Abstract
Recently, myxoma virus was shown to encode an additional member of the serpin superfamily. The viral gene, called serp2, was cloned, and the Serp2 protein was shown to specifically bind to interleukin-1β (IL-1β)-converting enzyme (ICE), thus inhibiting the cleavage of pro-IL-1β by the protease (F. Petit, S. Bertagnoli, J. Gelfi, F. Fassy, C. Boucraut-Baralon, and A. Milon, J. Virol. 70:5860–5866, 1996). Here, we address the role of Serp2 in the development of myxomatosis, a lethal infectious disease of the European rabbit. A Serp2 mutant myxoma virus was constructed by disruption of the single-copy serp2 gene and insertion of the Escherichia coli gpt gene serving as the selectable marker. A revertant virus was obtained by replacing the E. coli gpt gene by the intact serp2 open reading frame. The Serp2− mutant virus replicated with wild-type kinetics both in rabbit fibroblasts and a rabbit CD4+ T-cell line (RL5). Moderate reduction of cell surface levels of major histocompatibility complex I was observed after infection with wild-type or Serp2− mutant myxoma virus, and both produced white pocks on the chorioallantoic membrane of the chick embryo. After the infection of European rabbits, the Serp2− mutant virus proved to be highly attenuated compared to wild-type myxoma virus, as demonstrated by the clinical course of myxomatosis and the survival rates of infected animals. Pathohistological examinations revealed that infection with wild-type myxoma virus resulted in a blockade of the inflammatory response at the vascular level. In contrast, rapid inflammatory reactions occurred upon infection with the Serp2− mutant virus. Furthermore, lymphocytes in lymph nodes derived from animals inoculated with Serp2 mutant virus were shown to rapidly undergo apoptosis. We postulate that the virulence of myxoma virus in the European rabbit can be partially attributed to an impairment of host inflammatory processes and to the prevention of apoptosis in lymphocytes. The weakening of host defense is directly linked to serp2 gene function and is likely to involve the inhibition of IL-1β-converting-enzyme-dependent pathways.
Virus survival within immunocompetent hosts requires multiple defensive strategies to evade antiviral and inflammatory responses. Poxviruses, which are among the largest animal viruses, have developed specific and efficient strategies, including interference with cytokines and growth factors, inhibition of the complement cascade, reduction of inflammation, and repression of cellular immune recognition, to effectively propagate within the infected host (3, 38, 47, 57, 60).
The genome of poxviruses is a linear large, double-stranded DNA molecule, encoding all the enzymes required for replication and transcription of its DNA, in addition to virulence factors. Whereas the essential genes are located in the central part of the genome, the genes responsible for virulence and host-range (usually not essential) mostly map near the termini (17, 65, 66). Some viral proteins help circumvent the host immune response, usually by mimicking cytokines or cytokine receptors. Poxviruses produce homologues to tumor necrosis factor (TNF) receptor (27, 56), interleukin-1β (IL-1β) receptor (2, 59), gamma interferon (IFN-γ) receptor (4, 5, 61, 69), and chemokine inhibitors (25, 55). Poxviruses are also able to block the production of some important cytokines by inhibiting the enzymes required for their processing. This is the case for the Orthopoxvirus genus members, for which SPI-2 protein (also known as CrmA) is an inhibitor of the IL-1β-converting enzyme (ICE) (33, 48, 50, 58, 64).
Previously, we described the cloning and characterization of Serp2, a new myxoma virus-encoded serpin protein closely related to CrmA (46). Myxoma virus (MV), a member of the genus Leporipoxvirus, is responsible for myxomatosis, a disease fatal to the European rabbit (Oryctolagus cuniculus). After an incubation period of a few days following infection, the primary site of intradermal inoculation evolves as a lesion characterized by tissue degeneration and necrosis. Viral dissemination leads to a generalization of the symptoms in the skin, head, and genital region, together with the development of gram-negative infections of the nasal and conjunctival mucosae (20, 37). The disease is characterized by general dysfunction of cellular immunity and multiple interruptions of the host cytokine network, with death as the most common outcome due to extreme weakness and secondary respiratory tract infections.
A number of MV proteins have been described to function as virulence factors, including M-T1 (25), M-T5 (39), M-T7 (40), M-T2 (69), SERP-1 (34, 67), M11L (45), and myxoma growth factor (45); most of these are proteins that interfere directly with effectors of the host immune system. The importance of Serp2 to myxoma virus replication in vitro and in vivo has not been investigated. We report here the characterization of Serp2 as another critical virulence factor of MV. Disruption of the Serp2 open reading frame resulted in an MV mutant that replicated normally in vitro and in vivo but was highly attenuated upon infection of European rabbits. Loss of MV virulence was correlated with marked increase of inflammatory and apoptotic responses in animals inoculated with Serp2− mutant virus. These in vivo findings are well explained by the results from our previous in vitro experiments suggesting that Serp2 can specifically inhibit ICE.
MATERIALS AND METHODS
Cells and viruses.
The MV strains T1 and Lausanne, the Serp2− mutant virus, the Shope fibroma virus (SFV), the vaccinia virus MVA, and the recombinant vaccinia virus MVA-HIV-nef were grown in the rabbit kidney cell line RK13 maintained in Dulbecco’s minimum essential medium supplemented with 10% fetal calf serum. The revertant MV-Serp2 rev was selected in HGPRT− HeLa cells (30). Rabbit CD4+ T lymphocytes, RL5 (29), were maintained in RPMI 1640 (Gibco BRL) supplemented with 10% fetal calf serum.
Construction of MV-Serp2− mutant and revertant viruses.
The MV serp2 gene was cloned into the Bluescript phagemid expression vector as described previously (46). DNA of this plasmid was used as template to PCR amplify the DNA fragments MV-serp2L and MV-serp2R. The following primers were used: 5′serp2-1 5′-XhoI-CAG CTC GAG CGT CGG CAG TCT TCG TTT CTC CCCG-3′; 5′serp2-2 5′-PstI-CAG CTG CAG GCC CTC GTT CCT CAC GTC CACG-3′, 3′serp2-1 5′-ClaI-CAG ATC GAT CCC GTA CGA GTA CGG GTA CTCC-3′, and 3′serp2-2 5′-SacI-CAG GAG CTC CGC GTA CGG GGG ACT GTT TAA ACG CG-3′. Amplified MV-serp2L and MV-serp2R DNA was digested with XhoI/PstI and ClaI/SacI, respectively, and inserted into pRBgpt flanking an expression cassette containing the Escherichia coli guanosine phosphoribosyltransferase (gpt) gene under the control of the vaccinia virus early-late promoter 7.5K (18). The resulting plasmid, called pserp2:gpt, was used for transfection into MV-infected cells. MV-Serp2− mutant MV was isolated selecting for resistance against mycophenolic acid (19).
An MV-Serp2 rev revertant virus containing a wild-type Serp2 open reading frame was constructed by transfecting plasmid DNA containing the complete serp2 gene into MV-Serp2−-infected RK13 cells and by using reverse gpt selection on HeLa cells (28) to isolate revertants. PCR and Southern blot analysis of viral DNA was used to confirm the disruption of the Serp2 open reading frame by insertion of the p7.5 gpt expression cassette, the absence of detectable wild-type virus in preparations of MV-Serp2− mutant virus, and the restoration of the serp2 gene in MV-Serp2 rev revertant virus. Immunoprecipitation of labeled proteins with anti-Serp2 antibodies was used to confirm the presence of a 34-kDa protein in extracts of cells infected with the wild-type or the MV-Serp2 rev revertant viruses, as well as the absence of any specific protein in extracts from MV-Serp2− mutant virus-infected cells.
Single-step growth analysis in cell culture.
RL5 cells (5 × 105) were infected with MV strain T1 or MV-Serp2− at a multiplicity of infection of 5 for 2 h. Unadsorbed free virus was removed, cells were washed with serum-free medium three times, growth medium was added, and cells were incubated at 37°C. Cultures were harvested at multiple time points postinoculation, and virus was released by freeze-thawing and brief sonication. Virus titers in these lysates were determined by standard plaque titration on RK13 cells monolayers. Single-step growth experiments were performed in triplicate.
Apoptosis assays.
