Abstract
A prior systematic review on the efficacy of halofuginone (HFG) treatment to prevent or treat cryptosporidiosis in bovine calves was inconclusive. We undertook an updated synthesis and meta-analyses on key outcomes for the treatment of calves with HFG. Evaluated outcomes were oocyst shedding, diarrhoea, mortality and weight gain. Experiments had to describe results for same age animals in contemporary arms. Most doses were 100–150 mcg kg−1 day−1. Results were subgrouped by study design, experiments with the lowest risk of bias and lack of industry funding. Eighteen articles were found that described 25 experiments. Most evidence came from randomized controlled trials in Europe. Significantly lower incidence of oocyst shedding, diarrhoea burden and mortality was reported when treatment started before calves were 5 days old. Most studies reported on outcomes for animals up to at least 28 days old. Publication bias was possible in all outcomes and seemed especially likely for diarrhoea outcomes. Beneficial results when HFG treatment was initiated in calves older than 5 days were also found. Prophylactic treatment to prevent cryptosporidiosis is effective in preventing multiple negative outcomes and is beneficial to calf health and will result in a reduction of environmental contamination by Cryptosporidium oocysts.
Key words: Bovine calves, cryptosporidiosis, dairy, diarrhoea, halofuginone
Introduction
Cryptosporidium parvum is a common protozoan parasite in cattle. It is a frequently diagnosed cause of acute diarrhoea (scour) in neonatal calves worldwide leading to delayed growth, considerable morbidity and potentially death (Thomson et al., 2017; Manzoor et al., 2018). Young calves (under 6 weeks old) are at the greatest risk of both catching and spreading pathogenic infections (Silverlås et al., 2009; Wells and Thomson, 2014). Economic costs in Great Britain were estimated in 2014 to be £100–£200 per infected calf (Shaw, 2014), arising mostly from veterinary treatment, need for higher nutritional inputs and reduced weight gain. A more recent study of beef calves in Scotland found that calves with severe cryptosporidiosis in the first 16 days of life were on average 34 kg lighter aged 6 months old, compared to calves in the same housing who had had no clinical signs of the disease (Shaw et al., 2020). The weight difference between the two groups was statistically significant and the authors calculated that the 34 kg in weight difference would account for a loss of £128/head based on market prices of cattle in 2018 (Shaw et al., 2020). Bovine cryptosporidiosis is widespread in Europe and the prevalence of C. parvum in stool samples of European cattle herds were reported to range from 13 to 100% (Imre and Dărăbus, 2011). Cattle are recognized as an especially important reservoir for C. parvum, which can spread from cattle to other animals or to humans through many routes (Hunter and Thompson, 2005; Wells and Thomson, 2014; Brankston et al., 2018). Higher infectious doses are linked to more severe disease and potentially higher losses (Zambriski et al., 2013). Globally, infections from C. parvum and other Cryptosporidium species (e.g. C. hominis) are considered important contributors to combined total human deaths from diarrhoeal illness (Vermeulen et al., 2017). Large outbreaks in humans (affecting dozens or even hundreds of people) from pathogenic C. parvum infection regularly occur in Europe (Cacciò and Chalmers, 2016). Control of C. parvum is therefore highly desirable for good animal welfare, to reduce risks to human health and to limit economic losses in affected industries.
An evidence review written for livestock managers (Wells and Thomson, 2014) reiterated that prophylactic and treatment options for C. parvum infection are limited. One of the few products widely licensed for the treatment of calves is halofuginone lactate, marketed as Halocur®. Halofuginone (HFG) is a coccidiostat that was identified as having promise for cryptosporidiosis prophylaxis and treatment in calves by the early 1990s (Villacorta et al., 1991). HFG is most often administered as halofuginone lactate. It is a synthetic biological agent derived from the alkaloid Febrifugine isolated from roots and leaves of Dichroa febrifuga plants (Jang et al., 1948; Pines and Spector, 2015). HFG targets several cell processes, including ATP-dependent inhibition of tRNA synthetase (Keller et al., 2012; Zhou et al., 2013). The piperidine ring on HFG binds to both the Proline-AMP binding pocket, stabilized by the quinazolinone group which mimics the terminal adenosine of tRNA. Subsequently, the biological activity of tRNA synthetase is inhibited, resulting in arrested cell growth and reduced viability (Francklyn and Mullen, 2019). In vitro work by McDonald et al. (1990) found that HFG lactate suppressed 90% of C. parvum growth using 4 μg mL−1. In vitro studies of HFG lactate using C. parvum-infected HCT-8 cells by Shahiduzzaman et al. (2009) found that on average, 98.05% of sporozoites were inhibited using 25 μm at 27 h after exposure. Experiments in Wistar rats demonstrated HFG lactate promoted metalloproteinase −3 and −13, mediated by the activation of p38 mitogen-activated protein kinase and nuclear factor NFκB, in turn inhibiting T-helper cell-17 activity (Popov et al., 2006). This enables the extracellular matrix to be broken down (Kamberov et al., 2011), reducing fibrosis in the liver and other organs in mammalian systems.
A prior systematic review on the efficacy of halofuginone lactate to prevent or reduce symptoms caused by C. parvum infection was published 11 years ago (Silverlås et al., 2009). The meta-analyses in this previous review were based on only seven comparison groups in six publications and concluded that evidence was inadequate and too heterogenous to say if HFG products were effective. The authors were concerned that the evidence base was limited (just 3–4 studies for most outcomes), heavily influenced by industry funding and suffered from publication bias. Publication bias occurs when researchers decline to publish results believing they are not important due to no findings of significant association (Deeks et al., 2011). Research may be unlikely to proceed to publication after insignificant findings when the study was at least part-funded by an intervention sponsor. Longer duration studies (with outcomes beyond 3 weeks of age) were also few in the 2009 synthesis. Silverlås et al. (2009) recommended that HFG should only be used in severe cases of existing disease, not least to prevent the development of drug resistance. Structured reviews to assess the efficacy of HFG for prevention or treatment have not been published since, although many trials since 2008 have been published. The aim of our study was to investigate the effects of HFG treatment using updated trial data (published up until early 2020) on four outcomes: diarrhoeal intensity, oocyst shedding intensity, mortality and weight gain when used as prophylactic or therapeutic treatment against calf cryptosporidiosis. This was done by performing a systematic review and meta-analysis of available data.
Materials and methods
PRISMA literature search reporting guidelines were followed (Toews, 2017). The search strategy is available in Supplementary Material 1.
Population and other inclusion and exclusion criteria
The population of interest were cows (Bos taurus). Articles on humans, related species such as buffalo or yaks and other animals were ineligible. Studies on hybrids of cattle with other animals (e.g. beefalo) or mixed species herds (of B. taurus mixed with others) were considered individually, in case they provided sufficient cattle-specific information. Selected studies had to address outcomes related to C. parvum infection. Cryptosporidium parvum infection in the animals was based on clinical diagnosis. Other Cryptosporidium spp. infections were excluded because of evidence that other Cryptosporidium species are likely to be pathogenic in calves is weak (Wells and Thomson, 2014; Thomson et al., 2017; Åberg et al., 2020).
Intervention
The intervention had to be HFG treatment, in the form of halofuginone lactate or halofuginone hydrobromide, in an attempt to reduce the incidence or severity of cryptosporidiosis in young cows. Interventions could be either relatively prophylactic because very early in life (before 5 days old) or relatively late and therefore more likely to be treatment for existing or developing symptoms (age 5 days+). The vast majority of calves suffering from cryptosporidiosis are under 3–4 weeks old (Castro-Hermida et al., 2002; Wells and Thomson, 2014). The threshold of 5 days was to distinguish prophylaxis from treatment and was chosen to reflect a likely point of onset of symptoms, typically on day 4 or 5 of life (Erbe, 2010).
