Tran HA, Epps KC, Singh R, Psotka M, Shah P, Cooper LB, et al. Standardized team‐based care for cardiogenic shock. J Am Coll Cardiol. 2019;73:1659–1669. doi: 10.1016/j.jacc.2018.12.084 [DOI] [PubMed] [Google Scholar]
- 9. Moghaddam N, van Diepen S, So D, Lawler PR, Fordyce CB. Cardiogenic shock teams and centres: a contemporary review of multidisciplinary care for cardiogenic shock. ESC Heart Fail. 2021;8:988–998. doi: 10.1002/ehf2.13180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Provias T, Dudzinski DM, Jaff MR, Rosenfeld K, Channick R, Baker J, Weinberg I, Donaldson C, Narayan R, Rassi AN, et al. The Massachusetts General Hospital Pulmonary Embolism Response Team (MGH PERT): creation of a multidisciplinary program to improve care of patients with massive and submassive pulmonary embolism. Hosp Pr. 2014;42:31–37. doi: 10.3810/hp.2014.02.1089 [DOI] [PubMed] [Google Scholar]
- 11. Berg DD, Bohula EA, Morrow DA. Epidemiology and causes of cardiogenic shock. Curr Opin Crit Care. 2021;27:401–408. doi: 10.1097/MCC.0000000000000845 [DOI] [PubMed] [Google Scholar]
- 12. Jentzer JC, Baran DA. The changing face of cardiogenic shock: definitions, epidemiology, and severity assessment. Curr Opin Crit Care. 2023;29:363–370. doi: 10.1097/MCC.0000000000001065 [DOI] [PubMed] [Google Scholar]
- 13. Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, Hollenberg SM, Kapur NK, O'Neill W, Ornato JP, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock. Catheter Cardiovasc Interv. 2019;94:29–37. doi: 10.1002/ccd.28329 [DOI] [PubMed] [Google Scholar]
- 14. Naidu SS, Baran DA, Jentzer JC, Hollenberg SM, van Diepen S, Basir MB, Grines CL, Diercks DB, Hall S, Kapur NK, et al. SCAI SHOCK stage classification expert consensus update: a review and incorporation of validation studies. J Am Coll Cardiol. 2022;79:933–946. doi: 10.1016/j.jacc.2022.01.018 [DOI] [PubMed] [Google Scholar]
- 15. Jentzer JC, Burstein B, van Diepen S, Murphy J, Holmes DR Jr, Bell MR, Barsness GW, Henry TD, Menon V, Rihal CS, et al. Defining shock and preshock for mortality risk stratification in cardiac intensive care unit patients. Circ Heart Fail. 2021;14:e007678. doi: 10.1161/CIRCHEARTFAILURE.120.007678 [DOI] [PubMed] [Google Scholar]
- 16. Vallabhajosyula S, Verghese D, Henry TD, Katz JN, Nicholson WJ, Jaber WA, Jentzer JC. Contemporary management of concomitant cardiac arrest and cardiogenic shock complicating myocardial infarction. Mayo Clin Proc. 2022;97:2333–2354. doi: 10.1016/j.mayocp.2022.06.027 [DOI] [PubMed] [Google Scholar]
- 17. Jentzer JC, Bhat AG, Patlolla SH, Sinha SS, Miller PE, Lawler PR, van Diepen S, Khanna AK, Zhao D, Vallabhajosyula S. Concomitant sepsis diagnoses in acute myocardial infarction‐cardiogenic shock: 15‐year national temporal trends, management, and outcomes. Crit Care Explor. 2022;4:e0637. doi: 10.1097/CCE.0000000000000637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Venkataraman S, Bhardwaj A, Belford PM, Morris BN, Zhao DX, Vallabhajosyula S. Veno‐arterial extracorporeal membrane oxygenation in patients with fulminant myocarditis: a review of contemporary literature. Medicina. 2022;58:215. doi: 10.3390/medicina58020215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vallabhajosyula S, Bhopalwala HM, Sundaragiri PR, Dewaswala N, Cheungpasitporn W, Doshi R, Prasad A, Sandhu GS, Jaffe AS, Bell MR, et al. Cardiogenic shock complicating non‐ST‐segment elevation myocardial infarction: an 18‐year study. Am Heart J. 2022;244:54–65. doi: 10.1016/j.ahj.2021.11.002 [DOI] [PubMed] [Google Scholar]
