Abstract
Background
Sudden cardiac death (SCD) is a significant global public health problem accounting for 15% to 20% of all deaths. A great majority of SCD is associated with coronary heart disease, which may first be detected at autopsy. The ankle–brachial index (ABI) is a simple, noninvasive measure of subclinical atherosclerosis. The purpose of this study was to examine the relationship between ABI and SCD in a middle‐aged biracial general population.
Methods and Results
Participants of the ARIC (Atherosclerosis Risk in Communities) study with an ABI measurement between 1987 and 1989 were included. ABI was categorized as low (≤0.90), borderline (0.90–1.00), normal (1.00–1.40), and noncompressible (>1.40). SCD was defined as a sudden pulseless condition presumed to be caused by a ventricular tachyarrhythmia in a previously stable individual and was adjudicated by a committee of cardiac electrophysiologists, cardiologists, and internists. Cox proportional hazards models were used to evaluate the associations between baseline ABI and incident SCD. Of the 15 081 participants followed for a median of 23.5 years, 556 (3.7%) developed SCD (1.96 cases per 1000 person‐years). Low and borderline ABIs were associated with an increased risk of SCD (demographically adjusted hazard ratios [HRs], 2.27 [95% CI, 1.64–3.14] and 1.52 [95% CI, 1.17–1.96], respectively) compared with normal ABI. The association between low ABI and SCD remained significant after adjustment for traditional cardiovascular risk factors (HR, 1.63 [95% CI, 1.15–2.32]).
Conclusions
Low ABI is independently associated with an increased risk of SCD in a middle‐aged biracial general population. ABI could be incorporated into future SCD risk prediction models.
Keywords: ankle–brachial index, epidemiology, subclinical atherosclerosis, sudden cardiac death
Subject Categories: Epidemiology, Sudden Cardiac Death
Nonstandard Abbreviations and Acronyms
- ARIC
Atherosclerosis Risk in Communities
- SCD
sudden cardiac death
Clinical Perspective.
What Is New?
In a middle‐aged biracial cohort, subclinical atherosclerosis, measured by ankle–brachial index, was associated with an increased risk of sudden cardiac death over a median follow‐up of 23.5 years.
Low ankle–brachial index (≤0.9) was independently associated with an increased risk of sudden cardiac death in the ARIC (Atherosclerosis Risk in Communities) study.
What Are the Clinical Implications?
The ankle–brachial index is a simple, noninvasive measure of subclinical atherosclerosis, and detecting subclinical atherosclerosis by ankle–brachial index could potentially aid health care professionals in providing patient‐centered care to reduce the risk of sudden cardiac death.
Sudden cardiac death (SCD) represents a significant public health concern, with an estimated 356 500 occurrences annually in the United States, 1 , 2 accounting for 50% of all cardiovascular death. 3 SCD is a leading cause of death in the United States, and on a global scale, an estimated 4–5 million SCDs occur each year. 4 Furthermore, SCD is associated with ≈80% of coronary heart disease (CHD) cases, often first detected at autopsy. 5 There is an increasing need to prevent SCD at both population and global levels. 6
Subclinical atherosclerosis has been associated with risk of SCD. We previously showed that carotid intima‐media thickness is associated with the risk of SCD in 2 biracial community‐based cohorts. 7 However, measurement of carotid intima‐media thickness requires special equipment such as ultrasound and trained readers. 8 In contrast, the ankle–brachial index (ABI) is a simple, noninvasive measure of subclinical atherosclerosis. 9 , 10 By comparing blood pressure in the upper and lower limbs, ABI can be seamlessly incorporated into routine physical examinations, making it more widely available compared with carotid intima‐media thickness. Indeed, ABI is known to predict CHD, 11 stroke, 12 cardiovascular death, 13 , 14 and cancer death. 15 The association between subclinical atherosclerosis, measured by ABI, and the risk of SCD remains unexplored. Therefore, the purpose of this study is to determine whether ABI is associated with SCD in the general population, independently of traditional cardiovascular risk factors.