To determine whether Serp2 could inhibit TNF-α-mediated apoptosis, 2 × 104 HeLa cells in microtiter plates were infected with wild-type or MV-Serp2− MV at a multiplicity of infection of 100, in RPMI containing 10 μM BrdU (bromodeoxyuridine). At 15 h postinfection, the cells were rinsed twice and fresh medium supplemented with serum was added. TNF-α (10 ng/ml; Boehringer-Mannheim) and cycloheximide (40 μg/ml; Sigma) were added, and apoptosis was assessed 8 h later by cellular DNA fragmentation enzyme-linked immunosorbent assay (ELISA) (Boehringer-Mannheim) according to the recommendations of the manufacturer. The cells were harvested by the addition of lysis buffer, which leads to a release of fragmented DNA from the cytoplasm to the supernatant. After an incubation of 30 min at room temperature, followed by a centrifugation at 250 × g for 10 min, the supernatants were transferred to an ELISA plate precoated with anti-DNA antibodies, and the amount of BrdU present in each sample was determined by using anti-BrdU peroxidase conjugate solution and its substrate with a spectrophotometer at 450 nm. Mock-infected cells, in the presence of TNF-α and cycloheximide, were used as a positive control, and cells cultured for 8 h with cycloheximide in the absence of TNF-α were used as a negative control.
Antibody binding and flow cytometry analysis.
RL5 cells (2 × 106) were infected with MV strain T1, MV-Serp2−, MV strain Lausanne, SFV, vaccinia virus MVA, or recombinant vaccinia virus MVA-HIV-nef at a multiplicity of infection of 5 for 2 h. Unadsorbed virus was removed, and the cells were washed with serum-free medium three times and then with medium supplemented with serum; the cultures were then harvested at 24 h postinfection. Cells were rinsed twice in RPMI supplemented with 1% serum and resuspended in 100 μl RPMI or binding buffer (137 mM NaCl, 12 mM NaHCO3, 2.6 mM KCl, 2 mM MgCl2, 5.6 mM glucose; pH 7.4) (11). CD4 monoclonal antibodies (Spring Valley Laboratories) were added at a concentration of 25 μg/ml to the cells in RPMI, and class I major histocompatibility complex (MHC) monoclonal antibodies (Spring Valley Laboratories) were added at a concentration of 25 μg/ml to the cells in binding buffer. After an incubation of 20 min at 4°C, the cells were rinsed in RPMI before addition of fluorescein isothiocyanate-conjugated goat anti-mouse antibody. The mixture was incubated for a further 20 min at 4°C. The cells were then rinsed twice in RPMI and resuspended in phosphate-buffered saline for analysis on a fluorescence-activated cell sorter Calibur flow cytometer (Becton Dickinson). Data were acquired from 20,000 cells and analyzed with CellQuest software. Experiments using isotypic immunoglobulin G1 were used as nonspecific binding controls. Analysis with all antibodies and all viruses were performed in triplicate.
Infection of rabbits with MV-Serp2− mutant virus.
Eight-week-old male New Zealand White rabbits (Oryctolagus cuniculus) were obtained from a local supplier and housed in biocontainment facilities under the guidelines of the European Community Council on Animal Care. Injections were performed intradermally in the right ear with 5 × 103 PFU of virus per animal. Rabbits which became moribund were sacrificed after anesthetization with T61 (Distrivet) administered intravenously. For histological studies, six rabbits each were inoculated with MV T1 strain or MV-Serp2− as described above. At 4, 8, and 11 days postinoculation, two animals from each group were sacrificed. Two mock-infected rabbits were sacrificed and used as controls.
Histological examination.
All animals were subjected to a complete postmortem examination. Tissue material from the injection site (ear; primary site) and ocular conjunctiva, parotid lymph node, spleen, lungs, and testis were taken and stored in 10% neutral formalin for further analysis. After fixation, tissues were processed routinely into paraffin blocks, sectioned at 4 μm, and stained with hematoxylin and eosin for microscopic examination. Histologic lesions were assessed and graded as follows: +, minimal; ++, light; +++, moderate; ++++, marked; and +++++, severe. The TUNEL method was used to assess apoptosis of lymphocytes in parotid lymph nodes and spleen. For this reaction, two thymuses of young mice treated with corticoids were used as positive controls. Conventional histologic sections, pretreated with 20 μg of proteinase K per ml (Boehringer-Mannheim) for 15 min at 37°C, were incubated with digoxigenin-labeled dUTP and terminal deoxytransferase (ONCOR), according to the recommendations of the manufacturer. The samples were stained using anti- digoxigenin peroxidase-conjugated antibodies. The localized peroxidase then catalytically generated a signal from a chromogenic substrate (3,3′-diaminobenzidine with nickel). The following form of grading, based on the number of apoptotic bodies for each microscopic field at a ×400 magnification, was used: +, minimal apoptosis, 25 to 50 apoptotic bodies; ++, light apoptosis, 50 to 75 apoptotic bodies; +++, moderate apoptosis, 75 to 100 apoptotic bodies; ++++, marked apoptosis, 75 to 100 apoptotic bodies; and +++++, severe apoptosis, 125 to 150 apoptotic bodies.
Immunostaining of histologic sections.
Viral antigens in paraffin-embedded sections of ear (primary site) and parotid lymph node were reactivated by using 0.1% trypsin in phosphate-buffered saline (pH 7.6) for 30 min at 37°C. After neutralization of endogenous peroxidase and before incubation with the specific primary antibody (rabbit hyperimmune serum anti-MV), the samples were incubated in goat preimmune serum. This step was performed in order to minimize the background generated by the secondary antibody (biotinylated goat anti-rabbit immunoglobulin G) provided by Dako (kit K492). Horseradish peroxidase-streptavidin complex was added and revealed by DAB (3,3′-diaminobenzidine tetrahydrochloride), which formed a brown precipitate. Nuclei were counterstained with Mayer’s hematoxylin.
RESULTS
Construction and characterization of MV-Serp2− mutant and revertant.
The Serp2 open reading frame is present as a single-copy gene in the MV genome (46) located within the EcoRI-F restriction fragment. To construct MV mutant virus, we disrupted the Serp2 open reading frame by targeting insertion of the Ecogpt marker gene under the control of the vaccinia virus p7.5 promoter by homologous recombination precisely to a site within the Serp2 coding sequence, resulting in a 30-bp deletion in the coding sequence. Using a standard dominant selection method (12, 19, 68), we isolated an MV-Serp2− mutant virus able to replicate in the presence of mycophenolic acid. As a control we constructed a revertant virus derived from MV-Serp2− in which the complete Serp2 open reading frame was restored and which was referred to as MV-Serp2 rev.
To ensure correct disruption of the serp2 gene and its authentic restoration in MV-Serp2 rev, viral DNA was analyzed by PCR with the specific primers 5′serp2-1 and 3′serp2-2. Agarose gel electrophoresis of DNA amplified from wild-type- and MV-Serp2 rev-specific template DNA revealed fragments corresponding in size (1,130 bp) to the expected molecular weight of the complete Serp2 coding sequence (data not shown). In contrast, a DNA fragment of 2,850 bp could be amplified from MV-Serp2− DNA, which indicated the presence of additional genomic sequence due to the integration of the gpt expression cassette. Furthermore, this analysis confirmed the genetic purity of MV-Serp2− mutant virus as shown by the failure to amplify a detectable DNA band corresponding in size to the wild-type Serp2 coding sequence. Additionally, Southern blot analysis of viral DNA revealed characteristic restriction patterns for the wild-type, MV-Serp2−, and MV-Serp2 rev genomes (data not shown). Since only 30 bp of the serp2 open reading frame were missing, we wanted to make sure that the protein could not be produced, even as a truncated form. Immunoprecipitation with a specific anti-Serp2 antiserum (46) revealed a polypeptide of 34 kDa, corresponding to Serp2, in extracts from RK13 cells infected by either the wild-type or the MV-Serp2 rev virus, but no specific band could be visualized on extracts from cells infected with the MV-Serp2− mutant (data not shown).
Comparison of pocks formed on the chorioallantoic membrane.
The chorioallantoic membrane (CAM) of chick embryos has been widely used to study the acute inflammatory response to poxvirus infection. In that system the wild-type cowpox virus produces hemorrhages, whereas a mutant lacking the crmA gene produces white pocks characterized by an influx of inflammatory cells (22, 48). To compare the lesions produced on CAM, 9-day-old embryonated eggs were infected with 105 PFU of wild-type MV or MV-Serp2− mutant. After 5 days of incubation at 33°C, an examination of the CAM revealed small white pocks (ca. 1 mm in diameter) which were present on CAM infected with either virus. No hemorrhage was detected. Furthermore, the pocks formed by both viruses did not differ in number or other morphology.
MV-Serp2− mutant efficiently replicates in rabbit kidney cell and CD4+ T-cell lines.
No defects in the ability of MV-Serp2− to replicate in cultured rabbit RK13 fibroblasts in vitro were noted in a single-step growth curve analysis. Similar results were also found in a cultured rabbit RL5 CD4+ T-cell line (data not shown).
