Abstract
Background
Patent foramen ovale (PFO) is causally associated with stroke in some patients younger than 60 years, especially when it is large or associated with an atrial septal aneurysm (ASA). After 60 years of age, this association is less well understood. We assessed the relationships between detailed atrial septal anatomy and the cryptogenic nature of stroke in this population.
Methods and Results
We reviewed all patients aged 60 to 80 years admitted to our stroke center for ischemic stroke who underwent contrast echocardiography between 2016 and 2021. The atherosclerosis, small‐vessel disease, cardiac pathology, other causes, and dissection (ASCOD) classification was used to reevaluate the etiological workup. Associations between cryptogenic stroke and (1) PFO presence or (2) categories of PFO anatomy (nonlarge PFO without ASA, nonlarge PFO with ASA, large PFO without ASA, and large PFO with ASA) were assessed using logistic regression. Among 533 patients (median National Institutes of Health Stroke Scale score=1), PFO was present in 152 (prevalence, 28.5% [95% CI, 24.9–32.5]). Compared with noncryptogenic stroke, cryptogenic stroke (n=218) was associated with PFO presence (44.5% versus 17.5%; P<0.0001). Among patients with a PFO, septal anatomy categories were associated with cryptogenic stroke (P=0.02), with a strong association for patients with both large PFO and ASA (38.1% versus 14.5%, P=0.002).
Conclusions
PFO presence remains strongly associated with cryptogenic stroke between 60 and 80 years of age. Large PFO, ASA, and their association were strongly associated with cryptogenic stroke in this age group. Our results support performing contrast echocardiography even after 60 years of age, although the optimal secondary prevention therapy in this population remains to be determined in randomized trials.
Keywords: cryptogenic stroke, etiological assessment, older patients, patent foramen ovale
Subject Categories: Ischemic Stroke, Echocardiography, Etiology
Nonstandard Abbreviations and Acronyms
- ASA
atrial septal aneurysm
- ASCOD
atherosclerosis, small‐vessel disease, cardiac pathology, other causes, and dissection
- PFO
patent foramen ovale
- TEE
transesophageal echocardiography
- TTE
transthoracic echocardiography
Clinical Perspective.
What Is New?
This study provides novel evidence regarding the associations between detailed atrial septal anatomy and stroke etiological assessment in patients aged 60 to 80 years.
Approximately 45% of patients with a cryptogenic stroke have a patent foramen ovale (PFO), compared with 18% of patients with a stroke of identified cause.
Atrial septal anatomy is significantly associated with the cryptogenic nature of the stroke in this age group, with the strongest association observed in patients with both large PFO and atrial septal aneurysm.
What Are the Clinical Implications?
These exploratory findings suggest that even in this age group, some PFOs may be related to stroke and that detailed analysis of septal anatomy may help distinguish culprit from incidental PFOs.
It is unlikely that the presence of a PFO can be reliably predicted on the basis of clinical criteria, suggesting that a contrast echocardiography should be performed even after 60 years of age.
Randomized controlled trials are warranted to determine the optimal secondary prevention therapy in patients older than 60 years with PFO and otherwise cryptogenic stroke.
Several randomized trials have demonstrated that percutaneous patent foramen ovale (PFO) closure reduces the risk of recurrent ischemic stroke by 60% compared with medical therapy alone in patients younger than 60 years of age for whom no alternative cause of stroke has been identified. 1 , 2 , 3 , 4 , 5 Septal anatomic features, including the presence of a large shunt or an atrial septal aneurysm (so‐called “high‐risk PFOs”), can help distinguish culprit from incidental PFOs and identify patients up to 60 years of age who may benefit the most from PFO closure. 6 , 7 , 8 , 9 , 10 The existence of a causal relationship between PFO and stroke remains debated in older patients due to the higher prevalence of vascular risk factors and alternative causes of stroke. 11 However, the prevalence of PFO remains higher in cryptogenic than in noncryptogenic stroke patients older than 60 years, 12 , 13 suggesting that some PFOs are responsible for stroke even in this age group. We hypothesize that atrial septal anatomy may help identify patients with culprit PFO beyond age 60.
Our aim was to determine the prevalence of PFO and the associations between septal anatomy and cryptogenic stroke in a thoroughly phenotyped cohort of patients aged 60 to 80 years with recent ischemic stroke who underwent routine transthoracic or transesophageal contrast echocardiography.
METHODS
Participants
This retrospective study was prepared in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement. 14 We reviewed the patients admitted to a single comprehensive stroke center (Sainte‐Anne Hospital, Paris, France) for acute ischemic stroke between January 2016 and October 2021. In this center, patients aged up to 80 years with no contraindication to transesophageal echocardiography (TEE) are routinely scheduled for both transthoracic echocardiography (TTE) and TEE with contrast study, 1 , 15 , 16 unless (1) they are deemed unable to withstand TEE (eg, swallowing disorders, vomiting, altered consciousness, severe stroke, cognitive impairment, or frailty) or (2) a major alternative cause is immediately identified (eg, severe stenosis [>70%] or occlusion attributed to atherosclerosis in the stroke territory, atrial fibrillation, dissection). Inclusion criteria for the present study were (1) age between 60 and 80 years; (2) recent ischemic stroke demonstrated on imaging (diffusion weighted‐imaging lesion on magnetic resonance imaging or hypodensity on computed tomography); (3) intention to perform TEE. We did not exclude patients who could not tolerate TEE and only underwent contrast TTE (n=19). Patients with a transient ischemic attack (ie, transient episode of focal neurological dysfunction caused by focal brain ischemia without acute infarction) or who did not undergo contrast echocardiography were excluded.
