Key words: Cytokines, MyD88, non-archetypal strains, T cells, toxoplasmosis, virulence
Abstract
In most of the world Toxoplasma gondii is comprised of archetypal types (types I, II and III); however, South America displays several non-archetypal strains. This study used an experimental mouse model to characterize the immune response and parasite kinetics following infection with different parasite genotypes. An oral inoculation of 50 oocysts per mouse from T. gondii M4 type II (archetypal, avirulent), BrI or BrIII (non-archetypal, virulent and intermediate virulent, respectively) for groups (G)2, G3 and G4, respectively was used. The levels of mRNA expression of cytokines, immune compounds, cell surface markers and receptor adapters [interferon gamma (IFNγ), interleukin (IL)-12, CD8, CD4, CD25, CXCR3 and MyD88] were quantified by SYBR green reverse transcription-quantitative polymerase chain reaction. Lesions were characterized by histology and detection by immunohistochemistry established distribution of parasites. Infection in G2 mice was mild and characterized by an early MyD88-dependent pathway. In G3, there were high levels of expression of pro-inflammatory cytokines IFNγ and IL-12 in the mice showing severe clinical symptoms at 8–11 days post infection (dpi), combined with the upregulation of CD25, abundant tachyzoites and tissue lesions in livers, lungs and intestines. Significant longer expression of IFNγ and IL-12 genes, with other Th1-balanced immune responses, such as increased levels of CXCR3 and MyD88 in G4, resulted in survival of mice and chronic toxoplasmosis, with the occurrence of tissue cysts in brain and lungs, at 14 and 21 dpi. Different immune responses and kinetics of gene expression appear to be elicited by the different strains and non-archetypal parasites demonstrated higher virulence.
Introduction
Toxoplasmosis is a zoonotic disease occurring worldwide with a very high prevalence of infection in warm-blooded animals (Dubey, 2010) and is considered a parasitic cause of congenital disease in humans, while in livestock the parasite is a major cause of ovine abortion (Watson and Beverley, 1971; Dubey and Beattie, 1988; Hide et al., 2009; Tzanidakis et al., 2012). Genetic studies have shown that Toxoplasma gondii comprises mostly three archetypal (clonal) genotypes, types I, II and III (Howe and Sibley, 1995), although South America and Asia are dominated by genetically distinct non-archetypal strains (Pena et al., 2008; Shwab et al., 2014). Type II genotypes of T. gondii are largely found in humans with congenital toxoplasmosis in Europe (Ajzenberg et al., 2002) whereas the non-archetypal types BrI, BrII and BrIII represent the most predominant genotypes found within animal reservoirs in Brazil (Pena et al., 2006; Dubey et al., 2012). Previous research has highlighted that non-archetypal genotypes of Toxoplasma may be related to a severe syndrome associated with pulmonary infection including in immunocompetent patients (Elbez-Rubinstein et al., 2009). In mice, archetypal type I genotypes are considered virulent and lethal, whereas genotypes II and III usually present with intermediate and low virulence (Sibley et al., 1999). Most of the non-archetypal isolates experimentally studied in mice are virulent, causing high levels of morbidity (Pena et al., 2008). Virulence of four Brazilian isolates of T. gondii, two BrI and two BrIII genotypes, were studied in mice infected with oocysts. The BrI isolates caused large levels of mortality indicating high virulence. Although for the BrIII isolates low to medium levels of dose-dependent mortality was observed, characterizing the virulence as intermediate (Chiebao et al., 2016).
From previous studies in mice it is known that a primary infection with T. gondii stimulates the innate immune system, in particular the Toll-like receptors (TLRs), associated with adaptor proteins such as myeloid differentiation primary response protein 88 (MyD88) (Kawai and Akira, 2005), to produce interleukin (IL)-12 and interferon gamma (IFNγ). These cytokines will help to elicit an adaptive Th1 type immune response mediated by the proliferation of the CD4+ and CD8+ T-cells (Innes and Vermeulen, 2006; Miller et al., 2009). However, an uncontrolled inflammatory response can lead to immune-mediated pathology, so the host must balance the immune response to minimize tissue damage (Shaw et al., 2006), by the activation of a complex set of immune response components, among them the regulatory T cells (Tregs).
The aim of this study was to characterize inflammatory markers and their association with virulence in mice infected with different isolates of T. gondii: either M4 (type II variant, archetypal), BrI or BrIII (non-archetypal) oocysts. The study sheds light on the pathology inflicted by these different genotypes and the role they play in clinical manifestations and parasite kinetics during toxoplasmosis. Novel results are presented addressing the effects of different field isolates of T. gondii on the immune response of mice and it correlates this difference with virulence.
Materials and methods
Toxoplasma gondii oocysts
Sporulated oocysts of T. gondii South American non-archetypal genotypes BrI (ToxoDB RFLP#6, isolate TgCatBr71) and BrIII (ToxoDB RFLP#8, isolate TgCatBr60) were obtained from the feces of cats fed brains containing tissue cysts from experimentally infected mice of a previous study (Chiebao et al., 2019), as previously described (Dubey, 2010). The BrI and BrIII strains were originally obtained from naturally infected cats (Pena et al., 2006) and the sporulated oocysts were characterized (Chiebao et al., 2016). The sporulated oocysts of the archetypal M4 strain (type II variant – ToxoDB RFLP#3) were obtained as previously described (Benavides et al., 2011). The concentration of oocysts in the suspension was determined using a Neubauer counting chamber and the inoculum was prepared 1 h before use.
Animals and experimental design
One hundred and ten 8 weeks-old female Swiss Webster mice were randomly divided into four groups, one control group (group G1, n = 5) and three inoculated groups (groups G2, G3 and G4, n = 35 each) with five mice per cage being provided with food and water ad libitum. Animals were kept at a containment level 2 facility.
All mice from G2, G3 and G4 were orally infected with ~50 T. gondii oocysts in 100 μL of phosphate-buffered saline of either M4, BrI or BrIII strain, respectively. Animals were monitored twice a day for signs of clinical disease (Table 1). As part of a serial analysis, five animals from groups G2, G3 and G4 were euthanized at 24, 48, 72, 96 h, 7-, 14- and 21-days post infection (dpi) by CO2 inhalation followed by cervical dislocation. The five mice from the control group (G1) were euthanized at the end of the experiment, at 21 dpi. Mice were scored twice daily for coat condition (A) and demeanor (B) and any mouse that reached a morbidity predetermined humane endpoint (A + B ⩾ 3) was euthanized (Table 1).
Table 1.
Mice scoring system listing the criteria to euthanize an animal after experimental infection (total score = A + B)
| Category | Description | Score |
|---|---|---|
| A = Coat condition | Sleek/glossy coat | 0 |
| Ruffled coat | 1 | |
| Stary stiff coat | 2 | |
| B = Demeanor | Bright and active | 0 |
| Hunched | 1 | |
| A reluctance to move | 1 | |
| Tottering gait | 1 |
At post-mortem examination samples of spleen and mesenteric lymph nodes (MLNs) were collected for RNA analysis; additionally, samples of small intestine (SI), heart, both ocular globes (OGs) and brain (cut along central line: ½ for DNA extraction and ½ for pathology) were collected for DNA analysis. Brain, kidneys, lungs, liver, diaphragm and the remaining gastrointestinal tract (stomach, SI and large intestine) were fixed in 10% buffered formalin and paraffin-wax embedded for histological and immunohistochemical studies. Samples for mRNA isolation were immediately placed in a CK28 Precellys tissue homogenization tube (Stretton Scientific, Stretton, UK) containing 1 mL of Trizol reagent (Applied Biosystems, Foster City, USA). Samples for DNA extraction were placed in sterile 1.5 mL microcentrifuge tubes. All samples for RNA and DNA extraction were stored at −80°C prior to analysis.
RNA isolation and cDNA synthesis
The spleen and MLN samples were disrupted using a Precellys 24 tissue homogenizer (Stretton Scientific, Stretton, UK) at 5000 rpm for 60 s. Thereafter, RNA isolation was carried out according to the manufacturer's instructions for the Direct-zol® RNA MiniPrep Kit (Zymo Research Corporation, Irvine, USA). The concentration of the RNA was measured using the Nanodrop ND1000 spectrophotometer (ThermoFisher Scientific, Winsford, UK), cDNA was synthesized from 2 μg of total RNA using the advised protocol (MultiScribe® Life Technologies, Waltham, USA) as previously described (Bartley et al., 2013a).
Quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR)
Primers were designed to evaluate mRNA expression of IFNγ, IL-12, T-cells surface markers CD8, CD4 and CD25 as well as receptor adapters MyD88 and CXCR3 were studied (Table 2). Primers for the control gene hypoxanthine phosphoribosyltransferase (HPRT) were also generated. Amplification of HPRT from cDNA was used as the control for RNA integrity and cDNA synthesis. The amplifications of all analytes were normalized using HPRT. Each cDNA (20 ng/reaction) sample was analysed by RT-PCR in triplicate using standard amplification conditions (Bartley et al., 2013b) using a real-time PCR instrument (ABI 7500, Applied Biosystems, Foster City, USA). Each 20 μL reaction contained 10 μL of 2× fast SYBR green master mix (Applied Biosystems, Foster City, USA), 1 μL each forward and reverse primer (10 mm), 4 μL distilled water (dH2O) and 4 μL (20 ng) cDNA with the resultant data being analysed using SDS software.
Table 2.
