Abstract
Background
Among individuals with hypertension and low diastolic blood pressure (DBP), the optimal BP target remains controversial due to concerns that BP lowering may reduce coronary perfusion. We determined the impact of intensive BP control among individuals with elevated systolic BP who have low DBP and elevated hs‐cTnT (high‐sensitivity cardiac troponin T) levels.
Methods and Results
A total of 8828 participants in SPRINT (Systolic Blood Pressure Intervention Trial) were stratified by baseline DBP. Those with low DBP (<70 mm Hg) were further stratified by elevated hs‐cTnT (≥14 ng/L) at baseline. The effects of intensive versus standard BP lowering on a cardiovascular disease composite end point, all‐cause death, and 1‐year change in hs‐cTnT were determined. The combination of low DBP/high hs‐cTnT was independently associated with a higher risk for cardiovascular disease and all‐cause death, as well as greater 1‐year increases in hs‐cTnT, compared with DBP ≥70 mm Hg. However, randomization to intensive versus standard BP lowering led to similar reductions in cardiovascular disease risk among individuals with low DBP/high hs‐cTnT (hazard ratio [HR], 0.82 [95% CI, 0.57–1.19]), low DBP/low hs‐cTnT (HR, 0.48 [95% CI, 0.29–0.79]), and DBP ≥70 mm Hg (HR, 0.73 [95% CI, 0.60–0.89]; P for interaction=0.20). Intensive BP lowering also led to a reduction in all‐cause death that was similar across groups (P for interaction=0.57).
Conclusions
In this nonprespecified subgroup analysis of SPRINT, individuals with low DBP and elevated hs‐cTnT, low DBP and nonelevated hs‐cTnT, and DBP ≥70 mm Hg derived similar cardiovascular disease and mortality benefits from intensive BP lowering. These findings warrant confirmation in other studies.
Keywords: diastolic blood pressure, hypertension, J curve, SPRINT, troponin
Subject Categories: Hypertension, Cardiovascular Disease, Blood Pressure, Biomarkers
Nonstandard Abbreviations and Acronyms
- PP
pulse pressure
- SPRINT
Systolic Blood Pressure Intervention Trial
Clinical Perspective.
What Is New?
In SPRINT (Systolic Blood Pressure Intervention Trial, the baseline combination of low diastolic blood pressure (DBP) and elevated levels of hs‐cTnT (high‐sensitivity cardiac troponin T), a marker of myocardial injury, was associated with greater risk of cardiovascular disease and death compared with those without low baseline DBP, whereas those with low DBP and nonelevated hs‐cTnT levels did not experience greater risk.
Randomization to intensive‐treatment (targeting systolic blood pressure <120 mm Hg) versus standard‐treatment (targeting systolic blood pressure <140 mm Hg) led to similar decreases in cardiovascular disease and death and similar minor increases in hs‐cTnT across groups.
What Are the Clinical Implications?
Among individuals with hypertension and low DBP, the presence of elevated hs‐cTnT levels identifies a high‐risk group that may account for the J‐curve relationship between DBP and cardiovascular disease risk.
The findings from the present analysis reinforce the concept that low DBP does not have a directly causal relationship with cardiovascular disease and death.
Low DBP should not be a barrier to intensive blood pressure management among SPRINT‐eligible individuals.
Hypertension affects nearly half of all US adults and is the leading modifiable contributor to cardiovascular disease (CVD) and early death. 1 , 2 Among nondiabetic individuals with hypertension and at high CVD risk, the SPRINT trial (Systolic Blood Pressure Intervention Trial) demonstrated that targeting a systolic blood pressure (SBP) of <120 mm Hg versus <140 mm Hg significantly reduced CVD events and all‐cause death. 3 The findings from SPRINT led to practice guidelines recommending lower BP targets for hypertension treatment, but controversy remains regarding the optimal BP target when low diastolic blood pressure (DBP) is present. 4 Previous observational studies demonstrate a J‐shaped relationship between DBP and myocardial infarction, fatal and nonfatal CVD, and all‐cause death. 5 , 6 , 7 , 8 , 9 , 10 This has been hypothesized to result from reduced coronary perfusion. However, recent ancillary analyses of several large BP target trials observed that the benefits of intensive versus standard BP lowering are similar regardless of baseline DBP, 11 , 12 , 13 , 14 , 15 , 16 and Mendelian randomization studies show no evidence of a J‐curve relationship between genetically predicted DBP and CVD risk. 17 , 18 These results indicate that the J‐curve phenomenon likely instead represents confounding from factors that are related both to the presence of low DBP and CVD risk, such as arterial stiffness or coronary atherosclerosis.
To our knowledge, prior analyses from BP target trials have not used an intermediary marker of myocardial damage to identify a subset of individuals with low DBP who may be particularly susceptible to the consequences of reduced coronary perfusion from additional BP lowering. Minimal elevations in hs‐cTnT (high‐sensitivity cardiac troponin T) levels, a marker of chronic myocardial injury, are common in the ambulatory setting and strongly associated with CVD and all‐cause death. 19 , 20 , 21 , 22 , 23 , 24 In addition, increases in hs‐cTnT over time are associated with increased CVD risk. 25 Low DBP is strongly associated with elevated hs‐cTnT levels, and individuals with both low DBP and elevated hs‐cTnT are at substantially elevated CVD risk. 26 , 27 Evaluating the effects of intensive BP lowering in those with low DBP and elevated hs‐cTnT levels may help to reconcile the clinical importance and biological mechanisms of the J‐curve, which could have implications for hypertension management.
