Abstract
Background
Subclinical myocardial injury in form of hs‐cTn (high‐sensitivity cardiac troponin) levels has been associated with cognitive impairment and imaging markers of cerebral small vessel disease (SVD) in population‐based and cardiovascular cohorts. Whether hs‐cTn is associated with domain‐specific cognitive decline and SVD burden in patients with stroke remains unknown.
Methods and Results
We analyzed patients with acute stroke without premorbid dementia from the prospective multicenter DEMDAS (DZNE [German Center for Neurodegenerative Disease]‐Mechanisms of Dementia after Stroke) study. Patients underwent neuropsychological testing 6 and 12 months after the index event. Test results were classified into 5 cognitive domains (language, memory, executive function, attention, and visuospatial function). SVD markers (lacunes, cerebral microbleeds, white matter hyperintensities, and enlarged perivascular spaces) were assessed on cranial magnetic resonance imaging to constitute a global SVD score. We examined the association between hs‐cTnT (hs‐cTn T levels) and cognitive domains as well as the global SVD score and individual SVD markers, respectively. Measurement of cognitive and SVD‐marker analyses were performed in 385 and 466 patients with available hs‐cTnT levels, respectively. In analyses adjusted for demographic characteristics, cardiovascular risk factors, and cognitive status at baseline, higher hs‐cTnT was negatively associated with the cognitive domains “attention” up to 12 months of follow‐up (beta‐coefficient, −0.273 [95% CI, −0.436 to −0.109]) and “executive function” after 12 months. Higher hs‐cTnT was associated with the global SVD score (adjusted odds ratio, 1.95 [95% CI, 1.27–3.00]) and the white matter hyperintensities and lacune subscores.
Conclusions
In patients with stroke, hs‐cTnT is associated with a higher burden of SVD markers and cognitive function in domains linked to vascular cognitive impairment.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01334749.
Keywords: acute stroke, cardiac troponin, cognitive impairment, heart and brain axis
Subject Categories: Cognitive Impairment, Ischemic Stroke, Cerebrovascular Disease/Stroke
Nonstandard Abbreviations and Acronyms
- CERAD
Consortium to Establish a Registry for Alzheimer's Disease Plus
- CMB
cerebral microbleeds
- DEMDAS
DZNE [German Center for Neurodegenerative Disease]‐Mechanisms of Dementia After Stroke study
- NIHSS
National Institutes of Health Stroke Scale
- PVS
perivascular spaces
- SVD
small vessel disease
- WMH
white matter hyperintensities
Research Perspective.
What Is New?
Higher levels of hs‐cTnT (high‐sensitivity cardiac troponin T) are associated with cognitive outcome in the domains attention and executive function up to 12 months after acute ischemic stroke, suggesting that hs‐cTnT is more closely associated with cognitive domains typically affected by vascular cognitive impairment.
Higher levels of hs‐cTnT are associated with a higher burden of cerebral small vessel disease in acute ischemic stroke, which is mainly driven by an association with higher severity of white matter hyperintensities.
What Question Should Be Addressed Next?
Future studies should address whether hs‐cTnT is linked to progression of small vessel disease and long‐term cognitive outcome after stroke.
Cognitive impairment and dementia are common complications following stroke and can lead to significant disability. 1 Previous studies have shown an association between heart disease and cognitive decline as well as incident dementia. 2 , 3 Additionally, data from the general population have provided evidence that (subclinical) myocardial injury, reflected by higher levels of hs‐cTnT (high‐sensitivity cardiac troponin T), is also associated with poor cognitive performance cross‐sectionally as well as incident dementia and cognitive decline even in the absence of manifest cardiac comorbidities. 4 , 5 Hs‐cTnT is a sensitive and specific biomarker of myocardial injury. Routine measurement of hs‐cTn is currently not recommended as a screening tool for cognitive impairment in the general population or in a memory clinic setting. However, current guidelines by the American Heart Association recommend routine measurement of hs‐cTn in patients with ischemic stroke. 6 This recommendation is based on previous studies that have shown an association between hs‐cTn and higher mortality and adverse cardiovascular events after stroke. 7 , 8 At the same time, there is limited evidence on the association between elevated hs‐cTn and cognitive function after stroke. In a study with patients with first‐ever ischemic stroke, we have previously demonstrated that hs‐cTnT is associated with worse cognitive performance at baseline and during follow‐up but not with more severe or faster cognitive decline. 6 However, in the PROSCIS (Prospective Cohort with Incident Stroke) study, longitudinal cognitive data were collected using a screening test via telephone interview, which did not allow for domain‐specific assessment. Furthermore, data on prestroke cognitive status were not available. 9 Because hs‐cTn indicates myocardial injury, one possible explanation for the link between cognitive outcome and hs‐cTn levels is that patients with chronic myocardial injury (reflected in higher levels of cardiac biomarkers such as hs‐cTnT) may also have chronic vascular damage in the brain (eg, cerebral small vessel disease [SVD]) due to common underlying cardiac and cerebrovascular risk factors. 5 Indeed, hs‐cTnT has been associated with white matter hyperintensities (WMH), a marker of cerebral SVD, both in the general population and in patients with acute ischemic stroke. 10 , 11 However, previous studies of hs‐cTnT and SVD have generally examined individual markers rather than the global burden of SVD. The magnetic resonance imaging (MRI)‐based global SVD score, 12 which considers 4 different markers of cerebral SVD, has been linked to cognitive performance both in the general population and in patients with stroke. 13 , 14 , 15
In this study, we aimed to explore the association of hs‐cTnT with longitudinal outcome in different cognitive domains and with SVD burden in patients with stroke without prestroke cognitive impairment or dementia. We assessed data from a prospective multicenter study that was specifically designed to identify predictors of long‐term cognitive outcomes in different cognitive domains post stroke. 16
METHODS
Study Population
The anonymized data that support the findings of this study are available from the principal investigator upon reasonable request.
This study is an exploratory analysis of the ongoing DEMDAS (DZNE [German Center for Neurodegenerative Disease]‐Mechanisms of Dementia After Stroke) study (NCT01334749). DEMDAS is an investigator‐initiated, prospective, multicenter cohort study. The study protocol has been described in detail before. 16 Between January 2014 and January 2019, 600 patients ≥18 years with acute ischemic or hemorrhagic stroke (onset of symptoms within 5 days before inclusion) were enrolled in 7 stroke centers across Germany. The diagnosis of stroke was confirmed by neuroimaging (ie, a diffusion‐weighted imaging‐positive lesion on cranial MRI or a new ischemic lesion on a delayed cranial computed tomography or an intracerebral hemorrhage on cranial computed tomographyor MRI). Due to a low number of patients with hemorrhagic stroke, we included only patients with ischemic stroke into our analyses (see Figure S1). Stroke severity at baseline was measured using the National Institutes of Health Stroke Scale (NIHSS). 17 Prestroke level of function was assessed using the modified Rankin Scale. In order to determine the prestroke modified Rankin Scale level, patients and their informants were questioned about the patient's living situation, need for assistance in activities of daily life, and limited physical abilities before the stroke during the baseline study visit. Patients who were not able to undergo cranial MRI or had a preexisting diagnosis of dementia or an Informant Questionnaire on Cognitive Decline in the Elderly score>64 (indicating preexisting cognitive impairment) at baseline were excluded. 18 For this substudy, we additionally excluded all patients with unavailable hs‐cTnT values (n=87). For the analysis of imaging data on SVD, we excluded all patients with incomplete MRI assessment (n=33).
Study participants and their informants were invited for in‐person follow‐up visits 6 and 12 months after the initial event. At each follow‐up visit, patients and their informants underwent comprehensive cognitive assessments; details are in Table S1.
The DEMDAS study was conducted according to the Declaration of Helsinki and was approved by local ethics committees of all participating sites. All patients or their legal guardians provided written informed consent before study inclusion. Reporting of this substudy follows the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
Blood Tests
Hs‐cTnT was measured from the blood samples collected during the baseline visit using the Elecsys assay (Roche Elecsys Troponin Ths, Mannheim, Germany). This test has a cutoff at 14 ng/L as its upper reference limit (based on the 99th percentile of a healthy population) and a limit of blank at 3 ng/L, a limit of detection at 5 ng/L, and a coefficient of variation of 9% at the upper reference limit. 19
Neurocognitive Testing
During the follow‐up visits, a comprehensive battery of neuropsychological tests classified in 5 cognitive domains (executive function, memory, language, attention, visuospatial function) was performed. Classification of cognitive domains has been published earlier. 20 The Trail Making Test Part B from the Consortium to Establish a Registry for Alzheimer's Disease Plus (CERAD‐Plus) battery and the Stroop Colour‐Word‐Interference Test were used to examine executive function. 21 , 22 Word List Learning/Recall and Recognition and Figure Recall from CERAD‐Plus and immediate and delayed recall of the Rey‐Osterrieth Complex Figure were used to examine memory function. 23 Semantic and Phonemic Fluency and Boston Naming Test, which were subtests of the CERAD‐Plus as well as language items from the Mini‐Mental State Examination were used to examine language. 24 The Trail Making Test Part A from CERAD‐Plus and the Digit‐Symbol‐Substitution Test of the Wechsler Intelligence Scale were used to examine attention. 25 The Figure Drawing Test from CERAD‐Plus and the copy test of Rey‐Osterrieth Complex Figure were used to examine visual spatial function. 21 , 23
First, a Z score was calculated for each individual test based on published norms corrected for age, sex, and education. 15 In a second step, the test‐specific Z scores were averaged for each domain to calculate the 5 domain‐specific Z scores. Lastly, the 5 domain‐specific Z scores were averaged to calculate the global cognitive score. Cognitive impairment for any given domain was defined as a domain‐specific Z score lower than −1.5.
