Abstract
Background
This study aimed to establish and validate a nomogram model for predicting 90‐day mortality in patients with acute basilar artery occlusion receiving endovascular thrombectomy.
Methods and Results
A total of 242 patients with basilar artery occlusion undergoing endovascular thrombectomy were enrolled in our study, in which 172 patients from 3 stroke centers were assigned to the training cohort, and 70 patients from another center were assigned to the validation cohort. Univariate and multivariate logistic regression analyses were adopted to screen prognostic predictors, and those with significance were subjected to establish a nomogram model in the training cohort. The discriminative accuracy, calibration, and clinical usefulness of the nomogram model was verified in the internal and external cohorts. Six variables, including age, baseline National Institutes of Health Stroke Scale score, Posterior Circulation–Alberta Stroke Program Early CT (Computed Tomography) score, Basilar Artery on Computed Tomography Angiography score, recanalization failure, and symptomatic intracranial hemorrhage, were identified as independent predictors of 90‐day mortality of patients with basilar artery occlusion and were subjected to develop a nomogram model. The nomogram model exhibited good discrimination, calibration, and clinical usefulness in both the internal and the external cohorts. Additionally, patients were divided into low‐, moderate‐, and high‐risk groups based on the risk‐stratified nomogram model.
Conclusions
Our study proposed a novel nomogram model that could effectively predict 90‐day mortality of patients with basilar artery occlusion after endovascular thrombectomy and stratify patients with high, moderate, or low risk, which has a potential to facilitate prognostic judgment and clinical management of stroke.
Keywords: basilar artery occlusion, endovascular thrombectomy, mortality, nomogram
Subject Categories: Ischemic Stroke
Nonstandard Abbreviations and Acronyms
- BAO
basilar artery occlusion
- BATMAN
Basilar Artery on Computed Tomography Angiography
- EVT
endovascular thrombectomy
- NIHSS
National Institutes of Health Stroke Scale
- pc‐ASPECTS
Posterior Circulation–Alberta Stroke Program Early CT (Computed Tomography) Score
- sICH
symptomatic intracranial hemorrhage
Clinical Perspective.
What Is New?
This study established and validated a novel risk stratification nomogram model for predicting 90‐day mortality in patients with acute basilar artery occlusion receiving endovascular thrombectomy.
What Are the Clinical Implications?
This nomogram model has the potential to facilitate the prognostic prediction of basilar artery occlusion stroke in a clinical application.
This new nomogram model can better target the clinical environment and improve stroke management.
Basilar artery occlusion (BAO) is a catastrophic cause of acute stroke with high mortality rates. 1 Two recent randomized controlled trials have confirmed the efficacy of endovascular thrombectomy (EVT) performed within 12 or 24 hours of symptom onset in patients with BAO. 2 , 3 Despite the progress made in the management of acute stroke, the 90‐day mortality rate of patients with BAO remains high, with a range of 30.9% to 47.3%. 2 , 3 , 4 , 5 , 6 Therefore, it is critical to identify clinical characteristics for prediction of the mortality and risk stratification of patients.
Several risk models have been proposed to predict mortality in acute ischemic stroke; however, the accuracy of these scores in patients receiving thrombectomy remains elusive. 7 , 8 , 9 Moreover, the predictors of mortality and an effective stratifying system for patients with BAO after EVT have not been extensively addressed to date. 10 , 11 Thus, we retrospectively analyzed the clinical data of patients with acute BAO after EVT, incorporating demographic characteristics, clinical and imaging data, serological markers, and procedural‐related indicators. A novel predictive nomogram model was constructed to better target the clinical environment and improve stroke management. The predictive and discriminative efficiency was verified in an external validation cohort to ensure the robustness of this nomogram model in clinical application.
METHODS
Study Cohort
This study was performed in accordance with the Declaration of Helsinki and was approved by the ethics committee. Because of the retrospective study design, the written informed consent from patients was waived by the ethics committee, and all medical data were collected and processed anonymously. The data that support the findings of this study are available from the corresponding author upon reasonable request. From January 2015 to November 2022, we retrospectively analyzed the clinical data of patients with BAO after EVT from 4 comprehensive stroke centers. Inclusion criteria for this study were adult patients (aged ≥18 years) who were eligible for EVT within 24 hours of the BAO onset and had a prestroke modified Rankin Scale score of 0 to 2, an admission National Institutes of Health Stroke Scale (NIHSS) score of ≥6, and a Posterior Circulation–Alberta Stroke Program Early CT (Computed Tomography) score (pc‐ASPECTS) of ≥6. Exclusion criteria were patients with spontaneous recanalization of the BAO revealed by angiography, combined anterior circulation occlusion or vertebral artery occlusion, or unavailable clinical data (eg, imaging and follow‐up data). We selected the center with the largest sample size among the other 3 medical centers as the external validation cohort. Finally, a total of 242 patients were enrolled in this study, in which 172 patients from 3 stroke centers were included in the training cohort and 70 patients from another stroke center were used as an external validation cohort. The flowchart of patient enrollment is presented in Figure 1.
Figure 1. Flow diagram for patient selection.
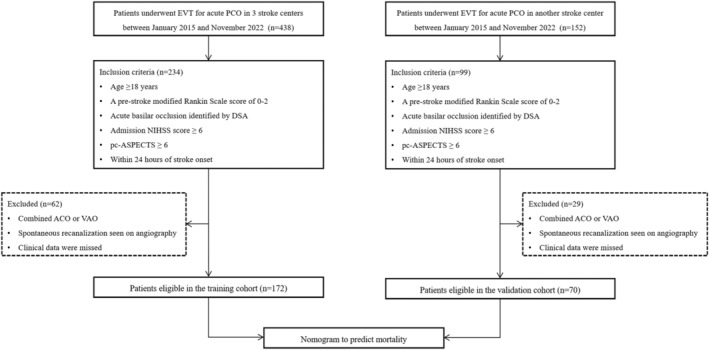
ACO indicates anterior circulation occlusion; DSA, digital subtraction angiography; EVT, endovascular thrombectomy; NIHSS, National Institutes of Health Stroke Scale; pc‐ASPECTS, Posterior Circulation–Alberta Stroke Program Early CT (Computed Tomography) Score; PCO, posterior circulation occlusion; and VAO, vertebral artery occlusion.