MV-Serp2− mutant fails to induce apoptosis upon cell culture infection.
Since we have demonstrated previously that Serp2 exhibits ICE inhibiting activity and that the ICE family, recently named the caspases, plays a central role in the execution of apoptosis, we were interested in investigating apoptotic cell death after infection with MV-Serp2− mutant virus.
Since MV-Serp2− could replicate with wild-type kinetics in RL5, these cells were not expected to be a good model for investigating an antiapoptotic role for Serp2. Indeed, when using DNA fragmentation ELISA, we found no apoptosis in RL5 cells, infected with either wild-type or MV-Serp2− MV.
MV-Serp2− mutant virus inhibits TNF-α-mediated apoptosis.
In a similar way we also studied HeLa cells, which are susceptible to TNF-α-mediated apoptosis. We measured the cellular DNA fragmentation (ELISA test) after infection with wild-type MV or MV-Serp2− and after treatment with TNF-α and cycloheximide. Mock-infected cells, in the absence of TNF-α, showed no sign of apoptosis. Incubation with TNF-α and cycloheximide induced apoptosis, as measured 8 h after treatment. There was no significative apoptosis in cells infected with wild-type MV. The results were the same with cells infected with MV-Serp2−, indicating that Serp2 is not necessary for the inhibition of TNF-induced apoptosis in HeLa cells (data not shown).
Effect of Serp2 on the level of cell surface antigens.
Since it has been shown that there is a decrease of the class I MHC surface expression at 24 h after infection with MV strain Lausanne (11), we investigated whether disruption of the Serp2 open reading frame could influence the expression of these antigens at the cell surface. Having checked that mock-infected RL5 cells showed strongly positive surface staining with monoclonal antibody against the class I MHC, we saw no striking difference in the downregulation of MHC I on the surface of RL5 cells infected with wild-type MV strain T1 or with MV-Serp2− mutant virus (data not shown). These results suggest that Serp2 is not implicated in the downregulation of MHC I antigens on the surface of T-lymphocyte cells.
It has also been published that upon infection with MV, the level of CD4 surface antigens was markedly reduced (8). In a similar way, we also investigated the influence of serp2 disruption on the described phenotype. Mock-infected RL5 cells showed a strong positive surface staining with anti-CD4 antibodies. After infection with wild-type MV strain T1 or Lausanne, there was a slight decrease in surface CD4 levels; the same observation could be made after infection of RL5 cells with MV-Serp2− mutant. Infection of RL5 cells with vaccinia virus expressing HIV-nef led to a severe downregulation of CD4 molecules and served as a positive control (data not shown). These results suggest that Serp2 has no impact on the downregulation of CD4 antigens on the surface of RL5 cells.
MV-Serp2 is an important virulence factor in the European rabbit.
European rabbits infected with wild-type MV (strain T1 or Lausanne) rapidly develop a routinely 100% fatal disease known as myxomatosis. As well as being able to activate CPP32, an important cell death protease, ICE is known to liberate bioactive IL-1β, a proinflammatory lymphokine that can alert neighboring cells of the immune system. This signaling may allow inflammatory cells to activate and accumulate at a site where cell suicide is being used as an antiviral defense (70). Therefore, the putative anti-ICE activity of Serp2 tempted us to determine the effects of a serp2 disruption on MV virulence in vivo. Three groups of animals were infected with wild-type MV (n = 4), MV-Serp2− mutant virus (n = 10), or MV-Serp2 rev (n = 4). We observed a marked reduction in virulence in rabbits infected with MV-Serp2− compared to rabbits infected with wild-type MV or MV-Serp2 rev, respectively (Table 1). On day 4 postinfection, the inoculation site was a diffuse inflammation in rabbits infected with MV-Serp2−, larger and less circumscribed than in rabbits infected with wild-type MV or MV-Serp2 rev, in which a red and soft nodule, known as the primary myxoma, was present. At day 7 postinfection, when rabbits inoculated with either wild-type MV or MV-Serp2 rev demonstrated classical symptoms of myxomatosis and were prostrated, rabbits infected with MV-Serp2− had no skin lesions, such as secondary myxomas, on the head, body, or legs. They behaved normally and presented only a mild conjunctivitis. The ears were thickened and red. By day 11 postinfection, all eight rabbits infected with wild-type MV or MV-Serp2 rev had to be sacrificed, whereas in MV-Serp2−-infected rabbits, clinical signs of gram-negative infections in the conjunctiva and respiratory tract had developed, although the usual state of health remained unchanged (Fig. 1). The respiratory symptoms worsened between days 10 and 15, but no classical myxomas were observed. Seven of ten animals infected with MV-Serp2− mutant virus completely recovered within 30 days. Only three rabbits had to be sacrificed because of respiratory infection (on days 14, 15, and 25), which yields an overall survival rate of 70% in animals infected with MV-Serp2− virus. In contrast, a 100% mortality was found in both groups of control rabbits.
TABLE 1.
Pathogenicity of MV-Serp2− in European rabbits
| Day | Virulence effects of:
|
|
|---|---|---|
| Wild-type MV and MV-Serp2 reva | MV-Serp2− mutantb | |
| 0 | Inoculation of four rabbits with wild-type MV strain T1 and four rabbits with MV-Serp2 rev intradermally with 5,000 PFU | Inoculation of 10 rabbits intradermally with 5,000 PFU |
| 4 | Primary lesions at inoculation sites: raised, soft, red, ca. 2 cm | Primary lesions at inoculation sites: red, diffuse, ca. 3 cm |
| 7 | Emaciated; prostrated; gram-negative bacterial infections of nasal and conjunctival mucosa; multiple secondary lesions on face and ears; edema of the testicles | Normal fatness; regular activity; mild conjunctivitis; no gram-negative bacterial infections detected; diffuse inflammation of the ears |
| 11 | Emaciated; ataxia; dyspnea; severe gram-negative bacterial infections in conjunctiva and respiratory tract; secondary lesions turning necrotic; all rabbits sacrificed because of increased severity of the symptoms | Regular activity; purulent blepharoconjunctivitis and nasal discharge; marked inflammation of the ears; no secondary myxomas (6/10) or minute myxomas on the back and nose (4/10) |
| 15 | Gram-negative bacterial infections of nasal and conjunctival mucosa; no secondary or minute myxomas; one rabbit sacrificed at day 14; one rabbit sacrificed at day 15 | |
| 30 | Full regression of the symptoms, except for one rabbit sacrificed at day 25 due to respiratory bacterial infection | |
Rabbit survival: 0 of 4 and 0 of 4.
Rabbit survival: 7 of 10.
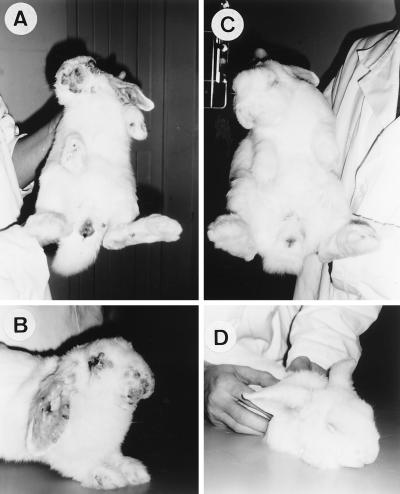
FIG. 1.
Clinical course of rabbits at 10 days postinoculation. Rabbits infected with the wild-type MV (A and B) show secondary skin lesions turning necrotic on the head, the back, and the legs and edema of the testicles (A) and show primary and secondary myxomas on the ears, leading to abnormal posture, and on the nose and around the eyes, and blepharoconjunctivitis (B). Rabbits infected with the MV-Serp2− mutant (C and D) show no secondary skin lesions and little edema of the genital region (C); they also show marked diffuse inflammation of the ears and blepharoconjunctivitis (D).
Histologic analysis of lesions from wild-type virus and MV-Serp2−-infected rabbits.
In view of the significant difference in virulence of the MV-Serp2− mutant and the wild-type MV, we performed a more detailed histologic examination of tissue material taken from both primary (ear) and secondary (ocular conjunctiva and parotid lymph node) infection sites at various times during the course of infection. Other sample sites (spleen, lungs, and testis) were realized to detect possible histologic lesions linked to a systemic spread of the virus. In all animals, no lesion was observed in these tissues, whatever the time after infection. The results of the complete analysis are summarized in Table 2.
TABLE 2.
