Key words: Cryptic species, cytb gene, Haemosporida, mtDNA sequencing
Abstract
Delimiting and describing Plasmodium species in reptiles remains a pressing problem in Haemosporida taxonomy. The few morphological characters used can overlap, and the significance of some life-history traits is not fully understood. Morphologically identical lizard Plasmodium forms have been reported infecting different cell types (red and white blood cells) in the same host and have been considered the same species. An example is Plasmodium tropiduri tropiduri, a species known to infect erythrocytes, thrombocytes and lymphocyte-like cells. Here, both forms of P. t. tropiduri were analysed using light microscope-based morphological characteristics and phylogenetic inferences based on almost complete mitochondrial genomes of parasites naturally infecting lizards in southeastern Brazil. Although morphologically similar, two distinct phylogenetic lineages infecting erythrocytes and non-erythrocytic cells were found. The lineage found in the erythrocytes forms a monophyletic group with species from Colombia. However, the non-erythrocytic lineage shares a recent common ancestor with Plasmodium leucocytica, which infects leucocytes in lizards from the Caribbean islands. Here, Plasmodium ouropretensis n. sp. is described as a species that infects thrombocytes and lymphocyte-like cells.
Introduction
Morphological characters in parasites are of limited value for evolutionary inferences, particularly when species cannot be delimited (Poulin, 2011; Pacheco and Escalante, 2020). For example, the description of malaria parasites (genus Plasmodium) mainly relied on morphological evaluation via light microscopy of the stages infecting blood cells. Recent studies using genetic sequencing have revealed that morphologically similar parasites may have independent evolutionary histories (Perkins, 2000; Palinauskas et al., 2015). As a result, the phylogenetic relationships of some groups with scarcely available molecular data, such as lizard haemosporidians, are still a work in progress (Telford, 2009; Perkins et al., 2011).
Lizards are commonly infected with non-erythrocytic haemosporidians (Telford, 2009). The family Garniidae was proposed by Lainson et al. (1971) to include erythrocytic parasites that do not produce haemozoin pigment, genus Garnia; and to include those parasites that undergo merogony and gametocytogenesis in non-erythrocytic blood cells, genus Fallisia. Non-erythrocytic parasites also have been interpreted as a life cycle trait of Plasmodium parasites when asexual stages (trophozoites and merozoites) were found in lizards harbouring erythrocytic Plasmodium infections (Telford, 2009). However, molecular data are required to define whether these non-erythrocytic parasites form a distinct family or if they are simply related Plasmodium species (Telford, 1973, 2009; Lainson et al., 1974; Perkins, 2000).
Molecular tools have proven essential in uncovering cryptic species and understanding the diversity and phylogenetic relationships of lizard Plasmodium infecting erythrocytic and non-erythrocytic blood cells (Perkins, 2000; Falk et al., 2011). Perkins (2000) used molecular data and demonstrated that, despite the morphological similarity of erythrocytic and non-erythrocytic forms, Plasmodium azurophilum was composed of two genetically divergent lineages within Plasmodiidae. These parasites were divided into two species: P. azurophilum, which infects erythrocytes, and another species later named Plasmodium leucocytica by Telford (2009), which infects white blood cells.
Plasmodium tropiduri is a species complex with four recognized subspecies infecting lizards from Mexico to southeastern Brazil (Telford, 1979, 2009). Plasmodium tropiduri tropiduri is a widespread parasite in Brazil and Venezuela and infects at least five lizard species of four families (Telford, 2009; Picelli et al., 2020). This parasite produces trophozoites, meronts, and gametocytes in thrombocytes. A comparison between erythrocytic and non-erythrocytic parasites found no significant differences in their ultrastructure (Scorza, 1971). Thus, a thrombocytic cycle in the life history of P. t. tropiduri was suggested (Scorza, 1971; Telford, 2009). Non-erythrocytic parasites in co-occurrence with P. t. tropiduri were identified as Fallisia modesta (Telford, 2009), which infects thrombocytes and lymphocytes of T. hispidus from Pará, Brazil (Lainson et al., 1974; Telford, 2009). However, there are no quantitative morphological data associated with the molecular information of these forms. Therefore, whether non-erythrocytic forms are a part of the phenotypic plasticity of P. t. tropiduri or a different parasite species altogether remains to be solved. Here, erythrocytic and non-erythrocytic forms of P. t. tropiduri were studied in samples collected in free-ranging Amazon Lava Lizards (Tropidurus torquatus) from southeastern Brazil. Our study included morphometric analyses and molecular phylogenetic inferences based on almost complete mitochondrial genomes (5420 bp out of 5896 bp).
Materials and methods
Our study was conducted at Andorinhas Municipal Park in Ouro Preto, Minas Gerais, Brazil (43°29′13.90″W, 20°33′1.22″S), between December 2015 and January 2016. Amazon Lava lizards were caught using a slip noose on the end of a fishing pole. Blood samples were collected from the ventral caudal vein. Then, two blood smears per lizard were prepared, and two drops of blood were stored in filter paper for molecular analysis.
Blood smears were fixed with absolute methanol within 24 h of preparation and stained within 1 week with 10% buffered Giemsa solution (pH 7.2) for 60 min. An Olympus CX31 light microscope equipped with an Olympus Q-Color5 imaging system (Olympus, Tokyo, Japan) and Q-Capture Pro7 imaging software (QImaging, Surrey, Canada) was used to examine blood films and capture images. A minimum of 200 microscopic fields at high magnification (1000×) were examined to detect erythrocytic and non-erythrocytic forms of P. t. tropiduri. The two parasite forms were compared based on merozoite number, length, and width of meronts and gametocytes. Also, cell and nucleus sizes (length × width) of infected and non-infected host cells were compared for both parasite forms. Differences in morphology were tested using the Wilcoxon rank-sum test for each set of measures. Before all comparisons, homoscedasticity in their variances were tested using Levene's test. R's stats and car packages were used for these analyses, respectively (R Core Team, 2017, Gouy et al., 2010).