Study design and comparators
Only deliberate experiments with concurrent comparison animals were included. Pre-post and case–control designs were not eligible. There were no limits on location or publication date. Studies were excluded if not available in a language known to the authors (English, German, Spanish or French) or if the article could not be easily translated into English using Google Translate. Articles without abstracts or available full text were excluded.
Outcomes of experiments
To be eligible, studies had to address at least one of these outcomes:
Clinically detectable infection in (shedding from) live animals, of C. parvum.
Fecal scoring consistency: usually on scales of 1–3, 0–3, 0–4, etc., to describe the severity of diarrhoea
Measures of weight gain
Mortality
Reference sources
The search was mostly within peer-review research. Literature databases were chosen following recommendations about the most comprehensive bibliographic sources for veterinary science research (Grindlay et al., 2012). Searched databases were: Scopus, CAB International abstracts, PubMed and Embase. A limited grey literature search was undertaken of three government databases via websites: The UK Department for Food and Rural Affairs, The US Department of Agriculture library (at Cornell University) and The European Commission, Agricultural and Rural Development section. Conference proceedings were not searched.
Search strategy
From preliminary literature scoping, we selected two exemplar articles that met our inclusion criteria (De Waele et al., 2010; Trotz-Williams et al., 2011). The search terms were developed and validated by making sure searching using the below terms found both exemplar articles with a minimum of extraneous results (irrelevant search return results). Within the peer-review bibliographic databases, we searched title/abstract/keywords:
At least one of (Cryptosporidium, C. parvum, cryptosporidiosis)
AND
At least one of (calf, cattle, cow, bull, dam, dairy, beef, herd, calves)
Grey literature search terms were cryptosporidium, cryptosporidiosis and parvum. Some especially thorough and recent review papers about cryptosporidiosis (Silverlås et al., 2009; Johnson et al., 2011; Taylor and Bartram, 2012; Olias et al., 2018; Beaver et al., 2019) were checked for eligible articles missed by our search strategy. Forward and backward citation searches of included articles were not done to look for additional studies.
Study selection, quality assessment and data extraction
After de-duplication, titles and abstracts were independently screened by two investigators (JB and CCH). Items were chosen for full-text review or excluded. Selection disagreements were resolved by discussion or on the verdict of a third reviewer (PRH). Full texts were obtained where possible. Decisions about final inclusion or exclusion were made after full-text review by one or more authors.
Quality assessment
The reliability and consistency of the original experimental information were assessed using a customized-quality assessment form. Focused questions were written to assess quality using consistent decision criteria. The standardized extraction form and quality assessment decision criteria are available in Supplementary Material 2. Full-text review, data extraction and quality assessment were undertaken by a single author (either JB or CCH). Low-risk studies were deemed to be those who had a low risk of bias assigned for at least five of the seven possible quality checklist questions. Trial quality is shown by colour coding (green = low risk of bias, yellow = unclear, red = high risk of bias). Because C. parvum infection in the animals was based on clinical diagnosis, we report narratively what the diagnostic or clinical evidence was for C. parvum infection among the calves in each study.
Reporting and synthesis
Meta-analysis was with random-effects due to expected high heterogeneity, using REVMAN version 5.3 (Deeks et al., 2011). Meta-analyses are described narratively with reference to forest plots. Studies were all either randomized controlled trials (RCTs) or controlled clinical trials (CCTs) (Deeks et al., 2011). The advantage of RCTs is that animals are assigned randomly to treatment/not treatment, which means that any co-variates should be randomly distributed and are less likely to bias the experiment. CCTs are structured similarly but animals were not randomized to treatment, which means that there are more likely to be unobserved confounders that explain any differences rather than the treatment regime. The forest plots distinguish results by study design (RCT vs CCT) for main results with combined study designs in subgroupings. Significance level was set at P ≤ 0.05. Funnel plots were generated and visually examined for evidence of publication bias, such as missing small trials with negative results. Funnel plots were only generated where at least ten studies provided relevant data (either/both CCTs or RCTs) for any outcome stratified by early/late treatment phase as described below. Funnel plots are discussed narratively in this manuscript and provided in graphic format in Supplementary Material 3.
How to best pool extract the data depended on the specific outcome. Mortality was simple: of the animals that started the trials in each arm, how many died during the monitoring period could be the input to calculate pooled risk ratios. However, data for any amount (or severity) of oocyst shedding or diarrhoea incidence were reported using a variety of scales. Shedding of oocysts was reported (for instance) as the prevalence of animals with any detected oocysts, the prevalence of animals shedding above a certain threshold or by an average score for the arm on specific dates (scoring from 0 to higher levels, where higher level numbers meant more oocysts detected). Because all these forms of reporting were the ways of comparing oocyst detection between groups, we pooled these outcomes into a combined outcome which we describe under the umbrella term ‘oocyst shedding’. Fecal consistency was typically reported on multi-level scales (from 0 to 2, 3 or 4, where higher levels were more liquid). Weight might be reported as average daily weight gain, total weight at trial end or weight change since birth. These diverse metrics were measuring the same outcomes but on different scales. They were therefore compared in the meta-analysis using standard mean differences (SMDs). SMDs standardize for differences between arms rather than rely on the same instrument being used to measure an outcome in all trials. Lower SMD was a better outcome for calves with regard to diarrhoea or oocyst shedding but higher SMD value (above 0) was the preferred outcome with respect to weight gain. However, rarely was variance reported with either oocyst counts or diarrhoea outcomes. These outcomes were usually presented as simple averages without variance at single points in the monitoring period. For those studies, where an entire-period average and standard deviation value was unavailable, the mean period value was used with standard deviations calculated from the daily averages. For example, if the only fecal scores supplied for a group of animals for just three dates during the monitoring period were daily averages from three scores = 1, 2, 3, then the mean for the monitoring period was assigned to equal 2, s.d. 1. For transparency, the data extracted and used to calculate the oocyst/diarrhoea scores are available in Supplementary Material 4. Comparing transformed scores was valid as the original metrics fundamentally measured the same outcome (e.g. weight gain, intensity of oocyst shedding or diarrhoea) and were compared between studies using SMDs.
Stratification and subgrouping
Exposure soon after birth for most animals means that most untreated calves are symptomatic by 5 days old (Erbe, 2010). The pathology of disease is worse in young animals (Thomson et al., 2019); older infected animals may be more resilient and have less morbidity and mortality. Results were therefore divided by whether treatment was relatively early (before calves were 5 days old), or relatively ‘late’: started when animals were age 5 days or older. Where at least four eligible trials were available, we analysed subgroups for when studies were funded by known or likely sellers of HFG or rival products, or had a low risk of bias. For diarrhoea only, we also tested whether the efficacy of HFG varied depending on whether dams received vaccination against other pathogens that can cause diarrhoea (e.g. rotavirus, coronavirus or E. coli). We did not subgroup by daily dosage or duration of treatment because these varied relatively little between trials (see Results, Table 1).
Table 1.