Methods
Study Cohort
We studied the participants of the ARIC (Atherosclerosis Risk in Communities) study. Details of the ARIC study have been described elsewhere. 16 Briefly, the ARIC study is a prospective cohort study that enrolled 15 792 adults aged 45–64 years between 1987 and 1989 from 4 US communities (suburbs of Minneapolis, Minnesota; Forsyth County, North Carolina; Washington County, Maryland; and Jackson, Mississippi). Of those 15 792 ARIC participants at visit 1 (1987–1989), we excluded participants with missing ABI data or covariates (n=585), those with a history of lower‐extremity revascularization (presence of clinical peripheral artery disease; n=25), those with self‐reported race other than Black or White (n=47), and Black participants from the Minnesota and Maryland centers (n=54), resulting in a study population of 15 081 participants. The participants were followed for a median of 23.5 years. The study was approved by the institutional review boards at all institutions involved in the study, and informed consent was obtained from all participants. Anonymized data and materials have been made publicly available at the database of Genotypes and Phenotypes and can be accessed at https://www.ncbi.nlm.nih.gov/gap/.
Exposure Measurement
ABI was measured at baseline. ABI measurement in the ARIC study has been previously described. 17 , 18 Briefly, resting ankle and brachial pressures were measured by a standard protocol using a DIANAMAP 1846 SX automated oscillometric device (Critikon, Tampa, FL). Ankle blood pressure was measured with the individual in the prone position, in only 1 leg, which was selected on the basis of the fifth digit of the ARIC ID. If the number was even, the right leg was used; if the number was odd, the left leg was used. Following a first manually triggered measurement to calibrate the occlusion pressure, 2 readings of ankle blood pressure were taken 5 to 8 minutes apart. Two brachial blood pressure measurements were measured in the supine position, 5 minutes apart. The ABI was calculated as the average of the 2 resting ankle systolic pressure readings divided by the average of the 2 resting brachial systolic pressure readings. Participants were categorized on the basis of baseline ABI per a scientific statement from the American Heart Association (low, ≤0.90; borderline, 0.90 to <1.00; normal, 1.00–1.40; and high, indicating noncompressible arteries, >1.40). 19
Other Variables of Interest and Covariates
Participants reported information on smoking, alcohol intake, and physical activity. Seated blood pressure was measured 3 times, and the average of the last 2 was used for the analysis. In addition, sociodemographic variables were obtained at baseline, including age, race, sex, education, and field center. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or treatment for hypertension. Diabetes was defined as fasting glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, treatment for diabetes, or a self‐reported physician diagnosis of diabetes. Prevalent CHD was defined as self‐reported CHD or the presence of a previous myocardial infarction by electrocardiography at baseline. Prevalent heart failure (HF) was defined as self‐reported use of HF medications within 2 weeks (“Were any of the medications you took during the last 2 weeks for HF?”) or manifest” HF by Gothenburg criteria. 20 Cornell voltage for left ventricular hypertrophy was defined as the sum of S‐wave amplitude in V3 and R‐wave amplitude in aVL on ECG. High‐density lipoprotein cholesterol level was determined using enzymatic methods, and low‐density lipoprotein cholesterol level was calculated using the Friedewald equation.
Sudden Cardiac Death
SCD was defined as a sudden pulseless condition presumed to be due to a ventricular tachyarrhythmia in a previously stable individual without evidence of a noncardiac cause of cardiac arrest. All events classified as fatal CHD (out of hospital definite myocardial infarction, definite fatal CHD, or possible fatal CHD) that occurred by December 2012 were adjudicated by a committee of cardiac electrophysiologists, cardiologists, and internists. After a review of available data, cases were classified as definite SCD, possible SCD, not sudden arrhythmic death, or unclassifiable. For this analysis, all patients with definite or possible SCD were considered as SCD. 7
Statistical Analysis
Participants were categorized on the basis of baseline ABI by the commonly used guideline‐based categories noted above. 19 Kaplan–Meier curves for SCD based on these ABI categories were generated. Age, race, and sex‐adjusted Kaplan–Meier curves were subsequently generated. Then, log‐rank testing was used to compare among the groups. Association between ABI as a continuous variable and risk of SCD was also evaluated using Cox proportional hazards models.
Cox proportional hazards models were used to evaluate associations of incident SCD with baseline ABI. ABI was treated as a continuous variable (per 0.10 decrement) and a categorical variable. We constructed 3 adjustment models, including model 1: adjusted for age, sex, race, education, and field center; model 2: model 1+CHD, HF, diabetes, body mass index (BMI), high‐density lipoprotein and low‐density lipoprotein cholesterols, current alcohol use, and current smoking; and model 3: model 2+hypertension, Cornell voltage, and heart rate.