Marker name, primer sequence, identification and references of the primers used for qRT-PCR in mice tissues
| Marker | Sequence | GenBank accession | Reference |
|---|---|---|---|
| HPRT | 5′-TGCTGACCTGCTGGATTACA-3′ 5′-TATGTCCCCCGTTGACTGAT-3′ |
J00423 | Konecki et al. (1982) |
| CD4 | 5′-AGGAAGTGAACCTGGTGGTG-3′ 5′-CTCCTGCTTCAGGGTCAGTC-3′ |
NM013488 | Ganesan et al. (2013) |
| CD8 | 5′-CCGTGAGGGACACGAATAAT-3′ 5′-GACTGGCACGACAGAACTGA-3′ |
MMU34881 | Johnson-Tardieu et al. (1996) |
| CD25 | 5′-AGAACACCACCGATTTCTGG-3′ 5′-CTGTGGGTTGTGGGAAGTCT-3′ |
BC114437 | Strausberg et al. (2002) |
| IFNγ | 5′-GCGTCATTGAATCACACCTG-3′ 5′-TGAGCTCATTGAATGCTTGG-3′ |
NM008337 | Shibata et al. (2014) |
| IL-12 | 5′-AAGCCACCAGTCCCAGTATG-3′ 5′-GAACAGGCCACAGTTCCATT-3′ |
NM008354 | Hoeman et al. (2013) |
| CXCR3 | 5′-GCAAGTTCCCAACCACAAGT-3′ 5′-CAAAGTCCGAGGCATCTAGC-3′ |
NM009910 | Chiu et al. (2013) |
| MyD88 | 5′-ACTGGCCTGAGCAACTAGGA-3′ 5′-TGTCCCAAAGGAAACACACA-3′ |
NM010851 | Wang et al. (2014) |
To determine changes in the levels of transcription, we compared the ΔΔCt values created after normalization of data of the ΔCt of each analyte against the ΔCt value of the HPRT, as a control reference (Bartley et al., 2019). Markers with higher levels of gene expression result in lower Ct values. In some cases, results are displayed as negative values, as the levels of transcription for their genes were greater than the levels of HPRT. Spleen samples from G2 at 48 h showed low levels of cDNA, probably due to technical problems during sample homogenization and were therefore not included.
Standard curves were generated using known concentrations of each immunological component to analyse the data using SDS system software and statistically analysed by one way analysis of variance (ANOVA) using Statistical Package for the Social Sciences (SPSS) software, version 16 (SPSS Inc., SPSS for Windows, Chicago, USA).
DNA extraction
DNA was extracted from approximately 1 g of homogenized brain tissue as described previously (Bartley et al., 2013b). Briefly, thawed tissue was processed using the Wizard® genomic DNA purification protocol (Promega Corporation, Madison, USA) and the final DNA pellet was resuspended in 200 μL of RNase/DNase free water. For all of the other tissues, samples were finely chopped using sterile scalpel blades and 0.5 g was transferred into 2.0 mL microtubes containing 0.8 mL lysis buffer (50% KCl 1 m, 20% Tris-HCl 500 mm, 10% gelatin A.R., 0.5% NP40 100% and 0.5% Tween 20, pH 8.3) and incubated overnight at 55°C with 20 mg mL−1 proteinase K (ThermoFisher Scientific, Waltham, USA). After this incubation, the proteinase K was inactivated (98°C for 10 min) and the samples were centrifuged, the resultant supernatant was then removed and processed through to DNA (as above). The final DNA suspensions in 200 μL RNase/DNase free water were stored at −20°C prior to PCR analysis. Samples of water were processed along with each batch of samples; these were used not only as additional negative controls but also acted as extraction controls to ensure no contamination occurred during the extraction process.
Diagnostic internal transcribed spacer (ITS)-1 nested-PCR and host actin inhibition control PCR
The nested ITS1 PCR targets the multicopy 18S-5.8S rRNA ITS1 region of the Toxoplasma parasite. Primers and methodology were previously described by Burrells et al. (2013). To improve the sensitivity of the assay, each DNA sample was analysed in triplicate. A sample was considered positive if at least 1/3 replicates returned a positive result.
First round reactions were conducted as described: standard 20 μL PCR reactions contained 2 μL 10× PCR master mix (45 mm Tris-HCl, 11 mm (NH4)2SO4, 4.5 mm MgCl2, 0.113 mg mL−1 BSA, 4.4 μm EDTA and 1.0 mm dATP, dATC, dGTP, dTTP), 0.75 units BioTaq (Bioline, London, UK), using 3.75 μm of primers NN1-ext-F and NN1-ext-R (Buxton et al., 2001), listed in Table 2 and 2 μL DNA. Positive control DNA was included with all batches of samples and consisted of T. gondii DNA derived from tachyzoites grown in tissue culture, negative controls were also included these consisted of sterile DNase/RNase free water and DNA extraction controls. All reactions were made to a final volume of 20 μL with sterile DNase/RNase free water. Reaction conditions were 5 min at 95°C, followed by 35 cycles of 1 min at 95°C, 1 min at 55°C, 1 min at 72°C and a final extension period of 5 min at 72°C. The reaction conditions for the second-round amplification were identical to the first, with the following exceptions, the first round amplicon was diluted with 100 μL of DNase/RNase free water and 2 μL of this diluted amplicon was used as the template and 3.75 μm of primers Toxo_NP-1_F and Toxo_NP-2_R (Hurtado et al., 2001) were used. All second round PCR products were visualized under UV light following electrophoresis in 2% agarose gels incorporating Biotum Gel Red™ (1:10 000 dilution) (Cambridge Bioscience Ltd., Cambridge, UK).
To ensure the integrity of the DNA in each sample and to ensure that any negative result was not due to PCR inhibition, a PCR reaction which amplified host actin was carried out using the diluted ITS1 first round products. The actin PCR used the same standard master mix as previously described above for the ITS1 PCR, but with the following differences: 2 μL of diluted first round ITS1 PCR product was used as a template and 4 μm of the primers Uni_Actin_F3 and Uni_Actin_R3 were used per reaction (Table 2). Cycling conditions were as previously described for the nested ITS1 PCR. All PCR products were visualized following electrophoresis in 2% agarose gels incorporating Gel Red™.
To determine any statistical differences in the incidence of DNA detection in the different groups a chi-square test was performed (for each day and organ separately) (Massad et al., 2004; Minitab, 2019). The significance level of α = 0.05 was adopted. In the case of the pairwise comparisons between proportions (post-hoc test assuming an equal proportion of positive results in each group), the Bonferroni correction significance level αbonf = 0.016 (Hochberg, 1988) was applied. All statistical analyses were performed in R-Studio software, Version 1.2.1335.
Histopathology and immunohistochemistry (IHC)
Formalin-fixed paraffin-embedded samples as described in the ‘Animals and experimental design’ section were processed as reported in Hamilton et al. (2019). Briefly, 5 μm sections were cut then processed immediately with haematoxylin and eosin (HE) for histological examination. For IHC, heat-induced epitope retrieval was used by adding slides to citrate buffer (0.01 m citric acid, pH 6) and autoclaving them for 10 min at 121°C. Toxoplasma gondii rabbit polyclonal antibody (1:600 dilution, catalogue no. PA5-16638, ThermoFisher Scientific, Waltham, USA) and normal rabbit serum (HKV1; 1:500 dilution) for the negative control was applied to slides for 18–24 h at 4°C. Next, goat anti-rabbit horseradish peroxidase polymer from the Dako Envision Kit K4001 (Dako North America Incorporation, Carpinteria, USA) was applied for 30 min at room temperature then AEC (3-amino-9-ethylcarbazole) red chromogen solution (Vector SK-4200) was applied for 30 min at room temperature (Benavides et al., 2011). All tissues were then counterstained with haematoxylin and mounted with coverslips (Marienfeld Microscope Cover Glasses, Lauda-Königshofen, Germany) using Shandon Consul-Mount® Histology Formulation (ThermoFisher Scientific, Waltham, USA). Histology was carried out on all samples collected and the severity of pathology was based on the number and size of lesions observed in the tissues examined. The presence of T. gondii tachyzoites and cysts was assessed by IHC.
Results
Clinical observations
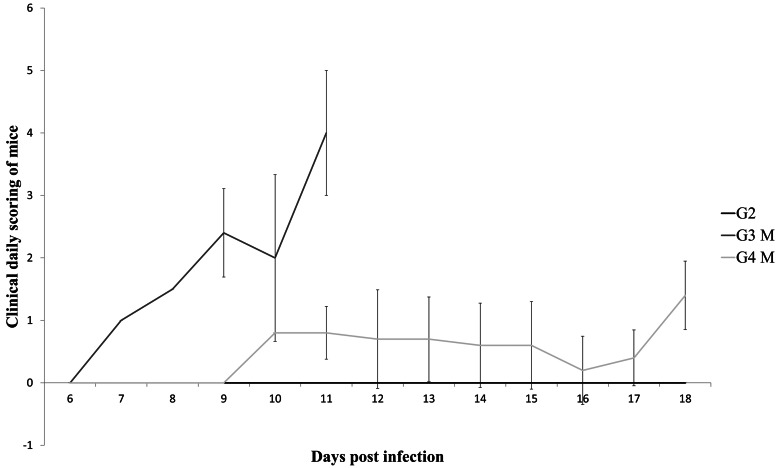
Clinical signs of acute toxoplasmosis were observed only in mice of G3 (stary stiff coat and poor demeanor) and G4 (ruffled coat and hunched back). The scoring of the mice is graphically summarized in Fig. 1. In G3, eight mice were euthanized between 8 and 11 dpi due to their clinical score; this was earlier than the scheduled time points of 14 and 21 dpi. These samples were labelled as 8–11 dpi for identification purposes and differentiation from mice euthanized at their allocated time points as part of the serial analysis. Two mice in G3 recovered from acute disease, these mice were euthanized at 14 dpi as scheduled. Nine mice in G4 displayed mild clinical signs of infection, five were symptomatic during the experiment but were euthanized at their scheduled time point and four recovered to being asymptomatic before being euthanized at their scheduled time point.
