Abstract
Background
Although accumulating data indicate that IL‐6 (interleukin‐6) can promote heart rate‐corrected QT interval (QTc) prolongation via direct and indirect effects on cardiac electrophysiology, current evidence comes from basic investigations and small clinical studies only. Therefore, IL‐6 is still largely ignored in the clinical management of long‐QT syndrome and related arrhythmias. The aim of this study was to estimate the risk of QTc prolongation associated with elevated IL‐6 levels in a large population of unselected subjects.
Methods and Results
An observational study using the Veterans Affairs Informatics and Computing Infrastructure was performed. Participants were US veterans who had an ECG and were tested for IL‐6. Descriptive statistics and univariate and multivariate regression analyses were performed to study the relationship between IL‐6 and QTc prolongation risk. Study population comprised 1085 individuals, 306 showing normal (<5 pg/mL), 376 moderately high (5–25 pg/mL), and 403 high (>25 pg/mL) IL‐6 levels. Subjects with elevated IL‐6 showed a concentration‐dependent increase in the prevalence of QTc prolongation, and those presenting with QTc prolongation exhibited higher circulating IL‐6 levels. Stepwise multivariate regression analyses demonstrated that increased IL‐6 level was significantly associated with a risk of QTc prolongation up to 2 times the odds of the reference category of QTc (e.g. QTc >470 ms men/480 ms women ms: odds ratio, 2.28 [95% CI, 1.12–4.50] for IL‐6 >25 pg/mL) regardless of the underlying cause. Specifically, the mean QTc increase observed in the presence of elevated IL‐6 was quantitatively comparable (IL‐6 >25 pg/mL:+6.7 ms) to that of major recognized QT‐prolonging risk factors, such as hypokalemia and history of myocardial infarction.
Conclusions
Our data provide evidence that a high circulating IL‐6 level is a robust risk factor for QTc prolongation in a large cohort of US veterans, supporting a potentially important arrhythmogenic role for this cytokine in the general population.
Keywords: arrhythmic risk, general population, IL‐6, QTc prolongation
Subject Categories: Arrhythmias, Sudden Cardiac Death
Nonstandard Abbreviations and Acronyms
- APD
action potential duration
- LQTS
long QT‐syndrome
- QRS
QRS interval
- QTc
heart rate‐corrected QT interval
- TdP
torsades de pointes
- VINCI
Veterans Affairs Informatics and Computing Infrastructure
Clinical Perspective.
What Is New?
In a large cohort of US veterans, subjects with elevated interleukin‐6 (IL‐6) levels showed a concentration‐dependent increase in the prevalence of heart rate‐corrected QT interval (QTc) prolongation; moreover, those presenting with QTc prolongation exhibited higher levels of circulating IL‐6.
IL‐6 level increase was significantly associated with an up to ≈2 times higher risk of QTc prolongation, regardless of the underlying cause.
The mean QTc increase observed in the presence of elevated IL‐6 was quantitatively comparable to that of major recognized QT‐prolonging risk factors, such as hypokalemia and history of myocardial infarction.
What Are the Clinical Implications?
Our data provide evidence that an elevated circulating IL‐6 level is an independent and robust risk factor for QTc prolongation in a large cohort of US veterans, supporting a potentially important arrhythmogenic role for this cytokine in the general population.
It is recommended that inflammatory markers, specifically IL‐6, should be included the clinical management of patients with long QT syndrome/torsades de pointes, also because cytokine‐targeting therapies are increasingly recognized as an essential tool to achieve a comprehensive treatment of cardiovascular disease, possibly including cardiac arrhythmias.
The prolongation of heart rate‐corrected QT interval (QTc) on the surface ECG, representing a surrogate of ventricular action potential duration (APD), is recognized to be an important risk factor for life‐threatening ventricular arrhythmias, particularly torsades de pointes (TdP) ventricular tachycardia, and sudden cardiac death in the general population. 1 , 2 This clinical condition, also known as long‐QT syndrome (LQTS), can be the result of a wide number of causative factors, inherited or acquired, in all cases responsible for a dysfunction of 1 or more of the ion channels which determine ventricular APD, specifically a decrease of potassium (K+) channel outward repolarizing currents or an enhancement of sodium (Na+) and calcium (Ca++) channel inward depolarizing currents. 2 Although congenital LQTS is not common, affecting ≈1:2000 apparently healthy newborns, 3 acquired LQTS is frequently observed in clinical practice occurring in up to 25% to 30% of hospitalized patients. 4 Electrolyte imbalances, specifically hypokalemia, hypocalcemia, and hypomagnesemia, medications blocking the hERG (human‐ether‐à‐go‐go)‐related gene‐K+ channel, and structural heart diseases are the most commonly involved recognized causes of acquired LQTS. 2
Although numerous, it is ever more evident that these and other less common conventional risk factors 2 are not able to explain all cases of LQTS/TdP observed in the general population. 5 In this regard, immune‐inflammatory activation is increasingly emerging in the recent years as a novel nonconventional QT‐prolonging risk factor, 6 , 7 , 8 , 9 , 10 whose relevance was dramatically understood worldwide during COVID‐19 pandemic. 11 In fact, an accumulating body of basic and clinical studies has demonstrated that systemic inflammation can actively promote QTc prolongation, as a result of a number of direct and indirect prolonging effects of inflammatory cytokines on ventricular APD. 12 A particularly important role seems to be played by IL‐6 (interleukin‐6), 12 a pleiotropic cytokine that is central in most of the inflammatory reactions, either infective, immune mediated, or other. 13
Although the aforementioned data point to IL‐6 as a novel and potentially important QT‐prolonging risk factor, its actual impact on the general population is unknown, as clinical evidence presently comes from studies involving relatively small samples or selected patients only. 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 As a result, inflammatory markers, including IL‐6, are still largely ignored in the diagnostic and therapeutic management of LQTS/TdP, and more in general of cardiac arrhythmias. 12 This may be due, at least in part, to the fact that no cohort studies investigating the relationship between QTc prolongation and IL‐6 levels in the general population are currently available. Nevertheless, large investigations demonstrated that higher IL‐6 levels predicted sudden cardiac death in unselected individuals, 22 , 23 and ventricular tachycardia/ventricular fibrillation in recipients of implantable cardioverter‐defibrillators. 24 Based on such evidence, it is reasonable to hypothesize that these associations may be mediated, at least in part, by the aptitude of IL‐6 to promote QTc prolongation, thereby increasing the risk of QT‐associated life‐threatening ventricular arrhythmias such as TdP.
To fill this gap in knowledge, we here performed an observational cohort study using US Veterans Affairs Informatics and Computing Infrastructure to evaluate whether high IL‐6 levels are associated with an increased risk of QTc prolongation in a large population of subjects.
Methods
The authors declare that all supporting data are available within the article (and its online supplementary files).
Study Design and Setting
We performed a cross‐sectional study based on Veterans Affairs Informatics and Computing Infrastructure, an analytic research platform using data collected from 1400 US veterans' points of care. The Veterans Affairs Informatics and Computing Infrastructure Clinical Data Warehouse, which is a relational database, was interrogated using structured query language. Logical Observation Identifiers Names and Codes were used to identify all subjects who have been tested for circulating IL‐6 levels and who had at least 1 ECG from January 2003 to December 2023. By using a unique subject identifier, the following data were obtained: demographic data, laboratory data including IL‐6, electrolytes (potassium, calcium, magnesium), and anti‐Ro/Sjögren's‐syndrome‐related antigen A antibody positivity, medications, including QT interval prolonging drugs, cardiovascular risk factors and diseases, and ECG parameters (heart rate, RR interval, QRS interval, QTc). QTc was automatically measured using Bazett's formula [QT/RR interval1/2] and then confirmed by a cardiologist who was independent of the study and blinded to IL‐6 levels. Patient‐related ECG parameters and laboratory values were computed by averaging multiple available measurements. Specifically, the mean number of measurements was 8.0 and 1.3 for ECG and IL‐6 testing, respectively.
Inclusion and Exclusion Criteria
All subjects who were tested for IL‐6 and underwent ECG were included in the study. Exclusion criteria were a QRS interval >120 ms (complete bundle‐branch block, ventricular paced rhythm), profound brady‐ and tachycardia <50 bpm and >120 bpm, respectively, or the unavailability of a quantitative result for IL‐6 levels. Patients with atrial fibrillation were not excluded as multiple RR intervals were averaged.
IL‐6 Level Assessment
Circulating IL‐6 levels were measured by quantitative ELISA. In most cases, the results were provided quantitatively (pg/mL), whereas a qualitative result only (normal: <5 pg/mL; elevated: ≥5 pg/mL) was available in a small set of subjects (8%). IL‐6 levels were defined as normal when <5 pg/mL (as it was largely the most common cutoff used throughout the different US veterans points of care), and then the elevated interval (≥5 pg/mL) was further divided in moderately high (5–25 pg/mL), and high (>25 pg/mL) according to the median of this range. In this way, we were able to divide the population into 3 numerically well‐balanced groups.
Repeated IL‐6 measurements were available for 196 subjects, in most cases showing stable levels over time. In fact, in ≈2/3 of cases (132 subjects, 67.3%) IL‐6 remained in the same concentration range. Moreover, in the other one‐third of subjects the IL‐6 level changed always toward the nearest range.