Subgroup analyses were performed by age (below versus above median, 54 years), sex, race, the status of CHD, HF, diabetes, hypertension, and obesity (defined as BMI ≥30 kg/m2). Stratified analyses were performed in each stratum. Difference in hazard ratio (HR) between 2 strata was examined from stratified models. In sensitivity analyses, we explored a different ABI cut point for high ABI (ABI, 1.30) as used in the previous ARIC study. 10 P<0.05 was considered significant. All statistical analyses were performed using Stata 17.0 (StataCorp, LP, College Station, TX).
Results
Baseline Characteristics of the Cohort
The mean age of study participants was 54 years (SD, 5.8), and a minority were men (n=6747; 44.8%). Of the 15 081 participants, there were 628 (4.2%) with low ABI and 1641 (10.9%) with borderline ABI. Baseline characteristics of the cohort based on ABI status are shown in Table 1. Participants with lower ABI were more likely to be older, men, Black, and more likely to have diabetes, hypertension, CHD, and HF (P<0.001).
Table 1.
Baseline Characteristics of the Cohort Based on ABI Status
| Total (n=15 081) | ABI | ||||
|---|---|---|---|---|---|
| Low (≤0.90; n=628) | Borderline (0.90 to <1.00; n=1641) | Normal (1.00–1.40; n=12 440) | Noncompressible (>1.40; n=372) | ||
| Age, y | 54.16 (5.76) | 55.76 (5.91) | 54.10 (5.81) | 54.06 (5.73) | 55.21 (5.67) |
| Male, n (%) | 6747 (45) | 208 (33) | 474 (29) | 5854 (47) | 211 (57) |
| Black, n (%) | 4018 (27) | 207 (33) | 434 (26) | 3308 (27) | 69 (19) |
| Education, n (%) | |||||
| Below high school | 3567 (24) | 221 (35) | 436 (27) | 2827 (23) | 83 (22) |
| High school | 6149 (41) | 250 (40) | 694 (42) | 5058 (41) | 147 (40) |
| College or more | 5341 (35) | 157 (25) | 510 (31) | 4533 (37) | 141 (38) |
| Current drinking, n (%) | 8396 (56) | 308 (49) | 886 (54) | 7011 (57) | 191 (51) |
| Current smoking, n (%) | 3914 (26) | 251 (40) | 479 (29) | 3101 (25) | 83 (22) |
| Physical activity index | 2.36 (0.57) | 2.26 (0.62) | 2.36 (0.59) | 2.36 (0.57) | 2.40 (0.56) |
| Diabetes, n (%) | 1770 (12) | 117 (19) | 213 (13) | 1394 (11) | 46 (12) |
| Hypertension, n (%) | 5227 (35) | 303 (48) | 626 (38) | 4179 (34) | 119 (32) |
| Coronary heart disease, n (%) | 616 (4) | 47 (8) | 73 (5) | 477 (4) | 19 (5) |
| Heart failure, n (%) | 704 (5) | 66 (11) | 98 (6) | 526 (4) | 14 (4) |
| Systolic blood pressure, mm Hg | 121.20 (18.77) | 125.81 (21.38) | 123.18 (20.11) | 120.81 (18.46) | 118.08 (16.11) |
| Diastolic blood pressure, mm Hg | 73.66 (11.25) | 72.85 (12.06) | 73.35 (11.56) | 73.80 (11.18) | 71.52 (10.73) |
| Heart rate, min | 66.66 (10.31) | 69.14 (11.66) | 68.71 (11.17) | 66.32 (10.06) | 64.72 (10.35) |
| Corrected QT interval, ms | 0.40 (0.42) | 0.38 (0.08) | 0.38 (0.03) | 0.40 (0.47) | 0.38 (0.04) |
| Body mass index, kg/m2 | 27.68 (5.32) | 27.98 (6.08) | 28.35 (6.10) | 27.54 (5.15) | 28.83 (5.61) |
| Cornell voltage, µV | 1225.18 (551.11) | 1225.10 (586.45) | 1186.75 (549.24) | 1228.40 (549.60) | 1289.00 (539.97) |
| LDL cholesterol, mg/dL | 137.59 (39.29) | 146.24 (42.12) | 139.63 (39.49) | 136.89 (39.07) | 137.38 (39.39) |
| HDL cholesterol, mg/dL | 51.64 (17.15) | 51.14 (17.29) | 52.80 (17.23) | 51.61 (17.18) | 48.46 (15.29) |
Data are presented as mean (SD) or n (%). P <0.001 for all variables except corrected QT interval (0.67). ABI indicates ankle–brachial index; HDL, high‐density lipoprotein; and LDL, low‐density lipoprotein.
