Abstract
Cutaneous leishmaniasis (CL) is one of the most disregarded tropical neglected disease with the occurrence of self-limiting ulcers and triggering mucosal damage and stigmatizing scars, leading to huge public health problems and social negative impacts. Pentavalent antimonials are the first-line drug for CL treatment for over 70 years and present several drawbacks in terms of safety and efficacy. Thus, there is an urgent need to search for non-invasive, non-toxic and potent drug candidates for CL. In this sense, we have implemented a shape-based virtual screening approach and identified a set of 32 hit compounds. In vitro phenotypic screenings were conducted using these hit compounds to check their potential leishmanicidal effect towards Leishmania amazonensis (L. amazonensis). Two (Cp1 and Cp2) out of the 32 compounds revealed promising antiparasitic activities, exhibiting considerable potency against intracellular amastigotes present in peritoneal macrophages (IC50 values of 9.35 and 7.25 μm, respectively). Also, a sterile cidality profile was reached at 20 μm after 48 h of incubation, besides a reasonable selectivity (≈8), quite similarly to pentamidine, a diamidine still in use clinically for leishmaniasis. Cp1 with an oxazolo[4,5-b]pyridine scaffold and Cp2 with benzimidazole scaffold could be developed by lead optimization studies to enhance their leishmanicidal potency.
Key words: Cutaneous leishmaniasis, in vitro experimental chemotherapy, Leishmania amazonensis, shape-based virtual screening
Introduction
Cutaneous leishmaniasis (CL) is a vector-borne tropical neglected disease caused by over 20 different species of kinetoplastid parasites of the genus Leishmania. This disfiguring and stigmatizing disease occurs through the injection of promastigote forms into the mammalians by infected female sandflies, triggering ulcers and permanent scars on the skin and/or oral and nasal mucosa injuries, thus contributing to high social stigmatization and public health issue (Bilgic-Temel et al., 2019; WHO, 2020). Although about 1.2 million new cases occur annually, CL does not have adequate treatment that are mostly based on old and highly toxic drugs besides the occurrence of high number of parasite species with drug-resistance profile (Alvar et al., 2012; de Vries et al., 2015; Bailey et al., 2019; ; Van Bocxlaer et al., 2019).
Computer-aided drug design is an efficient strategy to identify active compounds. Shape-based screening has been successfully employed for the development of anti-fungal and anti-bacterial agents (Swinney and Anthony, 2011). The purpose of the shape-based screening is to identify chemically diverse compounds that show similar biological activity as the query compound. This is based on the principle that diverse structures that share similar shape and electrostatic potential surface or topology will have highest probability to bind to the same pocket and consequently share a similar activity (Kumar and Zhang, 2018).
Due to the limited information on targeted proteins of Leishmania amazonensis, we have chosen ligand-based shape screening approach for the hit identification process. The main advantages of this approach are that it only needs a single active compound as the starting point, and that a target crystal structure is not a requirement. This approach aids in the identification of diverse scaffolds other than the query compound scaffold, hence patent issues associated with the chemical space can be avoided.
Ligand-based shape screening was carried out using an active compound GNF5343 (Fig. 1) that displayed activity against the kinetoplastid parasites Trypanosoma cruzi, Trypanosoma brucei and Leishmania donovani (Khare et al., 2016). To our knowledge, GNF5343 activity against L. amazonensis has not yet been reported. It has been suggested that the kinetoplastid parasites (Leishmania species and Trypanosoma species) share comparative genomic features (EI-Sayed et al., 2005). GNF5343 activity against the three parasites suggests that this compound could be acting through a common receptor target. Hence, we have hypothesized that compounds that show shape similarity with GNF5343 should inhibit the common target and display activity against the L. amazonensis. Based on this hypothesis, we presently used GNF5343 as a query compound.
Fig. 1.
Shape-based virtual screening results. (A) Illustrating the good alignment of query compound GNF5343 (orange sticks) with compound 2 (atom type coloured sticks). (B) Displaying maximum volume overlap that indicates good shape complementarity between the query compound (represented with mesh with an area of 300 Å2) and the compound 2 (represented with van der Waals molecular surface area of 326.5 Å2). (C) Molecular structures and their associated activity data. 1a and 1b images are generated using Phase-Schrödinger drug design software.