- 20. Vallabhajosyula S, Dewaswala N, Sundaragiri PR, Dewaswala N, Cheungpasitporn W, Doshi R, Prasad A, Sandhu GS, Jaffe AS, Bell MR, et al. Cardiogenic shock complicating ST‐segment elevation myocardial infarction: an 18‐year analysis of temporal trends, epidemiology, management, and outcomes. Shock. 2022;57:360–369. doi: 10.1097/SHK.0000000000001895 [DOI] [PubMed] [Google Scholar]
- 21. Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med. 1999;341:625–634. doi: 10.1056/NEJM199908263410901 [DOI] [PubMed] [Google Scholar]
- 22. Damluji AA, van Diepen S, Katz JN, Menon V, Tamis‐Holland JE, Bakitas M, Cohen MG, Balsam LB, Chikwe J. Mechanical complications of acute myocardial infarction: a scientific statement from the American Heart Association. Circulation. 2021;144:e16–e35. doi: 10.1161/CIR.0000000000000985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vallabhajosyula S, Vallabhajosyula S, Vaidya VR, Patlolla SH, Desai V, Mulpuru SK, Noseworthy PA, Kapa S, Egbe AC, Gersh BJ, et al. Venoarterial extracorporeal membrane oxygenation support for ventricular tachycardia ablation: a systematic review. ASAIO J. 2020;66:980–985. doi: 10.1097/MAT.0000000000001125 [DOI] [PubMed] [Google Scholar]
- 24. Hernandez‐Montfort J, Kanwar M, Sinha SS, Garan AR, Blumer V, Kataria R, Whitehead EH, Yin M, Li B, Zhang Y, et al. Clinical presentation and in‐hospital trajectory of heart failure and cardiogenic shock. JACC: Heart Fail. 2023;11:176–187. [DOI] [PubMed] [Google Scholar]
- 25. Abraham J, Blumer V, Burkhoff D, Pahuja M, Sinha SS, Rosner C, Vorovich E, Grafton G, Bagnola A, Hernandez‐Montfort JA, et al. Heart failure‐related cardiogenic shock: pathophysiology, evaluation and management considerations. J Card Fail. 2021;27:1126–1140. doi: 10.1016/j.cardfail.2021.08.010 [DOI] [PubMed] [Google Scholar]
- 26. Berg DD, Bohula EA, van Diepen S, Katz JN, Alviar CL, Baird‐Zars VM, Barnett CF, Barsness GW, Burke JA, Cremer PC, et al. Epidemiology of shock in contemporary cardiac intensive care units. Circ Cardiovasc Qual Outcomes. 2019;12:e005618. doi: 10.1161/CIRCOUTCOMES.119.005618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kanwar MK, Everett KD, Gulati G, Brener MI, Kapur NK. Epidemiology and management of right ventricular‐predominant heart failure and shock in the cardiac intensive care unit. Eur Heart J Acute Cardiovasc Care. 2022;11:584–594. doi: 10.1093/ehjacc/zuac063 [DOI] [PubMed] [Google Scholar]
- 28. Bansal M, Mehta A, Balakrishna AM, Sundaram AK, Kanwar A, Singh M, Vallabhajosyula S. Right ventricular dysfunction in sepsis: an updated narrative review. Shock. 2023;59:829–837. doi: 10.1097/SHK.0000000000002120 [DOI] [PubMed] [Google Scholar]
- 29. Korabathina R, Heffernan KS, Paruchuri V, Patel AR, Mudd JO, Prutkin JM, Orr NM, Weintraub A, Kimmelsteil CD, Kapur NK. The pulmonary artery pulsatility index identifies severe right ventricular dysfunction in acute inferior myocardial infarction. Catheter Cardiovasc Interv. 2012;80:593–600. doi: 10.1002/ccd.23309 [DOI] [PubMed] [Google Scholar]
