Abstract
The increasing distribution and prevalence of fasciolosis in both human and livestock are concerning. Here, we examine the various types of factors influencing fasciolosis transmission and burden and the interrelations that may exist between them. We present the arsenal of molecules, ‘adjusting’ capabilities and parasitic strategies of Fasciola to infect. Such features define the high adaptability of Fasciola species for parasitism that facilitate their transmission. We discuss current environmental perturbations (increase of livestock and land use, climate change, introduction of alien species and biodiversity loss) in relation to fasciolosis dynamics. As Fasciola infection is directly and ultimately linked to livestock management, living conditions and cultural habits, which are also changing under the pressure of globalization and climate change, the social component of transmission is also discussed. Lastly, we examine the implication of increasing scientific and political awareness in highlighting the current circulation of fasciolosis and boosting epidemiological surveys and novel diagnostic techniques. From a joint perspective, it becomes clear that factors weight differently at each place and moment, depending on the biological, environmental, social and political interrelating contexts. Therefore, the analyses of a disease as complex as fasciolosis should be as integrative as possible to dissect the realities featuring each epidemiological scenario. Such a comprehensive appraisal is presented in this review and constitutes its main asset to serve as a fresh integrative understanding of fasciolosis.
Key words: Climate change, environmental degradation, Fasciola, human activities, livestock production, neglected tropical diseases, parasite biology, poverty, water and food security
Introduction
Among parasitic zoonoses, fasciolosis is particularly interesting due to its worldwide distribution, altitudinal transmission and the increasing impact on public and veterinary health that has come with its (re-)emergence. This parasitosis is caused by the liver flukes Fasciola hepatica and Fasciola gigantica (Trematoda: Digenea: Fasciolidae). The former has a major role in the global burden of the disease with the largest distribution (from temperate to tropical regions of all continents except Antarctica) and host range. More restricted, F. gigantica is largely distributed within the tropical and subtropical regions of Asia and Africa (Mas-Coma et al., 2009a). Incomplete reproductive isolating mechanisms have favoured interspecific cross-hybridization in regions of Asia and Africa where both species overlap, imposing an additional challenge for morphological and genetic identification of their intermediate forms, and for the epidemiological management of the disease (Itagaki et al., 2011; Shoriki et al., 2016). A third species, Fasciola nyanzae, infects only hippos generating scarce interest (Dinnik and Dinnik, 1961), whereas genetic analysis currently locates former Fasciola jacksoni (significant parasite of Asian elephants), into the genus Fascioloides (Heneberg, 2013; Rajapakse et al., 2020). Therefore, in the present paper, only F. hepatica and F. gigantica will be discussed.
Both parasites present a similar two-host life cycle in which freshwater snails of the family Lymnaeidae and a large variety of mammals (i.e. domestic ruminants, wildlife, and humans) serve, respectively, as intermediate and definitive hosts. In the snail host, Fasciola spp. undergoes development and asexual reproduction; from the infective miracidium to sporocyst and then, to several generations of redia and to thousands of cercariae. The mammalian host gets infected after consuming edible plants or water contaminated with encysted cercariae (=metacercariae) and, once in the liver and biliary ducts of the host, the parasites reach adulthood and sexual maturity to produce thousands of eggs. The eggs embryonate and hatch in the environment after being expelled within the feces, and the resulting miracidia actively infect the snail host, continuing the life cycle (see Andrews, 1999). The migration of the parasite to the liver and through the liver parenchyma in the mammal host coincides with the acute phase, whereas a chronic or obstructive phase may occur once the adult flukes are established within the bile ducts (see Behm and Sangster, 1999 for details on the synthomatology and disease progression).
The progression of the disease and the number and intensity of the symptoms in the definitive host depend on the quantity of ingested cysts, the stage of the infection and the species involved, as well as the immunity and nutritional status of the host (Mas-Coma et al., 2009a). Morbidity is mostly unspecific and extremely variable; some people may have only mild symptomatology early in the infection or none at all, while others only feel sick later in the infection, during the chronic phase, when the damage to the liver and the gallbladder is significant (Kaya et al., 2011; Ramachandran et al., 2012). This is particularly important as it can have a direct epidemiological impact on both diagnosis (incidence and prevalence) and transmission (parasite circulation), as discussed further on. The death rate could be significant in both sheep and cattle, as they usually harbour higher numbers of parasites, but it is generally low among humans (Torgerson and Claxton, 1999). Although significantly fewer, ectopic infections can occur and, in humans, adult flukes have been reported in intestines and peritoneal cavity, skin and subcutaneous tissues, eyes, brain, pancreas, neck and lymph node, lung and dorsal spine (see Taghipour et al., 2019 for review).
Long considered mostly of veterinary concern, the global increase in the prevalence and distribution of fasciolosis in livestock is worrying (Pritchard et al., 2005; Rojas et al., 2010; Caminade et al., 2015; Kelley et al., 2020). Similarly, at the public health level, the pattern of fasciolosis shifted from an occasional zoonotic disease, to today's estimations from 2.4 to 50 million of infected people from all inhabited continents (World Health Organization, 2013; Nyindo and Lukambagire, 2015). In this sense, some recent papers have reviewed different aspects of fasciolosis epidemiology and its transmission (e.g. Beesley et al., 2018; Mas-Coma et al., 2019), whilst independent studies have been made relating fasciolosis impact to factors such as climate change or direct human activities (Mas-Coma et al., 2009b; Caminade et al., 2015; Sabourin et al., 2018). Nonetheless, given the complexity of fasciolosis, it is essential to examine the (re)-emergence of fasciolosis through a more integrative scrutiny of the concomitant factors that affect transmission and may have contributed to such significant increase.
In this review, we attempt an integrative and multicentred analysis of the disease considering both species of Fasciola and all elements involved in transmission (natural or anthropic) at a global scale. It is aimed at providing a comprehensive overview of fasciolosis (re)emergence from all accountable aspects underlying the transmission. To accomplish this goal, we bring forward the various types of factors impacting the burden of fasciolosis and the interrelations that may exist between them to dissect the realities and complexities behind this phenomenon. From biological and ecological to social and political factors, past and current trends of fasciolosis dynamics are analysed at the light of adaptation for parasitism and ecology of transmission, and further contextualized in past and current trends of environmental degradation and of scientific awareness.
Fasciolosis (re-)emergence: an overview
Overall, animal fasciolosis is highly prevalent in livestock worldwide, particularly in bovines, and accounts for most transmissions causing 29% of zoonoses (Nyindo and Lukambagire, 2015). An estimate of over 600 million domestic ruminants are infected with Fasciola parasites (Toet et al., 2014), causing worldwide losses in animal production of US$3.2 billion per year (Mehmood et al., 2017). Such economic losses are mainly due to direct death (Fiss et al., 2013), liver condemnation (Nyirenda et al., 2019), reduced milk, wool and meat production (Charlier et al., 2014; Arenal et al., 2018), and as a result of reduced fertility and high costs of drug therapy (Schweizer et al., 2005; Mehmood et al., 2017). Traditionally, it has been a serious veterinary health concern in areas with diverse socio-economic scenarios and epidemiological contexts such as Europe (Beesley et al., 2018), East Africa (Gryseels, 1988), South Asia (Gupta and Singh, 2002) and the Bolivian Altiplano (Ueno et al., 1975). Moreover, increasing trends in the prevalence and/or spreading of animal fasciolosis are presumed at a global scale; in the Americas and the Caribbean (Rojas et al., 2010; Molento et al., 2018; Villa-Mancera and Reynoso-Palomar, 2019), in Europe (Pritchard et al., 2005; Caminade et al., 2015), in Africa (Jajaa et al., 2017), in Asia (Nguyen et al., 2017; Meshgi et al., 2019) and in Australia and Oceania (Haydock et al., 2016; Kelley et al., 2020).
A retrospective analysis of human fasciolosis carried out in the early 1990s over a 20-year period of published literature computed a prevalence of merely 2594 clinical cases from 42 countries (Chen and Mott, 1990). Such numbers contrast with the highest current estimates of around 50 million people infected and 180 million living at risk (World Health Organization, 2013; Nyindo and Lukambagire, 2015). In addition, a median number of total disability-adjusted life years due to human fasciolosis is nowadays calculated to be 90 041 (95% uncertainty interval; range 58 050–209 097; Torgerson et al., 2015). Importantly, even though the highlands of South America, the Nile valley, the Caspian Sea basin and East and Southeast Asia are recognized hyperendemic areas of human fasciolosis, human cases occur all over the globe and probably no country can be considered free from the risk of fascioliasis (World Health Organization, 2013).
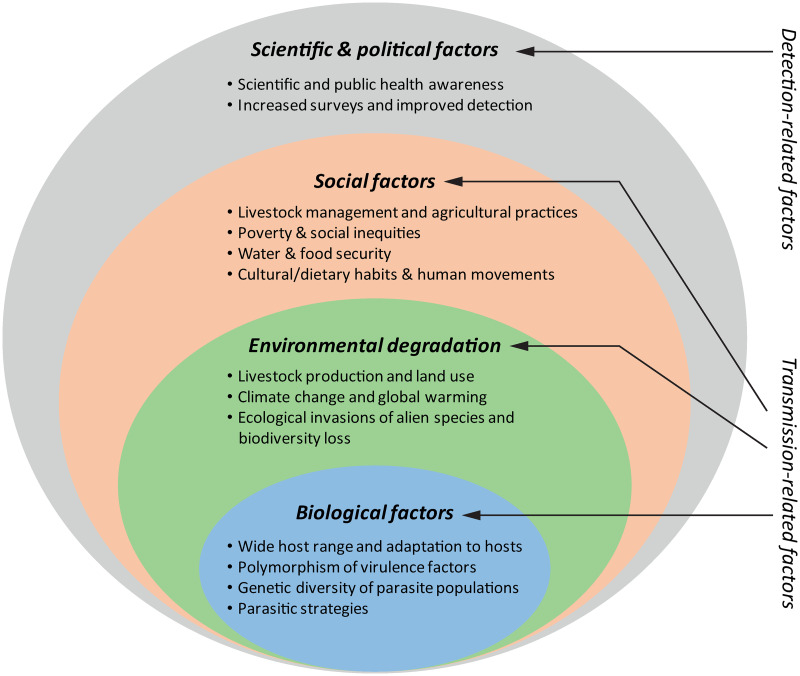
Previous data show an alarming increase of fasciolosis burden in the last decades. Interestingly, the disease has likely been sub-diagnosed, resulting in lower past and present prevalence (Toet et al., 2014), and due to its complex transmission, the rapid rise of fasciolosis as a global health concern is most likely a consequence of different concomitant factors. Therefore, as it was previously mentioned, it should be revised in an integrative manner. In 2012, Cabada and White reviewed the epidemiological data on fasciolosis at the time and suggested that the current burden was related to two (non-exclusive) hypotheses: (i) either the endemic areas are expanding and/or (ii) the disease is being identified in areas where it was not previously observed (Cabada and White, 2012). Here, we discuss this topic from both perspectives to give a comprehensive and integrative overview of this phenomenon. On the one hand, the increase of fasciolosis burden lies on the high adaptability of the causative parasites, and has been promoted by the expansion of the livestock industry and the increasing environmental degradation associated with the plethora of human activities, whereas social factors will ultimately define the extent of infection risks, especially within human populations. On the other hand, while a neglected problem in the past, increased scientific and public health awareness have drawn attention to human and animal fasciolosis. The latter has boosted the significance and global burden of this parasitosis as it has promoted epidemiological surveys and improvements in diagnosis (see Fig. 1 for details).
Fig. 1.
Factors that have contributed to the rise of fasciolosis burden in the last decades. The biological features of Fasciola parasites determining their high adaptability together with the increased environmental degradation and social risk-related factors are in the core of faciolosis (re-)emergence. The increasing scientific awareness and the boosting of health policies aiming at recognizing and investigating the so-called neglected tropical diseases concomitantly with improvements in diagnosis have contributed to highlight the global burden of this largely ignored disease.
Biological factors: a glance at highly evolved and adaptable parasites
Wide range of and adaptation to hosts
The earliest recorded observation of F. hepatica dates back to 1379 when leaf-shaped parasites found in the liver of a sheep were mistaken for a ‘nuysant et dommageuse herbe’ called dauve that was believed to adhere to the liver after its consumption by the animal and to cause disease (De Brie, 1379). Further works from different European scientists rendered more accurate morphological and biological descriptions of the parasite as well as the elucidation of its complex life cycle. The snail Galba truncatula and domestic herbivores, particularly cattle and sheep, were respectively referenced as the typical intermediate and definitive hosts (details on timeline discovery of F. hepatica in Andrews, 1999). Today, the number of reports of different species serving as hosts of F. hepatica and F. gigantica is significant. Overall, around 30 species of freshwater lymnaeid snails are recognized as intermediate hosts (reviewed in Vázquez et al., 2018). Furthermore, more than 50 species of mammals from seven different orders, mainly Artiodactyla but also Diprotodontia, Lagomorpha, Perissodactyla, Rodentia, and two examples from Primate and Carnivora, can get naturally infected and transmit at least one of the two species (see Table 1 for details). Even if certain regions may have their own local intermediate and definitive host species, it is evident that fasciolosis can be transmitted almost anywhere in the world (see Table 1 in Vázquez et al., 2018).
Table 1.
Animal species reported to naturally host adult stages of Fasciola hepatica (F. hep) and F. gigantica (F. gig).
| Definitive host | Status | Distribution | Species of Fasciola | |
|---|---|---|---|---|
| Bovids | African buffalo (Syncerus caffer) | Wildlife | Africa | F. hep/F. gig (Haamond, 1972) |
| Bushbuck (Tragelaphus scriptus) | Wildlife | Africa | F. gig (Malatji et al., 2019) | |
| Cattle (Bos spp.) | Domestic | Worldwide | F. hep/F. gig (Mehmood et al., 2017) | |
| Common duiker (Sylvicapra grimmia) | Wildlife | Africa | F. hep/F. gig (Haamond, 1972; Malatji et al., 2019) | |
| Eland (Taurotragus oryx) | Wildlife | Africa | F. gig (Haamond, 1972; Malatji et al., 2019) | |
| European bison (Bison bonasus) | Wildlife | Europe | F. hep (Kiziewicz, 2013) | |
| Hartebeest (Alcelaphus buselaphus) | Wildlife | Africa | F. hep/F. gig (Haamond, 1972) | |
| Impala (Aepyceros melampus) | Wildlife | Africa | F. gig (Haamond, 1972; Malatji et al., 2019) | |
| Kob (Kobus kob) | Wildlife | Africa | F. hep/F. gig (Haamond, 1972) | |
| Kudu (Tragelaphus strepsiceros) | Wildlife | Africa | F. hep/F. gig (Haamond, 1972; Malatji et al., 2019) | |
| Lechwe (Kobus leche) | Wildlife | Africa | F. gig (Malatji et al., 2019) | |
| Sable antelope (Hippotragus niger) | Wildlife | Africa | F. hep/F. gig (Malatji et al., 2019) | |
| Tsessebe (Damaliscus lunatus) | Wildlife | Africa | F. gig (Haamond, 1972; Malatji et al., 2019) | |
| Water buffalo (Bubalus bubalis) | Domestic | Worldwide | F. hep/F. gig (Mehmood et al., 2017) | |
| Waterbuck (Kobus ellipsiprymnus) | Wildlife | Africa | F. hep/F. gig (Haamond, 1972) | |
| Wildebeest (Connochaetes taurinus) | Wildlife | Africa | F. gig (Haamond, 1972; Malatji et al., 2019) | |
| Camelids | Alpaca (Vicugna pacos) | Domestic | South America, North America*, Europe* | F. hep (Neyra et al., 2002) |
| Camel (Camelus spp.) | Domestic | Africa East Asia |
F. hep/F. gig (Haridy and Morsy, 2000; Sazmand and Joachim, 2017) | |
| Guanaco (Lama guanicoe) | Wildlife | South America | F. hep (Issia et al., 2009) | |
| Llama (L. glama) | Domestic | South America, North America*, Europe*, Australia* | F. hep (Cafrune et al., 1996a) | |
| Vicugna (V. vicugna) | Wildlife | South America | F. hep (Cafrune et al., 1996b) | |
| Caprids | Goat (Capra hircus) | Domestic | Worldwide | F. hep/F. gig (Mehmood et al., 2017) |
| Pyrenean chamois (Rupicapra pyrenaica) | Wildlife | Pyrenees, Cantabrian mountains, Apennine mountains | F. hep (Roldán et al., 2020) | |
| Sheep/mouflon (Ovis spp.) | Domestic | Worldwide | F. hep/F. gig (Mehmood et al., 2017) | |
| Carnivores | Otter (Lutra lutra) | Wildlife | Europe, Asia, North Africa | F. hep (Shimalov and Shimalov, 2000) |
| Cervids | Elk (Alces alces) | Wildlife | Europe, North America | F. hep (Shimalov and Shimalov, 2000) |
| Fallow deer (Dama dama) | Wildlife | Europe, Australia*, some regions of Africa* and the Americas* | F. hep (Jenkins et al., 2020) | |
| Marsh deer (Blastocerus dichotomus) | Wildlife | South America | F. hep (Orozco et al., 2020) | |
| Red deer (Cervus elaphus) | Wildlife | Europe, North Africa and South-eastern Asia, Australia*, the Americas* | F. hep (Shimalov and Shimalov, 2000) | |
| Roe deer (Capreolus capreolus) | Wildlife | Europe and South-eastern Asia | F. hep (Shimalov and Shimalov, 2000) | |
| Sika deer (Cervus nippon) | Wildlife | East Asia | ‘Hybrid’ Fasciola sp. (Ichikawa-Seki et al., 2017a) | |
| Equids | Donkey (Equus africanus asinus) | Domestic | Worldwide | F. hep/F. gig (Haridy et al., 2002; Badawy et al., 2014) |
| Horse (E. ferus caballus) | Worldwide | F. hep (Haridy et al., 2002) | ||
| Giraffids | Giraffa (Giraffa camelopardalis) | Wildlife | Africa | F. gig (Haamond, 1972; Malatji et al., 2019) |
| Hominids | Human (Homo sapiens) | – | Worldwide | F. hep/F. gig (Mas-Coma et al., 2009a) |
| Lagomorphs | Cape hare (Lepus capensis) | Wildlife | Africa, middle Asia | F. hep (Rondelaud et al., 2001) |
| Eastern cottontail rabbit (Sylvilagus floridanus) | Wildlife | North and Central America, northern South America, Caribbean*, Europe* | F. hep (Rondelaud et al., 2001) | |
| European hare (L. europaeus) | Wildlife | Europe, Asia, the Americas*, Australia | F. hep (Shimalov and Shimalov, 2000) | |
| European rabbit (Oryctolagus cuniculus) | Wildlife and domestic | Worldwide | F. hep (Ménard et al., 2000; Rondelaud et al., 2001) | |
| Marsupials | Brushtail possum (Trichosurus vulpecula) | Wildlife | Australia | F. hep (Spratt and Presidente, 1981) |
| Common wombat (Vombatus ursinus) | ||||
| Eastern grey kangaroo (Macropus giganteus) | ||||
| Red-necked wallaby (M. rufogriseus banksianus) | ||||
| Swamp wallaby (Wallabia bicolor) | ||||
| Swine | Pigs/wild boar (Sus scrofa) | Domestic/wildlife | Worldwide/Europe, Asia, North Africa, Australia*, the Americas*, the Caribbean* | F. hep (Shimalov and Shimalov, 2000; Capucchio et al., 2009; Mezo et al., 2013) |
| Rodents | Beaver (Castor fiber) | Wildlife | Europe, Asia | F. hep (Shimalov and Shimalov, 2000) |
| Black rat (Rattus rattus) | Wildlife | Worldwide | F. hep (Mas-Coma et al., 1988) | |
| Bush rat (Rattus fuscipes) | Wildlife | Australia | F. hep (Spratt and Presidente, 1981) | |
| Capybara (Hydrochoerus hydrochaeris) | Wildlife | South America | F. hep (Dracz et al., 2016) | |
| Coypu (Myocastor coypus) | Wildlife | South America, North America*, Europe* Asia*, Africa* | F. hep (Ménard et al., 2000; Issia et al., 2009) | |
| Mouse (Mus musculus) | Wildlife | Worldwide | F. hep (Mas-Coma et al., 1988) | |
| Wild guinea pig (Cavia aperea) | Wildlife | South America | F. hep (Dittmar, 2002) |
*regions where the host species is introduced.
Concerning snails, besides the family Lymnaeidae (usual intermediate hosts), successful infection (i.e. intramolluscan developed parasite larvae) was observed in Bulinus truncatus (Bulinidae) and Biomphalaria alexandrina (Planorbidae) (Dar et al., 2005; Hamed et al., 2009). The possible role of Anisus leucostoma (Planorbidae) as an intermediate host of F. hepatica in France has also been suggested, but only when co-infected with Calicophoron daubneyi (Abrous et al., 1998). However, the transmission capacity of these snail species is not clear as infection events may occur at random but do not necessarily translate into successful cercarial shedding and metacercarial development.