Included article characteristics
| Article | Dosea; industry sponsored? | Study design location | Calf age (days) when HFG administered | Oldest age data used in meta-analysis; detection method | Outcomes used in meta-analysis |
|---|---|---|---|---|---|
| Al Mawly et al. (2013) | 8 mL Halocur® day−1 Yes | RCT New Zealand | 0–6 | 20 days fluor. microsc. | Diarrhoea, mortality, oocysts |
| De Waele et al. (2010) | 100 μg kg−1 day−1 No | RCT Ireland | 0–6 | 28 days fluor. microsc. | Diarrhoea, mortality, oocysts |
| Erbe (2010) | 8 mL Halocur® day−1 Yes | RCT Germany | 0–6 | 22+ days stain microsc. | Diarrhoea, oocysts, weight |
| Jarvie et al. (2005) | 5 mg day−1 Yes | RCT Canada | 0–6 | 26+ days microscopy | Diarrhoea, mortality, oocysts, weight |
| Joachim et al. (2003) | 2 mL Halocur®/10 kg No | RCT Germany | 0–6 | 21 days stain microsc. and ELISA | Diarrhoea, mortality, oocysts |
| Keidel and Daugschies (2013) | 120 μg kg−1 day−1 Unclear | RCT, CCT Germany | 1–7 | 20 days stain microsc. and ELISA/PCR | Diarrhoea, oocysts |
| Klein (2008) | 100 μg kg−1 day−1 No | RCT Czech Rep. | 1–7 and 8–16 | 27 days microscopy | Diarrhoea, oocysts |
| Lallemond et al. (2006) | 100 μg kg−1 day−1 Yes | RCT Canada | 8–14 | 34 days microscopy | Diarrhoea, oocysts, mortality, weightb |
| Lefay et al. (2001) | 120 μg kg−1 day−1 Yes | RCT France | 1–7 | 22 days microscopy | Diarrhoea, mortality |
| Martins et al. (2007) | 120μg kg−1 day−1 Unclear | CCT Portugal | 0/1 to 6/7 | 14 days stain microsc. | Oocysts |
| Naciri et al. (1993) | 120 μg kg−1 day−1 Unclear | CCT Belgium | 2–8 | 31 days microscopy | Oocysts, mortality, weight |
| Niine et al. (2018) | Unclear dose No | CCT Estonia | 1–7 or 3–9 | 47 days fluor. microsc. | Mortality, oocysts, weight |
| Peeters et al. (1993) | 120 μg kg−1 day−1 Yes | CCT France | 2–8 and 5–11 | 29 and 53 days microscopy | Mortality, oocysts |
| Pilarczyk et al. (2008) | 2 mL Halocur ®/10 kg Unclear | CCT Germany | 0–6 and variable to +6 day | 18 days stain microsc. | Mortality, oocysts |
| Trotz-Williams et al. (2011) | 100 μg kg−1 day−1 Yes | RCT Canada | 0–6 | 21 days microscopy | Diarrhoea, mortality, oocysts |
| Vélez et al. (2019) | 8 or 12 mL day−1 Yesc | CCT Germany | 0–6 | 28+ days stain microsc. | Diarrhoea, mortality, oocysts, weight |
| Villacorta et al. (1991) | 125 μg kg−1day−1 No | CCT Spain | 1–7 | 28 days stain microsc. | Oocysts, weight |
| Wiedemann et al. (2012) | 100 μg kg−1 day−1 Yes | CCT Germany | 1–7 | 35 days stain microsc. | Diarrhoea, oocysts, weight |
CCT, controlled clinical trial; ELISA, enzyme-linked immunoassay; fluor, fluorescent or immunofluorescence-enhanced microscopy; microsc, microscopy; PCR, polymerase chain reaction (test); RCT, randomized controlled trial.
Studies were deemed to be sponsored by industry if they stated an HFG distributor was their funder, a co-author worked for a pharmaceutical company that was possibly a distributor of HFG or potential rival products for controlling cryptosporidium (Intervet, Delimax, Roussel-Uclaf) or if authors stated that a company had ‘supplied’ the HFG. Sponsor was deemed to be ‘unclear’ if no funding statement was made.
Some trials had arms receiving other doses; results from other doses were not used in our pooling.
Weight was reported with unclear variance in Lallemond et al. (2006) so not suitable for pooling in the meta-analysis.
Vélez et al. (2019) was funded by commercial developer of a potential rival product to HFG that was also trialled in a third arm not summarized in this review.
Results
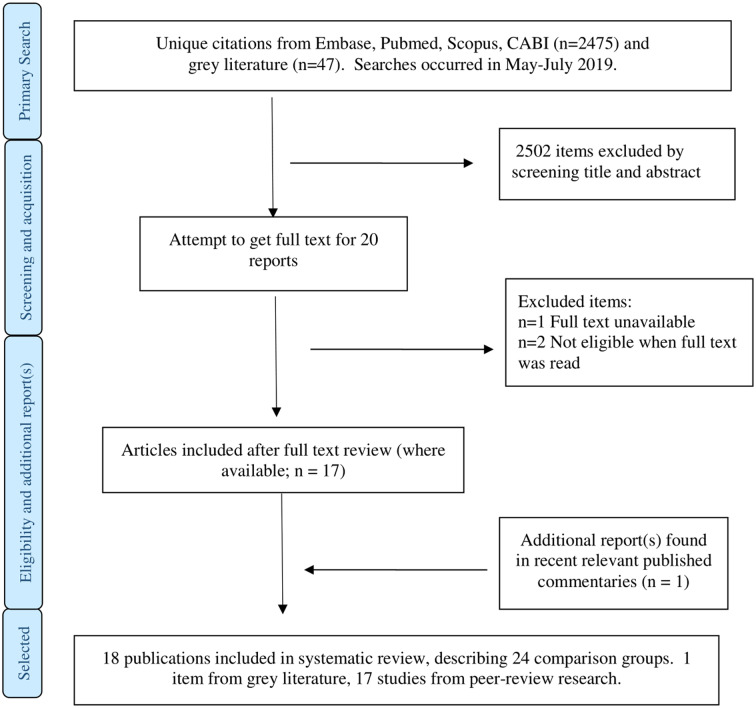
Figure 1 shows the study selection process. There were 2475 mostly unique articles found in scientific databases. There were no hits on the USDA library site and 14 hits on the EC site (none of which were eligible for inclusion). On the UK DEFRA site, there were 33 hits, most of which related to prevalence in humans or human disease risk factors; none related to disease prevention in cattle.
Fig. 1.
Study selection procedure.
Twenty articles were selected for full-text review. Of these reports, one was unavailable. One write-up (available as a thesis; Erbe, 2010) was added because it is published online and was mentioned in recent literature reviews. Two articles were excluded after full-text review leaving 18 articles that were included in this review. Most articles described RCTs, but some described experiments where it was not stated that animals had been assigned to treatment randomly (CCTs). Often, more than one experiment was documented within a single report. There were 15 RCT comparison arms described in 11 articles. One article (Keidel and Daugschies, 2013) described two RCTs and a CCT. Seven other articles described ten comparisons in CCTs. Most reports addressed early treatment with HFG, but four articles described tests of HFG when treatment started mostly after 4 days of age. Table 1 lists the characteristics of the 18 included scientific articles.
Four trials were assessed to have a low risk of bias (De Waele et al., 2010; Trotz-Williams et al., 2011; Al Mawly et al., 2013; Vélez et al., 2019). Diagnosis of C. parvum infection was mostly based on clinical presentation, sometimes informed by herd history. Confirmation of Cryptosporidium spp. oocyst shedding was done in all studies using microscopy, sometimes enhanced with staining or immunofluorescence (see detection methods for each study listed in Table 1). Two studies (Joachim et al., 2003; Keidel and Daugschies, 2013) also used an immunological test (ELISA) as a diagnosis aid.
All reports described treatment on the herds of B. taurus (no mixed species or hybrids). The vast majority were Holstein breed or Holstein crosses, from dairy herds. There was a mix of sexes. Most (14/18) of the included articles described research that took place in Western Europe.