- 30. Esposito ML, Kapur NK. Acute mechanical circulatory support for cardiogenic shock: the “door to support” time. F1000. Research. 2017;6:737. doi: 10.12688/f1000research.11150.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vallabhajosyula S, Dunlay SM, Kashani K, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR, Jaffe AS, Barsness GW. Temporal trends and outcomes of prolonged invasive mechanical ventilation and tracheostomy use in acute myocardial infarction with cardiogenic shock in the United States. Int J Cardiol. 2019;285:6–10. doi: 10.1016/j.ijcard.2019.03.008 [DOI] [PubMed] [Google Scholar]
- 32. Vallabhajosyula S, Dunlay SM, Prasad A, Kashani K, Sakhuja A, Gersh BJ, Jaffe AS, Holmes DR Jr, Barsness GW. Acute noncardiac organ failure in acute myocardial infarction with cardiogenic shock. J Am Coll Cardiol. 2019;73:1781–1791. doi: 10.1016/j.jacc.2019.01.053 [DOI] [PubMed] [Google Scholar]
- 33. Vallabhajosyula S, Kashani K, Dunlay SM, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR, Gersh BJ, Jaffe AS, Barsness GW. Acute respiratory failure and mechanical ventilation in cardiogenic shock complicating acute myocardial infarction in the USA, 2000‐2014. Ann Intensive Care. 2019;9:96. doi: 10.1186/s13613-019-0571-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vallabhajosyula S, Dunlay SM, Barsness GW, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR, Gersh BJ, Jaffe AS, Kashani K. Temporal trends, predictors, and outcomes of acute kidney injury and hemodialysis use in acute myocardial infarction‐related cardiogenic shock. PLoS ONE. 2019;14:e0222894. doi: 10.1371/journal.pone.0222894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chouairi F, Vallabhajosyula S, Mullan C, Mori M, Geirsson A, Desai HR, Ahmad T, Miller PE. Transition to advanced therapies in elderly patients supported by extracorporeal membrane oxygenation therapy. J Card Fail. 2020;26:1086–1089. doi: 10.1016/j.cardfail.2020.07.019 [DOI] [PubMed] [Google Scholar]
- 36. Vallabhajosyula S, Dunlay SM, Prasad A, Sangaralingham LR, Kashani K, Shah ND, Jentzer JC. Cardiogenic shock and cardiac arrest complicating ST‐segment elevation myocardial infarction in the United States, 2000‐2017. Resuscitation. 2020;155:55–67. doi: 10.1016/j.resuscitation.2020.07.022 [DOI] [PubMed] [Google Scholar]
- 37. Vallabhajosyula S, Prasad A, Bell MR, Sandhu GS, Eleid MF, Dunlay SM, Schears GJ, Stulak JM, Singh M, Gersh BJ, et al. Extracorporeal membrane oxygenation use in acute myocardial infarction in the United States, 2000 to 2014. Circ: Heart Fail. 2019;12:e005929. doi: 10.1161/CIRCHEARTFAILURE.119.005929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fernando SM, Mathew R, Sadeghirad B, Brodie D, Belley‐Cote EP, Thiele H, van Diepen S, Fan E, Di Santo P, Simard T, et al. Inotropes, vasopressors, and mechanical circulatory support for treatment of cardiogenic shock complicating myocardial infarction: a systematic review and network meta‐analysis. Can J Anesthesia. 2022;69:1537–1553. doi: 10.1007/s12630-022-02337-7 [DOI] [PubMed] [Google Scholar]
- 39. Ostadal P, Roykta R, Karasek J, Kruger A, Vondrakova D, Janotka M, Naar J, Smalcova J, Hubatova M, Hromadka M, et al. Extracorporeal membrane oxygenation in the therapy of cardiogenic shock: results of the ECMO‐CS randomized clinical trial. Circulation. 2023;147:454–464. doi: 10.1161/CIRCULATIONAHA.122.062949 [DOI] [PubMed] [Google Scholar]
- 40. Jentzer JC, Schrage B, Holmes DE, Dabboura S, Anavekar NS, Kirchhof P, Barsness GW, Blankenberg S, Bell MR, Westermann D. Influence of age and shock severity on short‐term survival in patients with cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2021;10:604–612. doi: 10.1093/ehjacc/zuaa035 [DOI] [PubMed] [Google Scholar]
