Abstract
In order to elucidate the infection pathways of third stage larvae (L3) of Angiostrongylus cantonensis, we performed experiments to assess: (i) the shedding of L3 from two species of experimental veronicellid slugs drowned in water and the ratio of emerged larvae, (ii) the transmission of viable L3 from drowned terrestrial gastropods to aquatic snails, and (iii) the transmission of viable L3 between terrestrial snails. Molluscs were experimentally infected by first stage larvae (L1) of A. cantonensis. Significantly more L3 larvae were released from Veronicella cubensis than from Veronicella sloanei. Numerous L3 were observed in the muscular foot, and also in the connective tissue between internal organs. Experimental exposure of P. maculata to L3 of A. cantonensis liberated from other gastropod species led to their infection and the infectivity of larvae after intermediesis was demonstrated by infection of laboratory rats (Rattus norvegicus). The transmission of L3 was observed in three out of four experiment replications and L3 were retrieved from 6 out of 24 Subulina octona snails. The infected synanthropic molluscs represent a key component in the epidemiology of human infections by A. cantonensis. Escape of L3 larvae from bodies of dead snails or slugs and their ability to infect further gastropod hosts (intermediesis) represents a public health risk. Thus, control of molluscs living in peri-domestic environment is an essential part of prevention of human infections.
Key words: Angiostrongylus cantonensis, disease, emerging, experimental infection, intermediesis, zoonosis
Introduction
The majority of metastrongyloid nematodes (Strongylida: Metastrongyloidea) develop from the first (L1) to the third (infective) larval stage (L3) in various terrestrial and aquatic gastropods (Grewal et al., 2003; Morley, 2010). The host specificity of angiostrongylids for mollusc intermediate hosts is rather low and parasites tend to infect a range of snail and slug genera living in suitable habitats. Usually, the infection of the vertebrate hosts (including humans) occurs by ingestion of L3 through consumption of molluscs (obligate intermediate hosts) as well as of amphibians and reptiles as paratenic hosts (Mendoza et al., 2020) or crustaceans or arthropods as transport hosts. However, the importance of free-living L3 escaping the intermediate host either while alive or after its death was demonstrated for metastrongylids of carnivores (Giannelli et al., 2015; Conboy et al., 2017) and repeatedly discussed also in case of transmission of Angiostrongylus cantonensis to humans (reviewed by Cowie, 2013a). Indeed, when terrestrial gastropods drown in an aquatic environment, L3 may spontaneously emerge into the water, as in A. cantonensis (Cheng and Alicata, 1964; Crook et al., 1971; Kramer et al., 2018; Howe et al., 2019) and feline lungworms Aelurostrongylus abstrusus (Railliet, 1898) and Troglostrongylus brevior (Gerichter, 1949; Giannelli et al., 2015). In the case of A. cantonensis, the survival of L3 in water was investigated and their penetration into sources of drinking water was hypothesized in relation to the epidemiology of human infections in Hawaii (Howe et al., 2019). Meanwhile, the apparent survival of L3 in water had opened a range of questions about the fate of emerged infective-stage larvae of metastrongylids from a perspective of their biology and the life cycle plasticity. Colella et al. (2015) described the potential for horizontal transmission of L3 of A. abstrusus and T. brevior from experimentally infected to naive snails sharing the same environment, therefore proposing a term intermediesis for such a mode of transmission between intermediate hosts. However, no studies have confirmed the infectivity of L3 from the second intermediate host to the definitive host, therefore validating the intermediesis as an effective way of parasite transmission.
The present study aimed to investigate: (i) the shedding of L3 from two species of experimental veronicellid slugs drowned in water and the ratio of emerged larvae, (ii) the transmission of viable L3 from drowned terrestrial gastropods to aquatic snails (=intermediesis sensu Colella et al., 2015), (iii) transmission of viable L3 between terrestrial snails, and tested (iv) the infectivity of L3 for the definitive host (rat) following the intermediesis.
Materials and methods
The experimental strain of A. cantonensis was brought from Fatu Hiva, French Polynesia in 2017 and has been maintained in laboratory conditions, circulating among laboratory rats (Rattus norvegicus, Wistar strain), the experimental gastropods Subulina octona (Bruguière, 1789) and Veronicella spp. as an intermediate host. The identity of the isolate was confirmed based on the morphology of adult nematodes from infected rats as well as by sequencing cox1, with the haplotype identified as part of the A. cantonensis clade 2 (Červená et al., 2019).