Our objectives were (1) to evaluate in SPRINT the associations of low DBP and elevated hs‐cTnT levels at baseline with CVD, all‐cause death, and changes in hs‐cTnT over time; (2) to determine whether the effects of intensive BP lowering on CVD, all‐cause death, and changes in hs‐cTnT varied among individuals with low DBP and elevated hs‐cTnT levels, low DBP and nonelevated hs‐cTnT levels, and those without low DBP. We hypothesized that compared with SPRINT participants without low DBP at baseline and participants with low DBP and nonelevated hs‐cTnT levels, those with low DBP and elevated hs‐cTnT levels would (1) be at greater risk for CVD and all‐cause death and have greater increases in hs‐cTnT over time and (2) derive similar relative CVD and mortality benefits from intensive BP lowering.
Methods
Study Design
The design and protocol of SPRINT have been reported previously. 3 , 28 In brief, SPRINT was a National Institutes of Health‐funded open‐label clinical trial that randomized participants with hypertension and at elevated CVD risk to an intensive SBP target of <120 mm Hg versus a standard SBP target of <140 mm Hg, with individual patient management at the discretion of the trial investigators. A total of 9361 participants were enrolled in SPRINT between November 2010 and March 2013 across 102 sites in the United States and Puerto Rico. Inclusion criteria were age ≥50 years; systolic BP 130–180 mm Hg; and high CVD risk (defined as prior clinical or subclinical CVD other than stroke, chronic kidney disease [estimated glomerular filtration rate (eGFR) 20–59 mL/min per 1.73 m2], age ≥75 years, or 10‐year CVD risk >15% based on the Framingham risk score). Key exclusion criteria included diabetes, prior stroke or transient ischemic attack, eGFR <20 mL/min per 1.73 m2, symptomatic heart failure, or a left ventricular ejection fraction <35%. The SPRINT protocol comprised a baseline visit and follow‐up visits monthly for the first 3 months and every 3 months thereafter. The trial was stopped early on the recommendation of the Data and Safety Monitoring Board, which noted substantive evidence of treatment benefit during their regular scheduled interim evaluation of the data. The SPRINT study was approved by the institutional review board at each participating study site, and all participants provided written informed consent.
This analysis was approved by the committees on human research at the University of California, Davis, the University of California, San Francisco, and the San Francisco Veterans Affairs HealthCare System. The data that support the findings of this study are available from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repositories and the corresponding author upon request.
BP and hs‐cTnT Measurement
Baseline and follow‐up BPs were recorded by trained study coordinators using an automated oscillometric measurement system (model 907XL, Omron Healthcare) during clinic visits after participants were seated for 5 minutes. The recorded BP was the mean of 3 BP readings taken 1 minute apart, as previously described. 29 Pulse pressure (PP) for each participant was calculated as SBP minus DBP.
Blood samples used for hs‐cTnT measurement were obtained at the time of study entry and at the 12‐month study visit, processed immediately, and stored at −80°C until measurements were performed at the SPRINT Central Laboratory (University of Minnesota, Minneapolis, MN). The hs‐cTnT was measured from freshly thawed samples using an electrochemiluminescence immunoassay on the Roche Cobas 6000 platform (Roche Diagnostics, Indianapolis, IN) as previously described. 30 The hs‐cTnT assay (5th generation) has an interassay coefficient of variation of 3.4% at 28.3 ng/L and 2.3% at 2076 ng/L, with a lower limit of quantitation of 6 ng/L.
Combined DBP and hs‐cTnT Groups
Consistent with previous studies in SPRINT, low DBP was defined as <70 mm Hg and elevated hs‐cTnT was defined as ≥14 ng/L. 30 , 31 , 32 Participants were categorized at baseline as low DBP with elevated hs‐cTnT (low DBP/high hs‐cTnT), low DBP with non‐elevated hs‐cTnT (low DBP/low hs‐cTnT), and DBP ≥70 mm Hg, because the beneficial treatment effects of intensive BP lowering are established among those with normal and high DBP. 13
Outcomes
The primary outcome of this ancillary analysis was SPRINT's CVD composite end point, which included nonfatal myocardial infarction, acute coronary syndrome not resulting in myocardial infarction, acute decompensated heart failure, stroke, and death from CVD. As previously described, SPRINT outcomes were defined, ascertained, and formally adjudicated by a central committee of investigators blinded to treatment assignment. 3 , 28 Secondary clinical outcomes included all‐cause death and 1‐year change in hs‐cTnT.
Covariates
Age, sex, race, ethnicity, medical history, medications, and smoking status were obtained by questionnaire. Body mass index was calculated as weight in kilograms divided by height in meters squared. Fasting serum low‐density lipoprotein cholesterol, serum creatinine, and urine albumin and creatinine were measured at the SPRINT Central Laboratory. The eGFR was calculated according to the 2021 Chronic Kidney Disease‐Epidemiology Collaboration creatinine estimating equation without race. 33
Statistical Analysis
We evaluated the associations of baseline DBP/hs‐cTnT categories with risk of the CVD composite outcome and all‐cause death using multivariable Cox proportional hazards models. Models were adjusted for demographics (age, sex, race), treatment assignment, and baseline clinical characteristics (body mass index, smoking status, prevalent CVD, eGFR, urine albumin‐to‐creatinine ratio, low‐density lipoprotein cholesterol, SBP, and statin use).
Event proportions were then compared between the intensive and standard SBP treatment arms among participants with low DBP/low hs‐cTnT, low DBP/high hs‐cTnT, and DBP ≥70 mm Hg. Cox proportional hazards models were used to evaluate the effect of intensive versus standard BP lowering on the CVD composite outcome and all‐cause death stratified by these groups. CIs were calculated using robust variance estimators. There was no evidence that the proportional hazards assumption for each outcome was violated in tests using Schoenfeld residuals. Variation of the treatment effect across groups for each outcome was assessed using a Wald test for multiplicative interaction terms in models that included main effects.
