Abstract
Visceral leishmaniasis (VL) is endemic in 70 countries and has been reported in 12 countries of Latin America, with over 90% of the cases reported in Brazil, where epidemics have occurred since 1980. The objective of this review is to describe the factors associated with the occurrence of VL epidemics in humans in urban areas. A systematic review was conducted according to the PRISMA-P guidelines. The databases PubMed (by Medline), Cochrane Library, Embase, Amed, LILACS and grey literature [Google Scholar and handsearch of the database of the Information System for Notifiable Diseases (SINAN) of Brazil's Unified Health System] were used. The protocol was registered under PROSPERO (CRD42019128998). Climatic, environmental factors and indicators of urban social structure were described as influencing the outbreaks in the North and Northeast regions. Gender and age characteristics were related to a greater chance of developing VL in the Central-West, Northeast and Southeast regions. Vector indicators showed a positive correlation with the incidence of VL in studies in the Northeast region. In the Southeast and Northeast regions, studies revealed the presence of dogs with positive correlation with VL. Knowledge gaps remain regarding the contribution to the increase in the risk factors described in ecological approaches, as no analysis was performed at the individual level, and it is still necessary to discuss the influence of other associated elements in epidemic episodes in the spread of VL.
Key words: Epidemic, Leishmania infantum, systematic review, visceral leishmaniasis
Introduction
Visceral leishmaniasis (VL) is a neglected tropical disease that accounts for 200 000–400 000 new cases each year worldwide, and is endemic in 70 countries on the five continents (Alvar et al., 2012; WHO, 2018). Currently, VL is present in 12 countries of the Americas, with 96% of the cases being reported in Brazil (4200–6300 cases per year), an incidence rate of 2/100 000 inhabitants and a case fatality rate of around 7% (Romero and Boelaert, 2010; Menon et al., 2016). In the Americas, the aetiologic agent is the protozoan Leishmania (Leishmania) infantum, which is usually transmitted by the bite of the sandfly Lutzomyia longipalpis (Romero and Boelaert, 2010), although there is already a study that has identified other species as transmitters of leishmania (Rêgo et al., 2020). Dogs represent the main urban reservoir (Romero and Boelaert, 2010).
In Brazil, the first urban epidemic was reported in Teresina (Piauí state) between 1981 and 1985, when the disease, initially limited to rural areas, expanded to peripheral areas of the city (Costa et al., 1990). Since then, the disease has been expanding from cities in the Northeast to other regions of the country (Badaro et al., 1986; Costa et al., 1990; Evans et al., 1992; Jerônimo et al., 2004; Moreno et al., 2005; de Oliveira et al., 2008; Falqueto et al., 2009).
The literature reports occurrences of VL epidemics in different countries worldwide (Imamura et al., 2016). However, there is no literature record of systematic reviews and meta-analysis regarding factors associated with occurrences of VL epidemics. In this respect, our study sets out to elucidate the knowledge gaps regarding the factors associated with VL occurrence in different urban contexts during epidemic processes. This systematic review aims to describe the factors associated with the occurrence of VL epidemics in humans in urban areas.
Materials and methods
This study was conducted using the guideline Preferred Reporting Items for Systematic Review and Meta-Analysis (Moher et al., 2015). We registered the protocol before its execution in the International Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD42019128998.
Selection criteria
In this review, we included epidemiological ecological, case series, cross-sectional and case-control studies that described associations among individual characteristics (demographic variables), socioeconomic and environmental variables (climates, household characteristics and social, urban and population structures), presence of vectors and animals (dogs and other animals) and the occurrence of any outcome related to VL. This outcome could be the infection by L. infantum, the clinical disease or the notification of cases to the health services.
Eligibility criteria
We included studies that investigated factors associated with VL infection and clinical disease confirmed and notified in situations of epidemics in Brazil. No restrictions were applied regarding language for the inclusion of the studies.
Research and selection of studies
The search was carried out in the electronic databases PubMed (by Medline), Cochrane Library, Embase, Amed, LILACS and grey literature [Google Scholar and handsearch of the database of the Information System for Notifiable Diseases (SINAN), Department of Informatics of the Brazilian Unified Health System (DATASUS)]. Papers cited in the selected studies were included as grey literature. Pre-defined descriptors were used for each database to increase the sensitivity of the search. The search strategy was defined following prior consultation of the uniterms leishmaniasis, association and occurrence. This consultation was carried out in MeSH (PubMed by Medline) and adapted for other databases. For LILACS, we used the Health Sciences Descriptors (DeCS), and for Embase, we used Emtree.
The references retrieved in the search strategies were exported to an Endnote® X7 file and the duplicates were removed. The studies were independently selected by two researchers who used a form previously tested in five selected articles to identify any adjustments needed before their application in the remaining studies. Any disagreements were resolved by consensus.
Studies were included according to our criteria for each section following the order of evaluation in three phases: 1st: titles; 2nd: abstracts and 3rd: main text.
An epidemic was defined as the occurrence of a health-related event that exceeds normal expectations, with a number of cases higher than expected in a specific area and time, in the presence of an epidemiological link (Braga and Werneck, 2008).
Studies analysing factors associated with VL that did not specify whether they were carried out at the time of an epidemic and were investigated using the database of the Information System for Notifiable Diseases (SINAN), Department of Informatics of the Brazilian Unified Health System (DATASUS). For each study, we searched for the number of confirmed cases in the places and years in which the study was carried out, including an interval of 3 years before and after, to verify the number of cases of the disease. Therefore, if an above-expected increase in the number of cases that would characterize an epidemic was detected, even if the article did not describe the occurrence of an epidemic, it was included in the present review.
Analysis and presentation of results
The results of the synthesis of the articles were presented qualitatively and quantitatively with the factors related to the occurrence of VL pointing out the specificities and regional differences of the occurrence of the disease considering the context of the study (Table 1). The characteristics of the included studies were described considering each group of factors by region studied.
Table 1.