It should be noted that >60% of IL‐6 measurements were performed in the past few years, that is, in the period 2020 to 2023, because before the COVID‐19 pandemic IL‐6 testing was available for routine clinical assessment in only a few institutions. This aspect was a key determinant of the study size.
Definition of QTc Prolongation
The presence of QTc prolongation was defined following the recommendations by the American Heart Association, American College of Cardiology, Heart Rhythm Society, and European regulatory guidelines. 2 , 25 , 26 Specifically, 4 different, progressively more stringent cutoffs were employed: (1) >440 ms regardless of sex, representing the classic cutoff for QTc prolongation, although this value is exceeded by up to ≈20% of the general population; 2 (2) >450 ms in men or >470 in women; 26 (3) >470 ms in men or >480 in women, representing approximate 99th percentile QTc values for otherwise healthy postpubertal individuals; 2 (4) >500 ms, which is defined by American Heart Association/American College of Cardiology as marked or highly abnormal QTc prolongation and recognized to be associated with a significant arrhythmic risk in both sexes. 2
Statistical Analysis
Descriptive statistics was carried out and quantitative variables were summarized with mean and SD or with median and interquartile range, and qualitative variables with absolute frequency and percentages. Kolmogorov–Smirnov test was applied to verify normality distribution. When normality was refused, nonparametric tests were used to compare groups. Specifically, to compare patients with or without QTc prolongation based on different cutoffs, the t test or the Mann–Whitney U test were used. To compare the 3 groups of subjects stratified by circulating IL‐6 levels, ANOVA or Kruskal–Wallis test were used. Post hoc tests with Holm correction were then carried out. Chi‐square test was applied to 2×2 contingency tables to evaluate the association between frequencies of qualitative variables.
Multivariate stepwise logistic regression analysis was carried out to identify, among all risk factors included in the model, a statistically significant minimum subset of factors with the highest possible accuracy to predict prolonged QTc. Odds ratios (ORs) and their 95% CI were calculated for significant variables. In addition, a stepwise linear regression was estimated to predict the average QTc. Coefficients and their 95% CI were calculated for significant variables. In both cases the stepwise procedure was a backward‐forward stepwise based on Akaike information criteria. A statistical significance level of 95% (P<0.05) was always considered. R software, version 4.1.1, was used for all statistical computations.
Ethics
The data were deidentified and the study was approved by the Veterans Affairs New York Harbor Healthcare System Institutional Review Board under ID#01810.
Results
Characteristics of the Study Population
In the database, we identified 1652 subjects who underwent both ECG and IL‐6 testing. Among them, 567 were excluded for having complete bundle‐branch block, ventricular paced rhythm, profound brady‐ or tachycardia, or because quantitative IL‐6 level assessment was not available. The resulting study population consisted of 1085 subjects, primarily males (92.0%) and White (66.0%), with a median age of 71 years (Table 1). Depending on the specific cutoff considered, a prolonged QTc was present in 49.4% (QTc >440 ms; n=536), 31.2% (QTc >450 ms men/470 ms women; n=338), 11.2% (QTc >470 ms men/480 ms women; n=122), and 1.7% (QTc >500 ms; n=18) of subjects, respectively (Table 1). Median IL‐6 level was 12.7 pg/mL, with 28.2% of the subjects having normal levels (<5 pg/mL), 34.7% moderately high levels (5–25 pg/mL), and 37.1% high levels (>25 mg/dL) (Table 1). Although circulating anti‐Sjögren's syndrome‐related antigen A‐antibodies are increasingly recognized as a nonconventional risk factor for QTc prolongation in the general population, 27 , 28 , 29 they were tested in only 121 out of 1085 subjects, and 5 of them (4.1%) showed a positive result. Given the high percentage of missing values, anti‐Sjögren's syndrome‐related antigen A‐antibody positivity was not considered in this study but has been previously reported. 27
Table 1.
Demographic and Health Characteristics of the Overall Study Population
| Characteristic | |
|---|---|
| Total, n | 1085 |
| Demographics | |
| Age, y | 71 [63–75] |
| Male sex, n | 998 (92.0%) |
| White race, n | 716 (66.0%) |
| Black race, n | 291 (26.8%) |
| Other race, n‡ | 78 (7.2%) |
| ECG findings | |
| QTc, ms | 439 [421–456] |
| Heart rate, bpm | 79 [70–90] |
| QRS interval, ms | 89 [82–97] |
| QTc >440 ms, n | 536 (49.4%) |
| QTc >450 ms men/>470 ms women, n | 338 (31.2%) |
| QTc >470 ms men/>480 ms women, n | 122 (11.2%) |
| QTc >500 ms, n | 18 (1.7%) |
| Laboratory data | |
| Potassium, mEq/l | 4.2 [4.0–4.4] |
| Potassium <4.0 mEq/L, n | 236 (25.2%) |
| Calcium, mg/dL | 9.2 [8.9–9.4] |
| Calcium <8.5 mg/dL, n | 51 (5.5%) |
| Magnesium, mg/dL | 2.0 [1.9–2.1] |
| Magnesium <1.8 mg/dL, n | 107 (11.5%) |
| IL‐6, pg/mL | 12.7 [4.4–47.2] |
| IL‐6 <5 pg/mL, n | 306 (28.2%) |
| IL‐6 5–25 pg/mL, n | 376 (34.7%) |
| IL‐6 >25 pg/mL, n | 403 (37.1%) |
| Anti‐Ro/Sjögren's syndrome‐related antigen A antibody positivity, n* | 5 (4.1%) |
| Cardiovascular risk factors/diseases | |
| Smoking, n | 471 (43.4%) |
| Hypertension, n | 256 (23.6%) |
| Diabetes mellitus, n | 614 (56.6%) |
| Myocardial infarction, n | 252 (23.2%) |
| Angina pectoris, n | 204 (18.8%) |
| Left ventricular hypertrophy/heart failure, n | 134 (12.4%) |
| Autoimmune rheumatic diseases, n† | 92 (8.5%) |
| Drugs | |
| Alpha blockers, n | 675 (62.2%) |
| Anesthetics, n | 94 (8.7%) |
| Antianginal, n | 335 (30.9%) |
| Antiarrhythmics, n | 121 (11.2%) |
| Antibiotics, n | 1054 (97.1%) |
| Anticonvulsants, n | 774 (71.3%) |
| Antidepressants, n | 851 (78.4%) |
| Antiemetics, n | 603 (55.6%) |
| Antifungals, n | 375 (34.6%) |
| Antihistamines, n | 868 (80.0%) |
| Antihypertensives, n | 458 (42.2%) |
| Antimalarials, n | 160 (14.7%) |
| Antipsychotics, n | 409 (37.7%) |
| Antivirals, n | 453 (41.8%) |
| Beta blockers, n | 734 (67.6%) |
| Bronchodilators, n | 732 (67.5%) |
| Calcium blockers, n | 681 (62.8%) |
| Diuretics, n | 787 (72.5%) |
| Muscle relaxants, n | 726 (66.9%) |
| Sedatives, n | 751 (69.2%) |
Values are expressed as median [interquartile range], or frequency count (percentage). IL‐6 indicates interleukin‐6; and QTc, heart rate‐corrected QT‐interval.
Data available for only 121 subjects.
Including rheumatoid arthritis, systemic lupus erythematosus, Sjögren's syndrome, systemic sclerosis, and other connective tissue diseases.
Other includes: Asian, American Indian, Alaska native, Hawaiian or other Pacific Islander, and unknown.
As shown in Tables 2 and 3 and Table S1, several variables were found to be significantly associated with the risk of QTc prolongation throughout the different cutoffs or increased IL‐6 levels in the whole population, including demographic factors (age, sex), ECG findings (heart rate, QRS), laboratory data (potassium, calcium), cardiovascular risk factors/diseases (history of hypertension, myocardial infarction, left ventricular hypertrophy/heart failure), and medications (alpha blockers, anesthetics, antiarrhythmics, antiemetics, antihypertensives, antipsychotics, antivirals, beta blockers, bronchodilators, calcium blockers, diuretics, and sedatives).
Table 2.