For analyses with change in hs‐cTnT as the outcome, linear regression models were constructed with change in hs‐cTnT defined as log(year 1 hs‐cTnT) minus log(baseline hs‐cTnT). Because 21% of hs‐cTnT levels were below the limit of detection, we imputed undetectable values using a Tobit regression model. 34 Geometric mean ratios and 95% CIs were estimated. These models were additionally adjusted for the baseline hs‐cTnT level.
In sensitivity analyses, we used multivariable Fine–Gray subdistribution hazard regression models to account for the competing risk of death (data not shown). 35 We also evaluated the effects of intensive versus standard BP lowering on the CVD composite outcome and all‐cause death using a widened baseline PP (>70 mm Hg) instead of low DBP; using prevalent CVD instead of baseline hs‐cTnT level; and using a nonstroke CVD composite outcome because low DBP is associated with adverse CVD outcomes and death but not stroke. 6 , 7 , 10
All analyses were conducted using Stata software, version 17 (StataCorp LP, College Station, TX).
Results
Study Population and Baseline Characteristics
Of the 9361 SPRINT participants, 8828 (94.3%) had baseline hs‐cTnT measurements. In this study sample, 6757 (74.5%) had a baseline DBP ≥70 mm Hg, 1287 (14.6%) had low DBP and nonelevated hs‐cTnT levels, and 784 (8.9%) had low DBP and elevated hs‐cTnT levels. Mean baseline DBP in the DBP ≥70 mm Hg, low DBP/low hs‐cTnT, and low DBP/high hs‐cTnT groups were 83, 63, and 62 mm Hg, respectively, and median hs‐cTnT levels were 8.8, 8.7, and 19.9 ng/L, respectively. Compared with the DBP ≥70 mm Hg group, those with low DBP/high hs‐cTnT were on average older, more often male, less often Black or Hispanic, less often current smokers, more likely to be on a statin, and had a higher PP and lower eGFR. Similarly, those with low DBP/low hs‐cTnT were less often Black or current smokers, more likely to be on a statin, and had a higher PP; however, they were more likely to be female or Hispanic, and their mean eGFR and age fell between the other 2 groups (Table 1).
Table 1.
Baseline Characteristics of SPRINT Participants Stratified by Combined Baseline DBP and hs‐cTnT Categories
| Baseline characteristic | DBP ≥70 mm Hg (N=6757) | DBP <70 mm Hg+hs‐cTnT <14 ng/L (N=1287) | DBP <70 mm Hg+hs‐cTnT ≥14 ng/L (N=784) |
|---|---|---|---|
| Age, y | 66.1±9.0 | 72.1±8.0 | 77.5±7.7 |
| Female sex | 2396 (35%) | 646 (50%) | 208 (27%) |
| Black race | 2140 (32%) | 277 (22%) | 168 (21%) |
| Hispanic ethnicity | 718 (11%) | 168 (13%) | 58 (7%) |
| hs‐cTnT, ng/L | 8.9 (6.2–13.1) | 8.7 (6.4–11.1) | 19.9 (16.2–27.1) |
| DBP, mm Hg | 83±9.1 | 63±5.0 | 62± 5.9 |
| Systolic BP, mm Hg | 142±15.0 | 131±14.8 | 134±15.3 |
| Pulse pressure, mm Hg | 59±13.4 | 67±14.9 | 72±15.7 |
| Intensive BP arm | 3377 (50%) | 635 (49%) | 405 (52%) |
| Current smoker | 996 (15%) | 106 (8%) | 46 (6%) |
| Body mass index, kg/m2 | 30.3±5.8 | 28.3±5.2 | 28.4±5.6 |
| Prevalent cardiovascular disease | 1154 (17%) | 332 (26%) | 266 (34%) |
| Low density lipoprotein cholesterol, mg/dL | 115±35 | 107±34 | 98±30 |
| Statin use | 2705 (40%) | 679 (53%) | 432 (56%) |
| Number of BP meds | 1.8±1.1 | 2.1±1.1 | 2.5±1.1 |
| Estimated glomerular filtration rate, mL/min per 1.73m2 | 75±19 | 72±18 | 58±19 |
| Urine albumin‐to‐creatinine ratio, mg/g | 9 (6v21) | 9 (6–17) | 15 (8–43) |
Data displayed as N (%), mean±SD, or median (interquartile range). BP indicates blood pressure; DBP, diastolic blood pressure; hs‐cTnT, high‐sensitivity cardiac troponin T; and SPRINT, Systolic Blood Pressure Intervention Trial.
Associations With CVD, All‐Cause Death, and Change in hs‐cTnT
The CVD composite outcome occurred in 5.9% of participants with DBP ≥70 mm Hg, 5.5% with low DBP/low hs‐cTnT, and 14.4% with low DBP/high hs‐cTnT (Figure S1). In multivariable models, compared with the DBP ≥70 mm Hg group, participants in the low DBP/high hs‐cTnT group had a significantly increased risk for the CVD composite outcome, whereas those with low DBP/low hs‐cTnT had numerically lower risk (Table 2). Deaths occurred in 3.4% of participants with DBP ≥70 mm Hg, 3.4% with low DBP/low hs‐cTnT, and 9.7% with low DBP/high hs‐cTnT. In multivariable models, compared with the DBP ≥70 mm Hg group, participants in the low DBP/high hs‐cTnT group had a numerically higher risk of death, whereas the low DBP/low hs‐cTnT group were at similar risk (Table 2).
Table 2.