EVT Procedure
The EVT was usually conducted under regional anesthesia or conscious sedation. For patients with poor consciousness and poor respiratory status, EVT was conducted under general anesthesia or tracheal intubation to protect the airways. In most cases, transfemoral access was preferred for EVT. If transfemoral access was not available, transradial access was the alternative approach. The EVT process included direct distal aspiration using an aspiration catheter or stent‐retriever thrombectomy combined with continuous distal aspiration.
Patients who had residual stenosis revealed by angiography during the procedure were administered tirofiban. Rescue therapy was considered for patients with >70% apparent stenosis, moderate stenosis with insufficient distal vascular blood flow, or reocclusion after thrombectomy. The rescue therapy strategies, such as balloon‐assisted angioplasty or stenting, were conducted in accordance with the protocol of each stroke center.
Data Collection and Evaluation
The clinical data, including age, sex, medical history, stroke severity, procedure details, and clinical outcomes, were extracted from medical records of the 4 stroke centers. NIHSS score was used to assess the severity of stroke‐related neurologic deficits. The follow‐up might vary in each center, but all entailed a nonenhanced CT immediately after the EVT to detect intracranial hemorrhage. Additional CT or magnetic resonance imaging was obtained within 1 to 7 days after EVT. Symptomatic intracranial hemorrhage (sICH) was defined as any hemorrhage leading to an impaired neurologic function (eg, drowsiness and worsening of hemiparesis) and an increase of the NIHSS score of at least 4 points from baseline to within 7 days after onset. 12 The causes of stroke were classified into large‐artery atherosclerosis, cardiac embolism, and other or unknown cause according to the Trial of Org 10172 in Acute Stroke Treatment classification system following a comprehensive diagnostic workup.
The pc‐ASPECTS derived from the nonenhanced 5‐mm thickness cranial CT scans was used to assess the early ischemic changes in 10 specific regions of the posterior circulation of the brain. 13 The collateral circulation was assessed using the Basilar Artery on Computed Tomography Angiography (BATMAN) score, which is a 10‐point scoring system based on initial CT angiography. 14 The modified Thrombolysis in Cerebral Infarction score was used to assess the recanalization efficiency of EVT, in which a modified Thrombolysis in Cerebral Infarction score of 2b or 3, based on the final angiography, indicates successful recanalization. Procedural‐related adverse events, such as vessel dissection, new embolization, and vessel perforation, were recorded. The imaging data were independently examined by 2 experienced neuroradiologists (ZLB and LS) blinded to the outcomes. In cases of disagreements between the 2 neuroradiologists, a third neuroradiologist (SHB) helps them reach the consensus.
The follow‐up data were collected by experienced neurologists during a clinical visit or telephone interview with patients or their relatives. The modified Rankin Scale score was used to assess functional disability and individual dependence after a stroke. A 90‐day modified Rankin Scale score of 0 to 3 was considered a favorable clinical outcome. The primary outcome of this study was all‐cause mortality at 90 days after BAO.
Sample Size
The events per variable of 10 is the accepted minimal sample size for logistic regression analysis. On the basis of preliminary data from the principal center, 6 variables were included in the multivariate logistic regression analysis, including age, baseline NIHSS score, pc‐ASPECTS, BATMAN score, recanalization failure, and sICH. Because the primary outcome of mortality occurs in ≈35.5% of BAO strokes, 15 an adequate sample size of at least 169 should be collected in the training cohort. In this study, 172 patients were assigned to the training cohort, and 53 patients (30.8%) died within 90 days. The events per variable of this study failed to reach the expected 10 because of the slightly lower mortality rate and the inclusion of 2 additional covariates (rescue therapy and procedural duration) in the multivariate logistic regression analysis. Previous studies have suggested a minimal events per variable of 5 for reliable results when developing a predictive model using logistic regression analysis. 16 Given the fact that this study was an exploratory study and the results were robust in the training and validation cohorts, we did not include additional patients.
Development and Validation of the Nomogram Model
In the training cohort, the parameters with a P<0.10 in the univariate analysis were selected for the first multivariate analysis (model 1). The backward stepwise method based on likelihood ratio test was used in multivariate analyses to screen the independent risk factors of mortality. In the second multivariate analysis (model 2), sICH was included to determine whether sICH would change the predictive efficiency of the above baseline characteristics as predictors. Receiver operating characteristic was used to determine the optimal cutoff value of age, baseline NIHSS score, pc‐ASPECTS, and BATMAN score. The variance inflation factor was calculated to evaluate the collinearity between the included variables, in which variance inflation factor >5 was considered having collinearity. The independent predictors identified in model 2 were used to develop a nomogram model. We also used the bootstrap technique for resampling to enhance the robustness of our model outcomes. The discriminative efficiency of the nomogram model was evaluated by concordance index. The calibration curve and Hosmer‐Lemeshow test were used to evaluate the goodness of fit of the nomogram model. The decision curve analysis was applied to estimate the value of the nomogram model in clinical application by quantifying the net benefits across a range of threshold probabilities. The nomogram model was internally validated using 1000 bootstrap resamples in the training cohort. The predictive and discriminative efficiency of the nomogram model was further verified in the external validation cohort.
In addition, we also compared the predictive efficiency of several previously developed scales using receiver operating characteristic curve analysis: the Predicting 90 Days Mortality of Acute Ischemic Stroke With Mechanical Thrombectomy scale was proposed to predict the 90‐day mortality of patients with acute stroke receiving mechanical thrombectomy 7 ; the modified Stroke Subtype, Oxfordshire Community Stroke Project Classification, Age, and Prestroke Modified Rankin scale was proposed to predict early mortality after acute stroke 9 ; and the Predicting Early Mortality From Ischemic Stroke scale was also developed to estimate early mortality of patients with ischemic stroke. 8 The area under the curve (AUC) value of each scale was compared using the DeLong method.