Thus, the urgent need for safer and selective potent drugs associated with promising aspects of identified diverse hit compounds from our ligand-based shape-screening programme encouraged us to perform in vitro phenotypic screening on amastigotes of L. amazonensis, which is one of the main agents of CL in the Americas (Martins et al., 2014; de Vries et al., 2015).
Methods
Compounds
All 32 identified hit compounds (Figs 1 and 2) that were purchased from Asinex commercial vendor and the reference drug, pentamidine (Pt), were dissolved in 99.99% dimethyl sulphoxide (DMSO) (stock solutions at 20 mm) and fresh dilutions prepared extemporaneously, with the final concentration never exceeding 0.6% DMSO for in vitro experiments, which does not induce host cell toxicity (Santos et al., 2018).
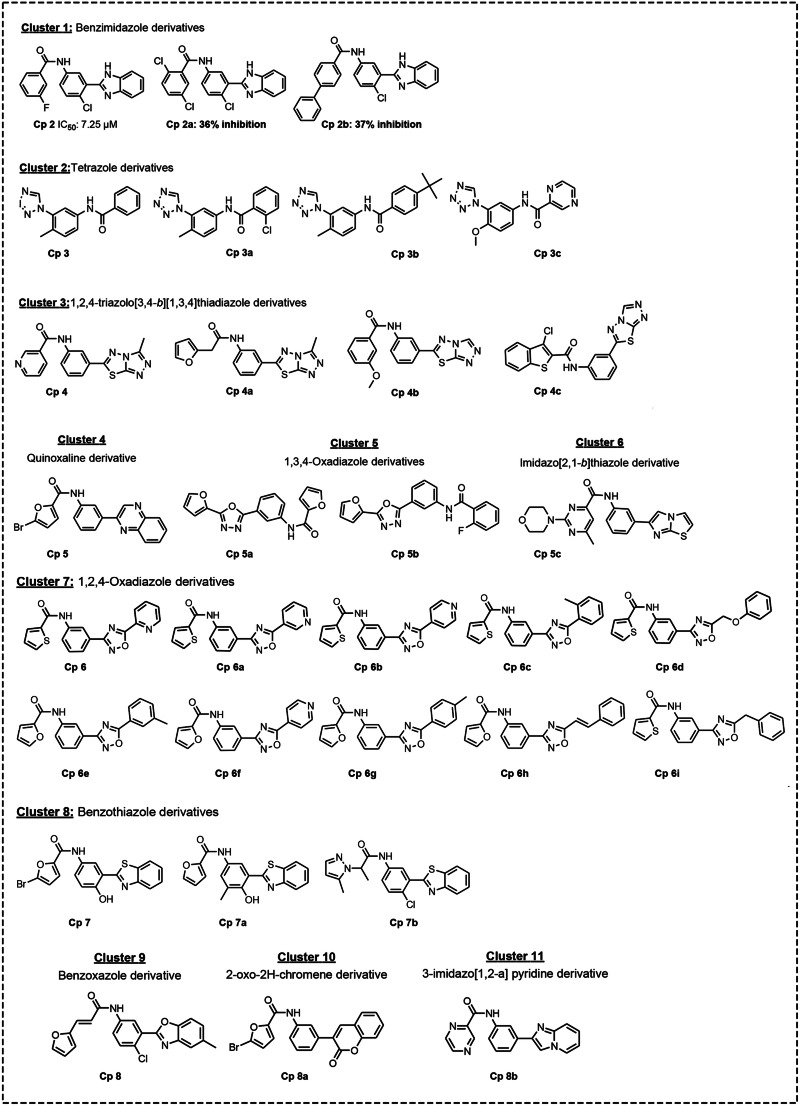
Fig. 2.
Clusters of hit compounds identified by shape-based virtual screening strategy. Cp 2a and Cp2b: % inhibition at 10 μm.