Demographic, ECG, Laboratory, and Clinical Findings in Subjects With or Without QTc Prolongation Based on Different Cutoffs
| Cutoff 440 ms | Cutoff 450 ms men/470 ms women | Cutoff 470 ms men/480 ms women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | QTc <440 | QTc >440 | P value | QTc <450/470 | QTc >450/470 | P value | QTc <470/480 | QTc >470/480 | P value |
| Total, n | 549 (50.6%) | 536 (49.4%) | … | 747 (68.8%) | 338 (31.2%) | … | 963 (88.8%) | 122 (11.2%) | … |
| Demographics | |||||||||
| Age, y | 70 [63–75] | 71 [63–76] | 0.556 | 70 [63–75] | 71 [63–76] | 0.367 | 71 [63–75] | 70 [64–76] | 0.601 |
| Male sex, n | 518 (94.4%)* | 480 (89.6%)* | 0.005* | 676 (90.5%)* | 322 (95.3%)* | 0.010* | 881 (91.5%) | 117 (95.9%) | 0.130 |
| Race, n | |||||||||
| White | 355 (64.7%) | 361 (67.4%) | 0.568 | 499 (66.8%) | 217 (64.2%) | 0.700 | 647 (67.2%) | 69 (56.6%) | 0.016* |
| Black | 151 (27.5%) | 140 (36.1%) | 196 (26.2%) | 95 (28.1%) | 245 (25.4%) | 46 (37.7%) | |||
| Others† | 43 (7.8%) | 35 (6.5%) | 52 (7.0%) | 26 (7.7%) | 71 (7.4%) | 7 (5.7%) | |||
| ECG findings | |||||||||
| Heart rate, bpm | 74 [66–86]* | 83 [74–93]* | <0.001* | 76 [68–87]* | 84 [74–93]* | <0.001* | 79 [69–89]* | 84 [76–93]* | <0.001* |
| QRS interval, ms | 88 [82–94]* | 91 [84–100]* | <0.001* | 88 [82–96]* | 92 [84–101]* | <0.001* | 88 [82–96]* | 94 [86–104]* | <0.001* |
| Laboratory data | |||||||||
| Potassium <4.0 mEq/L, n | 82 (18.1%)* | 154 (32.0%)* | <0.001* | 135 (21.5%)* | 101 (33.0%)* | <0.001* | 197 (23.8%)* | 39 (36.1%)* | 0.008* |
| Calcium <8.5 mg/dL, n | 10 (2.2%)* | 41 (8.5%)* | <0.001* | 14 (2.2%)* | 37 (12.1%)* | <0.001* | 31 (3.7%)* | 20 (18.5%)* | <0.001* |
| Magnesium <1.8 mg/dL, n | 57 (12.6%) | 50 (10.4%) | 0.344 | 75 (11.9%) | 32 (10.5%) | 0.576 | 97 (11.7%) | 10 (9.3%) | 0.547 |
| Cardiovascular risk factors/diseases | |||||||||
| Smoking, n | 229 (41.7%) | 242 (45.1%) | 0.280 | 321 (43.0%) | 150 (44.4%) | 0.714 | 414 (43.0%) | 57 (46.7%) | 0.493 |
| Hypertension, n | 110 (20.0%)* | 146 (27.2%)* | 0.006* | 154 (20.6%)* | 102 (30.2%)* | 0.001* | 216 (22.4%)* | 40 (32.8%)* | 0.015* |
| Diabetes, n | 302 (55.0%) | 312 (58.2%) | 0.316 | 413 (55.3%) | 201 (59.5%) | 0.222 | 543 (56.4%) | 71 (58.2%) | 0.777 |
| Myocardial infarction, n | 109 (19.9%)* | 143 (26.7%)* | 0.010* | 152 (20.3%)* | 100 (29.6%)* | 0.001* | 207 (21.5%)* | 45 (36.9%)* | <0.001* |
| Angina pectoris, n | 106 (19.3%) | 98 (18.3%) | 0.723 | 141 (18.9%) | 63 (18.6%) | 0.993 | 176 (18.3%) | 28 (23.0%) | 0.262 |
| Left ventricular hypertrophy/heart failure, n | 63 (11.5%) | 71 (13.2%) | 0.427 | 79 (10.6%)* | 55 (16.3%)* | 0.011* | 109 (11.3%)* | 25 (20.5%)* | 0.006* |
| Autoimmune rheumatic diseases, n | 38 (6.9%) | 54 (10.1%) | 0.079 | 56 (7.5%) | 36 (10.7%) | 0.107 | 81 (8.4%) | 11 (9.0%) | 0.957 |
| Drugs | |||||||||
| Alpha blockers, n | 344 (62.7%) | 331 (61.8%) | 0.806 | 452 (60.5%) | 223 (66.0%) | 0.098 | 588 (61.1%)* | 87 (71.3%)* | 0.036* |
| Anesthetics, n | 34 (6.2%)* | 60 (11.2%)* | 0.005* | 52 (7.0%)* | 42 (12.4%)* | 0.004* | 71 (7.4%)* | 23 (18.9%)* | <0.001* |
| Antianginal, n | 161 (29.3%) | 174 (32.5%) | 0.293 | 226 (30.3%) | 109 (32.2%) | 0.557 | 292 (30.3%) | 43 (35.2%) | 0.315 |
| Antiarrhythmics, n | 33 (6.0%)* | 88 (16.4%)* | <0.001* | 54 (7.2%)* | 67 (19.8%)* | <0.001* | 83 (8.6%)* | 38 (31.1%)* | <0.001* |
| Antibiotics, n | 529 (96.4%) | 525 (97.9%) | 0.164 | 724 (96.9%) | 330 (97.6%) | 0.649 | 934 (97.0%) | 120 (98.4%) | 0.570 |
| Anticonvulsants, n | 394 (71.8%) | 380 (70.9%) | 0.802 | 539 (72.2%) | 235 (69.5%) | 0.415 | 694 (72.1%) | 80 (65.6%) | 0.165 |
| Antidepressants, n | 429 (78.1%) | 422 (78.7%) | 0.871 | 594 (79.5%) | 257 (76.0%) | 0.226 | 757 (78.6%) | 94 (77.0%) | 0.781 |
| Antiemetics, n | 274 (49.9%)* | 329 (61.4%)* | <0.001* | 408 (54.6%) | 195 (57.7%) | 0.380 | 528 (54.8%) | 75 (61.5%) | 0.195 |
| Antifungals, n | 181 (33.0%) | 194 (36.2%) | 0.292 | 243 (32.5%)* | 132 (39.1%)* | 0.043* | 327 (34.0%) | 48 (39.3%) | 0.281 |
| Antihistamines, n | 425 (77.4%)* | 443 (82.6%)* | 0.038* | 597 (79.9%) | 271 (80.2%) | 0.987 | 769 (79.9%) | 99 (81.1%) | 0.829 |
| Antihypertensives, n | 210 (38.3%)* | 248 (46.3%)* | 0.009* | 286 (38.3%)* | 172 (50.9%)* | <0.001* | 390 (40.5%)* | 68 (55.7%)* | 0.002* |
| Antimalarials, n | 79 (14.4%) | 81 (15.1%) | 0.803 | 105 (14.1%) | 55 (16.3%) | 0.389 | 139 (14.4%) | 21 (17.2%) | 0.496 |
| Antipsychotics, n | 190 (34.6%)* | 219 (40.9%)* | 0.039* | 264 (35.3%)* | 145 (42.9%)* | 0.021* | 355 (36.9%) | 54 (44.3%) | 0.136 |
| Antivirals, n | 209 (38.1%)* | 244 (45.5%)* | 0.015* | 306 (41.0%) | 147 (43.5%) | 0.474 | 398 (41.3%) | 55 (45.1%) | 0.487 |
| Beta blockers, n | 351 (63.9%)* | 383 (71.5%)* | 0.010* | 488 (65.3%) | 246 (72.8%)* | 0.018* | 639 (66.4%)* | 95 (77.9%)* | 0.014* |
| Bronchodilators, n | 353 (64.3%)* | 379 (70.7%)* | 0.029* | 498 (66.7%) | 234 (69.2%) | 0.444 | 648 (67.3%) | 84 (68.9%) | 0.807 |
| Calcium blockers, n | 338 (61.6%) | 343 (64.0%) | 0.445 | 451 (60.4%)* | 230 (68.0%)* | 0.019* | 597 (62.0%) | 84 (68.9%) | 0.169 |
| Diuretics, n | 378 (68.9%)* | 409 (76.3%)* | 0.007* | 519 (69.5)* | 268 (79.3)* | 0.001* | 682 (70.8%)* | 105 (86.1%)* | 0.001* |
| Muscle relaxants, n | 365 (66.5%) | 361 (67.4%) | 0.811 | 512 (68.5) | 214 (63.3) | 0.104 | 651 (67.6%) | 75 (61.5%) | 0.210 |
| Sedatives, n | 358 (65.2%)* | 393 (73.3%)* | 0.005* | 502 (67.2)* | 249 (73.7)* | 0.039* | 656 (68.1%)* | 95 (77.9%)* | 0.036* |
QTc indicates heart rate‐corrected QT‐interval. Values are expressed as median [interquartile range], or frequency count (percentage). Differences between the 2 groups were evaluated by the Mann–Whitney test or the chi‐square test.
Statistically significant (P<0.05) differences in variables.
Other includes: Asian, American Indian, Alaska native, Hawaiian or other Pacific Islander, and unknown.
Table 3.