In the case of definitive hosts, several examples exist in the literature about the substantial contribution of different species on Fasciola parasites circulation. Such examples vary from local endemic species such as marsupials in Australia (Spratt and Presidente, 1981), to wide-range wildlife serving as reservoirs, and worldwide introduced domestic mammals (Haamond, 1972; Ménard et al., 2000; Rondelaud et al., 2001; Issia et al., 2009). As an add-on, effective experimental infections of small (mice, hamsters, Winstar rats, rabbits; Itagaki et al., 1994; Terasaki et al., 2003; Phalee et al., 2015; Khan et al., 2017) and large mammals (e.g. bighorn sheep; Foreyt, 2009, the American bison; Foreyt and Drew, 2010) are also indistinctively reported for both parasites. There are also isolated reports of the presence of F. hepatica adults and eggs in ratite birds (Palaeognathae), particularly among native South American rheas (Soares et al., 2007; Martínez-Díaz et al., 2013) and emus (Vaughan et al., 1997). Overall, F. hepatica is considered more successful than F. gigantica in exploiting diverse species of mammals and lymnaeid snails (see Table 1 in Vázquez et al., 2018), a fact that has certainly contributed to its spread and global burden.
Overall, both Fasciola parasites can be considered generalists in terms of host choice. However, the landscape of parasite specificity differs between intermediate and definitive hosts and across species. The specificity of the intermediate host is narrower considering that all reports of successful Fasciola transmission are only linked to certain snail species within a single family. In addition, several studies have pointed out the occurrence of differential affinities of each Fasciola species to distinct genus and species within the Lymnaeidae at phenotypical (e.g. higher susceptibility in F. gigantica–Radix s.l. combinations; Dar et al., 2004; Kaset et al., 2010; Ashrafi and Mas-Coma, 2014), eco-epidemiological (differential distribution of Fasciola spp. in relation with snail distribution; Walker et al., 2008; Mas-Coma et al., 2009a) and population levels (e.g. local acclimation and adaptation; Vázquez et al., 2014; Alba et al., 2018).
While it is suggested that F. gigantica prefers bovids as definitive hosts, F. hepatica's infectivity, life span, egg shedding and immunity data in ovine species are proposed as evidence of higher adaptability of F. hepatica in the latter (Mas-Coma et al., 2009a). In fact, higher parasite prevalence and intensity are documented in F. hepatica-infected in relation to F. gigantica-infected ovine hosts, with also an enhanced ability to evade and modulate the immune response in sheep (Zhang et al., 2005; Raadsma et al., 2007). On the other hand, the lateral transfer of F. hepatica to small rodents in the island of Corsica constitutes an example of F. hepatica's significant adaptation to small non-ruminant mammals driven by the insular enlarging of the ecological niche of the wild rat (highly abundant and markedly herbivorous in the island; Magnanou and Morand, 2006). The latter, as well as other examples such as the finding of natural infection of F. hepatica in the carnivore Lutra lutra (Shimalov and Shimalov, 2000), suggest an ecological rather than phylogenetical compatibility, driving leap and acclimation to new definitive hosts in Fasciola spp., particularly F. hepatica.
High polymorphism of virulence factors
Part of the success of Fasciola species relies on a fast and critical ‘sensing’ of the different environments along their life cycle, which is followed by rapid and highly effective metabolic and physiological adjustments to the new conditions (including hosts; Cwiklinski et al., 2015; Cwiklinski et al., 2018; Zhang et al., 2019b). The genomes of F. hepatica and F. gigantica are among the largest pathogen genomes known (around 1.1–1.3 Gb; Cwiklinski et al., 2015; McNulty et al., 2017; Choi et al., 2020). Non-repeating genome sizes are similar to other trematodes but there are genomic regions containing interspersed repetitive elements that are exceptionally long in Fasciola spp. compared to other digenean, even within the Fasciolidae (Choi et al., 2020). Fasciola spp. diverged from the phylogenetically related Fascioloides buski (planorbid snails, and pigs and human as intermediate and definitive hosts), through adaptive radiation that involved genomic changes mediated by transposable elements (Choi et al., 2020). According to the authors, they appear to be lineage-specific and may have played a central role in Fasciola spp. genome size evolution and adaptation capacity to different hosts (Choi et al., 2020). Moreover, substantial genome-wide polymorphism is noticeable in F. hepatica (48% of genes exhibited at least one non-synonymous SNP; Cwiklinski et al., 2015). Comparative analysis with other parasitic species reveals phylogenetic conservation and diversification of orthologous protein families among trematodes (McNulty et al., 2017), with high non-synonymous polymorphism found in genes related to other parasitic taxa and involved in infection and survival within the host (Cwiklinski et al., 2015).
In this sense, a unique feature of Fasciola trematodes is their almost exclusive reliance on a family of secreted–excreted papain-like cysteine peptidases, called cathepsins (L and B) to parasitize the hosts, facilitating tissue migration, food intake and immune evasion/modulation (Cwiklinski et al., 2019). They are among the most significantly over-expressed genes in the different developmental stages of Fasciola parasites (McNulty et al., 2017; Zhang et al., 2019b) and, whilst within Trematoda they have expanded, the differential expansion and diversification that occurred in Fasciola resulted in more families and gene copy numbers of proteins with overlapping but broad functions (McNulty et al., 2017; Cwiklinski et al., 2019). So far, 37 and 24 transcript clusters have been, respectively, recognized as cathepsin L and B sequences from transcriptomic analyses in different stages of F. gigantica, including intramolluscan larvae (Zhang et al., 2019b). In F. hepatica, at least 11 sequence clusters of cathepsin B and 13 of cathepsin L comprising 29 and 44 sequences, respectively, have been identified from only 13 adult F. hepatica (McNulty et al., 2017). In both species, cathepsins L and B are strictly regulated during development (Di Maggio et al., 2016; McNulty et al., 2017; Zhang et al., 2019b). Different clades of cathepsin proteases have evolved distinctive peptidolytic activity specific to the requirements of different lifecycle stages (Robinson et al., 2008), which highlights the biological significance of these molecules for infection and survival, and their occurrence in host-specific and stage-specific ‘winning combinations’.
Furthermore, a wide-genome comparison of F. hepatica and F. gigantica has underlined the differential expansion and positive selection, along with cathepsins, of the fatty-acid-binding proteins and protein disulphide-isomerases. Such molecular compounds are also significant for infection and survival of the parasites within the hosts along with G-protein-coupled receptors, which may have key roles in physiological and behavioural adaptation to new ecological niches (Choi et al., 2020). These pronounced differential expansions in biologically relevant excretory–secretory proteins leading to lineage-specific adaptation occurred at or after the split between Fasciolinae (Fasciola spp.) and Fasciolopsinae (F. buski) and have been associated with host switch capabilities and to the wide range of hosts related to Fasciola spp. (Choi et al., 2020).
Genetic diversity of parasite populations
In addition to the high genomic polymorphism, perhaps one of the most advantageous features of Fasciola spp. is its ability to generate and maintain an above-fair amount of intra-population genetic diversity (Hurtrez-Boussès et al., 2004). For instance, F. hepatica has shown high multilocus genotypic diversity using highly polymorphic markers (e.g. microsatellites) in analysed populations from the UK (Beesley et al., 2017), Spain (Vilas et al., 2012), Bolivia (Hurtrez-Boussès et al., 2004), France (Vázquez et al., 2020) and Cuba (Vázquez et al., 2016). Similar results in this species were observed when other variable markers were explored (e.g. mitochondrial or nuclear) in Spain (Thang et al., 2020), Austria (Husch et al., 2020), Armenia (Aghayan et al., 2019), Iran (Bozorgomid et al., 2020) and Australia (Elliott et al., 2014). In the case of F. gigantica, high genetic diversity is also reported from Pakistan (Rehman et al., 2020), Cambodia (Loeurng et al., 2019) and Nigeria (Ichikawa-Seki et al., 2017b) using mitochondrial markers. However, other countries have shown lower haplotype diversity generally associated with recent introductions from particular regions, such as in Indonesia (Hayashi et al., 2016).
In any case, in both F. hepatica and F. gigantica, although a globally high genetic diversity is observed, a lack of between-population differentiation within the same country or region has been found. This is generally explained by a continuous movement of livestock that facilitates the mixing of populations (Vázquez et al., 2016; Beesley et al., 2017; Ichikawa-Seki et al., 2017b). However, several studies assert that some structuration may exist in the definitive hosts, which raise questions on host specificity and the general epidemiology of fasciolosis. For example, significant differentiation between F. hepatica isolates was observed in Spain and Iran when hosts such as cattle, buffalo or sheep were explored (Meshgi et al., 2008; Vilas et al., 2012).
Such a marked tendency towards maintaining very high genetic diversity is usually sustained by a preferential outcrossing over a self-mating strategy of the hermaphroditic adult flukes (Hurtrez-Boussès et al., 2004; Beesley et al., 2017). The existence of high within-population diversity translates into greater plasticity and fast adaptability to new environmental (intra or extra-hosts) variations and constraints, and thus, to higher chances of initiating and maintaining transmission, even in unusual hosts. In a worldwide scenario of fasciolosis (re-)emergence, genetically diversified parasite populations may also facilitate the appearance of resistance to anthelmintic drugs, further hindering disease control (Dreyfuss and Rondelaud, 2008).
Parasitic strategies
Experimental infections of intermediate hosts indicate that their interaction with Fasciola parasites is usually characterized by relatively high survival of infected snails during the mid-late days of pre-patent infection and early-patent period (Sorensen and Minchella, 2001; Dar et al., 2004; Ashrafi and Mas-Coma, 2014; Alba et al., 2018; Vázquez et al., 2019). In the definitive hosts, particularly in humans, fasciolosis is frequently non-fatal and clinically mild especially in the case of low-intensity infections (Behm and Sangster, 1999). As a result, the lifespan of most infected hosts is long enough for Fasciola trematodes to develop into rediae and shed cercariae (in the snail) or into egg-producing sexually mature parasites (in the definitive hosts).
The predominant infection strategy of fasciolids involves the immune evasion/modulation of the immune system of the hosts starting at early infection, which derives into a parasite-driven hyporesponsive or inhibitory immune phenotype in both definitive and intermediate hosts. Overall, polarized antigen-specific Th (lymphocytes T helper) 2 pattern and/or downregulation of Th1 and Th17 immune responses are detected in splenocytes within 7 days in F. hepatica-murids/ruminants and in F. gigantica-buffalo models, and are featured by a mixed pattern of elevated anti-inflammatory cytokines [e.g. interleukin (IL)-10, tumour growth factor (TGF)-β]. Higher IL-4/interferon-γ ratio can be also observed with the increase of the parasitic burden and/or infection progress (Mulcahy et al., 1999; O'Neill et al., 2000; Donnelly et al., 2008; Walsh et al., 2009; Shi et al., 2017; Sheng et al., 2019). Towards chronicity, immunomodulation of inflammation and a significant cytokine dysregulation are also present as Th2/Treg immune responses become more dominant, with increasing levels of Treg lymphocytes, TGF-β and IL-10 and neutralizing inflammatory Th1/Th2 cytokines in different Fasciola-mammal models (Walsh et al., 2009; Escamilla et al., 2016; Shi et al., 2017). On the intermediate host side, a few hours following infection, there is no significant haemocyte (snail immune cells) proliferation. In addition, lower expression of the proliferative cytokine granulin and lower levels of in vitro encapsulation activity (=protective immune response towards Fasciola) of haemocytes against F. hepatica larvae have been found in susceptible over resistant Pseudosuccinea columella snails (Alba et al., 2020).
These are some of the fine strategies aimed at avoiding/diminishing the defence response of the hosts that not only support the parasite's successful establishment but also could directly contribute to the balance of energetic trade-offs between the two partners, mitigating the fitness costs of the infection for the host and building up host tolerance. Furthermore, both Fasciola species also appear to induce wound-healing immune mechanisms in their hosts (Ruiz-Campillo et al., 2018; Zhang et al., 2020). Controlling inflammation and inducing tissue repair mechanisms are also certainly needed to assure the migratory activity of Fasciola trematodes without seriously compromising vital tissues and thus, supporting the overall long-term survival of the parasites (Adelman and Hawley, 2017; Motran et al., 2018).
The rapid and significant modulation of host immunity exerted by the parasites is possibly linked to the lack of concomitant immunity following Fasciola primo infection. In this sense, as pre-exposure to fasciolids does not confer complete protection in trickle/challenge infection of intermediate (Alba et al., 2018) or definitive hosts (Bossaert et al., 2000; Wiedosari et al., 2006), it is certainly an add-on factor for transmission.
Finally, the longevity of adult parasites, which could range from 9–12 months up to 11 years depending on the combination of host species-Fasciola (Mas-Coma et al., 2009a), the large egg outputs (Walker et al., 2006) and the environmental resilience of eggs and metacercariae (Luzón-Peña et al., 1994; Suhardono et al., 2006a) are also pivotal strategies for parasitism. Furthermore, the significant clonal expansion within the snail host in which tens of rediae and thousands of cercariae can be generated from single miracidium infection (Rondelaud et al., 2009; Ashrafi and Mas-Coma, 2014; Hodgkinson et al., 2018) is another aspect to consider when discussing transmission success of Fasciola parasites.
Environmental degradation: ecological impact of human activities and climate factors as major determinants of fasciolosis dynamics
The transmission of a parasite by its hosts is a probabilistic event that depends on biological determinants as well as on environmental constraints (suitable ecological conditions, availability of compatible hosts, etc.; Sandland and Minchella, 2003). Therefore, different environments in which hosts and parasites develop strongly affect specificity and genotype selection and determine the close link between host–parasite relationship and natural or anthropic changes (Wolinska and King, 2009). Particularly, human activities and the unsustainable exploitation of natural resources that have dramatically increased over the past decades are leading to a serious environmental degradation that influences current and future epidemiological patterns of infectious diseases including that of Fasciola spp. transmission.
Livestock production and land use
Since the 1960s, livestock systems have increasingly developed to respond to the food demands of an ever-growing human population. Today, livestock occupies between 22 and 26% of the ice-free terrestrial surface of the planet (Phelps and Kaplan, 2017), and it is increasingly affected by fasciolosis (Mehmood et al., 2017). Changes in distribution and/or increase of animal fasciolosis over the last decades have been discussed elsewhere (see Mehmood et al., 2017; Beesley et al., 2018 for review on animal fasciolosis). Reports of active transmission foci of the parasites and naturally infected snails occurring in lands associated with farming and agricultural activities are also frequent (e.g. Schweizer et al., 2007; Nguyen et al., 2012; Grabner et al., 2014; Vázquez et al., 2015). Other authors have informed of an increase in parasite prevalence among field populations of lymnaeid hosts over time (Dreyfuss et al., 2003; Alba et al., 2016).
Livestock-related land use has drastically modified terrestrial ecosystems into extensive pasture fields that include natural water bodies but also drains, ditches and large-scale irrigation systems. These systems are often built to manage water supply and/or flash floods. Indeed, flood-based practices are occasionally used to support grazing and bolster the food industry (Robinson et al., 2011). This anthropomorphic degradation of natural habitats impacts fasciolosis transmission as it fosters lymnaeid snails-prone ecosystems in proximity to definitive hosts. Ecological features within the Lymnaeidae family vary between genus and species. For instance, several Galba species (generally highly associated with F. hepatica transmission) can thrive from cold to more tropical regions and present a considerably amphibious nature, whilst Radix snails are aquatic species. Thus, Galba species are frequently found in shallower and/or temporary waters such as ditches, puddles, banks of slowly-moving streams, spring swamps, reeds, cattle watering tanks and irrigation channels (Hammami and Ayadi, 1999; de Kock et al., 2003; Kleiman et al., 2007; Schweizer et al., 2007; Vázquez et al., 2009). Nevertheless, beyond the differences, a general pattern is evident within the family Lymnaeidae: a preference for stagnant, slow-flowing water ecosystems where they usually reach high densities and occur close to the shoreline (Utzinger and Tanner, 2000; de Kock et al., 2003; Dreyfuss et al., 2003; Kleiman et al., 2007; Schweizer et al., 2007; Vázquez et al., 2009; Dida et al., 2014). In particular, different authors report a positive association between the presence and abundance of different lymnaeid host species in anthropized sites such as farms and pasture lands (Pfukenyi et al., 2006; Kleiman et al., 2007; Schweizer et al., 2007; Vázquez et al., 2009; Novobilský et al., 2013; Alba et al., 2016), and man-made irrigation systems (Diaw et al., 1990; Hammami and Ayadi, 1999; Esteban et al., 2002; Chlyeh et al., 2006; Novobilský et al., 2013; Grabner et al., 2014). Therefore, under such conditions, the probability of contact between Fasciola parasites and their intermediate and definitive hosts increases.
Increase land use has led to deforestation and land overexploitation, significantly increasing the risk of floods and/or drought in the different areas (Food and Agriculture Organization, 2020). Therefore, the growing need to install infrastructure to manage freshwater supplies for the livestock industry has resulted in 20% increase of global land area equipped for irrigation from 1995 to 2016 (Food and Agriculture Organization, 2020). These conditions can further promote the establishment, growth and development of lymnaeid snail populations and the survival of parasite free-living stages (even stating certain seasonality of transmission). Furthermore, man-made irrigation systems can also greatly influence the genetic structure of snail populations as snails can be frequently dispersed throughout the hydrological network (Sabourin et al., 2018). The relationship between the drainage basin structure and genetic population structure of lymnaeid snails was studied on G. truncatula. Significant genetic differentiation increasing with hydrographic distance and consistent with a dispersion dependent on the direction of water flow has been observed (Hurtrez-Boussès et al., 2010). This can affect the epidemiology of fasciolosis transmission as the ability for Fasciola parasites to infect and develop in the snail hosts can vary among populations (e.g. Vignoles et al., 2012; Vázquez et al., 2014). The installation of artificial irrigation networks may favour the rapid spread of snail genotypes sensitive to F. hepatica and may therefore be a concomitant factor on the (re-)emergence of fasciolosis in certain areas (Hurtrez-Boussès et al., 2010).
Concerning transmission to humans, sporadic cases and epidemic outbreaks of human fasciolosis usually occur where the presence of infected local livestock is also acknowledged, mainly in association with rural environments (e.g. Mas-Coma et al., 1999; Esteban et al., 2002, 2003; Parkinson et al., 2007; Rojas et al., 2010; Nguyen et al., 2012; Chen et al., 2013; Ashrafi et al., 2015). Therefore, the ongoing circulation of Fasciola parasites in domestic animals is associated with higher risks of human infection. Nonetheless, a highly significant prevalence of animal fasciolosis does not necessarily associate with areas where human infection is also a highly significant problem (Mas-Coma et al., 2009a). The latter highlights the significance of social factors as the ultimate drivers of Fasciola spp. transmission, which will be further discussed.
Climate change and global warming
The impact of human activities is driving drastic changes in long-term weather patterns with global warming being one of its more patent effects. The livestock industry is a major contributor of heat-trapping gases to the atmosphere, accounting for around 20% of total anthropogenic emissions, and more CO2 production than the entire world's transportation systems combined (Thornton et al., 2008). The impacts of climate change throughout the globe will vary from region to region but overall, mean temperature will rise, as well as the frequency of extreme weather events (e.g. droughts, rainfalls), all of which will affect the spatial and temporal dynamics of infectious diseases such as fasciolosis (Utaaker and Robertson, 2015).
In the current global climate scenario, it is crucial to delineate the causality between climate change and the transmission of parasites, particularly of those like Fasciola spp. which have heteroxenous life cycles and development stages both in the environment and/or in ectothermic hosts or vectors (Mas-Coma et al., 2009b). Climate can influence population dynamics (e.g. host/parasite abundance, population genetic structure and diversity, adaptation), the parasite's life cycle (e.g. physiology, metabolism, development rate) and disease transmission (e.g. host/parasite distribution and behaviour, transmission windows, susceptibility/virulence, prevalence, intensity, morbidity and mortality). In turn, such changes may lead to community-wide impacts such as shifts in species composition of host and parasite faunas (Polley and Thompson, 2009; Mas-Coma et al., 2009b).
The presence and abundance of lymnaeid host populations are dependent on environmental factors such as water velocity, soil evapotranspiration, rainfall and temperature, depicting different seasonality patterns (Goumghar et al., 2001; Prepelitchi et al., 2011). Even in compatible snail–digenean systems, changes in ecological variables such as temperature and droughts may directly and indirectly alter the outcome of parasite exposure. Average daily temperatures above 10 or 16°C combined with high moisture levels are required for the reproduction of F. hepatica or F. gigantica within the snail; thus, if these conditions are not met, the transmission is halted (Mas-Coma et al., 2009a). Furthermore, the dependency of temperature and moisture levels in egg development and metacercarial infectivity span is widely acknowledged for Fasciola spp. (Andrews, 1999). Therefore, an increase in temperature within the optimal range for parasites and snail hosts is associated with a shorter pre-patent infection (the period before cercarial shedding) and increase in cercarial output and metacercariae production (Polley and Thompson, 2009; Mas-Coma et al., 2009b).