DNA from blood samples was extracted using a conventional phenol−chloroform method with isopropanol precipitation (Sambrook and Russell, 2001). Samples with the highest parasitaemia levels were submitted to mitochondrial genome amplification. The mtDNA was amplified using TaKaRa LA Taq Polymerase (TaKaRa Mirus Bio Inc.) following manufacturer directions using oligos forward AE170-5′-GAGGATTCTCTCCACACTTCAATTCGTACTTC-3′ and reverse AE171-5′-CAGGAAAATWATAGACCGAACCTTGGACTC-3′. A nested PCR was performed using the internal oligos forward AE176-5′-TTTCATCCTTAAATCTCGTAAC-3′ and reverse AE136-5′-GACCGAACCTTGGACTCTT-3′ for samples that did not initially amplify (Pacheco et al., 2018). Both outer and inner PCR were performed in 50 μL using 8 μL of total genomic DNA. PCR conditions were a partial denaturation at 94°C for 1 min and 30 cycles with 30 s at 94°C, and 7 min at 67°C, followed by a final extension of 10 min at 72°C. PCR products (50 μL for each sample) were ran in agarose gels (1%) and later, excised bands of approximately 6 kb were purified using a QIAquick® Gel extraction kit (Qiagen, GmbH, Hilden, Germany). Finally, these products were cloned using pGEM®-T Easy Vector systems (Promega, Madison, USA) following the manufacturer's directions. A minimum of three clones and both strands were sequenced per sample bi-directionally in an Applied Biosystems 3730 capillary sequencer. In this study, inconsistencies between the clones were not found so, four mtDNA genomes (one for each positive lizard sample) are reported and submitted to GenBank under accession numbers MW491387−MW491390.
Mitochondrial genome sequences from the following four lizards were obtained: a lizard (voucher: UFMG141) with parasites infecting only erythrocytes; a lizard (voucher: UFMG123) with parasites infecting only thrombocytes and lymphocyte-like cells; a lizard (voucher: UFMG29) infected predominantly with erythrocytic infection (17 erythrocytic parasites vs three thrombocytic parasites in 200 high magnification fields); and a lizard (voucher: UFMG37) infected with a predominant infection in thrombocytes and lymphocyte-like cells (six erythrocytic parasites vs 11 non-erythrocytic parasites in 200 high magnification fields). Sequences were compared with the GenBank database using BLAST (Altschul et al., 1990). The molecular data obtained from samples with both parasites was linked to the dominant morphospecies infection identified by microscopy using sample 141 (parasites infecting only erythrocytes) and sample 123 (parasites infecting only non-erythrocytic cells).
Two alignments were constructed using ClustalX v2.0.12 and Muscle as implemented in SeaView v4.3.5 (Gouy et al., 2010) with manual editing. The first alignment included 52 partial cytb gene sequences (479 bp) belonging to three genera (Haemoproteus, Haemocystidium, and Plasmodium) available from GenBank for some well-identified parasite species using morphology plus the four new cytb (from the mtDNA genomes) sequences obtained in this study. The 479 bp cytb fragment (Hellgren et al., 2004) was chosen as it is one of the most commonly used in studies involving lizard and bird Plasmodium species to compare data with a larger dataset. The second alignment (5420 bp excluding gaps) was constructed using 13 almost full mitochondrial genomes (mtDNA) sequences belonging to Haemoproteus and Plasmodium genera available from GenBank plus the four new mtDNA sequences reported in this study.
Then, both alignments were used to infer the parasites’ phylogenetic relationships applying Bayesian methods implemented in MrBayes v3.2.7a with the default priors (Ronquist et al., 2012). In both phylogenetic trees, Haemoproteus species were used as an outgroup. In the case of the mtDNA alignment, the phylogenetic relationships were inferred using six partitions (Pacheco et al., 2018). These partitions correspond to the three non-protein-coding regions between the ORFs (fragmented SSU rRNA and LSU rRNA) and the three protein-coding genes, keeping their order in the mtDNA genome (nonprotein coding, cox3, nonprotein coding, cox1, cytb, nonprotein coding). A general time-reversible model was used with gamma-distributed substitution rates and a proportion of invariant sites (GTR + Γ + I) for the first and each partition of the second alignments. This model was the one with the lowest Bayesian Information Criterion (BIC) scores for both alignments and each partition estimated by MEGA v7.0.14 (Kumar et al., 2016). The Bayesian support for the nodes was inferred by sampling every 1000 generations from two independent chains lasting 106 Markov chain Monte Carlo steps. Convergence between chains was assumed once the average standard deviation of the posterior probability was <0.01, and the value of the potential scale reduction factor was between 1.00 and 1.02 (Ronquist et al., 2012). Then, as a ‘burn-in’, 25% of the sample was discarded once convergence was reached.
The average evolutionary divergence over all sequence pairs was estimated using both alignments (mtDNA and partial cytb gene) and the Kimura 2-parameter model (Kimura, 1980). The analysis involved 10 nucleotide sequences, and all positions were included (1st + 2nd + 3rd). Positions containing gaps and missing data were eliminated. These evolutionary analyses were conducted using MEGA7 (Kumar et al., 2016), and the mtDNA genome final dataset had 5420 positions while the partial cytb gene final dataset had 452 positions.
Results
Microscopic detection and morphological analysis
Plasmodium parasites were detected in 56 lizards (64%); 42 were single infections of the erythrocytic form, six were single infections of non-erythrocytic parasites, and eight were mixed infections.
Meront size, merozoite number, and gametocyte width did not differ between parasites infecting erythrocytes and non-erythrocytic cells (Table 1). However, parasites infecting erythrocytes were longer than parasites infecting non-erythrocytic cells (Table 1). Erythrocytes infected by meronts were similar in size (L × W) to uninfected cells, whereas the nuclei of erythrocytes infected with gametocytes were smaller when similarly compared (P value = 0.01, w = 36). In contrast, meront-infected non-erythrocytic cells were markedly bigger (P value <0.0005, w = 63) and had a slightly bigger nucleus (P value = 0.09, w = 96) compared to uninfected cells. As expected, haemozoin pigment was not observed in parasites infecting non-erythrocytic cells.