Gastropods used in experiments, namely Veronicella sloanei (Cuvier, 1817), Veronicella cubensis (Pfeiffer, 1840), S. octona (Bruguière, 1789) and Pomacea cf. maculata (Perry, 1810; from Bangkok) from experimental colonies kept at the facilities of the Department of Pathology and Parasitology, University of Veterinary and Pharmaceutical Sciences Brno, Czech Republic, and Lissachatina fulica (Férusaac, 1821) purchased from private breeders. Molluscs were previously identified based on morphological features (Thomé, 1972) or their identification was accompanied by barcoding using 16S and 28S genes (Kim et al., 2016) to avoid ambiguities with identification in case of used Pomacea. The 16S and 28S sequences are deposited in the GenBank under accession numbers MT985557 and MT985558, respectively.
Shedding of larvae in slug drowning experiment and histology (experimental series A)
Subadults V. cubensis (n = 21; average weight 2.12 g) and V. sloanei (n = 22; average weight 1.69 g) were divided into five groups for each species (Table 1) housed in 2L plastic boxes, fed commercially grown lettuce and cucumber and fasted for three consecutive days prior the infection. The first three groups (single infection; A1, A2, A3) were fed over the course of four consecutive days with feces from an experimental rat heavily shedding A. cantonensis L1. Every day, unconsumed rat feces were removed and fresh ones were offered. To increase the loads of L3 and homogeneity of infection among individuals of the same group, an extended period of exposure to feces was implemented in two groups (continuous infection; A4, A5). Slugs in these two groups were fed feces with L1 from a heavily shedding infected rat over the course of four consecutive days in the same manner as previous groups; this was repeated during four consecutive weeks. The shedding and viability of L1 in rat feces was confirmed by a modified Baermann technique and microscopy.
Table 1.
Overview of groups and experimental design of drowning experiments (experimental series A); dpi = days post infection
| Experiment | Veronicella cubensis | Veronicella sloanei | |
|---|---|---|---|
| A1 | Single infection, examined 45 dpi | 4 | 4 |
| A2 | Single infection, histology 45 dpi | 2 | 3 |
| A3 | Single infection, examined 80 dpi | 5 | 5 |
| A4 | Continuous infection, examined 45 dpi | 5 | 5 |
| A5 | Continuous infection, examined 80 dpi | 5 | 5 |
Slugs were drowned at 45 days (A1, A2, A4) and 80 days (A3, A5) post infection (dpi). Prior to drowning, slugs in all groups were washed in lukewarm tap water to remove the soil. Slugs from groups A1, A3, A4 and A5 were placed individually in 50 mL falcon tubes fully filled with lukewarm tap water and tightly closed with a lid. The slugs were left to drown and decompose at room temperature (20°C). They were all removed from water at different time points (i.e. after 7, 22, 26, 30 and 46 h) each placed back into new falcon tubes filled with clean tap water to continue decomposing. At each time point, released larvae in the sediment of the previously occupied tube were counted under light microscope. Slugs were finally removed from tubes after 52 h and digested individually for 1 h at 37°C in digestion liquid (i.e. 0.3 g pepsin and 100 mL 0.7% HCl on a magnetic stirrer set to 600 RPM). The digested liquid was filtered through a sieve and centrifuged, and released L3 in the sediment were counted under the microscope. Slugs in group A2 were treated in a similar way, but fixed in buffered formaldehyde for histology. Snails of this group that were not drowned prior to the fixation were euthanized by submersion in 5% ethanol for at least 15 min or until their movement stopped. The sediment from ethanol solution was checked for the presence of L3. After at least 24 h fixation in 10% buffered formalin, bodies of slugs of the A2 groups were embedded in paraffin and 6 μm sections were cut transversally in four planes, stained with haematoxylin–eosin (H&E) for histology. For a summary of this experimental part, see Table 1.
GraphPad Prism 8.0.1 was used to perform the statistical analysis of the data. The Mann–Whitney two-tailed test was used to compare total numbers of released L3, and two-sided Fisher's exact test was used to compare the proportion of larvae released to water and the proportion of L3 released via digestion from V. cubensis and V. sloanei during the experimental series A.
