Abstract
This study aimed to evaluate the performance of the point-of-care circulating cathodic antigen (POC-CCA) test in a highly endemic area in Brazil, comparing it to the Kato-Katz (KK) technique for sensitivity, specificity and the intensity of the reaction of the test in relation to the parasitic load. The community in Sergipe, Brazil, participated in the study, providing three stool samples, one of urine (POC-CCA) and fingers tick blood sample was tested by enzyme-linked immunosorbent assay (ELISA). Sensitivity, specificity, positive predictive value, negative predictive value, accuracy, kappa coefficient and Spearman's correlation were calculated for the POC-CCA test using the KK as the reference. The prevalence of schistosomiasis by KK testing was 48.82%; POC-CCA (t+) 66.14%; POC-CCA (t−) 45.24%. ELISA results showed 100% agreement in individuals with high and moderate eggs per gram (EPG). POC-CCA presented good diagnostic performance in individuals with medium and high EPG, but there were a high number of false negatives in individuals with low intensity infections. As observed, POC-CCA-filter test improves accuracy and sensitivity compared to a conventional test.
Key words: Diagnosis, high endemicity, immunochromatography, Kato-Katz, POC-CCA, Schistosoma mansoni
Introduction
Schistosomiasis is one of the most prevalent neglected tropical diseases in developing countries and is closely associated with poor sanitation, lack of clean water and low socioeconomic conditions (Danso-Appiah et al., 2016). According to data from the World Health Organization (WHO, 2020a), this parasitosis is endemic in 78 countries. It is estimated that there are more than 200 million individuals with schistosomiasis globally and approximately 1 billion people live in areas at risk for transmission of the disease (Oliveira et al., 2018). In Brazil, data from the Ministry of Health's National Survey on Prevalence of Schistosomiasis and Geo-Helminths (INPEG) estimated about 1.5 million people infected with Schistosoma mansoni in the country (almost 1% of the population) between 2011 and 2014 (Katz, 2018). According to data from the Information System of the Schistosomiasis Control Program (SISPCE), Department of Informatics of the Unified Health System (DATASUS), analysis of the results of the examinations conducted over a longer period of time (2010–2015) estimated the positivity rate in the country at 4.4% (Brasil, 2017).
The diagnostic method most commonly used in epidemiological surveys for schistosomiasis mansoni is the Kato-Katz (KK) technique. However, the KK sensitivity decreases in individuals with low parasite loads. Thus, KK cannot be considered a ‘gold standard’. The WHO also recommends circulating cathodic antigen (CCA) testing in areas of active disease transmission (World Health Organization, 2020b).
The point-of-care (POC)-CCA test is a rapid qualitative diagnostic test for detection of S. mansoni infection. It detects a specific carbohydrate antigen associated with the parasite's gut. Adult worms regurgitate this antigen, which circulates in the bloodstream of the infected individual, is eliminated by the kidneys and can be detected in urine (van Dam et al., 2004). The use of urine rather than stool increases the acceptance of the testing in affected populations. In addition, the possibility of performing the testing in the field increases the speed with which results can be obtained and reduces requirements for modern equipment and advanced training (Utzinger et al., 2015).
In recent years, POC-CCA has been used in several endemic areas for schistosomiasis in Africa (Bärenbold et al., 2018; Rubaba et al., 2018). Studies in areas where KK has a prevalence of ⩾50%, POC-CCA positivity is similar, showing a good performance when compared to KK (Kittur et al., 2016; Sanneh et al., 2017; Fuss et al., 2018; Okoyo et al., 2018). However, in areas with prevalence of ⩽50%, the POC-CCA is much more sensitive compared to KK, with an increase of 1.5- to 6-fold higher in the detection power as the prevalence by KK decreases (Tchuem Tchuenté et al., 2012; Adriko et al., 2014; World Health Organization, 2020a, 2020b). As a result, there are still many uncertainties regarding the specificity and sensitivity of the POC-CCA test, especially with reference to the interpretation of weak ‘trace’ results, which are considered positive by the manufacturer.
In Africa, most studies find that POC-CCA is more sensitive compared to KK, but in Brazil the situation is different, with low sensitivity of POC-CCA in areas that have low KK prevalence (Bezerra et al., 2018; Oliveira et al., 2018). For further investigation of this phenomenon, we compared the performance of POC-CCA with KK, in an area of high endemicity, and we evaluated whether there is a correlation between the intensity of the immunochromatographic reaction of the POC-CCA test with the parasite load.
Materials and methods
Study area
The study was conducted in 2018 in the locality of Siebra, in the municipality of Maruim, Sergipe state, Brazil. This rural community has 179 residents, with similar numbers of men and women. The age range varies from 1 to 82 years (mean age 27.6 years), most people live in brick homes and are predominantly subsistence farmers. Siebra (10°44′6″ latitude and 37°04′35″ longitude) is located 32 km from the state capital, Aracaju (IBGE, 2016). According to data from the State Health Department, the last schistosomiasis assessment in Siebra was in 2008, showing a prevalence of 37.9% of S. mansoni infection in the community.
Ethical considerations
The study was approved by the ethics committee of the Federal University of Ceará – UFC (2647566) and by the ethics committee of the Federal University of Sergipe UFS (2806891). It was conducted in accordance with Resolution 466/12 of the National Health Council. District health authorities and the entire community were informed about the purpose, procedures, risks and potential benefits of the study. Prior to enrolment and collection of biological materials, written consent was obtained from participants. Parents/guardians and their children provided written consent for to participate in the research.