Multivariable‐Adjusted Associations Between Baseline Combined DBP and hs‐cTnT Categories and Risk of the CVD Composite End Point and All‐Cause Death
| Outcome | No. of events/total (%) | Model 1* HR (95% CI) | Model 2† HR (95% CI) |
|---|---|---|---|
| CVD composite | |||
| DBP ≥70 mm Hg | 399/6757 (5.9%) | Reference | Reference |
| DBP <70 mm Hg + hs‐cTnT <14 ng/L | 71/1287 (5.5%) | 0.94 (0.73–1.21) | 0.79 (0.60–1.03) |
| DBP <70 mm Hg + hs‐cTnT ≥14 ng/L | 113/784 (14.4%) | 2.74 (2.22–3.38) | 1.45 (1.13–1.87)‡ |
| All‐cause death | |||
| DBP ≥70 mm Hg | 231/6757 (3.4%) | Reference | Reference |
| DBP <70 mm Hg + hs‐cTnT <14 ng/L | 44/1287 (3.4%) | 1.01 (0.73–1.39) | 0.87 (0.61–1.25) |
| DBP <70 mm Hg + hs‐cTnT ≥14 ng/L | 76/784 (9.7%) | 3.08 (2.37–3.99) | 1.33 (0.96–1.84)‡ |
CVD composite end point includes myocardial infarction, acute coronary syndrome, heart failure, stroke, and cardiovascular death. CVD indicates cardiovascular disease; DBP, diastolic blood pressure; HR, hazard ratio; and hs‐cTnT, high‐sensitivity cardiac troponin T.
Model 1 is unadjusted.
Model 2 adjusts for baseline age, sex, race, treatment assignment, current smoking, prior CVD, body mass index, low‐density lipoprotein cholesterol, statin use, systolic blood pressure, total number of antihypertensive medications, and estimated glomerular filtration rate.
Comparing those with DBP <70 mm Hg and hs‐cTnT <14 ng/L (referent group) vs those with DBP <70 mm Hg and hs‐cTnT ≥14 ng/L, the adjusted HR=1.96 (95% CI, 1.39–2.78) for the CVD composite outcome and the adjusted HR=1.82 (95% CI, 1.18–2.81) for all‐cause death.
There were 8027 (85.7%) SPRINT participants who also had hs‐cTnT measured at year 1. In general, there were only minimal increases in hs‐cTnT levels from baseline to year 1 across groups (Table 3). In multivariable models, compared with the DBP ≥70 mm Hg group, the low DBP/high hs‐cTnT had a 5% greater 1‐year increase in hs‐cTnT levels, whereas the low DBP/low hs‐cTnT group had similar 1‐year changes in hs‐cTnT (Table 3).
Table 3.
Multivariable‐Adjusted Associations Between Baseline Combined DBP and hs‐cTnT Categories and 1‐Year Change in hs‐cTnT
| Baseline DBP category | hs‐cTnT level, ng/L | 1‐y Change in hs‐cTnT | ||
|---|---|---|---|---|
| Baseline, median (IQR) | Year 1, median (IQR) | Model 1* GMR (95% CI) | Model 2† GMR (95% CI) | |
| DBP ≥70 mm Hg | 8.9 (6.2–13.1) | 8.9 (6.2–13.4) | Reference | Reference |
| DBP <70 mm Hg + hs‐cTnT <14 ng/L | 8.7 (6.4–11.1) | 8.8 (6.5–11.6) | 1.04 (1.02–1.06) | 1.01 (0.99–1.04) |
| DBP <70 mm Hg + hs‐cTnT ≥14 ng/L | 19.9 (16.2–27.1) | 20.3 (16.3–28.1) | 1.07 (1.03–1.11) | 1.05 (1.01–1.09)‡ |
DBP indicates diastolic blood pressure; GMR, geometric mean ratio; hs‐cTnT, high sensitivity cardiac troponin T; and IQR, interquartile range.
Model 1 adjusts for baseline log(hs‐cTnT).
Model 2 adjusts for model 1+baseline age, sex, race, treatment assignment, current smoking, prior cardiovascular disease, body mass index, low‐density lipoprotein cholesterol, statin use, systolic blood pressure, total number of antihypertensive medications, and estimated glomerular filtration rate.
Adjusted GMR, 1.11 (95% CI, 0.92–1.33) comparing those with DBP <70 mm Hg and hs‐cTnT <14 ng/L vs those with DBP <70 mm Hg and hs‐cTnT ≥14 ng/L.
Effects of Intensive BP Lowering on Change in SBP, DBP, and hs‐cTnT
The distribution of achieved SBP at year 1 in the standard BP control arm (SBP target <140 mm Hg) was similar across the DBP ≥70 mm Hg, low DBP/low hs‐cTnT, and low DBP/high hs‐cTnT groups (Figure 1). A similar pattern was observed for the intensive BP control arm (SBP target <120 mm Hg). The mean DBP at year 1 was 79, 68, and 66 mm Hg in the standard BP control arm of the DBP ≥70 mm Hg, low DBP/low hs‐cTnT, and low DBP/high hs‐cTnT groups, respectively, and 71, 62, and 60 mm Hg in the intensive BP control arm across the 3 groups, respectively. Median hs‐cTnT levels at year 1 were similar in the DBP ≥70 mm Hg and low DBP/low hs‐cTnT groups in the standard and intensive BP control arms, whereas year 1 hs‐cTnT levels were higher in the low DBP/high hs‐cTnT group across both treatment arms (Figure 1).
Figure 1. One‐year changes in SBP, DBP, and hs‐cTnT stratified by randomized treatment assignment and combined DBP and hs‐cTnT categories.