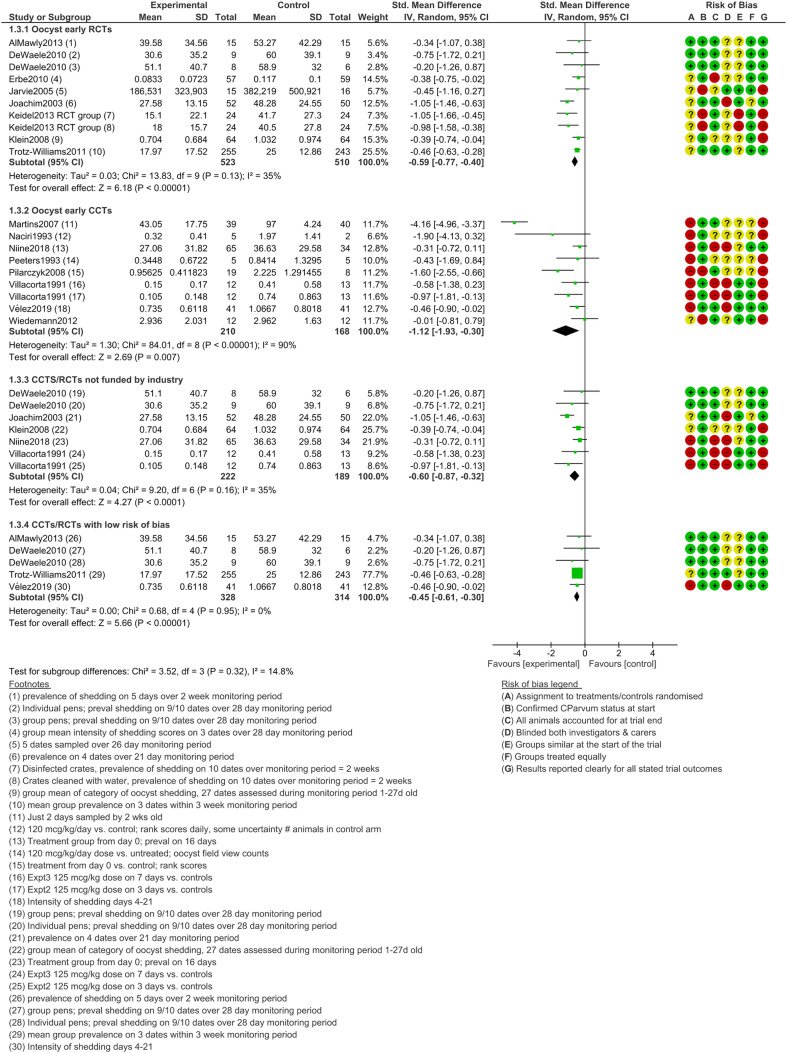
Oocyst shedding, early treatment
Most of the included trials provided data to compare how many animals in each arm shed oocysts. The meta-analysis results for early treatment (Fig. 2) are consistent in both RCTs and CCTs in showing significantly less oocyst shedding in the treatment arms (both P < 0.01). Similar levels of significance were retained even when the data were subgrouped to only include experiments at a low risk of bias (SMD −0.45, 95% CI −0.61 to −0.30, I2 = 0%) or those not funded by industry (SMD −0.60, 95% CI −0.87 to −0.32, I2 = 35%). The funnel plot (Fig. S3.1 in Supplementary Material 3) for these trials showed outliers but not large imbalances on either side of the mean effect.
Fig. 2.
Oocyst shedding following early (prophylactic) treatment with halofuginone products.
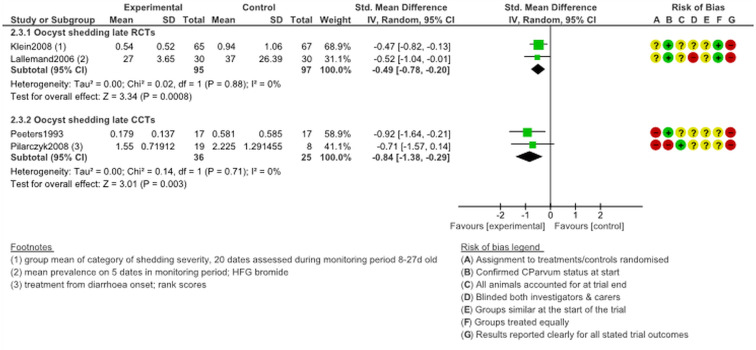
Oocyst shedding, late treatment
Information about oocyst shedding among animals who started treatment at age 5 days+ was available in four studies. All of the studies reported significantly less oocyst shedding by calves in experimental arms (P ≤ 0.01; Fig. 3). None of these trials had a low risk of bias and only one trial (Klein, 2008) was clearly not funded by industry.
Fig. 3.
Oocyst shedding following late treatment with halofuginone products.
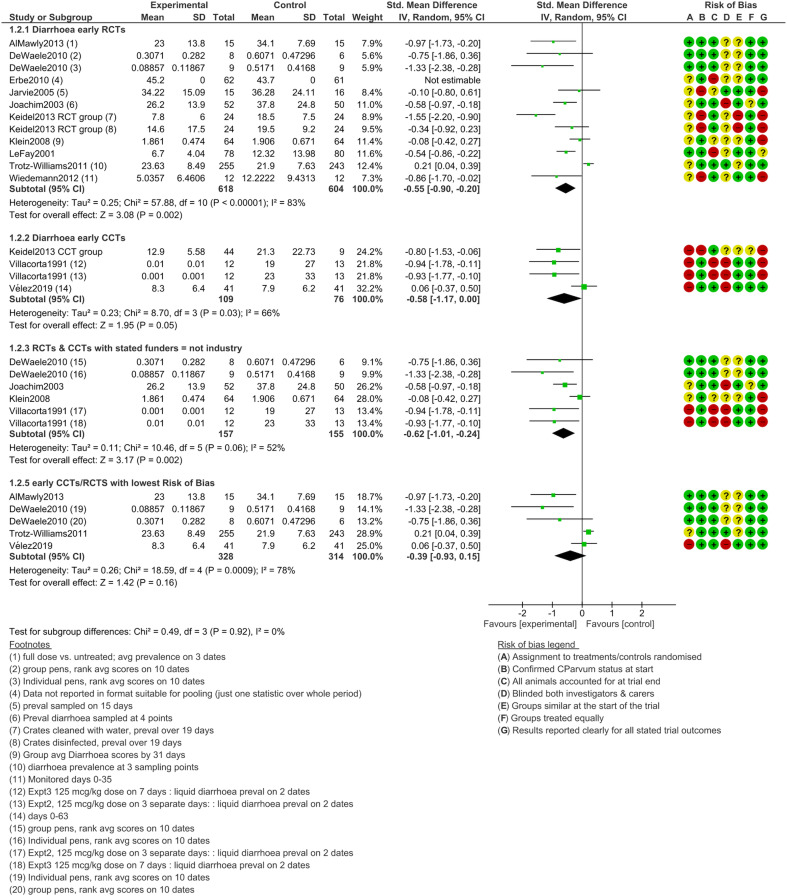
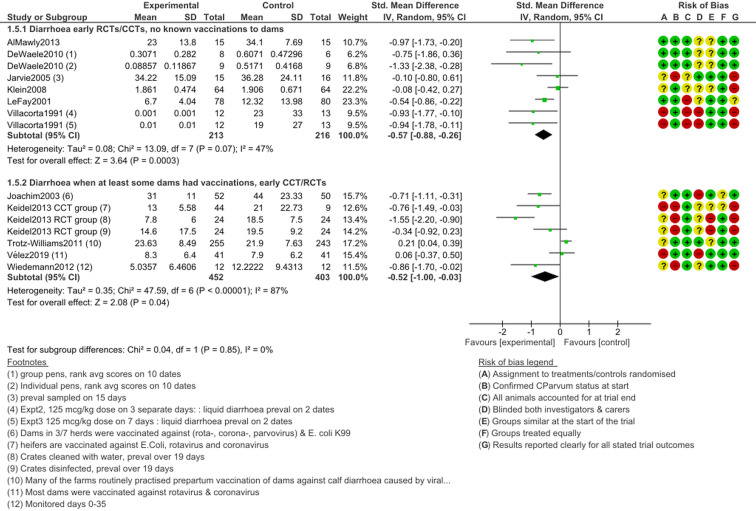
Diarrhoea, early treatment
Figure 4 shows outcomes after halofuginone lactate treatment initiation before 5 days old. Diarrhoeal burden was significantly lower in treated animals in the main results grouped by study design (RCT or CCT) and among studies with stated funders that were not sponsored by industry (P ≤ 0.05 in all of these; Fig. 4). However, among the four trials at a low risk of bias, the pooled effect SMD did not reach the significance threshold (SMD −0.39, 95% CI −0.93 to 0.15, I2 = 78%, P = 0.16). Heterogeneity was relatively high in all of the comparisons (I2 > 50%), which indicates a lack of consistency in results.