Histologic observations of lesions from rabbits infected with the wild-type MV or the MV-Serp2− mutant
| Site | Lesion intensity (lesion topography)a at:
|
|||||
|---|---|---|---|---|---|---|
| Day 4 p.i.
|
Day 8 p.i.
|
Day 11 p.i.
|
||||
| Wild type | MV-Serp2− | Wild type | MV-Serp2− | Wild type | MV-Serp2− | |
| Primaryb | ||||||
| Perivascular dermatitis with: | ||||||
| Epidermal spongiosis and vesicles | +++++ | + (f) | ++++ | +++ | ||
| Edema | ++ (f) | + (f) | +++++ (d) | + | +++ (d) | ++ (d) |
| Focal interstitial microhemorrhage | ++ | + | +++ | + | ||
| Focal thrombosis | ++ | |||||
| Inflammatory infiltrates of: | ||||||
| Heterophils (perivascular and interstitial) | ++ (s) | + | +++ | + (s) | +++++ | ++ (s) |
| Histiocytes, lymphocytes (interstitial) | + | ++ (s) | ++++ | |||
| Activated fibroblasts | + | + | ++++ | ++ | ++++ | ++ |
| Interstitial mucinosis | + | + | ++++ | +++ | ++++ | ++ |
| Secondaryc | ||||||
| Superficial perivascular conjunctivitis with: | ||||||
| Inflammatory infiltrates of: | ||||||
| Heterophils | ++ (f) | + | +++ (f) | + (s) | ++++ (m) | ++ (s) |
| Mononuclear cells (histiocytes, lymphocytes) | ++ (s) | ++ | ||||
| Focal lymphoid hyperplasia | ++ | ++ | ||||
| Focal lymphoid depletion | ++ | |||||
| Activated fibroblasts | ++++ | ++ | ++++ | + | ||
| Interstitial mucinosis | ++++ | ++ | ++++ | + | ||
| Lymphadenitis with: | ||||||
| Lymphodepletion | +++ (f) | +++++ (m) | +++++ (e) | |||
| Focal microhemorrhage | ++ | |||||
| Focal histiocytosis | +++ | ++ | ++++ (e) | + | ++++ (e) | ++ |
| Infiltration by heterophils | ++ | +++ | +++ | + (s) | ++ | + (s) |
| Activated fibroblasts | ++++ | +++++ | ||||
| Interstitial mucinosis | ++++ | ++++ | ||||
Lesion intensity: +, minimal; ++, light; +++, moderate; ++++, marked; +++++, severe. Lesion topography: s, scattered; f, focal; m, multifocal; e, extensive; d, diffuse. p.i., postinoculation.
Samples were taken from lesions at the inoculation site.
Samples were taken from lesions on the ocular conjunctiva and parotid nodes.
At day 4 postinoculation, the injection site of animals infected with wild-type MV demonstrated a light perivascular dermatitis with focal edema and scattered heterophils. At the site of injection of the MV-Serp2− mutant, the inflammatory response was qualitatively the same but was less intense. A superficial perivascular conjunctivitis was present in each case, though slightly more pronounced for the wild-type virus. It should be noted that one rabbit inoculated with MV-Serp2− mutant showed a light lymphodepletion, while small lymphonodules were present in the lamina propria of the ocular conjunctiva in all other rabbits.
In the parotid lymph nodes, lesions induced by the two types of viruses differed significantly. A marked lymphadenitis with histiocytosis and infiltration by heterophils was seen after infection with the wild-type MV, whereas the lesions caused by MV-Serp2− mutant virus were characterized by extensive focal depletions of lymphocytes. The latter finding could well be the consequence of vigorous cell death triggered by the viral infection even if this process cannot be determined on conventional histologic sections.
At day 8 postinoculation, the differences in the resulting pathologic pattern became even more evident. In primary sites, the lesions associated with the wild-type virus were a marked perivascular dermatitis with diffuse edema, focal interstitial hemorrhages, accumulation of heterophils, and a well-developed myxoma (i.e., activated fibroblasts and interstitial mucinosis). In contrast, the lesions associated with the MV-Serp2− mutant presented two striking differences: they were much less intense, and the sequential inflammatory cellular reactions progressed more rapidly, as attested to by the presence of infiltrates of mononuclear cells (histiocytes and lymphocytes). These observations are also valid for the conjunctival lesions. The parotid lymph nodes of rabbits inoculated with the wild-type MV showed a marked lymphadenitis with secondary myxomas. The parotid lymph nodes of rabbits inoculated with MV-Serp2− mutant exhibited a severe lymphodepletion and no secondary myxoma was observed.
At day 11, the same major differences were noted, and some features must be pointed out: (i) a severe infiltration by heterophils persisted at the primary sites of infection with the wild-type virus, whereas for the MV-Serp2− mutant, mononuclear cells were predominant (Fig. 2); (ii) well-developed secondary myxomas were seen only in secondary sites of rabbits inoculated with wild-type MV; and (iii) a severe lymphodepletion was present only in the parotid lymph node of MV-Serp2− mutant-inoculated rabbits.
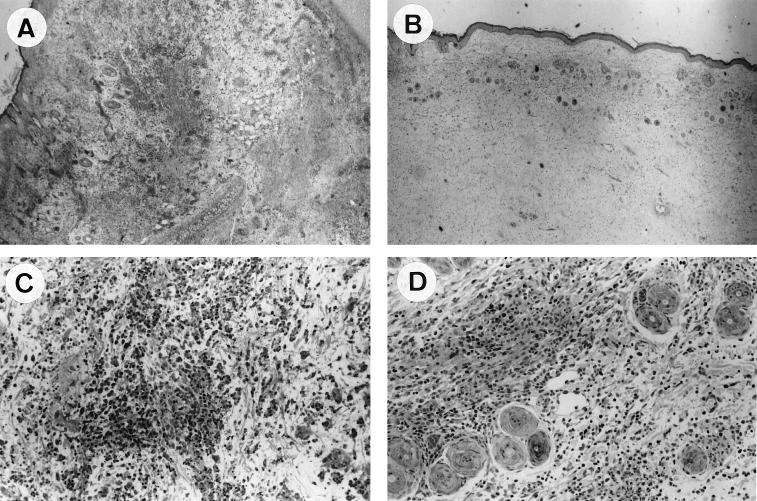
FIG. 2.
Hematoxylin and eosin-stained skin samples from rabbits at 11 days postinoculation with wild-type MV (A and C) or with MV-Serp2− mutant (B and D). Magnification: ×40 (A and B); ×200 (C and D). The panels show perivascular dermatitis with diffuse edema, thrombosis and perivascular accumulation of heterophils (A); perivascular dermatitis with a less-severe edema and interstitial infiltrates of mononuclear cells (B); severe perivascular and interstitial accumulation of heterophils (C); and interstitial infiltrates of mononuclear cells (C).
From these observations we can conclude that the inflammatory reaction is far less hemorrhagic and more rapidly progressive in lesions induced by the MV-Serp2− mutant. This virus leads to a progressive, severe lymphodepletion in the parotid lymph node. No secondary myxoma was seen in the secondary sites.
Histologic assessment of lymphoid apoptosis.
The TUNEL method was used to assess apoptosis of lymphocytes in the parotid lymph node and spleen of 12 inoculated rabbits and 2 control animals. The main results for the parotid lymph node are summarized in Table 3. The two controls showed minimal apoptosis (25 to 50 apoptotic bodies for each microscopic field at a ×400 magnification), mainly located in the germinal centers. For rabbits inoculated with wild-type MV, apoptosis of lymphocytes was at the same level and showed the same localization as controls, whatever the day of the experiment (Fig. 3A). The foci of apoptotic heterophils were seen; they could be easily distinguished from apoptotic lymphocytes by cytologic criteria.
TABLE 3.
Histologic assessment by the TUNEL method of lymphoid apoptosis in the parotid lymph nodes of rabbits infected with wild-type MV or MV-Serp2− mutant
| Day | Histologic assessment (lesion intensity)a
|
|
|---|---|---|
| Wild-type | MV-Serp2− | |
| 4 | A few apoptotic cells in germinal centers (as in controls). | Focal extensive apoptosis. |
| Score: homogeneous (+) | Score: variable (++/+++++). | |
| 8 | A few apoptotic lymphocytes in germinal centers (as in controls). | Focal extensive lymphoid apoptosis. |
| Score: homogeneous (+) | Score: homogeneous (+++++). | |
| 11 | A few apoptotic lymphocytes in germinal centers (as in controls). | Large foci of remnants of apoptotic bodies. |
| Score: homogeneous (+) | Score: homogeneous (+++++). | |
| Residual focal extensive apoptosis. | ||
| Score: homogeneous (+++++). | ||
| Extension of apoptosis in remaining lymphoid tissue. | ||
| Score: homogeneous (++). | ||
Lesion intensity: +, minimal; ++, light; +++, moderate; ++++, marked; +++++, severe.