Clinical Data
The following variables were collected from medical records: vascular risk factors (age, sex, history of hypertension, diabetes, current smoking, use of lipid‐lowering therapy), medical history (ischemic stroke, transient ischemic attack, coronary heart disease, atrial fibrillation or flutter, venous thromboembolic disease, and cancer), antithrombotic treatment on admission (antiplatelets, anticoagulants), stroke severity according to the National Institutes of Health Stroke Scale (NIHSS) score on admission. The diagnosis of previous stroke was based on a review of the patient's medical record. It was operationally defined as a history of focal neurological deficit with a corresponding infarct documented on imaging.
Echocardiographic Studies and Definition of PFO Anatomy Categories
TTEs and TEEs were performed by cardiologists using the same PHILIPS IU22 echocardiography machine throughout the study period. TTEs were performed in dorsal decubitus, or in left lateral decubitus, according to standard practice. After TTE, TEE was performed under local anesthesia with lidocaine and if necessary, after mild sedation with a 1‐mg intravenous bolus of midazolam. Contrast study was performed during TTE and repeated during TEE using direct intravenous injection of manually agitated saline solution into the upper limb. The presence of a PFO was defined by the appearance of at least 3 microbubbles in the left atrium within the first 3 cardiac cycles after complete opacification of the right atrium either spontaneously or after a provocative Valsalva maneuver. For the present study, we reviewed the echocardiographic examination report of each patient and defined large PFO as the appearance of >20 microbubbles in the left atrium. 17 Atrial septal aneurysm (ASA) was defined as a phasic septal excursion of >10 mm. 17 In case of discordance between TTE and TEE findings for the presence of PFO or quantification of right‐to‐left shunt, the modality with the higher value was selected. The reference standard for assessing the presence of an ASA was TEE. Presence of left atrial enlargement (>20 cm2) was also systematically investigated using planimetry in the apical 4‐chamber view. 18 , 19 In case of discordance between TTE and TEE for this measurement, the TTE result was retained. 20 , 21 , 22 Patients with a PFO were retrospectively classified into 4 prespecified categories, 9 as follows: nonlarge PFO without ASA, nonlarge PFO with ASA, large PFO without ASA, and large PFO with ASA.
Etiological Workup and Definition of Cryptogenic Stroke
The extent and results of each patient's etiological workup were reviewed and retrospectively classified according to the atherosclerosis, small‐vessel disease, cardiac pathology, other causes and dissection (ASCOD) criteria. 23 We decided a priori to define cryptogenic stroke as any ischemic stroke that did not fulfill any grade 1 (potentially causal) or grade 2 (uncertain causal link) ASCOD category, excluding all PFO‐related items (see Table S1 for details). Presence of a PFO was therefore never considered to be a cause of stroke for the purpose of this study. We performed a sensitivity analysis by classifying patients with grade 1, 2, or 3 for any ASCOD category (except for PFO‐related items) as having a noncryptogenic stroke.
Statistical Analysis
Categorical variables were expressed as numbers and percentages and compared using Pearson's χ 2 test or Fisher's exact test, as appropriate. Quantitative variables were expressed as mean and SD or median and interquartile range and compared using Student's t test, or Mann–Whitney U test, as appropriate. P values were not adjusted for multiple hypothesis testing. The prevalence of a PFO and each septal anatomy category was calculated in the whole cohort and in the subgroups of patients with cryptogenic and noncryptogenic stroke. The 95% CIs were estimated using the Wilson method. Associations between cryptogenic stroke and the presence of (1) a PFO, or (2) each PFO anatomic category were assessed by calculating crude and adjusted odds ratios (OR) and their 95% CI. The multivariable model included the following potential confounders: age, sex, vascular risk factors significantly associated with cryptogenic stroke in univariable analysis, and antithrombotic treatment (anticoagulants, antiplatelets).
We also investigated factors (ie, potential predictors) associated with the presence of a PFO in the subgroup of patients with cryptogenic stroke. We conducted post hoc subgroup analyses stratified by age groups: 60 to 69 and 70 to 80 years old. All tests were 2 tailed and P<0.05 was considered statistically significant. Statistical analysis was conducted with SAS 9.4 (SAS Institute, Cary, NC).
Ethical Considerations
According to French legislation, as this study involved only retrospective analysis of pseudonymized data collected as part of routine care, neither formal approval by an ethics committee nor written informed consent was required. Each patient was informed of their participation in this study and given the opportunity to withdraw. The raw, anonymized data that support the findings of this study are available from the corresponding author upon reasonable request and after signing a data transfer and use agreement.
RESULTS
Population Characteristics and Prevalence of PFO
During the study period, 703 patients aged 60 to 80 years were admitted to our center for a recent stroke and scheduled for TEE. Among them, 163 patients (23.2%) did not undergo contrast study, mostly because a cause of stroke was identified before echocardiography (see Figure S1 for details). Seven patients declined to have their data used for this study, leaving 533 patients for analysis. Of note, 19 (3.6%) of these 533 patients failed to tolerate and therefore could not undergo TEE (see Methods). Mean age was 68.4±5.4 years, and 344 (64.5%) patients were men. Most patients had at least 1 vascular risk factor (Table 1) and 33 (6.2%) had a history of atrial fibrillation or flutter. The median NIHSS score on admission was 1 (interquartile range, 0–3). Brain magnetic resonance imaging was performed in 517 (97.0%) patients. All patients had intra‐ and extracranial arterial imaging (Table 1). The prevalence of PFO was 28.5% (95% CI, 24.9–32.5).
Table 1.