Studies carried out in periods of epidemics in Brazil, according to the location of the study, year of execution and period of epidemics, from 1980 to 2019
| Study (author/year) | Region | Study design | Location of the study municipalities and state | Year(s) of execution of the study | Year(s) of epidemics record |
|---|---|---|---|---|---|
| Costa et al. (1990)a | Northeast | Ecological | Municipalities of Piauí | 1971–1986 | 1980–1986 |
| Lima et al. (2017)a | Northeast | Cross-sectional | Natal, Rio Grande do Norte | 1990–2014 | Peak between 1990 and 1994 |
| Jerônimo et al. (2004)a | Northeast | Cross-sectional | Natal, Rio Grande do Norte | 1994–2000 | 1991 |
| Cerbino-Neto et al. (2009)a | Northeast | Ecological | Teresina, Piauí | 1991–2000 | Peak between 1992 and 1995 and in 1998 |
| Werneck et al. (2002)a | Northeast | Cross-sectional | Teresina, Piauí | 1993–1996 | 1993–1996 |
| Werneck and Maguire (2002)a | Northeast | Ecological | Teresina, Piauí | 1995–1996 | 1993–1996 |
| Costa et al. (2005)a | Northeast | Case control | Teresina, Piauí | 1995–1996 | 1993–1996 |
| Werneck et al. (2007)a | Northeast | Ecological | Teresina, Piauí | 1993–1996 | 1993–1996 |
| Ximenes et al. (2007)a | Northeast | Ecological | Municipalities from Rio Grande do Norte | 1995–2005 | Peak in 1995 and 2000 |
| Araújo et al. (2018)a | Northeast | Cross-sectional | State of Ceará | 1986–2017 | Peak in 1995, 2000, 2006, 2011 and 2014 |
| Bavia et al. (2005)a | Northeast | Ecological | Municipalities of Bahia | 1990–1998 | 1997 |
| de Oliveira et al. (2008)a | Central West | Cross-sectional | Três Lagoas, Mato Grosso do Sul | 2002 | Epidemic since 2000 |
| de Almeida et al. (2011)b | Northeast | Ecological | Teresina, Piauí | 2001–2006 | Peak in 2003 |
| Borges et al. (2008)b | Southeast | Case control | Belo Horizonte, Minas Gerais | 2006 | Peak from 2004 to 2006 |
| Borges et al. (2009)b | Southeast | Case control | Belo Horizonte, Minas Gerais | 2004 | Peak from 2004 to 2006 |
| Viana et al. (2011)b | Northeast | Cross-sectional | São Luis, Maranhão | 2002–2010 | Peak from 2004 to 2006 |
| Brazuna et al. (2012)b | Central West | Case series | Campo Grande, Mato Grosso do Sul | 2002–2009 | Peak from 2004 |
| Oliveira et al. (2014)b | North | Cross-sectional | Araguaína, Tocantins | 2007–2012 | Peak in 2007 and 2008 |
| Carranza-Tamayo et al. (2016)b | Central West | Cross-sectional | Brasília, Distrito Federal | 2007–2008 | Peak in 2007 and 2008 |
| dos Reis et al. (2019) | North | Ecological | Municipalities of Tocantins | 2007–2014 | Peak in 2007–2011 |
| de Toledo et al. (2017)a | North | Ecological | Araguaína, Tocantins | 2007–2012 | Peak in 2008 and 2011 |
| Rocha et al. (2018)b | Northeast | Ecological | Teresina, Piauí | 2007–2016 | Peak in 2008 and 2014 |
| de Freitas et al. (2013)b | Northeast | Case series | Fortaleza, Ceará | 2006–2016 | Peak in 2009, 2010 and 2011 |
Studies carried out during periods of an epidemic as described in the paper.
Studies identified in the periods of epidemic according to the analysis of the data from the DATASUS.
Results
Description of included studies
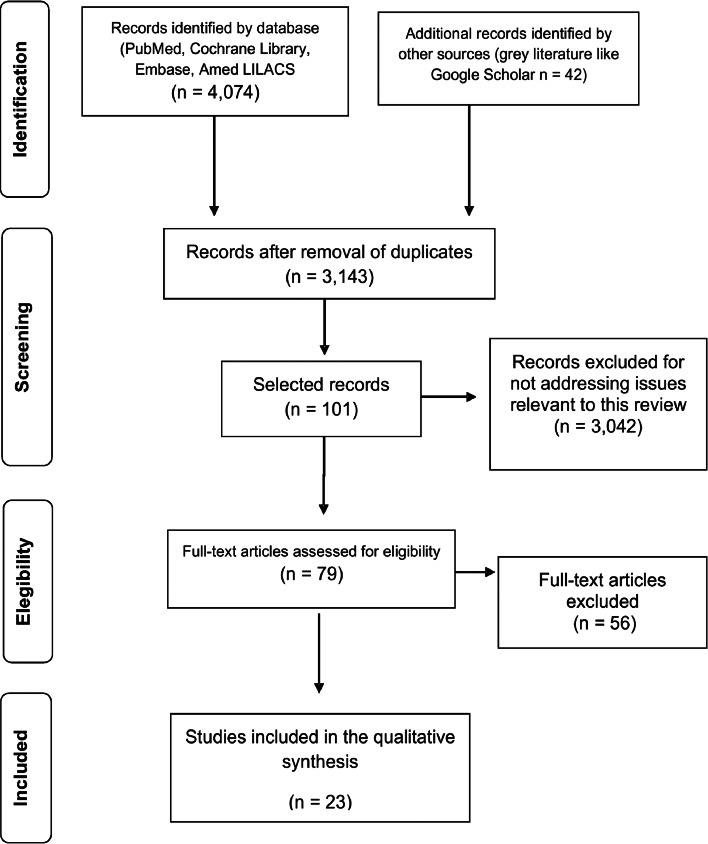
The search strategies retrieved 3143 titles, out of which 23 studies met the eligibility criteria and were included in this systematic review (Fig. 1). Studies have shown the factors present in the context of epidemics between the years 1980 and 2014 in Brazil. In the 1980s, two epidemics were analysed, and in the 1990s epidemics were described between 1990 and 1998. In the first decade of the 2000s, the studies analysed included epidemics that occurred in all years, except in 2001 and 2002. As of 2010, studies described epidemics in 2010, 2011 and 2014. The first urban epidemic occurred in 1980 in the state of Piauí, and the most recent epidemic process included in this review occurred in 2014 in the state of Ceará.
Fig. 1.
Systematic review on factors associated with urban epidemics of human VL in Brazil.
Of the 23 selected studies, nine (39%) were ecological studies, nine (39%) were cross-sectional studies, two (9%) were case series studies and three (13%) were case-control studies. Regarding the coverage areas, 15 (65%) studies were carried out in the Northeast region of Brazil, three (13%) in the Central-West, three (13%) in the North and two (9%) in the Southeast (Table 1 and Supplementary material 1).
Four studies (17%) evaluated factors associated with asymptomatic L. infantum infection, and the others analysed factors associated with the VL clinical disease.
From 1980 to 2000, VL epidemics were described and analysed in five Brazilian states, four in the Northeast and one in the Central-West. An increase in the epidemic record was observed in the first decade of the 2000s, with expansion to the North and Southeast regions, and records in seven states. Between 2010 and 2019, the studies in this review presented epidemic records in three states in the North and Northeast regions (Fig. 2).