ABI and SCD
Over a median of 23.5 years of follow‐up, there were 556 (3.7%) SCD events (1.96 cases per 1000 person‐years). Figure 1 shows Kaplan–Meier curves by ABI status with the end point of SCD. The overall P value for all 4 categories were significant (P<0.001 by log‐rank testing). Age, race, and sex‐adjusted Kaplan–Meier curves by ABI status with the end point of SCD are shown in Figure S1. The overall P value remained significant (P<0.001 by log‐rank testing). Association between ABI as a continuous variable and risk of SCD are shown in Figure 2. HRs were estimated from Cox proportional hazard regression models. Risk of SCD is gradually increased with decrease in ABI.
Figure 1. Kaplan–Meier curve with the end point of SCD based on ABI status.
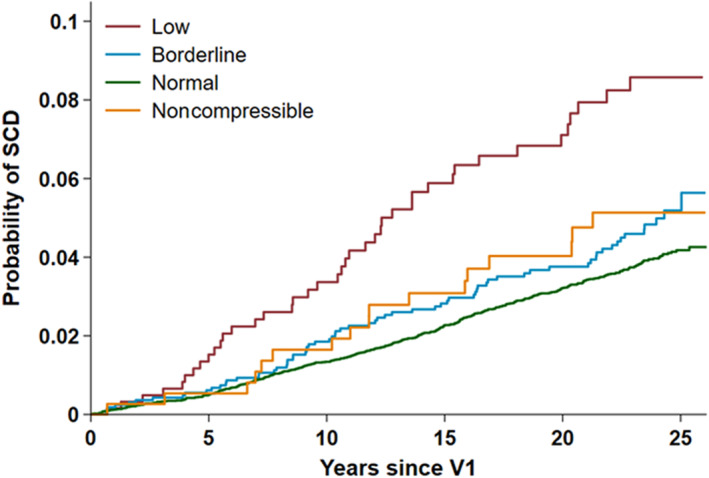
ABI indicates ankle–brachial index; SCD, sudden cardiac death; and VI, visit 1.
Figure 2. Association between ABI as a continuous variable and risk of SCD.
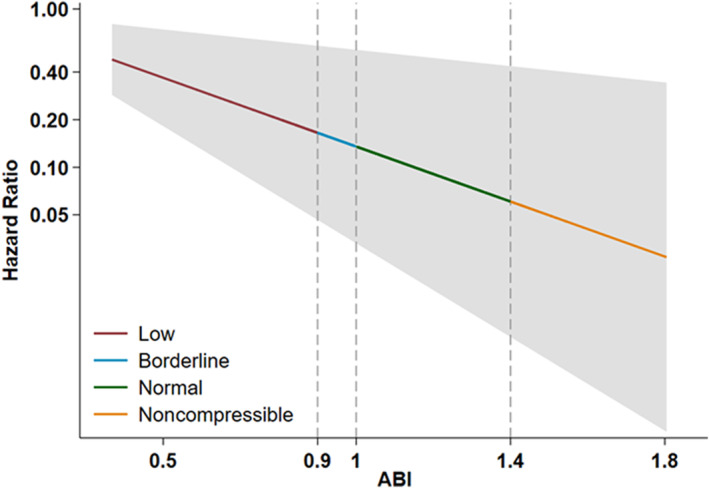
Hazard ratio was estimated from Cox proportional hazards models. The gray zone represents 95% CI. ABI indicates ankle–brachial index; and SCD, sudden cardiac death.
HRs with 95% CIs of SCD based on ABI status are shown in Table 2. Low and borderline ABIs were associated with an elevated risk of SCD (HR, 2.27 [95% CI, 1.64–3.14] and HR, 1.52 [95% CI, 1.17–1.96], respectively) in comparison with normal ABI. The association between low ABI and SCD remained significant after adjustment for traditional cardiovascular risk factors but not for borderline ABI (HR, 1.63 [95% CI, 1.15–2.32] and HR, 1.24 [95% CI, 0.94–1.63]; Table 2).
Table 2.