Snail-to-snail transmission in an aquatic environment (experimental series B)
To investigate the infectivity of L3 in gastropods (i.e., intermediesis sensu Colella et al., 2015) in an aquatic environment, a series of five experiments (experiment B1–B5) was performed. Slugs (V. sloanei, n = 30, subadults, ~40–50 mm long when extended) and snails Lissachatina fulica (n = 10, subadults, ~50–65 mm shell height) were infected by daily ingestion of fresh faces of rats shedding the L1 larvae of the Fatu Hiva experimental strain of A. cantonensis over the course of 4 days. Forty to fifty days later, the L3 were released either by spontaneous emergence after snail drowning in 50 mL Falcon tube or by artificial digestion (as described above). Juvenile aquatic snails Pomacea cf. maculata (~25–30 mm shell height) originated from a captive colony established from a breeding stock from Thailand (n = 42) were kept in 3 L (B1–3, B5) and 10 L (B4) plastic aquaria with aeration and offered washed carrot and lettuce at the bottom of the tank throughout the experiments to encourage consumption of the L3. The water in aquaria was changed every second day. At the end of the experiment, the snails were euthanized and the number of L3 obtained from each snail was counted after individual digestion 14 days post infection (dpi) (experiments B1, B2, B4) or 22 dpi (experiment B3). A single adult female of Wistar rat was infected by 50 L3 obtained by digestion at the end of experiment B5 to test the infectivity of received L3 for the definitive host. For a summary of this experimental part, see Table 2.
Table 2.
Overview of water intermediasis experiments (experimental series B); larvae retrieved from snails in experiment B5 were used for infection of a control Wistar rat
| Experiment | Number of experimental P. maculata | Source of L3 larvae and how obtained | Method of infection | Euthanized and examined at dpi |
|---|---|---|---|---|
| B1 | 10 | V. sloanei, spontaneous emergence | 200 free L3 | 14 |
| B2 | 10 | A. fulica, spontaneous emergence | 500 free L3 | 14 |
| B3 | 5 | A. fulica, artificial digestion | 500 free L3 | 14 |
| B4 | 12 | A. fulica, N.A. | 12 snails drowned in exp. tank | 14 |
| B5 | 5 | A. fulica, artificial digestion | 10.000 free L3 | 22 |
Snail-to-snail transmission in terrestrial environment (experimental series C)
Two experiments were designed to test the snail-to-snail transmission of L3 from infected to naïve, susceptible gastropods in terrestrial experimental design. Terrestrial snails S. octona originated from a captive breeding colony established in 2018 from individuals obtained from a private mollusc breeder (10–15 mm shell height) served as a source of infective L3; they were fed feces with L1 from infected rats 35 days prior to the experiment.
Infected and co-habiting S. octona (n = 8) marked with paint were co-housed with uninfected specimens (n = 8), two with two, in four 250 mL containers (experiment C1). In experiment C2, mechanically killed and crushed infected snails were placed into 250 mL containers with uninfected snails (each container with two infected and six uninfected). All snails from a container were artificially digested at one of four different time-points (i.e., 1, 4, 8, 12 days) and examined for the presence of L3 as described above. The presence of alive L3 in every experimental setting was confirmed by digestion of infected snails (in case of the experiment C1) or remnant of dead ‘bait’ snails (the experiment C2).
Results
Drowning experiment
All experimental slugs that were submerged into water died within 3 h. In both slug species, we found the L3 actively escaping the body after drowning. This phenomenon was observed in all V. cubensis, but only in some V. sloanei. L3 larvae were not detected in the water in 4 of the 16 V. sloanei even though three of them were positive at the digestion; a single V. sloanei was negative following digestion. The numbers of L3 detected in individual slugs showed enormous variability (data shown in Supplementary material 1); thus, we present them as sums of larvae retrieved from all individuals of a group (Table 3). In general, more larvae were retrieved from slugs that were fed rat feces for extended periods, presumably because of a cumulative effect. However, the total number of larvae retrieved was significantly higher in V. cubensis (P < 0.0001). The dynamics of numbers of larvae that escaped drowned slugs in each interval is shown in Table 4.
Table 3.
Summarized data from the drowning experiment with V. cubensis and V. sloanei – the sum of L3 larvae released by all slugs in experimental groups A1–A5 and the percentage of larvae that spontaneously emerged into the water when compared to the total number of retrieved larvae (drowning and artificial digestion)
| A1 | A3 | A4 | A5 | ||
|---|---|---|---|---|---|
| Veronicella cubensis | Sum of L3 released by group (range in individuals) | 4156 (201–2507) | 13 736 (648–4446) | 32 550 (5684–10 127) | 30 750 (5118–8880) |
| Average % of L3 released to water (range in %) | 5.82 (3.48–7.01) | 4.6 (2.0–7.5) | 39.6 (34.7–47.9) | 2.60 (0.68–7.39) | |
| Veronicella sloanei | Sum of L3 released by group (range in individuals) | 253 (33–181) | 331 (2–251) | 1962 (8–1918) | 223 (0–206) |
| Average % of L3 released to water (range in %) | 22.1 (0–78.8) | 5.1 (0–37.5) | 43.1 (0–43.9) | 8.5 (0–33.3) |
Brackets show the maximum and minimum values recorded in individuals of a given experimental group of slugs.