Fig. 2.
Distribution of studies on VL epidemics in Brazil from 1980 to 2019, by state.
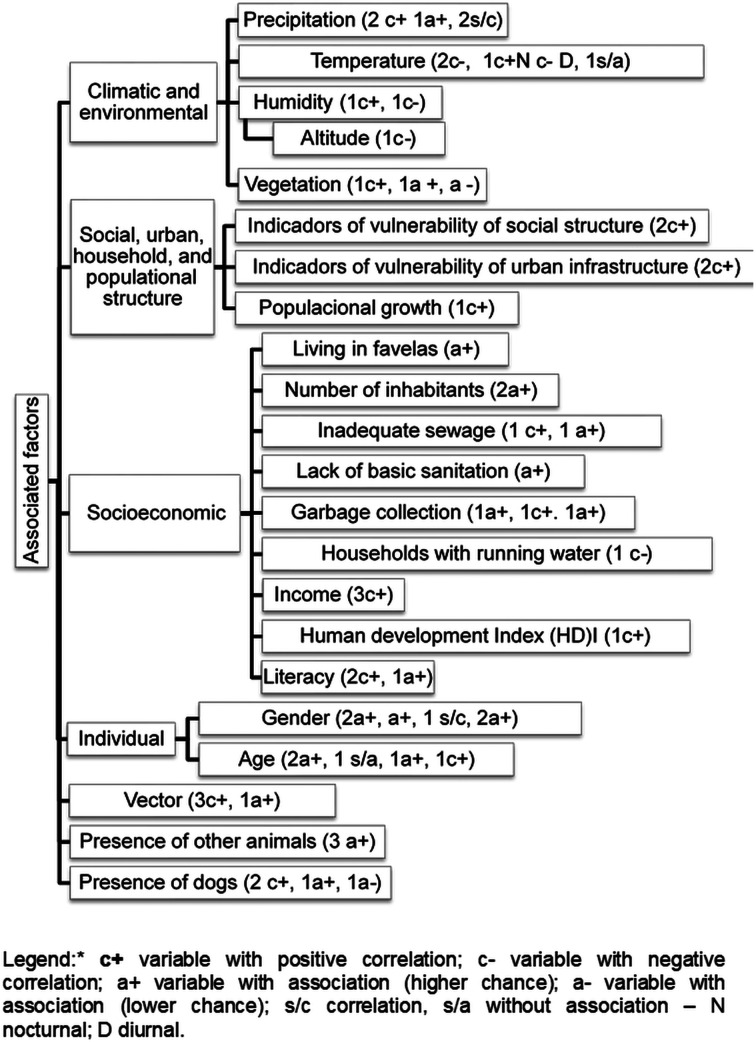
The factors related to VL in urban epidemics are shown in Table 2, Fig. 3 and Supplementary material 1 by author and occurrence of visceral leishmaniasis by region of Brazil.
Table 2.
Categorization of studies on factors associated with the occurrence of VL in the context of epidemics, according to the geographical area and type of factor
| Study (author/year) | Brazilian region | Climatic and environmental | Social, urban, household and populational structure | Socioeconomic | Individual | Vector | Presence of dogs | Presence of other animals |
|---|---|---|---|---|---|---|---|---|
| Costa et al. (1990) | Northeast | |||||||
| Werneck et al. (2002) | Northeast | |||||||
| Werneck and Maguire (2002) | Northeast | |||||||
| Jerônimo et al. (2004) | Northeast | |||||||
| Bavia et al. (2005) | Northeast | |||||||
| Costa et al. (2005) | Northeast | |||||||
| Werneck et al. (2007) | Northeast | |||||||
| Ximenes et al. (2007) | Northeast | |||||||
| Borges et al. (2008) | Southeast | |||||||
| de Oliveira et al. (2008) | Central West | |||||||
| Borges et al. (2009) | Southeast | |||||||
| Cerbino-Neto et al. (2009) | Northeast | |||||||
| de Almeida et al. (2011) | Northeast | |||||||
| Viana et al. (2011) | Northeast | |||||||
| Brazuna et al. (2012) | Central West | |||||||
| de Freitas et al. (2013) | Northeast | |||||||
| Oliveira et al. (2014) | North | |||||||
| Carranza-Tamayo et al. (2016) | Central West | |||||||
| de Toledo et al. (2017) | North | |||||||
| Lima et al. (2017) | Northeast | |||||||
| Araújo et al. (2018) | Northeast | |||||||
| Rocha et al. (2018) | Northeast | |||||||
| dos Reis et al. (2019) | North |
Fig. 3.
Factors related to human VL described in the studies included in this systematic review.
Climate and environmental factors
Six studies pointed out climatic and environmental factors related to VL in the context of an epidemic in the Northeast region. Living in areas covered by green vegetation (Werneck and Maguire, 2002), and increased vegetation have been associated with a high incidence of human VL (Werneck et al., 2007). VL was also correlated with locations with the presence of green areas (Cerbino-Neto et al., 2009). Bavia et al. (2005) demonstrated that low values of the normalized difference vegetation index (NDVI) in municipalities at high risk for VL were related to the high number of positive cases of the disease.
Precipitation showed a positive correlation with the incidence rate of VL (dos Reis et al., 2019), and also with the incidence of VL in the previous year (Lima et al., 2017). There was an association between the number of cases of VL and precipitation (Viana et al., 2011).
The average temperature was negatively correlated with the incidence rate of VL (de Freitas et al., 2013; Oliveira et al., 2014). Similarly, daytime temperature also showed a negative correlation with VL (dos Reis et al., 2019). However, night-time temperature showed a positive correlation with the incidence rate of VL (dos Reis et al., 2019).
The relative humidity of the air showed divergent behaviour patterns in the studies of the North and Northeast regions. It presented a negative correlation with the incidence rate of VL (de Freitas et al., 2013) and a positive correlation between the incidence rate of VL minimum and maximum humidity (dos Reis et al., 2019).
The effects of altitude were analysed in the municipalities of the state of Tocantins and the results showed a negative correlation between the municipalities with higher altitudes and the incidence rate of VL (dos Reis et al., 2019).
Factors related to social, urban and population structure
Studies conducted in the North and Northeast states showed a positive correlation between indicators of social and urban structure and the incidence of VL (de Toledo et al., 2017; Rocha et al., 2018). Rocha et al. (2018) pointed out statistically significant clusters between the incidence of the disease and the indicators of vulnerability of social structure, household structure, urban infrastructure and composite index of vulnerability. Cerbino-Neto et al. (2009) demonstrated that the incidence of VL was positively correlated with population growth.