- 41. Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–1296. doi: 10.1056/NEJMoa1208410 [DOI] [PubMed] [Google Scholar]
- 42. Thiele H, Akin I, Sandri M, Fuernau G, de Waha S, Meyer‐Saraei R, Nordbeck P, Geisler T, Landmesser U, Skurk C, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377:2419–2432. doi: 10.1056/NEJMoa1710261 [DOI] [PubMed] [Google Scholar]
- 43. Mathew R, Di Santo P, Jung RG. Milrinone as compared with dobutamine in the treatment of cardiogenic shock. N Engl J Med. 2021;385:516–525. doi: 10.1056/NEJMoa2026845 [DOI] [PubMed] [Google Scholar]
- 44. Thiele H, Zeymer U, Akin I, Behnes M, Rassaf T, Mahabadi AA, Lehmann R, Eitel I, Graf T, Seidler T, et al. Extracorporeal life support in infarct‐related cardiogenic shock. N Engl J Med. 2023;389:1286–1297. doi: 10.1056/NEJMoa2307227 [DOI] [PubMed] [Google Scholar]
- 45. Jaamaa‐Holmberg S, Salmela B, Suojaranta R, Lemstrom KB, Lommi J. Cost‐utility of venoarterial extracorporeal membrane oxygenation in cardiogenic shock and cardiac arrest. Eur Heart J Acute Cardiovasc Care. 2019;9:333–341. doi: 10.1177/2048872619900090 [DOI] [PubMed] [Google Scholar]
- 46. Vetrovec GW, Lim MJ, Needham KA. Cost savings for pVAD compared to ECMO in the management of acute myocardial infarction complicated by cardiogenic shock: an episode‐of‐care analysis. Catheter Cardiovasc Interv. 2021;98:703–710. doi: 10.1002/ccd.29181 [DOI] [PubMed] [Google Scholar]
- 47. Miller PE, Bromfield SG, Ma Q, Crawford G, Whitney J, DeVries A, Desai NR. Clinical outcomes and cost associated with an intravascular microaxial left ventricular assist device vs intra‐aortic balloon pump in patients presenting with acute myocardial infarction complicated by cardiogenic shock. JAMA Intern Med. 2022;182:926–933. doi: 10.1001/jamainternmed.2022.2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Strom JB, Zhao Y, Shen C, Chung M, Pinto DS, Popma JJ, Cohen DJ, Yeh RW. Hospital variation in the utilization of short‐term nondurable mechanical circulatory support in myocardial infarction complicated by cardiogenic shock. Circ Cardiovasc Interv. 2019;12:e007270. doi: 10.1161/CIRCINTERVENTIONS.118.007270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shah RU, de Lemos JA, Wang TY, Chen AY, Thomas L, Sutton NR, Fang JC, Scirica BM, Henry TD, Granger CB. Post‐hospital outcomes of patients with acute myocardial infarction with cardiogenic shock: findings from the NCDR. J Am Coll Cardiol. 2016;67:739–747. doi: 10.1016/j.jacc.2015.11.048 [DOI] [PubMed] [Google Scholar]
- 50. Sterling LH, Fernando SM, Talarico R, Qureshi D, van Diepen S, Herridge MS, Price S, Brodie D, Fan E, Di Santo P, et al. Long‐term outcomes of cardiogenic shock complicating myocardial infarction. J Am Coll Cardiol. 2023;82:985–995. doi: 10.1016/j.jacc.2023.06.026 [DOI] [PubMed] [Google Scholar]
- 51. Berg KM, Cheng A, Panchal AR, Topjian AA, Aziz K, Bhanji F, Bigham BL, Hirsch KG, Hoover AV, Kurz MC, et al. Part 7: Systems of Care: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020;142:S580–S604. doi: 10.1161/CIR.0000000000000899 [DOI] [PubMed] [Google Scholar]
- 52. Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, et al. Guidelines on myocardial revascularization. Task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS); European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2010;31:2501–2555. doi: 10.1093/eurheartj/ehq277 [DOI] [PubMed] [Google Scholar]