Table 4.
The total number and percentage of larvae retrieved from all experimental slugs Veronicella cubensis and V. sloanei at 7, 22, 26, 30, 46 and 52 h
| Hours in water | V. cubensis (n = 17) | V. sloanei (n = 16) | ||
|---|---|---|---|---|
| Sum of larvae released into water | % of total larvae | Sum of larvae released into water | % of total larvae | |
| 0–7 | 1064 | 1.3 | 433 | 15.6 |
| 7–22 | 6437 | 7.9 | 336 | 12.1 |
| 22–26 | 3324 | 4.1 | 93 | 3.4 |
| 26–30 | 2179 | 2.7 | 32 | 1.2 |
| 30–46 | 1121 | 1.4 | 19 | 0.7 |
| 46–52 | 449 | 0.5 | 25 | 0.9 |
| Total L3 retrieved from water | 14 574 | 938 | ||
| Total L3 retrieved by digestion | 66 618 | 1831 | ||
| Total L3 retrieved | 81 192 | 2769 | ||
| Average L3 per snail | 4776.0 | 173.1 | ||
Total numbers of larvae retrieved by both used techniques (drowning and artificial digestion).
The total number of larvae that emerged spontaneously into the water was significantly higher in V. cubensis (P < 0.0001). In both Veronicella spp., a majority of larvae escaped from the snails within the first 26 h (i.e. 74.3% in V. cubensis and 91.9% in V. sloanei). However, the proportion of larvae that escaped from dead slugs and those retrieved in total differed significantly (P < 0.0001) between species, being higher in V. sloanei (34%) than in V. cubensis (18%).
Histology of V. cubensis and V. sloanei
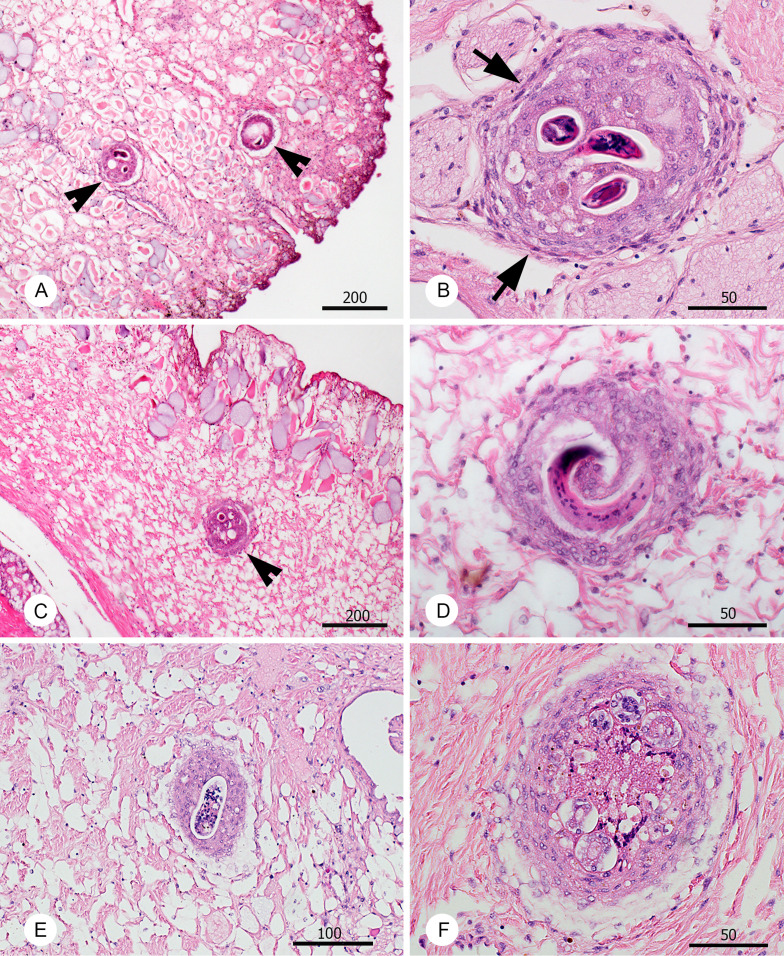
At the histological examination of slugs euthanized 45 dpi (exp. A2), numerous L3 were observed in the muscular foot. However, individual larvae were found also in the connective tissue between internal organs. There were differences in the viability of observed L3 in tissues of the two species. In the two V. cubensis individuals, all observed larvae (30 and 8) in histological sections were in well-demarked granulomas surrounded by organized connective tissue (fibroblasts) and none were lysed (Fig. 1A and B). However, in the three V. sloanei, the larvae (8, 22 and 11) were surrounded by proliferation of cellular tissue involving amoebocytes and few fibroblasts (Fig. 1C and D), giving the granulomas less demarcated appearance with diffuse margins. In addition, 37% of A. cantonensis larvae in V. sloanei were found lysed, surrounded by intense tissue reaction composed of a mixture of cell types (Fig. 1E and F).