Parasite strain and mammalian host cell cultures
Leishmania amazonensis (strain LTB0016) was used throughout the study. Male BALB/c mice were infected (106 amastigotes 20 μL−1 culture medium, via subcutaneous) at their foot paws, using a BD ultrafineTM 6 mm syringe (15/64″) × 31 G, following previous reported protocol, with minor modifications (Van Bocklaer et al., 2019). After 30 days post infection, the animal skin lesions were aseptically removed, and the parasites obtained by mechanic dissociation (pipetting). The purified amastigotes were then assayed directly with the studied compounds to check the activity upon free amastigote (FA) forms, or used to infect primary cultures of peritoneal macrophages (PMM) to investigate their potency against intracellular forms (IA) (Feitosa et al., 2019). Swiss male mice (18–20 g) were inoculated with 3% thioglycolate and after 4 days, PMM collected by rinsing the animals' peritoneum with RPMI 1640. Mammalian cells were seeded at 24 (3 × 105 cells well−1) and 96-well (5 × 104 cells well−1) plates and used for in vitro infection and host cell cytotoxicity analysis, respectively. The cultures were sustained at 37°C with 5% CO2 atmosphere in RPMI 1-640 medium (pH 7.2−7.4) without phenol red (Gibco BRL) but supplemented with 1% l-glutamine, penicillin−streptomycin 10 000 U mL−1, 10% fetal bovine serum (FBS). Assays using FA were also maintained at 32°C using the same RPMI culture medium but adding 5% FBS instead of 10%.
Cytotoxicity upon mammalian host cells and leishmanicidal analysis
For cytotoxicity analysis, PMM were incubated for 48 h with increasing concentrations of the tested compounds (up to 500 μm). Cellular viability was evaluated by AlamarBlue tests (Invitrogen) following the manufacturer's instructions (Da Silva et al., 2007; Romanha et al., 2010). The leishmanicidal activity was explored in two steps: in the first set of assays, amastigotes (106 parasites per well in 0.2 mL) purified from animal lesions (FA) were exposed for 48 h using a fixed concentration (10 μm) and then, drug activity assessed by AlamarBlue tests (Mikus & Sterverding, 2000). Then, in a second set of phenotypic screenings, the activity of the compounds was further validated on IA. In these assays, PMM (3 × 105) were infected with amastigotes (9 × 105 amastigotes) using multiplicity of infection (MOI) 3:1 (Van Bocxlaer et al., 2019). After 48 h of compound incubation (0–20 μm), infected PMM were rinsed with saline buffered with phosphate (PBS), fixed with Bouin's solution and stained with Giemsa for light microscopy analysis (Santos et al., 2018). Then, the percentage of infected host cells and the number of parasites per infected cells were scored for determination of the corresponding infection index (II) that represents the multiplication factor of both parameters. Only characteristic parasite nuclei and kinetoplasts were counted as surviving parasites since irregular structures could mean parasites undergoing death. The results were expressed as % of reduction of the parasite burden and the IC50 and IC90 calculated (Santos et al., 2018). All assays were run in at least twice in three independent repeats.
Compound database preparation
Using the LigPrep module of the Schrödinger drug design software, a database (Asinex gold) of commercially available compounds was prepared by performing two-dimensional (2D) to three-dimensional (3D) conversion, addition of hydrogens, generation of ionization states, tautomeric states, stereoisomers and ring conformations at the physiological pH 7.0 ± 2.0. Further, energy minimization of all the compounds was carried out using the molecular mechanics OPLS3 force field (Harder et al., 2016). Compound selection was carried out by applying the Lipinski filter (molecular weight (mol. wt.) ≤500; calculated log P (cLogP) ≤5; H-bond acceptors (HBA) ≤10; H-bond donors (HBD) ≤5), besides removing the compounds with nitro groups and reactive functional groups (QikProp, Schrödinger release, 2017). A database was computed with a final set of 60 000 compounds.
Shape-based screening approach
The shape screening was performed using the phase module (Schrödinger-Phase, 2017). We used single 3D structure of GNF5343 compound as the query structure (Fig. 1). Compounds were screened by using the pharmacophore volume scoring setting which treats each compound as a collection of pharmacophore features. These features are aromatic groups, HBA groups, HBD groups, hydrophobic groups, positive charge groups and negative charge groups. Pharmacophoric tolerance was represented by a sphere of radius 2 Å. During the screening, 100 conformers were generated for each compound and the best 10 conformers were retained for matching pharmacophore features similarity. Phase similarity score (phase sim score) was computed based on the maximum aligned features for these conformers.
Cluster analysis
Schrödinger Canvas module was used to perform the cluster analysis as described (Schrödinger-Canvas manual, 2017). All the 31 compounds (Fig. 2) were processed using the hierarchical clustering algorithm with tanimoto similarity as a distance matrix. 2D fingerprints and atom pairs were used as metrics to quantify the chemical diversity.