Fig. 1.
Variation of mean value of scoring (M) and standard deviation according to the clinical findings in mice experimentally infected with type II, genotypes BrI and BrIII (G2, G3 and G4, respectively) of Toxoplasma gondii. The vertical axis stands for the sum of daily scoring in each mouse according to the following criteria: 1 = ruffled coat or hunched back; 2 = ruffled coat and hunched back or reluctance to move; 3 = stary stiff coat and hunched back; 4 = stary stiff coat, hunched back, reluctance to move and dehydration.
Post-mortem findings
Enlargement of MLNs and spleen was the main finding in mice from all experimentally infected groups immediately after infection (24 h). Organs were considered enlarged when they were at least 1.5 times larger than in the control group. Mice from G2 showed splenomegaly and lymph adenomegaly until 7 dpi, after then only MLN enlargement was observed at 14 dpi and nothing abnormal was noted at 21 dpi. Enlargement of the spleen and MLN were consistently observed until 14 and 21 dpi in G3 and G4 mice, respectively. The eight mice from G3 that were euthanized on welfare grounds at 8–10 dpi showed darker lungs than the control mice; at 8–11 dpi, the livers, kidneys and spleens were either paler or showed white spots in the surface. At 10 and 11 dpi, the intestines were congested and empty and all of the mice showed symptoms of dehydration (lethargic and skin tightened). G4 mice presented with darker coloured lungs at 14 and 21 dpi. The most severe splenomegaly was present in G4 mice at 14 dpi, with spleens three times larger than those in G1 control mice.
Cell and marker phenotypes after T. gondii infection
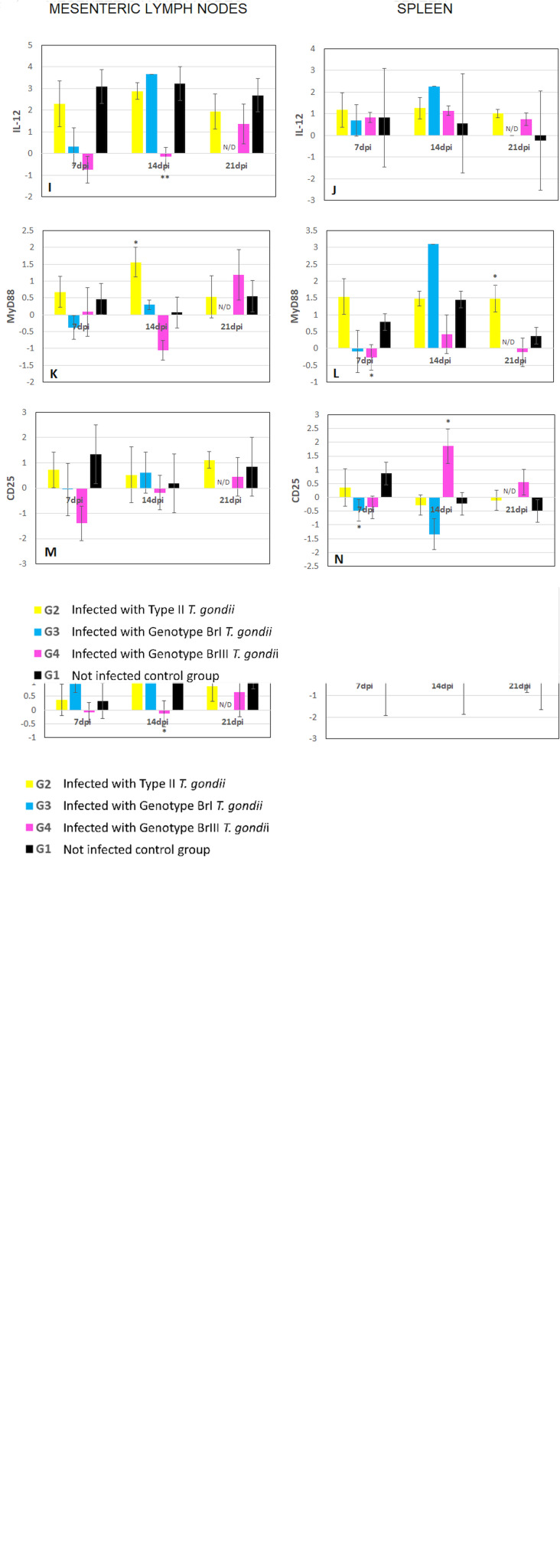
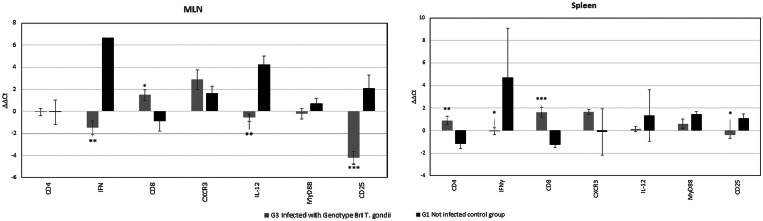
Mean levels of expression (cDNA) for immune markers from experimentally infected groups (G2, G3 and G4) and uninfected G1 mice were plotted against time, which was established as the horizontal axis for graphic representation (Fig. 2). Results from mice euthanized at 8–11 dpi were shown separately in Fig. 3. All expression comparisons were made to those of G1.
Fig. 2.
Mean levels and standard deviation of ΔΔCt values for mRNA expression of immune response genes normalized against HPRT from samples of spleen and MLNs of mice at different time points (hours = h, and days post infection = dpi, not determined = N/D) after challenge with M4, BrI and BrIII oocysts (G2, G3 and G4, respectively) of T. gondii. Results are compared with negative control (G1). P value was determined using the one-way ANOVA (*P < 0.05, **P < 0.01, ***P < 0.001). (A, B) CD4; (C, D) IFNγ; (E, F) CD8; (G, H) CXCR3; (I, J) IL-12; (K, L) MyD88; (M, N) CD25.
Fig. 3.
Mean levels and standard deviation of mRNA expression of CD4, IFNγ, CD8, CXCR3, IL-12, MyD88 and CD25 markers in the spleen and MLNs of mice euthanized due to severity of symptoms at 8–11 dpi after challenge with BrI oocysts (G3) of T. gondii. Results are compared with negative control (G1). P value was determined using the one-way ANOVA (*P < 0.05, **P < 0.01, ***P < 0.001).
The marker for which most significant different expression profiles were seen in G2 mice was MyD88 in the MLN at 14 dpi and the spleen at 21 dpi (Table 3). For G3, altered expression of the studied markers was demonstrated earlier at 48 h after infection in the spleen and continued until 8–11 dpi in both MLN and spleen, in which increased significant results were observed (Table 3) most frequently for CD8, CD25 and IFNγ. The increase in expression of markers in G4 mice started at 96 h after infection onwards and remained upregulated until the end of the experiment, the MLN was the tissue for which most significant results were observed at 14 dpi (Table 3). Detailed results by specific markers and organs are described below.
Table 3.
One way ANOVA significant results obtained after qRT-PCR analysis of markers from immunological components expressed by tissues of mice experimentally infected with different strains of Toxoplasma gondii against the ΔCt value of HPRT from non-infected mice
| Groups compared with G1 (control) | |||
|---|---|---|---|
| G2 (type II) | G3 (BrI) | G4 (BrIII) | |
| MLN |
14 dpi ↓MyD88 (P = 0.047) |
8–11 dpi ↓CD8 (P = 0.045) ↑IL-12 (P = 0.001) ↑CD25 (P < 0.0001) ↑IFNγ (P = 0.004) |
4 dpi ↓IFNγ (P = 0.009) 7 dpi ↑IFNγ (P = 0.009) 14 dpi ↑CXCR3 (P = 0.047) ↑IL-12 (P = 0.009) ↑IFNγ (P = 0.003) |
| Spleen |
7 dpi ↓CD4 (P = 0.006) 21 dpi ↓MyD88 (P = 0.042) |
2 dpi ↓CD8 (P = 0.032) 4 dpi ↓MyD88 (P = 0.050) 7 dpi ↓CD8 (P = 0.018) ↑CD25 (P = 0.044) 8–11 dpi ↓CD4 (P = 0.004) ↑CD8 (P < 0.0001) ↑CD25 (P = 0.022) ↑IFNγ (P = 0.046) |
7 dpi ↑MyD88 (P = 0.046) 14 dpi ↓CD4 (P = 0.008) ↓CD25 (P = 0.023) 21 dpi ↑CD4 (P = 0.049) |
Up and down arrows represent up and downregulation, respectively.
CD4
Alterations in the expression of this T cell marker were observed in the spleen of mice from all experimental groups (Fig. 2B). For G2 (type II) there was a tendency for upregulation of CD4 expression at 72 h, 96 h (Supplementary material) but significantly decreased (P = 0.006) at 7 dpi compared to the control group. It was observed that the CD4 marker expression was also significantly decreased (P = 0.004) in G3 (Genotype BrI) mice that were euthanized due severe toxoplasmosis at 8–11 dpi. The G4 (genotype BrIII) mice demonstrated significant decreases in CD4 expression in spleen samples at 14 and 21 dpi (P = 0.008 and P = 0.049, respectively) compared to the control mice (Fig. 3).