Fig. 1.
Histopathological changes related to the presence of L3 larvae of A. cantonensis (arrowheads) in the tissues of experimental slugs infected in experiment A2 and examined 45 dpi. Veronicella cubensis (A–B) and V. sloanei (C–F). The nodules surrounding the L3 larvae in case of V. cubensis are surrounded by a layer of fibroblasts (arrows) and filled with mixed inflammatory cells (B). The nodules surrounding A. cantonensis L3 larvae in V. sloanei were less compact with minimum fibroblasts (D). Notably, numerous larvae were found dead in V. sloanei, partly (E) or almost entirely disintegrated (F). All scale bars in μm.
Snail-to-snail transmission in aquatic environment
Experimental exposure of P. maculata to L3 of A. cantonensis liberated from other gastropod species led to the infection of 18 out of 41 snails (Table 5). Transmission occurred in all but one experimental setting, regardless of the L3 source. Infection of the Wistar rat led to the presence of L1, 45 dpi and onwards, demonstrating the infectivity of the L3 exhibiting the intermediesis.
Table 5.
Number of live L3 retrieved by digestion from Pomacea maculata individuals previously exposed to L3 of Angiostrongylus cantonensis
| B1 | B2 | B3 | B4 | B5 | ||
|---|---|---|---|---|---|---|
| L3 dose per group | 200 | 500 | 500 | – | 10.000 | |
| L3 retrieval | Snail 1 | 0 | 1 | 0 | 0 | 3 |
| Snail 2 | 2 | 2 | 0 | 0 | 19 | |
| Snail 3 | 0 | 1 | 0 | 1 | 16 | |
| Snail 4 | 0 | 2 | 0 | 0 | 10 | |
| Snail 5 | 0 | 1 | - | 0 | 6 | |
| Snail 6 | 0 | 3 | 0 | |||
| Snail 7 | 2 | 1 | 1 | |||
| Snail 8 | 0 | 0 | 0 | |||
| Snail 9 | 0 | 1 | 0 | |||
| Snail 10 | 1 | 0 | 0 | |||
| Snail 11 | 0 | |||||
| Snail 12 | 0 | |||||
| Total | 5 | 12 | 0 | 2 | 54 | |
One snail from the exp. B3 died before the examination. The dose of L3 is not available for exp. B4, as the infection was mimicking the natural method of exposure without the possibility of counting the larvae released from drowned snails.
Snail-to-snail transmission in terrestrial environment
None of the naïve S. octona snails that were co-habiting with infected snails (experiment C1) were infected by A. cantonensis. We observed experimental naïve S. octona snails being attracted by the crushed bodies of previously infected snails (experiment C2) in all four experimental groups. Transmission of L3 was observed in three out of four experiment replicates and live L3 were retrieved from 6 out of 24 experimental S. octona snails (Table 6).
Table 6.
Results of experiment C2 – live L3 larvae counted after digestion from individual land snails Subulina octona in group examined 1, 4, 8 and 12 days after contact with two crushed infected S. octona snails
| Group | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| Examined at (days post exposure) | 1 | 4 | 8 | 12 | |
| L3 retrieval | Snail 1 | 0 | 0 | 2 | 0 |
| Snail 2 | 0 | 3 | 0 | 0 | |
| Snail 3 | 0 | 0 | 7 | 1 | |
| Snail 4 | 0 | 9 | 0 | 0 | |
| Snail 5 | 0 | 0 | 0 | 0 | |
| Snail 6 | 0 | 9 | 0 | 0 | |
| Total larvae | 0 | 21 | 9 | 2 | |
| Larvae in remnants | 148 | 46 | 24 | 19 | |
The remnants of dead Subulina used as a bait were examined at the same time as the experimental snails to prove the presence of L3 in the bait.