Population Characteristics
| Variable | All (n=533) | Cryptogenic stroke (n=218) | Noncryptogenic stroke (n=315) | P value |
|---|---|---|---|---|
| Age, y, mean (SD) | 68.4 (5.4) | 68.3 (5.2) | 68.4 (5.5) | 0.71 |
| Male sex | 344 (64.5%) | 142 (65.1%) | 202 (64.1%) | 0.85 |
| Hypertension | 321 (60.2%) | 107 (49.1%) | 214 (67.9%) | <0.0001 |
| Diabetes | 124 (23.3%) | 35 (16.1%) | 89 (28.3%) | <0.0001 |
| Current smoking | 118 (22.1%) | 42 (19.3%) | 76 (24.1%) | 0.18 |
| Lipid lowering medication | 217 (40.7%) | 82 (37.6%) | 135 (42.9%) | 0.23 |
| Antiplatelet medication | 180 (33.8%) | 61 (28.0%) | 119 (37.8%) | 0.02 |
| Vitamin K antagonists | 20 (3.8%) | 2 (0.9%) | 18 (5.7%) | <0.0001 |
| Direct oral or other anticoagulant | 22 (4.1%) | 6 (2.8%) | 16 (5.1%) | 0.19 |
| Previous ischemic stroke | 58 (10.9%) | 21 (9.6%) | 37 (11.7%) | 0.44 |
| Previous transient ischemic attack | 41 (7.7%) | 18 (8.3%) | 23 (7.3%) | 0.68 |
| Previous coronary heart disease | 83 (15.6%) | 22 (10.1%) | 61 (19.4%) | <0.0001 |
| Atrial fibrillation* | 32 (6.0%) | 1 (0.5%)† | 31 (9.8%) | <0.0001 |
| Previous deep vein thrombosis or pulmonary embolism | 20 (3.8%) | 9 (4.1%) | 11 (3.5%) | 0.7 |
| Previous cancer | 67 (12.6%) | 27 (12.4%) | 40 (12.7%) | 0.91 |
| NIHSS score on admission, median (IQR) | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) | 2.0 (0.0–4.0) | <0.0001 |
| Magnetic resonance imaging performed | 517 (97.0%) | 213 (97.7%) | 304 (96.5%) | 0.84 |
| Contrast‐enhanced magnetic resonance angiography performed | 447 (83.9%) | 189 (86.7%) | 258 (81.9%) | 0.14 |
| Computed tomography angiography performed | 173 (32.5%) | 55 (25.2%) | 118 (37.5%) | <0.0001 |
| Ultrasound performed | 221 (41.5%) | 73 (33.5%) | 148 (47.0%) | <0.0001 |
| 24‐h ECG monitoring | 88 (16.5%) | 38 (17.4%) | 50 (15.9%) | 0.63 |
| Prolonged ECG monitoring | 339 (63.6%) | 166 (76.1%) | 173 (54.9%) | <0.0001 |
| Anticoagulation at discharge | 91 (17.1%) | 30 (13.8%) | 61 (19.4%) | 0.09 |
| Time between event and echocardiography, d, median (IQR) | 5.0 (3.0, 7.0) | 4.0 (3.0, 7.0) | 5.0 (3.0, 7.0) | 0.98 |
| Dilated left atrium | 225 (42.2%) | 80 (36.7%) | 145 (46.0%) | 0.03 |
| PFO presence | 152 (28.5%) | 97 (44.5%) | 55 (17.5%) | <0.0001 |
| PFO categories | 0.02 | |||
| Large PFO with ASA | 45 (8.4%) | 37 (17.0%) | 8 (2.5%) | |
| Large PFO without ASA | 38 (7.1%) | 23 (10.6%) | 15 (4.8%) | |
| Nonlarge PFO with ASA | 20 (3.8%) | 11 (5.0%) | 9 (2.9%) | |
| Nonlarge PFO without ASA | 49 (9.2%) | 26 (11.9%) | 23 (7.3%) |
ASA indicates atrial septum aneurysm; IQR, interquartile range; NIHSS, National Institutes of Health Stroke Scale; and PFO, patent foramen ovale.
Diagnosed after transesophageal echocardiography was performed.
Two very brief episodes of atrial fibrillation were recorded but were not considered causal in this patient because they occurred in a postoperative setting with concomitant deep vein thrombosis and PFO.
Association Between PFO and Cryptogenic Stroke
A total of 218 (40.9%, [95% CI, 36.8–45.1]) and 315 (59.1% [95% CI, 54.9–63.2]) patients had a stroke classified as cryptogenic and noncryptogenic, respectively. Baseline characteristics according to the cryptogenic nature of the stroke are presented in Table 1. Compared with noncryptogenic stroke, patients with cryptogenic stroke were more likely to have a PFO (44.5% versus 17.5%, P<0.0001, Table 1). This association remained similar in the sensitivity analysis in which noncryptogenic stroke was defined as any ASCOD category of 1, 2, or 3 (data not shown) and in the post hoc subgroup analysis stratified by age (Data S1).
Factors Associated With the Presence of a PFO Among Patients With Cryptogenic Stroke
In the subgroup of patients with cryptogenic stroke (Table 2), the presence of a PFO was associated with a history of previous ischemic stroke (14.4% versus 5.8%, P=0.03) and inversely associated with diabetes (9.3% versus 21.5%, P=0.01). An inverse association between the presence of a PFO and history of hypertension failed to reach statistical significance (42.3% versus 54.5%, P=0.07). In the sensitivity analysis, no association remained significant (data not shown).
Table 2.