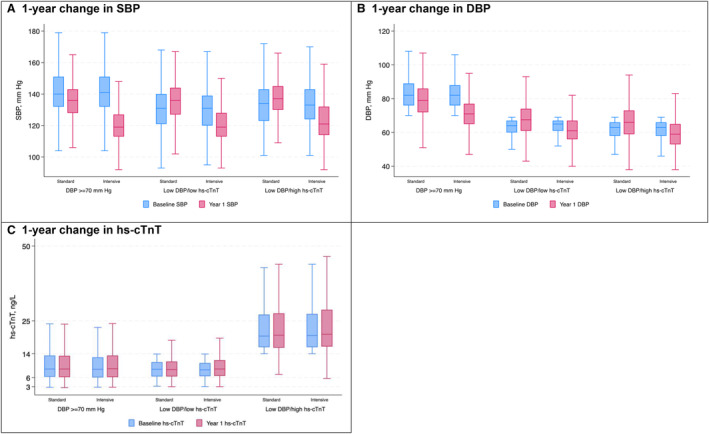
The boxplots display the median as well as 25th and 75th percentiles of patients' mean measurements in the standard and intensive treatment arms at baseline (blue) and 1‐year (red) for SBP (A), DBP (B), and hs‐cTnT (C) by baseline DBP/hs‐cTnT category. DBP indicates diastolic blood pressure; hs‐cTnT, high sensitivity cardiac troponin T; and SBP, systolic blood pressure.
Effects of Intensive BP Lowering on CVD and All‐Cause Death
The effect of randomization to intensive versus standard BP lowering on the CVD composite outcome was similar among participants with low DBP/high hs‐cTnT (hazard ratio [HR], 0.82 [95% CI, 0.57–1.19]) and those with DBP ≥70 mm Hg (HR, 0.73 [95% CI, 0.60–0.89]), and although the effect appeared stronger among participants with low DBP/low hs‐cTnT (HR, 0.48 [95% CI, 0.29–0.79]), the overall test for interaction across groups was not significant (P for interaction=0.21; Figure 2). Intensive BP lowering also led to a similar reduction in all‐cause death among participants with low DBP/high hs‐cTnT (HR, 0.83 [95% CI, 0.53–1.30]), low DBP/low hs‐cTnT (HR, 0.76 [95% CI, 0.42–1.39]), and DBP ≥70 mm Hg (HR, 0.65 [95% CI, 0.50–0.84], P for interaction=0.60; Figure 2). In addition, intensive BP lowering led to minor increases in hs‐cTnT at 1 year that was similar across groups (P for interaction=0.51; Table S1).
Figure 2. Effect of intensive BP lowering on the CVD composite end point and all‐cause death stratified by baseline combined DBP and hs‐cTnT categories.
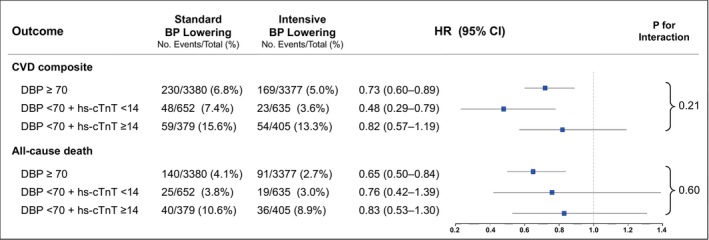
The composite CVD outcome includes nonfatal myocardial infarction, acute coronary syndrome not resulting in myocardial infarction, nonfatal acute decompensated heart failure, stroke, and death from CVD. HRs were obtained from multivariable Cox proportional hazards models that included age, sex, race, treatment assignment, current smoking, prior CVD, body mass index, low‐density lipoprotein cholesterol, SBP, statin use, total number of antihypertensive medications, and estimated glomerular filtration rate. CVD indicates cardiovascular disease; DBP, diastolic blood pressure; HR, hazard ratio; hs‐cTnT, high sensitivity cardiac troponin T; and SBP, systolic blood pressure.
In sensitivity analyses, results were similar when accounting for the competing risk of death using Fine–Gray subdistribution hazard regression models. A similar pattern of results was also observed categorizing participants by widened PP instead of low DBP and categorizing by prevalent CVD instead of elevated baseline hs‐cTnT levels (Table S2). Results were also similar using a nonstroke CVD composite outcome (Figure S2).
Discussion
In this ancillary analysis of SPRINT, participants with low DBP and elevated hs‐cTnT levels at baseline had a higher risk of the CVD composite outcome and all‐cause death compared with those with DBP ≥70 mm Hg. They also had greater increases in hs‐cTnT over time. However, randomization to intensive versus standard BP lowering led to similar reductions in the CVD composite outcome and all‐cause death.
Previous studies demonstrate that low DBP is associated with elevations in hs‐cTnT and that this combination is strongly associated with increased risk for CVD and death, as well as greater increases in hs‐cTnT over time. 26 , 27 We extend these findings to SPRINT and show in a large study sample that, compared with participants with DBP ≥70 mm Hg, those with low DBP and elevated hs‐cTnT levels were at higher risk of CVD, and those with low DBP and nonelevated hs‐cTnT levels were at lower risk. These findings suggest that hs‐cTnT can identify a high‐risk group that may account for the J‐curve relationship between DBP and CVD risk.