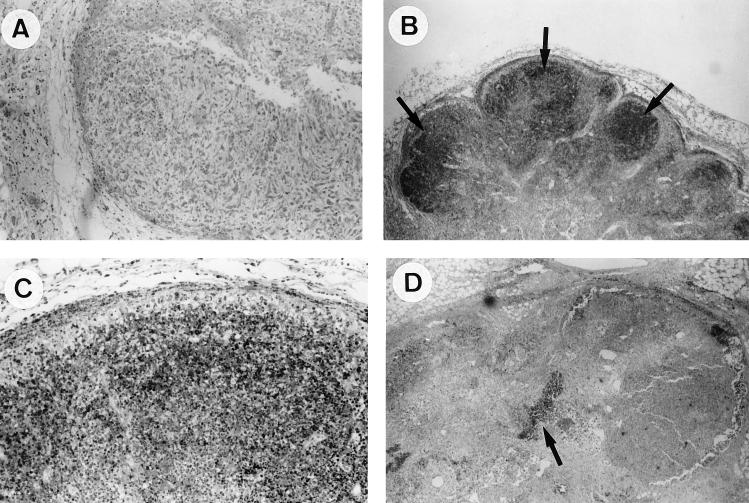
FIG. 3.
Parotid lymph node (TUNEL method). The panels show samples at 8 days postinoculation with the wild-type MV, with minimal lymphocytic apoptosis (A), and infection with the MV-Serp2− mutant at 4 days postinoculation, with focal extensive lymphocytic apoptosis in cortical areas (arrows) (B); at 4 days postinoculation, with focal extensive lymphocytic apoptosis at a higher magnification (C); and at 11 days postinoculation, with remnants of apoptotic foci in a lymphodepleted parenchyma (arrow) (D). Magnification: ×100 (A); ×40 (B); ×100 (C); ×40 (D).
The parotid lymph nodes of rabbits inoculated with the MV-Serp2− mutant underwent apoptosis, with major differences from the controls: at day 4 postinoculation, a focal extensive apoptosis was initiated in an area of the lymph node (Fig. 3B and C). The grade of the two rabbits was different (light versus severe). Foci of apoptotic heterophils were also seen. At day 8 postinoculation, the parotid lymph node of both rabbits showed a severe focal extensive apoptosis. At day 11, large areas of the parotid lymph node contained only remnants of apoptotic bodies for each animal (Fig. 3D). Traces of localized extensive foci persisted. In intact remaining lymphoid tissue, a light increase in the number of apoptotic lymphocytes was noticed.
From these results we conclude that lymphodepletion in the lymph node draining the primary site is imputable to apoptosis of lymphocytes. Apoptosis of lymphocytes in the spleen was the same in rabbits injected either with the wild-type MV or with the MV-Serp2− mutant and was identical to the controls.
Virus load in tissues.
In order to make sure that the striking phenotypic differences between the wild-type and the MV-Serp2− mutant could not be attributed to an impairment of the latter virus to replicate in vivo, we quantified the level of viral replication at the inoculation site and the parotid lymph node. Standard immunohistochemical studies were carried out, and the levels of virus replication in tissues stained with anti-MV antibodies were quantified by counting the number of infected cells per square millimeter.
At days 4, 8, and 11 postinoculation, there was the same level of virus replication at the injection site of either the wild-type or the MV-Serp2− mutant (not shown); comparable results were obtained in the parotid lymph node (Fig. 4). Eight days after infection with the wild-type MV, the cells in the lymph node (mostly fibroblasts and histiocytes, which are characteristic of the secondary myxomas) were positively stained with the anti-MV antibodies (Fig. 4A). With the MV-Serp2− mutant, the cell populations were different (mostly mononuclear cells, which from morphologic criteria could be defined as lymphocytes), but the viral load, according to our semiquantitative measure, was on the same order of magnitude (Fig. 4B). From these results we conclude that the MV-Serp2− mutant is able to replicate in vivo at a level comparable to that of the wild-type MV.
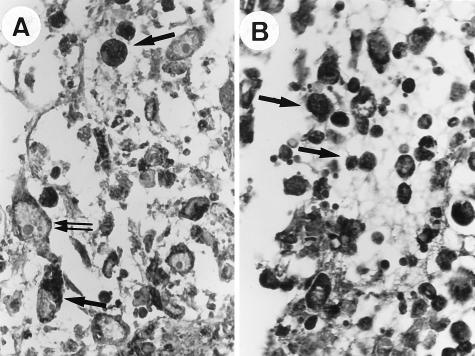
FIG. 4.
Parotid lymph node (immunohistochemistry) from rabbits at 8 days postinoculation (×400). Infected cells are visualized with DAB (arrow). (A) Infection with the wild-type MV. The cells present in the lymph node are mostly histiocytes and activated fibroblasts (large nucleus, double arrows). These and other cells are infected. (B) Infection with the MV-Serp2− mutant. Most cells are infected, and mononuclear cells are predominant.
DISCUSSION
In this study we report data to elucidate the functions of the MV Serp2 protein, which is 35% identical to the cowpox virus CrmA protein. CrmA has been characterized as viral inhibitor of ICE (caspase 1), which can prevent the onset of both inflammation and apoptosis by reducing the levels of the active proinflammatory lymphokines IL-1β and IL-18 and by preferentially inhibiting the proximal components of the ICE/CED-3 protease cascade in the cell death process (33, 48, 50, 58, 64). CrmA also has a high affinity for caspase 8 or FLICE (72) and Granzyme B (49). More importantly, Serp2 was also previously shown to bind to human ICE and prevent it from processing the pro-IL-1β into its bioactive form (46). Therefore, Serp2 was another candidate poxvirus-encoded factor to counteract host defenses and to potentially contribute to the pathology associated with MV infection.
To assess the role of Serp2 in the pathogenesis of myxomatosis, we successfully engineered a fully replication-competent MV mutant deleted in the Serp2 open reading frame. Revealing a wild-type-like phenotype when grown in vitro, the mutant virus was found to be highly attenuated upon in vivo infection in rabbits. The clinical course of infection with MV-Serp2− mutant was characterized by the development of mild and more-diffuse primary myxoma lesions at the site of inoculation, the absence or benignity of rare secondary lesions and, most impressively, by the high rate of complete recovery seen in the infected animals.
Detailed histologic examination led to three major findings. First, the inflammatory response upon inoculation with wild-type MV seemed to be arrested at the vascular level (with heterophils predominant even at a late time), whereas with the MV-Serp2 mutant the inflammation proceeded to the cellular phase (with lymphocytes and histiocytes infiltrating the lesion). In this latter case the overall reaction was less intense and less hemorrhagic than when Serp2 was present. The inhibition of IL-1β processing by Serp2 is in accordance with these observations. IL-1 is a potent proinflammatory cytokine that can affect the function of many physiologic systems (for a review, see reference 15). Among the pleiotropic effects of IL-1β, including thrombosis and inflammation, is an increased expression of the immunoglobulin superfamily molecules ICAM-1 and VCAM-1, which bind to integrins on lymphocytes and monocytes (14, 24). By blocking IL-1β processing, Serp2 would diminish the ability of these cells to migrate into the inflamed tissue. Moreover, ICE is involved in the processing of pro-IL-18 into its active form (23, 26). IL-18, also known as IFN-γ-inducing factor, stimulates the production of IFN-γ by lymphocytes (42, 44). As for IL-1β, the effects of IFN-γ are numerous, including the chemoattraction of macrophages (10, 43, 71). This phenomenon was also occurs after infection with a mutant lacking a secreted homolog of the IFN-γ receptor; Mossman et al. (40) reported that infection with the IFN-γ receptor-deficient MV resulted in an inversion of the heterophil/mononuclear cell ratio at the secondary sites. Our histologic findings, although not definitely proving which cytokine defect is involved, are consistent with the hypothesis that the presence of Serp2 leads to a reduced amount of both IL-1β and IFN-γ at the infection sites.