Results
A subset of 31 compounds was selected based on the structural diversity and phase similarity score. Phase similarity score was computed from the maximum overlapping pharmacophore features as described in the method section. Clustering analysis was carried out on the selected subset of 31 compounds using a hierarchical clustering algorithm. A total of 11 clusters were identified (Fig. 2), of which five were singletons with diverse chemical structures such as quinoxaline derivative (cluster 4), imidazo[2,1-b]thiazole derivative (cluster 6), benzoxazole derivative (cluster 9), 2-oxo-2H-chromene derivative (cluster 10) and 3-imidazo[1,2-a] pyridine derivative (cluster 11). As the query compound GNF5343 was not available for purchase, we have selected one of its close analogue Cp1 (Fig. 1) for the comparative studies along with the selected set of 31 hit compounds. Cp1 differs from GNF5343 in having a thiophenyl ring substitution instead of furyl ring. All 32 compounds were purchased from Asinex and assayed using different protocols in vitro. A fixed concentration (10 μm) was first assessed on FA and findings demonstrated that two (Cp1 and Cp2) out of the 32 compounds reduced (≥50%) the number of live parasites. Analogues of Cp2 from cluster 1 (Fig. 1: Cps 2a and 2b) displayed weak inhibition (Fig. 2). Compounds from other clusters did not show any activity. While Cp1 is a close analogue of query compound, Cp2 is structurally diverse from GNF5343 in having benzimidazole scaffold. These active compounds (Cp1 and Cp2) and the reference drug pentamidine (Pt) were further analysed against FA using increasing concentrations of the tested compounds and Pt. The findings showed moderate leishmanicidal effect, with IC50 values of 13.03 ± 2.69 and 14.09 ± 2.25 μm for Cp1 and Cp2, respectively, being less potent than Pt (0.71 ± 0.05 μm).
In the second round of assays, the compounds were further evaluated against IA present in the cytoplasm of PPM, which represent the gold models for in vitro screening of leishmanicidal agents (DNDi, 2018). Our data showed that both compounds were active upon IA, exhibiting IC50 values of 9.35 ± 1.87 and 7.25 ± 1.46 μm, respectively, while Pt yielded 1.94 ± 0.50 μm. Against the IA, Cp1 and Cp2 reached low IC90 values (17.25 ± 0.21 μm and 18.54 ± 0.96 μm), exhibiting a leishmanicidal profile since both drastically dropped the number of parasites per cell as well as the percentage of infected PMM (Fig. 3). Regarding the mammalian host cell toxicity, we found that after 48 h of exposure, Cp1 and Cp2 were about 3-fold less toxic as compared to Pt, giving IC50 of 62.75 ± 0.27 and 65.39 ± 0.61, with selectivity indexes of 6 and 9, respectively, in similar range than the reference drug (SI = 8).
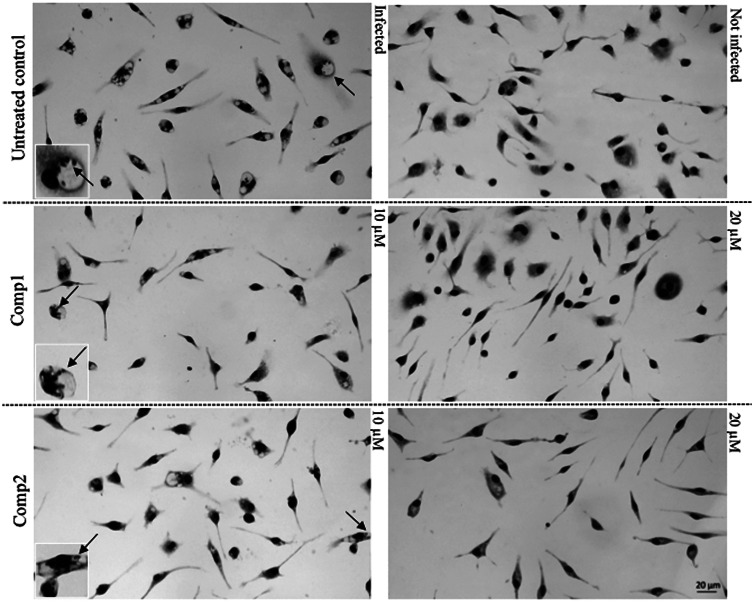
Fig. 3.