Prognostic Risk Stratification
The cutoff points for low‐, moderate‐, and high‐risk groups were determined using the X‐tile software, version 3.6.1 (http://www.tissuearray.org).
Statistical Analysis
Nine patients (3.8%) with missing data (6 patients with no survival data and 3 patients with missing imaging data) were excluded from the analyses to maintain the integrity of the data. The continuous variables were presented as mean±SD or median with interquartile range (IQR), whereas the categorical variables were presented as frequencies (percentages). The normality of data distribution was assessed by the Shapiro‐Wilk test, whereas the homogeneity of variance was evaluated using the Levene test. The comparisons between the 2 groups were conducted using Student t test or Mann‐Whitney U test for continuous variables and the Fisher exact test or χ2 tests for categorical variables. The interrater reproducibility for the pc‐ASPECTS and BATMAN score evaluation was assessed using Cohen κ statistics. Results of the Cohen κ coefficient were interpreted as follows: κ<0.4 was considered as poor reproducibility, 0.4<κ<0.75 was considered as fair to good reproducibility, and 0.75<κ<1.00 was considered excellent reproducibility. Statistical analyses were performed using SPSS, version 26 (IBM, Armonk, NY), and R software, version 4.2.3 (https://www.r‐project.org). The receiver operating characteristic curve analysis was conducted using Medcalc software. P<0.05 was deemed statistically significant.
RESULTS
Baseline Characteristics of Enrolled Patients
A total of 242 patients with acute BAO were enrolled in this study based on the inclusion and exclusion criteria, including 172 in the training cohort and 70 in the validation cohort (Figure 1). The baseline characteristics of the training cohort were shown as follows: the median age of the patients was 70 years (IQR, 60–78 years); 33.7% of patients were women; the median baseline NIHSS score, pc‐ASPECTS, and BATMAN score were 21 (IQR, 15–35), 9 (IQR, 7–9), and 7 (IQR, 5–8), respectively; general anesthesia was used in 22 patients (12.8%); intravenous thrombolysis with alteplase was administrated in 48 patients (27.9%) before EVT; rescue therapy was performed in 60 patients (30.9%); 152 patients (88.4%) achieved successful recanalization, and 58 patients (33.7%) had a favorable clinical outcome; and the sICH and mortality rates after 90 days of EVT were 6.4% and 30.8%, respectively. The assessment of pc‐ASPECTS and BATMAN score showed excellent interrater agreement (κ=0.838 and 0.825, respectively). There was no significant difference of clinical characteristics between the training and validation cohorts (Table S1).
Univariate and Multivariate Analyses
To screen predictors of 90‐day mortality of patients with BAO after EVT, we conducted univariate analysis in the training cohort. Results indicated that patients with an older age (median age, 75 versus 67 years; P=0.006), a higher baseline NIHSS score (30 versus 18; P=0.006), a lower pc‐ASPECTS (7 versus 9; P<0.001), and a lower BATMAN score (6 versus 7; P<0.001) tended to have a high risk of mortality (Table 1). In addition, there were significant differences in the recanalization failure (P=0.003) and sICH (P<0.001) between the survival and nonsurvival groups. Receiver operating characteristic curve analysis was conducted to determine the optimal cutoff value of age (71 years), NIHSS score (20), pc‐ASPECTS (7), and BATMAN score (6) (Figure S1).
Table 1.
Comparison of Clinical Characteristics of Survival and Nonsurvival for Patients in the Training Cohort
| Variable | Nonsurvival (n=53) | Survival (n=119) | P value |
|---|---|---|---|
| Demographic characteristics | |||
| Age, median (IQR), y | 75 (64–82) | 67 (59–76) | 0.006 |
| Female sex, n (%) | 18 (34.0) | 40 (33.6) | 0.964 |
| Medical history, n (%) | |||
| Hypertension | 38 (71.7) | 90 (75.6) | 0.585 |
| Arterial fibrillation | 17 (32.1) | 37 (31.1) | 0.898 |
| Diabetes | 11 (20.8) | 24 (20.2) | 0.930 |
| Coronary artery disease | 6 (11.3) | 16 (13.4) | 0.700 |
| Dyslipidemia | 11 (20.8) | 34 (28.6) | 0.282 |
| Previous ischemic stroke | 10 (18.9) | 23 (19.3) | 0.944 |
| History of antiplatelet/anticoagulant use | 20 (37.7) | 37 (31.1) | 0.393 |
| Smoking | 11 (20.8) | 28 (23.5) | 0.688 |
| Clinical data | |||
| Admission SBP, mean±SD, mm Hg | 150.3±26.7 | 152.1±23.5 | 0.667 |
| Baseline NIHSS score, median (IQR) | 30 (17–35) | 18 (13–32) | 0.006 |
| Baseline pc‐ASPECTS, median (IQR) | 7 (7–9) | 9 (8–9) | <0.001 |
| BATMAN score, median (IQR) | 6 (4–7) | 7 (6–8) | <0.001 |
| Prestroke mRS score of 0–1, n (%) | 51 (96.2) | 115 (96.6) | 0.892 |
| White blood cell count, median (IQR), ×109/L | 11.2 (8.0–15.4) | 10.9 (8.5–14.3) | 0.867 |
| Baseline glucose level, median (IQR), mmol/L | 5.9 (5.4–7.2) | 6.0 (5.0–7.4) | 0.997 |
| Cause, n (%) | 0.207 | ||
| Cardioembolism | 19 (35.8) | 45 (37.8) | |
| Large‐artery atherosclerosis | 17 (32.1) | 50 (42.0) | |
| Undetermined cause or others | 17 (32.1) | 24 (20.2) | |
| Occlusion site, n (%) | 0.731 | ||
| Proximal‐middle | 30 (56.6) | 64 (53.8) | |
| Distal | 23 (43.4) | 55 (46.2) | |
| General anesthesia, n (%) | 9 (17.0) | 13 (10.9) | |
| Accepted intravenous thrombolysis, n (%) | 16 (30.2) | 32 (26.9) | 0.656 |
| Rescue treatment, n (%) | 13 (24.5) | 47 (39.5) | 0.057 |
| Successful recanalization, n (%) | 41 (77.4) | 111 (93.3) | 0.003 |
| Passes, median (IQR), n | 1 (1–3) | 1 (1–2) | 0.562 |
| OTP time, median (IQR), min | 300 (236–486) | 297 (190–428) | 0.435 |
| Procedural duration, median (IQR), min | 89 (45–116) | 67 (45–85) | 0.054 |
| Procedural adverse events, n (%) | 5 (9.4) | 9 (7.6) | 0.679 |
| sICH, n (%) | 9 (17.0) | 2 (1.7) | <0.001 |
BATMAN indicates Basilar Artery on Computed Tomography Angiography; IQR, interquartile range; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; OTP, onset to puncture; pc‐ASPECTS, Posterior Circulation–Alberta Stroke Program Early CT (Computed Tomography) Score; SBP, systolic blood pressure; and sICH, symptomatic intracranial hemorrhage.