Our second important histologic observation was that lymphocytes, in the absence of Serp2, undergo apoptosis which can be quantified in the parotid lymph nodes. According to the spatial distribution of the apoptotic foci in the lymph nodes, it is reasonable to assume that apoptosis occurred in the lymph node itself, as well as in lymphocytes drained from the inoculation site. ICE has been widely recognized as an important mediator of the apoptotic process (reviewed in references 7 and 41). The impairing of ICE by Serp2 would be in accordance with an inhibition of the apoptotic process by the wild-type virus, but not by the MV-Serp2− mutant virus. However, in our in vitro experiments, the defect in Serp2 production did not lead to apoptosis of RL5 T-helper lymphocytes. This cell line has been widely used to study other MV genes. MV lacking the TNF receptor homologue (53), the M11L-gene (35), and the host-range superfamily member M-T5 (39) have all been shown to induce apoptosis in this cell line. The reason why all these mutants display the same phenotype in RL5 cells is not clear. M-T5 might counteract the shutoff of protein synthesis after MV infection (39), but M-T2 does probably not block apoptosis via its TNF binding domain (53). M11L is a transmembrane protein involved in the repression of inflammation, but its target is unknown (43). On the other hand, the cell-type dependence of apoptosis inhibition by viral products has been widely reported (6, 13, 21, 51). In that context, it is clear that RL5 cells are not the best model for checking Serp2 inhibition of ICE-mediated apoptosis.
There are several reports that either cowpox CrmA (62) or its homologue vaccinia virus SPI-2 protein (16, 32) can inhibit Fas-mediated apoptosis. However, in our experiments Serp2 was not required for the inhibition of TNF-induced apoptosis in HeLa cells. Several hypotheses underlie our findings. The first possibility is that, although reported to be unable to bind to human TNF-α (52), the MV TNF receptor homologue was responsible for this inhibition; another possibility is that there exists an as-yet-unidentified antiapoptotic factor encoded by MV, whose role would be to prevent TNF-induced apoptosis. It has recently been shown that the MC159 protein of Molluscum contagiosum, another member of the poxvirus family, could inhibit Fas- and TNFR1-induced apoptosis through its death effector domain (9). It is thus possible that MV encodes one or more proteins that would prevent ICE activation in cell culture.
Since it has been reported that MV could downregulate the expression of CD4 (8) and MHC I molecules (11) on the surface of infected cells, we checked whether Serp2 could account for this phenotype. In fact, we could see no difference in the relative decrease of either CD4 or MHC I antigens of RL5 cells infected with the wild-type or the MV-Serp2− mutant. The effect of MV on the expression of these surface molecules was light in both cases compared to the more drastic effect observed upon the vaccinia virus-mediated expression of the human immunodeficiency virus type 1 nef gene (1, 54). It was particularly relevant to check for a possible implication of Serp2 in MHC I downregulation, since this would have interfered with the apoptosis process. Any decrease in MHC I expression at the surface of infected cells would result in the inability of the cytotoxic T lymphocytes to bind to these cells and to recognize them as targets. Since Serp2 is not interfering with MHC I expression, the marked difference in the lymphocyte apoptosis observed in vivo cannot be attributed to a modification of the immune effectors on the membrane of the infected cells. More likely, the difference is due to an antiapoptotic effect of Serp2.
The last histologic finding was that in the secondary sites (ocular conjunctiva and lymph nodes) there were no so-called secondary myxomas upon infection with the MV-Serp2− mutant, contrasting with the wild-type MV. The molecular mechanism by which MV induces the formation of myxomas is unclear, but it should not be attributed only to the presence of the virus in the tissue. Indeed, we were able to show that the MV-Serp2− mutant can replicate as efficiently as the wild-type virus at the inoculation site and in the parotid lymph node. The comparable level of viral loads between both viruses indicates that the clinical and histologic differences observed cannot be attributed simply to a more poorly growing mutant virus. We were able to show that the lymphocytes in the lymph node of rabbits infected with the MV-Serp2− mutant are indeed infected.
Our conclusions from these observations are that in the MV, Serp2 is able to block both the processing of the inflammatory reaction at an early stage and apoptosis of the lymphocytes. It seems relevant to associate these phenomena with the inhibition of caspase 1 and/or another aspartic acid-specific protease.
It was previously reported that inactivation of the crmA gene of cowpox virus or its equivalent SPI-2 gene of rabbitpox virus resulted in an attenuation of the clinical course, as measured by the body weight of mice after intranasal infection (63). However, the cowpox and rabbitpox viruses differed in the inflammatory response. An acute inflammatory response was described as associated with disruption of the SPI-2 gene in the rabbitpox virus, whereas disruption of the open reading frame on the cowpox virus seemingly resulted in a decreased influx of inflammatory cells. Using the same murine intranasal model, other authors have reported that inactivation of the SPI-2 gene of the vaccinia virus had no effect on virus virulence (31). These contradictory results concerning three members of the Orthopoxvirus genus may be attributed to either true differences in the viruses or to mutations acquired elsewhere in the genomes, since neither cowpox nor rabbitpox revertant viruses were produced (63). However, in each case the mice had been inoculated intranasally. Here, we have been using the intradermal route, which seems more relevant since rabbits get contaminated through fleas or mosquito bites (20). It is also unlikely that mutations acquired elsewhere in the genome are responsible for our results since restoration of the wild-type phenotype was observed with the revertant virus. So we can state that what we describe here truly reflects the role of Serp2 during the course of a natural infection.
The actual targets of Serp2 in vivo have not been found yet. Indeed, in previous experiments we were able to visualize a complex between Serp2 and human ICE, and the processing of pro-IL-1β by ICE was inhibited by Serp2 produced in a baculovirus expression system (46). It is also reported, however, that CrmA can form a complex with ICE (caspase 1) (50), Cpp32 (caspase 3), FLICE (caspase 8), and Mch2 (caspase 6) (72); CrmA also has some inhibitory effect upon these four proteases (33, 72). However, kinetics analysis indicates that caspase 1 and caspase 8, but not caspase 3 and caspase 6, are direct targets of CrmA in vivo (72).
It has recently been demonstrated that, although the products of SPI-2 and crmA were thought to be equivalent, these two serpins actually have distinct effects on the caspases (36). Both proteins can block ICE activity in vitro (36). However, SPI-2 is unable to prevent apoptosis in pig kidney cells, whereas CrmA is a potent inhibitor. These results suggest that SPI-2 and CrmA have different targets in vivo.
It is likely that, like CrmA, Serp2 has more than one target. Our in vivo data support the hypothesis that ICE is one of them. Unlike CrmA and SPI-2, which are only expressed early during infection, Serp2 is expressed both at early and late times (46). It is not surprising that its extra target(s) might be different from those of CrmA or SPI-2.
There is currently a better knowledge of the viral genetic functions important for the pathogenesis of the leporipoxvirus genus, and several factors have been characterized to date. These include the MV TNF receptor (68); the IFN-γ receptor homolog (69); M-T5, which is a member of the host range superfamily (39); M11L and myxoma growth factor (MGF) (45); SERP-1, which is a serpin-like protein that interferes with inflammation (34, 67); and M-T1, a 35-kDa protein recently described as a chemokine-binding protein (25). Serp2 appears as a critical component of the immune evasion strategy elicited by MV. Further dissection of the multiple consequences of the inhibition of ICE and/or other caspases by Serp2 will be informative regarding the relative importance of inflammation and apoptosis for eliminating the virus.
ACKNOWLEDGMENTS
We are grateful to G. McFadden and G. L. Smith for kindly providing us with RL5 cells and HGPRT− HeLa cells, respectively. We also thank V. Lourec for monitoring the rabbits.
This work was supported by a grant from Institut National de la Recherche Agronomique.