At baseline (ie, during the acute in‐hospital stay) we performed the Mini‐Mental State Examination and the Montreal Cognitive Assessment to screen for global cognitive impairment in the acute poststroke phase. 22 , 26
Neuroimaging
Upon study inclusion 3 Tesla cranial MRI imaging (Siemens, Erlangen, Germany) was performed. Details on the neuroimaging protocol may be found in Data S1. The global SVD burden was examined by assembling the individual SVD markers into a global score from 0 to 4. 9 One point is given for each of the following lesions: (1) presence of lacunes, (2) presence of WMH (periventricular WMH Fazekas grade 3 or deep WMH Fazekas grade≥2), (3) presence of cerebral microbleeds (CMB), and (4) presence of moderate to severe perivascular spaces (PVS) (grade≥2). 12 , 15 SVD markers that were found within the stroke lesion were not incorporated into imaging analysis. 15
Statistical Analysis
Data are shown as median with interquartile range (25th and 75th percentile) for continuous and as absolute (N) and relative (%) frequencies for categorical variables. In order to examine the association between hs‐cTnT and longitudinal cognitive outcome (ie, cognitive trajectories between 6 and 12 months after stroke), we calculated unadjusted and adjusted generalized linear regression models for continuous cognitive data (ie Z scores for global cognitive performance and the 5 individual domains) and logistic regression for dichotomous outcomes (domain‐specific Z scores dichotomized at <−1.5) using generalized estimating equations. In addition to longitudinal cognitive outcome, we assessed cross‐sectional cognitive data at 6 and 12 months separately by performing linear and penalized logistic regression (using the firthlogit command in STATA) analyses. Both longitudinal and cross‐sectional analyses were performed with different levels of adjustment: after running an unadjusted analysis (model 1), we performed our analyses after adjusting for age, sex, and years of education (model 2). In the fully adjusted model (model 3), we additionally adjusted for history of hypertension, diabetes, coronary artery disease, atrial fibrillation, baseline NIHSS score, prestroke modified Rankin Scale score, and cognitive impairment at baseline defined as a Montreal Cognitive Assessment score <26 points or a Mini‐Mental State Examination score <24 points if no Montreal Cognitive Assessment was performed in the subacute stroke phase. Because hs‐cTnT levels were not normally distributed in our study population, we used log‐transformed values for all analyses.
As sensitivity analyses, we reran model 3 with (1) additional adjustment for total SVD score to assess whether the link between hs‐cTnT levels and cognitive performance may be mediated by SVD burden, (2) additional adjustment for stroke localization in the left anterior territory, and (3) after exclusion of patients with stroke affecting more than 1 territory.
To investigate the associations between log‐transformed hs‐cTnT and SVD, we used the following SVD parameters as dependent variables: (1) the global SVD score (range 0–4), (2) the 4 SVD subscores, and (3) the 5 separate SVD markers (lacune counts, periventricular WMH grade, deep WMH grade, CMB counts, PVS grade). For the association with ordinal‐scaled variables (ie, global SVD score, periventricular and deep WMH grade as well as with PVS grade), we calculated ordinal logistic regression models. To assess count variables (ie, lacune count and CMB count), we performed negative binomial regression analyses because both lacune count and CMB count data were overdispersed. Finally, to assess the 4 constituent SVD subscores, we used binary logistic regression analyses. All analyses with regard to SVD markers were performed using 3 models with different levels of adjustment: (1) model 1 unadjusted, (2) model 2 adjusted for age and sex, and (3) model 3 with additional adjustment for hypertension, diabetes, hyperlipidemia, coronary artery disease, atrial fibrillation, smoking status and baseline NIHSS score. In a sensitivity analysis, we reran model 3 after exclusion of patients with stroke affecting more than 1 territory.
To account for multiple comparisons, we calculated corrected P values for all analyses using false discovery rate according to the Benjamini–Hochberg method. The false discovery rate adjustment of P values was based on the sum total of all the tests. We defined statistical significance as a corrected P value <0.05. We performed all statistical calculations using SPSS Statistics 26.0 (IBM, Armonk, NY) and STATA 14.0. The corresponding author had full access to all the data from this substudy and takes responsibility for its integrity and the data analysis.
RESULTS
Baseline Characteristics
We included 385 patients in the analysis of cognitive data and 466 patients in the analysis of imaging data (see Figure S1). The study population included in the analysis of cognitive outcome consisted of patients with mostly mild to moderate strokes (median NIHSS score at baseline=2, interquartile range 1–5), the median age was 68 (interquartile range 59–75) years, and 124 (32.3%) of patients were female. Median hs‐cTnT levels in our study population were 7 ng/L (interquartile range 4–12 ng/L). Hs‐cTnT values were above the upper reference limit of 14 ng/L in 73 (19.0%) of patients. Median time from stroke symptom onset to hs‐cTnT measurement was 1 day (interquartile range 1–2 days). Cognitive impairment at baseline was present in 174 (45.2%) patients with available cognitive follow‐up data. Detailed patients' baseline characteristics are shown in Table, including differences with respect to patients who were not included in the analysis of cognitive outcome due to missing data. Patients with missing cognitive follow‐up data were older, more often had cognitive impairment at baseline, and more often had a history of coronary artery disease or diabetes (see Table). There were no statistically significant differences in baseline characteristics between patients who were included in the analyses of hs‐cTnT and SVD markers and those who were excluded from these analyses due to missing data (see Table S2).
Table .
Baseline Characteristics of Patients Included in and Excluded From Cognitive Analyses
| Patients included in cognitive analyses (n=385) | Patients excluded from cognitive analyses (n=215) | P | |
|---|---|---|---|
| Age, y, median (IQR) | 68 (59–75) | 71 (62–78) | 0.010 |
| Female sex, n (%) | 124 (32.3%) | 76 (35.3%) | 0.434 |
| Years of education, median (IQR) | 13 (12–17) | 13 (11–15) | 0.012 |
| History of hypertension, n (%) | 208 (54.0%) | 128 (59.5%) | 0.165 |
| History of diabetes, n (%) | 50 (13.0%) | 43 (20.0%) | 0.021 |
| History of coronary artery disease, n (%) | 17 (4.4%) | 18 (8.4%) | 0.047 |
| History of atrial fibrillation, n (%) | 36 (9.4%) | 30 (14.0%) | 0.067 |
| Cognitive impairment at baseline, n (%) | 174 (45.2%) | 130 (60.5%) | <0.001 |
| Hs‐cTnT, median (IQR) | 7 (4–12) | ||
| Hs‐cTnT>upper reference limit, n (%) | 73 (19.0%) | ||
| Days from symptom onset to blood draw, median (IQR) | 1 (1–2) | ||
| Stroke cause | |||
| Large artery atherosclerosis, n (%) | 98 (25.5%) | 65 (30.2%) | 0.066 |
| Cardioembolism, n (%) | 80 (20.8%) | 53 (24.7%) | 0.110 |
| Small artery occlusion, n (%) | 50 (13.0%) | 16 (7.4%) | 0.074 |
| Other cause, n (%) | 50 (13.0%) | 15 (7.0%) | 0.052 |
| Undetermined cause, n (%) | 107 (27.8%) | 50 (23.3%) | 0.491 |
| Informant Questionnaire on Cognitive Decline in the Elderly score, median (IQR) | 48 (48–49) | 48 (48–50) | 0.538 |
| Baseline National Institutes of Health Stroke Scale score, median (IQR) | 2 (1–5) | 3 (1–5) | 0.332 |
| Cognitive impairment at 6 mo, n (%) | 112 (29.1%) | ||
| Language | 17 (4.4%) | ||
| Memory | 26 (6.8%) | ||
| Executive function | 30 (7.8%) | ||
| Attention | 35 (9.1%) | ||
| Visuospatial function | 69 (17.9%) | ||
| Cognitive impairment at 12 mo, n (%) | 90 (23.4%) | ||
| Language | 15 (3.9%) | ||
| Memory | 20 (5.2%) | ||
| Executive function | 21 (5.5%) | ||
| Attention | 22 (5.7%) | ||
| Visuospatial function | 64 (16.6%) | ||
| Stroke localization | |||
| Anterior left | 110 (28.6%) | 51 (23.7%) | 0.199 |
| Anterior right | 90 (23.4%) | 56 (26.0%) | 0.465 |
| Posterior cerebral artery left | 29 (7.5%) | 13 (6.0%) | 0.494 |
| Posterior cerebral artery right | 23 (6.0%) | 16 (7.4%) | 0.484 |
| Brainstem | 36 (9.4%) | 18 (8.4%) | 0.688 |
| Cerebellum | 31 (8.1%) | 12 (5.6%) | 0.261 |
| Multiple | 48 (12.5%) | 30 (14.0%) | 0.604 |
Baseline characteristics of patients included in cognitive analyses and excluded from cognitive analyses. Univariable comparisons were performed using chi‐square test for dichotomous variables and Mann–Whitney U test for linear variables. Patients with missing cognitive follow‐up data were older, more often had cognitive impairment at baseline and more often had a history of coronary artery disease or diabetes. hs‐cTnT indicates high‐sensitivity cardiac troponin T; and IQR, interquartile range.