Demographic, ECG, Laboratory, and Clinical Findings in Subjects of the Study Population Stratified by IL‐6 Level
| IL‐6, pg/mL | ||||
|---|---|---|---|---|
| Characteristic | <5 | 5–25 | >25 | P value |
| Total, n | 306 | 376 | 403 | … |
| Demographics | ||||
| Age, y | 68 [61–74] | 71 [63–76] * | 72 [64–77] * , † | <0.001 |
| Male sex, n | 277 (90.5%) | 343 (91.2%) | 378 (93.8%) | 0.226 |
| Race, n | ||||
| White | 200 (65.4%) | 262 (69.7%) | 254 (63.0%) | 0.406 |
| Black | 83 (27.1%) | 91 (24.2%) | 117 (29.0%) | |
| Others‡ | 23 (7.5%) | 23 (6.1%) | 32 (7.9%) | |
| ECG findings | ||||
| Heart rate, bpm | 73 [66–85] | 79 [70–88] * | 83 [73–94] * , † | <0.001 |
| QRS interval, ms | 90 [82–96] | 90 [84–98] | 88 [82–96] | 0.161 |
| Laboratory data | ||||
| IL‐6, pg/mL | 2.9 [2.1–3.7] | 10.7 [7.3–15.8] * | 64.8 [40.7–135.6] * , † | <0.001 |
| Potassium <4.0 mEq/L, n | 63 (25.7%) | 95 (29.3%) | 78 (21.3%) | 0.053 |
| Calcium <8.5 mg/dL, n | 4 (1.6%) | 10 (3.1%) | 37 (10.1%) * , † | <0.001 |
| Magnesium <1.8 mg/dL, n | 34 (13.9%) | 40 (12.3%) | 33 (9.0%) | 0.144 |
| Cardiovascular risk factors/diseases | ||||
| Smoking, n | 138 (45.1%) | 171 (45.5%) | 162 (40.2%) | 0.259 |
| Hypertension, n | 47 (15.4%) | 101 (26.9%) * | 108 (26.8%) * | <0.001 |
| Diabetes mellitus, n | 138 (45.1%) | 235 (62.5%) * | 241 (59.8%) * | <0.001 |
| Myocardial infarction, n | 45 (14.7%) | 87 (23.1%) * | 120 (29.8%) * , † | <0.001 |
| Angina pectoris, n | 62 (20.3%) | 67 (17.8%) | 75 (18.6%) | 0.714 |
| Left ventricular hypertrophy/heart failure, n | 30 (9.8%) | 52 (13.8%) | 52 (12.9%) | 0.258 |
| Autoimmune rheumatic diseases, n | 25 (8.2%) | 36 (9.6%) | 31 (7.7%) | 0.625 |
| Drugs | ||||
| Alpha blockers, n | 186 (60.8%) | 233 (62.0%) | 256 (63.5%) | 0.752 |
| Anesthetics, n | 9 (2.9%) | 19 (5.1%) | 66 (16.4%) * , † | <0.001 |
| Antianginal, n | 93 (30.4%) | 112 (29.8%) | 130 (32.3%) | 0.740 |
| Antiarrhythmics, n | 26 (8.5%) | 38 (10.1%) | 57 (14.1%) | 0.044 |
| Antibiotics, n | 291 (95.1%) | 363 (96.5%) | 400 (99.3%) * , † | 0.003 |
| Anticonvulsants, n | 241 (78.8%) | 273 (72.6%) | 260 (64.5%) * , † | <0.001 |
| Antidepressants, n | 257 (84.0%) | 302 (80.3%) | 292 (72.5%) * , † | 0.001 |
| Antiemetics, n | 169 (55.2%) | 206 (54.8%) | 228 (56.6%) | 0.872 |
| Antifungals, n | 93 (30.4%) | 132 (35.1%) | 150 (37.2%) | 0.160 |
| Antihistamines, n | 246 (80.4%) | 304 (80.9%) | 318 (78.9%) | 0.779 |
| Antihypertensives, n | 113 (36.9%) | 172 (45.7%) | 173 (42.9%) | 0.064 |
| Antimalarials, n | 38 (12.4%) | 49 (13.0%) | 73 (18.1%) | 0.054 |
| Antipsychotics, n | 102 (33.3%) | 131 (34.8%) | 176 (43.7%) * , † | 0.007 |
| Antivirals, n | 102 (33.3%) | 165 (43.9%) * | 186 (46.2%) * | 0.002 |
| Beta blockers, n | 191 (62.4%) | 253 (67.3%) | 290 (72.0%) * | 0.026 |
| Bronchodilators, n | 189 (61.8%) | 264 (70.2%) | 279 (69.2%) | 0.041 |
| Calcium blockers, n | 178 (58.2%) | 229 (60.9%) | 274 (68.0%) * | 0.018 |
| Diuretics, n | 195 (63.7%) | 275 (73.1%)* | 317 (78.7%) * | <0.001 |
| Muscle relaxants, n | 226 (73.9%) | 250 (66.5%) | 250 (62.0%) * | 0.004 |
| Sedatives, n | 207 (67.6%) | 256 (68.1%) | 288 (71.5%) | 0.464 |
IL‐6 indicates interleukin‐6. Values are expressed as median [interquartile range], or frequency count (percentage). Differences between the groups were evaluated by the Kruskal–Wallis test or chi‐squard test.
Statistically different from IL‐6 <5 pg/mL group.
Statistically different from IL‐6 5–25 pg/mL group (post hoc tests: multiple Mann–Whitney U test or chi‐square test with Holm correction).
Other includes: Asian, American Indian, Alaska native, Hawaiian or other Pacific Islander, and unknown.
Association Between IL‐6 Levels and QTc
Subjects presenting with QTc prolongation showed significantly higher IL‐6 levels when compared with those without, approximately 2 times higher. This finding was consistently observed throughout the different cutoffs used, with a progressive increase in median IL‐6 levels as the cutoff became more stringent, until approaching 24 pg/mL in subjects with a QTc>470 ms men/480 ms women (Table 4). Accordingly, in subjects with QTc prolongation, the prevalence of moderately high/high IL‐6 levels was significantly higher than in those without, and gradually increased throughout the different cutoffs analyzed. Notably, among subjects with a QTc >470 ms men/480 ms women, moderately high or high IL‐6 levels were present in over 85% of cases, including ≈50% of subjects having IL‐6 levels >25 pg/mL (Table 4).
Table 4.
IL‐6 Levels in Subjects With or Without QTc Prolongation Based on Different Cutoffs
| Cutoff 440 ms | Cutoff 450 ms men/470 ms women | Cutoff 470 ms men/480 ms women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | QTc <440 | QTc >440 | P value | QTc <450/470 | QTc >450/470 | P value | QTc <470/480 | QTc >470/480 | P value |
| Total, n | 549 (50.6%) | 536 (49.4%) | … | 747 (68.8%) | 338 (31.2%) | … | 963 (88.8%) | 122 (11.2%) | … |
| IL‐6, pg/mL | 9.5 [3.4–37.5] | 18.9 [6.1–55.6] | <0.001 | 9.9 [3.7–38.2] | 22.0 [7.5–60.6] | <0.001 | 11.7 [4.0–44.3] | 23.8 [8.5–66.4] | <0.001 |
| IL‐6 level | |||||||||
| <5 pg/mL, n | 192 (35.0%) | 114 (21.3%) | <0.001 | 244 (32.7%) | 62 (18.3%) | <0.001 | 289 (30.0%) | 17 (13.9%) | <0.001 |
| 5–25 pg/mL, n | 186 (33.9%) | 190 (35.4%) | 260 (34.8%) | 116 (34.3%) | 331 (34.4%) | 45 (36.9%) | |||
| >25 pg/mL, n | 171 (31.1%) | 232 (43.3%) | 243 (32.5%) | 160 (47.3%) | 343 (35.6%) | 60 (49.2%) | |||
IL‐6 indicates interleukin‐6; and QTc, heart rate‐corrected QT‐interval. Values are expressed as median [interquartile range], or frequency count (percentage). Differences between the 2 groups were evaluated by the Mann–Whitney test or chi‐square test.
The existence of an association between IL‐6 and QTc was further confirmed by the significant QTc differences observed when subjects were stratified by IL‐6 level (Figure). In fact, while the median QTc value in the normal IL‐6 group was distinctly lower than all cutoffs considered (429 ms), the median QTc significantly increased to 440 ms and 444.5 ms in the moderately high IL‐6 group and in the high IL‐6 group, respectively (Table 5).
Figure . Empirical distribution of QTc values in the overall cohort, stratified by IL‐6 level.
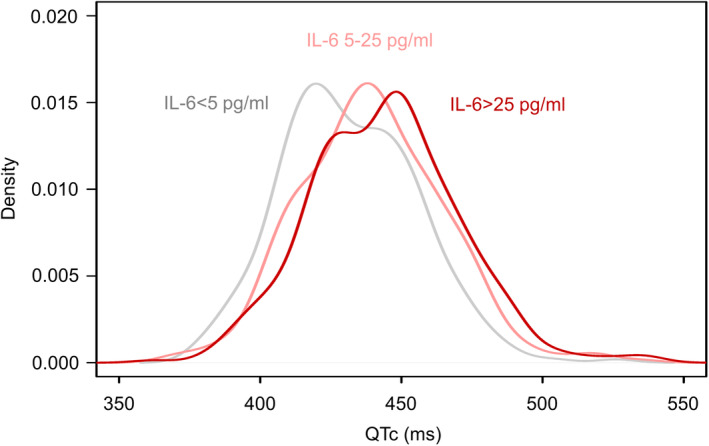
IL‐6 indicates interleukin‐6; and QTc, heart rate‐corrected QT interval.
Table 5.
Median QTc Values and QTc Prolongation Frequencies in Subjects Stratified by IL‐6 Level
| IL‐6, pg/mL | ||||
|---|---|---|---|---|
| Characteristic | <5 | 5–25 | >25 | P value |
| Total, n | 312 | 379 | 414 | … |
| QTc, ms | 429 [414–447.25] | 440 [422–456]* | 444.5 [426–461]* , † | <0.001 |
| QTc>440 ms, n | 116 (37.2%) | 190 (50.1%)* | 236 (57.0%)* | <0.001 |
| QTc >450 ms men/470 ms women, n | 63 (20.2%) | 116 (30.6%)* | 163 (39.4%)* , † | <0.001 |
| QTc >470 ms men/480 ms women, n | 17 (5.4%) | 45 (11.9%)* | 61 (14.7%)* | <0.001 |
IL‐6 indicates interleukin‐6; and QTc, heart rate‐corrected QT‐interval. Values are expressed as median [interquartile range], or frequency count (percentage). Differences between the groups were evaluated by the Kruskal–Wallis test or chi‐square test.