REFERENCES
- 1.Aiken C, Konner J, Landau N R, Lenburg M E, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 2.Alcami A, Smith G L. A soluble receptor for interleukin-1β encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992;71:153–167. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]
- 3.Alcami A, Smith G L. Cytokine receptors encoded by poxviruses: a lesson in cytokine biology. Immunol Today. 1995;16:474–478. doi: 10.1016/0167-5699(95)80030-1. [DOI] [PubMed] [Google Scholar]
- 4.Alcami A, Smith G L. Vaccinia, cowpox, and camelpox viruses encode soluble gamma interferon receptors with novel broad species specificity. J Virol. 1995;69:4633–4639. doi: 10.1128/jvi.69.8.4633-4639.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alcami A, Smith G L. Soluble interferon-gamma receptors encoded by poxviruses. Comp Immunol Microbiol Infect Dis. 1996;19:305–317. doi: 10.1016/0147-9571(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 6.Ali A N, Turner P C, Brooks M A, Moyer R W. The SPI-1 gene of rabbitpox virus determines host range and is required for hemorrhagic pock formation. Virology. 1994;202:305–314. doi: 10.1006/viro.1994.1347. [DOI] [PubMed] [Google Scholar]
- 7.Anderson P. Kinase cascades regulating entry into apoptosis. Microbiol Mol Biol Rev. 1997;61:33–46. doi: 10.1128/mmbr.61.1.33-46.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barry M, Lee S F, Boshkov L, McFadden G. Myxoma virus induces extensive CD4 downregulation and dissociation of p56lck in infected rabbit CD4+ T lymphocytes. J Virol. 1995;69:5243–5251. doi: 10.1128/jvi.69.9.5243-5251.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertin J, Armstrong R C, Ottilie S, Martin D A, Wang Y, Banks S, Wang G-H, Senkevitch T G, Alnemri E S, Moss B, Leonardo M J, Tomaselli K J, Cohen J I. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:1172–1176. doi: 10.1073/pnas.94.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boehm U, Klamp T, Groot M, Howard J C. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 11.Boshkov L K, Macen J L, McFadden G. Virus-induced loss of class 1 MHC antigens from the surface of cells infected with myxoma virus and malignant rabbit fibroma virus. J Immunol. 1992;148:881–887. [PubMed] [Google Scholar]
- 12.Boyle D B, Coupar B E H. A dominant selectable marker for the construction of recombinant poxviruses. Gene. 1988;65:123–128. doi: 10.1016/0378-1119(88)90424-6. [DOI] [PubMed] [Google Scholar]
- 13.Brooks M A, Ali A N, Turner P C, Moyer R W. A rabbitpox virus serpin gene controls host range by inhibiting apoptosis in restrictive cells. J Virol. 1995;69:7688–7698. doi: 10.1128/jvi.69.12.7688-7698.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dianzani U, Malavasi F. Lymphocyte adhesion to endothelium. Crit Rev Immunol. 1995;15:167–200. doi: 10.1615/critrevimmunol.v15.i2.40. [DOI] [PubMed] [Google Scholar]
- 15.Dinarello C A. The biological properties of interleukin-1. Eur Cytokine Netw. 1994;5:517–531. [PubMed] [Google Scholar]
- 16.Dobbelstein M, Shenk T. Protection against apoptosis by the vaccinia virus SPI-2 (B13R) gene product. J Virol. 1996;70:6479–6485. doi: 10.1128/jvi.70.9.6479-6485.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drillien R, Spehner D, Villeval D, Lecoq J-P. Similar genetic organization between a region of fowlpox virus DNA and the vaccinia virus HindIIIJ fragment despite divergent location of the thymidine kinase gene. Virology. 1987;160:203–209. doi: 10.1016/0042-6822(87)90061-4. [DOI] [PubMed] [Google Scholar]
- 18.Falkner F G, Moss B. Escherichia coli gpt gene provides dominant selection for vaccinia virus open reading frame expression vectors. J Virol. 1988;62:1849–1854. doi: 10.1128/jvi.62.6.1849-1854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falkner F G, Moss B. Transient dominant selection of recombinant vaccinia viruses. J Virol. 1990;64:3108–3111. doi: 10.1128/jvi.64.6.3108-3111.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fenner F, Ratcliff F N. Myxomatosis. Cambridge, England: Cambridge University Press; 1965. [Google Scholar]
- 21.Foghsgaard L, Jaattela M. The ability of BHRF1 to inhibit apoptosis is dependent on stimulus and cell type. J Virol. 1997;71:7509–7517. doi: 10.1128/jvi.71.10.7509-7517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fredrickson T N, Sechler J M G, Palumbo G J, Albert J, Khairallah L H, Buller R M L. Acute inflammatory response to cowpox virus infection of the chorioallantoic membrane of the chick embryo. Virology. 1992;187:693–704. doi: 10.1016/0042-6822(92)90472-2. [DOI] [PubMed] [Google Scholar]
- 23.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Skut L, Talanian R, Paskind M, Wong W, Kamen R, Tracey D, Allen H. Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN-γ production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 24.Girard J-P, Springer T A. High endothelial venules (HEV): specialized endothelium for lymphocyte migration. Immunol Today. 1995;16:449–457. doi: 10.1016/0167-5699(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 25.Graham K A, Lalani A S, Macen J L, Ness T L, Barry M, Liu L-Y, Lucas A, Clark-Lewis I, Moyer R W, McFadden G. The T1/35kDa family of poxvirus-secreted proteins bind chemokines and modulate leukocyte influx into virus-infected tissues. Virology. 1997;229:12–24. doi: 10.1006/viro.1996.8423. [DOI] [PubMed] [Google Scholar]
- 26.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming M A, Hayashi N, Higashino K, Okamura H, Nakanishi K, Kurimoto M, Tanimoto T, Flavell R A, Sato V, Harding M W, Livingston D J, Su M S. Activation of interferon-γ inducing factor mediated by interleukin-1β converting enzyme. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 27.Hu F-Q, Smith C A, Pickup D J. Cowpox virus contains two copies of an early gene encoding a soluble secreted form of the type II TNF receptor. Virology. 1994;204:343–356. doi: 10.1006/viro.1994.1539. [DOI] [PubMed] [Google Scholar]
- 28.Isaacs S N, Kotwal G J, Moss B. Reverse guanine phosphoribosyltransferase selection of recombinant vaccinia viruses. Virology. 1990;178:626–630. doi: 10.1016/0042-6822(90)90367-z. [DOI] [PubMed] [Google Scholar]
- 29.Kaschka-Dierich C, Werner F J, Bauer I, Fleckenstein B. Structure of nonintegrated, circular herpesvirus saimiri and herpesvirus ateles genomes in tumor cell lines and in vitro-transformed cells. J Virol. 1982;44:295–310. doi: 10.1128/jvi.44.1.295-310.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerr S M, Johnston L H, Odel M, Duncan S A, Law K M, Smith G L. Vaccinia DNA ligase complements Saccharomyces cerevisiae cdc9, localizes in cytoplasmic factories and affects virulence and virus sensitivity to DNA damaging agents. EMBO J. 1991;10:4343–4350. doi: 10.1002/j.1460-2075.1991.tb05012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kettle S, Blake N W, Law K M, Smith G L. Vaccinia virus serpins B13R (SPI-2) and B22R (SPI1) encode Mr38.5 and 40K, intracellular polypeptides that do not affect virus virulence in a murine intranasal model. Virology. 1995;206:136–147. doi: 10.1016/s0042-6822(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 32.Kettle S, Alcami A, Khanna A, Ehret R, Jassoy C, Smith G L. Vaccinia virus serpin B13R (SPI-2) inhibits interleukin-1β-converting enzyme and protects virus-infected cells from TNF- and Fas-mediated apoptosis, but does not prevent IL-1β-induced fever. J Gen Virol. 1997;78:677–685. doi: 10.1099/0022-1317-78-3-677. [DOI] [PubMed] [Google Scholar]
- 33.Komiyama T, Ray C A, Pickup D J, Howard A D, Thornberry N A, Peterson E B, Salvesen G. Inhibition of interleukin-1β converting enzyme by the cowpox virus serpin CrmA. An example of cross-class inhibition. J Biol Chem. 1994;269:19331–19337. [PubMed] [Google Scholar]
- 34.Macen J L, Upton C, Nation N, McFadden G. SERP-1, a serine proteinase inhibitor encoded by myxoma virus, is a secreted glycoprotein that interferes with inflammation. Virology. 1993;195:348–363. doi: 10.1006/viro.1993.1385. [DOI] [PubMed] [Google Scholar]
- 35.Macen J L, Graham K A, Lee S F, Schreiber M, Boshkov L K, McFadden G. Expression of the myxoma virus tumor necrosis factor receptor homologue and M11L genes is required to prevent virus-induced apoptosis in infected rabbit T lymphocytes. Virology. 1996;218:232–237. doi: 10.1006/viro.1996.0183. [DOI] [PubMed] [Google Scholar]