Hs‐cTnT and Cognitive Outcome
Overall, cognitive outcomes improved in all domains between month 6 and 12 after stroke. The number of individuals with global cognitive impairment was 112 (29.1%) and 90 (23.4%) at 6 and 12 months, respectively. Hs‐cTnT was associated with global cognitive performance in the unadjusted longitudinal analysis as well as in the unadjusted cross‐sectional analysis at 12 months after stroke. Both associations were no longer statistically significant after full adjustment.
With regard to specific cognitive domains, hs‐cTnT was negatively associated with performance in the domain “attention” in the longitudinal analysis after adjustment for demographic characteristics, cardiovascular risk factors, and clinical outcome at baseline as well as correction for multiple comparisons (Figure 1). When we examined cognitive outcome at 6 and 12 months separately in cross‐sectional analyses, we found an association with the domain “attention” both at 6 and 12 months (Figures 2 and 3) and with executive function at 12 months (Figure 3). The associations we found with the domains “attention” and “executive function” remained significant in the sensitivity analysis after additional adjustment for total SVD burden (see Table S3) and for stroke localization in the left anterior territory (see Table S4). After exclusion of patients with strokes in multiple territories, the association between hs‐cTnT and performance in the domain “attention” at 6 months of follow‐up was no longer significant (see Table S5). Apart from that, the results remained unchanged compared with the main analyses.
Figure 1. Log‐transformed hs‐cTnT and cognitive domains (continuous) across 12 months of follow‐up according to generalized linear regression models using generalized estimating equations.
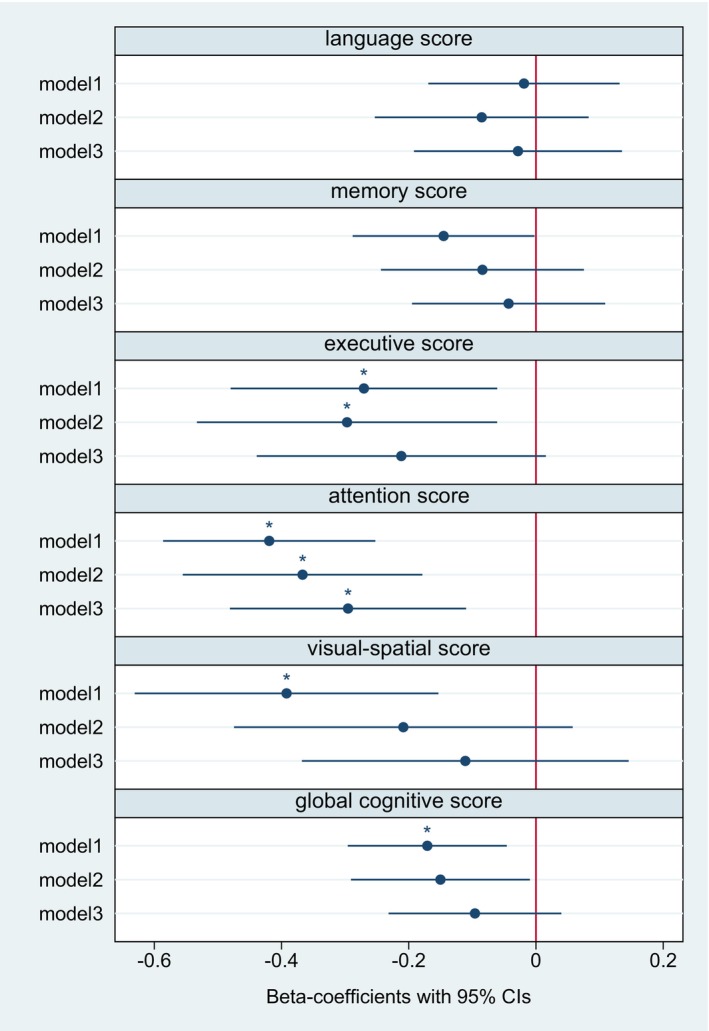
The figure displays the respective regression coefficients and 95% CIs. Model 1: unadjusted. Model 2: adjusted for age, sex, and years of education. Model 3: additional adjustment for hypertension, diabetes, coronary artery disease, atrial fibrillation, cognitive impairment at baseline, baseline NIHSS score, and prestroke mRS score. *P corr<0.05. After full adjustment, hs‐cTnT was associated with a decline in performance in the cognitive domain “attention” between 6 and 12 months after stroke. Hs‐cTnT indicates high‐sensitivity cardiac troponin T; mRS, modified Rankin Scale; and NIHSS, National Institutes of Health Stroke Scale.
Figure 2. Log‐transformed hs‐cTnT and cognitive domains (continuous) at 6 months of follow‐up according to linear regression models.
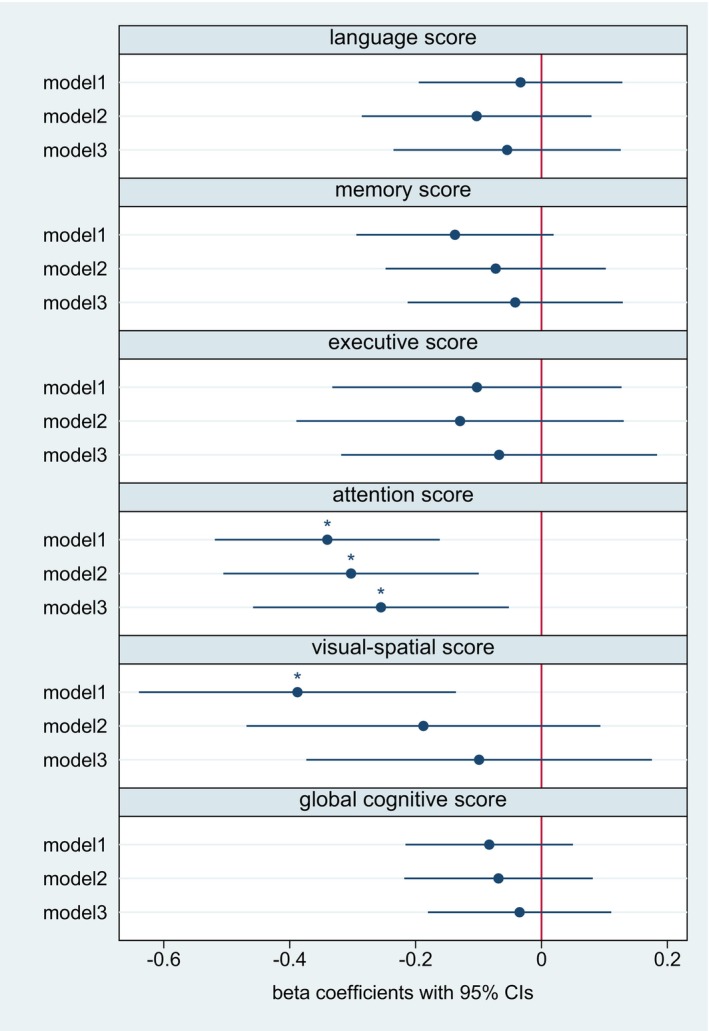
The figure displays the respective regression coefficients and 95% CIs. Model 1: unadjusted. Model 2: adjusted for age, sex, and years of education. Model 3: additional adjustment for hypertension, diabetes, coronary artery disease, atrial fibrillation, cognitive impairment at baseline, baseline NIHSS score, and prestroke mRS score. *P corr<0.05. After full adjustment, hs‐cTnT was negatively associated with performance in the cognitive domain “attention” in the cross‐sectional analyses 6 months after stroke. Hs‐cTnT indicates high‐sensitivity cardiac troponin T; mRS, modified Rankin Scale; and NIHSS, National Institutes of Health Stroke Scale.