Fig. 4.
Diarrhoea intensity following early (prophylactic) treatment with halofuginone products, subgroups by funder and risk of bias.
The funnel plot of the early treatment and diarrhoea intensity outcome after halofuginone lactate treatment (Supplementary Material Fig. S3.2) is strongly not symmetrical and suggests there are many missing small studies with negative results (i.e. suggesting publication bias). The funnel plot highlights that the Trotz-Williams et al. (2011) study is an outlier; it was especially large and unusually found that untreated calves had less diarrhoea. Another trial (Klein, 2008) was relatively large while finding little evidence of differences in diarrhoea intensity between experimental arms.
Diarrhoea after early treatment, whether dams had any vaccinations against diarrhoeal diseases
HFG seemed most effective when the studies did not state that dams had relevant vaccinations (SMD −0.57, 95% CI −0.88 to −0.26, I2 = 47%, P = 0.0003; Fig. 5). However, strong efficacy was also observed when dams were reported to have had vaccinations relevant to diarrhoeal disease (second subgroup Fig. 5). These findings suggest that vaccination status of dams did not prevent diarrhoea in very young calves, but this is caveated with the observation that the pooled data were highly heterogeneous and therefore inconsistent (I2 ≥ 47%).
Fig. 5.
Diarrhoea intensity following early (prophylactic) treatment with halofuginone products, subgroup by vaccination history of the dam.
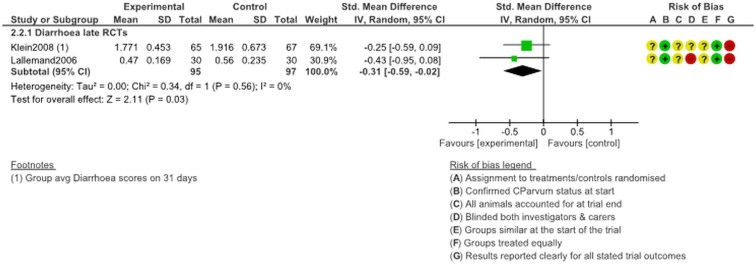
Diarrhoea, treatment from 5 days of age
Two RCTs reported data about diarrhoea in calves following treatment initiation at age 5+ days. The trial by Klein (2008) used halofuginone lactate while the trial by Lallemond et al. (2006) used halofuginone hydrobromide. Both seemed at least somewhat effective at reducing the severity of diarrhoea. In the pooled analysis, diarrhoeal intensity scores were significantly lower among treated animals (SMD −0.31, 95% CI −0.59 to −0.02, P = 0.03; Fig. 6). Neither trial had a low risk of bias, while only the trial by Klein (2008) was clearly not funded by industry.
Fig. 6.
Diarrhoea intensity following late treatment (at age 5 days+) with halofuginone products.
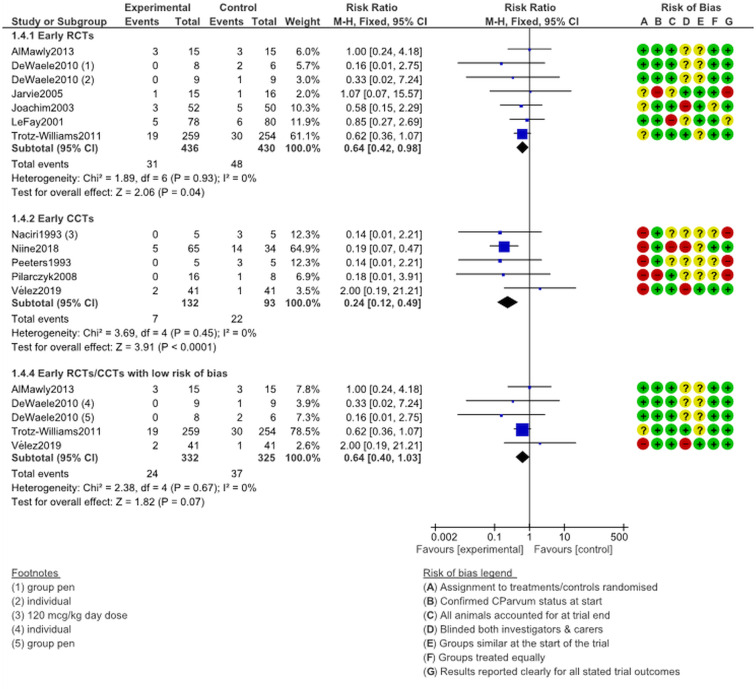
Mortality, prophylactic (early) treatment
Pooled risk ratios were calculated for mortality outcomes at the end of monitoring periods and are presented in the chart below. Mortality was significantly lower in treatment arms (RCT RR 0.64, 95% CI 0.42–0.98, I2 = 0%; CCT RR 0.24, 95% CI 0.12–0.49, I2 = 0%). In Fig. 7, a lower risk ratio is good (meaning fewer deaths in that arm). Heterogeneity was low (low I2) which means that the results from different trials are fairly similar. The evidence base is somewhat problematic in that many trials made no explicit mention whether there were deaths or not. Many other trials were explicit in saying that no deaths occurred in neither arm. The data below are drawn from trials where at least one death occurred in either arm.
Fig. 7.
Mortality following early (mortality) treatment with halofuginone products.
Four studies provided data on five early-treatment comparisons with a low risk of bias reported on mortality data; the RR almost reached significance threshold (RR 0.64, 95% CI 0.40–1.03, I2 = 0%, P = 0.07). The near significance is due to the results of a single large trial (Trotz-Williams et al., 2011). The funnel plot (Supplementary Material Fig. S3.3) did not suggest strong publication bias: effects were scattered equally around the mean difference and roughly in a triangular shape.
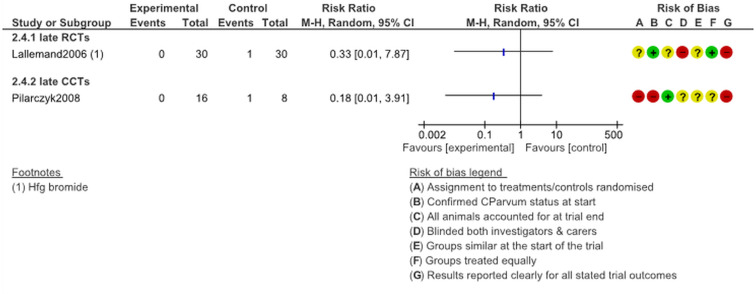
Mortality, late treatment
Only two trials, one RCT and one CCT, reported on mortality when HFG treatment was initiated to animals aged ≥5 days. Again, mortality was lower in the treatment arms although the risk ratios are less significant than they were for early treatment experiments (see Fig. 8).
Fig. 8.
Mortality following late (age 5 days+) treatment with halofuginone products.