- 36.Macen J L, Takahashi A, Moon K B, Nathaniel R, Turner P C, Moyer R W. Activation of caspases in pig kidney cells infected with wild-type and CrmA/SPI-2 mutants of cowpox and rabbitpox viruses. J Virol. 1998;72:3524–3533. doi: 10.1128/jvi.72.5.3524-3533.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McFadden G. Poxviruses of rabbits, 37–62. In: Darai G, editor. Virus diseases in laboratory and captive animals. Boston, Mass: Martinus Nijhoff; 1988. [Google Scholar]
- 38.McFadden G, editor. Viroceptors, virokines and related immune modulators encoded by DNA viruses. R. G. Austin, Tex: Landes Co.; 1995. [Google Scholar]
- 39.Mossman K, Lee S F, Barry M, Boshkov L, McFadden G. Disruption of M-T5, a novel myxoma virus gene member of the poxvirus host range superfamily, results in dramatic attenuation of myxomatosis in infected European rabbits. J Virol. 1996;70:4394–4410. doi: 10.1128/jvi.70.7.4394-4410.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mossman K, Nation P, Macen J, Garbutt M, Lucas A, McFadden G. Myxoma virus M-T7, a secreted homolog of the interferon-γ receptor, is a critical virulence factor for the development of myxomatosis in European rabbits. Virology. 1996;215:17–30. doi: 10.1006/viro.1996.0003. [DOI] [PubMed] [Google Scholar]
- 41.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura K, Okamura H, Nagata K, Komatsu T, Tamura T. Purification of a factor which provides a costimulatory signal for gamma interferon production. Infect Immun. 1993;61:64–70. doi: 10.1128/iai.61.1.64-70.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohh M, Takei F. New insights into the regulation of ICAM-1 gene expression. Leuk Lymphoma. 1996;20:223–228. doi: 10.3109/10428199609051611. [DOI] [PubMed] [Google Scholar]
- 44.Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, Akita K, Namba M, Tanabe F, Konishi K, Fukuda S, Kurimoto M. Cloning of a new cytokine that induces IFN-γ production by T-cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 45.Opgenorth A, Graham K, Nation N, Strayer D, McFadden G. Deletion analysis of two tandemly arranged virulence genes in myxoma virus, M11L and myxoma growth factor. J Virol. 1992;66:4720–4731. doi: 10.1128/jvi.66.8.4720-4731.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petit F, Bertagnoli S, Gelfi J, Fassy F, Boucraut-Baralon C, Milon A. Characterization of a myxoma virus-encoded serpin-like protein with activity against interleukin-1β-converting enzyme. J Virol. 1996;70:5860–5866. doi: 10.1128/jvi.70.9.5860-5866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pickup D J. Poxviral modifiers of cytokines responses to infection. Infect Agents Dis. 1994;3:116–127. [PubMed] [Google Scholar]
- 48.Pickup D J, Ink B S, Ray C A, Joklik W K. Hemorrhage in lesions caused by cowpox virus is induced by a viral protein that is related to plasma protein inhibitors of serine proteases. Proc Natl Acad Sci USA. 1986;83:7698–7702. doi: 10.1073/pnas.83.20.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quan L T, Caputo A, Bleakley R C, Pickup D J, Salvesen G. Granzyme B is inhibited by the cowpox virus serpin cytokine response modifier A. J Biol Chem. 1995;270:10377–10379. doi: 10.1074/jbc.270.18.10377. [DOI] [PubMed] [Google Scholar]
- 50.Ray C A, Black R A, Kronheim S R, Greenstreet T A, Sleath P R, Salvesen G S, Pickup D J. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1β converting enzyme. Cell. 1992;69:597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- 51.Ray C A, Pickup D J. The mode of death of pig kidney cells infected with cowpox virus is governed by the expression of the crmA gene. Virology. 1996;217:384–391. doi: 10.1006/viro.1996.0128. [DOI] [PubMed] [Google Scholar]
- 52.Schreiber M, McFadden G. The myxoma virus TNF-receptor homologue (T2) inhibits tumor necrosis factor α in a species-specific fashion. Virology. 1994;204:692–705. doi: 10.1006/viro.1994.1585. [DOI] [PubMed] [Google Scholar]
- 53.Schreiber M, Sedger L, McFadden G. Distinct domains of M-T2, the myxoma virus tumor necrosis factor (TNF) receptor homolog, mediate extracellular TNF binding and intracellular apoptosis inhibition. J Virol. 1997;71:2171–2181. doi: 10.1128/jvi.71.3.2171-2181.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwartz O, Marechal V, Gall S L, Lemonnier F, Heard J M. Endocytosis of major histocompatibility complex class I molecules is induced by HIV-1 Nef protein. Nature Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 55.Smith C A, Davis Smith T, Smolak P J, Friend D, Hagen H, Gerhart M, Park L, Pickup D J, Torrance D, Mohler K, Schooley K, Goodwin R. Poxvirus genomes encode a secreted, soluble protein that preferentially inhibits β chemokine activity yet lacks sequence homology to known chemokine receptors. Virology. 1997;236:316–327. doi: 10.1006/viro.1997.8730. [DOI] [PubMed] [Google Scholar]
- 56.Smith C A, Davis T, Wignall J M, Din W S, Farrah T, Upton C, McFadden G, Goodwin R G. T2 open reading frame from the Shope fibroma virus encodes a soluble form of the TNF receptor. Biochem Biophys Res Commun. 1991;176:335–342. doi: 10.1016/0006-291x(91)90929-2. [DOI] [PubMed] [Google Scholar]
- 57.Smith G L. Virus strategies for evasion of the host response to infection. Trends Microbiol. 1994;2:81–88. doi: 10.1016/0966-842x(94)90539-8. [DOI] [PubMed] [Google Scholar]
- 58.Smith G L, Howard S T, Chan Y S. Vaccinia virus encodes a family of genes with homology to serine proteinase inhibitors. J Gen Virol. 1989;70:2333–2343. doi: 10.1099/0022-1317-70-9-2333. [DOI] [PubMed] [Google Scholar]
- 59.Spriggs M K, Hruby D E, Maliszewski C R, Pickup D J, Sims J E, Buller R M L, Van Slyke J. Vaccinia and cowpox viruses encode a novel secreted interleukin-1 binding protein. Cell. 1992;71:145–152. doi: 10.1016/0092-8674(92)90273-f. [DOI] [PubMed] [Google Scholar]
- 60.Spriggs M K. One step ahead of the game: viral immunomodulatory molecules. Ann Rev Immunol. 1996;14:101–130. doi: 10.1146/annurev.immunol.14.1.101. [DOI] [PubMed] [Google Scholar]
- 61.Symons J A, Alcami A, Smith G L. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell. 1995;81:551–560. doi: 10.1016/0092-8674(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 62.Tewari M, Dixit V M. Fas- and tumor necrosis factor-induced apoptosis is inhibited by the poxvirus crmA gene product. J Biol Chem. 1995;270:3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- 63.Thompson J P, Turner P C, Ali A N, Crenshaw B C, Moyer R W. The effects of serpin gene mutations on the distinctive pathobiology of cowpox and rabbitpox virus following intranasal inoculation of BALB/c mice. Virology. 1993;197:328–338. doi: 10.1006/viro.1993.1594. [DOI] [PubMed] [Google Scholar]
- 64.Turner P C, Musy P Y, Moyer R W. Poxvirus serpins. In: McFadden G, editor. Viroceptors, virokines and related immune modulators encoded by DNA viruses. R. G. Austin, Tex: Landes Co.; 1995. pp. 67–88. [Google Scholar]
- 65.Upton C, McFadden G. Tumorigenic poxviruses: analysis of viral DNA sequences implicated in the tumorigenicity of Shope fibroma virus and malignant rabbit virus. Virology. 1986;61:1271–1275. doi: 10.1016/0042-6822(86)90134-0. [DOI] [PubMed] [Google Scholar]
- 66.Upton C, Macen J L, McFadden G. Mapping and sequencing of a gene from myxoma virus that is related to those encoding epidermal growth factor and transforming growth factor α. J Virol. 1987;152:308–321. doi: 10.1128/jvi.61.4.1271-1275.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Upton C, Macen J L, Wishard D S, McFadden G. Myxoma virus and malignant rabbit fibroma virus encode a serpin-like protein important for virus virulence. Virology. 1990;179:618–631. doi: 10.1016/0042-6822(90)90129-f. [DOI] [PubMed] [Google Scholar]
- 68.Upton C, Macen J L, Schreiber M, McFadden G. Myxoma virus expresses a secreted protein with homology to the tumor necrosis factor receptor gene family that contributes to viral virulence. Virology. 1991;184:370–382. doi: 10.1016/0042-6822(91)90853-4. [DOI] [PubMed] [Google Scholar]
- 69.Upton C, Mossman K, McFadden G. Encoding of a homolog of the gamma-IFN receptor by myxoma virus. Science. 1992;258:1369–1372. doi: 10.1126/science.1455233. [DOI] [PubMed] [Google Scholar]
- 70.Vaux D L, Haecker G, Strasser A. An evolutionary perspective on apoptosis. Cell. 1994;76:777–779. doi: 10.1016/0092-8674(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 71.Young H A, Hardy K J. Role of interferon-gamma in immune cell regulation. J Leukoc Biol. 1995;58:373–381. [PubMed] [Google Scholar]
- 72.Zhou Q, Snipas S, Orth K, Muzio M, Dixit V M, Salvesen G S. Target protease specificity of the viral serpin CrmA. Analysis of five caspases. J Biol Chem. 1997;272:7797–7800. doi: 10.1074/jbc.272.12.7797. [DOI] [PubMed] [Google Scholar]