The results of multivariate logistic regression analysis in the training cohort are shown in Table 2. In the first multivariate model (model 1), age >71 years (odds ratio [OR], 2.37 [95% CI, 1.08–5.22]; P=0.032), baseline NIHSS score >20 (OR, 3.40 [95% CI, 1.46–7.92]; P=0.004), pc‐ASPECTS ≤7 (OR, 3.19 [95% CI, 1.34–7.58]; P=0.009), BATMAN score ≤6 (OR, 2.64 [95% CI, 1.09–6.38]; P=0.031), and recanalization failure (OR, 0.61 [95% CI, 0.40–0.92]; P=0.002) were identified as independent risk factors of 90‐day mortality. In the second multivariate model (model 2), sICH was included to determine whether sICH would change results revealed by model 1. Results showed that sICH was an independent risk factor for 90‐day mortality (OR, 7.02 [95% CI, 1.09–45.10]; P=0.040), and the OR value of other variables did not substantially change. The bootstrap results, after 1000 resamples, demonstrated more robust estimates, reducing the impact of small sample bias and providing more reliable statistical inferences (Table S2). The new 95% CIs were tighter than the original analysis, reflecting a more accurate range of the parameter estimates, thereby rendering the model's interpretation more credible.
Table 2.
Multivariate Logistic Analysis for the Risk Factors Associated With Mortality in the Training Cohort
| Variables | Model 1* | Model 2† | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | β | OR (95% CI) | P value | VIF | |
| Aged >71 y | 2.37 (1.08–5.22) | 0.032 | 0.846 | 2.33 (1.04–5.22) | 0.040 | 1.08 |
| Baseline NIHSS score >20 | 3.40 (1.46–7.92) | 0.004 | 1.136 | 3.12 (1.32–7.34) | 0.009 | 1.14 |
| pc‐ASPECTS ≤7 | 3.19 (1.34–7.58) | 0.009 | 1.091 | 2.95 (1.22–7.12) | 0.016 | 1.42 |
| BATMAN score ≤6 | 2.64 (1.09–6.38) | 0.031 | 1.080 | 2.98 (1.21–7.34) | 0.018 | 1.31 |
| Failed recanalization | 7.12 (2.09–24.28) | 0.002 | 1.522 | 4.58 (1.22–17.28) | 0.025 | 1.35 |
| Procedural duration | … | 0.232 | … | … | 0.430 | 1.34 |
| Rescue therapy | … | 0.356 | … | … | 0.354 | 1.15 |
| sICH | Not included | … | 1.948 | 7.02 (1.09–45.10) | 0.040 | 1.22 |
BATMAN indicates Basilar Artery on Computed Tomography Angiography; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; pc‐ASPECTS, Posterior Circulation–Alberta Stroke Program Early CT (Computed Tomography) Score; sICH, symptomatic intracranial hemorrhage; and VIF, variance inflation factor.
Model 1: sICH was not included in the final multivariate model.
Model 2: sICH was included to determine whether sICH would change the predictive effect of risk factors for mortality in model 1.
Development and Validation of the Nomogram Model
On the basis of the results of the multivariate analysis of model 2, 6 independent risk factors were used to construct the predictive nomogram model (Figure 2). The nomogram point was the sum of the point of each variable based on its β value, and the sum point matched the scale to obtain the probability of 90‐day mortality. Furthermore, we established a dynamic online nomogram for easier use by clinicians, which is available online at https://online‐prediction.shinyapps.io/DynNomapp/. Clinicians can input the individual variables of patients on the web page to obtain the risk of mortality effortlessly (Figure S2).
Figure 2. Individualized 90‐day mortality nomogram and risk stratification for patients with acute basilar artery occlusion after endovascular thrombectomy.
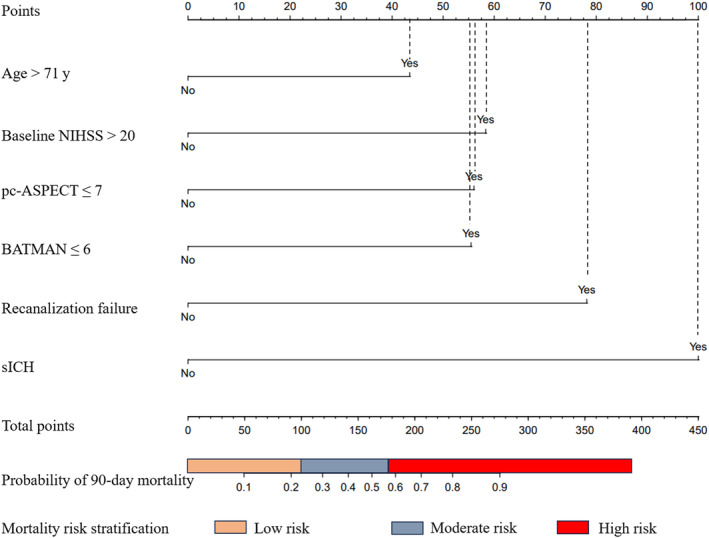
The cutoff points of low, moderate, and high risk were 100 and 176, respectively. BATMAN indicates Basilar Artery on Computed Tomography Angiography; NIHSS, National Institutes of Health Stroke Scale; pc‐ASPECTS, Posterior Circulation–Alberta Stroke Program Early CT (Computed Tomography) Score; and sICH, symptomatic intracranial hemorrhage.