Table 1.
Comparative table of characters of uninfected host cells, Fallisia modesta from Lainson et al. (1974), Plasmodium tropiduri tropiduri from this study and from Telford (2009), Plasmodium ouropretensis from this study and thrombocytic parasites from Scorza (1971), and the host cells these parasites infect
| Fallisia modesta from Brazil | P. t. tropiduri from Venezuela | P. tropiduri from Venezuela | P. t. tropiduri | P. ouropretensis | Wilcoxon test of P. t. tropiduri vs P. ouropretensis from this study | |
|---|---|---|---|---|---|---|
| Parasites | Lainson et al. (1974) | Telford (2009) | Scorza (1971) | This study | w value – P value (significance level of p < 0.05) | |
| Host cell | Lymphocytes and thrombocytes | Erythrocytes | Thrombocytes | Erythrocytes | Thrombocytes and lymphocyte-like cell | |
| Uninfected host cell | n = 10 | n = 10 | ||||
| Length | 14.7–19 (16.54 ± 1.12) | 6.13–12–6 (9.28 ± 2.04) | ||||
| Width | 8.7–11 (9.82 ± 0.78) | 4.3–9.34 (6.33 ± 2) | ||||
| Nucleus length | 6.1–8.2 (7.5 ± 0.64) | 4.47–9.78 (7.19 ± 1.65) | ||||
| Nucleus width | 4.1–5.1 (4.56–0.32) | 4.3–9.1 (5.78 ± 1.81) | ||||
| Meronts | n = 64 | n = 10 | n = 15 | |||
| Number of merozoites | 12–24 (16) | 4–24 (13.4 ± 4.7) | 10–26 | 10–25 (15.4 ± 4.45) | 3–36 (14.46 ± 11) | 91–0.3889 |
| Length | 5–9 (5.5) | 3–12 (7.2 ± 1.8) | 4 | 5.6–10.6 (7.5 ± 0.7) | 6–10.5 (7.94 ± 1.29) | 61–0.4611 |
| Width | 5–7.5 | 3–7 (5 ± 1.1) | 2 | 3.7–5.8 (4.9 ± 0.7) | 3.3–7.3 (5.17 ± 1.2) | 66–0.6433 |
| Length of infected host cell | 15–20 (17 ± 1.22) | 10–14.6 (12.2 ± 1.6) | ||||
| Width of infected host cell | 8–11.2 (10.3 ± 0.9) | 7.6–12.6 (9.20 ± 1.5) | ||||
| Length of infected host cell nucleus | 5.7–9 (7.66 ± 1) | 7.8–13 (9.04 ± 1.48) | ||||
| Width of infected host cell nucleus | 4.8–6.3 (5.23 ± 0.54) | 3.3–7.3 (5.86 ± 1.09) | ||||
| Relative size to uninfected host cell nucleus | 0.33–2.51 (1.40 ± 0.53) | 0.8–1–32 (1 ± 0.13) | 0.3–3 (1.2 ± 0.8) | |||
| Relative size to infected host cell nucleus | 0.51–1.17 (1.17 ± 0.56) | 0.16–1.2 (0.92 ± 0.1) | 0.43–1.46 (0.83 ± 0.36) | |||
| Effect on host cell | Hypertrophy | Hypertrophy | None | Hypertrophy | ||
| Gametocytes | n = 132 | n = 17 | n = 7 | |||
| Length | 6–8.5 (7.25) | 5–14 (7.9 ± 1.5) | 7–10 | 6.4–10.3 (7.7 ± 0.9) | 3.2–7.1 (6.29 ± 1.22) | 95–0.02 |
| Width | 5.5–6 (5.5) | 4–8 (5.6 ± 1) | 6–8 | 4.4–6.8 (5.07 ± 0.83) | 1.98–6.08 (4.35 ± 1.37) | 72–0.4551 |
| Length of infected host cell | 15–19 (17.3 ± 1.24) | 9.6–12.4 (11.27 ± 1.05) | ||||
| Width of infected host cell | 8.3–10.8 (9.4 ± 0.8) | 4.6–9.9 (7.58 ± 1.73) | ||||
| Length of infected host cell nucleus | 3.5–5.1 (7.13 ± 0.8) | 5.5–8.7 (7.40–1.12) | ||||
| Width of infected host cell nucleus | 3.8–5.1 (4.1 ± 0.45) | 4.5–7 (5.63 ± 0.85) | ||||
| Relative size to uninfected host cell nucleus | 0.73–2.54 (1.58 ± 0.37) | 0.8–1.7 (1.12 ± 0.27) | 0.33–1 (0.62 ± 0.23) | |||
| Relative size to infected host cell nucleus | 0.70–2.92 (1.51 ± 0.46) | 0.61–1.63 (0.98 ± 0.2) | 0.25–1.04 (0.67 ± 0.27) | |||
| Effect on host cell | Indentednucleus | Hypertrophy | Nucleuscompression | None | ||
| Micro | n = 7 | |||||
| Pigment number | 12–16 (14.1 ± 1.67) | |||||
| n = 40 | ||||||
| Pigment diameter | 0.43–0.82 (0.67 ± 0.09) | |||||
| Macro | n = 10 | |||||
| Pigment number | 13–22 (16.5 ± 3.6) | |||||
| n = 42 | ||||||
| Pigment diameter | 0.52–1.19 (0.71 ± 0.13) | |||||
Measurements are in μm and are presented as ranges followed by mean values and standard deviations. Relative size to host cells were calculated by multiplying L × W and dividing it by host cell L × W. Statistical test to compare measures of P. t. tropiduri and P. ouropretensis are presented.