Light microscopy images of Giemsa-stained uninfected and infected PMM exposed or not (untreated control) to 10 and 20 μm of Cp1 and Cp2, demonstrating parasite sterilization at 20 μm. Arrows: intracellular parasites.
In silico assessment of drug likeness and drug metabolism and pharmacokinetics (DMPK) properties is the most effective way in reducing time, expenses and maximizes the success in drug discovery process (Lombardo et al., 2017). Therefore, drug likeness and DMPK properties were predicted for both Cp1 and Cp2 using the QikProp module (QikProp, Schrödinger release 2017–2). Recommended compliance scores are given in Table 1. The predicted properties of Cp1 and Cp2 showed compliance with ‘Lipinski rule of five’. According to ‘Jorgensen rule of three’ any compound that met the recommended criteria (Table 1) are more likely to be orally available. The predicted properties of both Cp1 and Cp2 displayed compliance with ‘Jorgensen rule of three’. Hence, these compounds could show good permeability and solubility properties. Both these properties are crucial for good oral bioavailability. QPPMDCK values are the prediction of MDCK cell permeability (nm/s), which is a good mimic for blood−brain permeation. According to this, both compounds displayed good blood−brain permeation. The efficiency of a drug may be affected by the extent at which it binds to human plasma protein. If compounds show high binding affinity to serum albumin this could lead to poor efficacy. Hence it is very crucial to understand the binding characteristics of Cp1 and Cp2. The predicted human serum albumin binding values (Table 1) for both Cp1 and Cp2 are within the permissible range indicating a lower binding affinity to the serum albumin protein.
Table 1.
In silico assessment of drug likeness and predicted DMPK properties of Cp1 and Cp2
| Recommended compliance score (range for 95% of drugs) | Compound ID | |||
|---|---|---|---|---|
| Cp1 | Cp2 | |||
| Lipinski rule of five | Mol. wt. | ≤500 | 355.80 | 365.794 |
| HBD | ≤5 | 1 | 2 | |
| HBA | ≤10 | 4 | 2 | |
| cLogP | ≤5 | 3.36 | 4.59 | |
| Jorgensen rule of three | QPlogS | −6.5 to 0.5 | −5.380 | −6.289 |
| QPCaco | <25 poor, >500 great | 1403 | 1721 | |
| Primary metabolites | <7 | 3 | 0 | |
| % Human oral absorption | – | >80% is high, <25% is low | 100 | 100 |
| QPPMDCK | – | >500 great, <25 poor | 2650 | 3285 |
| QPlogKhsa | – | −1.5 to 1.5 | 0.243 | 0.616 |
Mol. wt: molecular weight; HBD: hydrogen bond donor; HBA: hydrogen bond acceptor; cLogP: calculated logarithm of partition coefficient; QPlogS: the logarithm of aqueous solubility; QPCaco: Caco-2 cell permeability in nm/s, model for the gut−blood barrier; QPPMDCK: Madin−Darby canine kidney (MDCK) cell permeability in nm/s, model for the blood−brain barrier; QPlogKhsa: the logarithm of predicted binding constant to human serum albumin.
Discussion
Compound GNF5343, an oxazolo[4,5-b]pyridine derivative (Fig. 1) was reported to display activity against L. donovani with an EC50 of 7.3 ± 0.6 μm, T. cruzi with an EC50 of 75 ± 0.8 nm and T. brucei with an EC50 of 150 ± 7.5 nm (Khare et al., 2016). This active compound was used as a query compound to perform the shape-based virtual screening of a set of 60 000 chemically diverse compounds from the Asinex database. Compounds were ranked based on the phase similarity score. Phase similarity scores range from 0 (minimum similarity) to 1 (maximum similarity). Compounds with a phase similarity score above 0.85 were not considered in the selection as compounds with high similarity scores could be the close analogues of the query compounds. Compounds that are chemically diverse but similar shape to query compound GNF5343 with a shape similarity score between 0.85 and 0.6 were chosen for further analysis. These compounds were visually inspected to assess their structural diversity and synthetic accessibility. Compounds that showed poor shape similarity (phase similarity score less than 0.6) to query compound GNF5343 were not considered in the selection. Cp1 and Cp2 are the two active compounds identified from the selected set of 32 compounds as described under the results. Analysis of the predicted alignments of these two compounds indicate that the root-mean-square deviation (RMSD) value for the maximum common structure between GNF5343 and Cp1 is 0.147 Å (figure not shown) and the RMSD value for the Cp2 is 1.569 Å (Fig. 1a). Both GNF5343 and Cp2 displayed maximum volume overlap (Fig. 1b) indicating that these two compounds are having similar topology to form similar van der Waals surface interactions at the same region. It is interesting to note the varied distribution of HBD, HBA and hydrophobicity between the basic scaffolds (oxazolo[4,5-b]pyridine vs benzimidazole) of GNF5343 and Cp2. Both Cp1 and Cp2 displayed activity against L. amazonensis (Fig. 1c) with an EC50 of 9.35 ± 1.87 μm and EC50 of 7.25 ± 1.46 μm, respectively.