To test the fitness of the nomogram model, the calibration plot and the Hosmer‐Lemeshow test (P=0.476) were conducted, and results indicated good calibration ability of the nomogram model in the training and validation cohorts (Figure 3). The concordance index for the nomogram model was 0.83 (95% CI, 0.76–0.90) in the training cohort and 0.81 (95% CI, 0.79–0.83) in the internal bootstrapping validation. In addition, the concordance index in the validation cohort was 0.94 (95% CI, 0.88–0.99), which suggested good discriminative ability of the nomogram model. The decision curve analysis was applied to estimate the translational value of the nomogram model in clinical application, which demonstrated that when the threshold probability was >5.0% in the training cohort, the nomogram model was efficient to predict 90‐day mortality (Figure 4A). Also in the validation cohort, the nomogram had a good predictive efficiency when the threshold probability was 1.0% to 85.0% (Figure 4B). Previous studies have proposed multiple scales to predict the prognosis of patients with acute stroke. Herein, we found that the AUC of model 2 in predicting 90‐day mortality was 0.83 (95% CI, 0.77–0.89) and 0.94 (95% CI, 0.86–0.98) in the training and validation cohorts, respectively, which was superior to other scales (both P<0.001) (Figure 5).
Figure 3. Assessment of the nomogram model with the calibration curve.
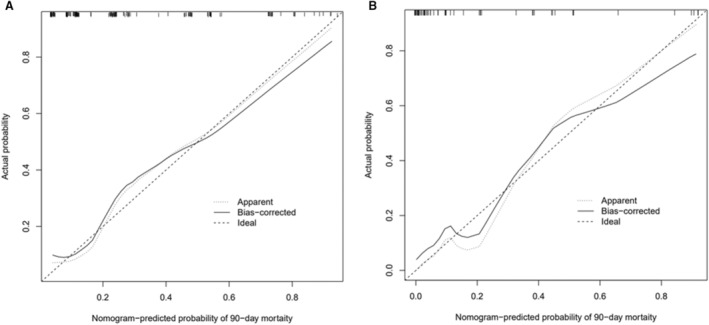
The calibration curves of the nomogram model in the training cohort (A) and the validation cohort (B). The x axis represents the predicted risk of mortality. The y axis represents the actual probability of mortality. The diagonal dashed line represents the perfect prediction of the ideal model. The solid line represents the performance of the nomogram model, where closer to the diagonal dashed line represents better prediction.
Figure 4. Assessment of the nomogram model with the decision curve analysis.
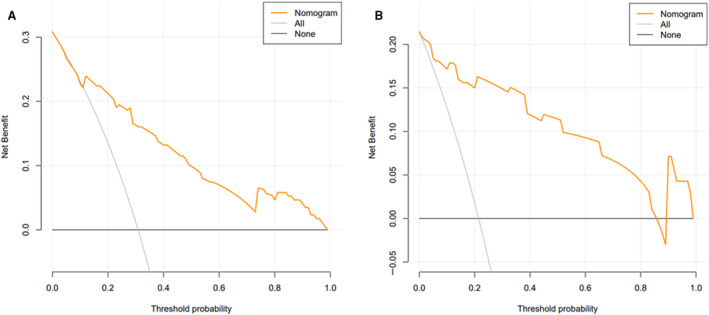
The decision curve analysis curves in the training (A) and validation (B) cohorts.
Figure 5. Comparison of different risk models using ROC curves.
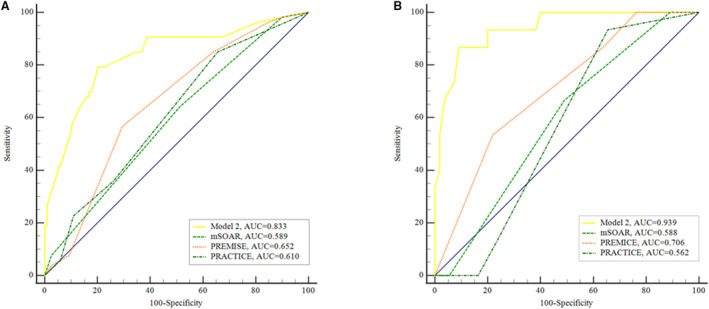
The ROC curves in the training cohort (A) and validation cohort (B). AUC indicates area under the ROC curve; mSOAR, modified Stroke Subtype, Oxfordshire Community Stroke Project Classification, Age, and Prestroke Modified Rankin; PREMISE, Predicting Early Mortality From Ischemic Stroke; PRACTICE, Predicting 90 Days Mortality of Acute Ischemic Stroke With Mechanical Thrombectomy; and ROC, receiver operating characteristic.
Risk Stratification Based on the Nomogram Model
Two optimal cutoff values of the total nomogram points were determined using the X‐tile software in the training cohort, which extended to the validation cohort and stratified patients into the low‐risk group (total nomogram points of ≤99, 86 cases in the training cohort and 31 cases in the validation cohort), the moderate‐risk group (total nomogram points of 100–176, 58 cases in the training cohort and 25 cases in the validation cohort), and the high‐risk group (total nomogram points of >176, 28 cases in the training cohort and 14 cases in the validation cohort). In the training cohort, the mortality rate among the high‐, moderate‐, and low‐risk groups had a significant difference (high‐ versus low‐risk group: OR, 6.41 [95% CI, 2.61–15.76]; P<0.001; moderate‐ versus low‐risk group: OR, 4.23 [95% CI, 2.08–8.63]; P<0.001). Similar results were obtained in the validation cohort. Hence, these results suggested a robust risk stratification ability of the nomogram model.