Molecular and phylogenetic analyses
Almost complete mitochondrial genomes (5896−5498 bp) were obtained from Plasmodium parasites infecting four lizards. Table 2 (mtDNA) and Table 3 (partial cytb gene) show the average pairwise divergence among lizard Plasmodium species over all sequence pairs that have been well-identified using morphology. Parasites from single erythrocytic infections and mixed infections dominated by erythrocyte-infecting parasites had 0.1% genetic divergence between them (Table 2). Likewise, parasites from single non-erythrocytic infections differed 0.1% from the mixed-infection sample dominated by parasites that infected thrombocytes and lymphocyte-like cells (Table 2). Using mtDNA genomes, pairwise comparisons between parasites infecting erythrocytes and non-erythrocytic cells revealed an average genetic distance between 2.3 and 2.5% (Table 2). The average genetic distance using only the cytb gene fragment was 2.7%.
Table 2.
Pairwise genetic distance using almost complete mtDNA genome among ten Reptilian Plasmodium species
| Pairwise genetic distance (Standard Error) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reptilian Plasmodium Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 1 | P. tropiduri tropiduri (erythrocytes – 29) | 0.000 | 0.002 | 0.002 | 0.003 | 0.004 | 0.002 | 0.002 | 0.003 | 0.003 | |
| 2 | P. tropiduri tropiduri (erythrocytes – 141) | 0.001 | 0.002 | 0.002 | 0.003 | 0.004 | 0.002 | 0.002 | 0.003 | 0.003 | |
| 3 | P. ouropretensis (thrombocytes – 37) | 0.023 | 0.024 | 0.000 | 0.003 | 0.004 | 0.002 | 0.002 | 0.003 | 0.003 | |
| 4 | P. ouropretensis (thrombocytes – 123) | 0.024 | 0.025 | 0.001 | 0.004 | 0.004 | 0.002 | 0.002 | 0.003 | 0.003 | |
| 5 | P. mexicanum (NC_009960) | 0.076 | 0.077 | 0.075 | 0.075 | 0.002 | 0.004 | 0.003 | 0.004 | 0.003 | |
| 6 | P. chiricahuae (KY653779) | 0.079 | 0.080 | 0.078 | 0.078 | 0.017 | 0.004 | 0.003 | 0.004 | 0.004 | |
| 7 | P. carmelinoi (KY653755) | 0.029 | 0.029 | 0.032 | 0.032 | 0.078 | 0.081 | 0.002 | 0.004 | 0.003 | |
| 8 | P. kentropyxi (KY653753) | 0.028 | 0.028 | 0.032 | 0.032 | 0.079 | 0.082 | 0.015 | 0.003 | 0.003 | |
| 9 | P. (Lacertamoeba) sp. (KY653796) | 0.057 | 0.057 | 0.059 | 0.059 | 0.072 | 0.075 | 0.060 | 0.062 | 0.002 | |
| 10 | P. floridense (NC_009961) | 0.057 | 0.057 | 0.057 | 0.057 | 0.073 | 0.075 | 0.060 | 0.060 | 0.016 | |
Distances (and Standard Error) among parasites infecting Amazon Lava Lizards (Tropidurus torquatus) from our study are shown in bold. Genetic divergence was estimated in MEGA 7.0.18 using mitochondrial genome sequence alignment (5420 bp without gaps). Standard Error estimate(s) are shown above the diagonal. Sample GenBank accession numbers are: Sample ID 29: MW491387, sample ID 141: MW491388, sample ID 37: MW491389, and sample ID 123: MW491390.
Table 3.
Average divergence over all cytb gene sequence pairs among 19 Reptilian Plasmodium species
| Reptilian Plasmodium Species | Pairwise genetic distance (s.e.) |
|---|---|
| P. carmelinoi (KY653755) vs P. kentropyxi (MN540144) | 0.025 (0.007) |
| P. carmelinoi (KY653755) vs P. kentropyxi (KY653753) | 0.026 (0.006) |
| P. fairchildi (KR477583) vs P. azurophilum (AY099055) | 0.020 (0.007) |
| P. megalotrypa (EU834705) vs P. koreafense (EU834704) | 0.052 (0.010) |
| P. megalotrypa (EU834705) vs P. minuoviride (EU834703) | 0.035 (0.008) |
| P. koreafense (EU834704) vs P. minuoviride (EU834703) | 0.027 (0.008) |
| P. chiricahuae (KY653779) vs P. mexicanum (NC_009960) | 0.017 (0.006) |
| P. intabazwe (KX121607) vs P. zonuriae (KX121609) | 0.035 (0.008) |
| P. floridense (NC_009961) vs P. hispaniolae (KR477594) | 0.048 (0.010) |
| P. agamae (AY099048) vs P. giganteum (AY099053 | 0.032 (0.008) |
| P. leucocytica (AY099058) vs P. azurophilum (AY099055) | 0.026 (0.007) |
| P. leucocytica (AY099058) vs P. ouropretensis (MW491390) | 0.022 (0.007) |
| P. leucocytica (AY099058) vs P. t. tropiduri (MW491388) | 0.028 (0.007) |
| P. ouropretensis (MW491390) vs P. t. tropiduri (MW491388) | 0.027 (0.007) |
Pairwise genetic distance (and standard error) among parasites infecting Amazon Lava Lizards (Tropidurus torquatus) in Brazil and P. ouropretensis (AY099058) are shown in bold. The genetic divergences were estimated in MEGA 7.0.18 using cytb fragment sequences alignment (452 bp without gaps). Sample GenBank accession numbers are in parentheses.
Phylogenetic analyses using cytb gene (Fig. 1A) and mtDNA genomes (Fig. 1B) showed that erythrocytic parasites shared their most recent common ancestor with erythrocyte-infecting Plasmodium species detected in Colombia (P. carmelinoi and P. kentropyxi, Matta et al., 2018). The erythrocyte-infecting parasite did not form a clade with the parasites infecting non-erythrocytes of lizards from same population. The two cytb sequences of erythrocytic parasites had 100% similarity with Plasmodium sp. infecting Tropidurus hispidus (TEC4576, TEC4983) and Hemidactylus mabouia (TEC4774, Fig. 1A) in Brazil (Harris et al., 2019), and had 99.6% similarity with parasites infecting Strobilurus torquatus in Brazil (STRTOR01, Fig. 1A; Ferreira et al., 2020). These parasites formed a monophyletic group with parasites infecting erythrocytes in lizards from Colombia (Matta et al., 2018) and Brazil (Harris et al., 2019, Ferreira et al., 2020). On the other hand, non-erythrocytic parasites formed a well-supported clade with Plasmodium leucocytica, a parasite that infects white blood cells of lizards in the Caribbean islands (Fig. 1, Table 3; Telford, 2009).