Figure 3. Log‐transformed hs‐cTnT and cognitive domains (continuous) at 12 months of follow‐up according to linear regression models.
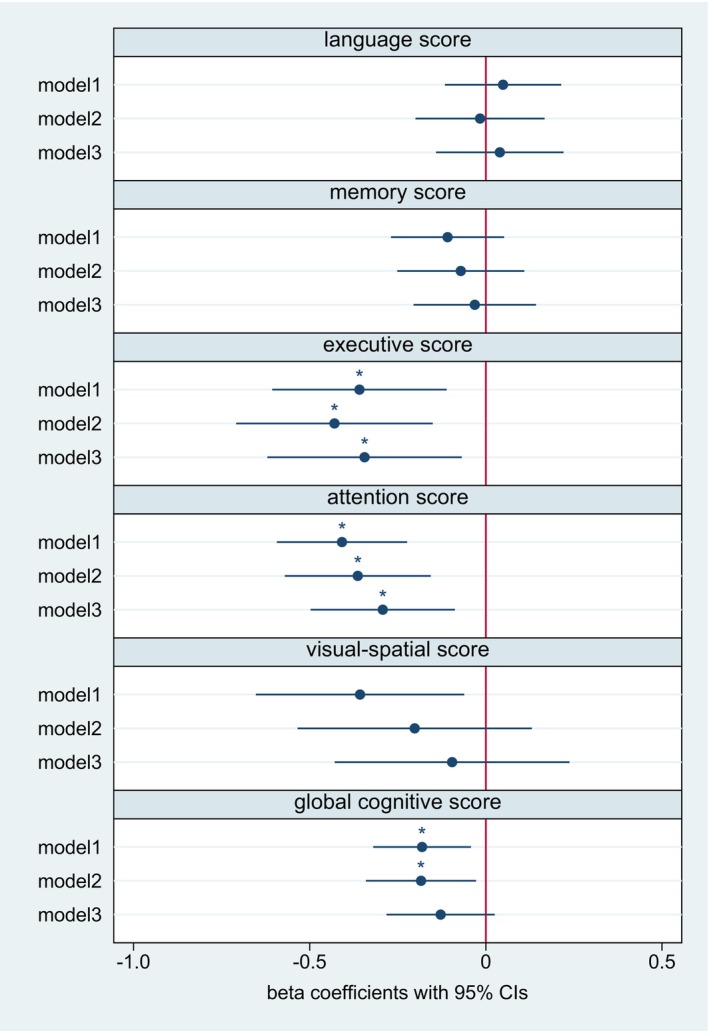
The figure displays the respective odds ratios and 95% CIs. Model 1: unadjusted. Model 2: adjusted for age, sex, and years of education. Model 3: additional adjustment for hypertension, diabetes, coronary artery disease, atrial fibrillation, cognitive impairment at baseline, baseline NIHSS score, and prestroke mRS score. *P corr<0.05. After full adjustment, hs‐cTnT was negatively associated with performance in the domains “attention” and “executive function” in the cross‐sectional analyses 6 months after stroke. Hs‐cTnT indicates high‐sensitivity cardiac troponin T; mRS, modified Rankin Scale; and NIHSS, National Institutes of Health Stroke Scale.
There were no statistically significant associations between hs‐cTnT and cognitive impairment in any specific domain in the binary outcome models (ie, after dichotomizing cognitive data at a Z score of −1.5) both in the longitudinal and in the cross‐sectional analyses (see Figures S2 through S4).
Hs‐cTnT and SVD Markers
The frequency and burden of SVD markers are displayed in Table S2. Most patients had an SVD score of either 0 (40.1%, no lesions fulfilling the score criteria) or 1 (29.4%, one lesion type fulfilling the score criteria). The SVD marker most frequently found to fulfill the score criteria was WMH (see Table S2).
Levels of hs‐cTnT were associated with the global SVD score (see Figure 4). This association remained statistically significant after full adjustment and correction for multiple testing (adjusted odds ratio for model 3, 1.87 [95% CI, 1.21–2.89], see Figure 4). In the unadjusted models, hs‐cTnT was associated with all 4 constituent SVD subscores except for the CMB subscore (see Figure 4). After full adjustment and correction for multiple testing, the association remained statistically significant for the WMH subscore and the lacune subscore (see Figure 4). However, after exclusion of patients with strokes in multiple territories, the association between hs‐cTnT and the lacune subscore was no longer statistically significant (see Table S6). When assessing individual SVD markers in their entire severity range, we found a statistically significant association with deep WMH grade after adjustment for potential confounders and correction for multiple testing (see Figure 5).
Figure 4. Log‐transformed hs‐cTnT and global cerebral small vessel disease score as well as 4 constituent SVD subscores.
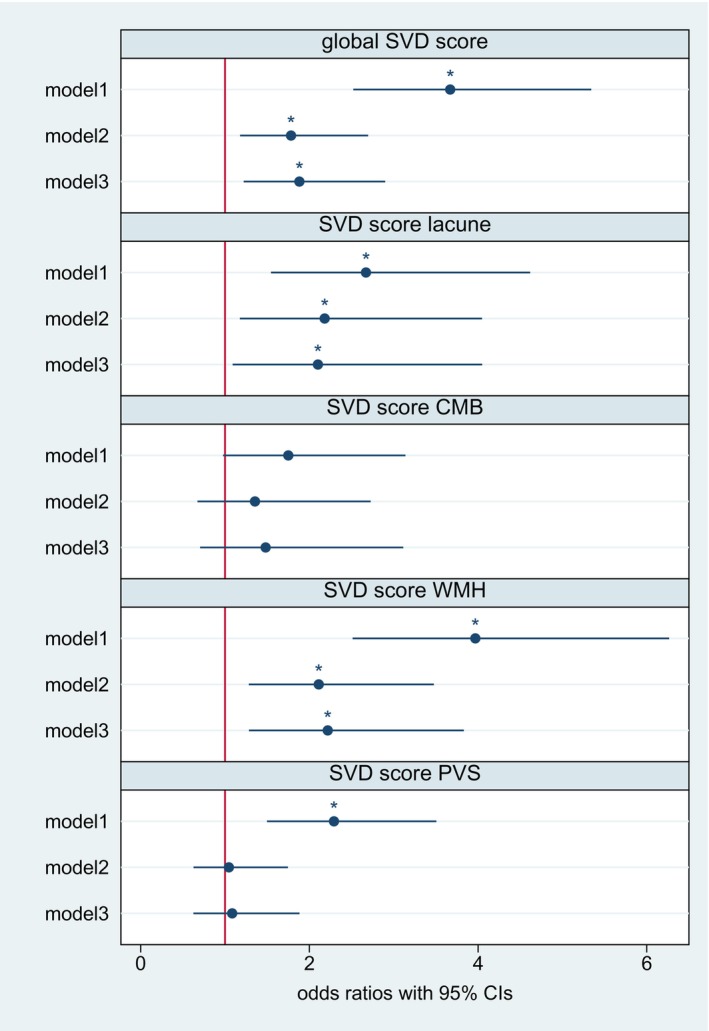
The figure displays odds ratios and 95% CIs derived from ordinal logistic regression models for the global SVD score and binary logistic regression models for each constituent subscore, respectively. Model 1: unadjusted. Model 2: adjusted for age and sex. Model 3: additional adjustment for hypertension, diabetes, hyperlipidemia, coronary artery disease, atrial fibrillation, smoking status, and baseline NIHSS score. After full adjustment, hs‐cTnT was associated with higher global SVD scores as well as the WMH and lacune subscores. *P corr<0.05. CMB indicates cerebral microbleeds; hs‐cTnT, high‐sensitivity cardiac troponin T; NIHSS, National Institutes of Health Stroke Scale; PVS, perivascular spaces; SVD, small vessel disease; and WMH, white matter hyperintensities.
Figure 5. Hs‐cTnT and individual cerebral small vessel disease markers in their entire range.