DISCUSSION
In recent years, emerging evidence advocates the efficacy of EVT in the treatment of BAO stroke; however, the overall mortality of patients with BAO remains high. In this study, we identified clinical characteristics that were associated with 90‐day mortality in patients with BAO after EVT and established a reliable nomogram model incorporating age, baseline NIHSS score, pc‐ASPECTS, BATMAN score, recanalization failure, and sICH. With the validation in an independent external cohort, the nomogram model exhibited a potent predictive and discriminative capacity. In addition, patients could be stratified into high‐, moderate‐, and low‐risk groups based on the nomogram point, and the mortality rate among different risk groups had a significant difference. Therefore, this nomogram model had a translation value for clinical application that facilitates the prediction of patients' prognoses and the management of treatment regimen.
Several studies have proposed risk models to predict the mortality of patients with stroke. The modified Stroke Subtype, Oxfordshire Community Stroke Project Classification, Age, and Prestroke Modified Rankin, 9 iScore, 17 based on preadmission comorbidities, level of consciousness, age, and neurologic deficit (PLAN), 18 and the Predicting Early Mortality from Ischemic Stroke (PRIMISE) 8 scores were developed for predicting the mortality of patients with all types of acute ischemic stroke; however, the accuracy of these scores in the thrombectomy cohort remains limited. Li et al validated the iScore and Predicting Early Mortality From Ischemic Stroke score in their cohort of patients with acute ischemic stroke treated with EVT, and results showed that the predictive ability and accuracy of the 2 scores were not satisfactory (AUC, <0.7 for both). Instead, they proposed a Predicting 90 Days Mortality of Acute Ischemic Stroke With Mechanical Thrombectomy score with relatively higher predictive ability (AUC, 0.74); however, most patients had anterior circulation occlusion stroke, and the predictive power was not verified in an independent cohort. Moreover, Huang et al estimated the performance of different risk models in patients with BAO receiving thrombectomy, which indicated that the Predicting Early Mortality From Ischemic Stroke score had the highest but limited predictive power (AUC, 0.64). Thus, it is necessary to develop a new model for predicting the mortality risk of BAO stroke after EVT. Herein, we established a risk model with validation in an independent cohort to demonstrate its potent ability for predicting the mortality of patients with BAO receiving EVT. Compared with modified Stroke Subtype, Oxfordshire Community Stroke Project Classification, Age, and Prestroke Modified Rankin, PRIMISE, and Predicting 90 Days Mortality of Acute Ischemic Stroke With Mechanical Thrombectomy scores, our model had the highest predictive accuracy.
In this study, the overall 90‐day mortality was relatively lower compared with previous studies (28.1% versus 35.5%), probably because of our strict inclusion criteria. 15 Taussky et al suggested that stratifying patients with high risk of mortality could help physicians decide whether the patients would benefit from EVT or not, especially for those who are eligible for EVT. 19 However, our study is aimed at understanding which patients may have a fatal outcome despite EVT and is not meant to choose patients in whom to offer thrombectomy. A randomized trial may be helpful for determining whether the patients would benefit from EVT or not. Similar to our results, age and baseline NIHSS score were risk factors for mortality after stroke. 11 , 20 Not surprisingly, elderly patients with stroke may have underlying diseases and had a higher incidence of complication, leading to higher mortality. Considering the potential benefit of EVT in elderly patients, previous research suggested physicians should tailor the treatment regimen for individuals with regard to existing risk factors. 21 A high NIHSS score represents a severe neurologic deficit and is a risk factor of 90‐mortality in patients with BAO. The mechanisms of increasing age and stroke severity leading to fatal outcome after EVT are poorly understood. Hence, more studies are needed to evaluate the pathophysiology and treatment options of these patients.
The development of noninvasive imaging has contributed to identifying neuroimaging markers for predicting the prognosis of patients with acute BAO. In the present study, pc‐ASPECTS was developed to assess early ischemic changes and was a risk factor of 90‐day mortality in patients with BAO. In line with our findings, Puetz et al analyzed 158 patients enrolled in the BASICS (Basilar Artery International Cooperation Study) and reported that pc‐ASPECTS could independently predict the mortality after adjustment for age, baseline NIHSS score, and thrombolysis (adjusted risk ratio, 0.7). 22 The BATMAN score reflects thrombus burden and collateral flow, which are associated with clinical outcome in patients with BAO. 14 , 23 Our results advocated this finding and confirmed that BATMAN score was able to predict mortality of patients with BAO stroke. Recently, several studies have identified imaging predictors and used the machine learning method to develop risk models for predicting clinical outcomes of patients with BAO. 24 , 25 However, an external validation was required to verify the robustness of these models. Meanwhile, future studies may incorporate radiographic indicators when constructing the risk model to predict mortality in patients with stroke.
Recanalization failure is a modifiable predictor of 90‐day mortality in patients with BAO. Previous studies have demonstrated that the recanalization rate of BAO differed on the basis of different pathogenesis. 26 Thus, the physician should design an individualized recanalization strategy to reduce the mortality risk. We further identified sICH as a predictor of 90‐day mortality. In this study, 6.4% of patients developed sICH after EVT, which was similar to the results of previous randomized controlled trials (range, 4.5%–7.6%). 2 , 3 , 4 , 5 Moreover, the inclusion of sICH did not substantially alter the predictive effect of baseline characteristics on 90‐day mortality in our study. Consistent with our findings, a retrospective study showed that intracranial hemorrhage was associated with long‐term mortality but not short‐term mortality for acute vertebrobasilar artery occlusion. 27 Gory et al revealed no association between sICH and 90‐day mortality of patients with acute BAO, but they only included 2 patients with sICH (1.7%) in the study. 11
Other variables may also potentially affect the mortality of patients with BAO after EVT. The inflammatory markers have been suggested to be associated with mortality after thrombectomy. 28 Moreover, a recent study reported that dynamic changes of high‐sensitivity cardiac troponin I were independently associated with 90‐day mortality after EVT. 29 Postprocedural complications, such as pneumonia, herniation, and gastrointestinal hemorrhage, were also considered potential prognostic factors in patients with BAO. 10 However, these factors are not obtained in all patients of this analysis, which requires future studies to characterize the role of these factors in patients with acute stroke.