This activity of both Cp1 and Cp2 supports our hypothesis. Herein, we report two novel compounds active against L. amazonensis.
Currently, our analysis was performed using primary cultures of PMM infected with L. amazonensis since professional phagocytes are the main source of host cells for those obligate intracellular parasites (Walker et al., 2014). This in vitro standardized experimental model for CL is claimed to closely reproduce in vivo conditions (Stacey, 2006), therefore, contributing to novel drug candidate screenings for this neglected illness (Chatelain and Ioset, 2011; Caridha et al., 2019). Another interesting point to be addressed is the use of protocols that enable the identification of antiparasitic drugs that induces rapid parasite lysis (Da Silva et al., 2007). This is especial characteristic as most of the CL patients live in very poor areas with difficult access to public health assistance and then, frequently display advanced pathologies, demanding a fast killer drug (Ruoti et al., 2013; Okwor and Uzonna, 2016). Aiming to fulfill this demand, we established a period of 48 h of drug exposure while testing the parasites and host mammalian cells, a shorter period of incubation as compared with others reported in the current literature for CL in vitro models (Van Bocklaer et al., 2019).
This study explored the leishmanicidal effect of Cp1 (an analogue of GNF5343) and Cp2 (a benzimidazole analogue) identified using shape-based virtual screening approach. Both Cp1 and Cp2 achieved quite relevant potency against amastigotes, especially those lodged inside macrophages, reaching IC50 values below 10 μm, a considerable characteristic preconized for a hit compound with anti-leishmania effect (Katsuno et al., 2015). Remarkably, clearance on L. amazonensis infection was found at 20 μm in the infected PMM, a relevant feature to mitigate the possible occurrence of parasite drug resistance and relapses after ceasing the drug administration (Cal et al., 2016). The Cp1 and Cp2 were less toxic than the reference drug (pentamidine) still in use for Leishmaniasis but their selectivity indexes (<10) discouraged to move them for in vivo proof of concept. However, their chemical optimization for a wider therapeutic window and promotion of potency is largely desirable to continue further studies using these compounds. Currently, we are performing structural modifications of Cp2 aiming to improve its potency, selectivity and satisfactory pharmacological profile, favouring future phenotypic studies in order to move its derivatives forward to new in vitro screening and in vivo evaluation, aiming to contribute for drug discovery process of new therapeutic approaches for CL. These analogues will also be tested against other kinetoplastid parasites to assess their activity.
Acknowledgments
The authors thank the Fortalecimento dos Programas de Gestão Estratégica de Pesquisa da Fiocruz Rede de Plataformas Fiocruz (VPPLR – 001 – Fio 14) and the Programa de Excelência Acadêmica (PROEX) from CAPES.
Financial support
This study was supported by grants from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional Desenvolvimento científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação Oswaldo Cruz, PDTIS, PAEF/CNPq/Fiocruz. MNCS is a research fellow of CNPq and CNE.
Ethical standards
All procedures were carried out in accordance with the guidelines established by the FIOCRUZ Committee of Ethics for the Use of Animals (CEUA L038/2017).
Conflicts of interest
None.