The putative mechanism underlying the J‐curve has been reduced coronary perfusion pressure from low DBP, but recent ancillary analyses from BP target trials show that individuals with low baseline DBP derive similar CVD benefits from more intensive BP lowering as those without low baseline DBP. 11 , 12 , 13 These findings suggested that the J‐curve is more likely explained by confounding or reverse causation. However, these studies lacked an intermediary marker of myocardial injury to better characterize those with low DBP. As an established marker of chronic myocardial injury, hs‐cTnT would hypothetically be able to distinguish individuals with low DBP who are particularly susceptible to decreased coronary perfusion and consequently at higher risk of CVD events from additional BP lowering. The present study demonstrates for the first time in a randomized trial that intensive BP lowering among individuals with low DBP and elevated hs‐cTnT levels leads to similar relative reductions in CVD and death and similar short‐term changes in hs‐cTnT, compared with individuals without low DBP and those with low DBP and nonelevated hs‐cTnT levels. SPRINT participants with low DBP and nonelevated hs‐cTnT levels appeared to have a stronger reduction in CVD risk with intensive BP lowering compared with the 2 other groups, although the overall test for interaction was not statistically significant. Taken together, these findings strengthen the evidence that low DBP does not have a directly causal relationship with CVD and death. This is reinforced by our sensitivity analyses using widened PP in place of low DBP and prevalent CVD in place of elevated hs‐cTnT levels.
Our findings may be explained by the counterbalancing of possibly reduced coronary perfusion pressure with an improved overall myocardial oxygen supply‐to‐demand ratio. Given that myocardial oxygen usage improves as the systolic pressure–time index decreases, the effect of improved demand may outweigh the potential supply constraint that occurs with decreased DBP. 36 , 37 This is supported by our observation that intensive BP lowering did not lead to major differential increases in hs‐cTnT in those with low DBP and elevated hs‐cTnT levels at baseline. Although intensive BP lowering led to small increases in hs‐cTnT across groups, recent work has shown that this is likely mediated by benign hemodynamic decreases in eGFR seen with intensive BP lowering. 38
The present study supports the use of lower BP targets in clinical practice, even among individuals with low DBP and evidence of subclinical myocardial injury or prevalent clinical CVD. Prior ancillary analyses of SPRINT and other BP target trials show that other potential harms of intensive BP lowering, including serious adverse events such as acute kidney injury, syncope, hypotension, and electrolyte abnormalities, are similar irrespective of baseline DBP. 11 , 12 , 13 In addition, the effects of intensive BP lowering on cognitive outcomes and cerebral perfusion appear to be similar among SPRINT participants with or without low DBP. 39
Our analysis benefited from the use of randomized data to minimize the impact of confounding, rigorously adjudicated CVD and death outcomes, and repeated hs‐cTnT measurements available in the majority of SPRINT participants. There were also several limitations. First, due to the relatively small proportion of participants with low DBP and elevated hs‐cTnT at baseline, the similar effects of intensive BP lowering on CVD and mortality across groups may be a chance finding and should be interpreted as exploratory. Further research is required to confirm these results. Second, elevations in hs‐cTnT may reflect multiple pathophysiologic mechanisms of chronic myocardial injury that may not confer similar susceptibility to reduced coronary perfusion pressure from low DBP. Third, our findings may not be generalizable to low DBP outside the baseline range in SPRINT, or to individuals who would not meet the inclusion criteria for SPRINT, including those with diabetes or prior stroke and those who are younger in age or at lower CVD risk.
Conclusions
In summary, we demonstrated that even though individuals with low DBP and elevated hs‐cTnT levels are at higher CVD and mortality risk and have greater increases in hs‐cTnT over time compared with those without low DBP, the CVD and mortality benefits of intensive BP lowering are similar. These novel findings strengthen the evidence that low DBP is not directly causal of CVD risk and reinforces the concept that low DBP among individuals with hypertension should not be a barrier to intensive BP control.
Sources of Funding
This ancillary study was supported by the National Heart, Lung, and Blood Institute (1R01HL144112‐01 for Jarett D. Berry); the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860 and linked award KL2 TR001859 (Simon B.Ascher); and the American Heart Association (14EIA18560026 for Joachim H. Ix and career development award 936281 for Simon B.Ascher). Analytical reagents for hs‐cTnT measurements were donated by Roche (Indianapolis, IN).
Disclosures
Jarett D. Berry reports grant support from the National Institutes of Health, Roche Diagnostics, and Abbott Diagnostics and consulting fees from Roche Diagnostics, AstraZeneca and the Cooper Institute. James A. de Lemos reports grant support from Roche Diagnostics and Abbott Diagnostics, consulting fees from Roche Diagnostics, Abbott Diagnostics, Ortho Clinical Diagnostics, Beckman Coulter, Quidel Cardiovascular, Inc., and Siemen's Health Care Diagnostics. He has been named a co‐owner on a patent awarded to the University of Maryland (US Patent Application Number: 15/309754) titled: Methods for Assessing Differential Risk for Developing Heart Failure. Joachim H. Ix holds an investigator‐initiated research grant from Baxter International Inc., serves as a member of a data safety monitoring board for Sanifit Therapeutics, is a member of the scientific advisory board for Alpha Young, and has served on advisory boards for AstraZeneca and Ardelyx. Michael G. Shlipak has received consulting income from Cricket Health, Inc; serves on advisory panels for Boehringer‐Ingelheim, AztraZeneca, and Bayer; and has received research support from Bayer. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
Figures S1–S2
Acknowledgments
The authors thank the participants and staff members of the SPRINT trial (Systolic Blood Pressure Intervention Trial), which was sponsored by the National Institutes of Health, including the National Heart, Lung, and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Institute of Neurological Disorders and Stroke, under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter‐Agency Agreement Number A‐HL‐13–002‐001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of article writing and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the US Department of Veterans Affairs, or the United States Government. For a full list of contributors to SPRINT, please see the supplementary acknowledgement list: https://www.sprinttrial.org/public/dspScience.cfm. We also acknowledge the support from the following Clinical and Translational Science Awards funded by National Center for Advancing Translational Science: Case Western Reserve University: UL1TR000439, Ohio State University: UL1RR025755, University of Pennsylvania: UL1RR024134& UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073 & UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, University of Texas Southwestern: 9U54TR000017–06, University of Utah: UL1TR000105–05, Vanderbilt University: UL1 TR000445, George Washington University: UL1TR000075, University of California, Davis: UL1 TR000002, University of Florida: UL1 TR000064, University of Michigan: UL1TR000433, Tulane University: P30GM103337 COBRE Award NIGMS, and Wake Forest University: UL1TR001420.