Our study has some limitations. Because of the retrospective study design, selection bias could not be avoided. In addition, we acknowledge that the wide CIs around the probability estimates may affect the reliability and interpretability of the results. Although each stroke center was highly specialized in performing EVT procedures and participated in large‐scale EVT clinical trials, there was no standardized management for patients with stroke across the different centers. Although this is technically a preliminary mortality prediction score, the importance of predicting fatal outcome becomes more essential as stroke becomes an increasing interventional disease. Moreover, our study excluded patients with mild stroke and mainly included Asian patients with atherosclerosis; therefore, our findings are required to be validated by additional large‐scale, multicenter trials.
In summary, we established a robust nomogram model to predict the prognosis and stratify mortality risk of patients with BAO receiving EVT. This model has the potential to facilitate the prognostic prediction and management of BAO stroke in a clinical application.
Sources of Funding
This work was supported by grants from the National Natural Science Foundation of China (grant No. 81971613) and Jiangsu Province Key Research and Development Plan of Social Development (grant No. BE2022809).
Disclosures
None.
Supporting information
Tables S1–S2
Figures S1–S2
This article was sent to Jose R. Romero, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.032107
For Sources of Funding and Disclosures, see page 10.
References
- 1. Meinel TR, Kaesmacher J, Chaloulos‐Iakovidis P, Panos L, Mordasini P, Mosimann PJ, Michel P, Hajdu S, Ribo M, Requena M, et al. Mechanical thrombectomy for basilar artery occlusion: efficacy, outcomes, and futile recanalization in comparison with the anterior circulation. J Neurointerv Surg. 2019;11:1174–1180. doi: 10.1136/neurintsurg-2018-014516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jovin TG, Li C, Wu L, Wu C, Chen J, Jiang C, Shi Z, Gao Z, Song C, Chen W, et al. Trial of thrombectomy 6 to 24 hours after stroke due to basilar‐artery occlusion. N Engl J Med. 2022;387:1373–1384. doi: 10.1056/NEJMoa2207576 [DOI] [PubMed] [Google Scholar]
- 3. Tao C, Nogueira RG, Zhu Y, Sun J, Han H, Yuan G, Wen C, Zhou P, Chen W, Zeng G, et al. Trial of endovascular treatment of acute basilar‐artery occlusion. N Engl J Med. 2022;387:1361–1372. doi: 10.1056/NEJMoa2206317 [DOI] [PubMed] [Google Scholar]
- 4. Langezaal LCM, van der Hoeven E, Mont'Alverne FJA, de Carvalho JJF, Lima FO, Dippel DWJ, van der Lugt A, Lo RTH, Boiten J, Lycklama à Nijeholt GJ, et al. Endovascular therapy for stroke due to basilar‐artery occlusion. N Engl J Med. 2021;384:1910–1920. doi: 10.1056/NEJMoa2030297 [DOI] [PubMed] [Google Scholar]
- 5. Liu X, Dai Q, Ye R, Zi W, Liu Y, Wang H, Zhu W, Ma M, Yin Q, Li M, et al. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (best): an open‐label, randomised controlled trial. Lancet Neurol. 2020;19:115–122. doi: 10.1016/S1474-4422(19)30395-3 [DOI] [PubMed] [Google Scholar]
- 6. Writing Group for the BG , Zi W, Qiu Z, Wu D, Li F, Liu H, Liu W, Huang W, Shi Z, Bai Y, et al. Assessment of endovascular treatment for acute basilar artery occlusion via a nationwide prospective registry. JAMA Neurol. 2020;77:561–573. doi: 10.1001/jamaneurol.2020.0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li H, Ye SS, Wu YL, Huang SM, Li YX, Lu K, Huang JB, Chen L, Li HZ, Wu WJ, et al. Predicting mortality in acute ischaemic stroke treated with mechanical thrombectomy: analysis of a multicentre prospective registry. BMJ Open. 2021;11:e043415. doi: 10.1136/bmjopen-2020-043415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gattringer T, Posekany A, Niederkorn K, Knoflach M, Poltrum B, Mutzenbach S, Haring HP, Ferrari J, Lang W, Willeit J, et al. Predicting early mortality of acute ischemic stroke. Stroke. 2019;50:349–356. doi: 10.1161/STROKEAHA.118.022863 [DOI] [PubMed] [Google Scholar]
- 9. Abdul‐Rahim AH, Quinn TJ, Alder S, Clark AB, Musgrave SD, Langhorne P, Potter JF, Myint PK. Derivation and validation of a novel prognostic scale (modified‐stroke subtype, oxfordshire community stroke project classification, age, and prestroke modified rankin) to predict early mortality in acute stroke. Stroke. 2016;47:74–79. doi: 10.1161/STROKEAHA.115.009898 [DOI] [PubMed] [Google Scholar]
- 10. Huang J, Wang M, Li F, Kong W, Liu D, Li H, Zhou P, Yan Z, Wang Y, Song J, et al. Clinical predictors for early mortality of patients with acute basilar artery occlusion. Cerebrovasc Dis. 2023;52:202–209. doi: 10.1159/000526124 [DOI] [PubMed] [Google Scholar]
- 11. Gory B, Mazighi M, Labreuche J, Blanc R, Piotin M, Turjman F, Lapergue B; ETIS (Endovascular Treatment in Ischemic Stroke) Investigators . Predictors for mortality after mechanical thrombectomy of acute basilar artery occlusion. Cerebrovasc Dis. 2018;45:61–67. doi: 10.1159/000486690 [DOI] [PubMed] [Google Scholar]
- 12. Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, et al. Randomised double‐blind placebo‐controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European‐Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–1251. doi: 10.1016/S0140-6736(98)08020-9 [DOI] [PubMed] [Google Scholar]