- 53. Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, Fonarow GC, Lange RA, Levine GN, Maddox TM, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation. 2014;130:1749–1767. doi: 10.1161/CIR.0000000000000095 [DOI] [PubMed] [Google Scholar]
- 54. Berg DD, Barnett CF, Kenigsberg BB, Papolos A, Alviar CL, Baird‐Zars VM, Barsness GW, Bohula EA, Brennan J, Burke JA, et al. Clinical practice patterns in temporary mechanical circulatory support for shock in the critical care cardiology trials network (CCCTN) registry. Circ: Heart Fail. 2019;12:e006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shaefi S, O'Gara B, Kociol RD, Joynt K, Mueller A, Nizamuddin J, Mahmood E, Talmor D, Shahul S. Effect of cardiogenic shock hospital volume on mortality in patients with cardiogenic shock. J Am Heart Assoc. 2015;4:e001462. doi: 10.1161/JAHA.114.001462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Na SJ, Park TK, Lee GY, Cho YH, Chung CR, Jeon K, Suh GY, Ahn JH, Carriere KC, Song YB, et al. Impact of a cardiac intensivist on mortality in patients with cardiogenic shock. Int J Cardiol. 2017;244:220–225. doi: 10.1016/j.ijcard.2017.06.082 [DOI] [PubMed] [Google Scholar]
- 57. de la Paz A, Orgel R, Hartsell SE, Pauley E, Katz JN. Getting cardiogenic shock patients to the right place–how initial intensive care unit triage decisions impact processes of care and outcomes. Am Heart J. 2020;230:66–70. doi: 10.1016/j.ahj.2020.09.008 [DOI] [PubMed] [Google Scholar]
- 58. Zweck E, Thayer KL, Helgestad OKL, Kanwar M, Ayouty M, Garan AR, Hernandez‐Montfort MC, Wencker D, Sinha SS, et al. Phenotyping cardiogenic shock. J Am Heart Assoc. 2021;10:e020085. doi: 10.1161/JAHA.120.020085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rab T, Ratanapo S, Kern KB, Basir MB, McDaniel M, Meraj P, King SB III, O'Neill W. Cardiac shock care centers: JACC review topic of the week. J Am Coll Cardiol. 2018;72:1972–1980. doi: 10.1016/j.jacc.2018.07.074 [DOI] [PubMed] [Google Scholar]
- 60. Beurtheret S, Mordant P, Paoletti X, Marijon E, Celermajer DS, Leger P, Pavie A, Combes A, Leprince P. Emergency circulatory support in refractory cardiogenic shock patients in remote institutions: a pilot study (the cardiac‐RESCUE program). Eur Heart J. 2013;34:112–120. doi: 10.1093/eurheartj/ehs081 [DOI] [PubMed] [Google Scholar]
- 61. Jaroszewski DE, Kleisli T, Staley L, Pierce C, Scott R, Steidley DE, DeValeria P, Arabia FA. A traveling team concept to expedite the transfer and management of unstable patients in cardiopulmonary shock. J Heart Lung Transplant. 2011;30:618–623. doi: 10.1016/j.healun.2010.11.018 [DOI] [PubMed] [Google Scholar]
- 62. Yuriditsky E, Horowitz JM. The role of the PERT in the management and therapeutic decision‐making in pulmonary embolism. Eur Heart J Acute Cardiovasc Care. 2022;11:693–694. doi: 10.1093/ehjacc/zuac102 [DOI] [PubMed] [Google Scholar]
- 63. Bejjani A, Khairani CD, Campia U, Piazza G. Pulmonary embolism response teams: theory, implementation, and unanswered questions. J Clin Med. 2022;11:6129. doi: 10.3390/jcm11206129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jimenez D, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543–603. doi: 10.1093/eurheartj/ehz405 [DOI] [PubMed] [Google Scholar]
- 65. Porres‐Aguilar M, Rosovsky RP, Rivera‐Lebron BN, Kaatz S, Mukherjee D, Anaya‐Ayala JE, Jimenez D, Jerjes‐Sanchez C. Pulmonary embolism response teams: changing the paradigm in the care for acute pulmonary embolism. J Thromb Haemost. 2022;20:2457–2464. doi: 10.1111/jth.15832 [DOI] [PubMed] [Google Scholar]