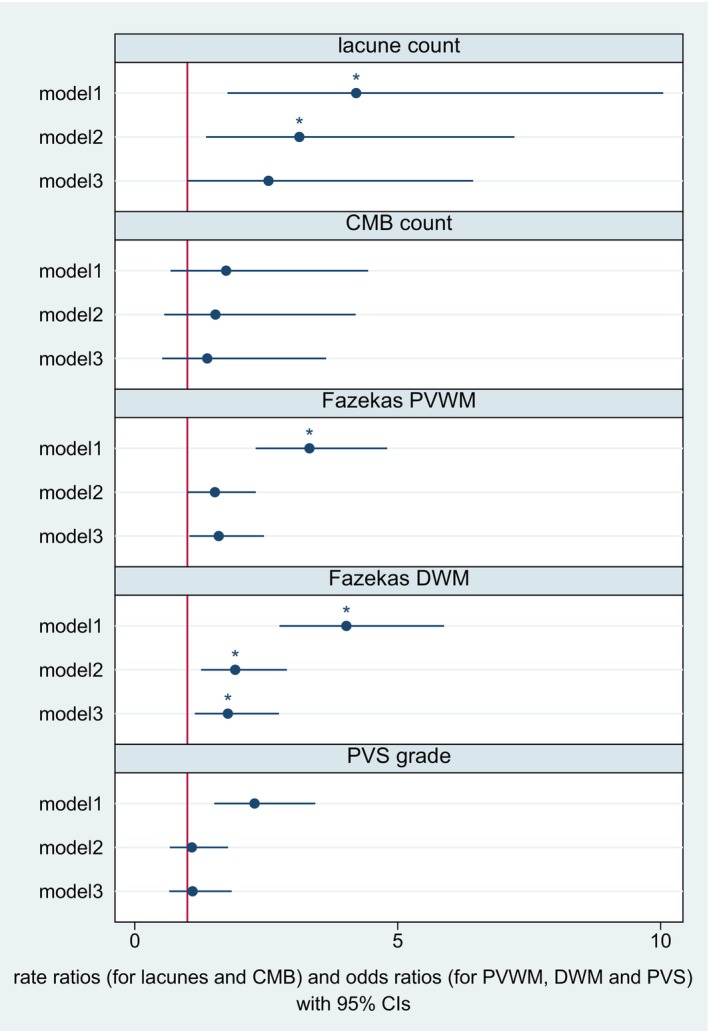
The figure displays odds ratios derived from ordinal regression models for periventricular WMH grade, deep WMH grade, and PVS grade. The figure displays rate ratios calculated using negative binomial regression models for lacune count and CMB count. Model 1: unadjusted. Model 2: adjusted for age and sex. Model 3: additional adjustment for hypertension, diabetes, hyperlipidemia, coronary artery disease, atrial fibrillation, smoking status, and baseline NIHSS score. After full adjustment, hs‐cTnT remained associated with higher deep WMH grade. *P corr<0.05. CMB indicates cerebral microbleeds; DWM, deep white matter; hs‐cTnT, high‐sensitivity cardiac troponin T; NIHSS, National Institutes of Health Stroke Scale; PVS, perivascular spaces; PVWM, periventricular white matter; SVD, small vessel disease; and WMH, white matter hyperintensities.
DISCUSSION
This exploratory analysis of the prospective multicenter DEMDAS study contains several important findings. First, hs‐cTnT levels were associated with poorer cognitive performance and decline in the domain “attention” up to 12 months of follow‐up. Associations were found in both longitudinal and cross‐sectional analyses and remained stable after adjustment for potential confounders, including prevalent cardiovascular risk factors, and after correction for multiple comparisons. Second, hs‐cTnT levels were associated with poorer performance in executive function at 12 months after the index stroke. Third, we found that hs‐cTnT levels were associated with cerebral SVD burden in patients with stroke, which was driven by the subscores of WMH burden and (to a lesser degree) lacunes. This highlights the potential interplay between subclinical myocardial injury, arteriolosclerotic SVD, and features of vascular dementia. However, because the association between hs‐cTnT levels and cognitive function remained statistically significant even after adjustment for SVD burden, the link between hs‐cTnT and cognition in patients with stroke seems to also be independently mediated by pathophysiological factors other than SVD.
To our knowledge, our study is the first to examine the association between hs‐cTnT levels and different cognitive domains in patients with stroke. Our results are in line with studies from the general population showing an association between hs‐cTnT levels and performance in the Digit‐Symbol‐Substitution Test, which tests mainly attention and processing speed and was also part of the cognitive tests used to assess attention in our study. 4 , 5 , 27 Attention and processing speed have typically been attributed to vascular pathology and vascular dementia. 28 Therefore, our results suggest that hs‐cTnT is associated with vascular pathology rather than the cognitive domains typically affected in Alzheimer's disease, such as memory or language. 29
Of note, we did not find a statistically significant association between hs‐cTnT levels and cognitive impairment when using the logistic model dichotomizing each score at −1.5. However, only a small percentage of patients (<10% in all cognitive domains except visual spatial function) had cognitive impairment for any given domain. Therefore, we might have missed a statistically significant association due to limited statistical power.
We found that hs‐cTnT levels were associated with the global SVD burden measured by the MRI‐based SVD score. Our results are in line with 2 previous studies in patients with hypertension and lacunar stroke, respectively, that found an association between NT‐proBNP (N‐terminal pro–brain natriuretic peptide) and global SVD burden. 30 , 31 We are, however, not aware of any other studies assessing the link between global SVD burden and hs‐cTnT.
When examining the four constituent SVD subscores and the respective SVD markers in their entire severity range, we found that the association with hs‐cTnT levels is largely driven by WMH, which was also the most common pathological SVD marker in our study population. Previous studies have also shown a link between hs‐cTnT levels and WMH both in the general population and in patients with ischemic stroke. 10 , 11 , 32 The association between cardiac biomarkers and other markers of SVD (ie, CMB, PVS, and lacunes) is less well described. We found an association between hs‐cTnT levels and the lacune subscore but not with lacune count as a linear variable. A possible explanation is that only a small proportion of our study population had lacunes and that lacune count as a linear variable was highly skewed. This may also be the reason why the association between hs‐cTnT and lacune subscore was no longer significant in the sensitivity analysis excluding patients with stroke in more than 1 territory. Concerning the PVS and CMB subscores, we did not find a statistically significant association with hs‐cTnT levels after full adjustment and correction for multiple testing. In line with our findings, Gyanwali et al. did not find an association between hs‐cTnT and incident CMBs on repeated MRI scans in 343 memory clinic patients. 33
The pathogenetic mechanisms that explain the association between markers of myocardial injury such as hs‐cTnT and cerebral SVD as well as cognitive function have not been fully elucidated. Importantly, it is unlikely that troponin itself causes cognitive impairment or SVD. Hs‐cTn is released into the bloodstream as a result of cardiomyocyte injury. 19 Both myocardial injury as well as SVD may result from common underlying vascular risk factors and vascular disease. 34 , 35 Apart from that, higher levels of hs‐cTn may also result from structural heart disease leading to chronic cerebral hypoperfusion. 36 , 37 Finally, acute stroke has been linked to autonomic cardiac dysfunction and stroke‐induced heart injury (so called stroke‐heart syndrome) that would explain increased cardiac biomarkers, too. 38 Stroke‐heart syndrome typically occurs in the (subacute) stroke phase. 38 It occurs more frequently in patients with higher stroke severity but also depending on stroke localization, for example, in strokes affecting the insular region. 38 , 39 Because blood draws for hs‐cTn measurement were taken relatively early after symptom onset in our study population, both chronic myocardial injury and stroke‐induced acute myocardial injury have likely contributed to hs‐cTnT levels measured in this study.
Our results suggest that hs‐cTnT levels may provide a more accurate determination of the cardiovascular‐associated risk for cognitive decline and SVD in patients with stroke than clinical history alone. Indeed, previous research has shown that although a history of cardiovascular comorbidities (such as ischemic heart disease, hypertension, or diabetes) is significantly associated with WMH, cardiovascular risk factors accounted for only a small amount of WMH variability. 40 Hs‐cTnT may be a useful parameter to identify patients at risk of cognitive decline because it is a sensitive biomarker for myocardial injury and can be measured in everyday clinical practice. 41 Moreover, current guidelines for the management of patients with acute ischemic stroke recommend the routine measurement of hs‐cTn. 8 Thus, hs‐cTn levels are widely available in patients with stroke in particular.
Our study benefits from the multicenter prospective design with the predefined aim to determine factors of cognitive impairment after stroke. To this end, patients underwent repeated face‐to‐face follow‐up examinations including detailed neuropsychological testing that provided an extensive, multidomain, and standardized assessment of cognitive performance. In addition, patients underwent 3T MRI imaging using a standardized protocol in accordance with the Standards for Reporting Vascular Changes on Neuroimaging recommendations for neuroimaging of SVD. 42 Interpretation of MRI was performed centralized and blinded to clinical data.