CD8
Significant increases in mean levels of transcription of CD8, a marker of cytotoxic T lymphocytes, were seen in mice from G3 in the spleen at 48 h (P = 0.032). However, CD8 was significantly downregulated in the spleen at 7 dpi (P = 0.018) (Table 3, Fig. 2F) and both in the spleen and MLN at 8–11 dpi (P = 0.045 and P ⩽ 0.001, respectively) of G3 mice compared to the G1 controls (Fig. 3). The mice in G2 demonstrated increased expression of CD8 in the spleen during early time points 72 h and 96 h pi (Supplementary material); however, as observed for G4, no statistical differences were demonstrated when comparing these results with the data from G1.
IFNγ
The mean expression levels of this cytokine were significantly increased in the MLN of mice from G4, at 96 h, 7 and 14 dpi (P = 0.009, P = 0.009 and P = 0.003, respectively), compared with the control group (Table 3, Fig. 2C). In mice from G3 with symptoms of acute toxoplasmosis, euthanized between 8 and 11 dpi, IFNγ showed significantly higher expression levels in both MLN and spleen (P = 0.004 and P = 0.046) (Fig. 3). Except for at 72 h pi (Supplementary material), in mice from G2 the IFNγ expression level observed was comparable with the control group and no significance was observed for this group.
IL-12
Significantly increased expression of IL-12 was only observed in MLN samples from the groups of mice infected with the non-archetypal strains of T. gondii. For G3 mice at 8–11 dpi (P = 0.004) and for G4 mice, at 7 and 14 dpi (P = 0.018 and P = 0.009, respectively) (Fig. 2I). In the spleen, the mean levels of expression of IL-12 were comparable with G1 (control), except for at 96 h for G2, but this difference was not significant.
MyD88
MLN and spleen samples from mice from G2 exhibited higher levels of MyD88 expression, an adapter molecule for receptors of antigen presenting cells, compared with the control group at 72 h (Supplementary material). However, at 14 and 21 dpi levels of MyD88 expression from the G2 mice were significant lower (P = 0.047 and P = 0.042, respectively) than G1. For G3 in the spleen at 96 h pi significantly less MyD88 expression was observed (P = 0.05) compared to G1. Only G4 showed significant upregulation at 7 dpi, and only for the spleen (P = 0.046). From 24 to 72 h, MyD88 expression levels were comparable to control in the non-archetypal groups (Supplementary material). In G3 mice that survived acute toxoplasmosis and were analysed at 14 dpi, decreased expression levels of MyD88 was observed (Fig. 2L) but without significance and it is noteworthy that, compared with G1, this marker was the least altered in mice with severe disease, euthanized between 8 and 11 dpi (Fig. 3).
CXCR3
Mean levels of the neutrophil surface activator marker CXCR3 were significantly increased only once in the studied time points, this was seen in the MLN of mice from G4 (BrIII) at 14 dpi (P = 0.047) (Fig. 2G). Apart from this, expression was comparable to the control group profile in the tissues of the other groups.
CD25
For CD25, a marker present on Tregs and early phase lymphocytes, significant higher levels of gene expression were observed in G3 mice (BrI) already showing clinical symptoms of toxoplasmosis (Fig. 1) at 7 dpi (P = 0.044) (Fig. 2N) and in animals euthanized at 8–11 dpi, in both MLN and spleen (P ⩽ 0.0001 and P = 0.022, respectively) (Table 3, Fig. 3). Expression levels in G2 mice (archetypal type II variant) were comparable with G1 (control) at all time points studied. In G4, significantly reduced levels of CD25 expression were seen in the spleen at 14 dpi (P = 0.023) (Fig. 2N) compared to the G1 controls.
Detection of parasite DNA in mouse tissues
Results obtained from brain, heart, SI and OG samples collected from all experimental groups of mice at all time points between 24 and 96 h were negative for the detection of parasite DNA. Positive results are shown in Table 4. A more widespread dissemination of parasite DNA was observed in G3 compared to the other experimental groups. The G2 mice infected with the archetypal strain M4 (type II variant, RFLP#3) demonstrated only three PCR positive results (brain), two at 7 dpi and one at 14 dpi. Parasite DNA was detected initially (7–14 dpi) in the brains of the G4 mice infected with the intermediate virulent genotype (BrIII) only being found in heart (1/5) and OG (1/5) on 21 dpi. For the G3 mice infected with the virulent isolate (BrI) OG samples from 4/5 mice tested PCR positive on 7 dpi and a further 8 out of 15 mice that tested positive between 7 and 14 dpi for at least one OG sample.
Table 4.
Toxoplasma gondii DNA detection by ITS1 nested-PCR in tissues from mice orally infected with oocysts from different genotypes
| Tissue | |||||
|---|---|---|---|---|---|
| T. gondii genotype | DPI | SI | Heart | Brain | OG |
| G2 – M4 (type II) | 7 | 0 (5) | 0 (5) | 2 (5) | 0 (5) |
| 14 | 0 (5) | 0 (5) | 1 (5) | 0 (5) | |
| 21 | 0 (5) | 0 (5) | 0 (5) | 0 (5) | |
| G3 – BrI | 7 | 0 (5) | 2 (5) | 4 (5) | 4 (5) |
| 8–11 | 0 (8) | 1 (8) | 8(8) | 3(8) | |
| 14 | 0 (2) | 0 (2) | 2 (2) | 1 (2) | |
| G4 – BrIII | 7 | 0 (5) | 0 (5) | 5 (5) | 0 (5) |
| 14 | 0 (5) | 0 (5) | 5 (5) | 0 (5) | |
| 21 | 0 (5) | 1 (5) | 3 (5) | 1 (5) | |
DPI, days post infection; SI, small intestine; OG, ocular globe.
( ) = number of mice tested.
Although the chi-square test for proportions (Table 5) pointed towards statistical association between the samples considering the OG at 7 dpi (P = 0.004) and the brain at 14 dpi (P = 0.015), none post-hoc test indicated association.
Table 5.
P value obtained from proportion chi-square test for each evaluated organ, considering the three genotypes of T. gondii
| SI | Heart | Brain | OG | ||
|---|---|---|---|---|---|
| Proportion chi-square test (α = 0.05) | |||||
| NA | 0.100 | 0.092 | 0.004 | ||
| Pairwise comparisons between proportions (αbonf = 0.016) | |||||
| 07 Days | G3 (BrI)–G2 (type II) | NA | 0.86 | 1.00 | 0.11 |
| G4 (BrIII)–G2 (type II) | NA | NA | 0.50 | NA | |
| G4 (BrIII)–G3 (BrI) | NA | 0.86 | 1.00 | 0.11 | |
| Proportion chi-square test (α = 0.05) | |||||
| NA | NA | 0.015 | 0.065 | ||
| Pairwise comparisons between proportions (αbonf = 0.016) | |||||
| 14 Days | G3 (BrI)–G2 (type II) | NA | NA | 0.28 | 1.00 |
| G4 (BrIII)–G2 (type II) | NA | NA | 0.11 | NA | |
| G4 (BrIII)–G3 (BrI) | NA | NA | NA | 1.00 | |
| Proportion chi-square test (α = 0.05) | |||||
| NA | 1.000 | 0.168 | 1.000 | ||
| Pairwise comparisons between proportions (αbonf = 0.016) | |||||
| 21 Days | G3 (BrI)–G2 (type II) | NA | NA | NA | NA |
| G4 (BrIII)–G2 (type II) | NA | 1.00 | 0.17 | 1.00 | |
| G4 (BrIII)–G3 (BrI) | NA | NA | NA | NA |
NA, not available.
Each statistical test was specifically performed for 7, 14 and 21 days. The significance level was assumed as α = 0.05 and αbonf = 0.016 for post-hoc test.
Histopathology and IHC
No lesions or parasites were identified in any section examined from any of the G1 or G2 mice throughout the course of the experiment.
The G3 mice euthanized at 7 dpi and between 8 and 11 dpi showed moderate to severe lesions in most tissues examined. The most severe lesions were seen in the liver (Fig. 4A), intestine, and in lungs of mice euthanized between 9 and 11 dpi. In the brain, meningoencephalitis was observed at 9 and 10 dpi and it was characterized by few small perivascular cuffs, glial foci and in one brain with central necrosis. In the other organs examined variable degrees of mononuclear inflammatory infiltrate was present at 7 dpi and from 8 to 11 dpi. At 7 and 11 dpi, parasites were found in high numbers in the majority of tissues, often in, predominantly in the form of tachyzoites (Fig. 4F). Mild lesions and few parasites were occasionally found in the G3 mice euthanized at 14 dpi.
Fig. 4.
Lesions and parasite detection in HE staining and specific labelling by IHC from tissues of mice infected with different strains of T. gondii. (A) and (E) Liver from a G3 (genotype BrI) mouse euthanized at 9 dpi. Focus of necrotizing mononuclear hepatitis (A, circle, HE, bar 100 μm), large number of immunolabelled tachyzoites and a moderately large cyst (arrow) within lesions (E, IHC, bar 100 μm). (B) and (F) Kidney from a G3 mouse euthanized at 9 dpi, small mononuclear focus of nephritis (B, circle, HE, bar 100 μm), and large number of immunolabelled T. gondii tachyzoites and cysts associated or not (arrow) with lesions (F, IHC, bar 100 μm). (C) and (G) Lungs from mice from G4 (genotype BrIII) euthanized at 14 dpi. Large focus of necrotizing interstitial pneumonia with mild central necrosis (C, HE, bar 100 μm), immunolabelled T. gondii cysts (arrow) not associated with lesions (G, IHC, bar 50 μm). (D) and (H) Brains from G4 mice euthanized at 14 dpi. Small mononuclear perivascular cuffs (D, circle, HE, bar 100 μm), immunolabelled T. gondii cysts and single tachyzoite not associated with lesions (H, IHC, bar 50 μm).