Extreme drought/rainfall events will also probably increase in frequency and yet its effect on transmission efficacy of Fasciola spp. may be difficult to ascertain. Even when extreme precipitations may result in deleterious effects for snail populations and wash away free-living stages (reducing the infection risk per individual host), they can also lead to extensive relocation of hosts and parasitic larvae in nearby lands through flooding and thus, to spatially expand transmission foci (Utaaker and Robertson, 2015). In East Anglia (UK), the emergence of fasciolosis in cattle at the beginning of the 21st century was associated with increased precipitation levels during summer months, favouring the thriving of snail hosts and the development of parasite free-living stages along with wetter grazing conditions (Pritchard et al., 2005). In Cuba, one of the largest outbreaks of human fasciolosis resulted from the consumption of lettuce contaminated with F. hepatica cysts following pastures run-off into the crop field due to heavy rainfall events (Rojas et al., 2010). Moderate droughts may impose a patchier distribution of transmission foci and higher risk for infection per individual hosts. These may result from an increase in the density of parasites and hosts as metacercariae-free herbage becomes sparse and the number and volume of remnant water bodies are reduced, promoting contact of all elements involved in transmission (Fox et al., 2011; Vázquez et al., 2015). Prolonged drought is intuitively related to a reduced risk of infection because free-living parasite stages and snail hosts may succumb to desiccation. However, during a severe water shortage, man-made irrigation systems, watering tanks and other artificial water sources might supply the necessary conditions to at least keep transmission afloat within domestic animals, and creating ‘artificial oasis’ for lymnaeid snails to colonize. The construction of man-made water resources to support farming and agriculture in a region within the Peruvian Altiplano during the third quarter of the 20th century resulted in the permanent transmission foci of F. hepatica, a current hot spot of human fasciolosis (Esteban et al., 2002).
Of note, even when major effects on transmission dynamics are expected to occur at the snail/parasite interface, it is already accepted that global warming, in combination with an increase in the frequency of weather extremes, also has serious direct and indirect effects on vertebrates including domestic animals. Even in moderate climate zones, such effects impact grassland production, fodder quality and nutrition of definitive hosts while also increasing environmental stress, and pathogens and vector loads. These, in turn, directly affect animal physiology, behaviour, welfare, health, and increase susceptibility to infections (Gauly and Ammer, 2020).
The influence of environmental factors on the distribution and dynamics of fasciolosis in different regions has been evidenced within recent years. For instance, the intensification and spreading of animal fasciolosis in the UK and acute fasciolosis outbreaks in southern Italy were related to simultaneous increases in temperature and rainfall (Pritchard et al., 2005; Fox et al., 2011; Bosco et al., 2015). Other examples include the diminishing of fasciolosis incidence in cattle after a heat wave in southwestern France (Thomas et al., 2007) or the emergence of human fasciolosis in Pakistan related to climate change and man-made irrigation systems (Afshan et al., 2014). Due to a growing understanding and acceptance of the effects of climate change (particularly global warming and rainfall patterns) on the incidence and spreading of fasciolosis, several models have attempted to assess (Malone et al., 1998; Fuentes et al., 2005; Afshan et al., 2014) and forecast (Fox et al., 2011; Caminade et al., 2015; Haydock et al., 2016) the risk for transmission based on climate data (latitude, altitude, local rainfall and temperature patterns, soil evapotranspiration, etc.). An overall increase of risk for transmission related to global warming in regions such as Europe (Caminade et al., 2015), Britain (Fox et al., 2011) and New Zealand (Haydock et al., 2016) is expected to occur in future years.
Ecological invasions of alien species and biodiversity loss
Host populations displaying high genetic diversity and occurring on stable environments are supposed to decrease the chances of parasite transmission (Sandland et al., 2007; Tavalire et al., 2016). However, the arrival of new invaders with different genotypes and usually depleted genetic diversity could drastically modify parasite success (Meunier et al., 2001; King and Lively, 2012). Therefore, processes related to human activity and to the effects of climate change such as the introduction and successful invasion of alien species and the loss of biodiversity are additional factors to consider when discussing the (re-)emergence of fasciolosis.
In addition to the global spread of production animals, the introduction of wild mammal species in new areas (e.g. South American rodent Myocastor coypus in Europe or the European hare Lepus europeaus in South America), along with successful parasite spillbacks, has concomitantly enlarged the repertoire of definitive hosts in the invaded regions (Ménard et al., 2000; Cuervo et al., 2015). However, given the generalized presence of livestock and wild native reservoirs of Fasciola spp. worldwide, the distribution and transmission of fasciolosis will ultimately be determined by the distribution of Lymnaeidae snails.
Several species of lymnaeid snails that are highly compatible with local liver flukes have been incidentally introduced out of their native ranges, promoting fasciolosis occurrence. In this sense, two of the most globally distributed and epidemiologically significant lymnaeids, i.e. G. truncatula and P. columella, are spread out of their native range (see Lounnas et al., 2017; Alda et al., 2020), definitely boosting fasciolosis transmission all over the world. For example, G. truncatula is known for transmitting F. hepatica at very high altitudes not only in the Andean Altiplano (Esteban et al., 2002) but also in the highlands of southern Tanzania (Walker et al., 2008) and in the alpine and subalpine ecosystems of the Pyrenees (Roldán et al., 2020). It is long recognized as the main intermediate host of F. hepatica in Europe (Mas-Coma et al., 2009a) and in Africa; G. truncatula is believed to be a significant host of F. gigantica as natural infection and significant larval production in experimental exposures have been recorded (Dar et al., 2005). The long-distance flash invasion of P. columella from North to South America and the Caribbean, Africa, Australia and the Pacific Islands, and Europe (see Lounnas et al., 2017) in the last 200 years is accompanied by reports of infection status or transmission capacity of the local F. hepatica (Prepelitchi et al., 2003; Dar et al., 2015; Alba et al., 2019) and F. gigantica (Grabner et al., 2014; Malatji and Mukaratirwa, 2019).
On the other hand, changes in pathogen complexes can also result from biological invasions and other human-related processes, and can alter the composition, abundance and timing of pathogen infection within the hosts. As co-infection by multiple parasites is the normal state in host populations, such modification of the already established equilibrium can drastically alter the effect of every single pathogen, increasing the complexity of transmission dynamics (Viney and Graham, 2013). There is scarce information on how exposure and susceptibility to other parasitic (or microbiota) organisms relate to F. hepatica transmission and surely further studies are needed to clarify this. However, as an example, the dual infection of F. hepatica and the rumen fluke C. daubneyi may decrease the mean number of metacercariae and the patent period for both digenean compared to single parasite infections in the snail P. columella (Dreyfuss et al., 2016).
Lastly, man-driven environmental degradation and introduction/spreading of alien species are also associated with biodiversity loss (Cardinale et al., 2012). In particular, the livestock industry plays an important role in the current biodiversity crisis at local and global levels. For instance, between 1900 and 2016, records show a 4-fold increase of human population, and with it, an equal increase of biomass of domestic animals and a biomass reduction of wild animals of a similar extent (Pozio, 2020). Furthermore, the domestication and inbreeding of livestock to increase production yield have led to a continuous drop of the genetic diversity within production animals, particularly in intensive production systems (Gauly and Ammer, 2020). The overall result of both effects is a decline in the genetic variability of wild and domestic animal populations, which directly render them more susceptible to changes in environmental conditions and to parasite infections (King and Lively, 2012). At the snail host level, the consequences of a reduced gene pool for fasciolosis transmission was observed in the Bolivian Altiplano as the hyperendemicity of human fasciolosis reported in this region is supported by the transmission capacity of the single genotype (at six polymorphic microsatellite loci) occurring in local G. truncatula populations (Meunier et al., 2001). Additionally, the relatively recent global spreading of a unique invasive P. columella genotype/haplotype illustrates the consequences of biological invasion in infectious diseases dynamics as it is supposed to be highly susceptible to F. hepatica infection and thus, to expand the epidemiological risk of fasciolosis transmission (Lounnas et al., 2017).
Social factors: human behaviour as ultimate driver of fasciolosis transmission
As in the case of other food-borne zoonoses, human infection with Fasciola spp. is directly and ultimately linked to livestock management and agricultural practices, environmental resources, population income and living conditions as well as consumption and cultural habits (Macpherson, 2005). Therefore, it is necessary to discuss not only the biological and environmental factors that promote Fasciola spp. circulation in nature but also the social factors that relate to the increased risk of fasciolosis.
Livestock management and agricultural practices
Livestock is a significant global asset providing many benefits and opportunities associated with rapid sector transformation and growth (Thornton, 2010). However, it also brings social, environmental and public health risks that differ among production systems, management strategies, and environmental and economic vulnerabilities (Robinson et al., 2011), determining current and future trends on fasciolosis dynamics (Bennema et al., 2011). Alongside climatic and environmental variations, management factors also affect the spatial distribution and transmission dynamics of Fasciola, and should always be considered, particularly in forecasting models (Bennema et al., 2011).
Parasite control in livestock systems is largely based on the use of chemoprophylactic anthelmintic agents, grazing management or a combination of both. On the one hand, proper pasture management (e.g. rotation, fencing, draining) is essential, especially in stationary husbandry and organic production, to diminish prevalence, to avoid (re-)infection and the high parasite burden that usually correlates with acute fasciolosis, and to contribute to a responsible use of flukicides (Knubben-Schweizer et al., 2010). Nevertheless, in high-risk areas, general guidelines of parasite control should be adapted to the specific conditions of each farm (infectious status, husbandry practices, wet habitats, etc.). In such areas, it is also recommended to organize the movement of livestock throughout the different pasture sections, according to the probability of occurrence of transmission foci. Here, it is also essential to consider the presence of lymnaeid snails and the characteristics of Fasciola spp. life cycle such as seasonality and length of the prepatent period within the mammal host (see Knubben-Schweizer et al., 2010; Knubben-Schweizer and Torgerson, 2015 for details). A study carried out in Switzerland puts theory into practice demonstrating the efficacy of this approach: a decrease in F. hepatica prevalence (from 30.7 to 9.3% during 3–4 years period) was solely observed in the herds where proper pasture management following these recommendations was applied (Knubben-Schweizer et al., 2010). Despite its advantages in the control of fasciolosis and other pasture-borne parasitoses, the design and application of appropriate (lymnaeid snail-based) pasture management can have some practical drawbacks that often threaten compliance. Technical (e.g. insufficient knowledge/information, insufficient suitable pastures for rotations) and behavioural (e.g. insufficient risk perception and/or motivation) are among the most cited challenges (Knubben-Schweizer et al., 2010; Knubben-Schweizer and Torgerson, 2015).
On the other hand, treatment with anthelmintics differently relates to the incidence of the disease, depending on the type of drug, its effect on the different parasite stages, the number and period of the applications in relation to the seasonality patterns of transmission (Bloemhoff et al., 2014; Munita et al., 2019), and to the history of chemotherapeutic pressure exerted on the parasite (McMahon et al., 2016). Thus, knowledge gaps between the scientific and farming communities could challenge the efficiency of this practice. Also, even when it can be relatively easy to accomplish and should reduce parasite load within the herds, the application of flukicides by itself does not prevent reinfections and other interventions are needed to lower the level of pasture infestation with parasite metacercariae (Knubben-Schweizer et al., 2010; Greter et al., 2016). Furthermore, the high selective pressure caused by the traditional overreliance on the use of triclabendazole in livestock has resulted in the appearance and spreading of resistant F. hepatica genotypes. This poses a major problem for the control of both human and animal fasciolosis and may significantly alter the epidemiology and transmission of the parasite over the coming decades (for review, see Kelley et al., 2016). Fluke resistance to other anti-flukicide treatments (albendazole, clorsulon) and to multiple treatments has also been reported (see Fairweather et al., 2020 for review).
Along with globalization and a growing demand for food, livestock movement (within or outside its current pasture lands) is also increasing. Movements may facilitate the dispersion of the parasites and the entanglement of ongoing transmission. For instance, this occurred during the New World colonization through the introduction of infected cattle and sheep from Europe (Mas-Coma et al., 2009a). Beyond mediating flash invasions of hosts and parasites into new areas, reports of pastural practices such as nomadism and transhumance as well as livestock trade (local or international) also promote genetic diversity of parasites through an increased genetic flow between domestic animals and/or wildlife. This immediately translates into an increase in parasite adaptability through a more diverse gene pool and has been also linked to the spreading of hybrid/introgressed forms of Fasciola spp. to new areas (see Vázquez et al., 2016; Beesley et al., 2017; Amor et al., 2020; Calvani et al., 2020 for some examples). This is particularly troublesome if we considered that resistance to available anti-flukicide drugs is on the rise worldwide, and can spread through improper cattle management (Beesley et al., 2018; Fairweather et al., 2020). Furthermore, such movement of hosts and parasites could facilitate contact with more compatible Fasciola–lymnaeid snail combinations, as it has been recorded from certain allopatric snail–parasite systems (high snail survival, infection rate and parasite burden; Gasnier et al., 2000; Vázquez et al., 2014) and hence, can increase the risk of liver fluke transmission.
Aside from livestock movements, other factors and management activities are highlighted as significant risk-increasing factors: (i) using unsafe water resources and prolonged grazing (for review, see Sabourin et al., 2018); (ii) feeding on wetland pastures vs feeding on forage/dry-land crop residues (Suon et al., 2006; Khan et al., 2009; Takeuchi-Storm et al., 2017); (iii) increased proportion of grazed grass and length of grazing season (Bennema et al., 2011); (iv) mixed farming of small and large ruminants; and (v) the use of stagnant pond bathing and river/canal bathing vs river/tap water bathing (Khan et al., 2009). In some cases, lower levels of literacy of farmers have been associated with higher infection risk, as proper knowledge and comprehension of control strategies in relation to the parasite cycle are essential for decreasing transmission in high-risk areas (Villa-Mancera and Reynoso-Palomar, 2019). Current trends have also led to an increase and expansion of organic ruminant production. In this context, parasite management remains a challenge as mandatory outdoor access for all age groups can increase exposure to pasture-borne parasites, whereas restrictions in the prophylactic use of anthelmintic drugs can limit parasite control (Takeuchi-Storm et al., 2019).
It is worth considering that even when the majority of world's livestock is under exclusive livestock farming (occupying more than 60% of the land devoted to livestock activities), mixed crop–livestock systems occur particularly in areas with the highest population density. This allows sharing and re-utilizing resources such as space and water supplies, with livestock frequently providing draught and manure for crops and control for herbs, whereas the residues and by-products of crops can be used for feeding livestock (Robinson et al., 2011). Wetland rice-based farming systems have been associated with animal fasciolosis transmission, particularly in regions of South and Southeast Asia where livestock manure is used to fertilize rice fields and animals are then fed with contaminated rice straws (Mahato and Harrison, 2005; Suon et al., 2006; Nguyen et al., 2011). In addition, the spatial proximity of infected animals, flooded vegetables usually consumed raw by humans (whether crops fields or small gardens) and shared water supplies may facilitate cross-contamination of crops and/or irrigation waters with parasite free-living stages leading to human infections in both high- and low-income countries (Esteban et al., 2002; Nguyen et al., 2011; Chen et al., 2013; Milas et al., 2020). Traditional practices such as the use of livestock manure as fertilizers might be risky if manure is not properly managed and stored to decrease the viability of Fasciola eggs (Suhardono et al., 2006b). Similarly, anecdotal reports of disease outbreaks in housed livestock in high-risk areas have documented the importance of appropriate management of forage and wetland crop residues to prevent livestock infection with metacercariae (John et al., 2019). In Indonesia, metacercarial attached to submerged rice plants showed the longest survival (5 weeks in water temperatures of 25–30°C); thus, the drying-out of rice fields for at least 2 weeks prior to harvest is needed to affect metacercariae infectivity as well as snail abundance (Suhardono et al., 2006a). The importance of maintaining a proper anaerobic ensiling environment to eliminate the risk of F. hepatica transmission from silage has been recently demonstrated (John et al., 2020).
Poverty and social inequities
Even though fasciolosis distribution, incidence and emergence are not exclusive of regions within low-income countries, it is considered an infectious disease of poverty (Zhou, 2012). Current hot spots of human fasciolosis are located in the Americas, North of Africa and Southern Asia (Mas-Coma et al., 2018), whereas animal fasciolosis is also more prevalent in some African, Asian and American countries with infection rates comparatively higher in developing than in developed countries (Mehmood et al., 2017).
Various factors determine the increased prevalence of fasciolosis in low- and middle-income countries. Agriculture and livestock production make important contributions to food security and poverty reduction and it is estimated that about 70% of the world's 1.4 billion persons living in extreme poverty depend on livestock (Food and Agriculture Organization, 2020). Nevertheless, conditions for livestock husbandry between developed and developing countries are usually different, with riskier conditions for transmission in terms of fasciolosis (and other infectious diseases) prevailing in low-income countries. In developed countries, each farmer usually grazes a large number of animals, has more control on the access to pasture and water, produces high-value products, operates applied strategic and tactical drenching programmes and anthelminthic treatments based on knowledge about the parasites (Roberts and Suhardono, 1996). Contrastingly, in low-income countries, scattered smallholder livestock systems usually have low- or no-input management with the animals of usually poor genetic breeds foraging for themselves, feeding on plants or waste that otherwise would not be used. Traditional and out-dated practices are usually coupled with a lack of knowledge among farmers, as literacy is usually lower in rural communities within developing countries. Limited capacity and resources, including limited services for disease detection and control, and unaffordable anthelmintic treatments hinder farmers' ability to control parasites in such settings (Roberts and Suhardono, 1996; Randolph et al., 2007; Nguyen et al., 2011).
While high-income countries are experiencing certain stagnation in the demand for livestock products and progress in efficient production systems that reduce environmental impact, low-income countries are reporting an increase in production mostly under low-resource livestock-keeping systems (Thornton, 2010). Furthermore, losses related to agriculture and livestock industry are a major factor in extending the cycle of poverty, driving low-resource farmers to overexploit the environment in the attempt to maximize production in already ecologically vulnerable areas (Thornton et al., 2008). As adaptation and mitigation could be significantly challenging in these settings, all these translate into further environmental degradation of certain areas (Thomas et al., 2019) and into a vicious cycle that perpetuates the ecological, economic and social conditions that favour fasciolosis transmission. In this scenario, low-income countries are expected to continue to experience a greater impact of fasciolosis and other infectious diseases (Roberts and Suhardono, 1996; Piedrafita et al., 2010).
In poor smallholder livestock communities, and particularly in developing countries, resources constrain, poor living conditions and low literacy level hinder the implementation of proper management practices and educational campaigns to increase awareness of food and water security as well as the risks of fasciolosis (Gray et al., 2008; Robinson and Dalton, 2009; Villa-Mancera and Reynoso-Palomar, 2019). Even if treatment-based interventions are applied and are proved effective (e.g. Curtale et al., 2005; Zhang et al., 2019a), reinfection of animals and/or human communities is likely to occur if not coupled with education on the aspects of parasite transmission, risky practices and infection sources, and on the importance of separating animals from crops (Robinson and Dalton, 2009; Greter et al., 2016). Therefore, it has become increasingly clear that options for effective management and sustainable control need to be developed locally with an understanding of the science underlying control mechanisms (Gray et al., 2008). Furthermore, resource-poor farmers and communities are more likely to need financial, resource assistance and other incentives to participate in control programmes and to adopt new practices. Thus, any control strategy needs to be accompanied by political commitment, ensuring mobilization of resources and multisectoral approaches (Curtale et al., 2005; Gray et al., 2008). This represents an additional challenge for developing countries experiencing economic instability and/or political crises.
Water and food security
Estimates indicate that 60% of the world's population lives in ecologically vulnerable areas in low-income countries where the adverse effects of climate change on health may manifest through direct pathways related to the shifting of climate patterns and weather extremes, and through indirect pathways like increasing social instability and inequality, such as food/water insecurity (Thornton et al., 2008). Even though access to safe food and water has increased in the last decades, around 30% of the world's population still suffers from food and water insecurity (Food and Agriculture Organization, 2020). This social inequality can also positively associate with fasciolosis transmission.
In addition to the pre-existing high probabilities of food/water contamination in rural communities from endemic areas (Esteban et al., 2002; Nguyen et al., 2011), scarce access to sufficient, safe and nutritious food may promote the consumption of wild edible plants to supplement domestic diets. This increases the risk of infection by Fasciola and other food-borne parasites, particularly in endemic areas (McGarry and Shackleton, 2009; Shumsky et al., 2014). Furthermore, selling wild plants in non-controlled places is a common practice also associated with the human transmission of fasciolosis and one that extends the epidemiological risk to urban dwellers (see Mas-Coma et al., 2018 for detail). To note, some of these risks have been also described in developed countries, which highlight the potential threat of fasciolosis transmission worldwide. Reports of fasciolosis in Europe through khat leaves consumption after non-controlled importation exist (Doherty et al., 1995; Chand et al., 2009). In addition, cases or outbreaks of human fasciolosis have been reported from eating locally-produced contaminated plants commercialized in urban markets within endemic areas of France (Mailles et al., 2006) and Australia (Hughes et al., 2003), or even cultivated in small house gardens (e.g. Milas et al., 2020 in Belgium).