Socioeconomic factors
Studies related to socioeconomic factors were carried out in states in the Northeast region. The factors that were associated with the incidence of VL in the context of epidemics were: living in a slum (Werneck and Maguire, 2002), percentage of literacy in the neighbourhood and homes without sanitation (Lima et al., 2017). The average percentage of households with at least one indoor bathroom connected to the sewage network was associated with the average annual incidence rate of VL (Cerbino-Neto et al., 2009).
As for the risk of developing VL, living in a house with more than four members doubled the risk of VL, and living in a house with an inadequate sewage network (without a bathroom) showed a high risk of VL (Costa et al., 2005). People living in households without regular garbage collection were more likely to develop VL (Costa et al., 2005).
The average number of residents per household (Lima et al., 2017), households with no garbage collection (Cerbino-Neto et al., 2009) and areas with a lower percentage of households with garbage collection (de Almeida et al., 2011; Lima et al., 2017) were positively correlated with the incidence of VL.
The lowest per capita income and the lowest human development index showed a positive correlation with the average number of cases of VL (Araújo et al., 2018). The annual incidence rates of VL correlated with the average income and illiteracy (Cerbino-Neto et al., 2009).
The average percentage of households with running water was negatively correlated with the incidence rate of VL (Cerbino-Neto et al., 2009). There was a spatial correlation between VL rates and the lowest average income of heads of households and illiteracy and a lower percentage of households with garbage collection (de Almeida et al., 2011).
However, in the Northeast, de Oliveira et al. (2008) found no relationship among accumulation of waste in the home, literacy of the head of household and infection by Leishmania.
Individual factors
A greater number of cases of VL in men were reported when compared to women (Brazuna et al., 2012) and men were more likely to contract the disease than women (Borges et al., 2008). VL was also associated with males and age groups (Lima et al., 2017). In the Northeast, de Oliveira et al. (2008) found no relationship between gender and infection by Leishmania.
The positivity in the skin test for Leishmania increased with age in the study by Werneck et al. (2002). Costa et al. (2005) pointed out that greater chances of developing VL were found in children aged 5–10 years, and children aged 1–4 years were 14 times more likely to develop the disease than those older than 10 years. The study by Borges et al. (2008) showed an increased risk of contracting VL in children under 10 years old. Carranza-Tamayo et al. (2016) demonstrated that VL infection in humans was associated with an age greater than 7 years. Oliveira et al. (2014) described that there was a higher prevalence of the disease in children under 15 years old than in the age group from 1 to 5 years. However, in the Northeast, de Oliveira et al. (2008) found no relationship between age and infection by Leishmania.
Factors related to vectors
Ximenes et al. (2007) found an association of vector species and distribution of diseases with demographic and physiognomic characteristics, disordered growth in the metropolitan region, living conditions and environmental degradation of the East Coast of the state of Rio Grande do Norte. The number of sandflies was associated with VL in the study by Bavia et al. (2005). Studies have demonstrated a correlation between the incidence of VL and vector infestation and relative abundance of L. longipalpis (Lima et al., 2017), as well as a correlation between households investigated and infested by L. longipalpis (Costa et al., 1990). The number of sandflies was associated with VL (Bavia et al., 2005).
Presence of dogs
Studies carried out in the states of the Northeast and Southeast regions showed divergent patterns for the presence of dogs and VL. Dog owners were more likely to contract VL when compared to individuals who did not have them, and an increased chance of contracting VL was observed for individuals with two dogs and for people who kept their dogs indoors during the day (Borges et al., 2009). The canine population negatively influenced the number of cases of VL and the percentage of positive dogs showed a correlation and positive influence on the incidence rate of VL (de Freitas et al., 2013). In a multilevel model study, Werneck et al. (2007) described an association between the prevalence of canine seropositivity and the incidence of the disease in humans. Jerônimo et al. (2004) found no association between LV and dog ownership.
Presence of other animals
The relationship between the presence of other animals and VL has been described in the Southeast and Central-West regions. The increase in the number of cases of VL was associated with the presence of ducks, rodents and chickens (Borges et al., 2009). The presence of opossums in the vicinity reduced the chances of falling ill with VL (Borges et al., 2009). In another study, the presence of opossums around the homes increased the chances of human infection (Carranza-Tamayo et al., 2016). However, in the Northeast region Jerônimo et al. (2004) did not find an association between LV and the possession of other animals.
Discussion
Urban VL has occurred in Brazil since 1980, but little is known about the dynamics of L. infantum transmission in urban centres or the variables that determine the distribution of the disease in these places in the context of an epidemic. As this is a relatively rare event, the context of epidemics provided us with more robust results in quantitative terms in analytical approaches in this review.
Factors related to the occurrence of VL in the context of epidemics were compared among regions and described on an individual and aggregate scale. In this scenario, the Northeast region stands out with the largest number of variables studied and related to the occurrence of the disease.
As for the individual factors analysed in this review, men were more likely to contract the disease or infection, which was also observed in studies carried out in the endemic period (de Delgado et al., 1998; Oliveira et al., 2006; Gouvêa et al., 2007; da Silva et al., 2016; Menezes et al., 2016). Other studies on VL and male sex suggest explanations for these results: hormonal or immunological problems can affect the progress of the infection (Sharma et al., 1990); men could be more exposed to vector bites (Costa et al., 1990) and men are more frequently involved in domestic chores or outdoor leisure activities during the period of greatest vector activity (Gouvêa et al., 2007).
Considering age as a factor related to VL, in studies carried out in the context of an epidemic, the results showed that children are more susceptible to both infection and illness. When analysing the difference between skin and serological tests in Teresina, the study showed that prevalence estimates based on the leishmanin skin test increased with age and that those based on serological tests showed a non-significant variation with age (Werneck et al., 2002). Still in Teresina in an endemic period, a study showed an association between a positive reaction to the Montenegro skin test and age (Gouvêa et al., 2007). The meta-analysis study by Belo et al. (2013) of VL in the Americas showed that children are more likely to develop the clinical disease and less likely to become infected. However, as it is known that children are less likely to have asymptomatic infection, the Montenegro test indicates infections that have occurred throughout an individual's life (Nascimento et al., 1993). However, the study by Silva et al. (2006) in Porteirinha, Minas Gerais, which must also be considered, demonstrated that serological tests for VL can also continue to be positive after the treatment of the disease, not indicating a bad prognosis or a poor therapeutic response.