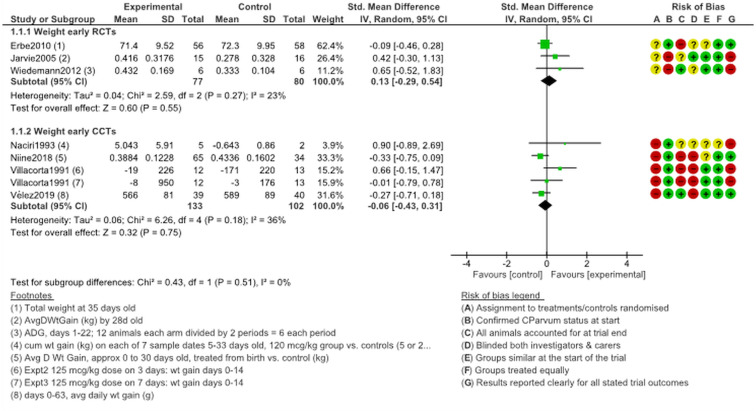
Weight gain
Relatively few reports (n = 7 providing data on eight comparisons) provided information about weight gain differences between arms following halofuginone lactate treatment (Fig. 9). Weight gain comparison data suitable for the meta-analysis were only available when calves had been treated relatively early (no data suitable for the meta-analysis were available for when treatment started at age 5 days plus). These data suggest no weight gain benefits for animals in either arm. RCT evidence for weight gain comparisons had SMD 0.13 (95% CI −0.29 to 0.54, I2 = 23%). Heterogeneity was relatively moderate among CCTs or RCTs. It is important to note that the weight comparisons mostly apply to surviving animals at the end of the monitoring periods. The monitoring periods were typically 28–33 days long (see Table 1 for specific durations).
Fig. 9.
Weight gain following early (prophylactic) treatment with halofuginone products.
There were no studies at a low risk of bias with weight gain data. Just two of the studies that reported on weight gain outcomes after halofuginone lactate treatment (Villacorta et al., 1991; Niine et al., 2018) were not sponsored by industry.
Discussion
This systematic review has shown that the prophylactic use of HFG products had significant effects on calf health and welfare as measured by reductions in oocyst shedding, diarrhoea and calf mortality. These findings were also upheld when the data were subdivided into randomized control treatment studies or clinical control treatment studies, showing that both study types provided consistent results on the efficacy of the prophylactic treatment of HFG. This current systematic review found more conclusive results on these characteristics than the earlier systematic review by Silverlås et al. (2009). This was possible because the earlier review could only use the data from six publications detailing seven comparison groups. In contrast, our review had data available from 18 publications involving 25 comparison groups. Our review separated the trials into the prophylactic and therapeutic use of HFG in order to reduce variation between studies due to the different treatment regimes. Our analysis offers more conclusive support for the efficacy of HFG as a prophylactic treatment against cryptosporidiosis. The previous systematic review by Silverlås et al. (2009) also highlighted the effect of potential bias in the available publications due to involvement by the funders. This was addressed in the current review by excluding data from publications which had industry sponsors. Our analysis showed significant differences between groups of calves that received prophylactic HFG treatments and the control groups for two outcomes: oocyst shedding and severity of diarrhoea. The evidence for reduced mortality was not as conclusive but did approach significance (P = 0.07). A further characteristic that did not give conclusive results was the prophylactic effect of HFG treatment on weight gain. This may be because only a few studies provided data suitable for the pooled analysis. For example, in an RCT using 60 animals and halofuginone bromide, Lallemond et al. (2006) reported nearly identical average daily weight gain in the period from 3 to 28 days old (0.31 kg day−1 for intervention arm, 0.33 kg day−1 for controls). Variance units for average daily weight gain in respective groups were unclearly reported in this study which made the calculation of an odds ratio unfeasible and the study had to be excluded from the meta-analysis. Weight gain as an outcome may be especially sensitive to the severity of the disease. Shaw et al. (2020) found no significant difference in weight gain between severely infected calves and calves that showed mild or moderate symptoms of cryptosporidiosis, by the age of 6 months. Calves grouped by symptom severity all had significantly lower weight gain by 6 months old than calves who had never presented symptoms of cryptosporidiosis. This suggests that preventive treatment may need to result in no signs of cryptosporidiosis to achieve a significant difference in weight gain results.
This current review also analysed the available data to see if there is any evidence for the efficacy for HFG as a therapeutic treatment, as defined by initiation of treatment date after calves were 5 days of age. Unfortunately, only four studies were available for these analyses. The CCT and the RCT studies did show significant reductions in oocyst shedding by the treated calves but one of the concerns is that only one study (Klein, 2008) did not have funding from industry sponsors. For the subsequent analyses (diarrhoea and mortality) only two studies provided data and none had data on weight gain, preventing comprehensive investigations into the therapeutic use of HFG against cryptosporidiosis. Since the review by Silverlås et al. was published in 2009, no new papers have been published with trials that describe the therapeutic use of HFG, leaving only four reports (Peeters et al., 1993; Lallemond et al., 2006; Klein, 2008; Pilarczyk et al., 2008). Four studies are unlikely to be sufficient to provide robust conclusions. Other areas that need more research that could not be addressed by the current review were the economic costs/benefits from using HFG and the efficacy of other treatments for cryptosporidiosis such as paromomycin-based products, which are licensed in some localities for use against cryptosporidiosis in neonatal calves. The environmental effect of HFG treatment was also not assessed in this review but treatment may be beneficial for calves born subsequently, as HFG treatment resulted in reduced oocyst shedding. There is evidence that increased infection doses lead to more morbidity due to cryptosporidiosis (Zambriski et al., 2013). This can explain why farmers often see more severe problems with cryptosporidiosis in the latter stages of the calving period as parasite loads are amplified due to greater environmental contamination.
One of the main limitations of HFG treatment is that it cannot be given to calves that have diarrhoea for more than 24 h or that are already weak or dehydrated (European Medicines Agency, 2007). This limits its suitability as a therapeutic treatment. As a result, farms may have to rely on a different treatment but still use HFG prophylactically for their other calves that do not show any symptoms of infection yet. Another complication with HFG treatment is that it is important to get the dose correct for each calf. HFG has been shown to have a toxic side at twice the recommended dose, which means that calves should be weighed in order to determine the correct dose (European Medicines Agency, 2007).
Almost all of the studies we found reported on the use of halofuginone lactate rather than halofuginone hydrobromide. This is probably because halofuginone hydrobromide (a derivative of halofuginone lactate) is less soluble. HFG products are typically administered as part of liquid feeds to very young calves, so it is impractical to use a poorly soluble product.
It is unlikely that C. parvum can be eliminated from an affected farm as infected animals shed oocysts in huge amounts while the infectious dose required for animals to contract cryptosporidiosis is very small (as low as 25 oocysts; Zambriski et al., 2013). However, disease severity seems to be dependent on the infectious dose (Zambriski et al., 2013) which means that many farmers have looked at livestock management strategies to reduce transmission and morbidity. Strategies involve improved hygiene, welfare, segregation and nutritional measures (Wells and Thomson, 2014). Hygiene encompasses the maintenance of rigorous cleaning and hygiene routines for both pens and animals with products that are efficacious for use against Cryptosporidium. Deep straw bedding may increase the cleanliness of the animals and reduce contact with contaminated feces. Bedding should be kept as dry as possible. Disinfection (buckets or pans) should be available to staff at entrances to calf sheds. Symptomatic treatment includes keeping animals warm and hydrated by rehydration with electrolytes if necessary. Healthy animals should be attended before sick animals as part of daily husbandry routines. Whether higher nutritional planes can make calves more resistant to morbidity from cryptosporidiosis has been tested in other experiments without conclusive advantages for any specific nutritional strategy (Meganck et al., 2014; Wells and Thomson, 2014; Vélez et al., 2019; Brainard et al., 2020a).
Segregation strategies encompass keeping healthy and sick animals separate as well as segregation by age groups. Young cows are the most at-risk group for developing illness from C. parvum. Sick calves should be quarantined as soon as possible after scouring begins and up to 7 days after scouring ends. Additionally, as older calves/cows can still shed C. parvum oocysts (Thomson et al., 2019), it may be best to keep older and pre-weaned animals separate. Individual housing of neonatal dairy calves does not seem to reduce calf-to-calf transmission, however (Brainard et al., 2020b). Within Europe, the possible management strategies are also governed by EU-side welfare regulations that ban the use of ‘veal crates’, for example (European Union, 2008). Such confinement in contrast to the use of large stalls that animals can move freely in was observed to increase morbidity due to cryptosporidiosis (Graef et al., 2018).