Water-borne transmission might represent another challenge to parasite control as a portion of shed cercariae can exist as free-floating metacercariae (Dreyfuss and Rondelaud, 1997; Rondelaud et al., 2004). The significance of secure water sources is pointed out by a study in Pakistan that aimed at assessing the contamination of different water sources with F. hepatica DNA and showed the lowest records from tap water (1.6%) over tube (10%) and open wells (8%), respectively (Khan et al., 2012). Indirect evidence of water-borne transmission has been indicated in particular epidemiological contexts where endemicity and infection rates of the Fasciola parasites are high among animals and snails, and access to safely managed drinking water is scarce (e.g. Esteban et al., 2002; Zumaquero-Ríos et al., 2013; Cabada et al., 2018). In the hyperendemic areas of the Northern Andean Altiplano, water and food security is a significant issue as inhabitants lack basic services such as piped potable water, sewage and waste disposal. On the Peruvian side of the Titicaca Lake, drinking water appears to be the predominant human infection source (Esteban et al., 2002), as the consumption of raw aquatic plants is uncommon. In contrast, eating contaminated vegetables is supposed to play a more important role than drinking unsafe water in the Bolivian part, eastward from Lake Titicaca (Parkinson et al., 2007). In such a setting, the possible differential epidemiological significance between water and food as the main vehicle of parasite transmission is yet to be linked to other social factors: cultural and dietary habits.
Cultural and dietary habits and human movements
Human behaviour is influenced by psycho-social factors such as ethnicity, culture and religion, which relate to every aspect of our lives including food choice and preparation, and determine the range and level of exposure to parasites (Macpherson, 2005). Several freshwater plants may be carriers of Fasciola metacercariae but their role as infection sources to humans will ultimately depend on the diet and traditions of the different groups (see Mas-Coma et al., 2018 for review on infection sources). Watercress (both common Nasturtium officinale and N. microphyllum and wild watercress Rorippa sylvestris and R. amphibia) are perennial aquatic or semi-aquatic herbs globally distributed and frequently consumed raw in salads. They are commonly associated with fasciolosis infection in humans worldwide (Mas-Coma et al., 2018) and have been found carrying large numbers of parasite metacercariae compared to those carried by other sympatric aquatic plants (Rondelaud et al., 2020). It is the most cited infection source of liver flukes to humans in endemic regions of Europe (e.g. Garcia et al., 1985; Mailles et al., 2006; Vignoles et al., 2019) and the Americas (e.g. Rojas et al., 2010; Mera y Sierra et al., 2011; Zumaquero-Ríos et al., 2013) and several field studies following fasciolosis outbreaks have reported lymnaeid snails in watercress beds (Ferrer et al., 1989; Rondelaud et al., 2001). Nevertheless, other edible plants have been also pointed out as infection sources in different regions. For instance, in Tunisia, the plant Apium nodiflorum (Fool's watercress) is recorded as the vehicle of parasite metacercariae into humans (Hammami et al., 2007), whereas in Southwest China, a human outbreak was associated with the consumption of contaminated fish mint Houttuynia cordata (Chen et al., 2013). In Iran, besides watercress, the species Mentha logifolia (mint) and M. spicata (spearmint) have been implicated in transmission as they are frequently eaten fresh in local traditional foods (Sarkari et al., 2012; Hosseini et al., 2015). Eating dandelion leaves (Taraxacum dens leonis or T. officinale) has been associated with human infection in France and Argentina (Mera y Sierra et al., 2011; Vignoles et al., 2019). In several regions of Latin America, the customary drinking of a beverage made from Alfalfa (Medicato sativa), particularly in rural populations, has been also related to an increased risk of infection (Mas-Coma et al., 1999; Zumaquero-Ríos et al., 2013). Other vegetables such as lettuce (Lactuca sativa), spinach (Spinacia oleracea), garden rocket (Eruca vesicaria syn. E. sativa) and leek (Allium porrum) have been also associated with infection (see Mas-Coma et al., 2018).
Cultural habits other than direct consumption of edible plants can also influence fasciolosis transmission in humans. For instance, sucking and/or chewing wild aquatic or semiaquatic plants has been pointed out as one of the possible explanations for the high infection rates in Aymara children in Bolivia (Mas-Coma et al., 1999). Several patients from Ethiopia and Yemen have become infected through the traditional chewing of khat (Catha edulis) leaves (Cats et al., 2000; Chand et al., 2009). The involvement in household and farm work is also considered a risky behaviour and it has been linked to a differential infection burden between women and men in certain human fascioliasis hyperendemic areas. Such differential risks have been observed in the Nile delta, Egypt (Esteban et al., 2003) and in the Bolivian Altiplano (Curtale et al., 2007), as these roles are usually assigned to women and girls in these regions and are presumed to increase exposure to the parasite. A common habit among farmers of the Nile Delta region that consists of picking vegetables and then leaving them immersed in the canals to keep them fresh while they continue picking has been identified as a possible reason for the high prevalence of fasciolosis in the area (Hotez et al., 2012).
Demographic movements towards metropolitan areas can also influence the incidence of human fasciolosis as Fasciola parasites mostly circulate within rural environments. The significant decrease in human cases in central France, from hundreds being diagnosed between 1956 and 1986 to a few tens between 1986 and 2006, has been associated with two factors: (i) an increased migration of the population towards highly urbanized cities (Vignoles et al., 2019) and (ii) changes in dietary and cultural habits such as the decrease of recollection and consumption of wild watercress among younger generations (Rondelaud et al., 2000). Nonetheless, even though human infection in city settlements occurs only sporadically, usually due to consumption of metacercariae-carrying vegetables acquired in urban establishments (see e.g. Hughes et al., 2003; Mailles et al., 2006), infection of urban dwellers is also possible during country field trips, as observed in Argentina (Mera y Sierra et al., 2011), or through international travel (Figtree et al., 2015; Salzer and Schmiedel, 2015). Similarly, human migration can also influence the prevalence of fasciolosis and its distribution. In Southwest Iran, a higher seroprevalence of fasciolosis (2.6%) was found among nomad communities that travel with their flocks twice a year between summer highlands and lower warmer winter pastures in comparison to the settled population (1.8%; Zoghi et al., 2019). In the USA and Germany where the incidence of human fasciolosis is relatively low, current trends show that, although some cases are local, most are associated with travellers or migrant populations (Chand et al., 2009; Fried and Abruzzi, 2010; Salzer and Schmiedel, 2015).
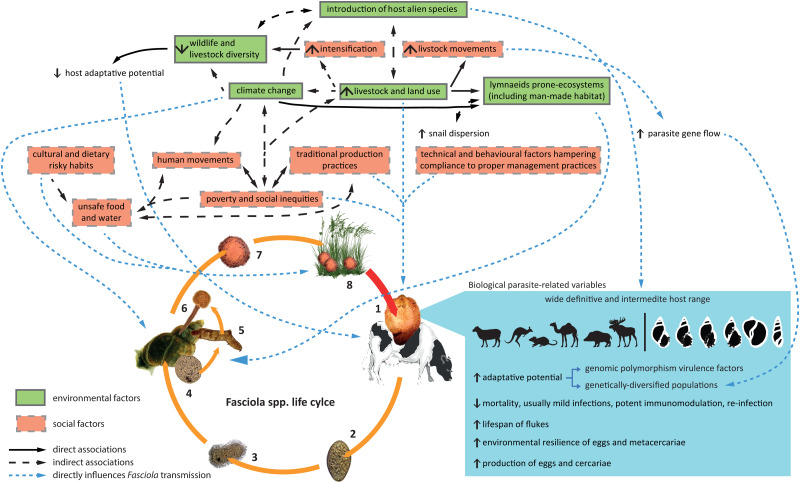
Figure 2 summarizes, in an integrative diagram, the interrelation of biological, environmental and social factors discussed so far at the view of Fasciola spp. transmission.
Fig. 2.
Integrative diagram showing the interrelation between biological (parasite-related), environmental and social factors directly influencing the different components participating in Fasciola spp. transmission. (1) Fasciola adult in the definitive host, (2) egg, (3) miracidium, (4, 5, 6) sporocyst development, and rediae and cercariae production in the snail host, (7) metacercariae formation following cercariae encystation, (8) water and vegetation contaminated with metacercariae.
Scientific and political factors: changing the view from sporadic diseases in humans to a significant (re)emergent public health problem
Scientific and public health awareness
Archaeological studies showing adult fragments and eggs of Fasciola within an Egyptian mummy (David, 1997) indicate the historical incidence of human fasciolosis. Nevertheless, even when records of the parasite in humans became more evident after their description in the 14th century, fasciolosis was traditionally (and unfortunately still is) considered a veterinary disease. For instance, whereas infection of human European population with Fasciola parasites during medieval and Renaissance periods is accountable today through paleoparasitological studies (Graff et al., 2020), by late 19th century, the following statement by Wilson (1879): ‘cases of its occurrence as a human parasite were by no means plentifully met with either in purely medical records on the one hand, or in helminthological treatises on the other’, clearly reflects that fasciolosis has been largely overlooked in humans. However, in the 20th century, this zoonosis began to acquire a new connotation within the scientific community due to indistinctive reports of significant numbers of infected people and/or epidemic outbreaks from different regions of the world. Some registered records are, for example, four outbreaks in Cuba between 1943 and 1983 with over 2000 people infected (Rojas et al., 2010), around 10 000 diagnosed cases between 1956 and 1982 (Gaillet, 1983) and one epidemic outbreak in 1989 in France (Chandenier et al., 1989), or a major outbreak in North Iran in 1988 affecting around 10 000 people (Rokni, 2008).
In 1990, Chen and Mott extensively reviewed the existing reports of clinical cases of human fasciolosis over a 20-year period (1970–1990) computing, for the first time, a worldwide prevalence of 2594 built on data from 42 countries of all continents (Chen and Mott, 1990). This investigation was highly significant as it redirected the attention towards human fasciolosis at a global scale and discussed the possibility of a higher overlooked impact on human populations. It raised awareness of fasciolosis in terms of its public health impact and preconized the (re-)emergence of fasciolosis in temperate and tropical regions of the world, triggering growth in epidemiological studies and a consequential drastic increase in the rate of case reports in the literature (Parkinson et al., 2011).
In parallel, the 1990s were characterized by an expansion of public health policies led by the World Health Organization (WHO) on diseases such as malaria, filariasis, lepra but also on other less notorious tropical diseases. Such policies promoted a greater emphasis on the provision of assistance for research and control in endemic countries, encouraging biomedical, social and science research institutions to devote greater attention to tropical diseases (World Health Organization, 1990). Particularly among these diseases, the significance of food-borne trematodoses was recognized in 1993 when a Study Group on the Control of Food-borne Trematode Infections held a debate about the burden of food-borne diseases and called for collaboration and potential strategies for their control. The report of the meeting officially recognized human fasciolosis, enlisted as part of the food-borne trematodoses, as a cosmopolitan infection. With an expanding distribution and clinical cases reported from 61 countries, a worldwide estimation of over 1.9 million people infected with fasciolosis was calculated but only counting the potential burdens of six countries: Bolivia, Ecuador, Peru, Egypt and Iran (World Health Organization, 1995). The WHO considered fasciolosis, along with the rest of food-borne trematode infections, as an emerging human disease of increasing impact and a severe public health problem ‘that made action imperative’. From this point forward, the WHO encouraged the establishment of integrative control programmes alongside agricultural and public health strategies, particularly within endemic countries, as well as the collection, monitoring and surveillance of epidemiological data at a global scale (World Health Organization, 1995). With more inclusive assessments, global prevalence quickly rose to 2.4 million (Rim et al., 1994) or to 17 million (Hopkins, 1992), and up to 50 or 72 million if underestimation is accounted for (Nyindo and Lukambagire, 2015), whereas 180 million people are currently considered to live at risk (World Health Organization, 2013). The lack of extensive public awareness of the transmission and impact of fasciolosis, the gaps in knowledge about the distribution and epidemiology of food-borne infections, the poorly assessed biology and ecology of the snails hosts and the particular features of the disease caused by the parasites were soon considered major constraints for the feasibility of control strategies (World Health Organization, 1995).
Additionally, in 1992, the Food and Agriculture Organization (FAO) also reviewed the geographic distribution of helminth infections (including Fasciola parasites) in livestock in developing countries based on the selection of relevant data from the literature published during the previous 15 years. Given the accumulated material, it considered liver fluke infections 'as an important regional threat to animal production in many developing countries’ (Over et al., 1992). Furthermore, with fasciolosis showing the greatest distribution of all zoonotic trematode infections and with predictably negative effects on the productivity of animals and in human health as a result of massive infection, FAO stated ‘that control measures should be introduced immediately’ (Over et al., 1992).
The 2000s have been characterized by a ‘momentum’ of the so-called neglected tropical diseases (NTD). Since then, largely ignored diseases have been given priority in the global health agenda. The first meeting of WHO on the control of NTD held in 2005 emphasized the urgency of gathering evidence and gaining understanding of particular NTDs including fasciolosis and other foodborne trematodoses (World Health Organization, 2011). These meetings also led to increased collaboration and concerted efforts with FAO in the development and research of common strategies and investments of the zoonoses. Such actions set the example for the need for coordinated and synergistic actions between animal and human health sectors when dealing with infections like fasciolosis to achieve ‘One Health’. Overall, the increasing scientific awareness and research evidence served as a call for attention to settle fasciolosis within the global landscape as a significant disease, and to stimulate research on Fasciola and fasciolosis.
To illustrate the worldwide interest in fasciolosis within the scientific community, we carried out a rapid search on Scopus and PubMed databases. We used (i) ‘Fasciola OR Fasciolosis OR Fascioliasis’ as the basic search criteria, and we also complemented it with: (ii) AND human, (iii) AND ruminants, (iv) AND epidemiology, (v) AND survey, (vi) AND diagnostic method. The compiled data evidence a growing trend of scientific interest and commitment that started rising in the 1960s and saw an exponential increase at the beginning of 2000s which continues to be present today (see Fig. 3A and B). The enhanced scientific production experienced in the last decades indirectly shows the interest from funding agencies and investors that followed the inclusion of fasciolosis in the international health agenda. A special mention ought to be given to the growing percentage of research within Fasciola and fasciolosis devoted to epidemiological surveys and detection methods (Fig. 3C and D). Given the long pre-patent period and normally insidious sub-clinical nature of the disease in the definitive hosts and the sensitivity issues of traditional parasitological diagnosis, these surveys and methods have significantly contributed to identifying endemic areas that were previously neglected.
Fig. 3.
The data compilation from searching results on Scopus (A, C) and Pubmed (B, D) databases using (i) Fasciola OR Fasciolosis OR Fascioliasis as basic search criterium, and complemented it with: (ii) AND human, (iii) AND ruminants OR livestock, (iv) AND epidemiology, (v) survey, (vi) diagnostic method OR detection method. (A, B) The absolute number of entries per year, between 1940 and 2019, for each search criteria. (C, D) The percentage of entries for each complementary criterion (from ii to vi) over the total of entries corresponding to the basic search criterion (i) in 10-year intervals.
Improved detection and increased surveys
The finding of Fasciola eggs in stool and/or adults in liver, gall bladder or biliary ducts is the classical and most widely used principle to diagnose infection and thus, it has been a significant contributor to prevalence assessments. However, parasitological detection has numerous drawbacks inherent to the different methods available, to the biology of Fasciola parasites, the progression of the infection and to the different epidemiological situations (see Mas-Coma et al., 2014 for review). Therefore, the incorporation of immunoassays, whether serological methods since the 1980s such as in-house methods (Chen and Mott, 1990) or commercial variants (e.g. Fas2-ELISA: Espinoza et al., 2007; Svanovir®: Charlier et al., 2009), and of Fasciola antigen capture-based immunodetection since the 1990s (e.g. FasciDig: Espino et al., 1998; Zumaquero-Ríos et al., 2013; MM3-ELISA: Mezo et al., 2004; Ubeira et al., 2009), has allowed faster, more sensible and complementary diagnoses of fasciolosis. This has proven to be particularly useful in non-endemic or hypo-endemic areas and/or for large-scale surveys (Mas-Coma et al., 2014). Lastly, the expansion of molecular techniques in the 2000s added a new dimension to the screening of fasciolosis transmission. Molecular tools solve the parasitological method's drawbacks of sensitivity and specificity, allow to discriminate both Fasciola species and the hybrid forms in a reliable way and to characterize genetically the different parasite and host populations (Martínez-Valladares and Rojo-Vázquez, 2016; Amor et al., 2020; Moghadamizad et al., 2020).
Different techniques within each type of non-classical detection method have been applied indistinctively in the monitoring of infection within definitive hosts such as ELISAs (Espino et al., 1998), rapid immunochromatographic tests (Xifeng et al., 2019), conventional PCR, loop-mediated isothermal amplification (Martínez-Valladares and Rojo-Vázquez, 2016) and high-resolution melting analysis (Moghadamizad et al., 2020). In addition, detection of snail infections also evolved from the classical parasitological dissection to immunoenzymatic assays (Alba et al., 2015a) and molecular methods (multiplex PCR, Alba et al., 2015b; real-time PCR, Schweizer et al., 2007; etc.). All these techniques have demonstrated improved diagnostic sensitivity and specificity (see e.g. Zumaquero-Ríos et al., 2013; Alba et al., 2016). Furthermore, other types of samples beyond blood, stool (definitive host) and snails (intermediate host) have been added to the panel of possibilities for active screening; from meat juice and bulk milk for serological assessment in livestock (Charlier et al., 2009; Villa-Mancera and Reynoso-Palomar, 2019) to water for environmental DNA detection of the parasite and its snail hosts (Jones et al., 2018; Davis et al., 2020).
Most of these newly developed methods have been implemented in epidemiological surveys during scientific investigations, and the conjunction of both factors has undoubtedly contributed to building up knowledge on fasciolosis burden in specific areas and its transmission by the different host systems. As the occurrence of most human endemic areas within poor rural populations is usually characterized by low attendance to health centres, frequent co-infection with other diseases, and a tricky diagnosis if it is based solely on clinical description, in the absence of sounded symptoms and/or active screening with sensitive methods, fasciolosis could go unnoticed. As reviewed by Chen and Mott (1990), before 1990, most scientific reports of human fasciolosis consisted of a small series of acute clinical cases seeking hospital attention. Furthermore, the highest numbers were reported from countries with strengthened public health systems and scientific tradition (e.g. France, Spain, Portugal; Chen and Mott, 1990). The authors alerted about the possibility of a serious underestimation of the global infection as only a few low-scale community-based surveys carried out at the time and applying serological screenings (in Peru, France, Portugal, Egypt and Puerto Rico) demonstrated improved capacity for assessing the impact of the disease (Chen and Mott, 1990). In this sense, a meta-analysis of epidemiological surveys from 38 communities in the Bolivian Altiplano established that fasciolosis is endemic there since at least 1984 (Parkinson et al., 2007). However, it was not until extensive surveys were conducted in the 1990s that it was pinpointed as a significant hot spot where the highest human prevalence was and continue to be reported (Esteban et al., 1999; Mas-Coma et al., 1999; Parkinson et al., 2007). Later, other significant endemic foci of human fasciolosis have been also found based on large-scale or community-based epidemiological studies, for example, in regions within Egypt (Esteban et al., 2003; Curtale et al., 2007), Iran (Sarkari et al., 2012), Peru (Esteban et al., 2002) and Mexico (Zumaquero-Ríos et al., 2013).
It should be mentioned that even though efforts have been increased, extensive epidemiological studies, especially in high-risk communities, remain scarce in most countries and the overall rates of infection are still being extrapolated from sporadic reports of cases or outbreaks (Parkinson et al., 2011). Factors such as: (i) fasciolosis can cause mild symptoms or go unnoticed for a long time; (ii) current diagnostic tools have sensitivity, reproducibility or availability and cost-associated issues; (iii) it is a disease of non-obligatory declaration in many countries; and (iv) coordinated and updated epidemiological information is usually scarce especially in low-income countries, imply that the real number of animals and humans infected with fasciolosis in some countries is presumed to be higher than their reports (Toet et al., 2014). At present, worldwide disease prevalence is still uncertain (Parkinson et al., 2011) and concerns on the real extent of human fasciolosis in certain geographical areas such as Argentina (Mera y Sierra et al., 2011), Nepal (Sah et al., 2018) and China (Chen et al., 2013) have been augmenting.
Realities and challenges towards the future
Herein we have exposed the different factors affecting fasciolosis dynamics and incidence in the context of its current (re)emergence but it is difficult to say with certainty up to when, where and for how long this trend will continue in the future. Global livestock population is predicted to almost double by 2050, especially in low- and middle-income countries and the intensification of livestock production in the humid and subhumid grazing systems of the world is also projected to increase (Thornton, 2010). The relationship between livestock production–environmental degradation–climate change (especially under poor management practices) may continue to do so in the near future. However, it is important to note that the effects of climate change are not homogeneous and straightforward in all regions and they are not altogether necessarily enhancers of transmission (see Mas-Coma et al., 2009b). Therefore, while it may be assumed that the current climate change has contributed to the overall spreading of fasciolosis and increasing prevalence in certain regions, a constant trend and a global homogeneous pattern cannot, by any chance, be expected. Therefore, regional projections and screenings must be put in place to assess the future impact of fasciolosis in different areas.