- 13. Puetz V, Sylaja PN, Coutts SB, Hill MD, Dzialowski I, Mueller P, Becker U, Urban G, O'Reilly C, Barber PA, et al. Extent of hypoattenuation on ct angiography source images predicts functional outcome in patients with basilar artery occlusion. Stroke. 2008;39:2485–2490. doi: 10.1161/STROKEAHA.107.511162 [DOI] [PubMed] [Google Scholar]
- 14. Alemseged F, Shah DG, Diomedi M, Sallustio F, Bivard A, Sharma G, Mitchell PJ, Dowling RJ, Bush S, Yan B, et al. The basilar artery on computed tomography angiography prognostic score for basilar artery occlusion. Stroke. 2017;48:631–637. doi: 10.1161/STROKEAHA.116.015492 [DOI] [PubMed] [Google Scholar]
- 15. Abdalkader M, Finitsis S, Li C, Hu W, Liu X, Ji X, Huo X, Alemseged F, Qiu Z, Strbian D, et al. Endovascular versus medical management of acute basilar artery occlusion: a systematic review and meta‐analysis of the randomized controlled trials. J Stroke. 2023;25:81–91. doi: 10.5853/jos.2022.03755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Puhr R, Heinze G, Nold M, Lusa L, Geroldinger A. Firth's logistic regression with rare events: accurate effect estimates and predictions? Stat Med. 2017;36:2302–2317. doi: 10.1002/sim.7273 [DOI] [PubMed] [Google Scholar]
- 17. Saposnik G, Kapral MK, Liu Y, Hall R, O'Donnell M, Raptis S, Tu JV, Mamdani M, Austin PC; Investigators of the Registry of the Canadian Stroke Network; Stroke Outcomes Research Canada (SORCan) Working Group . Iscore: a risk score to predict death early after hospitalization for an acute ischemic stroke. Circulation. 2011;123:739–749. doi: 10.1161/CIRCULATIONAHA.110.983353 [DOI] [PubMed] [Google Scholar]
- 18. O'Donnell MJ, Fang J, D'Uva C, Saposnik G, Gould L, McGrath E, Kapral MK; Investigators of the Registry of the Canadian Stroke Network . The plan score: a bedside prediction rule for death and severe disability following acute ischemic stroke. Arch Intern Med. 2012;172:1548–1556. doi: 10.1001/2013.jamainternmed.30 [DOI] [PubMed] [Google Scholar]
- 19. Taussky P, Agnoletto G, Grandhi R, Alexander MD, Wong KH, Albers GW, de Havenon A. Prediction of death after endovascular thrombectomy in the extended window: a secondary analysis of defuse 3. J Neurointerv Surg. 2021;13:805–808. doi: 10.1136/neurintsurg-2020-016548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cao J, Mo Y, Chen R, Shao H, Xuan J, Peng Y, Zhu X. Predictors of functional outcome and mortality in endovascular treatment for acute basilar artery occlusion: a single‐centre experience. Front Neurol. 2021;12:731300. doi: 10.3389/fneur.2021.731300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adcock AK, Schwamm LH, Smith EE, Fonarow GC, Reeves MJ, Xu H, Matsouaka RA, Xian Y, Saver JL. Trends in use, outcomes, and disparities in endovascular thrombectomy in US patients with stroke aged 80 years and older compared with younger patients. JAMA Netw Open. 2022;5:e2215869. doi: 10.1001/jamanetworkopen.2022.15869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Puetz V, Khomenko A, Hill MD, Dzialowski I, Michel P, Weimar C, Wijman CA, Mattle HP, Engelter ST, Muir KW, et al. Extent of hypoattenuation on ct angiography source images in basilar artery occlusion: prognostic value in the basilar artery international cooperation study. Stroke. 2011;42:3454–3459. doi: 10.1161/STROKEAHA.111.622175 [DOI] [PubMed] [Google Scholar]
- 23. Song K, Li F, Shi M, Yue F, Li C, Qi S, Wu Y, Yuan Z, Shi Q, Fu X, et al. Basilar artery on computed tomography angiography score and clinical outcomes in acute basilar artery occlusion. J Neurol. 2022;269:3810–3820. doi: 10.1007/s00415-022-11013-1 [DOI] [PubMed] [Google Scholar]
- 24. Kwak HS, Park JS. Mechanical thrombectomy in basilar artery occlusion: clinical outcomes related to posterior circulation collateral score. Stroke. 2020;51:2045–2050. doi: 10.1161/STROKEAHA.120.029861 [DOI] [PubMed] [Google Scholar]
- 25. Liu C, Huang J, Kong W, Chen L, Song J, Yang J, Li F, Zi W. Development and validation of machine learning‐based model for mortality prediction in patients with acute basilar artery occlusion receiving endovascular treatment: multicentric cohort analysis. J Neurointerv Surg. 2023;16:53–60. doi: 10.1136/jnis-2023-020080 [DOI] [PubMed] [Google Scholar]
- 26. Baik SH, Park HJ, Kim JH, Jang CK, Kim BM, Kim DJ. Mechanical thrombectomy in subtypes of basilar artery occlusion: relationship to recanalization rate and clinical outcome. Radiology. 2019;291:730–737. doi: 10.1148/radiol.2019181924 [DOI] [PubMed] [Google Scholar]
- 27. Wu M, Zha M, Zhang X, Yuan K, Huang K, Xie Y, Dai Q, Liu X. Predictors of mortality for acute vertebrobasilar artery occlusion receiving endovascular treatment. Acta Neurol Scand. 2021;144:433–439. doi: 10.1111/ane.13477 [DOI] [PubMed] [Google Scholar]
- 28. Irvine H, Krieger P, Melmed KR, Torres J, Croll L, Zhao A, Lord A, Ishida K, Frontera J, Lewis A. Markers of infection and inflammation are associated with post‐thrombectomy mortality in acute stroke. Clin Neurol Neurosurg. 2022;222:107467. doi: 10.1016/j.clineuro.2022.107467 [DOI] [PubMed] [Google Scholar]
- 29. Chen F, Bai X, Wang X, Zhang L, Wang F, Huang L, Deng J, Geng Z. Impact of high‐sensitivity troponin elevation and dynamic changes on 90‐day mortality in patients with acute ischemic stroke after mechanical thrombectomy: results from an observational cohort. J Neurointerv Surg. 2022;15:1142–1147. doi: 10.1136/jnis-2022-019682 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S2