The studies by Cunha et al. (1995); Desjeux (2001) and Albuquerque et al. (2009) showed that the occurrence of VL in the North and Northeast regions was related to socioeconomic and migratory factors. However, the results of this review indicate the occurrence of climatic and environmental factors, socioeconomic factors, vulnerability indicators of the social structure and urban infrastructure, individual characteristics, factors related to the vector, presence of dogs and other animals present during the context of the epidemic in the Northeast region of Brazil. These results corroborate with the observation of Sherlock (1996) in Bahia and other regions of the country in which poverty, malnutrition and high density of phlebotomines were associated with the presence of domestic animals, sanitary conditions and low socioeconomic level in the areas of transmission of VL. Among the factors present in the context of epidemics in Brazil, climatic and environmental factors deserve to be highlighted as the spread of VL may be related to these conditions normally found in poorer areas, with less urban infrastructure and with little sanitation.
Some points in the temperature and precipitation variables identified in this review should be considered as related to the increase in the number of cases of VL in the context of epidemics. Among the points, we highlight the influences of these climatic variables in the epidemiological cycle of VL, due to their influence on the activity of sandflies (Rivas et al., 2014; Lima et al., 2017; Sevá et al., 2017) which can influence the spread of the disease.
The influence of vegetation on VL cases can also be related to the different forms of urbanization and infrastructure of the studied places (or areas) and to the demographic and environmental characteristics, and also to the presence of the vector. As demonstrated in Teresina, the periods with high rates of the disease in the peripheral neighbourhoods were coincident with the expansion of the city area and population growth; and that green areas are positively associated with the occurrence of VL (Werneck and Maguire, 2002; Cerbino-Neto et al., 2009). These results were corroborated with the evidence from Feliciangeli et al. (2006) in which the proximity of houses to the forest is a probable risk factor for Leishmania infection. In the state of Bahia, the spatial comparison of human disease cases between NDVI and vegetation maps suggests that the highest incidence of VL is concentrated in areas with lower NDVI values, with the caatinga as the predominant vegetation (Bavia et al., 2005).
Other climatic and environmental factors, such as altitude and relative humidity, were discussed in only one of the studies included in this review, which limits the analysis of these variables. Studies that address these variables in other contexts and locations are still needed.
Studies have demonstrated socioeconomic factors related to VL in different locations (Werneck et al., 2002, Costa et al., 2005) through different mechanisms. The included studies pointed out the high incidence of VL associated with the worst living conditions of the populations and the lowest urban structure corroborating with the study by Araújo et al. (2018). Such factors are also responsible for the expansion of the disease in endemic regions (da Silva et al., 2008). The poor living conditions of the population contribute to the strengthening of the VL epidemiological chain (Ponte et al., 2011).
Studies carried out in epidemic periods point to a relationship between infestation and vector abundance and disease, which is also observed in the occurrence of VL in endemic periods. Ponte et al. (2011) showed that the presence of sandflies in the home was among the variables associated with infection in the municipality of Raposa (state of Maranhão). The fact that L. longipalpis is capable of adapting to different habitats (de Oliveira and Araújo et al., 2003; Barata et al., 2005) with evidence of the presence of sandflies in intra- and peri-domiciliary areas (Resende et al., 2006) may explain the relationship with the disease.
Cases of positive dogs have been linked to VL in humans in epidemic periods in the study by de Freitas et al. (2013) in Fortaleza (Ceará), from 2006 to 2012. Similar observations were reported in Belo Horizonte (Minas Gerais) (Oliveira et al., 2001; de Araújo et al., 2013; Bruhn et al., 2018) and in Feira de Santana (Bahia) (Carneiro et al., 2004) in endemic periods. In addition to the dog being considered the most important reservoir of infection in urban environments (Braga et al., 1986), the proximity between homes and shelters for domestic animals may be responsible for the presence of insects inside the home, together with their capacity for endophilia (Missawa and Dias, 2007).
For the presence of other animals, although there is no clear evidence about risk factors, and they are not reservoirs for Leishmania (Otranto et al., 2010), the studies in this review showed a relationship between VL and the presence of other animals. The presence of other animals in the household may be related to the favourable environment for the breeding of phlebotomine by the production of organic waste produced by these animals (Carvalho et al., 2000).
This study has some limitations that should be mentioned. In the sample of studies selected and analysed, there was no longitudinal study addressing the factors associated with VL, despite the importance of these studies to increase the strength of the scientific evidence of the results. Moreover, it was not possible to perform a meta-analysis due to the weaknesses of the measurements used and the differences in them performed in each study.
The studies included in this review took place in the states of the Northeast, North, Southeast and Central-West regions. The higher concentration and the absence of studies in certain regions reflect the distribution of the disease in Brazil. Studies that better clarify the risk factors are needed for intervention and planning of disease control policies in different regions of the country and to help design effective strategies to control the spread of VL in urban areas.
Despite the identification and description of the factors related to epidemics in Brazil presented here, more robust studies are needed to investigate the different behaviour patterns of the factors in different regions and within the same region. These studies will guide strategies to control VL transmission in epidemic contexts.
Author contributions
CSSC, DSB and MC conceptualized the study design. CSSC and NSG independently selected the studies. CSSC, VCO and DTC contributed to analysing the studies. CSSC, DSB, NSG and MC drafted the manuscript. All authors read and approved the final version of the manuscript.
Financial support
MC is grateful to CNPq-Brazil (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for the productivity research fellowships and Fundação de Amparo à Pesquisa de Minas Gerais – FAPEMIG for the financial support of Pesquisador Mineiro (PPM-00591-16). DSB is grateful for the research grant (409901/2016-4) from CNPq-Brazil (Conselho Nacional de Desenvolvimento Científico e Tecnológico).
Ethical standards
Not applicable.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182021000019.
click here to view supplementary material
Conflict of interest
None to declare.