Factors Associated With the Presence of PFO in the Subgroup of Patients With Cryptogenic Stroke
| Variable | All (n=218) | PFO (n=97) | No PFO (n=121) | P value |
|---|---|---|---|---|
| Age, y, mean (SD) | 68.0 (64.0–72.0) | 67.0 (64.0–72.0) | 68.0 (64.0–72.0) | 0.53 |
| Male sex | 142 (65.1%) | 63 (64.9%) | 79 (65.3%) | 0.96 |
| Hypertension | 107 (49.1%) | 41 (42.3%) | 66 (54.5%) | 0.07 |
| Diabetes | 35 (16.1%) | 9 (9.3%) | 26 (21.5%) | 0.01 |
| Current smoking | 42 (19.3%) | 17 (17.5%) | 25 (20.7%) | 0.56 |
| Lipid lowering medication | 82 (37.6%) | 39 (40.2%) | 43 (35.5%) | 0.48 |
| Antiplatelet medication | 61 (28.0%) | 28 (28.9%) | 33 (27.3%) | 0.79 |
| Vitamin K antagonists | 2 (0.9%) | 1 (1.0%) | 1 (0.8%) | 0.87 |
| Direct oral or other anticoagulant | 6 (2.8%) | 2 (2.1%) | 4 (3.3%) | 0.59 |
| Previous ischemic stroke | 21 (9.6%) | 14 (14.4%) | 7 (5.8%) | 0.03 |
| Previous transient ischemic attack | 18 (8.3%) | 11 (11.3%) | 7 (5.8%) | 0.14 |
| Previous coronary heart disease | 22 (10.1%) | 7 (7.2%) | 15 (12.4%) | 0.21 |
| Atrial fibrillation | 2 (0.9%) | 1 (1.0%) | 1 (0.8%) | 0.88 |
| Previous deep vein thrombosis or pulmonary embolism | 9 (4.1%) | 5 (5.2%) | 4 (3.3%) | 0.50 |
| Previous cancer | 27 (12.4%) | 14 (14.4%) | 13 (10.7%) | 0.41 |
| NIHSS score on admission, median (IQR) | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) | 0.96 |
| Magnetic resonance imaging performed | 213 (97.7%) | 94 (96.9%) | 119 (98.3%) | 0.48 |
| Contrast‐enhanced magnetic resonance angiography performed | 189 (86.7%) | 86 (88.7%) | 103 (85.1%) | 0.44 |
| Computed tomography angiography performed | 55 (25.2%) | 25 (25.8%) | 30 (24.8%) | 0.83 |
| Ultrasound performed | 73 (33.5%) | 27 (27.8%) | 46 (38.0%) | 0.11 |
| 24‐h ECG monitoring | 38 (17.4%) | 15 (15.5%) | 23 (19.0%) | 0.49 |
| Prolonged ECG monitoring | 166 (76.1%) | 79 (81.4%) | 87 (71.9%) | 0.10 |
| Anticoagulation at discharge | 30 (13.8%) | 19 (19.6%) | 11 (9.1%) | 0.03 |
| Time between event and echocardiography, d, median (IQR) | 4.0 (3.0–7.0) | 4.0 (3.0–6.0) | 5.0 (3.0–9.0) | 0.12 |
| Dilated left atrium | 80 (36.7%) | 30 (30.9%) | 50 (41.3%) | 0.11 |
IQR indicates interquartile range; NIHSS, National Institutes of Health Stroke Scale; and PFO, patent foramen ovale.
Detailed PFO Anatomy
In the whole cohort, the prevalence of the each PFO anatomic category was as follows (Table 1): large PFO with ASA: 8.4% (95% CI, 6.4–11.1), large PFO without ASA: 7.1% (95% CI, 5.2–9.6), nonlarge PFO with ASA: 3.8% (95% CI, 2.4–5.7), and nonlarge PFO without ASA: 9.2% (95% CI, 7.0–12.0).
In the subgroup of patients with PFO (n=152), the cryptogenic (versus noncryptogenic) nature of the stroke was associated with variation in septal anatomy (P=0.02; Table S2; Figure S1). Compared with noncryptogenic stroke, cryptogenic stroke was more frequent in the “large PFO with ASA” category (38.1% versus 14.5%, P=0.002) and was nonsignificantly less frequent in the “non‐large PFO without ASA category” (26.8 versus 41.8%, P=0.06, Figure 1; Table S2). Using the category “nonlarge PFO without ASA” as a reference, the category “large PFO with ASA” was strongly associated with cryptogenic stroke (crude OR, 4.09 [95% CI, 1.58–10.56], P=0.004; adjusted OR, 4.35 [95% CI, 1.61–11.75], P=0.004, Figure 2). All other variations of septal anatomy showed no statistical difference compared with the latter reference category. Post hoc subgroup analysis stratified by age yielded similar results (Data S1).
Figure 1. Prevalence of atrial septal anatomic features classified into 4 categories in the subgroup of patients with PFO.
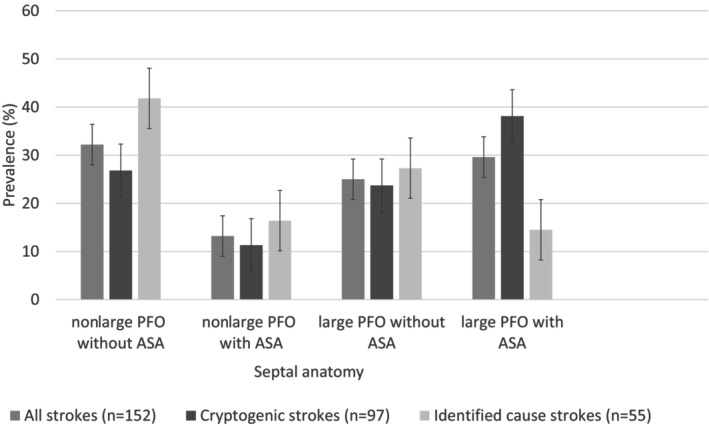
The “whiskers” represent the 95% CI of each prevalence. ASA indicates atrial septal aneurysm; and PFO, patent foramen ovale.