In G4 mice, moderate to severe lesions were usually seen at 14 and 21 dpi in most tissues examined. The brain and lungs were the most severely affected organs. In the brain, the main finding was meningoencephalitis (Fig. 4D), characterized by numerous small perivascular cuffs and few glial foci. In the lungs, all mice euthanized at 14 dpi and three of the five mice euthanized at 21 dpi had moderate to severe mononuclear inflammation and necrosis (Fig. 4G). In the liver, mild to severe lesions were present from 7 dpi until 21 dpi. Low to moderate numbers of T. gondii parasites were first identified in the lung, liver and intestine of G4 mice at 7 dpi. At 14 and 21 dpi, parasites were most abundant in the brain and lung. The presence of parasites in other tissues was sporadic and low. Tissue cysts were the predominant form of the parasite found in this group and they were often not associated with lesions (Fig. 4H).
Discussion
In the acute phase of infection caused by non-virulent strains of T. gondii the sporozoites penetrate the intestinal lamina propria, stage differentiates into tachyzoites and disseminates systemically. Once the parasite is controlled by the immune system, a persistent infection is established with tissue cysts forming in the central nervous system (Dubey, 2010). However, infection with virulent isolates in mice may lead to death (Hill et al., 2012) due to uncontrolled proliferation of the parasite or leading to immune imbalance of the Th1 response, as reported for European laboratory strains of T. gondii (Ajioka and Soldati, 2007). Previous information concerning host–pathogen immune response interactions examined by intraperitoneal inoculations of tachyzoites in mice have been obtained using archetypal strains type III (Tuladar et al., 2019), type II (Fox et al., 2016) and type I (Torres et al., 2013), but less data are available regarding oral infection (Detavernier et al., 2019). Currently, only a few studies have shown results of the responses between the host immune system with non-archetypal parasites (Meireles et al., 2015; Lu et al., 2018; Jin et al., 2019). To help understand differences in virulence between non-archetypal T. gondii strains, we report here novel information about tissue tropism, morbidity, mortality and the concomitant development of early immune responses of mice following an oral challenge with oocysts of genetically distinct T. gondii genotypes, including two field strains. The morbidity/mortality results of mouse infections with non-archetypal genotypes BrI (G3) and BrIII (G4) are comparable with clinical observations from a previous study in which 9 of 10 mice inoculated with 50 oocysts from isolate TgCatBr71 (BrI) died of acute toxoplasmosis and all mice that were infected with TgCatBr60 (BrIII) survived (Chiebao et al., 2016). This high and intermediate virulence for BrI and BrIII T. gondii, respectively, was also observed in our results. The current study also included more observations of lesions and the reactive spleens and MLN in experimental infected groups, which were more evident in G3 and G4; a high number of pro-inflammatory immune response markers significantly altered in G3 and seconded by G4; and more varied tissue distribution for the Brazilian strains.
During this current study the lack of statistical significance of the qRT-PCR for the immunological marker expression levels for mice from G2 in comparison with uninfected (G1) control mice may be due to variations in responses observed among the outbred individuals. Despite increased levels of expression for CD8 and MyD88 were observed in G2 at 48 h and for CD4 and IL-12 at 96 h pi (although these were not statistically significant), the only significant result for T cells markers was the splenic CD4 downregulation at 7 dpi. An important common finding for the infected mice (G2, G3 and G4) was the observation of lymph adenomegaly and splenomegaly at necropsy which is an indication of infection by the parasite. This is in agreement with a separate study in which spleen enlargement was also observed in BALB/c mice after 7 dpi with tachyzoites with different T. gondii laboratory strains (Zhang et al., 2018). The absence of any pathological lesions or demonstrable parasites from G2 (M4) shows that this isolate demonstrated lower virulence than the non-archetypal strains and it seems that oocyst infection with this type II parasite tends to be milder than tachyzoite infection (Sánchez-Sánchez et al., 2019). The signs of parasite dissemination (DNA) were evidenced by the PCR results of the brain samples at 7 and 14 dpi. Infection of outbred mice with T. gondii type II isolates does not usually lead to the development of severe disease and this was confirmed in the current study, in G2 mice. This could be further evidenced, since G2 mice did not show any pathological lesions or parasites when examined histologically. Similar results were observed by Bezerra et al. (2019), showing that mice infected with the ME49 strain (type II) had lower number of parasites in the central nervous system samples compared to the VEG strain (type III).
The lack of significant expression of activated T cells markers from G2 and G4, compared to G3 may be related to the low levels of pathogen-associated molecular pattern presentation after phagocytosis of sporozoites, which has been previously described in gut cells of mice (Guiton et al., 2017). Discrepancies with T cell expressions in this study, compared to previous results (Saeij et al., 2005), could be related to the infective stage, since oocysts were used in this study whereas others performed infection with cysts or tachyzoites, which could lead to differences in the timing and magnitude of the immune system stimulation.
There were significantly higher levels of pro-inflammatory cytokines for the spleen samples from the non-archetypal genotype G3 (BrI) mice euthanized at 8–11 dpi comparison to the control mice. In contrast, in the MLN at 7 dpi for G4 (BrIII) there was already upregulation of the pro-inflammatory cytokine marker IFNγ (P = 0.009), which remained significantly increased at 14 dpi (P = 0.003). The increase in IFNγ expression coincided with the significant activation of the IL-12 gene (P = 0.009) and increased expression levels of the neutrophil surface activator marker CXCR3 (P = 0.047). These observations suggest that a longer and controlled immune response at the initial site of infection balanced with an adequate expression of pro-inflammatory components at other lymphoid organs was helping to diminish clinical symptoms and in the establishment of chronic toxoplasmosis. Increased expression of IFNγ and IL-12 markers was also observed a little later in G3, at 8–11 dpi in the MLN (P = 0.004 and P = 0.001, respectively), whereas IFNγ expression was also shown to be significantly higher (P = 0.046) in the spleen. Previous studies using tachyzoites from archetypal strains of T. gondii (types I and II) observed differences in the expression levels of IL-12. These differences were observed at around 3–5 dpi, with higher levels of expression observed for the type II strain instead (Zhang et al., 2018). Type I genotypes induced a lethal infection in mice, which was combined with the production of high serum levels of IFNγ and IL-12 (Mordue et al., 2001). These results are in agreement with the findings of the current study for G3 (BrI). It would be interesting to examine the interplay between IL-12 and IFNγ further in determining clinical outcomes as it may offer a basis to evaluate if parasites from the BrIII non-archetypal genotype may have developed a mechanism to control excessive IFNγ production and thereby, avoiding the deleterious consequences of a strong Th1 response.
At 48 h to 96 h pi, lower expression levels of CXCR3 in G3 mice were observed in the MLN, compared to G1 (control) and this combination of results may suggest a predominance of a Th2 type immune response. As previously described, the lack of induction of cell surface receptors such as CXCR3 of Th1 cells can lead to increased susceptibility to disease and is observed when neutrophils are depleted early after infection (Miller et al., 2009).
The TLR-MyD88 signalling cascade was identified as essential to parasite detection by the innate immune response of mice infected with archetypal strains such as RH and ME49 (Gazzinelli et al., 2014). In the current study, this dependence was corroborated in mice from G4 (BrIII), with high levels of expression observed at 7 dpi (P = 0.046). A MyD88-dependent pathway required for mice survival was demonstrated previously, which occurred earlier than the current study (4 dpi) and when tachyzoites of the archetypal type II strain were used (Sukhumavasi et al., 2008).
Mice from G2 (type II) and G3 (BrIII) with acute toxoplasmosis showed a completely different result from G4. In mice from G3, a MyD88-independent pathway: with significantly lower expression of MyD88 was observed in spleen samples at 96 h pi (P = 0.050).
In G2, however, MyD88 expression was initially upregulated at 72 h pi and then decreased levels of expression were seen in the MLN at 14 dpi (P = 0.047) and in the spleen at 21 dpi (P = 0.042). This early MyD88 dependent pathway, followed by the decrease in expression, could explain the better control of the infection of the M4 strain. As suggested before, gene expression of proinflammatory response is different according to the virulence of the strain of Toxoplasma (Hill et al., 2012). Previously, it was also demonstrated that IFNγ production is delayed in the absence of MyD88 (Sukhumavasi et al., 2008), therefore the correlation between the downregulation of MyD88 and lack of IFNγ expression observed in G2 could also be a mechanism related to the low virulence of this parasite genotype.