References
- Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano, J, Jannin, J and den Boer, M., & WHO Leishmaniasis Control Team (2012) Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 7, e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey F, Mondragon-Shem K, Haines LR, Olabi A, Alorfi A, Ruiz-Postigo JA, Alvar J, Hotez P, Adams ER, Vélez ID, Al-Salem W, Eaton J, Acosta-Serrano Á and Molyneux DH (2019) Cutaneous leishmaniasis and co-morbid major depressive disorder: a systematic review with burden estimates. Cutaneous leishmaniasis and co-morbid major depressive disorder: a systematic review with burden estimates. PLoS Neglected Tropical Diseases 13, e0007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgic-Temel A, Murrell, DF and Uzun S (2019) Cutaneous leishmaniasis: a neglected disfiguring disease for women. International Journal of Women's Dermatology 5, 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cal M, Ioset J-R, Fügi MA, Mäser P and Kaiser M (2016) Assessing anti-T. cruzi candidates in vitro for sterile cidality. International Journal for Parasitology: Drugs and Drug Resistance 6, 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canvas (2017) Schrödinger Release 2017–2: Maestro, Schrödinger, LLC, New York, NY.
- Caridha D, Vesely B, van Bocxlaer K, Arana B, Mowbray CE, Rafati S, Uliana S, Reguera R, Kreishman-Deitrick M, Sciotti R, Buffet P and Croft SL (2019) Route map for the discovery and pre-clinical development of new drugs and treatments for cutaneous leishmaniasis. International Journal for Parasitology: Drugs and Drug Resistance 11, 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelain E and Ioset JR (2011) Drug discovery and development for neglected diseases: the DNDi model. Drug Design, Development and Therapy 16, 175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva CF, Batista MM, Mota RA, de Souza EM, Stephens CE, Som P, Boykin DW and Soeiro Mde N (2007) Activity of “reversed” diamidines against Trypanosoma cruzi “in Vitro”. Biochemical Pharmacology 73, 1939–46. [DOI] [PubMed] [Google Scholar]
- de Vries HJ, Reedijk SH and Schallig HD (2015) Cutaneous leishmaniasis: recent developments in diagnosis and management. American Journal of Clinical Dermatology 16, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DNDi Annual report (2018) Making medical history. Available at https://www.dndi.org/wp-content/uploads/2019/07/DNDi_2018_AnnualReport.pdf (Accessed 8 March 2020).
- El-Sayed N, Myler P, Blandin G, Berriman M, Crabtree J, Aggarwal G, Caler E, Renauld H, Worthey E, Hertz-Fowler C, Ghedi E, Peacock C, Bartholomeu D, Haas B, Tran A, Wortman J, Alsmark Ucm, Angiuoli S, Anupama A, Badger J, Bringaud F, Cadag E, Carlton J, Cerqueira G, Creasy T, Delcher A, Djikeng A, Embley T, Hauser C, Ivens A, Kummerfeld S, Pereira-Leal J, Nilsson D, Peterson J, Salzberg S, Shallom J, Silva J, Sundaram J, Westenberger S, White O, Metville S, Donelson J, Andersson B, Stuart K and Hall N (2005) Comparative genomics of trypanosomatid parasitic protozoa. Science 309, 404–409. [DOI] [PubMed] [Google Scholar]
- Feitosa LM, da Silva ER, Hoelz LVB, Souza DL, Come JAASS, Cardoso-Santos C, Batista MM, Soeiro MNC, Boechat N and Pinheiro LCS (2019) New pyrazolopyrimidine derivatives as Leishmania amazonensis arginase inhibitors. Bioorganic & Medical Chemistry 27, 3061–3069. [DOI] [PubMed] [Google Scholar]
- Harder E, Damm W, Maple J, Wu C, Reboul M, Xiang JY, Wang L, Lupyan D, Dahlgren MK, Knight JL, Kaus JW, Cerutti DS, Krilov G, Jorgensen WL, Abel R and Friesner RA (2016) OPLS3: a force field providing broad coverage of drug-like small molecules and proteins. Journal of Chemical Theory and Computation 12, 281–296. [DOI] [PubMed] [Google Scholar]
- Katsuno K, Burrows JN, Duncan K, van Huijsduijnen RH and Kaneko T (2015) Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nature Reviews Drug Discovery 14, 751–758. [DOI] [PubMed] [Google Scholar]