Statistically different from IL‐6 <5 pg/mL group.
Statistically different from IL‐6 5–25 pg/mL group (post hoc tests: multiple Mann–Whitney test or chi‐square test with Holm correction).
Consistently, the prevalence of QTc prolongation progressively increased throughout the 3 groups with all cutoffs, up to reaching a ≈3‐times higher risk of having a QTc >470 ms men/480 ms women in subjects with IL‐6 levels >25 pg/mL (14.7%) when compared with those with IL‐6 <5 pg/mL (5.4%) (Table 5).
Similar trends with further numerical increases in median IL‐6 levels and prevalence of moderately‐high/high IL‐6 levels were observed in subjects with QTc >500 ms. Moreover, the prevalence of QTc prolongation >500 ms progressively increased throughout the 3 IL‐6 ranges (up to >3 times). However, none of these differences reached statistical significance due to the very small sample size of subjects presenting with QTc>500 ms in the overall cohort (n=18) (Tables S2 and S3). Thus, this cutoff was therefore not considered for additional statistical analyses.
Association Between IL‐6 Levels and QTc: Multivariate Analysis
Based on the findings, the role of increased IL‐6 levels as an independent risk factor for QTc prolongation was then evaluated by a stepwise multivariate logistic regression analysis. We used a model in which all the variables found to be associated with QTc prolongation independently from IL‐6 levels (ie, sex, QRS, potassium <4.0 mEq/L, left ventricular hypertrophy/heart failure, alpha blockers, antiemetics, antihypertensives, antipsychotics, antivirals, beta blockers, bronchodilators, calcium blockers, diuretics) and the confounding factors (ie, variables associated concomitantly with QTc and IL‐6: age, heart rate, calcium <8.5 mg/dL, hypertension, myocardial infarction, anesthetics, antiarrhythmics, sedatives) were entered together with IL‐6 stratified as normal, moderately high, and high, to identify a minimum subset of factors with the highest possible prediction accuracy for QTc prolongation in our study population. As shown in Table 6, the presence of high IL‐6 levels (>25 pg/mL) showed a statistically significant association with increased risk of QTc prolongation for all the 3 cutoffs investigated. Particularly, ORs ranged from 1.66 for QTc >440 ms to 2.28 for QTc >470 ms men/480 ms women, indicating a ≈65% to 130% higher risk of developing QTc prolongation in subjects with IL‐6 levels >25 pg/mL when compared with those with IL‐6 levels <5 pg/mL. Moreover, specifically considering the QTc >470 ms men/480 ms women cutoff, even moderately high IL‐6 levels were found to have a statistically significant association with a ≈2 times higher risk of a prolonged QTc (Table 6).
Table 6.
Demographic, Clinical, Laboratory, and Therapeutic Variables Associated With QTc Interval Prolongation Based on Different Cutoffs: a Stepwise Multivariate Logistic Regression Analysis
| Dependent variable | Independent variables | OR (95% CI) | P value |
|---|---|---|---|
| QTc >440 ms | Heart rate | 1.04 (1.03–1.05) | <0.001 |
| QRS | 1.05 (1.04–1.07) | <0.001 | |
| Potassium <4.0 mEq/L | 2.17 (1.55–3.06) | <0.001 | |
| Antiarrhythmics | 3.11 (1.90–5.24) | <0.001 | |
| Calcium <8.5 mg/dL | 4.27 (2.08–9.55) | <0.001 | |
| Antiemetics | 1.72 (1.29–2.31) | <0.001 | |
| Female sex | 2.48 (1.43–4.38) | 0.001 | |
| IL‐6 (>25 pg/mL) | 1.66 (1.14–2.42) | 0.008 | |
| IL‐6 (5–25 pg/mL) | 1.34 (0.92–1.95) | 0.125 | |
| QTc > 450 ms men/470 ms women | Heart rate | 1.03 (1.02–1.04) | <0.001 |
| QRS | 1.04 (1.03–1.05) | <0.001 | |
| Calcium <8.5 mg/dL | 5.99 (3.11–12.13) | <0.001 | |
| Antiarrhythmics | 2.74 (1.77–4.26) | <0.001 | |
| Potassium <4.0 mEq/L | 1.88 (1.35–2.63) | <0.001 | |
| IL‐6>25 pg/mL | 1.81 (1.21–2.72) | 0.004 | |
| IL‐6 (5–25 pg/mL) | 1.39 (0.92–2.10) | 0.119 | |
| Antihypertensives | 1.37 (1.01–1.85) | 0.040 | |
| QTc >470 ms men/480 ms women | Antiarrhythmics | 3.58 (2.12–5.99) | <0.001 |
| Calcium <8.5 mg/dL | 5.99 (3.02–11.77) | <0.001 | |
| QRS | 1.04 (1.02–1‐06) | <0.001 | |
| Potassium <4.0 mEq/L | 1.98 (1.24–3.14) | 0.004 | |
| IL‐6 (>25 pg/mL) | 2.28 (1.19–4.65) | 0.017 | |
| IL‐6 (5–25 pg/mL) | 2.18 (1.12–4.50) | 0.026 | |
| Heart rate | 1.02 (1.00–1.04) | 0.026 | |
| Diuretics | 2.02 (1.10–3.96) | 0.031 |
IL‐6 indicates interleukin‐6; OR, odds ratio; QRS, QRS interval duration; and QTc, heart rate‐corrected QT interval. Variables entered in the model: age, sex, heart rate, QRS, potassium <4.0 mEq/L, calcium <8.5 mg/dL, IL‐6, hypertension, myocardial infarction, left ventricular hypertrophy/heart failure, alpha blockers, anesthetics, antiarrhythmics, antiemetics, antihypertensives, antipsychotics, antivirals, beta blockers, bronchodilators, calcium blockers, diuretics, and sedatives.
To further define the independent impact of increased IL‐6 levels on QTc length, the stepwise multivariate regression analysis was also performed by considering QTc as a linear variable. To perform this analysis an outlier was removed due to its high QTc value (650 ms; +6σ from mean). Again, both the high and moderately high IL‐6 ranges were found to have a statistically significant correlation with QTc duration. Specifically, having an IL‐6 level ranging from 5 to 25 pg/mL was associated with a mean QTc increase of 6 ms, increasing to almost 7 ms for IL‐6 >25 pg/mL, when compared with IL‐6 <5 pg/mL (Table 7). Notably, such increases were quantitatively comparable to those observed in the presence of 2 well‐recognized QT‐prolonging risk factors, such as hypokalemia (+ 8.8 ms) and history of myocardial infarction (+ 5.8 ms) (Table 7).
Table 7.
Demographic, Clinical, Laboratory, and Therapeutic Variables Associated With QTc Interval Duration: a Stepwise Multivariate Linear Regression Analysis
| Independent variable | Mean QTc increase, ms (95% CI) | P value |
|---|---|---|
| Calcium <8.5 mg/dL | 20.28 (13.63–26.93) | <0.001 |
| Female sex | 12.09 (6.40–17.80) | <0.001 |
| Antiarrhythmics | 11.55 (6.87–16.24) | <0.001 |
| Potassium <4 mEq/L | 8.82 (5.34–12.29) | <0.001 |
| IL‐6 >25 pg/mL | 6.70 (2.79–10.61) | <0.001 |
| IL‐6 5–25 pg/mL | 6.00 (2.11–9.89) | 0.003 |
| Myocardial infarction | 5.81 (2.24–9.37) | 0.001 |
| QRS interval duration (for 10‐ms increase) | 5.28 (3.89–6.67) | <0.001 |
| Heart rate (for 10‐bpm increase) | 4.41 (3.34–5.48) | <0.001 |
| Antiemetics | 3.98 (0.96–6.99) | 0.009 |
IL‐6 indicates interleukin‐6; and QTc, heart rate‐corrected QT interval. Variables entered in the model: age, sex, heart rate, QRS, potassium <4.0 mEq/L, calcium <8.5 mg/dL, IL‐6, hypertension, myocardial infarction, left ventricular hypertrophy/heart failure, alpha blockers, anesthetics, antiarrhythmics, antiemetics, antihypertensives, antipsychotics, antivirals, beta blockers, bronchodilators, calcium blockers, diuretics, and sedatives.
Some additional sensitivity analyses were performed to refine and strengthen the meaning of these findings. First, to provide support to the view that IL‐6 per se affects the risk of QTc prolongation, regardless of the specific underlying inflammatory process causing its elevation, the stepwise multivariate logistic and linear regression analyses were repeated also adding, among the variables considered, history of autoimmune rheumatic diseases (ie, rheumatoid arthritis, systemic lupus erythematosus, Sjögren's syndrome, systemic sclerosis, other connective tissue diseases), and the use of anti‐infective drugs (ie, antibiotics, antifungals, and antivirals) as a proxy of the occurrence of infectious inflammatory process. Also in this case, the analysis demonstrated that elevated IL‐6 levels were associated with an increased risk of QTc prolongation, throughout the 3 cutoffs investigated, without any change in the ORs observed (data not shown).