Discussion
In this study, we demonstrated that A. cantonensis escape from bodies of dead snails or slugs, potentially infecting other molluscs both in aquatic and terrestrial system, ultimately causing patent infection in definitive hosts. Indeed, gastropods are intermediate hosts of the majority of metastrongylid nematodes with a heteroxenous life cycle (Anderson, 2000; Grewal et al., 2003; Morley, 2010). Compared to other metastrongyloids, A. cantonensis infects a much broader spectrum of hosts other than the typical definitive (rodent) or intermediate (mollusc) hosts, which play an important role in the natural life cycle (Barratt et al., 2016), as well as being important as a source of human infection (reviewed in Mendoza et al., 2020). The complexity of the life cycle of A. cantonensis is reflected in huge geographic differences in the occurrence of eosinophilic meningitis in humans and other homoeothermic vertebrates. Thus, the epidemiology of the disease depends, among other factors, on the spectrum, abundance and ecology of the native and invasive definitive, intermediate and paratenic hosts as well as on human culture, culinary habits and the awareness level. However, in many situations, the source of the infection remains unknown (Epelboin et al., 2016; Dard et al., 2020) and alternative transmission pathways are a neglected part of the ecology of A. cantonensis.
We showed that L3 emerged from drowned slugs (the majority within the first 26 h) and those that remained in the slug tissue may survive long after its death. Detection of the largest number of larvae in water within the first 26 h is of practical importance as it may enhance the risk for human infection, as happened following drinking a traditional drink without realizing that there were drowned gastropods in the bottom of the container or as a result of drinking from garden hoses in which gastropods have taken refuge (Howe et al., 2019).
Dead snails may also represent a potential risk for human infection as indicated by L3 remaining alive in the tissue of intermediate hosts even 50 h after their death (Table 4). The differences in total numbers of L3 detected in experimentally infected V. cubensis and V. sloanei as well as in the percentage of larvae that spontaneously emerged could possibly be explained by different immune reactions of the slug species to A. cantonensis. In the case of V. cubensis, the nodules were well demarcated by fibroblasts, as for example in the infection case of infection of L. palustris (Held, 1836) (Rachford, 1976), and contained live larvae. In contrast, nodules containing L3 in tissues of V. sloanei were less separated from surrounding tissues with some of the larvae dead and in various stages of decomposition. Accordingly, previous field and experimental studies proved differing prevalence of A. cantonensis in various mollusc species as well as remarkable differences in numbers of larvae retrieved from their tissues (e.g. Richards and Merritt, 1967; Cowie, 2013a; Kim et al., 2014). The differences observed herein in the rate of spontaneous emergence of larvae might be caused by their firmer fixation in nodules in V. cubensis. Accordingly, the differences in total numbers of larvae retrieved between the two experimental slug species could relate to a significant proportion of dead and degraded larvae in V. sloanei.
The spontaneous release of L3 larvae of A. cantonensis from dead intermediate hosts and their extended survival in aquatic environment prompt a range of questions regarding their further fate. Thanks to its easy to maintain laboratory-based life cycle, A. cantonensis belongs among the most studied heteroxenous nematodes, and its life cycle is well described. Nonetheless, little is known about the circulation of A. cantonensis in the environment and about the routes of infection of rats in natural ecosystems. Limited experimental evidence with wild rats showed the inability of black rats to ingest larger gastropods (Noda et al., 1987). It is possible that free L3 larvae play a role not only in the epidemiology of human infections (Howe et al., 2019) but also in the infection of definitive rodent hosts. The differences between the two veronicellid slugs add to the knowledge of interspecific differences in the prevalence and abundance among mollusc species and suggest that fine aspects of the life cycle as well as local epidemiology of A. cantonensis infections depend on the composition of gastropod communities, as demonstrated in the case of Parmarion martensi (Simroth, 1893) on Hawaii when compared to other terrestrial gastropods in the same region (Jarvi et al., 2012; Kim et al., 2014).