In addition, the increase in livestock production could translate into economic growth. However, economic growth without consideration for social inequality does not automatically translate into human development. Therefore, poverty risks must be always considered because fasciolosis as other infectious diseases usually affects in low-income regions the most. This increased risk further constrains development at different levels (e.g. economic, environmental, social), reinforcing social inequities, environmental degradation and unsustainable exploitation of resources and condemning the population to a vicious cycle that perpetuates these very same socio-economic, ecological and epidemiological conditions. It should be mentioned that fasciolosis is a disabling disease (Torgerson et al., 2015) and, whilst all age groups can be affected, in human hyperendemic areas, children appear to be the most infected (Esteban et al., 2002, 2003; Zumaquero-Ríos et al., 2013). Hence, it directly constrains the development of the youngest generation.
With fasciolosis (re-)emergence and a growing livestock industry, the lack of vaccines places immediate pressure on the use of anthelmintic treatments to control F. hepatica infection leading to a significant problem to consider for the near future, such as parasite resistance (Kelley et al., 2016). Indeed, numerous reports of parasite resistance to triclabendazole, albendazole and clorsulon in infected livestock are growing in different regions of the world (Kelley et al., 2016; Fairweather et al., 2020), whereas the report of closantel failure (Novobilský and Höglund, 2015) is an additional wakeup call. The list of effective drugs to treat animal fasciolosis and decrease parasite circulation in nature may indeed quickly grow thinner. Even when the availability of treatment, and hence, the selective pressure, is expected to be higher in high-income countries, the worldwide trading of livestock and the F. hepatica–F. gigantica hybridization leave no region out of risk. Treatment failure with triclabendazole in human patients is already being documented in developed (Branco et al., 2020; in Portugal) as well as developing countries (Cabada et al., 2016; in Peru).
Other tendencies must be considered as they might become significant in increasing transmission risks, particularly in high-income regions where environmental education is usually higher. In this sense, future trends of livestock industry, such as those comprising the increase in intensification of pastoral animal production and organic ruminant production, will have positive and negative effects on animal welfare, including disease susceptibility. Here, the balance will be determined by the quality of management and stockmanship, as well as by the pressure on businesses to be profitable (Stafford and Gregory, 2008; Takeuchi-Storm et al., 2019). Concerning infection sources to humans through wild vegetables, whether directly collected from the field or from in-house gardens or acquired in urban markets, the recent drive to ‘go green’ as a healthy approach to the modern lifestyle in today's developed societies poses evident problems as it underlies an increase in the consumption of fresh, raw/green fruits and vegetables (Mas-Coma et al., 2018).
The complexity and heterogeneity of Fasciola spp. transmission make it difficult to know and forecast its burden with certainty across different regions. In addition, four aspects offer serious problems for the analysis of the epidemiological situations of this disease in the different countries; (i) diagnosis is still tricky in both developed and developing countries; (ii) as awareness of the disease in non-endemic areas and/or access to health or sensitive detection methods need to be improved in primary health centres (see e.g. Ramachandran et al., 2012; Perrodin et al., 2019); (iii) fasciolosis' usually mild or unspecific symptomatology; and (iv) the classification of fascioliasis as a non-reportable disease (Mas-Coma et al., 2014, 2018). Regional and international awareness remains fundamentally biased if human infections go unreported, keeping an underestimation of the real situation, about the risky areas and sources for human infection and, with it, deprioritizing much needed multi-sectoral approaches to control transmission.
Concluding remarks
Some zoonotic parasitic infections such as fasciolosis do not enjoy great notoriety among the general population, as they are far less understood and mostly unknown or forgotten. Regardless, they significantly affect or threat not only the poor long-neglected populations but also high-income countries. This review illustrates the different aspects involved in the transmission of fasciolosis and its current increasing burden within veterinary medicine and public health worldwide. It further demonstrates how interconnected biological, environmental and social factors have shaped the epidemiology of human and animal fasciolosis and how the shifting trends in scientific awareness and health policies have been pivotal to recognize the phenomenon of fasciolosis (re-)emergence.
Whereas simplification is usually essential to approach scientific topics, the complexity of fasciolosis and the numerous interacting aspects influencing transmission implies that all the different factors should be always pondered. Hypo-, meso- and hyperendemic regions of animal and human fasciolosis occur even within countries (Mas-Coma et al., 2009a), and such heterogeneity is determined by differences at biological, environmental, social and/or political levels. Considering them all at the view of each epidemiological context would be the best initial approximation to comprehend fasciolosis impact, to forecast parasite circulation and to successfully act upon it. Finally, through the integrative overview of the present review, the bridge among human and animal health and the environment becomes evident and, with it, the necessity to bring all these elements together in a single platform, i.e. One Health approach, to conduct interdisciplinary, collaborative, and socially relevant research and to develop control strategies that efficiently tackle fasciolosis and its (re-)emergence.
Acknowledgements
The authors would like to thank Dr Luis Fonte for insightful comments. We thank Dr Camila Perera for proofreading the manuscript. We are also grateful to the editor and two anonymous reviewers for proper corrections, suggestions and remarks that greatly helped to improve the manuscript.
Financial support
None.
Conflict of interest
None.
References
- Abrous M, Rondelaud D, Dreyfuss G and Cabaret J (1998) Unusual transmission of the liver fluke, Fasciola hepatica, by Lymnaea glabra or Planorbis leucostoma in France. Journal of Parasitology 84, 1257–1259. [PubMed] [Google Scholar]
- Adelman JS and Hawley DM (2017) Tolerance of infection: a role for animal behavior, potential immune mechanisms, and consequences for parasite transmission. Hormones and Behaviour 88, 79–86. [DOI] [PubMed] [Google Scholar]
- Afshan K, Fortes-Lima CA, Artigas P, Valero AM, Qayyum M and Mas-Coma S (2014) Impact of climate change and man-made irrigation systems on the transmission risk, long-term trend and seasonality of human and animal fascioliasis in Pakistan. Geospatial Health 8, 317–334. [DOI] [PubMed] [Google Scholar]
- Aghayan S, Gevorgian H, Ebi D, Atoyan H, Addy F, Mackenstedt U, Romig T and Wassermann M (2019) Fasciola spp. in Armenia: genetic diversity in a global context. Veterinary Parasitology 268, 21–31. [DOI] [PubMed] [Google Scholar]
- Alba A, Hernández H, Marcet R, Vázquez AA, Figueredo M, Sánchez J, Otero O and Sarracent J (2015a) A novel double monoclonal antibody based-immunoenzymatic assay for epidemiological surveillance of the vector snails of Fasciola hepatica (Trematoda: Digenea). International Journal for Parasitology 45, 113–119. [DOI] [PubMed] [Google Scholar]
- Alba A, Vázquez AA, Hernández H, Sánchez J, Marcet R, Figueredo M, Sarracent J and Fraga J (2015b) A multiplex PCR for the detection of Fasciola hepatica in the intermediate snail host Galba cubensis. Veterinary Parasitology 211, 195–200. [DOI] [PubMed] [Google Scholar]
- Alba A, Vázquez AA, Sánchez J, Fraga J, Martínez E, Hernández H, Marcet R, Figueredo M and Sarracent J (2016) Assessment of the FasciMol-ELISA in the detection of the trematode Fasciola hepatica in field-collected Galba cubensis: a novel tool for the malacological survey of fasciolosis transmission. Parasites & Vectors 9, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba A, Vázquez AA, Sánchez J, Duval D, Hernández H, Sabourin E, Vittecoq M, Hurtrez-Boussés S and Gourbal B (2018) Fasciola hepatica–Pseudosuccinea columella interaction: effect of increasing parasite doses, successive exposures and geographic origin on the infection outcome of naturally-resistant and susceptible snails from Cuba. Parasites & Vectors 11, 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba A, Vázquez AA, Sánchez J, Lounnas M, Pointier JP, Hurtrez-Boussès S and Gourbal B (2019) Patterns of distribution, population genetics and ecological requirements of field-occurring resistant and susceptible Pseudosuccinea columella snails to Fasciola hepatica in Cuba. Scientific Reports 9, 14359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba A, Duval D, Sánchez J, Pérez AB, Pinaud S, Galinier R, Vázquez AA and Gourbal B (2020) The immunobiological interplay between Pseudosuccinea columella resistant/susceptible snails with Fasciola hepatica: hemocytes in the spotlight. Developmental and Comparative Immunology 102, 103485. [DOI] [PubMed] [Google Scholar]
- Alda P, Lounnas M, Vázquez AA, Ayaqui R, Calvopiña M, Celi-Erazo M, Dillon RTJ, González Ramírez LC, Loker ES, Muzzio-Aroca J, Nárvaez AO, Noya O, Pereira AE, Martini Robles L, Rodríguez-Hidalgo R, Uribe N, David P, Jarne P, Pointier JP and Hurtrez-Boussès S (2020) Systematics and geographical distribution of Galba species, a group of cryptic and worldwide freshwater snails. Molecular Phylogenetics Evolution. 107035. doi: 10.1016/j.ympev.2020.107035. [DOI] [PubMed]
- Amor N, Farjallah S, Merella P, Alagaili AN and Mohammed OB (2020) Multilocus approach reveals discordant molecular markers and corridors for gene flow between North African populations of Fasciola hepatica. Veterinary Parasitology 278, 109035. [DOI] [PubMed] [Google Scholar]
- Andrews SJ (1999) The life cycle of Fasciola hepatica. In Dalton JP (ed.), Fasciolosis. Oxon, UK: CAB International, pp. 1–30. [Google Scholar]
- Arenal A, García Y, Quesada L, Velázquez D, Sánchez D, Peña M, Suárez A, Díaz A, Sánchez Y, Casaert S, van Dijk J, Vercruysse J and Charlier J (2018) Risk factors for the presence of Fasciola hepatica antibodies in bulk-milk samples and their association with milk production decreases, in Cuban dairy cattle. BMC Veterinary Research 14, 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi K and Mas-Coma S (2014) Fasciola gigantica transmission in the zoonotic fascioliasis endemic lowlands of Guilan, Iran: experimental assessment. Veterinary Parasitology 205, 96–106. [DOI] [PubMed] [Google Scholar]
- Ashrafi K, Valero MA, Peixoto RV, Artigas P, Panova M and Mas-Coma S (2015) Distribution of Fasciola hepatica and F. gigantica in the endemic area of Guilan, Iran: relationships between zonal overlap and phenotypic traits. Infection, Genetics and Evolution 31, 95–109. [DOI] [PubMed] [Google Scholar]
- Badawy A, Abouzaid N and Merwad A (2014) Occurrence of zoonotic fascioliasis in donkeys in Egypt with emphasis on PCR-RFLP of 28S rRNA gene. Revue de Médecine Vétérinaire 165, 167–171. [Google Scholar]
- Beesley NJ, Williams DJ, Paterson S and Hodgkinson J (2017) Fasciola hepatica demonstrates high levels of genetic diversity, a lack of population structure and high gene flow: possible implications for drug resistance. International Journal for Parasitology 47, 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesley NJ, Caminade C, Charlier J, Flynn RJ, Hodgkinson JE, Martinez-Moreno A, Martinez-Valladares M, Perez J, Rinaldi L and Williams DJL (2018) Fasciola and fasciolosis in ruminants in Europe: identifying research needs. Transboundary Emerging Diseases 65, 199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behm CA and Sangster NC (1999) Pathology, pathophysiology and clinical aspects. In Dalton JP (ed.), Fasciolosis. Oxon, UK: CAB International, pp. 185–224. [Google Scholar]
- Bennema SC, Ducheyne E, Vercruysse J, Claerebout E, Hendrickx G and Charlier J (2011) Relative importance of management, meteorological and environmental factors in the spatial distribution of Fasciola hepatica in dairy cattle in a temperate climate zone. International Journal for Parasitology 41, 225–233. [DOI] [PubMed] [Google Scholar]
- Bloemhoff Y, Danaher M, Forbes A, Morgan E, Mulcahy G, Power C and Sayers R (2014) Parasite control practices on pasture-based dairy farms in the Republic of Ireland. Veterinary Parasitology 204, 352–363. [DOI] [PubMed] [Google Scholar]
- Bosco A, Rinaldi L, Musella V, Amadesi A and Cringoli G (2015) Outbreak of acute fasciolosis in sheep farms in a Mediterranean area arising as a possible consequence of climate change. Geospatial Health 9, 319–324. [DOI] [PubMed] [Google Scholar]
- Bossaert K, Jacquinet E, Saunders J, Farnir F and Losson B (2000) Cell-mediated immune response in calves to single-dose, trickle, and challenge infections with Fasciola hepatica. Veterinary Parasitology 88, 17–34. [DOI] [PubMed] [Google Scholar]
- Bozorgomid A, Rouhani S, Harandi M, Ichikawa-Seki M and Raeghi S (2020) Genetic diversity and distribution of Fasciola hepatica haplotypes in Iran: molecular and phylogenetic studies. Veterinary Parasitology: Regional Studies Reports 19, 100359. [DOI] [PubMed] [Google Scholar]
- Branco EA, Ruas R, Nuak J and Sarmento A (2020) Treatment failure after multiple courses of triclabendazole in a Portuguese patient with fascioliasis. BMJ Case Reports 13, e232299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabada MM and White ACJ (2012) New developments in epidemiology, diagnosis, and treatment of fascioliasis. Current Opinion in Infectious Diseases 25, 518–522. [DOI] [PubMed] [Google Scholar]
- Cabada MM, Lopez M, Cruz M, Delgado JR, Hill V and White ACJ (2016) Treatment failure after multiple courses of triclabendazole among patients with fascioliasis in Cusco, Peru: a case series. PLoS Neglected Tropical Diseases 10, e0004361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabada MM, Morales ML, Webb CM, Yang L, Bravenec CA, Lopez M, Bascope R, White AC and Gotuzzo E (2018) Socioeconomic factors associated with Fasciola hepatica infection among children from 26 communities of the Cusco region of Peru. American Journal of Tropical Medicine and Hygiene 99, 1180–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafrune MM, Rebuffi GE, Cabrera RH and Aguirre DH (1996a) Fasciola hepatica en llamas (Lama glama) de la Puna Argentina. Revista Veterinaria Argentina 13, 570–574. [Google Scholar]
- Cafrune MM, Rebuffi GE, Gaido AB and Aguirre DH (1996b) Fasciola hepatica in semi-captive vicuñas (Vicugna vicugna) in north west Argentina. Veterinary Record 139, 97. [DOI] [PubMed] [Google Scholar]
- Calvani NED, Ichikawa-Seki M, Bush RD, Khounsy S and Šlapeta J (2020) Which species is in the faeces at a time of global livestock movements: single nucleotide polymorphism genotyping assays for the differentiation of Fasciola spp. International Journal for Parasitology 50, 91–101. [DOI] [PubMed] [Google Scholar]
- Caminade C, van Dijk J, Baylis M and Williams D (2015) Modelling recent and future climatic suitability for fasciolosis in Europe. Geospatial Health 9, 301–308. [DOI] [PubMed] [Google Scholar]
- Capucchio MT, Catalano D, Di Marco V, Russo M, Aronica V, Tomaselli A, Lazzara A, Amedeo S, Scaglione FE, Dore B and Guarda F (2009) Natural trematode infestation in feral Nebrodi black pigs: pathological investigations. Veterinary Parasitology 159, 37–42. [DOI] [PubMed] [Google Scholar]
- Cardinale BJ, Duffy JE, Gonzalez A, Hooper DU, Perrings C, Venail P, Narwani A, Mace GM, Tilman D, Wardle DA, Kinzig AP, Daily GC, Loreau M, Grace JB, Larigauderie A, Srivastava DS and Naeem S (2012) Biodiversity loss and its impact on humanity. Nature 486, 59–67. [DOI] [PubMed] [Google Scholar]
- Cats A, Scholten P, Meuwissen SG and Kuipers EJ (2000) Acute Fasciola hepatica infection attributed to chewing khat. Gut 47, 584–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chand MA, Herman JS, Partridge DG, Hewitt K and Chiodini PL (2009) Imported human fascioliasis, United Kingdom. Emerging Infectious Diseases 15, 1876–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandenier J, Bastard JP, Merioua A, Combes J and Thiebault C (1989) Première épidémie de distomatose à Fasciola hepatica dans le département de la Somme. Médecine et Maladies Infectieuses 20, 145–147. [Google Scholar]
- Charlier J, De Cat A, Forbes A and Vercruysse J (2009) Measurement of antibodies to gastrointestinal nematodes and liver fluke in meat juice of beef cattle and associations with carcass parameters. Veterinary Parasitology 166, 235–240. [DOI] [PubMed] [Google Scholar]
- Charlier J, Vercruysse J, Morgan E, van Dijk J and Williams DJ (2014) Recent advances in the diagnosis, impact on production and prediction of Fasciola hepatica in cattle. Parasitology 141, 326–335. [DOI] [PubMed] [Google Scholar]
- Chen MG and Mott KE (1990) Progress in assessment of morbidity due to Fasciola hepatica infection. A review of recent literature. Tropical Diseases Bulletin 87, 1–38. [Google Scholar]
- Chen JX, Chen MX, Ai L, Xu XN, Jiao JM, Zhu TJ, Su HY, Zang W, Luo JJ, Guo YH, Lv S and Zhou XN (2013) An outbreak of human Fascioliasis gigantica in southwest China. PLoS ONE 8, e71520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlyeh G, Dodet M, Delay B, Khallaayoune K and Jarne P (2006) Spatio-temporal distribution of freshwater snail species in relation to migration and environmental factors in an irrigated area from Morocco. Hydrobiologia 553, 129–142. [Google Scholar]
- Choi YJ, Fontenla S, Fischer PU, Le TH, Costábile A, Blair D, Brindley PJ, Tort JF, Cabada MM and Mitreva M (2020) Adaptive radiation of the flukes of the family Fasciolidae inferred from genome-wide comparisons of key species. Molecular Biology and Evolution 37, 84–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo PF, Cataldo SD, Fantozzi MC, Deis E, Isenrath GD, Viberti G, Artigas P, Peixoto R, Valero MA, Sierra RM and Mas-Coma S (2015) Liver fluke (Fasciola hepatica) naturally infecting introduced European brown hare (Lepus europaeus) in northern Patagonia: phenotype, prevalence and potential risk. Acta Parasitologica 60, 536–543. [DOI] [PubMed] [Google Scholar]
- Curtale F, Hassanein YA and Savioli L (2005) Control of human fascioliasis by selective chemotherapy: design, cost and effect of the first public health, school-based intervention implemented in endemic areas of the Nile Delta, Egypt. Transactions of the Royal Society of Tropical Medicine and Hygiene 99, 599–609. [DOI] [PubMed] [Google Scholar]
- Curtale F, Hassanein YA, Barduagni P, Yousef MM, Wakeel AE, Hallaj Z and Mas-Coma S (2007) Human fascioliasis infection: gender differences within school-age children from endemic areas of the Nile Delta, Egypt. Transactions of the Royal Society of Tropical Medicine and Hygiene 101, 155–160. [DOI] [PubMed] [Google Scholar]
- Cwiklinski K, Dalton JP, Dufresne PJ, La Course J, Williams DJL, Hodgkinson J and Paterson S (2015) The Fasciola hepatica genome: gene duplication and polymorphism reveals adaptation to the host environment and the capacity for rapid evolution. Genome Biology 16, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cwiklinski K, Jewhurst H, McVeigh P, Barbour T, Maule AG, Tort J, O'Neill SM, Robinson MW, Donnelly S and Dalton JP (2018) Infection by the helminth parasite Fasciola hepatica requires rapid regulation of metabolic, virulence, and invasive factors to adjust to its mammalian host. Molecular & Cellular Proteomics 17, 792–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cwiklinski K, Donnelly S, Drysdale O, Jewhurst H, Smith D, De Marco Verissimo C, Pritsch IC, O'Neill S, Dalton JP and Robinson MW (2019) The cathepsin-like cysteine peptidases of trematodes of the genus Fasciola. Advances in Parasitology 104, 113–164. [DOI] [PubMed] [Google Scholar]
- Dar Y, Vignoles P, Rondelaud D and Dreyfuss G (2004) Larval productivity of Fasciola gigantica in two lymnaeid snails. Journal of Helminthology 78, 215–218. [DOI] [PubMed] [Google Scholar]
- Dar Y, Rondelaud D and Dreyfuss G (2005) Update of fasciolosis-transmitting snails in Egypt (review and comment). Journal of the Egyptian Society of Parasitology 35, 477–490. [PubMed] [Google Scholar]
- Dar Y, Vignoles P, Rondelaud D and Dreyfuss G (2015) Role of the lymnaeid snail Pseudosuccinea columella in the transmission of the liver fluke Fasciola hepatica in Egypt. Journal of Helminthology 89, 699–706. [DOI] [PubMed] [Google Scholar]
- David AR (1997) Disease in Egyptian mummies: the contribution of new technologies. Lancet (London, England) 349, 1760–1763. [DOI] [PubMed] [Google Scholar]
- Davis CN, Tyson F, Cutress D, Davies E, Jones DL, Brophy PM, Prescott A, Rose MT, Williams M, Williams HW and Jones RA (2020) Rapid detection of Galba truncatula in water sources on pasture-land using loop-mediated isothermal amplification for control of trematode infections. Parasites & Vectors 13, 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brie J (1379) Le Bon Berger. https://gallica.bnf.fr/ark:/12148/bpt6k54694869.