This systematic review highlights the need for more targeted research of treatments against cryptosporidiosis. In particular, evidence about the therapeutic use of HFG as a treatment for cryptosporidiosis is available from relatively few publications. More studies (either therapeutic or prophylactic in design) not sponsored by industry would be preferable. This review also shows that there is a need for more data on the economic cost/benefit of HFG for farmers as well as better measures for evaluating the welfare benefit for the treated animals. The most reliable data available to date focused on oocyst counts and fecal consistency while fewer data on mortality and weight gain were available. Therefore, future drug treatment trials should be better designed to address these outcomes.
Conclusion
This systematic review has shown that the evidence in the scientific literature is getting stronger in support of the prophylactic treatment of calves with HFG because it has a beneficial impact on their health as it reduces diarrhoea, oocyst shedding and mortality. The prophylactic use of HFG, as per manufacturer instructions at 12–48 h old, on farms with confirmed cryptosporidiosis can be justified as it reduces morbidity and mortality. Subgrouping by sponsor or studies with the lowest risk of bias does not change the broad conclusion that HFG is protective when administered early. However, no conclusions could be reached that prophylactic HFG treatment resulted in higher weight gain among surviving animals. In addition, there is evidence of publication bias in the results and a dearth of utility calculations and reporting on adverse events, which means that it is difficult to establish that the treatment has economic benefits.
HFG is less effective in calves if treatment commences after bovine calves are 5 days old, although reduced oocyst shedding and diarrhoea intensity are still reduced by relatively late treatment. Evidence is quite limited about whether HFG is effective at reducing mortality when given late. No clear evidence was found whether late-treatment HFG affects weight gain. There is also inadequate evidence about whether other treatments (not HFG based) might be equally or even more effective (Brainard et al., 2020a) for improving important outcomes when given prophylactically or to symptomatic animals. Limitations of HFG use due to toxicity means that alternative products may have to be used, such as paromomycin, which were not evaluated within this systematic review.
Acknowledgements
We thank H4DC collaborators for their support. The opinions expressed here are those of the authors only. We also thank Chris Groom and Kate West (staff at the Norwich BioScience Institutes) for help with literature searches.
Author contributions
P.R.H. and K.T. conceived the study. J.B. and C.C.H. designed the study, screened and extracted data and undertook quality assessment. J.B. conducted the searches, undertook the analysis, wrote the first draft and assembled revisions. G.H. and K.T. researched and described in vitro and pharmaceutical properties of the drug. F.K. researched and described the veterinary significance of the treatment. All authors revised draft manuscripts and approved the final manuscript.
Financial support
This project received funding from the Interreg 2 Seas programme 2014–2020 co-funded by the European Regional Development Fund under subsidy contract No 2S05-043 H4DC.
Ethical standards
Not applicable.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182020002267.
click here to view supplementary material
Conflict of interest
None.
References
- Åberg M, Emanuelson U, Troell K and Björkman C (2020) A single-cohort study of Cryptosporidium bovis and Cryptosporidium ryanae in dairy cattle from birth to calving. Veterinary Parasitology: Regional Studies and Reports 20, 100400. [DOI] [PubMed] [Google Scholar]
- Al Mawly J, Prattley D, French N, Lopez-Villalobos N, Hedgespeth B and Grinberg A (2013) Utility of halofuginone lactate for the prevention of natural cryptosporidiosis of calves, in the presence of co-infection with rotavirus and Salmonella typhimurium. Veterinary Parasitology 197, 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver A, Meagher RK, von Keyserlingk MA and Weary DM (2019) A systematic review of the effects of early separation on dairy cow and calf health. Journal of Dairy Science 102, 5784–5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard J, Hammer CC, Tyler K and Hunter PR (2020a) Efficacy of non-halofuginone based strategies to prevent or treat cryptosporidiosis: a systematic review. preprints.org. [DOI] [PMC free article] [PubMed]
- Brainard J, Hooper L, McFarlane S, Hammer CC, Hunter PR and Tyler K (2020b) Systematic review of modifiable risk factors shows little evidential support for most current practices in Cryptosporidium management in bovine calves. Parasitology Research 119, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brankston G, Boughen C, Ng V, Fisman DN, Sargeant JM and Greer AL (2018) Assessing the impact of environmental exposures and Cryptosporidium infection in cattle on human incidence of cryptosporidiosis in Southwestern Ontario, Canada. PLoS ONE 13, e0196573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciò S and Chalmers RM (2016) Human cryptosporidiosis in Europe. Clinical Microbiology and Infection 22, 471–480. [DOI] [PubMed] [Google Scholar]
- Castro-Hermida JA, Gonzalez-Losada YA and Ares-Mazas E (2002) Prevalence of and risk factors involved in the spread of neonatal bovine cryptosporidiosis in Galicia (NW Spain). Veterinary Parasitology 106, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks JJ, Higgins J, Altman DG and Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1. 0 (updated March 2011). The Cochrane Collaboration, 2.
- De Waele V, Speybroeck N, Berkvens D, Mulcahy G and Murphy TM (2010) Control of cryptosporidiosis in neonatal calves: use of halofuginone lactate in two different calf rearing systems. Preventive Veterinary Medicine 96, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbe S (2010) Bovine cryptosporidiosis: analysis of an integrated control under the conditions of a natural infection position in a calf herd. Doctorate of Veterinary Medicine University of Leipzig. [Google Scholar]
- European Medicines Agency (2007) HALOCUR: EPAR Summary for the Public. Document number EMEA/V/C040, June. https://www.ema.europa.eu/en/documents/overview/halocur-epar-summary-public_en.pdf
- European Union (2008) Title. Directive 2008/119/EC – minimum standards for the protection of calves.