However, our study also has certain limitations: patients eligible for inclusion in DEMDAS had to be able to give informed consent and be willing and motivated to participate in a study with several years of follow‐up including repeated and extensive neuropsychological examinations and cerebral MRI. Therefore, the majority of our study population had mild stroke (median NIHSS score 2). In addition, most of our study population was highly educated (median 13 years of education) and had overall good cognitive outcome resulting in a low number of patients with cognitive impairment for every examined domain. This may have attenuated the association between hs‐cTnT levels and cognition, particularly in the dichotomous models and restricts the generalizability to more severely affected patients with stroke. Because hs‐cTnT levels were measured only once during the acute phase, we were not able to differentiate between acute and chronic myocardial injury and their respective associations with cognitive performance. Moreover, insular involvement was not systematically recorded in DEMDAS but may also have affected hs‐cTn levels in our study population. Stroke localization and the initial neurological deficit may affect the performance in cognitive tests. To account for this, we adjusted our analysis for initial NIHSS score and presence of global cognitive impairment at baseline. In addition, we performed a sensitivity analysis with additional adjustment for stroke located in the left anterior territory, which has been associated with an increased risk of poststroke cognitive impairment. 43 However, we cannot exclude residual confounding of our results due to stroke localization.
In addition, there was a considerable rate of loss to follow‐up in our study population. Because patients with poor cognitive function are less likely to take part in repeated follow‐up examinations, this might have led to selective attrition bias from loss to follow‐up. The analyses we report here were exploratory and not part of the prespecified DEMDAS study protocol. The DEMDAS study was not specifically powered to detect an association between hs‐cTnT and cognitive outcome. The long‐term follow‐up period of the DEMDAS study is still ongoing. Therefore, our current analysis on imaging data is restricted to the MRI at baseline and we were not able to examine the association between hs‐cTnT levels and SVD progression. However, the study protocol of DEMDAS includes repeated MRI imaging 16 during the follow‐up period so that this question may be addressed in future substudies.
CONCLUSIONS
Our results from this multicenter prospective study with comprehensive neuropsychological assessment show that hs‐cTnT levels at baseline is associated with performance in the cognitive domain “attention” and “executive function” in patients with stroke with up to 12 months of follow‐up. This suggests that hs‐cTnT is associated with vascular pathology and vascular dementia rather than the cognitive domains typically affected in Alzheimer's disease in patients with stroke. In this cohort, hs‐cTnT levels are also associated with the global SVD burden in general and severity of WMH as a marker of arteriolosclerotic atheropathy in particular.
APPENDIX
DEMDAS Investigators
Matthias Endres (Department of Neurology with Experimental Neurology, Charité—Univeristätsmedizin Berlin, Germany; German Center for Neurodegenerative Diseases (DZNE), Berlin, Germany; Center for Stroke Research Berlin (CSB), Charité—Universitätsmedizin Berlin, Germany; German Centre for Cardiovascular Research (DZHK), partner site Berlin, Germany), Thomas G. Liman (Department of Neurology with Experimental Neurology, Charité—Univeristätsmedizin Berlin, Germany; German Center for Neurodegenerative Diseases (DZNE), Berlin, Germany), Lucia Kerti (Center for Stroke Research Berlin (CSB), Charité—Universitätsmedizin Berlin, Germany; German Center for Neurodegenerative Diseases (DZNE), Berlin Germany), Christian H. Nolte (Department of Neurology with Experimental Neurology, Charité—Univeristätsmedizin Berlin, Germany; Center for Stroke Research Berlin (CSB), Charité—Universitätsmedizin Berlin, Germany; Berlin Institute of Health (BIH), Germany), Tatjana Wittenberg (Center for Stroke Research Berlin (CSB), Charité—Universitätsmedizin Berlin, Germany), Jan F. Scheitz (Department of Neurology with Experimental Neurology, Charité—Univeristätsmedizin Berlin, Germany; Center for Stroke Research Berlin (CSB), Charité—Universitätsmedizin Berlin, Germany; Berlin Institute of Health (BIH), Germany), Harald Prüß (Department of Neurology with Experimental Neurology, Charité—Univeristätsmedizin Berlin, Germany; German Center for Neurodegenerative Diseases (DZNE), Berlin, Germany), Pia Sophie Sperber (Department of Neurology with Experimental Neurology, Charité—Univeristätsmedizin Berlin, Germany; Center for Stroke Research Berlin (CSB), Charité—Universitätsmedizin Berlin, Germany); Alexander H. Nave (Department of Neurology with Experimental Neurology, Charité—Universitätsmedizin Berlin, Germany; Center for Stroke Research Berlin (CSB), Charité—Universitätsmedizin Berlin, Germany; Berlin Institute of Health (BIH), Germany), Anna Kufner Ibaroule (Department of Neurology with Experimental Neurology, Charité—Universitätsmedizin Berlin, Germany; Center for Stroke Research Berlin (CSB), Charité—Universitätsmedizin Berlin, Germany; Berlin Institute of Health (BIH), Germany), Gabor Petzold (Division of Vascular Neurology, Department of Neurology, University Hospital Bonn, Germany; German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany), Felix Bode (Division of Vascular Neurology, Department of Neurology, University Hospital Bonn, Germany; German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany), Sebastian Stösser (Division of Vascular Neurology, Department of Neurology, University Hospital Bonn, Germany; German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany), Julius Meissner (Division of Vascular Neurology, Department of Neurology, University Hospital Bonn, Germany), Taraneh Ebrahimi (Division of Vascular Neurology, Department of Neurology, University Hospital Bonn, Germany), Julia Nordsiek (Division of Vascular Neurology, Department of Neurology, University Hospital Bonn, Germany; German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany), Niklas Beckonert (Division of Vascular Neurology, Department of Neurology, University Hospital Bonn, Germany), Christine Kindler (Division of Vascular Neurology, Department of Neurology, University Hospital Bonn, Germany; German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany), Inga Zerr (Department of Neurology, University Medical Center Göttingen, Germany; German Center for Neurodegenerative Diseases (DZNE), Göttingen, Germany), Peter Hermann (Department of Neurology, University Medical Center Göttingen, Germany), Matthias Schmitz (Department of Neurology, University Medical Center Göttingen, Germany), Stefan Goebel (Department of Neurology, University Medical Center Göttingen, Germany), Timothy Bunck (Department of Neurology, University Medical Center Göttingen, Germany), Julia Schütte‐Schmidt (Department of Neurology, University Medical Center Göttingen, Germany), Sabine Nuhn (Department of Neurology, University Medical Center Göttingen, Germany), Corinna Volpers (Department of Neurology, University Medical Center Göttingen, Germany), Peter Dechent (Department of Neurology, University Medical Center Göttingen, Germany), Mathias Bähr (Department of Neurology, University Medical Center Göttingen, Germany; German Center for Neurodegenerative Diseases (DZNE), Göttingen, Germany; Cluster of Excellence Nanoscale Microscopy and Molecular Physiology of the Brain (CNMPB), Göttingen, Germany), Michael Görtler (Department of Neurology, University Hospital, Otto‐von‐Guericke University Magdeburg, Germany; German Center for Neurodegenerative Diseases (DZNE), Magdeburg, Germany), Wenzel Glanz (Department of Neurology, University Hospital, Otto‐von‐Guericke University Magdeburg, Germany; German Center for Neurodegenerative Diseases (DZNE), Magdeburg, Germany), Valentina Perosa (Department of Neurology, University Hospital, Otto‐von‐Guericke University Magdeburg, Germany; German Center for Neurodegenerative Diseases (DZNE), Magdeburg, Germany), Martin Dichgans (Institute for Stroke and Dementia Research (ISD), University Hospital, LMU Munich, Germany; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany), Frank Wollenweber (Institute for Stroke and Dementia Research (ISD), University Hospital, LMU Munich, Germany), Marios Georgakis (Institute for Stroke and Dementia Research (ISD), University Hospital, LMU Munich, Germany), Rong Fang (Institute for Stroke and Dementia Research (ISD), University Hospital, LMU Munich, Germany), Daniel Janowitz (Institute for Stroke and Dementia Research (ISD), University Hospital, LMU Munich, Germany), Karin Waegemann (Institute for Stroke and Dementia Research (ISD), University Hospital, LMU Munich, Germany; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany), Steffen Tiedt (Institute for Stroke and Dementia Research (ISD), University Hospital, LMU Munich, Germany), Silke Wunderlich (Department of Neurology, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany), Benno Ikenberg (Department of Neurology, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany), Kathleen Bernkopf (Department of Neurology, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany), Christiane Huber (Department of Neurology, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Germany), Holger Poppert (Neurology Department, Helios Klinikum München West, Munich, Germany), Marco Düring (Institute for Stroke and Dementia Research (ISD), University Hospital, LMU Munich, Germany; Medical Image Analysis Center (MIAC AG) and Quantitative Biomedical Imaging Group (qbig), Department of Biomedical Engineering, University of Basel, Switzerland), Miguel Ángel Araque Caballero (Institute for Stroke and Dementia Research (ISD), University Hospital, LMU Munich, Germany; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany), Benno Gieserich (Institute for Stroke and Dementia Research (ISD), University Hospital, LMU Munich, Germany), Anna Dewenter (Institute for Stroke and Dementia Research (ISD), University Hospital, LMU Munich, Germany), Laura Dobisch (German Center for Neurodegenerative Diseases (DZNE), Magdeburg, Germany), Katja Neumann (German Center for Neurodegenerative Diseases (DZNE), Magdeburg, Germany), Oliver Speck (Department of Biomedical Magnetic Resonance, Institute for Physics, Otto‐von‐Guericke University Magdeburg, Germany; German Center for Neurodegenerative Diseases (DZNE), Magdeburg, Germany; Leibniz Institute for Neurobiology, Magdeburg, Germany; Center for Behavioral Brain Sciences, Magdeburg, Germany), Annika Spottke (German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany; Division of Vascular Neurology, Department of Neurology, University Hospital Bonn, Germany), Tony Stöcker (German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany), Peter Bartenstein (Department of Nuclear Medicine, University Hospital, LMU Munich, Germany), Michael Wagner (German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany).