An additional sensitivity analysis was performed to exclude effects of temporality, that is, the fact that ECG acquisition and IL‐6 testing had not been always performed at the same time point, and it may have biased the results. Thus, we selected in the cohort only those subjects in whom both ECG and IL‐6 were evaluated within an interval not exceeding 7 days (n=260) or even in the same day (n=56), respectively. Again, in both subgroups median QTc levels progressively increased throughout the 3 IL‐6 ranges, similar to that observed in the whole population, and even more evidently (Table S4). In fact, although QTc in subjects with IL‐6 <5 pg/mL remained relatively stable independent of the time interval considered, in those with high IL‐6 levels median QTc values progressively increased as the time interval shortened, particularly when IL‐6 was >25 pg/mL (any time interval: 444.5 [426–461] ms; time interval ≤7 days: 450.8 [429.7–469.5] ms; time interval ≤1 day: 472.5 [454.8–505.0] ms) (Table S4). Such observation is consistent with the view that IL‐6 prolongs QTc as a result of direct and transient effects on cardiac electrophysiology. Overall, these data provide evidence that temporality of measurements did not significantly bias the findings of our analysis, at most underestimating the actual impact of IL‐6 on QTc.
Discussion
The key findings of the present study are the following: (1) in a cohort of ≈1000 US veterans, subjects with elevated IL‐6 levels showed a concentration‐dependent increase in the prevalence of QTc prolongation; moreover, those presenting with QTc prolongation exhibited higher levels of circulating IL‐6; (2) IL‐6 level increase was associated with an up to ≈2 times higher risk of QTc prolongation, regardless of the specific underlying cause; and (3) the mean QTc increase observed in the presence of elevated IL‐6 was quantitatively comparable (IL‐6 >25 pg/mL:+6.7 ms) to that of major recognized QT‐prolonging risk factors, such as hypokalemia and history of myocardial infarction.
In recent years, the key role of inflammatory cytokines in promoting arrhythmic events is ever more recognized. A large body of evidence indicates that these molecules, particularly IL‐6, can promote a wide range of cardiac arrhythmias due to multiple effects on arrhythmogenesis. 12 Specifically, many experimental studies on cell and animal models provided evidence that IL‐6 can prolong APD/QTc and increase ventricular arrhythmia susceptibility by inhibiting both the rapidly and slowly activating components of the delayed outward‐rectifying potassium current, and increasing the L‐type calcium current. 12 , 20 , 30 , 31 , 32 , 33 , 34 , 35 , 36 In addition, it has been demonstrated that IL‐6 can also indirectly promote APD/QTc prolongation via systemic effects, including cardiac sympathetic system activation, 12 increased bioavailability of QT‐prolonging drugs by inhibiting the hepatic cytochrome p450, 37 , 38 and reduction of testosterone levels in men due to an enhanced aromatase‐dependent androgen‐to‐estrogen conversion. 39 Consistently, several clinical studies demonstrated that in patients with different inflammatory diseases, including COVID‐19, circulating IL‐6 correlated with QTc duration and high levels were associated with TdP development. 14 , 15 , 16 , 17 , 18 , 19 , 20 Moreover, in patients with rheumatoid arthritis, treatment with the anti‐IL6‐receptor monoclonal antibody tocilizumab was associated with a rapid and significant QTc shortening, which correlated with C‐reactive protein and cytokine levels decrease. 15 , 17
However, although the background of basic investigations supporting the mechanistic role of IL‐6 in APD/QTc prolongation is strong, 12 the clinical evidence derives from only relatively small studies or studies involving selected groups of patients. 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 Thus, large data analyses aimed at evaluating the impact of IL‐6 on the risk of QTc prolongation in the general population are currently missing.
Here, we provide evidence, for the first time, that in a large population of over 1000 US veterans, subjects with elevated IL‐6 levels presented with a longer median QTc duration (+11.0 ms for the range 5–25 pg/mL, rising to +15.5 ms in those with levels >25 pg/mL) together with an increased prevalence of QTc prolongation when compared with subjects with normal IL‐6 levels. In the univariate analysis, the greater the QTc cutoff considered, the greater were the differences for QTc prolongation percentages with increasing ranges of IL‐6, up to a ≈3‐times higher risk for QTc >470 ms men/480 ms women in subjects with high versus normal levels. Moreover, among subjects with a QTc >470 ms men/480 ms women, ≈1 in every 2 subjects (49.2%) showed IL‐6 levels >25 pg/mL, and ≈6 in every 7 subjects (86.1%) >5 pg/mL.
In addition, it is important to note that in subjects falling in the moderately‐high IL‐6 range (5–25 pg/mL), the observed median IL‐6 levels were slightly higher than 10 pg/mL (10.7 pg/mL). Comparable serum IL‐6 levels were found (≈13–14 pg/mL) to be associated with of an increased prevalence of QTc >470 ms men/480 ms women ms in previous small clinical studies performed in patients with different inflammatory diseases. 16 , 19 Moreover, in a cohort of 40 consecutive patients with marked QTc prolongation who developed TdP, median IL‐6 levels at the moment of arrhythmia occurrence were 10 pg/mL. 16 Altogether, these findings support the view, as proposed during the COVID‐19 pandemic, that IL‐6 levels ≥10 pg/mL are already enough to induce significant QTc prolongation and even, in the presence of other concomitant QT‐prolonging risk factors, promote TdP development. 40 Our study also provides evidence that when IL‐6 rises over 25 pg/mL (median 64.8 pg/mL) the risk of QTc prolongation further increases. This suggests that the arrhythmogenic potential of IL‐6 has a threshold to emerge and then progressively augments with the increase in serum levels, as indeed expected considering the electrophysiological mechanisms by which this cytokine can prolong APD/QTc. 12 In particular, this is in agreement with the experimental evidence that IL‐6 blocking activity on hERG‐related gene and the related rapidly activating components of the delayed outward‐rectifying potassium current, in turn chiefly regulating APD in ventricular cardiomyocytes, shows a clear‐cut concentration dependence. 33
Although these findings seemed to point to an important role for IL‐6 in promoting QTc prolongation in the subjects under study, the univariate analysis showed that several other recognized QT‐prolonging risk factors were also significantly and consistently associated throughout the different cutoffs with the risk of presenting QTc prolongation in our population, specifically low electrolyte levels (potassium, calcium), several cardiovascular risk factors/diseases (history of hypertension, myocardial infarction, left ventricular hypertrophy/heart failure), and many drugs (anesthetics, antiarrhythmics, antihypertensives, antipsychotics, diuretics, sedatives). Given that some of these QT‐prolonging factors were also found to be more commonly present in subjects with elevated IL‐6 levels, that is, low calcium levels, history of hypertension and myocardial infarction, use of anesthetics, antiarrhythmics, and diuretics, it could not be ruled out that they were the true main drivers responsible for the association between IL‐6 and QTc observed in the univariate analysis. Based on these considerations, a multivariable analysis adjusting the data for all these confounders was performed, and again a significant association between elevated IL‐6 levels and QTc prolongation was confirmed with all the cutoffs considered. The independent association between elevated IL‐6 and QTc duration was quantitatively relevant (on average a ≈6–7 ms increase), as it was of a similar extent to that found to be associated with the presence of hypokalemia or history of myocardial infarction, 2 conventional risk factors whose QT‐prolonging role in the general population is well recognized. 2 Moreover, the independent association between elevated IL‐6 levels and QTc prolongation persisted after supplemental adjustment for history of autoimmune rheumatic diseases and the use of anti‐infective drugs, indicating that IL‐6 per se is associated with QTc lengthening, rather than the underlying inflammatory process responsible for its elevation. Overall, these findings provide evidence for a strong and independent role of IL‐6 in increasing the risk for having a prolonged QTc, thereby confirming that the direct electrophysiologic effects of this cytokine on the ventricular repolarization have a clinically relevant impact also in a large population of unselected individuals.
This study has several strengths, but also has some limitations. First, our population consisted of US veterans who are primarily male, White, and elderly. Although these characteristics might potentially represent a limitation in extending our results to the general population, on the other hand the impact of such a selection bias was significantly attenuated by the use of a network of integrated health care systems, based on national large‐scale data collected during routine medical care.
Other potential limitations of the study include the selection of subjects and the temporality of measurements. In fact, the retrospective selection of individuals in whom at least 1 measurement of serum IL‐6 was available, may have increased the probability to find augmented circulating levels, as in a number of cases IL‐6 testing was probably driven by the clinical suspicion of an active inflammatory process. Indeed, over two‐thirds of the subjects showed elevated IL‐6 concentrations, higher than 25 pg/mL in ≈37% of cases, with median IL‐6 levels of ≈13 pg/mL. Nevertheless, the stratification method that we used, based on increasing ranges of IL‐6, allowed to divide the population in 3 numerically well‐balanced groups, including one constituted by subjects with normal IL‐6 levels (despite the pretest clinical suspicion) used as the reference group. Thus, such apparent limitation might even represent a strength of the study, as it reinforces the idea that circulating IL‐6 levels independent of clinical manifestations of systemic inflammation are a key factor associated with QTc prolongation. Concerning the temporal bias, it should be stressed that its impact was significantly attenuated by using, for each variable, the mean value deriving from multiple measurements carried out in a single subject throughout the time. Accordingly, the sensitivity analysis focused on those subjects only in whom ECG and IL‐6 testing were temporally very close, confirmed and even emphasized the differences in terms of median QTc values throughout the 3 ranges of IL‐6 as observed in the whole population.