This article was sent to Samuel S. Gidding, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.032493
For Sources of Funding and Disclosures, see page 8.
References
- 1. Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, Alexander L, Estep K, Hassen Abate K, Akinyemiju TF, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990‐2015. JAMA. 2017;317:165–182. doi: 10.1001/jama.2016.19043 [DOI] [PubMed] [Google Scholar]
- 2. Muntner P, Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB, Colantonio LD. Trends in blood pressure control among US adults with hypertension, 1999‐2000 to 2017‐2018. JAMA. 2020;324:1190–1200. doi: 10.1001/jama.2020.14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wright JT, Whelton PK, Reboussin DM. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2016;374:2294–2295. doi: 10.1056/nejmc1602668 [DOI] [PubMed] [Google Scholar]
- 4. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324. doi: 10.1161/HYP.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 5. JohnM C, JeffreyM T, Zacharias FJ. Benefits and potential harm of lowering high blood pressure. Lancet. 1987;329:581–584. doi: 10.1016/S0140-6736(87)90231-5 [DOI] [PubMed] [Google Scholar]
- 6. Farnett L, Mulrow CD, Linn WD, Lucey CR, Tuley MR. The J‐curve phenomenon and the treatment of hypertension. Is there a point beyond which pressure reduction is dangerous? JAMA. 1991;265:489–495. doi: 10.1001/jama.1991.03460040065031 [DOI] [PubMed] [Google Scholar]
- 7. Messerli FH, Panjrath GS. The J‐curve between blood pressure and coronary artery disease or essential hypertension: exactly how essential? J Am Coll Cardiol. 2009;54:1827–1834. doi: 10.1016/j.jacc.2009.05.073 [DOI] [PubMed] [Google Scholar]
- 8. Bangalore S, Messerli FH, Wun CC, Zuckerman AL, DeMicco D, Kostis JB, LaRosa JC. Treating to New Targets Steering Committee and Investigators . J‐curve revisited: an analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. Eur Heart J. 2010;31:2897–2908. doi: 10.1093/eurheartj/ehq328 [DOI] [PubMed] [Google Scholar]
- 9. Böhm M, Schumacher H, Teo KK, Lonn EM, Mahfoud F, Mann JFE, Mancia G, Redon J, Schmieder RE, Sliwa K, et al. Achieved blood pressure and cardiovascular outcomes in high‐risk patients: results from ONTARGET and TRANSCEND trials. Lancet. 2017;389:2226–2237. doi: 10.1016/S0140-6736(17)30754-7 [DOI] [PubMed] [Google Scholar]
- 10. Gaffney B, Jacobsen AP, Pallippattu AW, Leahy N, McEvoy JW. The diastolic blood pressure J‐curve in hypertension management: links and risk for cardiovascular disease. Integr Blood Press Control. 2021;14:179–187. doi: 10.2147/IBPC.S286957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ilkun OL, Greene T, Cheung AK, Whelton PK, Wei G, Boucher RE, Ambrosius W, Chertow GM, Beddhu S. The influence of baseline diastolic blood pressure on the effects of intensive blood pressure lowering on cardiovascular outcomes and all‐cause mortality in type 2 diabetes. Diabetes Care. 2020;43:1878–1884. doi: 10.2337/dc19-2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shihab S, Boucher RE, Abraham N, Wei G, Beddhu S. Influence of baseline diastolic blood pressure on the effects of intensive systolic blood pressure lowering on the risk of stroke. Hypertension. 2022;79:785–793. doi: 10.1161/HYPERTENSIONAHA.121.18172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beddhu S, Chertow GM, Cheung AK, Cushman WC, Rahman M, Greene T, Wei G, Campbell RC, Conroy M, Freedman BI, et al. Influence of baseline diastolic blood pressure on effects of intensive compared with standard blood pressure control. Circulation. 2018;137:134–143. doi: 10.1161/CIRCULATIONAHA.117.030848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pareek M, Vaduganathan M, Biering‐Sørensen T, Byrne C, Qamar A, Almarzooq Z, Pandey A, Olsen MH, Bhatt DL. Pulse pressure, cardiovascular events, and intensive blood‐pressure lowering in the systolic blood pressure intervention trial (SPRINT). Am J Med. 2019;132:733–739. doi: 10.1016/j.amjmed.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 15. Foy AJ, Filippone EJ, Schaefer E, Nudy M, Ruzieh M, Dyer AM, Chinchilli VM, Naccarelli GV. Association between baseline diastolic blood pressure and the efficacy of intensive vs standard blood pressure‐lowering therapy. JAMA Netw Open. 2021;4:e2128980. doi: 10.1001/jamanetworkopen.2021.28980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang TI, Wei G, Boucher R, Kramer H, Chertow GM, Cheung AK, Greene T, Whelton PK, Beddhu S. Baseline diastolic blood pressure and cardiovascular outcomes in SPRINT participants with chronic kidney disease. Kidney360. 2020;1:368–375. doi: 10.34067/KID.0000982019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arvanitis M, Qi G, Bhatt DL, Post WS, Chatterjee N, Battle A, McEvoy JW. Linear and nonlinear Mendelian randomization analyses of the association between diastolic blood pressure and cardiovascular events. Circulation. 2021;143:895–906. doi: 10.1161/CIRCULATIONAHA.120.049819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Malik R, Georgakis MK, Vujkovic M, Damrauer SM, Elliott P, Karhunen V, Giontella A, Fava C, Hellwege JN, Shuey MM, et al. Relationship between blood pressure and incident cardiovascular disease: linear and nonlinear Mendelian randomization analyses. Hypertension. 2021;77:2004–2013. doi: 10.1161/HYPERTENSIONAHA.120.16534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Masson S, Latini R, Mureddu GF, Agabiti N, Miceli M, Cesaroni G, Forastiere F, Wienhues‐Thelen UH, Block D, Zaugg C, et al. High‐sensitivity cardiac troponin T for detection of subtle abnormalities of cardiac phenotype in a general population of elderly individuals. J Intern Med. 2013;273:306–317. doi: 10.1111/joim.12023 [DOI] [PubMed] [Google Scholar]