Sources of Funding
The DEMDAS study was funded by the German Center for Neurodegenerative Diseases (DZNE), reference number MC002. M.E. received funding from Deutsche Forschungsgemeinschaft (DFG) under Germany's Excellence Strategy—EXC‐2049—390688087, Collaborative Research Center ReTune TRR 295‐424778381, Bundesministerium für Bildung und Forschung, DZNE, DZHK, European Commission, Corona Foundation, and Fondation Leducq. G.C.P. received funding from the DFG (FOR 2795, PE1193/6‐2), European Research Area‐Network (MICRO‐BLEEDs and TACKLE‐CSVD), and Fondation Leducq (Transatlantic Network of Excellence 23CVD03), and is a member of the DFG‐funded Cluster of Excellence ImmunoSensation—EXC2151–390873048.
Disclosures
M.E. reports grants from Bayer and fees paid to the Charité from Abbot, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo, Sanofi, Novartis, and Pfizer, all outside the submitted work. C.H.N. reports speaker or consultation fees from Abbott, Alexion, Astra Zeneca, Bayer Pharma, Bristol‐Myers Squibb, Daiichi Sankyo, Novartis, Pfizer Pharma, Portola, and Takeda. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S6
Figures S1–S4
References 44,45
Acknowledgments
J.F.S. is participant in the Berlin Institute of Health‐Charité Advanced Clinician Scientist Program, funded by the Charité – Universitätsmedizin Berlin and the Berlin Institute of Health. SH is participant in the Berlin Institute of Health‐Charité Clinician Scientist Program funded by the Charité—Universitätsmedizin Berlin, and the Berlin Institute of Health at Charité.
This article was sent to Jose R. Romero, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.033439
For Sources of Funding and Disclosures, see page 14.
Contributor Information
Regina von Rennenberg, Email: regina-irene.freiin-von-rennenberg@charite.de.
for the DEMDAS investigators:
Matthias Endres, Thomas G. Liman, Lucia Kerti, Christian H. Nolte, Tatjana Wittenberg, Jan F. Scheitz, Harald Prüß, Pia Sophie Sperber, Alexander H. Nave, Anna Kufner Ibaroule, Gabor Petzold, Felix Bode, Sebastian Stösser, Julius Meissner, Taraneh Ebrahimi, Julia Nordsiek, Niklas Beckonert, Christine Kindler, Inga Zerr, Peter Hermann, Matthias Schmitz, Stefan Goebel, Timothy Bunck, Julia Schütte‐Schmidt, Sabine Nuhn, Corinna Volpers, Peter Dechent, Mathias Bähr, Michael Görtler, Wenzel Glanz, Valentina Perosa, Martin Dichgans, Frank Wollenweber, Rong Fang, Daniel Janowitz, Karin Waegemann, Steffen Tiedt, Silke Wunderlich, Benno Ikenberg, Kathleen Bernkopf, Christiane Huber, Holger Poppert, Marco Düring, Miguel Ángel Araque Caballero, Benno Gieserich, Anna Dewenter, Laura Dobisch, Katja Neumann, Oliver Speck, Annika Spottke, Tony Stöcker, Peter Bartenstein, and Michael Wagner
References
- 1. Pendlebury ST, Rothwell PM; Oxford Vascular Study . Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: analysis of the population‐based Oxford Vascular Study. Lancet Neurol. 2019;18:248–258. doi: 10.1016/S1474-4422(18)30442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rusanen M, Kivipelto M, Levälahti E, Laatikainen T, Tuomilehto J, Soininen H, Ngandu T. Heart diseases and long‐term risk of dementia and Alzheimer's disease: a population‐based CAIDE study. J Alzheimers Dis. 2014;42:183–191. doi: 10.3233/JAD-132363 [DOI] [PubMed] [Google Scholar]
- 3. Cannon JA, Moffitt P, Perez‐Moreno AC, Walters MR, Broomfield NM, McMurray JJV, Quinn TJ. Cognitive impairment and heart failure: systematic review and meta‐analysis. J Card Fail. 2017;23:464–475. doi: 10.1016/j.cardfail.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 4. von Rennenberg R, Liman T, Nolte CH, Nave AH, Scheitz JF, Düzel S, Regitz‐Zagrosek V, Gerstorf D, Steinhagen‐Thiessen E, Demuth I, et al. High‐sensitivity cardiac troponin T and cognitive decline in older adults: results of the Berlin Aging Study II. Gerontology. 2022;69:1–9. doi: 10.1159/000523845 [DOI] [PubMed] [Google Scholar]
- 5. Schneider AL, Rawlings AM, Sharrett AR, Alonso A, Mosley TH, Hoogeveen RC, Ballantyne CM, Gottesman RF, Selvin E. High‐sensitivity cardiac troponin T and cognitive function and dementia risk: the atherosclerosis risk in communities study. Eur Heart J. 2014;35:1817–1824. doi: 10.1093/eurheartj/ehu124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 7. Scheitz JF, Mochmann HC, Erdur H, Tütüncü S, Haeusler KG, Grittner U, Laufs U, Endres M, Nolte CH. Prognostic relevance of cardiac troponin T levels and their dynamic changes measured with a high‐sensitivity assay in acute ischaemic stroke: analyses from the TRELAS cohort. Int J Cardiol. 2014;177:886–893. doi: 10.1016/j.ijcard.2014.10.036 [DOI] [PubMed] [Google Scholar]
- 8. Hellwig S, Ihl T, Ganeshan R, Laumeier I, Ahmadi M, Steinicke M, Weber JE, Endres M, Audebert HJ, Scheitz JF. Cardiac troponin and recurrent major vascular events after minor stroke or transient ischemic attack. Ann Neurol. 2021;90:901–991. doi: 10.1002/ana.26225 [DOI] [PubMed] [Google Scholar]
- 9. Broersen LHA, Siegerink B, Sperber PS, von Rennenberg R, Piper SK, Nolte CH, Heuschmann PU, Endres M, Scheitz JF, Liman TG. High‐sensitivity cardiac troponin T and cognitive function in patients with ischemic stroke. Stroke. 2020;51:1604–1607. doi: 10.1161/STROKEAHA.119.028410 [DOI] [PubMed] [Google Scholar]
- 10. Dadu RT, Fornage M, Virani SS, Nambi V, Hoogeveen RC, Boerwinkle E, Alonso A, Gottesman RF, Mosley TH, Ballantyne CM. Cardiovascular biomarkers and subclinical brain disease in the atherosclerosis risk in communities study. Stroke. 2013;44:1803–1808. doi: 10.1161/STROKEAHA.113.001128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. von Rennenberg R, Siegerink B, Ganeshan R, Villringer K, Doehner W, Audebert HJ, Endres M, Nolte CH, Scheitz JF. High‐sensitivity cardiac troponin T and severity of cerebral white matter lesions in patients with acute ischemic stroke. J Neurol. 2019;266:37–45. doi: 10.1007/s00415-018-9085-3 [DOI] [PubMed] [Google Scholar]
- 12. Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM. Stroke subtype, vascular risk factors, and total MRI brain small‐vessel disease burden. Neurology. 2014;83:1228–1234. doi: 10.1212/WNL.0000000000000837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Staals J, Booth T, Morris Z, Bastin ME, Gow AJ, Corley J, Redmond P, Starr JM, Deary IJ, Wardlaw JM. Total MRI load of cerebral small vessel disease and cognitive ability in older people. Neurobiol Aging. 2015;36:2806–2811. doi: 10.1016/j.neurobiolaging.2015.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amin Al Olama A, Wason JMS, Tuladhar AM, van Leijsen EMC, Koini M, Hofer E, Morris RG, Schmidt R, de Leeuw FE, Markus HS. Simple MRI score aids prediction of dementia in cerebral small vessel disease. Neurology. 2020;94:e1294–e1302. doi: 10.1212/WNL.0000000000009141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Georgakis MK, Fang R, Düring M, Wollenweber FA, Bode FJ, Stösser S, Kindlein C, Hermann P, Liman TG, Nolte CH, et al. Cerebral small vessel disease burden and cognitive and functional outcomes after stroke: a multicenter prospective cohort study. Alzheimers Dement. 2023;19:1152–1163. doi: 10.1002/alz.12744 [DOI] [PubMed] [Google Scholar]