- De Kock KN, Wolmarans CT and Bornman M (2003) Distribution and habitats of the snail Lymnaea truncatula, intermediate host of the liver fluke Fasciola hepatica, in South Africa. Journal of the South African Veterinary Association 74, 117–122. [DOI] [PubMed] [Google Scholar]
- Diaw OT, Vassiliades G, Seye M and Sarr Y (1990) Prolifération des mollusques et incidence sur les trématodoses dans la région du delta et du lac de Guiers après la construction du barrage de Diama sur le fleuve Sénégal. Revue d'Elevage et de Médecine Vétérinaire des Pays Tropicaux 43, 499–502. [Google Scholar]
- Dida GO, Gelder FB, Anyona DN, Matano AS, Abuom PO, Adoka SO, Ouma C, Kanangire CK, Owuor PO and Ofulla AV (2014) Distribution and abundance of schistosomiasis and fascioliasis host snails along the Mara River in Kenya and Tanzania. Infection Ecology and Epidemiology 4, 24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maggio LS, Tirloni L, Pinto AF, Diedrich JK, Yates Iii JR, Benavides U, Carmona C, da Silva Vaz IJ and Berasain P (2016) Across intra-mammalian stages of the liver fluke Fasciola hepatica: a proteomic study. Scientific Reports 6, 32796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinnik JA and Dinnik NN (1961) On the morphology, and life history of Fasciola nyanzae Leiper, 1910 from the hippopotamus. Journal of Helminthology (Suppl.), 53–62. [DOI] [PubMed] [Google Scholar]
- Dittmar K (2002) Arthropod and helminth parasites of the wild guinea pig, Cavia aperea, from the Andes and the cordillera in Peru, South America. Journal of Parasitology 88, 409–411. [DOI] [PubMed] [Google Scholar]
- Doherty JF, Price N, Moody AH, Wright SG and Glynn MJ (1995) Fascioliasis due to imported khat. Lancet (London, England) 345, 462. [DOI] [PubMed] [Google Scholar]
- Donnelly S, Stack CM, O'Neill SM, Sayed AA, Williams DL and Dalton JP (2008) Helminth 2-Cys peroxiredoxin drives Th2 responses through a mechanism involving alternatively activated macrophages. FASEB Journal 22, 4022–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dracz RM, Ribeiro VM, Pereira CA and Lima W (2016) Occurrence of Fasciola hepatica (Linnaeus, 1758) in capybara (Hydrochoerus hydrochaeris) (Linnaeus, 1766) in Minas Gerais, Brazil. Revista Brasileira de Parasitologia Veterinária 25, 364–367. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G and Rondelaud D (1997) Fasciola gigantica and F. hepatica: a comparative study of some characteristics of Fasciola infection in Lymnaea truncatula infected by either of the two trematodes. Veterinary Research 28, 123–130. [PubMed] [Google Scholar]
- Dreyfuss G and Rondelaud D (2008) Biodiversity of flukes. Parasite 15, 282–285. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G, Vignoles P and Rondelaud D (2003) Natural infection of Omphiscola glabra (Lymnaeidae) with Fasciola hepatica in central France. Parasitology Research 91, 458–461. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G, Vignoles P and Rondelaud D (2016) Pseudosuccinea columella: experimental co-infections of juvenile and pre-adult snails with the digeneans Calicophoron daubneyi and Fasciola hepatica. Journal of Helminthology 90, 753–759. [DOI] [PubMed] [Google Scholar]
- Elliott T, Muller A, Brockwell Y, Murphy N, Grillo V, Toet H, Anderson G, Sangster N and Spithill T (2014) Evidence for high genetic diversity of NAD1 and COX1 mitochondrial haplotypes among triclabendazole resistant and susceptible populations and field isolates of Fasciola hepatica (Liver fluke) in Australia. Veterinary Parasitology 200, 90–96. [DOI] [PubMed] [Google Scholar]
- Escamilla A, Zafra R, Pérez J, McNeilly TN, Pacheco IL, Buffoni L, Martínez-Moreno FJ, Molina-Hernández V and Martínez-Moreno A (2016) Distribution of Foxp3+ T cells in the liver and hepatic lymph nodes of goats and sheep experimentally infected with Fasciola hepatica. Veterinary Parasitology 230, 14–19. [DOI] [PubMed] [Google Scholar]
- Espino AM, Díaz A, Pérez A and Finlay CM (1998) Dynamics of antigenemia and coproantigens during a human Fasciola hepatica outbreak. Journal of Clinical Microbiology 36, 2723–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza JR, Maco V, Marcos L, Saez S, Neyra V, Terashima A, Salmavides F, Gotuzzo E, Chavarry E, Huaman MC, Bargues MD, Valero MA and Mas-Coma S (2007) Evaluation of Fas2-ELISA for the serological detection of Fasciola hepatica infection in humans. American Journal of Tropical Medicine and Hygiene 76, 977–982. [PubMed] [Google Scholar]
- Esteban JG, Flores A, Angles R and Mas-Coma S (1999) High endemicity of human fascioliasis between Lake Titicaca and La Paz valley, Bolivia. Transactions of the Royal Society of Tropical Medicine and Hygiene 93, 151–156. [DOI] [PubMed] [Google Scholar]
- Esteban JG, Gonzalez C, Bargues MD, Angles R, Sanchez C, Naquira C and Mas-Coma S (2002) High fascioliasis infection in children linked to a man-made irrigation zone in Peru. Tropical Medicine & International Health 7, 339–348. [DOI] [PubMed] [Google Scholar]
- Esteban JG, Gonzalez C, Curtale F, Muñoz-Antoli C, Valero MA, Bargues MD, Mabrouk ES, el-Wakeel AA, Abdel-Wahab Y, Montresor A, Engels D, Savioli L and Mas-Coma S (2003) Hyperendemic fascioliasis associated with schistosomiasis in villages in the Nile Delta of Egypt. American Journal of Tropical Medicine and Hygiene 69, 429–437. [PubMed] [Google Scholar]
- Fairweather I, Brennan GP, Hanna REB, Robinson MW and Skuce PJ (2020) Drug resistance in liver flukes. International Journal for Parasitology: Drugs and Drug Resistance 12, 39–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer J, Perera G, Yong M and Amador O (1989) Estudios de densidad de hospederos intermediarios de enfermedades tropicales en berreras. Revista Cubana de Medicina Tropical 41, 341–354. [PubMed] [Google Scholar]
- Figtree M, Beaman MH, Lee R, Porter M, Torey E, Hugh TH and Hudson BJ (2015) Fascioliasis in Australian travellers to Bali. Medical Journal of Australia 203, 186–188. [DOI] [PubMed] [Google Scholar]
- Fiss L, de Lourdes Adrien M, Marcolongo-Pereira C, Assis-Brasil ND, Sallis ES, Riet-Correa F, Ruas JL and Schild AL (2013) Subacute and acute fasciolosis in sheep in southern Brazil. Parasitology Research 112, 883–887. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization (2020) Food and agriculture data. http://faostat.fao.org/static/syb/syb_5000.pdf.
- Foreyt WJ (2009) Experimental infection of bighorn sheep with liver flukes (Fasciola hepatica). Journal of Wildlife Diseases 45, 1217–1220. [DOI] [PubMed] [Google Scholar]
- Foreyt WJ and Drew ML (2010) Experimental infection of liver flukes, Fasciola hepatica and Fascioloides magna, in bison (Bison bison). Journal of Wildlife Diseases 46, 283–286. [DOI] [PubMed] [Google Scholar]
- Fox NJ, White PC, McClean CJ, Marion G, Evans A and Hutchings MR (2011) Predicting impacts of climate change on Fasciola hepatica risk. PLoS ONE 6, e16126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried B and Abruzzi A (2010) Food-borne trematode infections of humans in the United States of America. Parasitology Research 106, 1263–1280. [DOI] [PubMed] [Google Scholar]
- Fuentes MV, Sainz-Elipe S, Nieto P, Malone JB and Mas-Coma S (2005) Geographical information systems risk assessment models for zoonotic fascioliasis in the South American Andes region. Parasitologia 47, 151–156. [PubMed] [Google Scholar]
- Gaillet P (1983) Contribution à l’étude épidémiologique de la distomatose humaine à Fasciola hepatica en France métropolitaine depuis 1956. À propos de quelque 10.000 cas (MD thesis). Université Paris–Val-de-Marne, France, 163 pp. [Google Scholar]
- Garcia JA, Martin AM, Fernandez JM and Garcia EJ (1985) Fascioliasis in Spain: a review of the literature and personal observations. European Journal of Epidemiology 1, 121–126. [DOI] [PubMed] [Google Scholar]
- Gasnier N, Rondelaud D, Abrous M, Carreras F, Boulard C, Diez-Baños P and Cabaret J (2000) Allopatric combination of Fasciola hepatica and Lymnaea truncatula is more efficient than sympatric ones. International Journal for Parasitology 30, 573–578. [DOI] [PubMed] [Google Scholar]
- Gauly M and Ammer S (2020) Review: challenges for dairy cow production systems arising from climate changes. Animal: An International Journal of Animal Bioscience 14, s196–s203. [DOI] [PubMed] [Google Scholar]
- Goumghar MD, Vignoles P, Rondelaud D, Dreyfuss G and Benlemlih M (2001) Relationships between the annual generations of the snail Lymnaea trunculata (Mollusca Gastropoda: Lymnaeidae), altitude and the type of its habitats in central Morocco. Revue de Médecine Vétérinaire 152, 457–462. [Google Scholar]
- Grabner DS, Mohamed FA, Nachev M, Méabed EM, Sabry AH and Sures B (2014) Invasion biology meets parasitology: a case study of parasite spill-back with Egyptian Fasciola gigantica in the invasive snail Pseudosuccinea columella. PLoS ONE 9, e88537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff A, Bennion-Pedley E, Jones AK, Ledger ML, Deforce K, Degraeve A, Byl S and Mitchell PD (2020) A comparative study of parasites in three latrines from Medieval and Renaissance Brussels, Belgium (14th-17th centuries). Parasitology 147, 1443–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray GD, Copland RS and Copeman DB (2008) Overcoming Liver Fluke as a Constraint to Ruminant Production in South-East Asia, vol 133. Canberra, Australia: Australian Centre for International Agricultural Research, 155 pp. [Google Scholar]
- Greter H, Batil AA, Alfaroukh IO, Grimm F, Ngandolo BN, Keiser J, Utzinger J, Zinsstag J and Hattendorf J (2016) Re-infection with Fasciola gigantica 6-month post-treatment with triclabendazole in cattle from mobile pastoralist husbandry systems at Lake Chad. Veterinary Parasitology 230, 43–48. [DOI] [PubMed] [Google Scholar]
- Gryseels G (1988) Role of Livestock on Mixed Smallholder Farms in the Ethiopian Highlands. A Case Study from the Baso and Worena Wereda near Debre Berhan. Wageningen, The Netherlands: Agricultural University. [Google Scholar]
- Gupta SC and Singh BP (2002) Fasciolosis in cattle and buffaloes in India. Journal of Veterinary Parasitology 16, 139–145. [Google Scholar]
- Haamond JA (1972) Infections with Fasciola spp. in wildlife in Africa. Tropical Animal Health and Production 4, 1–13. [DOI] [PubMed] [Google Scholar]
- Hamed N, Hammami H, Khaled S, Rondelaud D and Ayadi A (2009) Natural infection of Fasciola hepatica (Trematoda: Fasciolidae) in Bulinus truncatus (Gastropoda: Planorbidae) in northern Tunisia. Journal of Helminthology 83, 271–273. [DOI] [PubMed] [Google Scholar]
- Hammami H and Ayadi A (1999) Écologie de Lymnaea truncatula Müller,hôte intermédiaire de Fasciola hepatica Linné dans le microclimat de Tozeur (sud-ouest de la Tunisie). Bulletin de la Société de Pathologie Exotique 92, 302–304. [PubMed] [Google Scholar]
- Hammami H, Hamed N and Ayadi A (2007) Epidemiological studies on Fasciola hepatica in Gafsa Oases (south west of Tunisia). Parasite 14, 261–264. [DOI] [PubMed] [Google Scholar]
- Haridy FM and Morsy TA (2000) Camel: a new Egyptian host for Fasciola gigantica. Journal of the Egyptian Society of Parasitology 30, 451–454. [PubMed] [Google Scholar]
- Haridy FM, Morsy TA, Gawish NI, Antonios TN and Abdel Gawad AG (2002) The potential reservoir role of donkeys and horses in zoonotic fascioliasis in Gharbia Governorate, Egypt. Journal of the Egyptian Society of Parasitology 32, 561–570. [PubMed] [Google Scholar]
- Hayashi K, Ichikawa-Seki M, Allamanda P, Wibowo PE, Mohanta UK, Sodirun, Guswanto A and Nishikawa Y (2016) Molecular characterization and phylogenetic analysis of Fasciola gigantica from western Java, Indonesia. Parasitology International 65, 424–427. [DOI] [PubMed] [Google Scholar]
- Haydock LAJ, Pomroy WE, Stevenson MA and Lawrence KE (2016) A growing degree-day model for determination of Fasciola hepatica infection risk in New Zealand with future predictions using climate change models. Veterinary Parasitology 228, 52–59. [DOI] [PubMed] [Google Scholar]
- Heneberg P (2013) Phylogenetic data suggest the reclassification of Fasciola jacksoni (Digenea: Fasciolidae) as Fascioloides jacksoni comb. nov. Parasitology Research 112, 1679–1689. [DOI] [PubMed] [Google Scholar]
- Hodgkinson JE, Cwiklinski K, Beesley N, Hartley C, Allen K and Williams DJL (2018) Clonal amplification of Fasciola hepatica in Galba truncatula: within and between isolate variation of triclabendazole-susceptible and -resistant clones. Parasites & Vectors 11, 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins DR (1992) Homing in on helminths. American Journal of Tropical Medicine and Hygiene 46, 626–634. [DOI] [PubMed] [Google Scholar]
- Hosseini G, Sarkari B, Moshfe A, Motazedian MH and Abdolahi Khabisi S (2015) Epidemiology of human fascioliasis and intestinal helminthes in rural areas of Boyer-Ahmad Township, southwest Iran: a population based study. Iranian Journal of Public Health 44, 1520–1525. [PMC free article] [PubMed] [Google Scholar]
- Hotez PJ, Savioli L and Fenwick A (2012) Neglected tropical diseases of the Middle East and North Africa: review of their prevalence, distribution, and opportunities for control. PLoS Neglected Tropical Diseases 6, e1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AJ, Spithill TW, Smith RE, Boutlis CS and Johnson PD (2003) Human fasciolosis acquired in an Australian urban setting. Medical Journal of Australia 178, 244–245. [DOI] [PubMed] [Google Scholar]
- Hurtrez-Boussès S, Durand P, Jabbour-Zahab R, Guégan J, Meunier C, Bargues M, Mas-Coma S and Renaud F (2004) Isolation and characterization of microsatellite markers in the liver fluke (Fasciola hepatica). Molecular Ecology 4, 689–690. [Google Scholar]
- Hurtrez-Boussès S, Hurtrez JE, Turpin H, Durand C, Durand P, De Meeüs T, Meunier C and Renaud F (2010) Hydrographic network structure and population genetic differentiation in a vector of fasciolosis, Galba truncatula. Infection, Genetics and Evolution 10, 178–183. [DOI] [PubMed] [Google Scholar]
- Husch C, Sattmann H, Haefeli I, Prosl H and Walochnik J (2020) Genetic diversity of Fasciola hepatica in Austria. Parasitology Research, 119, 1697–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa-Seki M, Shiroma T, Kariya T, Nakao R, Ohari Y, Hayashi K and Fukumoto S (2017a) Molecular characterization of Fasciola flukes from wild sika deer and domestic cattle in Hokkaido, Japan. Parasitology International 66, 519–521. [DOI] [PubMed] [Google Scholar]
- Ichikawa-Seki M, Tokashiki M, Opara MN, Iroh G, Hayashi K, Kumar UM and Itagaki T (2017b) Molecular characterization and phylogenetic analysis of Fasciola gigantica from Nigeria. Parasitology International 66, 893–897. [DOI] [PubMed] [Google Scholar]
- Issia L, Pietrokovsky S, Sousa-Figueiredo J, Stothard JR and Wisnivesky-Colli C (2009) Fasciola hepatica infections in livestock flock, guanacos and coypus in two wildlife reserves in Argentina. Veterinary Parasitology 165, 341–344. [DOI] [PubMed] [Google Scholar]
- Itagaki T, Sakamoto T, Tsutsumi Y and Itagaki H (1994) Infectivity of three species of Fasciola to Wistar rats. Journal of Veterinary and Medical Science 56, 977–979. [DOI] [PubMed] [Google Scholar]
- Itagaki T, Ichinomiya M, Fukuda K, Fusyuku S and Carmona C (2011) Hybridization experiments indicate incomplete reproductive isolating mechanism between Fasciola hepatica and Fasciola gigantica. Parasitology 138, 1278–1284. [DOI] [PubMed] [Google Scholar]
- Jajaa IF, Mushongab B, Greenc E and Muchenje V (2017) Seasonal prevalence, body condition score and risk factors of bovine fasciolosis in South Africa. Veterinary and Animal Science 4, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins DJ, Baker A, Porter M, Shamsi S and Barton DP (2020) Wild fallow deer (Dama dama) as definitive hosts of Fasciola hepatica (liver fluke) in alpine New South Wales. Australian Veterinary Journal 98, 546–549. [DOI] [PubMed] [Google Scholar]
- John BC, Davies DR, Williams DJL and Hodgkinson JE (2019) A review of our current understanding of parasite survival in silage and stored forages, with a focus on Fasciola hepatica metacercariae. Grass and Forage Science 74, 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John BC, Davies DR, Howell AK, Williams DJL and Hodgkinson JE (2020) Anaerobic fermentation results in loss of viability of Fasciola hepatica metacercariae in grass silage. Veterinary Parasitology 285, 109218. [DOI] [PubMed] [Google Scholar]
- Jones RA, Brophy PM, Davis CN, Davies TE, Emberson H, Rees Stevens P and Williams HW (2018) Detection of Galba truncatula, Fasciola hepatica and Calicophoron daubneyi environmental DNA within water sources on pasture land, a future tool for fluke control? Parasites & Vectors 11, 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaset C, Eursitthichai V, Vichasri-Grams S, Viyanant V and Grams R (2010) Rapid identification of lymnaeid snails and their infection with Fasciola gigantica In Thailand. Experimental Parasitology 126, 482–488. [DOI] [PubMed] [Google Scholar]
- Kaya M, Beştaş R and Çetin S (2011) Clinical presentation and management of Fasciola hepatica infection: single-center experience. World Journal of Gastroenterology 17, 4899–4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley JM, Elliott TP, Beddoe T, Anderson G, Skuce P and Spithill TW (2016) Current threat of triclabendazole resistance in Fasciola hepatica. Trends in Parasitology 32, 458–469. [DOI] [PubMed] [Google Scholar]
- Kelley JM, Rathinasamy V, Elliott TP, Rawlin G, Beddoe T, Stevenson MA and Spithill TW (2020) Determination of the prevalence and intensity of Fasciola hepatica infection in dairy cattle from six irrigation regions of Victoria, South-eastern Australia, further identifying significant triclabendazole resistance on three properties. Veterinary Parasitology 277, 109019. [DOI] [PubMed] [Google Scholar]
- Khan MK, Sajid MS, Khan MN, Iqbal Z and Iqbal MU (2009) Bovine fasciolosis: prevalence, effects of treatment on productivity and cost benefit analysis in five districts of Punjab, Pakistan. Research in Veterinary Science 87, 70–75. [DOI] [PubMed] [Google Scholar]
- Khan I, Khan AM, Ayaz S, Khan S, Anees M and Khan SA (2012) Molecular detection of Fasciola hepatica in water sources of District Nowshehra Khyber Pakhtunkhwa, Pakistan. International Journal of Advanced Research and Technology 1, 106–117. [Google Scholar]
- Khan MAH, Shareef PAA, Rehman A, Ullah R, Rehman L and Abidi SMA (2017) Genotoxic potential of Fasciola gigantica infection in experimentally infected rabbits. Journal of Parasitic Diseases 41, 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KC and Lively CM (2012) Does genetic diversity limit disease spread in natural host populations? Heredity 109, 199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiziewicz B (2013) Natural infection with Fasciola hepatica (Linnaeus, 1758) in the European bison (Bison bonasus) in Białowieża National Park, Poland. Helminthologia 50, 167–171. [Google Scholar]