References
- Albuquerque PLMM, da Silva Júnior GB, Freire CCF, Oliveira SBC, Daniel Medeiros Almeida DM, da Silva HF, Cavalcante MS and Sousa AQ (2009) Urbanization of visceral leishmaniasis (kala-azar) in Fortaleza, Ceará, Brazil. Revista Panamericana de Salud Pública 26, 330–336. [DOI] [PubMed] [Google Scholar]
- Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J and de Boer M (2012) Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 7, e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo WS, de Freitas-Neta MSB, Teixeira VJCL, e Silva-Neto JAL, Monteiro MA, Alves JWL, Cândido ELC and Firmino PRA (2018) Correlação entre leishmaniose visceral e indicadores socioeconômicos. Revista Multidisciplinar e de Psicologia 12, 817–824. [Google Scholar]
- Badaro R, Jones TC, Carvalho EM, Sampaio D, Reed SG, Barral A, Teixeira R and Johnson WD JR (1986) New perspectives on a subclinical form of visceral leishmaniasis. The Journal of Infectious Diseases 154, 1003–1011. [DOI] [PubMed] [Google Scholar]
- Barata RA, França-Silva JC, Mayrink W, Silva JC, Prata A, Lorosa ES, Fiúza JA, Gonçalves CM, de Paula KM and Dias ES (2005) Aspectos da ecologia e do comportamento de flebotomíneos em área endêmica de leishmaniose visceral, Minas Gerais. Revista da Sociedade Brasileira de Medicina Tropical 38, 421–425. [DOI] [PubMed] [Google Scholar]
- Bavia ME, Carneiro DD, Gurgel HC, Madureira Filho C and Barbosa MG. (2005) Remote sensing and geographic information systems and risk of American visceral leishmaniasis in Bahia, Brazil. Parasitologia 47, 165–169. [PubMed] [Google Scholar]
- Belo VS, Werneck GL, Barbosa DS, Simões TC, Nascimento BWL, Silva ES, da Silva ES and Struchiner CJ (2013) Factors associated with visceral leishmaniasis in the Americas: a systematic review and meta-analysis. PLoS Neglected Tropical Diseases 7, e2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges BKA, Silva JA, Haddad JPA, Moreira EC, Magalhães DF, Ribeiro LML and Fiúza VOP (2008) Assessment of knowledge and preventive attitudes concerning visceral leishmaniasis in Belo Horizonte, Minas Gerais State, Brazil. Cadernos de Saúde Pública 24, 777–784. [DOI] [PubMed] [Google Scholar]
- Borges BKA, Silva JA, Haddad JPA, Moreira EC, Magalhães DF, Ribeiro LML and Fiúza VOP (2009) Animal presence and the risk for transmission of visceral leishmaniasis in Belo Horizonte, Brazil. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 61, 1035–1043. [Google Scholar]
- Braga J and Werneck GL (2008) Vigilância epidemiológica. In RA Medronho, et al. (ed.), Epidemiologia. São Paulo: Atheneu, pp 103–121. [Google Scholar]
- Braga RR, Lainson R, Shaw JJ, Ryan L and Silveira FT (1986) Leishmaniasis in Brazil. XXII: characterization of Leishmania from man, dogs and the sandfly Lutzomyia longipalpis (Lutz & Neiva, 1912) isolated during an outbreak of visceral leishmaniasis in Santarém, Pará state. Transactions of the Royal Society of Tropical Medicine and Hygiene 80, 143–145. [DOI] [PubMed] [Google Scholar]
- Brazuna JCM, e Silva EA, Brazuna JM, Domingos IH, Chaves N, Honer MR, van Onselen VJ and de Oliveira ALL (2012) Profile and geographic distribution of reported cases of visceral leishmaniasis in Campo Grande, State of Mato Grosso do Sul, Brazil, from 2002 to 2009. Revista da Sociedade Brasileira de Medicina Tropical 45, 601–606. [DOI] [PubMed] [Google Scholar]
- Bruhn FRP, Morais MHF, Bruhn NCP, Cardoso DL, Ferreira F and Rocha CM BM (2018) Human visceral leishmaniasis: factors associated with deaths in Belo Horizonte, Minas Gerais state, Brazil from 2006 to 2013. Epidemiology and Infection 146, 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro D, Bavia ME, Rocha W, Lobão J, Madureira-Filho C, de Oliveira J B, da Silva CE, Barbosa MG and Rios R (2004) Identification of risk areas for visceral leishmaniasis through epidemiological studies and remote sensing data in Feira de Santana, Bahia, Brazil (2000–2002). Revista Baiana Saúde Pública 28, 19–32. [Google Scholar]
- Carranza-Tamayo CO, Werneck GL and Romero GAS (2016) Are opossums a relevant factor associated with asymptomatic Leishmania infection in the outskirts of the largest Brazilian cities? Brazilian Journal of Infectious Diseases 20, 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho ML, Rebêlo JMM, Araújo JC and Barros VLL (2000) Aspectos ecológicos dos flebotomíneos (Diptera: Psychodidae) do município de São José de Ribamar, MA, Brasil. Área endêmica de leishmanioses. Entomologia y Vectores 7, 19–32. [Google Scholar]
- Cerbino-Neto J, Werneck GL and Costa, CHN (2009) Factors associated with the incidence of urban visceral leishmaniasis: an ecological study in Teresina, Piauí State, Brazil. Cadernos de Saúde Pública 25, 1543–1551. [DOI] [PubMed] [Google Scholar]
- Costa CHN, Pereira HF and Araújo MV (1990) Visceral leishmaniasis epidemic in Piaui state, Brazil, 1980–1986. Revista de Saúde Pública 24, 361–372. [DOI] [PubMed] [Google Scholar]
- Costa CHN, Werneck GL, Rodrigues L JR, Santos MV, Araújo IB, Moura LS, Moreira S, Gomes RBB and Lima SS (2005) Household structure and urban services: neglected targets in the control of visceral leishmaniasis. Annals of Tropical Medicine & Parasitology 99, 229–236. [DOI] [PubMed] [Google Scholar]
- Cunha S, Freire M, Eulalio C, Cristovao J, Netto J, Johnson WD, Reed SG and Badaro R (1995) Visceral leishmaniasis in a new ecological niche near a major metropolitan area of Brazil. Transactions of the Royal Society of Tropical Medicine and Hygiene 89, 155–158. [DOI] [PubMed] [Google Scholar]
- da Silva AR, Tauil PL, Cavalcante MNS, Medeiros MN, Pires BN and Gonçalves EGR (2008) Situação epidemiológica da leishmaniose visceral, na Ilha de São Luís, Estado do Maranhão. Revista da Sociedade Brasileira de Medicina Tropical 41, 358–364. [DOI] [PubMed] [Google Scholar]