HRs and 95% CIs of SCD based on ABI Category
| Event | ABI category | ABI range | HR (95% CI); P value | ||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||
| SCD | Low | ≤0.90 | 2.27 (1.64–3.14); <0.001 | 1.68 (1.19–2.37); 0.003 | 1.63 (1.15–2.32); 0.006 |
| Borderline | 0.90–1.00 | 1.52 (1.17–1.96); 0.001 | 1.32 (1.01–1.72); 0.044 | 1.24 (0.94–1.63); 0.134 | |
| Normal | 1.00–1.40 | Ref | Ref | Ref | |
| Noncompressible | >1.40 | 1.19 (0.73–1.94); 0.481 | 1.10 (0.64–1.87); 0.733 | 1.15 (0.67–1.97); 0.606 | |
Model 1: adjusted for age, sex, education, race, and field center. Model 2: model 1+CHD, HF, diabetes, BMI, HDL and LDL cholesterols, current alcohol drinking, and current smoking. Model 3: model 2+hypertension, Cornell voltage, and heart rate. ABI indicates ankle–brachial index; BMI, body mass index; CHD, coronary heart disease; HDL, high‐density lipoprotein; HF, heart failure; HR, hazard ratio; LDL, low‐density lipoprotein; and SCD, sudden cardiac death.
Sensitivity Analysis
Stratified analysis was performed on the basis of age group, sex, race, CHD, HF, diabetes, hypertension, and obesity (defined as BMI ≥30 kg/m2) (Table 3). The associations of ABI with SCD were generally consistent across the subgroups except sex and diabetes. HRs for low ABI were higher in men (compared with women) and in participants with diabetes (compared with those without diabetes). When we used a different cut point for high ABI (ABI, 1.30), the results were largely similar (Table 4): low and borderline ABIs were associated with an elevated risk of SCD (HR, 2.23 [95% CI, 1.61–3.08] and HR, 1.49 [95% CI, 1.15–1.92], respectively) in comparison with normal ABI (model 1 in Table 4). The association between low ABI and SCD remained significant after adjustment for traditional cardiovascular risk factors but not for borderline ABI (HR, 1.62 [95% CI, 1.14–2.31] and HR, 1.23 [95% CI, 0.93–1.62]).
Table 3.
Stratified Analysis: HRs of SCD for Participants With Low ABI (Reference: Normal ABI)
| Variable | HR (95% CI) of SCD for low vs normal ABI; P value | P value for difference between 2 strata | |||
|---|---|---|---|---|---|
| Strata 1 | Strata 2 | ||||
| Age | Above median | 1.806 (1.221–2.671); 0.003 | Below median | 1.173 (0.509–2.703); 0.708 | 0.359 |
| Sex | Male | 2.701 (1.757–4.153); <0.001 | Female | 0.813 (0.443–1.490); 0.502 | 0.002 |
| Race | Black | 1.913 (1.170–3.127); 0.010 | White | 1.430 (0.860–2.379); 0.168 | 0.421 |
| CHD | Yes | 1.371 (0.654–2.872); 0.403 | No | 1.804 (1.215–2.678); 0.003 | 0.521 |
| HF | Yes | 1.760 (0.738–4.194); 0.202 | No | 1.753 (1.191–2.579); 0.004 | 0.994 |
| Diabetes | Yes | 2.720 (1.605–4.610); <0.001 | No | 1.286 (0.783–2.110); 0.320 | 0.042 |
| Hypertension | Yes | 1.743 (1.142–2.659); 0.010 | No | 1.570 (0.842–2.925); 0.156 | 0.785 |
| Obesity (BMI ≥30) | Yes | 1.865 (1.021–3.408); 0.043 | No | 1.546 (0.999–2.391); 0.050 | 0.621 |
Model: adjusted for age, sex, education, race, field center, CHD, HF, DM, BMI, HDL and LDL cholesterols, current drinking, current smoking, hypertension, Cornell voltage, and heart rate. The median age was 54 years. ABI indicates ankle–brachial index; BMI, body mass index; CHD, coronary heart disease; HDL, high‐density lipoprotein; HF, heart failure; HR, hazard ratio; LDL, low‐density lipoprotein; and SCD, sudden cardiac death.
Table 4.