- 20. de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. doi: 10.1001/jama.2010.1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. doi: 10.1001/jama.2010.1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eggers KM, Al‐Shakarchi J, Berglund L, Lindahl B, Siegbahn A, Wallentin L, Zethelius B. High‐sensitive cardiac troponin T and its relations to cardiovascular risk factors, morbidity, and mortality in elderly men. Am Heart J. 2013;166:541–548.e1. doi: 10.1016/j.ahj.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 23. Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Willeit P, Welsh P, Evans JDW, Tschiderer L, Boachie C, Jukema JW, Ford I, Trompet S, Stott DJ, Kearney PM, et al. High‐sensitivity cardiac troponin concentration and risk of first‐ever cardiovascular outcomes in 154,052 participants. J Am Coll Cardiol. 2017;70:558–568. doi: 10.1016/j.jacc.2017.05.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McEvoy JW, Chen Y, Ndumele CE, Solomon SD, Nambi V, Ballantyne CM, Blumenthal RS, Coresh J, Selvin E. Six‐year change in high‐sensitivity cardiac troponin T and risk of subsequent coronary heart disease, heart failure, and death. JAMA Cardiol. 2016;1:519–528. doi: 10.1001/jamacardio.2016.0765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McEvoy JW, Chen Y, Rawlings A, Hoogeveen RC, Ballantyne CM, Blumenthal RS, Coresh J, Selvin E. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol. 2016;68:1713–1722. doi: 10.1016/j.jacc.2016.07.754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bergmark BA, Scirica BM, Steg PG, Fanola CL, Gurmu Y, Mosenzon O, Cahn A, Raz I, Bhatt DL; SAVOR‐TIMI 53 Investigators . Blood pressure and cardiovascular outcomes in patients with diabetes and high cardiovascular risk. Eur Heart J. 2018;39:2255–2262. doi: 10.1093/eurheartj/ehx809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC, Johnson KC, Killeen AA, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532–546. doi: 10.1177/1740774514537404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson KC, Whelton PK, Cushman WC, Cutler JA, Evans GW, Snyder JK, Ambrosius WT, Beddhu S, Cheung AK, Fine LJ, et al. Blood pressure measurement in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension. 2018;71:848–857. doi: 10.1161/HYPERTENSIONAHA.117.10479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berry JD, Nambi V, Ambrosius WT, Chen H, Killeen AA, Taylor A, Toto RD, Soliman EZ, McEvoy JW, Pandey A, et al. Associations of high‐sensitivity troponin and natriuretic peptide levels with outcomes after intensive blood pressure lowering: findings from the SPRINT randomized clinical trial. JAMA Cardiol. 2021;6:1397–1405. doi: 10.1001/jamacardio.2021.3187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ascher SB, Scherzer R, de Lemos JA, Estrella MM, Jotwani VK, Garimella PS, Bullen AL, Ambrosius WT, Ballantyne CM, Nambi V, et al. Associations of high‐sensitivity troponin and natriuretic peptide levels with serious adverse events in SPRINT. J Am Heart Assoc. 2022;11:e023314. doi: 10.1161/JAHA.121.023314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sobieraj P, Lewandowski J, Siński M, Symonides B, Gaciong Z. Low diastolic blood pressure is not related to risk of first episode of stroke in a high‐risk population: a secondary analysis of SPRINT. J Am Heart Assoc. 2019;8:e010811. doi: 10.1161/JAHA.118.010811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. He H, Tang W, Kelly T, Li S, He J. Statistical tests for latent class in censored data due to detection limit. Stat Methods Med Res. 2020;29:2179–2197. doi: 10.1177/0962280219885985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.2307/2670170 [DOI] [Google Scholar]
- 36. Hoffman JIE, Buckberg GD. The myocardial oxygen supply:demand index revisited. J Am Heart Assoc. 2014;3:e000285. doi: 10.1161/JAHA.113.000285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beddhu S, Chertow GM, Cheung AK, Cushman WC, Greene T, Wei G, Boucher R, Whelton PK. Response by Beddhu et al to letters regarding article, "influence of baseline diastolic blood pressure on effects of intensive compared with standard blood pressure control". Circulation. 2018;137:2668–2669. doi: 10.1161/CIRCULATIONAHA.118.034738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Berry JD, Chen H, Nambi V, Ambrosius WT, Ascher SB, Shlipak MG, Ix JH, Gupta R, Killeen A, Toto RD, et al. Effect of intensive blood pressure control on troponin and natriuretic peptide levels: findings from SPRINT. Circulation. 2023;147:310–323. doi: 10.1161/CIRCULATIONAHA.122.059960 [DOI] [PubMed] [Google Scholar]
- 39. Jiang C, Li S, Wang Y, Lai Y, Bai Y, Zhao M, He L, Kong Y, Guo X, Li S, et al. Diastolic blood pressure and intensive blood pressure control on cognitive outcomes: insights from the SPRINT MIND trial. Hypertension. 2023;8:580–589. doi: 10.1161/HYPERTENSIONAHA.122.20112 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S2