Figure 2. Association between PFO anatomic features and cryptogenic stroke (multivariable analysis*).
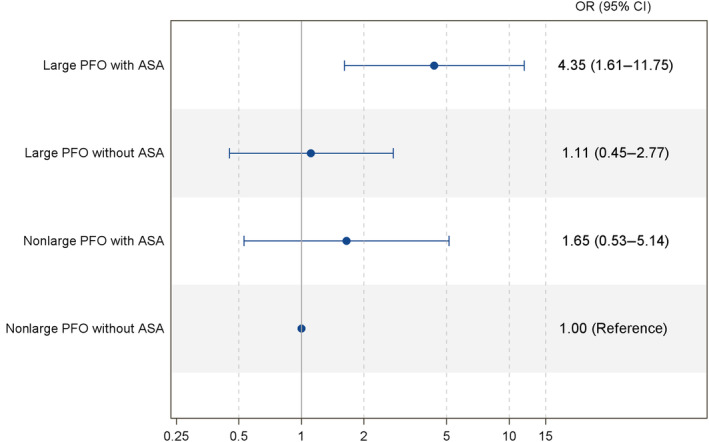
*Adjusted on history of age, sex, hypertension, diabetes, and antithrombotic treatment. ASA indicates atrial septal aneurysm; OR, odds ratio; and PFO, patent foramen ovale.
DISCUSSION
This study disclosed the following salient findings. Approximately 45% of patients aged 60 to 80 years with a cryptogenic stroke had a PFO, which is more than double the prevalence observed in noncryptogenic stroke. Presence of a PFO was associated with a history of previous stroke and inversely associated with diabetes. Among patients with a PFO, the association of large shunt and ASA was strongly associated with cryptogenic stroke.
Although alternative causes of stroke are more common after age 60 than in younger patients, the high prevalence of PFO in our cohort (28.5%) suggests that PFO may be involved in the occurrence of stroke in this age group. Conflicting results have been reported regarding the association between PFO and cryptogenic stroke in older patients, 24 , 25 until the publication of 2 large studies. 12 , 13 In a prospective single‐center observational study of patients who underwent contrast TTE and TEE, Handke et al showed that the prevalence of PFO was greater among patients with cryptogenic stroke than among those with noncryptogenic stroke, even in the subgroup of patients aged 55 to 85 years (n=372). 12 However, the accuracy of the classification of cryptogenic stroke in this study has been questioned as only half of included patients had an angiographic study of the intracranial arteries. 26 In a population‐based cohort of patients with transient ischemic attack (72%) or minor stroke (28%) who underwent contrast‐enhanced transcranial Doppler, Mazzucco et al showed that right‐to‐left shunting was more frequent in cryptogenic than in noncryptogenic stroke among patients aged over 60 (n=397). 13 Our results are consistent with these findings. The prevalence of PFO in the cryptogenic group was particularly high in our cohort (44.5%, compared with 28% in Handke et al 12 and 36% in Mazzucco et al). 13 This may be explained by the use of a different definition of cryptogenic stroke. Although not originally designed to define the cryptogenic nature of stroke, we decided to use the ASCOD classification because it allows detailed and comprehensive pathogenetic phenotyping. Of note, the proportion of cryptogenic strokes in our cohort (41%) was comparable to those reported in the literature. 12 , 13 , 27
Determining whether a PFO is likely to be stroke related is difficult in clinical practice. The Risk of Paradoxical Embolism (RoPE) score has been proposed for this purpose, 11 , 28 but it may be of limited interest for our population because it gives a very large weight to age, leading to a ceiling effect. Another potential limitation of the RoPE score is that patients with the highest scores are those with the lowest risk of recurrence, making its use difficult for selecting patients for targeted preventive therapy. More recently, the PFO‐Associated Stroke Causal Likelihood classification system has been proposed based on an individual participant data meta‐analysis of randomized trials of PFO closure in young patients (Systematic, Collaborative, PFO Closure Evaluation collaboration). 10 It combines the RoPE score with septal morphological characteristics (presence of a large PFO or associated ASA), which are thought to help identify those patients with stroke‐related PFOs in this age group. In young patients, the presence of either feature is associated with a higher absolute risk of recurrence with medical therapy 16 , 29 and a greater benefit from percutaneous closure. 30 In older patients, Mazzucco et al had observed an association between large PFO and cryptogenic stroke that did not quite reach statistical significance (adjusted OR, 1.94 [95% CI, 0.99–3.80]). 13 Of note, the imaging modality used in this study does not allow detection of ASA. 13 Handke et al observed an association between PFO‐ASA and cryptogenic stroke in older patients (OR, 3.88 [95% CI, 1.78–8.46]), but no quantification of shunt size was reported. 12 We decided to further investigate the potential importance of septal anatomy by using 4 PFO categories according to shunt size and ASA status. 9 , 29 Indeed, a recent analysis from the Systematic, Collaborative, PFO Closure Evaluation collaboration demonstrated that young patients with both large shunt and ASA derive the most benefit from PFO closure (number needed to treat at 2 years: 18, versus approximately 100 for other anatomic categories). 9 To our knowledge, our study is the first to provide estimates of the prevalence of each PFO anatomic category in older patients with cryptogenic and noncryptogenic stroke. The descriptive associations we observed are in line with the hypothesis that septal anatomy may also play an important role in determining whether a PFO is incidental or causally related in older patients. However, our cross‐sectional design precludes formal causal inference. Another important finding is that, even in this age group, there appears to be a “synergistic” association between large shunt and ASA, as this anatomic category was strongly associated with the cryptogenic nature of the stroke (OR, ≈4). However, it is worth noting that the majority of our population comprises patients with very low stroke severity (low NIHSS scores; see Limitations). It remains to be investigated whether comparable associations are observed in older patients with moderate‐to‐severe stroke.