HRs and 95% CIs of SCD Based on Alternative ABI Cut Point (ABI, 1.30)
| Event | ABI category | ABI range | HR (95% CI); P value | ||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||
| SCD | Low | ≤0.90 | 2.23 (1.61–3.08); <0.001 | 1.67 (1.18–2.35); 0.004 | 1.62 (1.14–2.31); 0.007 |
| Borderline | 0.90–1.00 | 1.49 (1.15–1.93); 0.003 | 1.31 (0.998–1.71); 0.052 | 1.23 (0.93–1.62); 0.150 | |
| Normal | 1.00–1.30 | Ref | Ref | Ref | |
| Noncompressible | >1.30 | 0.89 (0.67–1.19); 0.430 | 0.95 (0.70–1.28); 0.729 | 0.97 (0.71–1.32); 0.848 | |
Model 1: adjusted for age, sex, education, race, and field center. Model 2: model 1 adjustors+CHD, HF, diabetes, BMI, HDL and LDL cholesterols, current drinking, and current smoking. Model 3: model 2 adjustors+hypertension, Cornell voltage, and heart rate. ABI indicates ankle–brachial index; BMI, body mass index; CHD, coronary heart disease; HDL, high‐density lipoprotein; HF, heart failure; HR, hazard ratio; LDL, low‐density lipoprotein; and SCD, sudden cardiac death.
Discussion
In a middle‐aged biracial cohort, low ABI was independently associated with an increased risk of SCD over a median follow‐up of 23.5 years. This association remained significant after adjusting for traditional cardiovascular risk factors.
Previous studies have examined the associations between ABI and CHD, 11 stroke, 12 cardiovascular death, and all‐cause death. 13 , 14 However, to the best of our knowledge, this is the first study demonstrating the association between ABI and SCD. ABI is commonly used in clinical practice as a diagnostic method for lower‐extremity peripheral artery disease 19 , 21 and serves as a surrogate marker for generalized atherosclerosis. 22 In a previous study, we showed that carotid intima‐media thickness was associated with the risk of SCD. 7 Our current study extends this finding by showing that subclinical atherosclerosis, as measured by ABI, is associated with the risk of SCD.
Several possible mechanisms may link subclinical atherosclerosis and SCD. Subclinical atherosclerosis may indicate CHD and heart failure 8 , 23 ; both are associated with an increased risk of ventricular arrhythmia and SCD. 5 As CHD remains the dominant cause of SCD in the United States and about 50% of SCD occurs as the initial presentation of any heart disease, 24 it is most likely that progression of atherosclerosis plays an important role in developing SCD. In a previous autopsy study, >80% of the subjects with SCD had severe CHD, including previously undetected myocardial infarction. 5 Additionally, inflammation may serve as a link between subclinical atherosclerosis and SCD. Subclinical atherosclerosis has been shown to be associated with inflammation, 25 and inflammation has been linked to the risk of SCD. 26 , 27
Our results have important clinical and public health implications. As ABI is a simple, noninvasive measure of subclinical atherosclerosis, detecting subclinical atherosclerosis by ABI could potentially aid health care professionals in providing patient‐centered care to reduce the risk of SCD. From a public health perspective, most SCD occurs in the general population, rather than in high‐risk patients such as those with cardiomyopathy and channelopathy. Detecting subclinical atherosclerosis could help reduce the risk of SCD by promoting healthy lifestyle and initiating earlier pharmacologic therapies.
The strengths of this study include a large sample size with over 15 000 participants and the inclusion of a racially diverse population with a long follow‐up (median >23.5 years). Additionally, SCD was adjudicated by a panel of experts, which could have reduced the risk of misclassification. Limitations of the study should be acknowledged. First, our analyses relied on measurements of ABI from a single leg at baseline. Measurement of bilateral ABI might have provided a more robust association between ABI and SCD and could have reduced the risk of misclassification. We performed sensitivity analyses with a different ABI cut point and confirmed the robustness of our findings. Second, the ABI measurement was based on an oscillometer device. A Doppler probe was not used for the study. In one study comparing the diagnostic accuracy of an automated oscillometric ABI with the standard Doppler ABI, a small ABI overestimation was observed after oscillometry. 28 However, we believe that oscillometric ABI has been proven to be a good screening method, suitable in a general cohort. Third, given the study's observational design, there remains a possibility of residual confounding. However, we have adjusted for traditional risk factors, and sensitivity analyses were performed.
In summary, low ABI is independently associated with an increased risk of SCD in a biracial general population. Therefore, ABI could be incorporated into future models for predicting SCD risk.
Sources of Funding
The Atherosclerosis Risk in Communities study was funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services, under Contract Nos. 75N92022D00001, 75N92022D00002, 75N92022D00003, 75N92022D00004, and 75N92022D00005. Dr Alonso was supported by Grant No. K24HL148521 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
None.