Conclusions
In conclusion, our data provide evidence that circulating IL‐6 elevation is an independent and robust risk factor for QTc prolongation in a large cohort of US veterans. These findings support the hypothesis that the independent association between increased IL‐6 levels and life‐threatening ventricular arrhythmias/sudden cardiac death observed in the general population 22 , 23 , 24 is, at least in part, mediated by a higher propensity to develop QTc prolongation. Based on such evidence, it is recommended that inflammatory markers, specifically IL‐6, should be included in the clinical management of patients with LQTS/TdP. This also because accumulating data from recent clinical trials, 41 , 42 , 43 , 44 , 45 some very large, 46 are making it more and more realistic that cytokine‐targeting therapies could, in the near future, represent an essential tool to achieve a truly comprehensive treatment of cardiovascular disease, possibly including cardiac arrhythmias. 12 , 47
Sources of Funding
This work was supported by (1) Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR), Progetti di Rilevante Interesse Nazionale (PRIN), and Bando 2017, protocollo 2017XZMBYX (to P.E.L. and P.L.C.); (2) Regione Toscana, Bando Ricerca COVID‐19 2021, Progetto PRECARVID (to P.E.L. and P.L.C.); (3) Biomedical Laboratory Research & Development Service of Veterans Affairs Office of Research and Development, Merit Review grant I01 BX002137 (to M.B.); (4) National Heart, Lung, and Blood Institute 1R01HL164415‐01 (to M.B.); and (5) US Department of Defense award number W81XWH‐21‐1‐0424 (to M.B.).
Disclosures
Dr Pietro Enea Lazzerini received a grant from Roche Italia S.p.A. outside the submitted work in 2018. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S4
P. E. Lazzerini, M. Cupelli, A. Cartocci, F. Laghi‐Pasini, M. Acampa, P. L. Capecchi, N. El‐Sherif and M. Boutjdir contributed equally.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.032071
For Sources of Funding and Disclosures, see page 12.
References
- 1. Al‐Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary. Circulation. 2018;138:e210–e271. doi: 10.1161/CIR.0000000000000548 [DOI] [PubMed] [Google Scholar]
- 2. Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, Philippides GJ, Roden DM, Zareba W; American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology, the Council on Cardiovascular Nursing, and the American College of Cardiology Foundation . Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121:1047–1060. doi: 10.1161/CIRCULATIONAHA.109.192704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schwartz PJ, Stramba‐Badiale M, Crotti L, Pedrazzini M, Besana A, Bosi G, Gabbarini F, Goulene K, Insolia R, Mannarino S, et al. Prevalence of the congenital long‐QT syndrome. Circulation. 2009;120:1761–1767. doi: 10.1161/CIRCULATIONAHA.109.863209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tisdale JE. Prevalence and significance of acquired QT interval prolongation in hospitalized patients. Heart Rhythm. 2017;14:979–980. doi: 10.1016/j.hrthm.2017.03.036 [DOI] [PubMed] [Google Scholar]
- 5. El‐Sherif N, Turitto G, Boutjdir M. Acquired long QT syndrome and torsade de pointes. Pacing Clin Electrophysiol. 2018;41:414–421. doi: 10.1111/pace.13296 [DOI] [PubMed] [Google Scholar]
- 6. Lazzerini PE, Capecchi PL, Laghi‐Pasini F. Long QT syndrome: an emerging role for inflammation and immunity. Front Cardiovasc Med. 2015;2:26. doi: 10.3389/fcvm.2015.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lazzerini PE, Capecchi PL, Laghi‐Pasini F. Systemic inflammation and arrhythmic risk: lessons from rheumatoid arthritis. Eur Heart J. 2017;38:1717–1727. doi: 10.1093/eurheartj/ehw208 [DOI] [PubMed] [Google Scholar]
- 8. Lazzerini PE, Capecchi PL, Laghi‐Pasini F, Boutjdir M. Autoimmune channelopathies as a novel mechanism in cardiac arrhythmias. Nat Rev Cardiol. 2017;14:521–535. doi: 10.1038/nrcardio.2017.61 [DOI] [PubMed] [Google Scholar]
- 9. Lazzerini PE, Capecchi PL, El‐Sherif N, Laghi‐Pasini F, Boutjdir M. Emerging arrhythmic risk of autoimmune and inflammatory cardiac channelopathies. J Am Heart Assoc. 2018;7:e010595. doi: 10.1161/JAHA.118.010595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim E, Joo S, Kim J, Ahn J, Kimm K, Shin C. Association between C‐reactive protein and QTc interval in middle‐aged men and women. Eur J Epidemiol. 2006;21:653–659. doi: 10.1007/s10654-006-9034-9 [DOI] [PubMed] [Google Scholar]
- 11. Lazzerini PE, Laghi‐Pasini F, Boutjdir M, Capecchi PL. Inflammatory cytokines and cardiac arrhythmias: the lesson from COVID‐19. Nat Rev Immunol. 2022;22:270–272. doi: 10.1038/s41577-022-00714-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lazzerini PE, Abbate A, Boutjdir M, Capecchi PL. Fir(e)ing the rhythm: inflammatory cytokines and cardiac arrhythmias. JACC Basic Transl Sci. 2023;8:728–750. doi: 10.1016/j.jacbts.2022.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL‐6/IL‐6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond). 2012;122:143–159. doi: 10.1042/CS20110340 [DOI] [PubMed] [Google Scholar]
- 14. Adlan AM, Panoulas VF, Smith JP, Fisher JP, Kitas GD. Association between corrected QT interval and inflammatory cytokines in rheumatoid arthritis. J Rheumatol. 2015;42:421–428. doi: 10.3899/jrheum.140861 [DOI] [PubMed] [Google Scholar]
- 15. Lazzerini PE, Acampa M, Capecchi PL, Fineschi I, Selvi E, Moscadelli V, Zimbone S, Gentile D, Galeazzi M, Laghi‐Pasini F. Antiarrhythmic potential of anticytokine therapy in rheumatoid arthritis: tocilizumab reduces corrected QT interval by controlling systemic inflammation. Arthritis Care Res. 2015;67:332–339. doi: 10.1002/acr.22455 [DOI] [PubMed] [Google Scholar]
- 16. Lazzerini PE, Laghi‐Pasini F, Bertolozzi I, Morozzi G, Lorenzini S, Simpatico A, Selvi E, Bacarelli MR, Finizola F, Vanni F, et al. Systemic inflammation as a novel QT‐prolonging risk factor in patients with torsades de pointes. Heart. 2017;103:1821–1829. doi: 10.1136/heartjnl-2016-311079 [DOI] [PubMed] [Google Scholar]
- 17. Kobayashi H, Kobayashi Y, Yokoe I, Kitamura N, Nishiwaki A, Takei M, Giles JT. Heart rate‐corrected QT interval duration in rheumatoid arthritis and its reduction with treatment with the interleukin 6 inhibitor tocilizumab. J Rheumatol. 2018;45:1620–1627. doi: 10.3899/jrheum.180065 [DOI] [PubMed] [Google Scholar]
- 18. Wu KC, Zhang L, Haberlen SA, Ashikaga H, Brown TT, Budoff MJ, D'Souza G, Kingsley LA, Palella FJ, Margolick JB, et al. Predictors of electrocardiographic QT interval prolongation in men with HIV. Heart. 2019;105:559–565. doi: 10.1136/heartjnl-2018-313667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lazzerini PE, Acampa M, Laghi‐Pasini F, Bertolozzi I, Finizola F, Vanni F, Natale M, Bisogno S, Cevenini G, Cartocci A, et al. Cardiac arrest risk during acute infections: systemic inflammation directly prolongs QTc interval via cytokine‐mediated effects on potassium channel expression. Circ Arrhythm Electrophysiol. 2020;13:e008627. doi: 10.1161/CIRCEP.120.008627 [DOI] [PubMed] [Google Scholar]
- 20. Lazzerini PE, Accioli R, Acampa M, Zhang WH, Verrengia D, Cartocci A, Bacarelli MR, Xin X, Salvini V, Chen KS, et al. Interleukin‐6 elevation is a key pathogenic factor underlying COVID‐19‐associated heart rate‐corrected QT interval prolongation. Front Cardiovasc Med. 2022;9:893681. doi: 10.3389/fcvm.2022.893681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heravi AS, Etzkorn LH, Urbanek JK, Crainiceanu CM, Punjabi NM, Ashikaga H, Brown TT, Budoff MJ, D'Souza G, Magnani JW, et al. HIV infection is associated with variability in ventricular repolarization: the Multicenter AIDS Cohort Study (MACS). Circulation. 2020;141:176–187. doi: 10.1161/CIRCULATIONAHA.119.043042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Empana JP, Jouven X, Canouï‐Poitrine F, Luc G, Tafflet M, Haas B, Arveiler D, Ferrieres J, Ruidavets JB, Montaye M, et al. C‐reactive protein, interleukin 6, fibrinogen and risk of sudden death in European middle‐aged men: the PRIME study. Arterioscler Thromb Vasc Biol. 2010;30:2047–2052. doi: 10.1161/ATVBAHA.110.208785 [DOI] [PubMed] [Google Scholar]