- da Silva LB, de Aquino DMC, Bezerra JMT, Melo MN, Leonardo FS, e Silva ASG and Pinheiro VCS (2016) Factors associated with Visceral Leishmaniasis in an endemic area of Codó, State of Maranhão, Brazil. Revista de Epidemiologia e Controle de Infecção 6, 74–80. [Google Scholar]
- de Almeida AS, Medronho RA and Werneck GL (2011) Identification of risk areas for visceral leishmaniasis in Teresina, Piauí State, Brazil. American Journal of Tropical Medicine and Hygiene 84, 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araújo VEM, Pinheiro LC, Almeida MCM, de Menezes FC, Morais MHF, Reis IA, Assunção RM and Carneiro M (2013) Relative risk of visceral Leishmaniasis in Brazil: a spatial analysis in urban area. PLoS Neglected Tropical Diseases 7, e2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Delgado O, Feliciangeli MD, Gomez B, Alvarado J, Garcia I and Bello C (1998) The re-emergence of American visceral leishmaniasis in an old focus in Venezuela: present situation of human and canine infections. Parasite 5, 317–323. [DOI] [PubMed] [Google Scholar]
- de Freitas JCC, Sampaio-Filho AP, Santos GJLS, Lima AL and Nunes-Pinheiro DCS (2013) Analysis of seasonality, tendencies and correlations in human and canine visceral leishmaniasis. Acta Scientiae Veterimariae 41, 1151. [Google Scholar]
- de Oliveira SS and de Araújo TM (2003) Evaluation of control measures for visceral leishmaniasis (kala azar) in an endemic area in Bahia, Brazil (1995–2000). Cadernos de Saúde Pública 19, 1681–1690. [DOI] [PubMed] [Google Scholar]
- de Oliveira ALL, Paniago AMM, Sanches MA, Dorval MEC, Oshiro ET, Leal CRB, de Paula FH, Pereira LG, da Cunha RV and Bóia MN (2008) Asymptomatic infection in family contacts of patients with human visceral leishmaniasis in Três Lagoas, Mato Grosso do Sul State, Brazil. Cadernos de Saúde Pública, 24, 2827–2833. [DOI] [PubMed] [Google Scholar]
- Desjeux P. (2001) The increase in risk factors for leishmaniasis worldwide. Transactions of the Royal Society of Tropical Medicine and Hygiene 95, 239–243. [DOI] [PubMed] [Google Scholar]
- de Toledo CRS, de Almeida AS, Chaves SAM, Sabroza PC, Toledo LM and Caldas JP (2017) Vulnerability to the transmission of human visceral leishmaniasis in a Brazilian urban area. Revista de Saúde Pública 51, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Reis LL, Balieiro AAS, Fonseca FR and Gonçalves MJF (2019) Visceral leishmaniasis and its relationship with climate and environmental factors in the State of Tocantins, Brazil, from 2007 to 2014. Cadernos de Saúde Pública 35, e00047018. [DOI] [PubMed] [Google Scholar]
- Evans TG, Teixeira MJ, McAuliffe IT, Vasconcelos I, Vasconcelos AW, Sousa AA, Lima JW and Pearson RD (1992) Epidemiology of visceral leishmaniasis in northeast Brazil. The Journal of Infectious Diseases 166, 1124–1132. [DOI] [PubMed] [Google Scholar]
- Falqueto A, Ferreira AL, dos Santos CB, Porrozzi R, da Costa MVS, Teva A, Cupolillo E, Campos-Neto A and Grimaldi G JR (2009) Cross-sectional and longitudinal epidemiologic surveys of human and canine Leishmania infantum visceral infections in an endemic rural area of southeast Brazil (Pancas, Espírito Santo). The American Journal of Tropical Medicine and Hygiene 80, 559–565. [PubMed] [Google Scholar]
- Feliciangeli MD, Delgado O, Suarez B and Bravo B (2006) Leishmania and sand flies: proximity to woodland as a risk factor for infection in a rural focus of visceral leishmaniasis in west central Venezuela. Tropical Medicine and International Health 11, 1785–1791. [DOI] [PubMed] [Google Scholar]
- Gouvêa MV, Werneck GL, Costa CHN and Carvalho FAA (2007) Factors associated to Montenegro skin test positivity in Teresina, Brazil. Acta Tropica 104, 99–107. [DOI] [PubMed] [Google Scholar]
- Imamura H, Downing T, den Broeck FV, Sanders MJ, Rijal S, Sundar S, An Mannaert A, Vanaerschot M, Berg M, Muylder GD, Dumetz F, Cuypers B, Maes I, Domagalska M, Decuypere S, Rai K, Uranw S, Bhattarai NR, Khanal B, Prajapati VK, Sharma S, Stark O, Schönian G, Koning HPD, Settimo L, Vanhollebeke B, Roy S, Ostyn B, Boelaert M, Maes L, Berriman M, Dujardin JC and Cotton JA (2016) Evolutionary genomics of epidemic visceral leishmaniasis in the Indian subcontinent. eLife 5, e12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerônimo SMB, Duggal P, Braz FSR, Cheng C, Monteiro GRG, Nascimento ET, Martins DRA, Karplus TM, Ximenes MFFM, Oliveira CCG, Pinheiro VG, Pereira W, Peralta JM, Sousa J, Medeiros IM, Pearsoni RD, Burns TL, Pugh EW and Wilson ME (2004) An emerging peri-urban pattern of infection with Leishmania chagasi, the protozoan causing visceral leishmaniasis in northeast Brazil. Scandinavian Journal of Infectious Diseases 36, 443–449. [DOI] [PubMed] [Google Scholar]
- Lima ALM, de Lima ID, Coutinho JFV, de Sousa UPST and Rodrigues MAG (2017) Changing epidemiology of visceral leishmaniasis in northeastern Brazil: a 25-year follow-up of an urban outbreak. Transactions of the Royal Society of Tropical Medicine and Hygiene 111, 440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes JA, Luz TCB, de Sousa FF, Verne RN, Lima FP and Margonari C (2016) Peridomiciliary risk factors and knowledge concerning visceral leishmaniasis in the population of Formiga, Minas Gerais, Brazil. Revista Brasileira de Epidemiologia 19, 362–374. [DOI] [PubMed] [Google Scholar]
- Menon SS, Rossi R, Nshimyumukiza L and Zinszer K (2016) Decentralized control of human visceral leishmaniasis in endemic urban areas of Brazil: a literature review. Tropical Medicine and Health 44, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missawa NA and Dias ES (2007) Phlebotomine sand flies (Diptera: Psychodidae) in the municipality of Várzea Grande: an area of transmission of visceral leishmaniasis in the state of Mato Grosso, Brazil. Memória do Instituto Oswaldo Cruz 102, 913–918. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG and PRISMA and Group (2015) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Medicine 3123–130. [PMC free article] [PubMed] [Google Scholar]