- Kleiman F, Pietrokovsky S, Prepelitchi L, Carbajo AE and Wisnivesky-Colli C (2007) Dynamics of Fasciola hepatica transmission in the Andean Patagonian valleys, Argentina. Veterinary Parasitology 145, 274–286. [DOI] [PubMed] [Google Scholar]
- Knubben-Schweizer G and Torgerson PR (2015) Bovine fasciolosis: control strategies based on the location of Galba truncatula habitats on farms. Veterinary Parasitology 208, 77–83. [DOI] [PubMed] [Google Scholar]
- Knubben-Schweizer G, Rüegg S, Torgerson PR, Rapsch C, Grimm F, Hässig M, Deplazes P and Braun U (2010) Control of bovine fasciolosis in dairy cattle in Switzerland with emphasis on pasture management. Veterinary Parasitology 186, 188–191. [DOI] [PubMed] [Google Scholar]
- Loeurng V, Ichikawa-Seki M, Wannasan A, Sothyra T, Chaisowwong W and Tiwananthagorn S (2019) Genetic characterization of Cambodian Fasciola gigantica and dispersal direction of the species in Asia. Veterinary Parasitology 273, 45–51. [DOI] [PubMed] [Google Scholar]
- Lounnas M, Correa AC, Vázquez AA, Dia A, Escobar JS, Nicot A, Arenas J, Ayaqui R, Dubois MP, Gimenez T, Gutiérrez A, González-Ramírez C, Noya O, Prepelitchi L, Uribe N, Wisnivesky-Colli C, Yong M, David P, Loker ES, Jarne P, Pointier JP and Hurtrez-Boussès S (2017) Self-fertilization, long-distance flash invasion and biogeography shape the population structure of Pseudosuccinea columella at the worldwide scale. Molecular Ecology 26, 887–903. [DOI] [PubMed] [Google Scholar]
- Luzón-Peña M, Rojo-Vázquez FA and Gómez-Bautista M (1994) The overwintering of eggs, intramolluscal stages and metacercariae of Fasciola hepatica under the temperatures of a Mediterranean area (Madrid, Spain). Veterinary Parasitology 55, 143–148. [DOI] [PubMed] [Google Scholar]
- Macpherson CN (2005) Human behaviour and the epidemiology of parasitic zoonoses. International Journal for Parasitology 35, 1319–1331. [DOI] [PubMed] [Google Scholar]
- Magnanou E and Morand S (2006) Insularity and micromammals-macroparasites relationships In Morand S, Krasnov BR and Poulin R (eds), Micromammals and Macroparasites: From Evolutionary Ecology to Management. Tokyo, Japan: Springer-Verlag, pp. 295–315. [Google Scholar]
- Mahato SN and Harrison LJ (2005) Control of fasciolosis in stall-fed buffaloes by managing the feeding of rice straw. Tropical Animal Health and Production 37, 285–291. [DOI] [PubMed] [Google Scholar]
- Mailles A, Capek I, Ajana F, Schepens C, Ilef D and Vaillant V (2006) Commercial watercress as an emerging source of fascioliasis in Northern France in 2002: results from an outbreak investigation. Epidemiology and Infection 134, 942–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatji MP and Mukaratirwa S (2019) Molecular detection of natural infection of Lymnaea (Pseudosuccinea) columella (Gastropoda: Lymnaeidae) with Fasciola gigantica (Digenea: Fasciolidae) from two provinces of South Africa. Journal of Helminthology 94, e38. [DOI] [PubMed] [Google Scholar]
- Malatji MP, Pfukenyi DM and Mukaratirwa S (2019) Fasciola species and their vertebrate and snail intermediate hosts in East and Southern Africa: a review. Journal of Helminthology 94, e63. [DOI] [PubMed] [Google Scholar]
- Malone JB, Gommes R, Hansen J, Yilma JM, Slingenberg J, Snijders F, Nachtergaele F and Ataman E (1998) A Geographic Information System on the potential distribution and abundance of Fasciola hepatica and F. gigantica in East Africa based on Food and Agriculture Organization databases. Veterinary Parasitology 78, 87–101. [DOI] [PubMed] [Google Scholar]
- Martínez-Díaz RA, Martella MB, Navarro JL and Ponce-Gordo F (2013) Gastrointestinal parasites in greater rheas (Rhea americana) and lesser rheas (Rhea pennata) from Argentina. Veterinary Parasitology 194, 75–78. [DOI] [PubMed] [Google Scholar]
- Martínez-Valladares M and Rojo-Vázquez FA (2016) Loop-mediated isothermal amplification (LAMP) assay for the diagnosis of fasciolosis in sheep and its application under field conditions. Parasites & Vectors 9, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas-Coma S, Fons R, Feliu C, Bargues MD, Valero MA and Galan Puchades MT (1988) Small mammals as natural definitive hosts of the liver fluke, Fasciola hepatica Linnaeus, 1758 (Trematoda: Fasciolidae): a review and two new records of epidemiologic interest on the Island of Corsica. Rivista di Parassitologia 49, 73–78. [Google Scholar]
- Mas-Coma S, Anglés R, Esteban JG, Bargues MD, Buchon P, Franken M and Strauss W (1999) The Northern Bolivian Altiplano: a region highly endemic for human fascioliasis. Tropical Medicine & International Health 4, 454–467. [DOI] [PubMed] [Google Scholar]
- Mas-Coma S, Bargues MD and Valero MA (2009a) Chapter 2. Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Advances in Parasitology 69, 41–146. [DOI] [PubMed] [Google Scholar]
- Mas-Coma S, Valero MA and Bargues MD (2009b) Climate change effects on trematodiases, with emphasis on zoonotic fascioliasis and schistosomiasis. Veterinary Parasitology 163, 264–280. [DOI] [PubMed] [Google Scholar]
- Mas-Coma S, Bargues MD and Valero MA (2014) Diagnosis of human fascioliasis by stool and blood techniques: update for the present global scenario. Parasitology 141, 1918–1946. [DOI] [PubMed] [Google Scholar]
- Mas-Coma S, Bargues MD and Valero MA (2018) Human fascioliasis infection sources, their diversity, incidence factors, analytical methods and prevention measures. Parasitology 145, 1665–1699. [DOI] [PubMed] [Google Scholar]
- Mas-Coma S, Valero MA and Bargues MD (2019) Fascioliasis. Advances in Experimental Medicine and Biology 1154, 71–103. [DOI] [PubMed] [Google Scholar]
- McGarry DK and Shackleton CM (2009) Children navigating rural poverty: rural children's use of wild resources to counteract food insecurity in the Eastern Cape, South Africa. Journal of Children and Poverty 15, 19–37. [Google Scholar]
- McMahon C, Edgar HW, Hanna RE, Ellison SE, Flanagan AM, McCoy M, Kajugu PE, Gordon AW, Irwin D, Barley JE, Malone FE, Brennan GP and Fairweather I (2016) Liver fluke control on sheep farms in Northern Ireland: a survey of changing management practices in relation to disease prevalence and perceived triclabendazole resistance. Veterinary Parasitology 216, 72–83. [DOI] [PubMed] [Google Scholar]
- McNulty SN, Tort JF, Rinaldi G, Fischer K, Rosa BA, Smircich P, Fontenla S, Choi YJ, Tyagi R, Hallsworth-Pepin K, Mann VH, Kammili L, Latham P, Dell'Oca N, Dominguez F, Carmona C, Fischer PU, Brindley PJ and Mitreva M (2017) Genomes of Fasciola hepatica from the Americas reveal colonization with Neorickettsia endobacteria related to the agents of Potomac horse and human Sennetsu fevers. PLoS Genetics 13, e1006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmood K, Zhang H, Sabir AJ, Abbas RZ, Ijaz M, Durrani AZ, Saleem MH, Ur Rehman M, Iqbal MK, Wang Y, Ahmad HI, Abbas T, Hussain R, Ghori MT, Ali S, Khan AU and Li J (2017) A review on epidemiology, global prevalence and economical losses of fasciolosis in ruminants. Microbial Pathogenesis 109, 253–262. [DOI] [PubMed] [Google Scholar]
- Ménard A, L'Hostis M, Leray G, Marchandeau S, Pascal M, Roudot N, Michel V and Chauvin A (2000) Inventory of wild rodents and lagomorphs as natural hosts of Fasciola hepatica on a farm located in a humid area in Loire Atlantique (France). Parasite 7, 77–82. [DOI] [PubMed] [Google Scholar]
- Mera y Sierra R, Agramunt VH, Cuervo P and Mas-Coma S (2011) Human fascioliasis in Argentina: retrospective overview, critical analysis and baseline for future research. Parasites & Vectors 4, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshgi B, Karimi A and Shayan P (2008) Genetic variation of Fasciola hepatica from sheep, cattle and buffalo. Research Journal of Parasitology 3, 71–78. [Google Scholar]
- Meshgi B, Majidi-Rad M, Hanafi-Bojd AA and Fathi S (2019) Ecological niche modeling for predicting the habitat suitability of fascioliasis based on maximum entropy model in southern Caspian Sea littoral, Iran. Acta Tropica 198, 105079. [DOI] [PubMed] [Google Scholar]
- Meunier C, Tirard C, Hurtrez-Boussès S, Durand P, Bargues MD, Mas-Coma S, Pointier JP, Jourdane J and Renaud F (2001) Lack of molluscan host diversity and the transmission of an emerging parasitic disease in Bolivia. Molecular Ecology 10, 1333–1340. [DOI] [PubMed] [Google Scholar]
- Mezo M, González-Warleta M, Carro C and Ubeira FM (2004) An ultrasensitive capture ELISA for detection of Fasciola hepatica coproantigens in sheep and cattle using a new monoclonal antibody (MM3). Journal of Parasitology 90, 845–852. [DOI] [PubMed] [Google Scholar]
- Mezo M, González-Warleta M, Castro-Hermida JA, Manga-González MY, Peixoto R, Mas-Coma S and Valero MA (2013) The wild boar (Sus scrofa Linnaeus, 1758) as secondary reservoir of Fasciola hepatica in Galicia (NW Spain). Veterinary Parasitology 198, 274–283. [DOI] [PubMed] [Google Scholar]
- Milas S, Rossi C, Philippart I, Dorny P and Bottieau E (2020) Autochthonous human fascioliasis, Belgium. Emerging Infectious Diseases 26, 155–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadamizad Z, Hosseini-Safa A, Mohebali M, Heydarian P, Aryaeipour M and Rokni MB (2020) Specific detection of Fasciola hepatica and F. gigantica in infected domesticated animals using high-resolution melting analysis (HRM). Iranian Journal of Public Health 49, 521–529. [Google Scholar]
- Molento MB, Bennema S, Bertot J, Pritsch IC and Arenal A (2018) Bovine fascioliasis in Brazil: economic impact and forecasting. Veterinary Parasitology: Regional Studies Report 12, 1–3. [DOI] [PubMed] [Google Scholar]
- Motran CC, Silvane L, Chiapello LS, Theumer MG, Ambrosio LF, Volpini X, Celias D and Cervi L (2018) Helminth infections: recognition and modulation of the immune response by innate immune cells. Frontiers in Immunology 9, 664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy G, O'Connor F, Clery D, Hogan SF, Dowd AJ, Andrews SJ and Dalton JP (1999) Immune responses of cattle to experimental anti-Fasciola hepatica vaccines. Research in Veterinary Science 67, 27–33. [DOI] [PubMed] [Google Scholar]
- Munita MP, Rea R, Martinez-Ibeas AM, Byrne N, McGrath G, Munita-Corbalan LE, Sekiya M, Mulcahy G and Sayers RG (2019) Liver fluke in Irish sheep: prevalence and associations with management practices and co-infection with rumen fluke. Parasites & Vectors 12, 525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyra V, Chavarry E and Espinoza JR (2002) Cysteine proteinases Fas1 and Fas2 are diagnostic markers for Fasciola hepatica infection in alpacas (Lama pacos). Veterinary Parasitology 105, 21–32. [DOI] [PubMed] [Google Scholar]
- Nguyen TG, Le TH, Dao TH, Tran TL, Praet N, Speybroeck N, Vercruysse J and Dorny P (2011) Bovine fasciolosis in the human fasciolosis hyperendemic Binh Dinh province in Central Vietnam. Acta Tropica 117, 19–22. [DOI] [PubMed] [Google Scholar]
- Nguyen ST, Nguyen DT, Van Nguyen T, Huynh VV, Le DQ, Fukuda Y and Nakai Y (2012) Prevalence of Fasciola in cattle and of its intermediate host Lymnaea snails in central Vietnam. Tropical Animal Health and Production 44, 1847–1853. [DOI] [PubMed] [Google Scholar]
- Nguyen NT, Le TC, Vo MDC, Van Cao H, Nguyen LT, Ho KT, Nguyen QN, Tran VQ and Matsumoto Y (2017) High prevalence of cattle fascioliasis in coastal areas of Thua Thien Hue province, Vietnam. Journal of Veterinary and Medical Science 79, 1035–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novobilský A and Höglund J (2015) First report of closantel treatment failure against Fasciola hepatica in cattle. International Journal for Parasitology: Drugs & Drug Resistance 5, 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novobilský A, Kašný M, Beran L, Rondelaud D and Höglund J (2013) Lymnaea palustris and Lymnaea fuscus are potential but uncommon intermediate hosts of Fasciola hepatica in Sweden. Parasites & Vectors 6, 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyindo M and Lukambagire AH (2015) Fascioliasis: an ongoing zoonotic trematode infection. BioMed Research International 2015, 786195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyirenda SS, Sakala M, Moonde L, Kayesa E, Fandamu P, Banda F and Sinkala Y (2019) Prevalence of bovine fascioliasis and economic impact associated with liver condemnation in abattoirs in Mongu district of Zambia. BMC Veterinary Research 15, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill SM, Brady MT, Callanan JJ, Mulcahy G, Joyce P, Mills KH and Dalton JP (2000) Fasciola hepatica infection downregulates Th1 responses in mice. Parasite Immunology 22, 147–155. [DOI] [PubMed] [Google Scholar]
- Orozco MM, Argibay HD, Minatel L, Guillemi EC, Berra Y, Schapira A, Di Nucci D, Marcos A, Lois F, Falzone M and Farber MD (2020) A participatory surveillance of marsh deer (Blastocerus dichotomus) morbidity and mortality in Argentina: first results. BMC Veterinary Research 16, 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Over HJ, Jansen J and van Olm PW (1992. Distribution of Helminth Diseases of Livestock in Developing Countries. Rome: FAO Animal Production and Health Paper; 96
- Parkinson M, O'Neill SM and Dalton JP (2007) Endemic human fasciolosis in the Bolivian Altiplano. Epidemiology and Infection 135, 669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson M, Dalton JP and O'Neill SM (2011) Fasciolosis. In Palmer SR, Soulsby L, Torgerson P and Brown DWG (eds), Oxford Textbook of Zoonoses: Biology, Clinical Practice, and Public Health Control, 2nd Edn. Oxford, UK: Oxford University Press, pp. 864–872. [Google Scholar]
- Perrodin S, Walti L, Gottstein B, Kim-Fuchs C, Candinas D and Banz V (2019) Fasciola hepatica in a country of low incidence: a tricky diagnosis. Hepatobiliary Surgery and Nutrition 8, 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfukenyi DM, Mukaratirwa S, Willingham AL and Monrad J (2006) Epidemiological studies of Fasciola gigantica infections in cattle in the highveld and lowveld communal grazing areas of Zimbabwe. Onderstepoort Journal of Veterinary Research 73, 37–51. [PubMed] [Google Scholar]
- Phalee A, Wongsawad C, Rojanapaibul A and Chai JY (2015) Experimental life history and biological characteristics of Fasciola gigantica (Digenea: Fasciolidae). Korean Journal of Parasitology 53, 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps LN and Kaplan JO (2017) Land use for animal production in global change studies: defining and characterizing a framework. Global Change Biology 23, 4457–4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedrafita D, Spithill TW, Smith RE and Raadsma HW (2010) Improving animal and human health through understanding liver fluke immunology. Parasite Immunology 32, 572–581. [DOI] [PubMed] [Google Scholar]
- Polley L and Thompson RC (2009) Parasite zoonoses and climate change: molecular tools for tracking shifting boundaries. Trends in Parasitology 25, 285–291. [DOI] [PubMed] [Google Scholar]
- Pozio E (2020) How globalization and climate change could affect foodborne parasites. Experimental Parasitology 208, 107807. [DOI] [PubMed] [Google Scholar]
- Prepelitchi L, Kleiman F, Pietrokovsky SM, Moriena RA, Racioppi O, Alvarez J and Wisnivesky-Colli C (2003) First report of Lymnaea columella Say, 1817 (Pulmonata: Lymnaeidae) naturally infected with Fasciola hepatica (Linnaeus, 1758) (Trematoda: Digenea) in Argentina. Memórias do Instituto Oswaldo Cruz 7, 889– 891. [DOI] [PubMed] [Google Scholar]
- Prepelitchi L, Pietrokovsky S, Kleiman F, Rubel D, Issia L, Moriena R, Racioppi O, Álvarez J and Wisnivesky-Colli C (2011) Population structure and dynamics of Lymnaea columella (say, 1817) (Gastropoda: Lymnaeidae) in wetlands of northeastern Argentina. Zoological Studies 50, 164–176. [Google Scholar]
- Pritchard GC, Forbes AB, Williams DJ, Salimi-Bejestani MR and Daniel RG (2005) Emergence of fasciolosis in cattle in East Anglia. Veterinary Record 157, 578–582. [DOI] [PubMed] [Google Scholar]
- Raadsma HW, Kingsford N, Suharyanta M, Spithill TW and Piedrafita D (2007) Host responses during experimental infection with Fasciola gigantica or Fasciola hepatica in Merino sheep I. Comparative immunological and plasma biochemical changes during early infection. Veterinary Parasitology 143, 275–286. [DOI] [PubMed] [Google Scholar]
- Rajapakse RPVJ, Pham KLT, Karunathilake KJK, Lawton SP and Le TH (2020) Characterization and phylogenetic properties of the complete mitochondrial genome of Fascioloides jacksoni (syn. Fasciola jacksoni) support the suggested intergeneric change from Fasciola to Fascioloides (Platyhelminthes: Trematoda: Plagiorchiida). Infection, Genetics and Evolution 82, 104281. [DOI] [PubMed] [Google Scholar]
- Ramachandran J, Ajjampur SS, Chandramohan A and Varghese GM (2012) Cases of human fascioliasis in India: tip of the iceberg. Journal of Postgraduate Medicine 58, 150–152. [DOI] [PubMed] [Google Scholar]
- Randolph TF, Schelling E, Grace D, Nicholson CF, Leroy JL, Cole DC, Demment MW, Omore A, Zinsstag J and Ruel M (2007) Invited review: role of livestock in human nutrition and health for poverty reduction in developing countries. Journal of Animal Science 85, 2788–2800. [DOI] [PubMed] [Google Scholar]
- Rehman Z, Zahid O, Rashid I, Ali Q, Akbar M, Oneeb M, Shehzad W, Ashraf K, Sargison N and Chaudhry U (2020) Genetic diversity and multiplicity of infection in Fasciola gigantica isolates of Pakistani livestock. Parasitology International 76, 102071. [DOI] [PubMed] [Google Scholar]
- Rim H-J, Farag HF, Sornmani S and Cross JH (1994) Food-borne trematodes: ignored or emerging? Parasitology Today 10, 207–209. [Google Scholar]
- Roberts JA and Suhardono (1996) Approaches to the control of fasciolosis in ruminants. International Journal for Parasitology 26, 971–981. [DOI] [PubMed] [Google Scholar]
- Robinson MW and Dalton JP (2009) Zoonotic helminth infections with particular emphasis on fasciolosis and other trematodiases. Philosophical Transactions of the Royal Society B: Biological Science 364, 2763–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MW, Tort JF, Lowther J, Donnelly SM, Wong E, Xu W, Stack CM, Padula M, Herbert B and Dalton JP (2008) Proteomics and phylogenetic analysis of the cathepsin L protease family of the helminth pathogen Fasciola hepatica: expansion of a repertoire of virulence-associated factors. Molecular and Cellular Proteomics 7, 1111–1123. [DOI] [PubMed] [Google Scholar]
- Robinson TP, Thornton PK, Franceschini G, Kruska RL, Chiozza F, Notenbaert A, Cecchi G, Herrero M, Epprecht M, Fritz S, You L, Conchedda G and See L (2011) Global Livestock Production Systems. Rome: FAO and Nairobi, Kenya: ILRI [Google Scholar]
- Rojas L, Vázquez AA, Domenech I and Robertson L (2010) Fascioliasis: can Cuba conquer this emerging parasitosis? Trends in Parasitology 26, 26–34. [DOI] [PubMed] [Google Scholar]