Fig. 1.
Bayesian phylogenetic hypotheses of Plasmodium species infecting Amazon Lava Lizards (Tropidurus torquatus) in Brazil based on (A) partial sequences of the cytb gene (452 bp without gaps) and (B) partial mtDNA genome sequences (5420 bp without gaps). The values above branches are posterior probabilities (MrBayes v3.2.6). Parasites infecting erythrocytes (sample ID: 29 and 141) are shown in red (+), whereas those infecting thrombocytes and lymphocyte-like cells (sample ID: 37 and 123) are in blue (*). Haemoproteus genus (outgroup) is indicated in light grey. Parasites described as morphospecies are in bold. Genbank accession numbers for other parasite sequences used in the analyses are provided in parentheses.
Thus, the evidence presented here indicates two distinct lineages infecting T. torquatus, one related to the erythrocytic Plasmodium species distributed in South America (Fig. 2) and another related to a non-erythrocytic species from the Caribbean islands (Fig. 3). Therefore, we propose that the non-erythrocytic parasites detected in T. torquatus should be elevated to species level.
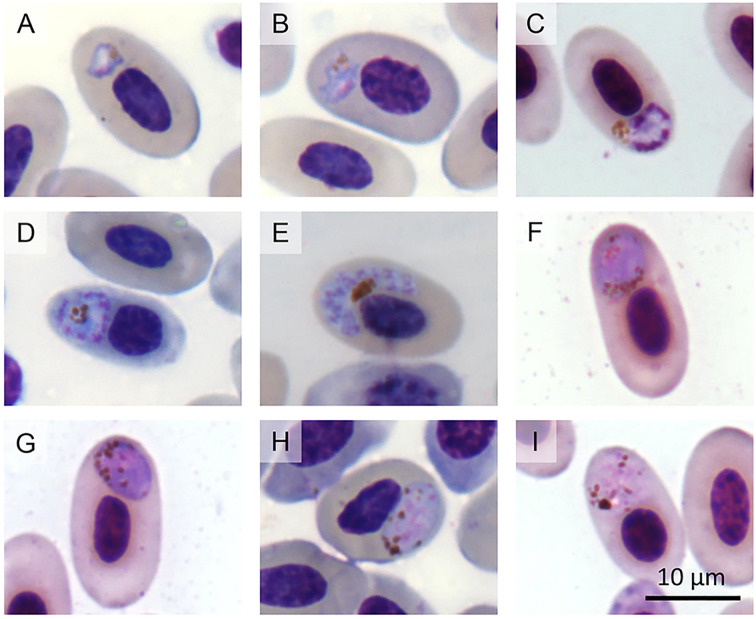
Fig. 2.
Plasmodium tropiduri tropiduri in erythrocytes from Tropidurus torquatus from Ouro Preto, Brazil. (A, B) trophozoites, (C−E) meronts, (F, G) macrogametocytes, H and I: microgametocytes.
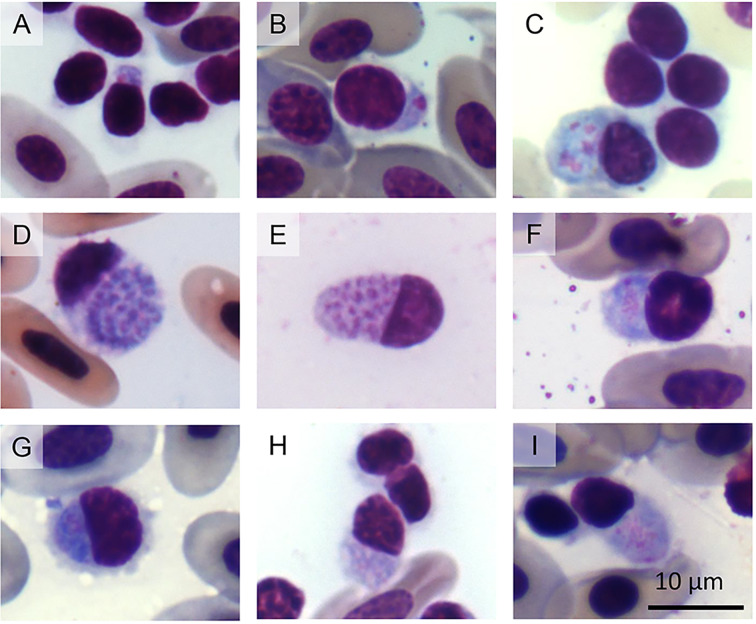
Fig. 3.
Plasmodium ouropretensis in thrombocyte and lymphocyte-like cells from Tropidurus torquatus from Ouro Preto, Brazil. (A, B) trophozoites, (C) oval meront, (D) round meront, (E) slightly elongated meront, (F, G) macrogametocytes, (H, I) microgametocytes.
Description of the parasite infecting non-erythrocytic blood cells
Plasmodium ouropretensis n. sp. (Fig. 3)
Type Host: Tropidurus torquatus (Tropiduridae).
Type Locality: Parque das andorinhas, Ouro Preto, Minas Gerais, Brazil (43°29′13.90″W, 20°33′1.22″S).
Type specimens: Hapantotype (UFMG37, UFMG123), intensities are 11 and 4 infected cells in 200 fields at 1.000×, respectively. Collected by O. D. Hernandes-Cordoba (Ouro Preto municipality, Brazil) were deposited in the Institute of Biological Sciences (Universidade Federal de Minas Gerais, Belo Horizonte, Brazil).
DNA Sequences: MW491389 and MW491390.