Previous studies on metastrongyloids parasitizing carnivores (A. abstrusus and T. brevior) have demonstrated the ability of snail-derived L3 larvae to infect other gastropods, and this infection pathway was termed intermediesis (Colella et al., 2015). In our series of experiments, we demonstrated the ability of A. cantonensis L3 larvae to infect snails in aquatic environment. However, in a terrestrial experimental system involving S. octona, the infection was achieved only by the consumption of infected dead snails. Similar infection route was described in case of predatory Euglandina rosea (Campbell and Little, 1988). We did not demonstrate the infection by simple co-habitation of naïve and infected snails, which contrast to previous studies (Colella et al., 2015). Survival of A. cantonensis L3 in humid terrestrial environment and their further transmission deserves more experimental investigation, involving elaborated experimental design and more gastropod species. Undoubtedly, infected synanthropic molluscs represent a key component in the epidemiology of human infections by A. cantonensis in areas where the terrestrial and aquatic snails, crustaceans or poikilothermic vertebrates are not intentionally consumed, such as in the Caribbean region and Hawaii (Dard et al., 2020; Cowie, 2013b). Local control of molluscs living in peri-domestic environment is an essential part of prevention of human infections in these regions (Hollingsworth et al., 2013). Escape of numerous L3 larvae from bodies of dead snails or slugs and their ability to infect further mollusc hosts via intermediesis has obvious practical implications. Although this may sound trivial, collecting and immediate removal of snails and slugs probably has a much bigger impact than simply killing them in situ.
Acknowledgements
The experimental work that includes the infection of rats was accredited according to Czech law Acts No. 246/1992, 419/2012 and 166/1999, as well as the European law of Council Regulation (EC) No 1099/2009. We are more than indebted to anonymous reviewers for constructive comments.
Ethical standards
Part of the study involving rats was authorized by the ethical committee for use of experimental animals at UVPS Brno (approval No. No. 40-2017) prior to the experiment. The authors have involved the minimum number of animals to produce statistically reproducible results.
Financial support
The study was, in part, supported by grants IGA VFU No. 123/2018/FVL and ITA VFU No. FVL/Celer/ITA 2020.
Conflicts of interest
The authors declare they have no conflicts of interest.
References
- Anderson RC (2000) Nematode Parasites of Vertebrates. Their Development and Transmission, 2nd Edn. Wallingford, Oxon (UK): CABI Publishing, 650 pp. [Google Scholar]
- Barratt J, Chan D, Sandaradura I, Malik R, Spielman D, Lee R, Mariott D, Harkness J, Ellis J and Stark D (2016) Angiostrongylus cantonensis: a review of its distribution, molecular biology and clinical significance as a human pathogen. Parasitology 143, 1087–1118. [DOI] [PubMed] [Google Scholar]
- Campbell BG and Little MD (1988) The finding of Angiostrongylus cantonensis in rats in new Orleans. American Journal of Tropical Medicine and Hygiene 38, 568–573. [DOI] [PubMed] [Google Scholar]
- Červená B, Modrý D, Fecková B, Hrazdilová K, Foronda P, Alonso AM, Lee R, Walker J, Niebuhr CN, Malik R and Šlapeta J (2019) Low diversity of Angiostrongylus cantonensis complete mitochondrial DNA sequences from Australia, Hawaii, French Polynesia and the Canary Islands revealed using whole genome next-generation sequencing. Parasites and Vectors 12, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng TC and Alicata JE (1964) Possible role of water in the transmission of Angiostrongylus cantonensis (Nematoda: Metastrongylidae). The Journal of Parasitology 50, 39–40. [Google Scholar]
- Colella V, Giannelli A, Brianti E, Ramos RAN, Cantacessi C, Dantas-Torres F and Otranto D (2015) Feline lungworms unlock a novel mode of parasite transmission. Scientific Reports 5, 13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy G, Guselle N and Schaper R (2017) Spontaneous shedding of metastrongyloid third-stage larvae by experimentally infected Limax maximus. Parasitology Research 116, 41–54. [DOI] [PubMed] [Google Scholar]
- Cowie RH (2013a) Biology, systematics, life cycle, and distribution of Angiostrongylus cantonensis, the cause of rat lungworm disease. Hawai'i Journal of Medicine & Public Health: A Journal of Asia Pacific Medicine & Public Health 72, 6–9. [PMC free article] [PubMed] [Google Scholar]