- Rokni MB (2008) The present status of human helminthic diseases in Iran. Annals of Tropical Medicine & Parasitology 102, 283–295. [DOI] [PubMed] [Google Scholar]
- Roldán C, Begovoeva M, López-Olvera JR, Velarde R, Cabezón O, Molinar AR, Pizzato F, Pasquetti M, Fernández X, Mentaberre G, Serrano E, Puig M, Espunyes J, Castillo-Contreras R, Estruch J and Rossi L (2020) Endemic occurrence of Fasciola hepatica in an alpine ecosystem, Pyrenees, Northeastern Spain. Transboundary and Emerging Diseases. doi: 10.1111/tbed.13865. [DOI] [PubMed] [Google Scholar]
- Rondelaud D, Dreyfuss G, Bouteille B and Dardé ML (2000) Changes in human fasciolosis in a temperate area: about some observations over a 28-year period in central France. Parasitology Research 86, 753–757. [DOI] [PubMed] [Google Scholar]
- Rondelaud D, Vignoles P, Abrous M and Dreyfuss G (2001) The definitive and intermediate hosts of Fasciola hepatica in the natural watercress beds in central France. Parasitology Research 87, 475–478. [DOI] [PubMed] [Google Scholar]
- Rondelaud D, Vignoles P, Vareille-Morel C, Abrous M, Mage C, Mouzet R and Dreyfuss G (2004) Fasciola hepatica and Fasciola hepatica: field observations on the transport and outcome of floating metacercariae in running water. Journal of Helminthology 78, 173–177. [DOI] [PubMed] [Google Scholar]
- Rondelaud D, Belfaiza M, Vignoles P, Moncef M and Dreyfuss G (2009) Redial generations of Fasciola hepatica: a review. Journal of Helminthology 83, 245–254. [DOI] [PubMed] [Google Scholar]
- Rondelaud D, Vignoles P and Dreyfuss G (2020) Fasciola hepatica: the dispersal of cercariae shed by the snail Galba truncatula. Parasite 27, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Campillo MT, Molina-Hernández V, Pérez J, Pacheco IL, Pérez R, Escamilla A, Martínez-Moreno FJ, Martínez-Moreno A and Zafra R (2018) Study of peritoneal macrophage immunophenotype in sheep experimentally infected with Fasciola hepatica. Veterinary Parasitology 257, 34–39. [DOI] [PubMed] [Google Scholar]
- Sabourin E, Alda P, Vázquez A, Hurtrez-Boussès S and Vittecoq M (2018) Impact of human activities on fasciolosis transmission. Trends in Parasitology 34, 891–903. [DOI] [PubMed] [Google Scholar]
- Sah R, Khadka S, Lakhey PJ, Pradhan S, Shah NP, Singh YP and Mas-Coma S (2018) Human case of Fasciola gigantica-like infection, review of human fascioliasis reports in Nepal, and epidemiological analysis within the South Central Asia. Acta Parasitologica 63, 435–443. [DOI] [PubMed] [Google Scholar]
- Salzer HJ and Schmiedel S (2015) Fasciola hepatica in a German traveler returning from Thailand. Journal of Travel Medicine 22, 285–286. [DOI] [PubMed] [Google Scholar]
- Sandland GJ and Minchella DJ (2003) Effects of diet and Echinostoma revolutum infection on energy allocation patterns in juvenile Lymnaea elodes snails. Oecologia 134, 479–486. [DOI] [PubMed] [Google Scholar]
- Sandland GJ, Foster AV, Zavodna M and Minchella DJ (2007) Interplay between host genetic variation and parasite transmission in the Biomphalaria glabrata-Schistosoma mansoni system. Parasitology Research 101, 1083–1089. [DOI] [PubMed] [Google Scholar]
- Sarkari B, Ghobakhloo N, Moshfea A and Eilami O (2012) Seroprevalence of human fasciolosis in a new-emerging focus of fasciolosis in Yasuj district, southwest of Iran. Iranian Journal of Parasitology 7, 15–20. [PMC free article] [PubMed] [Google Scholar]
- Sazmand A and Joachim A (2017) Parasitic diseases of camels in Iran (1931-2017) – a literature review. Parasite 24, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer G, Braun U, Deplazes P and Torgerson PR (2005) Estimating the financial losses due to bovine fasciolosis in Switzerland. Veterinary Record 157, 188–193. [DOI] [PubMed] [Google Scholar]
- Schweizer G, Meli ML, Torgerson PR, Lutz H, Deplazes P and Braun U (2007) Prevalence of Fasciola hepatica in the intermediate host Lymnaea truncatula detected by real time TaqMan PCR in populations from 70 Swiss farms with cattle husbandry. Veterinary Parasitology 150, 164–169. [DOI] [PubMed] [Google Scholar]
- Sheng ZA, Li J, Wang DY, Kang YQ, Wei ZY, Zhang FK, Zhu XQ, Luo HL and Huang WY (2019) Th2-related cytokines are associated with Fasciola gigantica infection and evasion in the natural host, swamp buffalo. Veterinary Parasitology 268, 73–80. [DOI] [PubMed] [Google Scholar]
- Shi W, Wei ZY, Elsheikha HM, Zhang FK, Sheng ZA, Lu KJ, Wang DY, Huang WY and Zhu XQ (2017) Dynamic expression of cytokine and transcription factor genes during experimental Fasciola gigantica infection in buffaloes. Parasites & Vectors 10, 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimalov VV and Shimalov VT (2000) Findings of Fasciola hepatica Linnaeus, 1758 in wild animals in Belorussian Polesie. Parasitology Research 86, 527. [DOI] [PubMed] [Google Scholar]
- Shoriki T, Ichikawa-Seki M, Suganuma K, Naito I, Hayashi K, Nakao M, Aita J, Mohanta UK, Inoue N, Murakami K and Itagaki T (2016) Novel methods for the molecular discrimination of Fasciola spp. on the basis of nuclear protein-coding genes. Parasitology International 65, 180–183. [DOI] [PubMed] [Google Scholar]
- Shumsky SA, Hickey GM, Pelletier B and Johns T (2014) Understanding the contribution of wild edible plants to rural socialecological resilience in semi-arid Kenya. Ecology and Society 19, 34. [Google Scholar]
- Soares MP, da Silva SS, Nizoli LQ, Felix SR and Schild AL (2007) Chronic fascioliasis in farmed and wild greater rheas (Rhea americana). Veterinary Parasitology 145, 168–171. [DOI] [PubMed] [Google Scholar]
- Sorensen RE and Minchella DJ (2001) Snail-trematode life history interactions: past trends and future directions. Parasitology 123, S3–18. [DOI] [PubMed] [Google Scholar]
- Spratt DM and Presidente PJ (1981) Prevalence of Fasciola hepatica infection in native mammals in southeastern Australia. Australian Journal of Experimental Biology and Medical Science 59, 713–721. [DOI] [PubMed] [Google Scholar]
- Stafford K and Gregory N (2008) Implications of intensification of pastoral animal production on animal welfare. New Zealand Veterinary Journal 56, 274–280. [DOI] [PubMed] [Google Scholar]
- Suhardono, Roberts JA and Copeman DB (2006a) The effect of temperature and humidity on longevity of metacercariae of Fasciola gigantica. Tropical Animal Health and Production 38, 371–377. [DOI] [PubMed] [Google Scholar]
- Suhardono, Roberts JA and Copeman DB (2006b) Variations in the survival of Fasciola gigantica eggs in bovine dung stored in the sun as opposed to the shade. Tropical Animal Health and Production 38, 379–382. [DOI] [PubMed] [Google Scholar]
- Suon S, Hol D, Siek S, McLean M and Copeman B (2006) Seasonal differences in the incidence of infection with Fasciola gigantica in Cambodian cattle. Tropical Animal Health and Production 38, 23–28. [DOI] [PubMed] [Google Scholar]
- Taghipour A, Zaki L, Rostami A, Foroutan M, Ghaffarifar F, Fathi A and Abdoli A (2019) Highlights of human ectopic fascioliasis: a systematic review. Infectious Diseases (London) 51, 785–792. [DOI] [PubMed] [Google Scholar]
- Takeuchi-Storm N, Denwood M, Hansen TVA, Halasa T, Rattenborg E, Boes J, Enemark HL and Thamsborg SM (2017) Farm-level risk factors for Fasciola hepatica infection in Danish dairy cattle as evaluated by two diagnostic methods. Parasites & Vectors 10, 555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi-Storm N, Moakes S, Thüer S, Grovermann C, Verwer C, Verkaik J, Knubben-Schweizer G, Höglund J, Petkevičius S, Thamsborg S and Werne S (2019) Parasite control in organic cattle farming: management and farmers’ perspectives from six European countries. Veterinary Parasitology: Regional Studies Reports 18, 100329. doi: 10.1016/j.vprsr.2019.100329 [DOI] [PubMed] [Google Scholar]
- Tavalire HF, Blouin MS and Steinauer ML (2016) Genotypic variation in host response to infection affects parasite reproductive rate. International Journal for Parasitology 46, 123–131. [DOI] [PubMed] [Google Scholar]
- Terasaki K, Noda Y, Shibahara T, Itagaki T, Fukuda K and Tsuchiya K (2003) Experimental fascioliasis in the rat-like hamster, Tscherskia triton, and other rodent hosts. Parasitology International 52, 147–154. [DOI] [PubMed] [Google Scholar]
- Thang T, Vázquez-Prieto S, Vilas R, Paniagua E, Ubeira F and Ichikawa-Seki M (2020) Genetic diversity of Fasciola hepatica in Spain and Peru. Parasitology International 76, 102100. [DOI] [PubMed] [Google Scholar]
- Thomas C, Jacquiet P and Dorchies P (2007) La prévalence des helminthoses bovines a-t-elle été modifiée par la canicule de l'été 2003 dans le sud-ouest de la France? Parasite 14, 265–268. [DOI] [PubMed] [Google Scholar]
- Thomas K, Hardy RD, Lazrus H, Mendez M, Orlove B, Rivera-Collazo I, Roberts JT, Rockman M, Warner BP and Winthrop R (2019) Explaining differential vulnerability to climate change: a social science review. Wiley Interdisciplinary Reviews: Climate Change 10, e565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton PK (2010) Livestock production: recent trends, future prospects. Philosophical Transactions of the Royal Society of London B: Biological Science 365, 2853–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton PK, van de Steeg J, Notenbaert A and Herrero M (2008) The Livestock–Climate–Poverty Nexus: A Discussion Paper on ILRI Research in Relation to Climate Change. Nairobi, Kenya: International Livestock Research Institute. [Google Scholar]
- Toet H, Piedrafita DM and Spithill TW (2014) Liver fluke vaccines in ruminants: strategies, progress and future opportunities. International Journal for Parasitology 44, 915–927. [DOI] [PubMed] [Google Scholar]
- Torgerson P and Claxton J (1999) Epidemiology and control. In Dalton JP (ed.), Fasciolosis. Oxon, UK: CAB International, pp. 113–150. [Google Scholar]
- Torgerson PR, Devleesschauwer B, Praet N, Speybroeck N, Willingham AL, Kasuga F, Rokni MB, Zhou XN, Fèvre EM, Sripa B, Gargouri N, Fürst T, Budke CM, Carabin H, Kirk MD, Angulo FJ, Havelaar A and de Silva N (2015) World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: a data synthesis. PLoS Medicine 12, e1001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeira FM, Muiño L, Valero MA, Periago MV, Pérez-Crespo I, Mezo M, González-Warleta M, Romarís F, Paniagua E, Cortizo S, Llovo J and Mas-Coma S (2009) MM3-ELISA detection of Fasciola hepatica coproantigens in preserved human stool samples. American Journal of Tropical Medicine and Hygiene 81, 156–162. [PubMed] [Google Scholar]
- Ueno H, Arandia R, Morales G and Medina G (1975) Fascioliasis of livestock and snail host for Fasciola in the Altiplano Region of Bolivia. National Institute of Animal Health Quarterly (Tokyo) 15, 61–67. [PubMed] [Google Scholar]
- Utaaker KS and Robertson LJ (2015) Climate change and foodborne transmission of parasites: a consideration of possible interactions and impacts for selected parasites. Food Research International 68, 16–23. [Google Scholar]
- Utzinger J and Tanner M (2000) Microhabitat preferences of Biomphalaria pfeifferi and Lymnaea natalensis in a natural and a man-made habitat in southeastern Tanzania. Memórias do Instituto Oswaldo Cruz 95, 287–294. [DOI] [PubMed] [Google Scholar]
- Vaughan JL, Charles JA and Boray JC (1997) Fasciola hepatica infection in farmed emus (Dromaius novaehollandiae). Australian Veterinary Journal 75, 811–813. [DOI] [PubMed] [Google Scholar]
- Vázquez AA, Hevia Y and Sánchez J (2009) Distribución y preferencia de hábitats de moluscos hospederos intermediarios de Fasciola hepatica en Cuba. Revista Cubana de Medicina Tropical 61, 248–253. [Google Scholar]
- Vázquez AA, Sánchez J, Pointier JP, Théron A and Hurtrez-Boussès S (2014) Fasciola hepatica in Cuba: compatibility of different isolates with two intermediate intermediate hosts, Galba cubensis and Pseudosuccinea columella. Journal of Helminthology 88, 434–440. [DOI] [PubMed] [Google Scholar]
- Vázquez AA, Sánchez J, Alba A, Pointier JP and Hurtrez-Boussés S (2015) Natural prevalence in Cuban populations of the lymnaeid snail Galba cubensis infected with the liver fluke Fasciola hepatica: small values do matter. Parasitology Research 114, 4205-4210. doi: 10.1007/s00436-015-4653-2. [DOI] [PubMed] [Google Scholar]
- Vázquez AA, Lounnas M, Sánchez J, Alba A, Milesi A and Hurtrez-Boussés S (2016) Genetic and infective diversity of the common liver fluke Fasciola hepatica (Trematoda: Digenea) from Cuba. Journal of Helminthology 14, 1–7. [DOI] [PubMed] [Google Scholar]
- Vázquez AA, Alda P, Lounnas M, Sabourin E, Alba A, Pointier JP and Hurtrez-Boussès S (2018) Lymnaeid snails hosts of Fasciola hepatica and Fasciola gigantica (Trematoda: Digenea): a worldwide review. CAB Reviews 13, 062. [Google Scholar]
- Vázquez AA, de Vargas M, Alba A, Sánchez J, Alda P, Sabourin E, Vittecoq M, Alarcón-Elbal PM, Pointier JP and Hurtrez-Boussès S (2019) Reviewing Fasciola hepatica transmission in the West Indies and novel perceptions from experimental infections of sympatric vs allopatric snail/fluke combinations. Veterinary Parasitology 275, 108955. [DOI] [PubMed] [Google Scholar]
- Vázquez AA, Sabourin E, Alda P, Leroy C, Leray C, Carron E, Mulero S, Caty C, Hasfia S, Boisseau M, Saugné L, Pineau O, Blanchon T, Alba A, Faugère D, Vittecoq M and Hurtrez-Boussès S (2020) Genetic diversity and relationships of the liver fluke Fasciola hepatica (Trematoda) with native and introduced definitive and intermediate hosts. Transboundary and Emerging Diseases. doi: 10.1111/tbed.13882. [DOI] [PubMed] [Google Scholar]
- Vignoles P, Dreyfuss G and Rondelaud D (2012) Larval development of Fasciola hepatica in experimental infections: variations with populations of Lymnaea truncatula. Journal of Helminthology 76, 179–183. [DOI] [PubMed] [Google Scholar]
- Vignoles P, Hourdin P, Dreyfuss G and Rondelaud D (2019) Epidémiologie de la fasciolose dans le Limousin: bilan des recherches effectuées depuis les années 1970. Annales Scientifiques du Limousin 28, 39–66. [Google Scholar]
- Vilas R, Vázquez-Prieto S and Paniagua E (2012) Contrasting patterns of population genetic structure of Fasciola hepatica from cattle and sheep: implications for the evolution of anthelmintic resistance. Infection Genetic & Evolution 12, 45–52. [DOI] [PubMed] [Google Scholar]
- Villa-Mancera A and Reynoso-Palomar A (2019) Bulk tank milk ELISA to detect IgG1 prevalence and clustering to determine spatial distribution and risk factors of Fasciola hepatica-infected herds in Mexico. Journal of Helminthology 93, 704–710. [DOI] [PubMed] [Google Scholar]
- Viney ME and Graham AL (2013) Advances in Parasitology 82, 321–370. [DOI] [PubMed] [Google Scholar]
- Walker SM, Hoey E, Fletcher H, Brennan G, Fairweather I and Trudgett A (2006) Stage-specific differences in fecundity over the life-cycle of two characterized isolates of the liver fluke, Fasciola hepatica. Parasitology 133, 209–216. [DOI] [PubMed] [Google Scholar]
- Walker SM, Makundi AE, Namuba FV, Kassuku AA, Keyyu J, Hoey EM, Prödohl P, Stothard JR and Trudgett A (2008) The distribution of Fasciola hepatica and Fasciola gigantica within southern Tanzania – constraints associated with the intermediate host. Parasitology 135, 495–503. [DOI] [PubMed] [Google Scholar]
- Walsh KP, Brady MT, Finlay CM, Boon L and Mills KH (2009) Infection with a helminth parasite attenuates autoimmunity through TGF-beta-mediated suppression of Th17 and Th1 responses. Journal of Immunology 183, 1577–1586. [DOI] [PubMed] [Google Scholar]
- Wiedosari E, Hayakawa H and Copeman B (2006) Host differences in response to trickle infection with Fasciola gigantica in buffalo, Ongole and Bali calves. Tropical Animal Health and Production 38, 43–53. [DOI] [PubMed] [Google Scholar]
- Wilson A (1879) On the occurrence of the common fluke (Fasciola hepatica) in the human subject. Edinburgh Medical Journal 25, 413–417. [PMC free article] [PubMed] [Google Scholar]
- Wolinska J and King KC (2009) Environment can alter selection in host-parasite interactions. Trends in Parasitology 25, 236–244. [DOI] [PubMed] [Google Scholar]
- World Health Organization (1990) Forty-third World Health Assembly, Geneva, 7–17 May 1990: resolutions and decisions, annexes., Geneva.
- World Health Organization (1995) WHO Study Group on the Control of Foodborne Trematode Infections. Geneva: WHO technical report series; 849. [PubMed] [Google Scholar]
- World Health Organization (2011) Annex 1: interagency roadmap for high-priority neglected zoonotic diseases: expected outcomes by objective by 2015 and 2020.
- World Health Organization (2013) Sustaining the Drive to Overcome the Global Impact of Neglected Tropical Diseases: Second WHO Report on Neglected Tropical Diseases. WHO Technical Report Series.
- Xifeng W, Mengfan Q, Kai Z, Guowu Z, Jing L, Lixia W, Jun Q, Qingling M, Shasha G, Yunfu H and Xuepeng C (2019) Development and evaluation of a colloidal gold immunochromatographic assay based on recombinant protein CatL1D for serodiagnosis of sheep fasciolosis. Journal of Helminthology 94, e98. [DOI] [PubMed] [Google Scholar]
- Zhang WY, Moreau E, Hope JC, Howard CJ, Huang WY and Chauvin A (2005) Fasciola hepatica and Fasciola gigantica: comparison of cellular response to experimental infection in sheep. Experimental Parasitology 111, 154–159. [DOI] [PubMed] [Google Scholar]
- Zhang JL, Si HF, Zhou XZ, Shang XF, Li B and Zhang JY (2019a) High prevalence of fasciolosis and evaluation of the efficacy of anthelmintics against Fasciola hepatica in buffaloes in Guangxi, China. International Journal for Parasitology: Parasites & Wildlife 8, 82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XX, Cwiklinski K, Hu RS, Zheng WB, Sheng ZA, Zhang FK, Elsheikha HM, Dalton JP and Zhu XQ (2019b) Complex and dynamic transcriptional changes allow the helminth Fasciola gigantica to adjust to its intermediate snail and definitive mammalian hosts. BMC Genomics 20, 729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Mei X, Liang Y, Zhu B, Sheng Z, Shi W, Wang D and Huang W (2020) Newly excysted juveniles (NEJs) of Fasciola gigantica induce mice liver fibrosis and M2 macrophage-like phenotype in vivo. Microbial Pathogenesis 139, 103909. [DOI] [PubMed] [Google Scholar]
- Zhou XN (2012) Prioritizing research for ‘One health – One world’. Infectious Diseases of Poverty 1, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghi S, Emami M, Shahriarirad S, Vahedi R, Cheraghi MR, Zamiri B, Arefkhah N, Ghorbani F and Sarkari B (2019) Human fascioliasis in nomads: a population-based serosurvey in southwest Iran. Infezioni in Medicina 27, 68–72. [PubMed] [Google Scholar]
- Zumaquero-Ríos JL, Sarracent-Pérez J, Rojas-García R, Rojas-Rivero L, Martínez-Tovilla Y, Valero MA and Mas-Coma S (2013) Fascioliasis and intestinal parasitoses affecting schoolchildren in Atlixco, Puebla State, Mexico: epidemiology and treatment with nitazoxanide. PLoS Neglected Tropical Diseases 7, e2553. [DOI] [PMC free article] [PubMed] [Google Scholar]