The mechanism by which a PFO would lead to stroke only after a period of more than 60 years is uncertain. Because the incidence of venous thromboembolism increases with age, 31 paradoxical embolism may be the main mechanism. However, our work did not show an overrepresentation of a history of venous thromboembolic disease or cancer in patients with cryptogenic stroke and PFO (Table 2). 32 , 33 These findings are consistent with several studies that have shown that concomitant deep vein thrombosis is seldomly diagnosed in patients with recent stroke and PFO, suggesting other underlying mechanisms, such as in situ thrombus formation within the septal aneurysm. 34 , 35 , 36 Another hypothesis would be an association between the presence of a PFO and the presence of an atrial cardiomyopathy. 37 However, we observed no signal of an overrepresentation of left atrial enlargement associated with PFO among patients with cryptogenic stroke (Table 2). This finding is consistent with recent results suggesting that atrial cardiopathy and probable pathogenic PFO are competing causes in patients with embolic stroke of undetermined source. 38 One explanation as to why a PFO becomes “symptomatic” only after age 60 could simply be that the PFO was either not considered causal or even not detected at the time of a previous ischemic stroke. Indeed, among patients with cryptogenic stroke, we observed a significant association between PFO presence and a previous history of stroke. We did not find any other association with baseline variables for the presence of a PFO, except for the absence of diabetes. Thus, it is highly unlikely that the presence of a PFO can be reliably predicted on the basis of clinical criteria in this age group. Our results suggest that it may be desirable to perform a contrast study even after the age of 60 years in patients with cryptogenic stroke, although the optimal secondary prevention therapy in this population is not yet known. The CLOSE‐2 (PFO Closure, Oral Anticoagulants or Antiplatelet Therapy After PFO‐Associated Stroke in Patients Aged 60 to 80 years) randomized trial (NCT05387954) and the COACH_ESUS (Prospective Registry of Elderly ESUS [Embolic Stroke of Undetermined Source] With PFO) prospective registry (NCT05238610) will address this issue. The high prevalence of PFO in this population in our cohort (45%) highlights the feasibility of such multicenter studies.
Our study has several strengths. To our knowledge, this is the first study to focus on the associations between detailed septal anatomy and detailed etiological assessment (using the ASCOD classification) in 60‐ to 80‐year‐old patients with acute ischemic stroke. We decided a priori not to include patients with transient ischemic attack because their inclusion would have inevitably led to a risk of classification bias, with some transient nonischemic events that might have diluted relevant associations. A very large proportion (97%) of the patients included had a magnetic resonance imaging‐confirmed diagnosis of stroke, and each patient had a thorough etiological workup performed, including both TTE and TEE in 96% of included patients. This approach allows a thorough analysis of the septal anatomy with both quantification of the PFO and assessment of the presence of an ASA.
Several limitations must be acknowledged. First, our results cannot be generalized to all patients in this age group with acute ischemic stroke. Indeed, we relied on an intention to perform TEE, which implied the judgment of the attending neurologist (which was not recorded) on (1) the ability of the patient to withstand TEE, therefore excluding patients with swallowing disorders, vomiting, altered consciousness, severe stroke, cognitive impairment, or frailty; and (2) the absence of an already identified cause of stroke. The intention to perform TEE depended on this judgment rather than a standardized prospective evaluation with ASCOD. Furthermore, only patients who underwent echocardiography at our institution were included, relying on data extracted from the local picture archiving and communicating system. Therefore, selection bias is possible and could account for the low median NIHSS score in our cohort (1, interquartile range, 0–3). Characteristics of patients deemed not eligible for TEE were not collected in this study. Second, there was no blinded analysis of the echocardiograms and the etiological workup. However, the variables were collected from the medical and examination reports, and the etiological classification was based on the rigid ASCOD framework. Third, it could be assumed that we misclassified some patients as cryptogenic stroke because of a less thorough workup: indeed, arterial exploration appears to be less detailed in patients with cryptogenic stroke (Table 1). However, it is likely that patients with a suspected arterial abnormality on initial vascular imaging were more likely to have had multiple arterial imaging modalities. All patients had satisfactory intracranial and extracranial vascular exploration according to ASCOD, including at least 1 imaging modality. Fourth, results of our univariable analyses should be considered as exploratory. Finally, some of the nonstatistically significant results could be affected by limited statistical power.
CONCLUSIONS
In conclusion, the prevalence of PFO is more than double that of noncryptogenic stroke, with approximately 45% of patients aged 60 to 80 years with cryptogenic stroke having a PFO. Among patients with a PFO, the association of large shunt and ASA was strongly associated with cryptogenic stroke, suggesting that these features may help identify patients older than 60 years with pathogenetic PFO, although our study design precludes formal causal inference. It is highly unlikely that the presence of a PFO can be reliably predicted on the basis of clinical criteria, suggesting that a contrast study should be performed even after 60 years of age. The optimal secondary prevention therapy in this age group remains to be determined in randomized trials.
Sources of Funding
None.
Disclosures
None.