- 66. Dudzinski DM, Piazza G. Multidisciplinary pulmonary embolism response teams. Circulation. 2016;133:98–103. doi: 10.1161/CIRCULATIONAHA.115.015086 [DOI] [PubMed] [Google Scholar]
- 67. Barnes GD, Kabrhel C, Courtney DM, Naydenov S, Wood T, Rosovsky R, Rosenfeld K, Giri J; for the national PERT consortium research committee . Diversity in the pulmonary embolism response team model: an organizational survey of the national PERT consortium members. Chest. 2016;150:1414–1417. doi: 10.1016/j.chest.2016.09.034 [DOI] [PubMed] [Google Scholar]
- 68. Sosa DF, Lehr AL, Zhao H, Roth S, Lakhther V, Bashir R, Cohen G, Panaro J, Maldonado TS, Horowitz J, et al. Impact of pulmonary embolism response teams on acute pulmonary embolism: a systematic review and meta‐analysis. Eur Respir Rev. 2022;31:220023. doi: 10.1183/16000617.0023-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Porres‐Aguilar M, Anaya‐Ayala JE, Heresi GA, Rivera‐Lebron BN. Pulmonary embolism response teams: a novel approach for the care of complex patients with pulmonary embolism. Clin Appl Thromb Hemost. 2018;24:48S–55S. doi: 10.1177/1076029618812954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. The PERT Consortium.
- 71. Rivera‐Lebron B, McDaniel M, Ahrar K, Alrifai A, Dudzinski DM, Fanola C, Blais D, Janicke D, Melamed R, Mohrien K, et al. Diagnosis, treatment and follow up of acute pulmonary embolism: consensus practice from the PERT consortium. Clin Appl Thromb Hemost. 2019;25:1076029619853037. doi: 10.1177/1076029619853037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hobohm L, Farmakis IT, Keller K, Scibior B, Mavromanoli AC, Sagoschen I, Munzel T, Ahrens I, Konstantinides S. Pulmonary embolism response team (PERT) implementation and its clinical value across countries: a scoping review and meta‐analysis. Clin Res Cardiol. 2023;112:1351–1361. doi: 10.1007/s00392-022-02077-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Klok FA, Piazza G, Sharp ASP, Ainle FN, Jaff MR, Chauhan N, Patel B, Barco S, Goldhaber SZ, Kucher N, et al. Ultrasound‐facilitated, catheter‐directed thrombolysis vs anticoagulation alone for acute intermediate‐high‐risk pulmonary embolism: rationale and design of the HI‐PEITHO study. Am Heart J. 2022;251:43–53. doi: 10.1016/j.ahj.2022.05.011 [DOI] [PubMed] [Google Scholar]
- 74. Comparison of two pulmonary embolism treatments . Accessed November 16, 2023. https://clinicaltrials.gov/study/NCT05684796
- 75. Evaluating the safety and efficacy of the AlphaVac Multipurpose Mechanical Aspiration (MMA) F1885 PE for treatment of acute pulmonary embolism (APEX‐AV). Accessed November 16, 2023. https://clinicaltrials.gov/study/NCT05318092.
- 76. Gonsalves CF, Gibson CM, Stortecky S, Alvarez RA, Beam DM, Horowitz JM, Silver MJ, Catalin T, Rundback JH, Rosenberg SP, et al. Randomized controlled trial of mechanical thrombectomy vs catheter‐directed thrombolysis for acute hemodynamically stable pulmonary embolism: rationale and design of the PEERLESS study. Am Heart J. 2023;266:128–137. doi: 10.1016/j.ahj.2023.09.002 [DOI] [PubMed] [Google Scholar]
- 77. Late phase clinical trial design and planning for pulmonary embolism–thrombus removal with adjunctive catheter‐directed therapy (PE‐TRACT). Accessed on November 16, 2023. https://reporter.nih.gov/search/I05Wh0xOe0agNBNSR5guhg/project‐details/9748145.