- Cowie RH (2013b) Pathways for transmission of angiostrongyliasis and the risk of disease associated with them. Hawai'i Journal of Medicine & Public Health: A Journal of Asia Pacific Medicine & Public Health 72, 70–74. [PMC free article] [PubMed] [Google Scholar]
- Crook JR, Fulton SE and Supanwong K (1971) The infectivity of third stage Angiostrongylus cantonensis larvae shed from drowned Achatina fulica snails and the effect of chemical agents on infectivity. Transactions of the Royal Society of Tropical Medicine and Hygiene 65, 602–605. [DOI] [PubMed] [Google Scholar]
- Dard C, Tessier E, Nguyen D, Epelboin L, Harrois D, Swale C, Cabié A, de Meuron K, Miossec C and Desbois-Nogard N (2020) First cases of Angiostrongylus cantonensis infection reported in Martinique 2002–2017. Parasite 27, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelboin L, Blondé R, Chamouine A, Chrisment A, Diancourt L, Villemant N, Atale A, Cadix C, Caro V, Malvy D and Collet L (2016) Angiostrongylus cantonensis infection on Mayotte Island, Indian Ocean, 2007–2012. PLoS Neglected Tropical Diseases 10, e0004635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannelli A, Colella V, Abramo F, do Nascimento Ramos RA, Falsone L, Brianti E, Varcasia A, Dantas-Torres F, Knaus M, Fox MT and Otranto D (2015) Release of lungworm larvae from snails in the environment: potential for alternative transmission pathways. PLoS Neglected Tropical Diseases 9, e0003722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal PS, Grewal SK, Tan L and Adams BJ (2003) Parasitism of molluscs by nematodes: types of associations and evolutionary trends. Journal of Nematology 35, 146–156. [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth RG, Howe K and Jarvi SI (2013) Control measures for slug and snail hosts of Angiostrongylus cantonensis, with special reference to the semi-slug Parmarion martensi. Hawai'i Journal of Medicine & Public Health: A Journal of Asia Pacific Medicine & Public Health 72, 75–80. [PMC free article] [PubMed] [Google Scholar]
- Howe K, Kaluna L, Lozano A, Torres Fischer B, Tagami Y, McHugh R and Jarvi S (2019) Water transmission potential of Angiostrongylus cantonensis: larval viability and effectiveness of rainwater catchment sediment filters. PLoS ONE 14, e0209813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvi SI, Farias MEM, Howe K, Jacquier S, Hollingsworth R and Pitt W (2012) Quantitative PCR estimates Angiostrongylus cantonensis (rat lungworm) infection levels in semi-slugs (Parmarion martensi). Molecular and Biochemical Parasitology 185, 174–176. 10.1016/j.molbiopara.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JR, Hayes KA, Yeung NW and Cowie RH (2014) Diverse gastropod hosts of Angiostrongylus cantonensis, the rat lungworm, globally and with a focus on the Hawaiian islands. PLoS ONE 9, e94969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JR, Hayes KA, Yeung NW and Cowie RH (2016) Identity and distribution of introduced slugs (Veronicellidae) in the Hawaiian and Samoan Islands. Pacific Science 70, 477–493. [Google Scholar]
- Kramer K, Posner J and Gosnell W (2018) The role of gastropod mucus in the transmission of Angiostrongylus cantonensis, a potentially serious neurological infection. ACS Chemical Neuroscience 9, 629–632. [DOI] [PubMed] [Google Scholar]
- Mendoza-Roldan JA, Modrý D and Otranto D (2020) Zoonotic parasites of reptiles: a crawling threat. Trends in Parasitology 36, 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley N (2010) Aquatic molluscs as auxiliary hosts for terrestrial nematode parasites: implications for pathogen transmission in a changing climate. Parasitology 137, 1041–1056. [DOI] [PubMed] [Google Scholar]
- Noda S, Uchikawa R, Matayoshi S, Watanabe Y and Sato A (1987) Observations on the transmission of Angiostrongylus cantonensis from snail to rodent. Journal of Helminthology 61, 241–246. [DOI] [PubMed] [Google Scholar]
- Rachford FW (1976) Host-parasite relationship of Angiostrongylus cantonensis in Lymnaea palustris. II. Histopathology. Experimental Parasitology 39, 382–392. [DOI] [PubMed] [Google Scholar]
- Richards CS and Merritt JW (1967) Studies on Angiostrongylus cantonensis In molluscan intermediate hosts. The Journal of Parasitology 53, 382. [PubMed] [Google Scholar]
- Thomé JW (1972) Redescrição dos tipos de Veronicellidae (Mollusca, Gastropoda) neotropicais: VIII. Espécies depositadas no ‘Institut für Spezielle Zoologie und zoologisches Museum’ de Berlim, Alemanha Oriental. Arquivos de Zoologia 21, 235–281. [Google Scholar]