Supporting information
Figure S1
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
This manuscript was sent to Tiffany M. Powell‐Wiley, MD MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.032008
For Sources of Funding and Disclosures, see page 7.
References
- 1. Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker‐Smith CM, Beaton AZ, Boehme AK, Buxton AE, et al. Heart disease and stroke statistics‐2023 update: a report from the American Heart Association. Circulation. 2023;147:e93–e621. doi: 10.1161/CIR.0000000000001123 [DOI] [PubMed] [Google Scholar]
- 2. Goldberger JJ, Basu A, Boineau R, Buxton AE, Cain ME, Canty JM Jr, Chen PS, Chugh SS, Costantini O, Exner DV, et al. Risk stratification for sudden cardiac death: a plan for the future. Circulation. 2014;129:516–526. doi: 10.1161/CIRCULATIONAHA.113.007149 [DOI] [PubMed] [Google Scholar]
- 3. Al‐Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018;138:e272–e391. doi: 10.1161/CIR.0000000000000549 [DOI] [PubMed] [Google Scholar]
- 4. Chugh SS, Reinier K, Teodorescu C, Evanado A, Kehr E, Al Samara M, Mariani R, Gunson K, Jui J. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis. 2008;51:213–228. doi: 10.1016/j.pcad.2008.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adabag AS, Peterson G, Apple FS, Titus J, King R, Luepker RV. Etiology of sudden death in the community: results of anatomical, metabolic, and genetic evaluation. Am Heart J. 2010;159:33–39. doi: 10.1016/j.ahj.2009.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wong CX, Brown A, Lau DH, Chugh SS, Albert CM, Kalman JM, Sanders P. Epidemiology of sudden cardiac death: global and regional perspectives. Heart Lung Circ. 2019;28:6–14. doi: 10.1016/j.hlc.2018.08.026 [DOI] [PubMed] [Google Scholar]
- 7. Suzuki T, Wang W, Wilsdon A, Butler KR, Adabag S, Griswold ME, Nambi V, Rosamond W, Sotoodehnia N, Mosley TH. Carotid intima‐media thickness and the risk of sudden cardiac death: the ARIC study and the CHS. J Am Heart Assoc. 2020;9:e016981. doi: 10.1161/JAHA.120.016981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nambi V, Chambless L, Folsom AR, He M, Hu Y, Mosley T, Volcik K, Boerwinkle E, Ballantyne CM. Carotid intima‐media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk in Communities) study. J Am Coll Cardiol. 2010;55:1600–1607. doi: 10.1016/j.jacc.2009.11.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greenland P, Abrams J, Aurigemma GP, Bond MG, Clark LT, Criqui MH, Crouse JR III, Friedman L, Fuster V, Herrington DM, et al. Prevention Conference V: beyond secondary prevention: identifying the high‐risk patient for primary prevention: noninvasive tests of atherosclerotic burden: Writing Group III. Circulation. 2000;101:E16–E22. doi: 10.1161/01.cir.101.1.e16 [DOI] [PubMed] [Google Scholar]
- 10. Weatherley BD, Nelson JJ, Heiss G, Chambless LE, Sharrett AR, Nieto FJ, Folsom AR, Rosamond WD. The association of the ankle‐brachial index with incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study, 1987–2001. BMC Ccardiovascular Ddisorders. 2007;7:3. doi: 10.1186/1471-2261-7-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McDermott MM, Liu K, Criqui MH, Ruth K, Goff D, Saad MF, Wu C, Homma S, Sharrett AR. Ankle‐brachial index and subclinical cardiac and carotid disease: the Multi‐Ethnic Study of Atherosclerosis. Am J Epidemiol. 2005;162:33–41. doi: 10.1093/aje/kwi167 [DOI] [PubMed] [Google Scholar]
- 12. Zheng ZJ, Sharrett AR, Chambless LE, Rosamond WD, Nieto FJ, Sheps DS, Dobs A, Evans GW, Heiss G. Associations of ankle‐brachial index with clinical coronary heart disease, stroke and preclinical carotid and popliteal atherosclerosis: the Atherosclerosis Risk In Communities (ARIC) study. Atherosclerosis. 1997;131:115–125. doi: 10.1016/S0021-9150(97)06089-9 [DOI] [PubMed] [Google Scholar]
- 13. Newman AB, Sutton‐Tyrrell K, Vogt MT, Kuller LH. Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index. JAMA. 1993;270:487–489. doi: 10.1001/jama.1993.03510040091035 [DOI] [PubMed] [Google Scholar]