- 23. Hussein AA, Gottdiener JS, Bartz TM, Sotoodehnia N, DeFilippi C, See V, Deo R, Siscovick D, Stein PK, Lloyd‐Jones D. Inflammation and sudden cardiac death in a community‐based population of older adults: the Cardiovascular Health Study. Heart Rhythm. 2013;10:1425–1432. doi: 10.1016/j.hrthm.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 24. Cheng A, Zhang Y, Blasco‐Colmenares E, Dalal D, Butcher B, Norgard S, Eldadah Z, Ellenbogen KA, Dickfeld T, Spragg DD, et al. Protein biomarkers identify patients unlikely to benefit from primary prevention implantable cardioverter defibrillators: findings from the Prospective Observational Study of Implantable Cardioverter Defibrillators (PROSE‐ICD). Circ Arrhythm Electrophysiol. 2014;7:1084–1091. doi: 10.1161/CIRCEP.113.001705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haverkamp W, Breithardt G, Camm AJ, Janse MJ, Rosen MR, Antzelevitch C, Escande D, Franz M, Malik M, Moss A, et al. The potential for QT prolongation and proarrhythmia by non‐antiarrhythmic drugs: clinical and regulatory implications. Report on a policy conference of the European Society of Cardiology. Eur Heart J. 2000;21:1216–1231. doi: 10.1053/euhj.2000.2249 [DOI] [PubMed] [Google Scholar]
- 26. Goldenberg I, Moss AJ. Long QT syndrome. J Am Coll Cardiol. 2008;51:2291–2300. doi: 10.1016/j.jacc.2008.02.068 [DOI] [PubMed] [Google Scholar]
- 27. Lazzerini PE, Cevenini G, Qu YS, Fabris F, El‐Sherif N, Acampa M, Cartocci A, Laghi‐Pasini F, Capecchi PL, Boutjdir M, et al. Risk of QTc interval prolongation associated with circulating anti‐Ro/SSA antibodies among US veterans: an observational cohort study. J Am Heart Assoc. 2021;10:e018735. doi: 10.1161/JAHA.120.018735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Akuka A, Ben‐Shabat N, Watad A, Tsur AM, Ehrenberg S, McGonagle D, Comaneshter D, Beinart R, Cohen AD, Amital H. Association of anti‐Ro seropositivity with cardiac rhythm and conduction disturbances. Eur Heart J. 2022;43:4912–4919. doi: 10.1093/eurheartj/ehac516 [DOI] [PubMed] [Google Scholar]
- 29. Lazzerini PE, Boutjdir M, Capecchi PL. Anti‐Ro/SSA‐antibodies and heart rhythm disturbances in the general population: the ‘dark side of the immune’. Eur Heart J. 2022;43:4920–4922. doi: 10.1093/eurheartj/ehac575 [DOI] [PubMed] [Google Scholar]
- 30. Murata M, Fukuda K, Ishida H, Miyoshi S, Koura T, Kodama H, Nakazawa HK, Ogawa S. Leukemia inhibitory factor, a potent cardiac hypertrophic cytokine, enhances L‐type Ca2+ current and [Ca2+]i transient in cardiomyocytes. J Mol Cell Cardiol. 1999;31:237–245. doi: 10.1006/jmcc.1998.0866 [DOI] [PubMed] [Google Scholar]
- 31. Takahashi E, Fukuda K, Miyoshi S, Murata M, Kato T, Ita M, Tanabe T, Ogawa S. Leukemia inhibitory factor activates cardiac L‐type Ca2+ channels via phosphorylation of serine 1829 in the rabbit Cav1.2 subunit. Circ Res. 2004;94:1242–1248. doi: 10.1161/01.RES.0000126405.38858.BC [DOI] [PubMed] [Google Scholar]
- 32. Hagiwara Y, Miyoshi S, Fukuda K, Nishiyama N, Ikegami Y, Tanimoto K, Murata M, Takahashi E, Shimoda K, Hirano T, et al. SHP2‐mediated signaling cascade through gp130 is essential for LIF‐dependent I CaL, [Ca2+]i transient, and APD increase in cardiomyocytes. J Mol Cell Cardiol. 2007;43:710–716. doi: 10.1016/j.yjmcc.2007.09.004 [DOI] [PubMed] [Google Scholar]
- 33. Aromolaran AS, Srivastava U, Alí A, Chahine M, Lazaro D, El‐Sherif N, Capecchi PL, Laghi‐Pasini F, Lazzerini PE, Boutjdir M. Interleukin‐6 inhibition of hERG underlies risk for acquired long QT in cardiac and systemic inflammation. PLoS One. 2018;13:e0208321. doi: 10.1371/journal.pone.0208321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhu X, Wang Y, Xiao Y, Gao Q, Gao L, Zhang W, Xin X, Chen K, Srivastava U, Ginjupalli VKM, et al. Arrhythmogenic mechanisms of interleukin‐6 combination with hydroxychloroquine and azithromycin in inflammatory diseases. Sci Rep. 2022;12:1075. doi: 10.1038/s41598-022-04852-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chowdhury MKH, Martinez‐Mateu L, Do J, Aromolaran KA, Saiz J, Aromolaran AS. Macrophage‐dependent Interleukin‐6‐production and inhibition of. Int J Mol Sci. 2021;22:11249. doi: 10.3390/ijms222011249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cupelli M, Ginjupalli VKM, Chen L, Capecchi PL, Lazzerini PE, Boutjdir M, El‐Sherif N. Contribution of cytokine‐mediated prolongation of QTc interval to the multi‐hit theory of torsade de pointes. Biochem Biophys Res Commun. 2023;655:82–89. doi: 10.1016/j.bbrc.2023.02.060 [DOI] [PubMed] [Google Scholar]
- 37. White CM. Inflammation suppresses patients' ability to metabolize cytochrome P450 substrate drugs. Ann Pharmacother. 2021;56:809–819. doi: 10.1177/10600280211047864 [DOI] [PubMed] [Google Scholar]
- 38. Dickmann LJ, Patel SK, Rock DA, Wienkers LC, Slatter JG. Effects of interleukin‐6 (IL‐6) and an anti‐IL‐6 monoclonal antibody on drug‐metabolizing enzymes in human hepatocyte culture. Drug Metab Dispos. 2011;39:1415–1422. doi: 10.1124/dmd.111.038679 [DOI] [PubMed] [Google Scholar]
- 39. Lazzerini PE, Cantara S, Bertolozzi I, Accioli R, Salvini V, Cartocci A, D'Errico A, Sestini F, Bisogno S, Cevenini G, et al. Transient hypogonadism is associated with heart rate‐corrected QT prolongation and torsades de pointes risk during active systemic inflammation in men. J Am Heart Assoc. 2022;11:e023371. doi: 10.1161/JAHA.121.023371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lazzerini PE, Laghi‐Pasini F, Acampa M, Boutjdir M, Leopoldo CP. IL‐6 (interleukin 6) blockade and heart rate corrected QT interval prolongation in COVID‐19. Circ Arrhythm Electrophysiol. 2020;13:e008791. doi: 10.1161/CIRCEP.120.008791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Van Tassell BW, Canada J, Carbone S, Trankle C, Buckley L, Oddi Erdle C, Abouzaki NA, Dixon D, Kadariya D, Christopher S, et al. Interleukin‐1 blockade in recently decompensated systolic heart failure: results from REDHART (Recently Decompensated Heart Failure Anakinra Response trial). Circ Heart Fail. 2017;10:e004373. doi: 10.1161/CIRCHEARTFAILURE.117.004373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Van Tassell BW, Trankle CR, Canada JM, Carbone S, Buckley L, Kadariya D, Del Buono MG, Billingsley H, Wohlford G, Viscusi M, et al. IL‐1 blockade in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2018;11:e005036. doi: 10.1161/CIRCHEARTFAILURE.118.005036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Abbate A, Trankle CR, Buckley LF, Lipinski MJ, Appleton D, Kadariya D, Canada JM, Carbone S, Roberts CS, Abouzaki N, et al. Interleukin‐1 blockade inhibits the acute inflammatory response in patients with ST‐segment‐elevation myocardial infarction. J Am Heart Assoc. 2020;9:e014941. doi: 10.1161/JAHA.119.014941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kleveland O, Kunszt G, Bratlie M, Ueland T, Broch K, Holte E, Michelsen AE, Bendz B, Amundsen BH, Espevik T, et al. Effect of a single dose of the interleukin‐6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non‐ST‐elevation myocardial infarction: a double‐blind, randomized, placebo‐controlled phase 2 trial. Eur Heart J. 2016;37:2406–2413. doi: 10.1093/eurheartj/ehw171 [DOI] [PubMed] [Google Scholar]
- 45. Broch K, Anstensrud AK, Woxholt S, Sharma K, Tøllefsen IM, Bendz B, Aakhus S, Ueland T, Amundsen BH, Damås JK, et al. Randomized trial of Interleukin‐6 receptor inhibition in patients with acute ST‐segment elevation myocardial infarction. J Am Coll Cardiol. 2021;77:1845–1855. doi: 10.1016/j.jacc.2021.02.049 [DOI] [PubMed] [Google Scholar]
- 46. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 47. Krisai P, Blum S, Schnabel RB, Sticherling C, Kühne M, von Felten S, Ammann P, Pruvot E, Albert CM, Conen D. Canakinumab after electrical cardioversion in patients with persistent atrial fibrillation: a pilot randomized trial. Circ Arrhythm Electrophysiol. 2020;13:e008197. doi: 10.1161/CIRCEP.119.008197 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4