Diagnosis: Plasmodium ouropretensis infects thrombocytes and lymphocyte-like cells. Trophozoites are usually located at the polar region of host-cells and have dark-stained nuclei with plentiful cytoplasm (Fig. 3A, B). Mature trophozoites may display cytoplasmatic outgrowths (Fig. 3B). Meronts have variable forms, including oval (Fig. 3C), round (Fig. 3D), and slightly elongated (Fig. 3E) shapes. Meronts produce an average of 14 merozoites, ranging from 3 to 36. Mature meronts cause hypertrophy to the host cell cytoplasm. Gametocytes are round to oval. The cytoplasm and the nuclei of macrogametocytes are slightly darker (Fig. 3F, G) when compared to microgametocytes (Fig. 3H, I). The nuclei of macrogametocytes are smaller and better defined than the nuclei of microgametocytes. Most parasite stages occupy almost all cytoplasmic space of the host cell and are markedly appressed to nuclei. Haemozoin pigment is absent in all parasite stages.
Site of infection: Thrombocytes and lymphocyte-like cells.
Prevalence: P. ouropretensis parasitized 14 of 87 lizards (16.1%) studied in Ouro Preto, Brazil.
Effects on host cells: P. ouropretensis meronts cause a slight host cell nucleus hypertrophy and a marked host cell distortion and hypertrophy.
Other Hosts: Tropidurus hispidus (Scorza, 1970; Telford, 2009).
Other Localities: Guanare, Venezuela (Scorza, 1971). São Paulo and Pará, Brazil (Lainson and Shaw, 1969; Rocha e Silva and Rodrigues, 1974).
Etymology: P. ouropretensis name is derived from the name of the municipality (Ouro Preto) where infected lizards were sampled.
Remarks: This species was placed within genus Plasmodium following the strong support of our genetic data and phylogenetic hypotheses. Due to morphological similarity and close host and geography association, P. ouropretensis parasites were reported as thrombocytic stages of P. tropiduri. Scorza (1971) studied and described the ultrastructure of these forms. Rocha e Silva and Rodrigues (1974) found similar parasites in T. torquatus, stating that these parasites were similar to those studied by Scorza (1971), except that haemozoin pigment was visualized in meronts. Morphological characters of P. ouropretensis overlap with Fallisia modesta (Lainson et al., 1974, Telford, 2009) described in T. hispidus. However, marked differences between F. modesta and P. ouropretensis were also observed. F. modesta rarely infects thrombocytes and its meronts and gametocytes produce severe host cell nucleus deformation. In contrast, P. ouropretensis causes a slight host cell nucleus hypertrophy and infects mainly thrombocytes.
Discussion
The analyses presented here indicate that erythrocytic and non-erythrocytic forms of P. t. tropiduri, as described by Telford (1988, 2009), are two different lineages. Thus, the latter group of parasites is proposed as a distinct species, Plasmodium ouropretensis n. sp.
Plasmodium t. tropiduri and P. ouropretensis are similar in their morphology. One distinction is the reduced gametocyte length observed for P. ouropretensis compared to Plasmodium t. tropiduri. Such a difference may relate to the host cell's reduced size since erythrocytes are longer than thrombocytes and lymphocyte-like cells.
Scorza (1971) found similar ultrastructure between the two forms of P. t. tropiduri. Scorza found thrombocytic forms eight months after experimentally infecting lizards with erythrocytic forms of P. t. tropiduri. His findings suggested that non-erythrocytic forms were always associated with erythrocytic infections. Consequently, Scorza (1971) hypothesized that P. t. tropiduri exhibits an intra-thrombocytic cycle, but sub-patent infections can remain undetected (Perkins et al., 1998). The apparent host cell switching observed by Scorza (1971) could be the result of a previously submicroscopic parasitaemia of thrombocyte-infecting parasites.
Our molecular data indicated that erythrocytic and non-erythrocytic forms belong to two distinct lineages. This finding supports the idea that phenotypic similarity hampers the distinction of lizard Plasmodium parasites (Perkins, 2000; Perkins and Schall, 2002). Plasmodium ouropretensis and P. t. tropiduri constitute different species based on three main observations derived from our phylogenetic analyses. First, P. t. tropiduri, infecting lizards in southeastern Brazil, is in a different subclade in relation to P. ouropretensis detected in the same lizard population. Second, P. t. tropiduri is part of a monophyletic group with erythrocyte-infecting Plasmodium species detected in Brazil and in Colombia. Finally, P. ouropretensis shares a recent common ancestor with P. leucocytica from the Caribbean islands. Both are part of a well-supported clade of non-erythrocytic parasites within the family Plasmodiidae. Thus, the parasites described here are suggested as another species of non-erythrocytic Plasmodium.
So far, molecular information from two species of lizard haemosporidians that infect white blood cells reinforces the idea that the family Garniidae may not be phylogenetically valid because they fall within the clade of lizard and avian Plasmodiidae. Telford (1973) suggested Garniidae as synonymy of Plasmodiidae, and called for a redefinition of lizard Plasmodium to include non-erythrocytic parasites, and this is supported by our data together with molecular information from Perkins (2000). However, more molecular data are necessary to evaluate evolutionary relationships among Plasmodium, Garnia, and Fallisia species, especially those from the Amazonia region, the type locality of the family Garniidae.
The limited data available indicates that P. ouropretensis is always found in sympatry with populations of P. t. tropiduri, particularly in the Venezuelan state of Guarico (Scorza, 1970), as well as in two Brazilian states: Sao Paulo (Rocha e Silva and Rodrigues, 1974), and Minas Gerais states (this study). Both parasites infect T. hispidus (Scorza, 1971) and T. torquatus (Rocha e Silva and Rodrigues, 1974). Positive interactions or facilitation effect (Dallas et al., 2019) may explain the co-occurrence pattern of these parasites exploiting different host resources. This has been observed in P. agame and P. giganteum (Schall and Bromwich, 1994), where the first parasite causes host immune alteration, allowing the successful infection of the second. However, Plasmodium t. tropiduri was also reported infecting T. hispidus, T. itambere, and Hemidactylus mabouya (Harris et al., 2019), and occurred in two other states in Venezuela (Telford, 1980) and Brazil (Carini, 1941; Pessôa and Lopes, 1963; Harris et al., 2019). Thus, P. ouropretensis may be less widespread than P. t. tropiduri.