Another important difference between groups was noted. Compared to G1, a significant increase in the expression of the T cell marker CD25 was observed in mice from G3 infected with the virulent strain BrI on days 7 (P = 0.044) and 8–11 in the spleen (P = 0.022) and at 8–11 dpi in the MLN (P < 0.001). This upregulation appeared to occur in severely ill mice just before they were euthanized in the acute phase of disease, but not in the G3 mice that recovered from acute toxoplasmosis. This may mean that the immune system of the mice showing severe clinical symptoms is trying to suppress the intense activation and proliferation of Th1 cells and the subsequent cascade of proinflammatory products. Alternatively, it could also reflect differences in parasite dissemination. In the groups with the more virulent parasites, more samples were positive for parasite DNA by PCR, indicating that the virulent parasites are disseminating more effectively. The increased immune responses represented by T cell markers CD8 (P < 0.001 at the spleen) and CD25 higher expression may be reflecting an increased parasite exposure. Previous in vitro studies (Saeij et al., 2005; Dardé, 2008) with an archetypal virulent type I strain showed that higher growth rates in cell culture may either be due to a higher reinvasion rate or to a shorter doubling time, both of which increase the number of parasites. Both facts could explain the increased incidence of parasite DNA positive tissues seen in mice from G3 in the current study. The invasion ability of T. gondii types I, II and III were compared in vitro before and cellular parasite penetration was enhanced in type I strains, compared to type II strains (Barragan and Sibley, 2002).
Histopathological data from the non-archetypal genotypes supports the significantly higher expression levels of IL-12 and IFNγ, since the great majority of lesions and parasites in the tissues and the significant expressions of the proinflammatory cytokines were observed between 7 and 14 dpi. The G3 mice had no pathology or Toxoplasma DNA in tissues up to a week (24–96 h) after infection. In this group from 7 to 11 dpi, parasites were found in high numbers in all organs but especially in the liver, lungs and intestine. Pathology was most consistently severe in the liver and intestine between 7 and 11 dpi and in the lungs from 9 to 11 dpi. Lesions and parasites were frequently found in the kidney, an organ not extensively studied in toxoplasmosis research. Most kidney samples with at least moderate lesions were also pale in appearance at post-mortem. At 14 dpi, only mild lesions and infrequent parasites were found in the lung and liver, respectively, by IHC. However, PCR analysis gave different results, identifying DNA parasite in the brains of most mice from the BrI (14/15) and BrIII (13/15) at 7 and 14 dpi. This difference may have occurred due to low numbers of parasites and their heterogeneous distribution resulting in the parasites being missed when sections were cut for IHC. The changes described in the most severely affected organs may explain some of the clinical signs observed in euthanized mice in this group, however, it is possible that the parasitaemia and immune response (also indicated by splenomegaly and lymphadenopathy detected at post-mortem) were also significant contributing factors to the mouse morbidity and mortality. In a previous study on Toxoplasma burden in tissue samples of mice infected with tachyzoites of the virulent RH strain using qPCR, it was reported that significantly higher parasite loads were observed earlier than in our study, at 24 and 48 h pi. The parasites were also mainly located in the liver and kidney, although the lungs were not analysed in their study (Dadimoghaddam et al., 2014). Tachyzoites appear to be the most frequent form of the parasite identified in BrI infected G3 mice, compared with G4 mice infected with BrIII in which bradyzoite (tissue cysts) were found more often. From our current results, it could be speculated that the virulent strain remains in the acute phase for longer. Therefore, the timing at which tachyzoites and bradyzoites begin to be found could be an indicator for strain virulence.
In this current mouse study, an important observation is that OG (eyes) appears to be a site of predilection for the virulent BrI strain (G3). This tropism is in accordance with a previous study in which T. gondii was quantified in high loads in eyes from mice after 3 days of infection with an archetypal type I strain (Dadimoghaddam et al., 2014). This early finding may be related to the intraperitoneal injection method and the use of tachyzoites (Dadimoghaddam et al., 2014) rather than using oocysts as described in this study. Collection of the OG may represent a viable sampling tissue for detection studies (saving the brain for isolation purposes) and indicate that ocular infection could be a virulence related trait, as observed in human toxoplasmosis in Brazil (Lopes et al., 2009; Herrmann et al., 2014; Mendes et al., 2014). Although no statistical significance was observed, the biological significance of the many PCR positive results in G3, at earlier time points, in specific organs, is clear. This positivity suggests that the virulent isolate (BrI) is driving a proinflammatory response that could lead to parasite evasion by dissemination to immune privileged organs, such as OG and brain.
Mice from G4 (BrIII) did not show severe pathology or high parasite dissemination in the majority of organs during the acute stage of infection, before 14 dpi. No parasites were detected with IHC in the brain before 14 dpi but parasite DNA was detected by PCR in all animals at 7 dpi. Again, it is possible that there were low numbers of parasites and they were not detected, or they were not present in the histological section. At 14 and 21 dpi, brains and lungs were the most affected organs. At 21 dpi, lesions were less severe and parasite invasion was reduced in organs compared to 14 dpi which may suggest a resolving infection as a result of an adequate immune response and may be further supported by the reduced incidence of splenomegaly and lymphadenopathy. This reduction in pathology was in lambs experimentally infected with oocysts of T. gondii (M4 type II variant), with a reduction in lesions in the central nervous system from 14 dpi (Katzer et al., 2014). Large genetic variability of T. gondii in Brazil has been shown both in animal and human reservoirs, which could be implicated in the clinical disease outcome. Recent studies in Brazil predominantly reported diverse new non-archetypal genotypes; the most common single genotype found was BrIII and it was identified in chickens, horses and wild mammals (Fournier et al., 2014; Silva et al., 2018; Pena et al., 2018a) in addition a type I genotype, although rare, was also described (Pena et al., 2018b). Finding such diverse strains supports the need for further studies comparing specific and cross protection immune responses raised against them by the hosts.
Mice infected with BrI (G3) failed to generate a sufficiently robust protective immune response to overcome the disease caused by the virulent BrI genotype. Two hypotheses are given as to why this may have happened. First, the virulent parasites induce an internal mechanism to suppress immunomodulation, as reported in the CTLA-4 blockage by Trypanosoma cruzi (Martins et al., 2004) and the excessive levels of resultant cytokines were lethal. Second, since the severely ill mice had higher expression levels of IFNγ than surviving mice and we demonstrated that its balanced production is important to overcome disease, then the virulent parasite could be stimulating its pathway. The mucosal immunity balanced with a controlled Th1 response in other tissues from the intermediate virulent strain (G4, BrIII) could be the optimal pathway stimulated to disease chronification and parasite propagation. The whole mechanism of action involved in the infection by different genotypes of T. gondii must be elucidated and future studies should also consider the role of the differences in growth rates of the parasite.
Overall, the results showed an overview of key molecules involved in the initial immune response against non-archetypal and archetypal strains of the parasite in mice and that virulence differs between strains, not only due to immune response stimuli variation but it is also due to different kinetics of gene expression. The current study has provided a basis for more evaluations of other possible pathways, for example host related regulatory compounds, such as Foxp3 and ocular cytokinomes, or alternatively parasite virulence genes such as polymorphic rhoptry protein kinases ROP18 and ROP5.
Acknowledgements
Thanks are due to CNPq (Brazilian National Council for Scientific and Technological Development) for supporting R.M.S. in this study (process number 310532/2017-5).
Author contributions
D.P.C., R.M.S. and E.A.I. conceived the experiment. D.P.C., P.M.B., E.A.I. and F.K. designed the experiment. D.P.C. and A.B. conducted the experiment. P.B. performed the statistical analysis. L.E.B. and F.C. completed the histopathological analysis. D.P.C., P.B., H.F.J.P., L.E.B., F.C. and F.K. contributed to the interpretation of data. D.P.C., P.M.B., A.B., L.E.B., F.C., E.A.I. and F.K. wrote the article.
Financial support
This study was funded by the Moredun Foundation, Scottish Government's Rural and Environment Science and Analytical Services Division (RESAS); FAPESP (Research Support Foundation of the State of São Paulo) (R.M.S., grant number 2011/19297-8) and CAPES (Higher Education Improvement Coordination) (D.P.C., grant number 0382/14-0).
Ethical standards
The animals were purchased for experimental purposes and all procedures were approved by the Moredun Research Institute Animal Welfare and Ethical Review Body (AWERB) (Experiment No. E22-14) and Biomathematics and Statistics Scotland (BIOSS). All experimental procedures complied fully with the regulations laid down by the Home Office of Great Britain and Northern Ireland for compliance with the Animals (Scientific Procedures) Act 1986. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that all of us have approved the order of authors listed in the manuscript.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182020002346.
click here to view supplementary material
Conflict of interest
The authors declare there are no conflicts of interest.