- Francklyn CS and Mullen P (2019) Progress and challenges in aminoacyl-tRNA synthetase-based therapeutics. Journal of Biological Chemistry 294, 5365–5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graef G, Hurst NJ, Kidder L, Sy TL, Goodman LB, Preston WD, Arnold SLM and Zambriski JA (2018) Impact of confinement housing on study end-points in the calf model of cryptosporidiosis. PLoS Neglected Tropical Diseases 12, e0006295. doi: 10.1371/journal.pntd.0006295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindlay DJ, Brennan ML and Dean RS (2012) Searching the veterinary literature: a comparison of the coverage of veterinary journals by nine bibliographic databases. Journal of Veterinary Medical Education 39, 404–412. [DOI] [PubMed] [Google Scholar]
- Hunter PR and Thompson RA (2005) The zoonotic transmission of Giardia and Cryptosporidium. International Journal for Parasitology 35, 1181–1190. [DOI] [PubMed] [Google Scholar]
- Imre K and Dărăbus G (2011) Distribution of Cryptosporidium species, genotypes and C. parvum subtypes in cattle in European countries. Revista Scientia Parasitologica 12, 1–9. [Google Scholar]
- Jang C, Fu F, Huang K and Wang C (1948) Pharmacology of Ch‘ang Shan (Dichroa febrifuga), a Chinese Antimalarial Herb. Nature 161, 400–401. [DOI] [PubMed] [Google Scholar]
- Jarvie BD, Trotz-Williams LA, McKnight DR, Leslie KE, Wallace MM, Todd CG, Sharpe PH and Peregrine AS (2005) Effect of halofuginone lactate on the occurrence of Cryptosporidium parvum and growth of neonatal dairy calves. Journal of Dairy Science 88, 1801–1806. [DOI] [PubMed] [Google Scholar]
- Joachim A, Krull T, Schwarzkopf J and Daugschies A (2003) Prevalence and control of bovine cryptosporidiosis in German dairy herds. Veterinary Parasitology 112, 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K, Burn CC and Wathes DC (2011) Rates and risk factors for contagious disease and mortality in young dairy heifers. Animal Science Reviews 205, 101–113. [Google Scholar]
- Kamberov YG, Kim J, Mazitschek R, Kuo WP and Whitman M (2011) Microarray profiling reveals the integrated stress response is activated by halofuginone in mammary epithelial cells. BMC Research Notes 4, 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keidel J and Daugschies A (2013) Integration of halofuginone lactate treatment and disinfection with p-chloro-m-cresol to control natural cryptosporidiosis in calves. Veterinary Parasitology 196, 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller TL, Zocco D, Sundrud MS, Hendrick M, Edenius M, Yum J, Kim Y-J, Lee H-K, Cortese JF and Wirth DF (2012) Halofuginone and other febrifugine derivatives inhibit prolyl-tRNA synthetase. Nature Chemical Biology 8, 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P (2008) Preventive and therapeutic efficacy of halofuginone-lactate against Cryptosporidium parvum in spontaneously infected calves: a centralised, randomised, double-blind, placebo-controlled study. Veterinary Journal 177, 429–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemond M, Villeneuve A, Belda J and Dubreuil P (2006) Field study of the efficacy of halofuginone and decoquinate in the treatment of cryptosporidiosis in veal calves. Veterinary Record 159, 672–677. [DOI] [PubMed] [Google Scholar]
- Lefay D, Naciri M, Poirier P and Chermette R (2001) Efficacy of halofuginone lactate in the prevention of cryptosporidiosis in suckling calves. Veterinary Record 148, 108–112. [DOI] [PubMed] [Google Scholar]
- Manzoor A, Nazir T, Untoo M, Fayaz A, Zaffer B, Afzal I, Akram T and Dar ZA (2018) Calf scour: an obstacle in successful dairying. Journal of Entomology and Zoology Studies 6, 2001–2005. [Google Scholar]
- Martins S, Sousa S, Madeira de Carvalho L, Bacelar J and Cannas da Silva J (2007) Prevalence of Cryptosporidium parvum infection in northwest Portugal dairy calves and efficacy of halofuginone lactate on the prevention of cryptosporidiosis. Cattle Practice 15, 152. [Google Scholar]
- McDonald V, Stables R, Warhurst D, Barer M, Blewett D, Chapman H, Connolly G, Chiodini P and McAdam K (1990) In vitro cultivation of Cryptosporidium parvum and screening for anticryptosporidial drugs. Antimicrobial Agents and Chemotherapy 34, 1498–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meganck V, Hoflack G and Opsomer G (2014) Advances in prevention and therapy of neonatal dairy calf diarrhoea: a systematical review with emphasis on colostrum management and fluid therapy. Acta Veterinaria Scandinavica 56, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naciri M, Mancassola R, Yvoré P and Peeters JE (1993) The effect of halofuginone lactate on experimental Cryptosporidium parvum infections in calves. Veterinary Parasitology 45, 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niine T, Dorbek-Kolin E, Lassen B and Orro T (2018) Cryptosporidium outbreak in calves on a large dairy farm: effect of treatment and the association with the inflammatory response and short-term weight gain. Research in Veterinary Science 117, 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olias P, Dettwiler I, Hemphill A, Deplazes P, Steiner A and Meylan M (2018) The significance of cryptosporidiosis for the health of calves in Switzerland. Schweizer Archiv fur Tierheilkunde 160, 363–374. [DOI] [PubMed] [Google Scholar]
- Peeters JE, Villacorta I, Naciri M and Vanopdenbosch E (1993) Specific serum and local antibody responses against Cryptosporidium parvum during medication of calves with halofuginone lactate. Infection and Immunity 61, 4440–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilarczyk B, Balicka-Ramisz A, Ramisz A, Pilarczyk R, Sablik P and Wójcik J (2008) The dynamics of Croptosporidium sp. infection in Halocur®-treated and-untreated calves. Tierarztliche Umschau 63, 656–661. [Google Scholar]
- Pines M and Spector I (2015) Halofuginone – the multifaceted molecule. Molecules 20, 573–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov Y, Patsenker E, Bauer M, Niedobitek E, Schulze-Krebs A and Schuppan D (2006) Halofuginone induces matrix metalloproteinases in rat hepatic stellate cells Via Activation of p38 and NFκB. Journal of Biological Chemistry 281, 15090–15098. [DOI] [PubMed] [Google Scholar]
- Shahiduzzaman M, Dyachenko V, Obwaller A, Unglaube S and Daugschies A (2009) Combination of cell culture and quantitative PCR for screening of drugs against Cryptosporidium parvum. Veterinary Parasitology 162, 271–277. [DOI] [PubMed] [Google Scholar]
- Shaw H (2014) Digest Paper – Cryptosporidiosis in calves, the economic impact and best control measures. British Cattle Breeders Club.
- Shaw HJ, Innes EA, Morrison LJ, Katzer F and Wells B (2020) Long-term production effects of clinical cryptosporidiosis in neonatal calves. International Journal for Parasitology 50, 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverlås C, Björkman C and Egenvall A (2009) Systematic review and meta-analyses of the effects of halofuginone against calf cryptosporidiosis. Preventive Veterinary Medicine 91, 73–84. [DOI] [PubMed] [Google Scholar]
- Taylor M and Bartram D (2012) The history of decoquinate in the control of coccidial infections in ruminants. Journal of Veterinary Pharmacology and Therapeutics 35, 417–427. [DOI] [PubMed] [Google Scholar]
- Thomson S, Hamilton CA, Hope JC, Katzer F, Mabbott NA, Morrison LJ and Innes EA (2017) Bovine cryptosporidiosis: impact, host-parasite interaction and control strategies. Veterinary Research 48, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson S, Innes EA, Jonsson NN and Katzer F (2019) Shedding of Cryptosporidium in calves and dams: evidence of re-infection and shedding of different gp60 subtypes. Parasitology 146, 1404–1413. [DOI] [PubMed] [Google Scholar]
- Toews LC (2017) Compliance of systematic reviews in veterinary journals with Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) literature search reporting guidelines. Journal of the Medical Library Association 105, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotz-Williams L, Jarvie B, Peregrine A, Duffield T and Leslie K (2011) Efficacy of halofuginone lactate in the prevention of cryptosporidiosis in dairy calves. Veterinary Record 168, 509–513. doi: 10.1136/vr.d1492 [DOI] [PubMed] [Google Scholar]
- Vélez J, Lange MK, Zieger P, Yoon I, Failing K and Bauer C (2019) Long-term use of yeast fermentation products in comparison to halofuginone for the control of cryptosporidiosis in neonatal calves. Veterinary Parasitology 269, 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen LC, Benders J, Medema G and Hofstra N (2017) Global Cryptosporidium loads from livestock manure. Environmental Science & Technology 51, 8663–8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villacorta I, Peeters JE, Vanopdenbosch E, Ares-Mazas E and Theys H (1991) Efficacy of halofuginone lactate against Cryptosporidium parvum in calves. Antimicrobial Agents and Chemotherapy 35, 283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells B and Thomson S (2014) Cryptosporidiosis in Cattle. Edinburgh, UK: Moredun Foundation. [Google Scholar]
- Wiedemann S, Prößler P, Möller-Holtkamp P, Kunz HJ and Kaske M (2012) Effects of prophylactic halofuginon administration during the first week of life on productivity and health of rearing calves. Tierarztliche Umschau 67, 339–344. [Google Scholar]
- Zambriski JA, Nydam DV, Wilcox ZJ, Bowman DD, Mohammed HO and Liotta JL (2013) Cryptosporidium parvum: determination of ID50 and the dose-response relationship in experimentally challenged dairy calves. Veterinary Parasitology 197, 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Sun L, Yang X-L and Schimmel P (2013) ATP-directed capture of bioactive herbal-based medicine on human tRNA synthetase. Nature 494, 121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182020002267.
click here to view supplementary material