- Khare S, Nagle AS, Biggart A, Lai YH, Liang F, Davis LC, Barnesn SW, Mathison CJN, Myburgh E, Gao MY, Gillespie JR, Liu X, Tan JL, Stinson M, Rivera IC, Ballard J, Yeh V, Groessl T, Federe G, Koh HXY, Venable JD, Bursulaya B, Shapiro M, Mishra PK, Spraggon G, Brock A, Mottram JC, Buckner FS, Rao SPS, Wen BG, Walker JR, Tuntland T, Molteni V, Glynne RJ and Supek F (2016) Proteasome inhibition for treatment of Leishmaniasis, Chagas disease and sleeping sickness. Nature 537, 229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A and Zhang KYJ (2018) Advances in the development of shape similarity methods and their application in drug discovery. Frontiers in Chemistry 6, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo F, Desai PV, Arimoto R, Desino KE, Fischer H, Keefer CE, Petersson C, Winiwarter S and Broccatelli F (2017) In silico absorption, distribution, metabolism, excretion, and pharmacokinetics (ADME-PK): utility and best practices. An industry perspective from the international consortium for innovation through quality in pharmaceutical development. Journal of Medical Chemistry 60, 9097–9113. [DOI] [PubMed] [Google Scholar]
- Martins AL, Barreto JA, Lauris JR and Martins AC (2014) American tegumentary leishmaniasis: correlations among immunological, histopathological and clinical parameters. Anais Brasileiros de Dermatologia 89, 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikus J and Sterverding D (2000) A simple colorimetric to screen drug cytotoxicity against Leishmania using the dye Alamar blue. Parasitology international 48, 265–269. [DOI] [PubMed] [Google Scholar]
- Okwor I and Uzonna J (2016) Social and economic burden of human leishmaniasis. American Journal of Tropical Medical Hygiene 94, 489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QikProp (2017) Virtual screening workflow, Schrödinger Release 2017-2: Maestro, Schrödinger, LLC, New York, NY.
- Romanha AJ, Castro SL, Soeiro Mde N, Lannes-Vieira J, Ribeiro I, Talvani A, Bourdin B, Blum B, Olivieri B, Zani C, Spadafora C, Chiari E, Chatelain E, Chaves G, Calzada JE, Bustamante JM, Freitas-Junior LH, Romero LI, Bahia MT, Lotrowska M, Soares M, Andrade SG, Armstrong T, Degrave W and Andrade Zde A (2010) In vitro and in vivo experimental models for drug screening and development for Chagas disease. Memórias do Instituto Oswaldo Cruz 105, 233–238. [DOI] [PubMed] [Google Scholar]
- Ruoti M, Oddone R, Lampert N, Orué E, Miles MA, Alexander N, Rehman AM, Njord R, Shu S, Brice S, Sinclair B and Krentel A (2013) Mucocutaneous leishmaniasis: knowledge, attitudes, and practices among Paraguayan communities, patients, and health professionals. Journal of Tropical Medicine 2013, 538629. 10.1155/2013/538629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos CC, Lionel JR, Peres RB, Batista MM, da Silva PB, de Oliveira GM, da Silva CF, Batista D, Souza S, Andrade CH, Neves BJ, Braga RC, Patrick DA, Bakunova SM, Tidwell RR and Soeiro M (2018) In vitro, in silico, and in vivo analyses of novel aromatic amidines against Trypanosoma cruzi. Antimicrobial Agents and Chemotherapy, 62: e02205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey G (2006) Primary cell cultures and immortal cell lines. Encyclopedia of Life Sciences. doi: 10.1038/npg.els.0003960. [DOI] [Google Scholar]
- Swinney DC and Anthony J (2011) How were new medicines discovered? Nature Reviews Drug Discovery 10, 507–519. [DOI] [PubMed] [Google Scholar]
- Van Bocxlaer K, Caridha D, Black C, Vesely B, Leed S, Sciotti RJ, Wijnant GJ, Yardley V, Braillard S, Mowbray CE, Ioset JR and Croft SL (2019) Novel benzoxaborole, nitroimidazole and aminopyrazoles with activity against experimental cutaneous leishmaniasis. International Journal for Parasitology: Drugs and Drug Resistance 11, 129–138. doi: 10.1016/j.ijpddr.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Oghumu S, Gupta G, McGwire BS, Drew ME and Satoskar AR (2014) Mechanisms of cellular invasion by intracellular parasites. Cellular and Molecular Life Sciences 71, 1245–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2020) Leishmaniasis factsheet – 2015 (last update 2 March 2020) Brazil. Available at https://www.who.int/en/news-room/fact-sheets/detail/leishmaniasis (Accessed 8 March 2020).