References
- Ajioka JW and Soldati D (2007). Genetics and genome organization of Toxoplasma gondii. In Ajioka JW and Soldati D (eds), Toxoplasma: Molecular and Cellular Biology. Wymondham: Horizon Bioscience, pp. 193–207. [Google Scholar]
- Ajzenberg D, Cogné N, Paris L, Bessières M-H, Thulliez P, Filisetti D, Pelloux H, Marty P and Dardé M-L (2002) Genotype of 86 Toxoplasma gondii isolates associated with human congenital toxoplasmosis, and correlation with clinical findings. The Journal of Infectious Diseases 186, 684–689. [DOI] [PubMed] [Google Scholar]
- Barragan A and Sibley LD (2002) Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence. Journal of Experimental Medicine 195, 1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley PM, Katzer F, Rocchi MS, Maley SW, Benavides J, Nath M, Pang Y, Cantón G, Thomson J, Chianini F and Innes EA (2013a) Development of maternal and foetal immune responses in cattle following experimental challenge with Neospora caninum at day 210 of gestation. Veterinary Research 44, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley PM, Wright SE, Zimmer IA, Roy S, Kitchener AC, Meredith A, Innes EA and Katzer F (2013b) Detection of Neospora caninum in wild carnivorans in Great Britain. Veterinary Parasitology 192, 279–283. [DOI] [PubMed] [Google Scholar]
- Bartley PM, Burrells A, Benavides J, Canton G, Garcia JL, Thomson J, Chianini F, Innes EA and Katzer F (2019) Cell mediated and innate immune responses in pigs following vaccination and challenge with Toxoplasma Parasites. Veterinary Parasitology 275, 108963. [DOI] [PubMed] [Google Scholar]
- Benavides J, Maley S, Pang Y, Palarea J, Eaton S, Katzer F, Innes EA, Buxton D and Chianini F (2011) Development of lesions and tissue distribution of parasite in lambs orally infected with sporulated oocysts of Toxoplasma gondii. Veterinary Parasitology 179, 209–215. [DOI] [PubMed] [Google Scholar]
- Bezerra ECM, dos Santos SV, dos Santos TCC, de Andrade HF and Meireles LR (2019) Behavioral evaluation of BALB/c (Mus musculus) mice infected with genetically distinct strains of Toxoplasma gondii. Microbial Pathogenesis 126, 279–286. [DOI] [PubMed] [Google Scholar]
- Burrells A, Bartley PM, Zimmer IA, Roy S, Kitchener AC, Meredith A, Wright SE, Innes EA and Katzer F (2013) Evidence of the three main clonal Toxoplasma gondii lineages from wild mammalian carnivores in the UK. Parasitology 140, 1768–1776. [DOI] [PubMed] [Google Scholar]
- Buxton D, Wright S, Maley SW, Rae AG, Lundén A and Innes EA (2001) Immunity to experimental neosporosis in pregnant sheep. Parasite Immunology 23, 85–91. [DOI] [PubMed] [Google Scholar]
- Chiebao DP, Pena HFDJ, Cabral AD, Rocca MP, Lopes EG, Valadas SYOB, Keid LB, Grisi Filho JHH and Soares RM (2016) Infection of mice with oocysts of Toxoplasma gondii by oral route showed differences of virulence from Brazilian RFLP genotypes BrI and BrIII. Research in Veterinary Science 107, 257–260. [DOI] [PubMed] [Google Scholar]
- Chiebao DP, Pena HF, Passarelli D, Santín T, Pulz LH, Strefezzi RF, Sevá AP, Martins CM, Lopes EG, Grisi-Filho JHH, Gennari SM and Soares RM (2019) Congenital transmission of Toxoplasma gondii after experimental reinfection with Brazilian typical strains in chronically infected sheep. Frontiers in Veterinary Science 6, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu BC, Martin BE, Stolberg VR and Chensue SW (2013) Cutting edge: central memory CD8T cells in aged mice are virtual memory cells. The Journal of Immunology 191, 5793–5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadimoghaddam Y, Daryani A, Sharif M, Ahmadpour E and Hossienikhah Z (2014) Tissue tropism and parasite burden of Toxoplasma gondii RH strain in experimentally infected mice. Asian Pacific Journal of Tropical Medicine 7, 521–524. [DOI] [PubMed] [Google Scholar]
- Dardé ML (2008) Toxoplasma gondii, ‘new’ genotypes and virulence. Parasite 15, 366–371. [DOI] [PubMed] [Google Scholar]
- Detavernier A, Azouz A, Shehade H, Splittgerber M, Van Maele L, Nguyen M, Thomas S, Achouri Y, Svec D, Calonne E, Fuks F, Oldenhove G and Goriely S (2019) Monocytes undergo multi-step differentiation in mice during oral infection by Toxoplasma gondii. Communications Biology 2, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP (2010) Toxoplasmosis of Animals and Humans, 2nd Edn. Boca Raton: CRC Press. [Google Scholar]
- Dubey JP and Beattie CP (1988) Toxoplasmosis of Animals and Man. Boca Raton: CRC Press. [Google Scholar]
- Dubey JP, Lago EG, Gennari SM, Su C and Jones JL (2012) Toxoplasmosis in humans and animals in Brazil: high prevalence, high burden of disease, and epidemiology. Parasitology 139, 1375–1424. [DOI] [PubMed] [Google Scholar]
- Elbez-Rubinstein A, Ajzenberg D, Dardé M-L, Cohen R, Dumètre A, Yera H, Gondon E, Janaud JC and Thulliez P (2009) Congenital toxoplasmosis and reinfection during pregnancy: case report, strain characterization, experimental model of reinfection and review. Journal of Infectious Diseases 199, 280–285. [DOI] [PubMed] [Google Scholar]
- Fournier GFDSR, Lopes MG, Marcili A, Ramirez DG, Acosta ICL, Giuli da Silva Ferreira JI, Cabral AD, Ribeiro de Lima JT, Fatima de Jesus Pena H, Dias RA and Gennari SM (2014) Toxoplasma gondii in domestic and wild animals from forest fragments of the municipality of Natal, Northeastern Brazil. Revista Brasileira de Parasitologia Veterinaria 23, 501–508. [DOI] [PubMed] [Google Scholar]
- Fox BA, Rommerein LM, Guevara RB, Falla A, Triana MAH, Sun Y and Bzik DJ (2016) The Toxoplasma gondii rhoptry kinome is essential for chronic infection. mBio 7, e00193–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan AP, Johansson M, Ruffell B, Yagui-Beltran A, Lau J, Jablons DM and Coussens LM (2013) Tumor-infiltrating regulatory T cells inhibit endogenous cytotoxic T cell responses to lung adenocarcinoma. The Journal of Immunology 191, 2009–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli RT, Mendonça-Neto R, Lilue J, Howard J and Sher A (2014) Innate resistance against Toxoplasma gondii: an evolutionary tale of mice, cats, and men. Cell Host and Microbe 15, 132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiton PS, Sagawa JM, Fritz HM and Boothroyd JC (2017) An in vitro model of intestinal infection reveals a developmentally regulated transcriptome of Toxoplasma sporozoites and a NF-κB-like signature in infected host cells. PLoS ONE 12, 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton C, Robins R, Thomas R, Oura C, Oliveira S, Villena I, Innes EA, Katzer F and Kelly PJ (2019) Prevalence and genetic diversity of Toxoplasma gondii in free-ranging chickens from the Caribbean. Acta Parasitologica 64, 738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann DC, Maksimov P, Hotop A, Groß U, Däubener W, Liesenfeld O, Pleyer U, Conraths FJ and Schares G (2014) Genotyping of samples from German patients with ocular, cerebral and systemic toxoplasmosis reveals a predominance of Toxoplasma gondii type II. International Journal of Medical Microbiology 304, 911–916. [DOI] [PubMed] [Google Scholar]
- Hide G, Morley EK, Hughes JM, Gerwash O, Elmahaishi MS, Elmahaishi KH, Thomasson D, Wright EA, Williams RH, Murphy RG and Smith JE (2009) Evidence for high levels of vertical transmission in Toxoplasma gondii. Parasitology 136, 1877–1885. [DOI] [PubMed] [Google Scholar]
- Hill RD, Gouffon JS, Saxton AM and Su C (2012) Differential gene expression in mice infected with distinct toxoplasma strains. Infection and Immunity 80, 968–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Y (1988) A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75, 800–802. [Google Scholar]
- Hoeman CM, Dhakal M, Zaghouani AA, Cascio JA, Wan X, Khairallah MT, Chen W and Zaghouani H (2013) Developmental expression of IL-12Rbeta2 on murine naive neonatal T cells counters the upregulation of IL-13Ralpha1 on primary Th1 cells and balances immunity in the newborn. The Journal of Immunology 190, 6155–6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe DK and Sibley LD (1995) Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. Journal of Infectious Diseases 172, 1561–1566. [DOI] [PubMed] [Google Scholar]
- Hurtado A, Aduriz G, Moreno B, Barandika J and Garcia-Perez AL (2001) Single tube nested PCR for the detection of Toxoplasma gondii in fetal tissues from naturally aborted ewes. Veterinary Parasitology 102, 17–27. [DOI] [PubMed] [Google Scholar]
- Innes EA and Vermeulen AN (2006) Vaccination as a control strategy against the coccidial parasites Eimeria, Toxoplasma and Neospora. Parasitology 133, 145–168. [DOI] [PubMed] [Google Scholar]
- Jin Y, Yao Y, El-Ashram S, Tian J, Shen J and Ji Y (2019) The neurotropic parasite Toxoplasma gondii induces astrocyte polarization through NFκB pathway. Frontiers in Medicine 6, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Tardieu JM, Walworth EW, Cornelius JG, Ye X, Schuster SM and Peck AB (1996) Autoimmune diabetes-prone NOD mice express the Lyt2 alpha (Lyt2.1) and Lyt3 alpha (Lyt3.1) alleles of CD8. Immunogenetics 43, 6–12. [DOI] [PubMed] [Google Scholar]
- Katzer F, Canton G, Burrells A, Palarea-Albaladejo J, Horton B, Bartley PM, Pang Y, Chianini F, Innes EA and Benavides J (2014) Immunization of lambs with the S48 strain of Toxoplasma gondii reduces tissue cyst burden following oral challenge with a complete strain of the parasite. Veterinary Parasitology 205, 46–56. [DOI] [PubMed] [Google Scholar]
- Kawai T and Akira S (2005) Tool-like receptor downstream signaling. Arthritis Research and Therapy 7, 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konecki DS, Brennand J, Fuscoe JC, Caskey CT and Chinault AC (1982) Hypoxanthine-guanine phosphoribosyl transferase genes of mouse and Chinese hamster: construction and sequence analysis of cDNA recombinants. Nucleic Acids Research 10, 6763–6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes FMR, Gonçalves DD, dos Reis CR, Breganó RM, Freire RL, de Freitas JC and Navarro IT (2009) Presence of domesticated cats and visual impairment associated to Toxoplasma gondii serum positive children at an elementary school in Jataizinho, state of Paraná, Brazil. Revista Brasileira de Parasitologia Veterinaria 17, 12–15. [DOI] [PubMed] [Google Scholar]
- Lu YY, Dong H, Feng YJ, Wang K, Jiang YB, Zhang LX and Yang YR (2018) Avirulence and lysozyme secretion in Paneth cells after infection of BALB/c mice with oocysts of Toxoplasma gondii strains TgCatCHn2 (ToxoDB#17) and TgCatCHn4 (ToxoDB#9). Veterinary Parasitology 252, 1–8. [DOI] [PubMed] [Google Scholar]
- Martins GA, Tadokoro CE, Silva RB, Silva JS and Rizzo LV (2004) CTLA-4 blockage increases resistance to infection with the intracellular protozoan Trypanosoma cruzi. The Journal of Immunology 172, 4893–4901. [DOI] [PubMed] [Google Scholar]
- Massad E, Menezes RX, Silveira PSP and Ortega NRS (2004) Métodos Quantitativos em Medicina. Barueri: Manole. [Google Scholar]
- Meireles LR, Ekman CCJ, De Andrade Júnior HF and Luna EJDA (2015) Human toxoplasmosis outbreaks and the agent infecting form. Findings from a systematic review. Journal of the Institute of Tropical Medicine of Sao Paulo 57, 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes NHD, Oliveira CBS, Garcia CA, Holanda CMXC and Andrade-Neto VF (2014) Epidemiological and serological profiles of ocular toxoplasmosis in the municipality of Natal, Northeastern Brazil. Transactions of the Royal Society of Tropical Medicine and Hygiene 108, 656–661. [DOI] [PubMed] [Google Scholar]
- Miller CM, Boulter NR, Ikin RJ and Smith NC (2009) The immunobiology of the innate response to Toxoplasma gondii. International Journal for Parasitology 39, 23–39. [DOI] [PubMed] [Google Scholar]
- Minitab (2019) Suporte ao Minitab 18. Available at https://support.minitab.com/pt-br/minitab/18/.