Similar to previous studies, non-erythrocytic parasites were less prevalent than the erythrocytic forms (Rocha e Silva and Rodrigues, 1974; Telford, 1980). The host cells required by P. ouropretensis are less abundant than erythrocytes. Therefore, if the abundance of host cells in circulating blood generated a barrier against successful transmission of these parasites (Schall, 2002), it may explain the low abundance of P. ouropretensis compared with P. t. tropiduri. Parasites infecting non-erythrocytic host cell types may exhibit differential infection traits and different pathologies in their hosts compared with the erythrocytic species. For example, P. t. tropiduri infection was associated with a higher proportion of monocytes, along with a decreased proportion of thrombocytes and an increased number of young erythrocytes in free ranging T. torquatus in Ouro Preto (Hernandes-Cordoba and Braga, 2019). Instead, P. ouropretensis infections were found to be associated uniquely with an increase in the monocyte proportion (Hernandes-Cordoba, unpublished data). More sampling and molecular data are needed to assess these species’ distribution and ecology across South America.
In conclusion, our study reports for the first time the mtDNA genomes of P. t. tropiduri and P. ouropretensis. The molecular phylogenies, including the parasites infecting different cell types, suggest that these two forms have different evolutionary histories based on mitochondrial genes. However, the two forms were found to share some morphological features and inhabit the same host, reinforcing the notion that morphological measurements may hinder species discovery and delimitation in Plasmodium parasites infecting lizards.
The first molecular characterization of lizard Plasmodium species infecting mainly thrombocytes was provided herein. This species formed a monophyletic clade with a Plasmodium species that infects the white blood cells of lizards from the Caribbean islands. These two parasite species were detected in distinct host species sampled more than 4000 km apart.
Interesting questions that emerge from studying non-erythrocytic Plasmodium parasites include: When did this life trait originate? Did this life trait appear more than once in the evolutionary history of lizard parasites? Are there more non-erythrocytic species encrypted by low sampling or morphological similarity with erythrocytic parasites? Answering such questions requires microscopy studies to discover the parasites infecting different cells and molecular methods to discover and delimit species.
Acknowledgements
We are grateful to Raquel Andrade and Daniela Dutra for their support during laboratory work at the Laboratorio de Parasitología (Universidade Federal de Minas Grais). We thank Scott Bingham from the DNA laboratory at the School of Life Sciences (Arizona State University) for their technical support.
Author contributions
OHC conceived, designed the study, and conducted field and laboratory work. EMB oriented and supervised the research. FCF analysed data and prepared figures. MAP and AE performed laboratory work and phylogenetic analysis. All authors wrote the article.
Financial support
OHC was supported by PAEC/OEA Fellowship Program. EMB was supported by Conselho Nacional de Desevolvimento da Pesquisa (CNPq), Brazil. FCF was supported by the National Postdoctoral Program/CAPES (PNPD/CAPES) and by National Science Foundation grant 1717498 as part of the joint NSF-NIH-USDA Ecology and Evolution of Infectious Diseases program.
Ethical standards
All applicable federal, state and institutional guidelines for the care and use of animals were followed in this study. This study was developed under the license from Brazilian authority: SISbio No. 51066-3.
Data
All sequences used here are available at GenBank.
Conflict of interest
The authors declare that they have no competing interests.
References
- Altschul SF, Gish W, Miller W, Myers EW and Lipman DJ (1990) Basic local alignment search tool. Journal of Molecular Biology 215, 403–410. [DOI] [PubMed] [Google Scholar]
- Carini A (1941) Sobre um Plasmodium endoglobular de um largarto. Arquivos do Instituto Biológico 25, 46–47. [Google Scholar]
- Dallas TA, Laine AL and Ovaskainen O (2019) Detecting parasite associations within multi-species host and parasite communities. Proceedings of the Royal Society B: Biological Sciences 286, 2019–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk B, Mahler DL and Perkins SL (2011) Tree-based delimitation of morphologically ambiguous taxa: a study of the lizard malaria parasites on the Caribbean island of Hispaniola. International Journal for Parasitology 41, 967–980. [DOI] [PubMed] [Google Scholar]
- Ferreira FC, Alves LGM, Jager GB, Franzini LD, Mesquita DO, Díaz-Delgado J, Catão-Dias JL and Martins-Braga ÉM (2020) Molecular and pathological investigations of Plasmodium parasites infecting striped forest whiptail lizards (Kentropyx calcarata) in Brazil. Parasitology Research 119, 2631–2640. [DOI] [PubMed] [Google Scholar]
- Gouy M, Guindon S and Gascuel O (2010) SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Molecular Biology and Evolution 27(2), 221–224. 10.1093/molbev/msp259 [DOI] [PubMed] [Google Scholar]
- Harris DJ, Santos JL, Borges-Nojosa DM and de Castro DP (2019) Molecular screening of Plasmodium (Haemosporidia: Plasmodiidae) Parasites from Reptiles in Brazil. Journal of Parasitology 105, 913. [PubMed] [Google Scholar]
- Hellgren O, Waldenström J and Bensch S (2004) A new pcr assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood a new PCR assay for simultaneous studies of Leucocytozoon. Journal of Parasitology 90, 797–802. [DOI] [PubMed] [Google Scholar]
- Hernandes-Cordoba O and Braga E (2019) Plasmodium tropiduri tropiduri in co-occurrence with Chigger Mites and Microfilaria in the Ground Lizard Tropidurus torquatus. Herpetological Conservation and Biology 14, 402–410. [Google Scholar]
- Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution 16, 111–120. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G and Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33, 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainson R and Shaw JJ (1969) New host records for Plasmodium diploglossi, P. tropiduri Aragao and Neiva, 1909, and P. cnemidophori Garini, 1941. Parasitology 59, 163–170. [DOI] [PubMed] [Google Scholar]
- Lainson R, Landau I and Shaw JJ (1971) On a new family of non-pigmented parasites in the blood of reptiles: Garniidae fam. nov. (Coccidiida: Haemosporidiidea). Some species of the new genus Garnia. International Journal for Parasitology 1, 241–250. [Google Scholar]
- Lainson R, Landau I and Shaw J (1974) Further parasites of the family Garniidae (Coccidiida: Haemosporidiidea) in Brazilian lizards. Fallisia effusa gen.nov., sp.nov. and Fallisia modesta gen.nov., sp.nov. Parasitology 68, 117–125. [PubMed] [Google Scholar]
- Matta NE, González LP, Pacheco MA, Escalante AA, Moreno AM, González AD and Calderón-Espinosa ML (2018) Plasmodium parasites in reptiles from the Colombia Orinoco-Amazon basin: a re-description of Plasmodium kentropyxi Lainson R, Landau I, Paperna I, 2001 and Plasmodium carmelinoi Lainson R, Franco CM, da Matta R, 2010. Parasitology Research 117, 1357–1370. [DOI] [PubMed] [Google Scholar]
- Pacheco, MA and Escalante AA (2020) Cophylogenetic patterns and speciation in Avian Haemosporidians. In Santiago-Alarcon D and Marzal A (eds), Avian Malaria and Related Parasites in the Tropics. Gewerbestrasse, SW: Springer Nature, pp. 401–427. [Google Scholar]
- Pacheco MA, Cepeda AS and Bernotiene R (2018) Primers targeting mitochondrial genes of avian haemosporidians: PCR detection and differential DNA amplification of parasites belonging to different genera. International Journal for Parasitology 45, 657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinauskas V, Žiegyte R, Ilgunas M, Iezhova TA, Bernotiene R, Bolshakov C and Valkiunas G (2015) Description of the first cryptic avian malaria parasite, Plasmodium homocircumflexum n. sp., with experimental data on its virulence and development in avian hosts and mosquitoes. International Journal for Parasitology 45, 51–62. [DOI] [PubMed] [Google Scholar]
- Perkins SL (2000) Species concepts and malaria parasites: detecting a cryptic species of Plasmodium. Proceedings of the Royal Society B: Biological Sciences 267, 2345–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins SL and Schall JJ (2002) A molecular phylogeny of malarial parasites recovered from cytochrome b gene sequences. Journal of Parasitology 88, 972–978. [DOI] [PubMed] [Google Scholar]
- Perkins SL, Osgood SM and Schall JJ (1998) Use of PCR for detection of subpatent infections of lizard malaria: implications for epizootiology. Molecular Ecology 7, 1587–1590. [Google Scholar]
- Perkins SL, Martinsen ES and Falk BG (2011) Do molecules matter more than morphology? Promises and pitfalls in parasites. Parasitology 138, 1664–1674. [DOI] [PubMed] [Google Scholar]
- Pessôa SB and Lopes J (1963) Nota sôbre alguns hematozoarios de lagartixas de Jacobina (Bahia, Brasil). Revista do instituto de medicina tropical de São Paulo 5, 133–139. [PubMed] [Google Scholar]
- Picelli AM, Ramires AC, Masseli GS, Pessoa FAC, Viana LA and Kaefer IL (2020) Under the light: high prevalence of haemoparasites in lizards (reptilia: Squamata) from central amazonia revealed by microscopy. Anais da Academia Brasileira de Ciencias 92, 1–19. [DOI] [PubMed] [Google Scholar]
- Poulin R (2011) The many roads to parasitism: a tale of convergence. Advances in Parasitology 74, 1–40. [DOI] [PubMed] [Google Scholar]
- R Core Team (2017) R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. Available at https://www.R-project.org/. [Google Scholar]
- Rocha e Silva E and Rodrigues D (1974) Encontro do Plasmodium (s) tropiduri no estado de São Paulo, Brasil. Revista de Saude Publica de Sao Paulo 8, 163–170. [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres D, Darling A, Höhna S, Larget B, Liu L, Suchard M and Huelsenbeck J (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 6, 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J and Russell DW (2001) Molecular Cloning. New York, USA: CSHL Press. [Google Scholar]
- Schall J (2002) Parasite virulence. In Lewis EE, Campell JF and Sukhdeo MVK (eds), The Behavioural Ecology of Parasites. Boston, USA: CAB International, pp. 283–313. [Google Scholar]
- Schall JJ and Bromwich CR (1994) Interspecific interactions tested: two species of malarial parasite in a West African lizard. Oecologia 97, 326–332. [DOI] [PubMed] [Google Scholar]
- Scorza J (1970) Unpigmented gametocytes of Plasmodium (Sauramoeba) tropiduri in thrombocytes of Tropidurus torquatus (Iguanidae). Journal of Parasitology 56, 470–471. [Google Scholar]
- Scorza J (1971) Asexual and sexual stages of a malaria parasite in the thrombocytes of Tropidurus torquatus (Iguanidae) infected with Plasmodium tropiduri. Eukaryotic Microbiology 18, 403–410. [DOI] [PubMed] [Google Scholar]
- Telford R (1973) Saurian malarial parasites from Guyana: their effect upon the validity of the family Garniidae and the genus Garnia, with descriptions of two new species. International Journal for Parasitology 3, 829–842. [Google Scholar]
- Telford SR (1979) A taxonomic reconsideration of some Plasmodium species from Iguanid lizards. Annales de Parasitologie Humaine et Comparée 54, 129–144. [DOI] [PubMed] [Google Scholar]
- Telford SR (1980) The saurian malarias of Venezuela: species from iguanid and teiid hosts. International Journal for Parasitology 10, 365–374. [DOI] [PubMed] [Google Scholar]
- Telford SR (1988) A contribution to the systematics of the reptilian malaria parasites, family Plasmodiidae (Apicomplexa: Haemospororina). Bulletin of the Florida State Museum. Biological Science 34, 65–97. [Google Scholar]
- Telford SR (2009) Hemoparasites of the Reptilia Color Atlas and Text. Boca Raton, USA: CRC Press; Taylor & Francis Group. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All sequences used here are available at GenBank.