Supporting information
Data S1
Tables S1–S3
Figure S1
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.031684
This article was sent to Jose R. Romero, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Mas J‐L, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L, Arquizan C, Béjot Y, Vuillier F, Detante O, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. 2017;377:1011–1021. doi: 10.1056/NEJMoa1705915 [DOI] [PubMed] [Google Scholar]
- 2. Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, Marks DS, Tirschwell DL. Long‐term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377:1022–1032. doi: 10.1056/NEJMoa1610057 [DOI] [PubMed] [Google Scholar]
- 3. Søndergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen‐Kudsk JE, Settergren M, Sjöstrand C, Roine RO, Hildick‐Smith D, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017;377:1033–1042. doi: 10.1056/NEJMoa1707404 [DOI] [PubMed] [Google Scholar]
- 4. Lee PH, Song J‐K, Kim JS, Heo R, Lee S, Kim D‐H, Song J‐M, Kang D‐H, Kwon SU, Kang D‐W, et al. Cryptogenic stroke and high‐risk patent foramen ovale: the DEFENSE‐PFO trial. J Am Coll Cardiol. 2018;71:2335–2342. doi: 10.1016/j.jacc.2018.02.046 [DOI] [PubMed] [Google Scholar]
- 5. Turc G, Calvet D, Guérin P, Sroussi M, Chatellier G, Mas J, the CLOSE Investigators . Closure, anticoagulation, or antiplatelet therapy for cryptogenic stroke with patent foramen ovale: systematic review of randomized trials, sequential meta‐analysis, and new insights from the CLOSE study. J Am Heart Assoc. 2018;7:e008356. doi: 10.1161/JAHA.117.008356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cabanes L, Mas JL, Cohen A, Amarenco P, Cabanes PA, Oubary P, Chedru F, Guérin F, Bousser MG, de Recondo J. Atrial septal aneurysm and patent foramen ovale as risk factors for cryptogenic stroke in patients less than 55 years of age. A study using transesophageal echocardiography. Stroke. 1993;24:1865–1873. doi: 10.1161/01.STR.24.12.1865 [DOI] [PubMed] [Google Scholar]
- 7. Serena J, Segura T, Perez‐Ayuso MJ, Bassaganyas J, Molins A, Dávalos A. The need to quantify right‐to‐left shunt in acute ischemic stroke: a case‐control study. Stroke. 1998;29:1322–1328. doi: 10.1161/01.STR.29.7.1322 [DOI] [PubMed] [Google Scholar]
- 8. Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke. Neurology. 2000;55:1172–1179. doi: 10.1212/WNL.55.8.1172 [DOI] [PubMed] [Google Scholar]
- 9. Mas J‐L, Saver JL, Kasner SE, Nelson J, Carroll JD, Chatellier G, Derumeaux G, Furlan AJ, Herrmann HC, Jüni P, et al. Association of atrial septal aneurysm and shunt size with stroke recurrence and benefit from patent foramen ovale closure. JAMA Neurol. 2022;79:1175–1179. doi: 10.1001/jamaneurol.2022.3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kent DM, Saver JL, Kasner SE, Nelson J, Carroll JD, Chatellier G, Derumeaux G, Furlan AJ, Herrmann HC, Jüni P, et al. Heterogeneity of treatment effects in an analysis of pooled individual patient data from randomized trials of device closure of patent foramen ovale after stroke. JAMA. 2021;326:2277. doi: 10.1001/jama.2021.20956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kent DM, Ruthazer R, Weimar C, Mas J‐L, Serena J, Homma S, Di Angelantonio E, Di Tullio MR, Lutz JS, Elkind MSV, et al. An index to identify stroke‐related vs incidental patent foramen ovale in cryptogenic stroke. Neurology. 2013;81:619–625. doi: 10.1212/WNL.0b013e3182a08d59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med. 2007;7:2262–2268. doi: 10.1056/NEJMoa071422 [DOI] [PubMed] [Google Scholar]
- 13. Mazzucco S, Li L, Binney L, Rothwell PM. Prevalence of patent foramen ovale in cryptogenic transient ischaemic attack and non‐disabling stroke at older ages: a population‐based study, systematic review, and meta‐analysis. Lancet Neurol. 2018;17:609–617. doi: 10.1016/S1474-4422(18)30167-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vandenbroucke JP, Poole C, Schlesselman JJ, Egger M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4:27. doi: 10.1371/journal.pmed.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lechat P, Mas JL, Lascault G, Loron PH, Theard M, Klimczac M, Drobinski G, Thomas D, Grosgogeat Y. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318:1148–1152. doi: 10.1056/NEJM198805053181802 [DOI] [PubMed] [Google Scholar]
- 16. Mas J‐L, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G, Coste J. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001;7:1740–1746. doi: 10.1056/NEJMoa011503 [DOI] [PubMed] [Google Scholar]
- 17. Mas J‐L. Transcatheter closure of patent foramen ovale to prevent stroke recurrence in patients with otherwise unexplained ischaemic stroke: expert consensus of the French Neurovascular Society and the French Society of Cardiology. Arch Cardiovasc Dis. 2019;112:532–542. doi: 10.1016/j.acvd.2019.06.002 [DOI] [PubMed] [Google Scholar]
- 18. Campbell BCV, Khatri P. Stroke. Lancet. 2020;396:129–142. doi: 10.1016/S0140-6736(20)31179-X [DOI] [PubMed] [Google Scholar]