- 14. Vogt MT, Cauley JA, Newman AB, Kuller LH, Hulley SB. Decreased ankle/arm blood pressure index and mortality in elderly women. JAMA. 1993;270:465–469. doi: 10.1001/jama.1993.03510040069031 [DOI] [PubMed] [Google Scholar]
- 15. Visona A, De Paoli A, Fedeli U, Tonello D, Zalunardo B, Zanatta N, Martini R, Pesavento R, Cuppini S, Prior M, et al. Abnormal ankle‐brachial index (ABI) predicts primary and secondary cardiovascular risk and cancer mortality. Eur J Intern Med. 2020;77:79–85. doi: 10.1016/j.ejim.2020.02.033 [DOI] [PubMed] [Google Scholar]
- 16. Wright JD, Folsom AR, Coresh J, Sharrett AR, Couper D, Wagenknecht LE, Mosley TH Jr, Ballantyne CM, Boerwinkle EA, Rosamond WD, et al. The ARIC (atherosclerosis risk in communities) study: JACC focus seminar 3/8. J Am Coll Cardiol. 2021;77:2939–2959. doi: 10.1016/j.jacc.2021.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hendriks EJ, Westerink J, de Jong PA, de Borst GJ, Nathoe HM, Mali WP, van der Graaf Y, van der Schouw YT, Beulens JW; Group SS . Association of high ankle brachial index with incident cardiovascular disease and mortality in a high‐risk population. Arterioscler Thromb Vasc Biol. 2016;36:412–417. doi: 10.1161/ATVBAHA.115.306657 [DOI] [PubMed] [Google Scholar]
- 18. Bekwelem W, Norby FL, Agarwal SK, Matsushita K, Coresh J, Alonso A, Chen LY. Association of peripheral artery disease with incident atrial fibrillation: the ARIC (atherosclerosis risk in communities) Study. J Am Heart Assoc. 2018;7:e007452. doi: 10.1161/JAHA.117.007452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jonsson B, Lacroix P, et al. Measurement and interpretation of the ankle‐brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–2909. doi: 10.1161/CIR.0b013e318276fbcb [DOI] [PubMed] [Google Scholar]
- 20. Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the atherosclerosis risk in communities study). Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061 [DOI] [PubMed] [Google Scholar]
- 21. Gerhard‐Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e686–e725. doi: 10.1161/CIR.0000000000000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ankle Brachial Index C, Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, et al. Ankle brachial index combined with Framingham risk score to predict cardiovascular events and mortality: a meta‐analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gupta DK, Skali H, Claggett B, Kasabov R, Cheng S, Shah AM, Loehr LR, Heiss G, Nambi V, Aguilar D, et al. Heart failure risk across the spectrum of ankle‐brachial index: the ARIC study (atherosclerosis risk in communities). JACC Heart Fail. 2014;2:447–454. doi: 10.1016/j.jchf.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mitrani RD, Myerburg RJ. Ten advances defining sudden cardiac death. Trends Cardiovasc Med. 2016;26:23–33. doi: 10.1016/j.tcm.2015.03.017 [DOI] [PubMed] [Google Scholar]
- 25. Libby P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc Res. 2021;117:2525–2536. doi: 10.1093/cvr/cvab303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hussein AA, Gottdiener JS, Bartz TM, Sotoodehnia N, DeFilippi C, See V, Deo R, Siscovick D, Stein PK, Lloyd‐Jones D. Inflammation and sudden cardiac death in a community‐based population of older adults: the Cardiovascular Health Study. Heart Rhythm. 2013;10:1425–1432. doi: 10.1016/j.hrthm.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 27. Albert CM, Ma J, Rifai N, Stampfer MJ, Ridker PM. Prospective study of C‐reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation. 2002;105:2595–2599. doi: 10.1161/01.cir.0000017493.03108.1c [DOI] [PubMed] [Google Scholar]
- 28. Hageman D, van den Houten MML, Pesser N, Gommans LNM, Scheltinga MRM, Teijink JAW. Diagnostic accuracy of automated oscillometric determination of the ankle‐brachial index in peripheral artery disease. J Vasc Surg. 2021;73:652–660. doi: 10.1016/j.jvs.2020.05.077 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