- Moreno EC, Melo MN, Genaro O, Lambertucci JR, Serufo JC, Andrade ASR, Antunes CMF and Carneiro M (2005) Risk factors for Leishmania chagasi infection in an urban area of Minas Gerais state. Revista da Sociedade Brasileira de Medicina Tropical 38, 456–463. [DOI] [PubMed] [Google Scholar]
- Nascimento MD, Alcântara-Neves NM, Muniz ME, Nunes SF, Paranhos M and Carvalho LC (1993) Induction and modulation of the immune response to Leishmania by Montenegro's skin test. Transactions of the Royal Society of Tropical Medicine and Hygiene 87, 91–93. [DOI] [PubMed] [Google Scholar]
- Oliveira CL, Assunção RM, Reis IA and Proietti FA (2001) Distribuição espacial da leishmaniose visceral humana e canina em Belo Horizonte, Minas Gerais, Brasil, 1994–1997. Cadernos de Saúde Pública 17, 1231–1239. [DOI] [PubMed] [Google Scholar]
- Oliveira CL, Diez-Roux A, César CC and Projetti FA (2006) A case-control study of microenvironmental risk factors for urban visceral leishmaniasis in a large city of Brazil, 1999–2000. Revista Panamericana de Salud Publica 20, 369–376. [DOI] [PubMed] [Google Scholar]
- Oliveira IBB, Batista HL, Peluzio JM, Pfrimer IAH, Rodrigues FM and Carmo-Filho JR (2014) Epidemiological and environmental aspects of visceral leishmaniasis in children under 15 years of age between 2007 and 2012 in the City of Araguaína, State of Tocantins, Brazil. Revista da Sociedade Brasileira de Medicina Tropical 47, 476–482. [DOI] [PubMed] [Google Scholar]
- Otranto D, Testini G, Buonavoglia C, Parisi A, Brandonisio O, Circella H, Dantas-Torres F and Camarda A (2010) Experimental and field investigations on the role of birds as hosts of Leishmania infantum, with emphasis on the domestic chicken. Acta Tropica 113, 80–83. [DOI] [PubMed] [Google Scholar]
- Ponte CB, Souza NC, Cavalcante MN, Barral AMP, de Aquino DMC and Caldas AJM (2011) Risk factors for Leishmania chagasi infection in an endemic area in Raposa, State of Maranhão, Brazil. Revista da Sociedade Brasileira de Medicina Tropical 44, 712–721. [DOI] [PubMed] [Google Scholar]
- Rêgo FD, Souza GD, Miranda JB, Peixoto LV and Andrade-Filho JD (2020) Potential vectors of Leishmania parasites in a recent focus of visceral Leishmaniasis in neighborhoods of Porto Alegre, State of Rio Grande do Sul, Brazil. Journal of Medical Entomology 57, 1286–1292. [DOI] [PubMed] [Google Scholar]
- Resende MC, Camargo MC, Vieira JR, Nobi RC, Porto MN, Oliveira CD, Pessanha JE, Cunga MDA, Brandão ST (2006) Seasonal variation of Lutzomyia longipalpis in Belo Horizonte, State of Minas Gerais Revista da Sociedade Brasileira de Medicina Tropical 39, 51-55.. [DOI] [PubMed]
- Rivas GB, de Souza NA, Peixoto AA and Bruno RV (2014) Effects of temperature and photoperiod on daily activity rhythms of Lutzomyia longipalpis (Diptera: Psychodidae). Parasites Vectors 7, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha ATF, de Espindola GM, Soares MRAS, Rocha JRS and Costa CHNC (2018) Visceral leishmaniasis and vulnerability conditions in an endemic urban area of northeastern Brazil. Transactions of the Royal Society of Tropical Medicine and Hygiene 112, 317–325. [DOI] [PubMed] [Google Scholar]
- Romero GA and Boelaert M (2010) Control of visceral leishmaniasis in Latin America – a systematic review. PLoS Neglected Tropical Diseases 4, e584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevá AP, Mao L, Galvis-Ovallos F, Lima JMT and Valle D (2017) Risk analysis and prediction of visceral leishmaniasis dispersion in São Paulo State, Brazil. PLoS Neglected Tropical Diseases 11, e0005353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma MC, Gupta AK, Saran R and Sinha SP (1990) The effect of age and sex on the incidence of kala-azar. The Journal of Communicable Diseases 22, 277–278. [PubMed] [Google Scholar]
- Sherlock IA 1996. Ecological interactions of visceral leishmaniasis in the State of Bahia, Brazil. Memórias do Instituto Oswaldo Cruz 91, 671–683. [DOI] [PubMed] [Google Scholar]
- Silva LDA, Romero HD, Prata A, Costa RT, Nascimento E, Carvalho SFG and Rodrigues V (2006). Immunologic tests in patients after clinical cure of visceral leishmaniasis. American Journal of Tropical Medicine and Hygiene 75, 739–743. [PubMed] [Google Scholar]
- Viana GMC, Nascimento MDSB, Rabelo EMF, Diniz-Neto JA, Binda-Júnior, JR, Galvão CSS, dos Santos AC, Santos-Júnior OM, de Oliveira RAS and Guimarães RS (2011) Relationship between rainfall and temperature: observations on the cases of visceral leishmaniasis in São Luis Island, State of Maranhão, Brazil. Revista da Sociedade Brasileira de Medicina Tropical 44, 722–724. [DOI] [PubMed] [Google Scholar]
- Werneck GL and Maguire JH (2002) Spatial modelling using mixed models: an ecological study of visceral leishmaniasis in Teresina, Piauí State, Brazil. Cadernos de Saúde Pública 18, 633–637. [DOI] [PubMed] [Google Scholar]
- Werneck GL, Rodrigue- Júnior L, Santos MV, Araújo IB, Moura LS, Lima SS, Gomes RBB, Maguire JH and Costa CHN (2002) The burden of Leishmania chagasi infection during an urban outbreak of visceral leishmaniasis in Brazil. Acta Tropica 83, 13–18. [DOI] [PubMed] [Google Scholar]
- Werneck GL, Costa CNH, Wlaker AM, David JR, Wand M and Maguire JH (2007) Multilevel modelling of the incidence of visceral leishmaniasis in Teresina, Brazil. Epidemiology and Infection 135, 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2018) Status of endemicity of visceral leishmaniasis worldwide. Available at https://www.who.int/docs/default-source/ntds/leishmaniasis/gho-vl-2018.pdf?sfvrsn=b748935a_4 (Accessed 29 June 2019).
- Ximenes MFFM, e Silva VPM, de Queiroz PVS, Rego MM, Cortez AM, Batista LMM, de Medeiros AS and Jeronimio SMB (2007) Flebotomíneos (Diptera: Psychodidae) e leishmanioses no Rio Grande do Norte, Nordeste do Brasil: reflexos do ambiente antrópico. Neotropical Entomology 36, 128–137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182021000019.
click here to view supplementary material