- 16. Wollenweber FA, Zietemann V, Rominger A, Opherk C, Bayer‐Karpinska A, Gschwendtner A, Coloma Andrews L, Bürger K, Duering M, Dichgans M. The determinants of dementia after stroke (DEDEMAS) study: protocol and pilot data. Int J Stroke. 2014;9:387–392. doi: 10.1111/ijs.12092 [DOI] [PubMed] [Google Scholar]
- 17. Adams HP Jr, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, Woolson RF, Hansen MD. Baseline NIH stroke scale score strongly predicts outcome after stroke: a report of the trial of org 10172 in acute stroke treatment (TOAST). Neurology. 1999;53:126–131. doi: 10.1212/WNL.53.1.126 [DOI] [PubMed] [Google Scholar]
- 18. Jorm AF, Korten AE. Assessment of cognitive decline in the elderly by informant interview. Br J Psychiatry. 1988;152:209–213. doi: 10.1192/bjp.152.2.209 [DOI] [PubMed] [Google Scholar]
- 19. Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high‐sensitivity cardiac troponin T assay. Clin Chem. 2010;56:254–261. doi: 10.1373/clinchem.2009.132654 [DOI] [PubMed] [Google Scholar]
- 20. Zietemann V, Georgakis MK, Dondaine T, Müller C, Mendyk AM, Kopczak A, Hénon H, Bombois S, Wollenweber FA, Bordet R, et al. Early MoCA predicts long‐term cognitive and functional outcome and mortality after stroke. Neurology. 2018;91:e1838–e1850. doi: 10.1212/WNL.0000000000006506 [DOI] [PubMed] [Google Scholar]
- 21. Berres M, Monsch AU, Bernasconi F, Thalmann B, Stähelin HB. Normal ranges of neuropsychological tests for the diagnosis of Alzheimer's disease. Stud Health Technol Inform. 2000;77:195–199. doi: 10.3233/978-1-60750-921-9-195 [DOI] [PubMed] [Google Scholar]
- 22. Bäumler G. Farb‐Wort‐Interferenztest (FWIT) nach J.R. Stroop. 1st ed. Hogrefe; 1985. [Google Scholar]
- 23. Somerville J, Tremont G, Stern RA. The Boston qualitative scoring system as a measure of executive functioning in Rey‐Osterrieth complex figure performance. J Clin Exp Neuropsychol. 2000;22:613–621. doi: 10.1076/1380-3395(200010)22:5;1-9;FT613 [DOI] [PubMed] [Google Scholar]
- 24. Tombaugh TN, McIntyre NJ. The mini‐mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x [DOI] [PubMed] [Google Scholar]
- 25. Young JC, Sawyer RJ, Roper BL, Baughman BC. Expansion and re‐examination of digit span effort indices on the WAIS‐IV. Clin Neuropsychol. 2012;26:147–159. doi: 10.1080/13854046.2011.647083 [DOI] [PubMed] [Google Scholar]
- 26. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 27. Pokharel Y, Mouhanna F, Schneider ALC, Rawlings AM, Knopman DS, Nambi V, Virani SS, Hoogeveen RC, Alonso A, Heiss G, et al. High‐sensitive troponin T, natriuretic peptide, and cognitive change. J Am Geriatr Soc. 2019;67:2353–2361. doi: 10.1111/jgs.16092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O'Brien JT, Thomas A. Vascular dementia. Lancet. 2015;386:1698–1706. doi: 10.1016/S0140-6736(15)00463-8 [DOI] [PubMed] [Google Scholar]
- 29. Salmon DP. Neuropsychological features of mild cognitive impairment and preclinical Alzheimer's disease. Curr Top Behav Neurosci. 2012;10:187–212. doi: 10.1007/7854_2011_171 [DOI] [PubMed] [Google Scholar]
- 30. Vilar‐Bergua A, Riba‐Llena I, Penalba A, Cruz LM, Jiménez‐Balado J, Montaner J, Delgado P. N‐terminal pro‐brain natriuretic peptide and subclinical brain small vessel disease. Neurology. 2016;87:2533–2539. doi: 10.1212/WNL.0000000000003423 [DOI] [PubMed] [Google Scholar]
- 31. Gómez‐Choco M, Mena L, Font MÀ, Mengual JJ, Garcia‐Sanchez SM, Avellaneda C, Montull C, Castrillo L, Blanch P, Lleixa M, et al. NT‐proBNP, cerebral small vessel disease and cardiac function in patients with a recent lacunar infarct. J Hum Hypertens. 2023;37:62–67. doi: 10.1038/s41371-021-00648-8 [DOI] [PubMed] [Google Scholar]
- 32. Veugen MGJ, Henry RMA, Brunner‐La Rocca HP, Dagnelie PC, Schram MT, van Agtmaal MJM, van der Kallen CJH, Sep SJS, van Boxtel MPJ, Bekers O, et al. Cross‐sectional associations between cardiac biomarkers, cognitive performance, and structural brain changes are modified by age. Arterioscler Thromb Vasc Biol. 2018;38:1948–1958. doi: 10.1161/ATVBAHA.118.311082 [DOI] [PubMed] [Google Scholar]
- 33. Gyanwali B, Lai MKP, Lui B, Liew OW, Venketasubramanian N, Richards AM, Chen C, Hilal S. Blood‐based cardiac biomarkers and the risk of cognitive decline, cerebrovascular disease, and clinical events. Stroke. 2021;52:2275–2283. doi: 10.1161/STROKEAHA.120.032571 [DOI] [PubMed] [Google Scholar]
- 34. Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the atherosclerosis risk in communities study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim BJ, Lee SH, Kim CK, Ryu WS, Kwon HM, Choi SY, Yoon BW. Advanced coronary artery calcification and cerebral small vessel diseases in the healthy elderly. Circ J. 2011;75:451–456. doi: 10.1253/circj.CJ-10-0762 [DOI] [PubMed] [Google Scholar]
- 36. de la Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol. 2012;2012:367516. doi: 10.1155/2012/367516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Januzzi JL Jr, Filippatos G, Nieminen M, Gheorghiade M. Troponin elevation in patients with heart failure: on behalf of the third universal definition of myocardial infarction global task force: heart failure section. Eur Heart J. 2012;33:2265–2271. doi: 10.1093/eurheartj/ehs191 [DOI] [PubMed] [Google Scholar]
- 38. Scheitz JF, Nolte CH, Doehner W, Hachinski V, Endres M. Stroke‐heart syndrome: clinical presentation and underlying mechanisms. Lancet Neurol. 2018;17:1109–1120. doi: 10.1016/S1474-4422(18)30336-3 [DOI] [PubMed] [Google Scholar]
- 39. Krause T, Werner K, Fiebach JB, Villringer K, Piper SK, Haeusler KG, Endres M, Scheitz JF, Nolte CH. Stroke in right dorsal anterior insular cortex is related to myocardial injury. Ann Neurol. 2017;81:502–511. doi: 10.1002/ana.24906 [DOI] [PubMed] [Google Scholar]
- 40. Wardlaw JM, Allerhand M, Doubal FN, Valdes Hernandez M, Morris Z, Gow AJ, Bastin M, Starr JM, Dennis MS, Deary IJ. Vascular risk factors, large‐artery atheroma, and brain white matter hyperintensities. Neurology. 2014;82:1331–1338. doi: 10.1212/WNL.0000000000000312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation. Eur Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575 [DOI] [PubMed] [Google Scholar]
- 42. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O'Brien JT, Barkhof F, Benavente OR, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. El Husseini N, Katzan IL, Rost NS, Blake ML, Byun E, Pendlebury ST, Aparicio HJ, Marquine MJ, Gottesman RF, Smith EE, et al. Cognitive impairment after ischemic and hemorrhagic stroke: a scientific statement from the American Heart Association/American Stroke Association. Stroke. 2023;54:e272–e291. doi: 10.1161/STR.0000000000000430 [DOI] [PubMed] [Google Scholar]
- 44. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351 [DOI] [PubMed] [Google Scholar]
- 45. Maclullich AM, Wardlaw JM, Ferguson KJ, Starr JM, Seckl JR, Deary IJ. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J Neurol Neurosurg Psychiatry. 2004;75:1519–1523. doi: 10.1136/jnnp.2003.030858 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S6
Figures S1–S4
References 44,45