- 19. Maceira AM, Cosín‐Sales J, Roughton M, Prasad SK, Pennell DJ. Reference left atrial dimensions and volumes by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2010;12:65. doi: 10.1186/1532-429X-12-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TSM. Left atrial size. J Am Coll Cardiol. 2006;47:2357–2363. doi: 10.1016/j.jacc.2006.02.048 [DOI] [PubMed] [Google Scholar]
- 21. Tops LF, van der Wall EE, Schalij MJ, Bax JJ. Multi‐modality imaging to assess left atrial size, anatomy and function. Heart. 2007;93:1461–1470. doi: 10.1136/hrt.2007.116467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, D'Hooge J, Donal E, Fraser AG, Marwick T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two‐dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2018;19:591–600. doi: 10.1093/ehjci/jey042 [DOI] [PubMed] [Google Scholar]
- 23. Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Wolf ME, Hennerici MG. The ASCOD phenotyping of ischemic stroke (updated ASCO phenotyping). Cerebrovasc Dis. 2013;36:1–5. doi: 10.1159/000352050 [DOI] [PubMed] [Google Scholar]
- 24. Hausmann D, Mügge A, Becht I, Daniel WG. Diagnosis of patent foramen ovale by transesophageal echocardiography and association with cerebral and peripheral embolic events. Am J Cardiol. 1992;70:668–672. doi: 10.1016/0002-9149(92)90210-P [DOI] [PubMed] [Google Scholar]
- 25. Luermans JG, Budts W, Ten Berg JM, Plokker HW, Suttorp MJ, Post MC. Comparison of outcome after patent foramen ovale closure in older versus younger patients. EuroIntervention. 2011;7:209–215. doi: 10.4244/EIJV7I2A35 [DOI] [PubMed] [Google Scholar]
- 26. Meyer MA. Patent foramen ovale and cryptogenic stroke. N Engl J Med. 2008;3:1518. [DOI] [PubMed] [Google Scholar]
- 27. Li L, Yiin GS, Geraghty OC, Schulz UG, Kuker W, Mehta Z, Rothwell PM. Incidence, outcome, risk factors, and long‐term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population‐based study. Lancet Neurol. 2015;14:903–913. doi: 10.1016/S1474-4422(15)00132-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. RoPE Study Investigators , Kent DM, Thaler DE. The Risk of Paradoxical Embolism (RoPE) study: developing risk models for application to ongoing randomized trials of percutaneous patent foramen ovale closure for cryptogenic stroke. Trials. 2011;12:185. doi: 10.1186/1745-6215-12-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Turc G, Lee J‐Y, Brochet E, Kim JS, Song J‐K, Mas J‐L. Atrial septal aneurysm, shunt size, and recurrent stroke risk in patients with patent foramen ovale. J Am Coll Cardiol. 2020;75:2312–2320. doi: 10.1016/j.jacc.2020.02.068 [DOI] [PubMed] [Google Scholar]
- 30. Elgendy AY, Saver JL, Amin Z, Boudoulas KD, Carroll JD, Elgendy IY, Grunwald IQ, Gertz ZM, Hijazi ZM, Horlick EM, et al. Proposal for updated nomenclature and classification of potential causative mechanism in patent foramen ovale–associated stroke. JAMA Neurol. 2020;77:878–886. doi: 10.1001/jamaneurol.2020.0458 [DOI] [PubMed] [Google Scholar]
- 31. Barco S, Klok FA, Mahé I, Marchena PJ, Ballaz A, Rubio CM, Adarraga MD, Mastroiacovo D, Konstantinides SV, Monreal M. Impact of sex, age, and risk factors for venous thromboembolism on the initial presentation of first isolated symptomatic acute deep vein thrombosis. Thromb Res. 2019;173:166–171. doi: 10.1016/j.thromres.2018.09.056 [DOI] [PubMed] [Google Scholar]
- 32. Stollberger C. The prevalence of deep venous thrombosis in patients with suspected paradoxical embolism. Ann Intern Med. 1993;119:461–465. doi: 10.7326/0003-4819-119-6-199309150-00003 [DOI] [PubMed] [Google Scholar]
- 33. Le Moigne E, Timsit S, Ben Salem D, Didier R, Jobic Y, Paleiron N, Le Mao R, Joseph T, Hoffmann C, Dion A, et al. Patent foramen ovale and ischemic stroke in patients with pulmonary embolism. Ann Intern Med. 2019;170:756–763. doi: 10.7326/M18-3485 [DOI] [PubMed] [Google Scholar]
- 34. Ranoux D, Cohen A, Cabanes L, Amarenco P, Bousser MG, Mas JL. Patent foramen ovale: is stroke due to paradoxical embolism? Stroke. 1993;24:31–34. doi: 10.1161/01.STR.24.1.31 [DOI] [PubMed] [Google Scholar]
- 35. Thaler DE, Ruthazer R, Weimar C, Mas J‐L, Serena J, Di Angelantonio E, Papetti F, Homma S, Mattle HP, Nedeltchev K, et al. Recurrent stroke predictors differ in medically treated patients with pathogenic vs other PFOs. Neurology. 2014;83:221–226. doi: 10.1212/WNL.0000000000000589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yan C, Li H. Preliminary investigation of in situ thrombus within patent foramen ovale in patients with and without stroke. JAMA. 2021;325:2116–2118. doi: 10.1001/jama.2021.4359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rigatelli G, Aggio S, Cardaioli P, Braggion G, Giordan M, Dell'avvocata F, Chinaglia M, Rigatelli G, Roncon L, Chen JP. Left atrial dysfunction in patients with patent foramen ovale and atrial septal aneurysm. JACC Cardiovasc Interv. 2009;2:655–662. doi: 10.1016/j.jcin.2009.05.010 [DOI] [PubMed] [Google Scholar]
- 38. Leventis IA, Sagris D, Strambo D, Perlepe K, Sirimarco G, Nannoni S, Korompoki E, Manios E, Makaritsis K, Vemmos K, et al. Atrial cardiopathy and likely pathogenic patent foramen ovale in embolic stroke of undetermined source. Thromb Haemost. 2021;121:361–365. doi: 10.1055/s-0040-1715831 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S3
Figure S1