- Mordue DG, Monroy F, La Regina M, Dinarello CA and Sibley LD (2001) Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. Journal of immunology (Baltimore, Md.: 1950) 167, 4574–4584. [DOI] [PubMed] [Google Scholar]
- Pena HFJ, Soares RM, Amaku M, Dubey JP and Gennari SM (2006) Toxoplasma gondii infection in cats from São Paulo state, Brazil: seroprevalence, oocyst shedding, isolation in mice, and biologic and molecular characterization. Research in Veterinary Science 81, 58–67. [DOI] [PubMed] [Google Scholar]
- Pena HFJ, Gennari SM, Dubey JP and Su C (2008) Population structure and mouse-virulence of Toxoplasma gondii in Brazil. International Journal for Parasitology 38, 561–569. [DOI] [PubMed] [Google Scholar]
- Pena HFJ, Pinheiro TM, Soares HS, Oliveira S, Alves BF, Ferreira MN and Gennari SM (2018a) Typical Brazilian genotype of Toxoplasma gondii isolated from a horse destined for human consumption in Europe from a slaughterhouse. Parasitology Research 117, 3305–3308. [DOI] [PubMed] [Google Scholar]
- Pena HFJ, Alves BF, Soares HS, Oliveira S, Ferreira MN, Bricarello PA, Machado TMP, Castro BBP and Gennari SM (2018b) Free-range chickens from Santa Catarina state, southern Brazil, as asymptomatic intermediate hosts for Toxoplasma gondii clonal type I and typical Brazilian genotypes. Veterinary Parasitology: Regional Studies and Reports 13, 55–59. [DOI] [PubMed] [Google Scholar]
- Saeij JPJ, Boyle JP and Boothroyd JC (2005) Differences among the three major strains of Toxoplasma gondii and their specific interactions with the infected host. Trends in Parasitology 21, 476–481. [DOI] [PubMed] [Google Scholar]
- Sánchez-Sánchez R, Ferre I, Regidor-Cerrillo J, Gutiérrez-Expósito D, Ferrer LM, Arteche-Villasol N, Moreno-Gonzalo J, Müller J, Aguado-Martínez A, Pérez V, Hemphill A, Ortega-Mora LM and Benavides J (2019) Virulence in mice of a Toxoplasma gondii type II isolate does not correlate with the outcome of experimental infection in pregnant sheep. Frontiers in Cellular and Infection Microbiology 9, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw MH, Freeman GJ, Scott MF, Fox BA, Bzik DJ, Belkaid Y and Yap GS (2006) Tyk2 negatively regulates adaptive Th1 immunity by mediating IL-10 signaling and promoting IFN-gamma dependent IL-10 reactivation. Journal of immunology (Baltimore, Md.: 1950) 176, 7263–7271. [DOI] [PubMed] [Google Scholar]
- Shibata K, Yamada H, Nakamura M, Hatano S, Katsuragi Y, Kominami R and Yoshikai Y (2014) IFN-gamma-producing and IL-17-producing gammadelta T cells differentiate at distinct developmental stages in murine fetal thymus. The Journal of Immunology 192, 2210–2218. [DOI] [PubMed] [Google Scholar]
- Shwab EK, Zhu XQ, Majumdar D, Pena HFJ, Gennari SM, Dubey JP and Su C (2014) Geographical patterns of Toxoplasma gondii genetic diversity revealed by multilocus PCR-RFLP genotyping. Parasitology 141, 453–461. [DOI] [PubMed] [Google Scholar]
- Sibley DL, Mordue D and Howe DK (1999) Experimental approaches to understanding virulence in toxoplasmosis. Immunobiology 201, 210–224. [DOI] [PubMed] [Google Scholar]
- Silva MA, Pena HFJ, Soares HS, Aizawa J, Oliveira S, Alves BF, Souza DS, Melo RPB, Gennari SM, Mota RA and Silva JCR (2018) Isolation and genetic characterization of Toxoplasma gondii from free-ranging and captive birds and mammals in Pernambuco State, Brazil. Revista Brasileira de Parasitologia Veterinaria 27, 481–487. [DOI] [PubMed] [Google Scholar]
- Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler GD, Altschul SF, Zeeberg B, Buetow KH, Schaefer CF, Bhat NK, Hopkins RF, Jordan H, Moore T, Max SI, Wang J, Hsieh F, Diatchenko L, Marusina K, Farmer AA, Rubin GM, Hong L, Stapleton M, Soares MB, Bonaldo MF, Casavant TL, Scheetz TE, Brownstein MJ, Usdin TB, Toshiyuki S, Carninci P, Prange C, Raha SS, Loquellano NA, Peters GJ, Abramson RD, Mullahy SJ, Bosak SA, McEwan PJ, McKernan KJ, Malek JA, Gunaratne PH, Richards S, Worley KC, Hale S, Garcia AM, Gay LJ, Hulyk SW, Villalon DK, Muzny DM, Sodergren EJ, Lu X, Gibbs RA, Fahey J, Helton E, Ketteman M, Madan A, Rodrigues S, Sanchez A, Whiting M, Madan A, Young AC, Shevchenko Y, Bouffard GG, Blakesley RW, Touchman JW, Green ED, Dickson MC, Rodriguez AC, Grimwood J, Schmutz J, Myers RM, Butterfield YS, Krzywinski MI, Skalska U, Smailus DE, Schnerch A, Schein JE, Jones SJ and Marra MA (2002) Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proceedings of the National Academy of Sciences of the United States of America 99, 16899–16903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhumavasi W, Egan CE, Amy L, Taylor GA, Fox BA, David J, Denkers EY, Warren AL and Bzik DJ (2008) TLR Adaptor MyD88 is essential for pathogen control during oral Toxoplasma gondii infection but not adaptive immunity induced by a vaccine strain of the parasite. The Journal of Immunology 181, 3464–3473. www.jimmunol.org/content/181/5/3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M, Guiton R, Lacroix-Lamandé S, Ryffel B, Leman S and Dimier-Poisson I (2013) Myd88 is crucial for the development of a protective CNS immune response to Toxoplasma gondii infection. Journal of Neuroinflammation 10, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuladar S, Kochanowsky JA, Bhaskara A, Ghotmi Y, Chandrasekaran S and Koshy AA (2019) The ROP16 III -dependent early immune response determines the subacute CNS immune response and type III Toxoplasma gondii survival. PLoS Pathogens 15, e1007856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzanidakis N, Maksimov P, Conraths FJ, Kiossis E, Brozos C, Sotiraki S and Schares G (2012) Toxoplasma gondii in sheep and goats: seroprevalence and potential risk factors under dairy husbandry practices. Veterinary Parasitology 190, 340–348. [DOI] [PubMed] [Google Scholar]
- Wang Z, Filgueiras LR, Wang S, Serezani AP, Peters-Golden M, Jancar S and Serezani CH (2014) Leukotriene B4 enhances the generation of proinflammatory microRNAs to promote MyD88-dependent macrophage activation. The Journal of Immunology 192, 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson WA and Beverley JKA (1971) Epizootics of toxoplasmosis causing ovine abortion. Veterinary Records 88, 120–124. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jiang N, Zhang T, Wang D, Feng Y, Sang X, Yang N and Chen Q (2018) Toxoplasma gondii genotype determines Tim-3 expression levels in splenic and circulatory T cells in mice. Frontiers in Microbiology 9, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182020002346.
click here to view supplementary material