Sample collection
After giving consent, study participants provided three stool samples on alternating days, one urine sample and one fingerstick blood sample. Individuals who did not provide one of the requested biological samples, or did not deliver samples of sufficient quality and quantity to perform the procedures were excluded. Two slides per stool were prepared in loco by the KK technique using the Helm-Test® kit produced by Bio-Manguinhos-Fiocruz (Rio de Janeiro, RJ, Brazil). Urines were aliquoted and transported under refrigeration to the laboratory where they were stored at −20°C. Capillary blood was collected on filter paper (Tropbio Pty Ltd, QLD, Australia), dried, and stored at −20°C.
Kato-Katz
The six KK slides prepared for each study participant were screened for the presence of S. mansoni eggs (Katz et al., 1972; Berhe et al., 2004). The intensity of infection was expressed as eggs per gram (EPG) feces and infected individuals were classified as having low (1–99 EPG), moderate (100–399) or heavy (⩾400 EPG) intensity infections according to WHO guidelines (World Health Organization, 2011).
Circulating cathodic antigen test (POC-CCA)
For POC-CCA diagnosis, all urine specimens were thawed and processed according to the manufacturer's instructions (Rapid Medical Diagnostics, Pretoria, South Africa). All kits used were part of the same lot (lot no. 180703067). The tests were classified using the G (G1–G10) score scale as standard, with G1 considered negative, G2 and G3 considered trace and G4–G10 being positive, according to the reaction intensity of the test band (Casacuberta-Partal et al., 2019). Positive results were stratified (Table 1) using a semi-quantitative scale at 1+ (G4 and G5), 2+ (G6 and G7) and 3+ (G8–G10) (Casacuberta-Partal et al., 2019). All tests were read by two trained investigators throughout the study. In cases of discordant results, a third investigator was consulted until a consensus was reached.
Table 1.
Prevalence of schistosomiasis according to KK and POC-CCA method, considering trace as positive (t+) and trace as negative (t−), in the 127 individuals tested
| Test | Samples | Positives | Positivity (%) |
|---|---|---|---|
| Kato-Katz | 1, 2 and 3 samples (6 slides) | 62 | 48.82 |
| POC-CCA (t+) | 1 sample | 84 | 66.14 |
| POC-CCA (t−) | 1 sample | 57 | 45.24 |
POC-CCA filter test (POC-CCA-FLT)
The samples that were negative or ‘trace’ by POC-CCA but were from persons who were egg positive by the KK technique, were concentrated by filtering 0.5 mL of urine through a 30-kDa membrane (MRCF0R030, Merck Millipore, Darmstadt, Germany), and then centrifuged for 30 min, at 2000 g. The pellet was resuspended in 0.05 mL distilled water, and then tested again by POC-CCA (Grenfell et al., 2018).
Enzyme-linked immunosorbent assay (ELISA)
For the ELISA, dried blood spots were sent to the Division of Parasitic Diseases and Malaria at the Centers for Disease Control and Prevention in Atlanta, GA USA. In brief, the sample of blood was transferred to a 1.5 mL microtube and incubated at 4°C overnight with 250 μL of 0.1 m phosphate-buffered saline (PBS) containing 0.3% Tween 20 and 5% nonfat milk. After overnight incubation on a shaker, the samples were transferred to Immulon 2HB plates (ThermoScientific, Rochester, NY) that had been coated with 100 μL of 2 μg mL−1 soluble schistosome egg antigen in 0.1 m sodium bicarbonate buffer, pH 9.6. Standards, controls and samples were diluted to a 1:50 concentration with 0.01 m PBS, pH 7.2/0.3% Tween-20/5% milk and allowed to incubate on the plate at room temperature for 30 min. All standards, controls and samples were run in duplicate. Plates were washed five times with PBS containing 0.3% Tween 20. The conjugate used was mouse anti-human IgG HRP (SouthernBiotech, Birmingham, AL) prepared at a dilution of 1:50 000 in 0.01 m PBS, pH 7.2/0.3% Tween-20 and incubated for 30 min. Following incubation with the conjugate and washing plate as above, room temperature SureBlue™ TMB substrate (SeraCare Life Sciences, Gaithersbug, MD) was allowed to incubate for 5 min prior to the addition of 1 N sulphuric acid to stop any further reaction. A standard curve of 0–500 arbitrary units (AU) using pooled positive sera was included on each plate. The cutoff used for positives is 40 AU.
Statistical analysis
All data were entered into Excel spreadsheets (Microsoft Corp., Redmond, WA, USA) for management and analysis. To determine the performance of each method, we calculated the sensitivity, specificity, positive (PPV) and negative (NPV) predictive values using MedCalc® statistical software, version 19.1 (Osten, Belgium). The agreement between the results of the KK and POC-CCA techniques, as well as the relationship of parasitic load (EPG) with the intensity of the reaction detected by POC-CCA was evaluated using the kappa index, calculated using the MedCalc® program. The graphics were created using GraphPad Prism® 6, version 6.1 for Windows (GraphPad Software, La Jolla California, USA).
Receiver operating characteristic (ROC) curves were used to compare sensitivity and specificity between tests, evaluating the true-positive rate vs the false-positive rate.
The correlations between the mean EPG, determined by KK egg counts and the intensity of the POC-CCA reaction, classified through the semi-quantitative scale, were calculated using the nonparametric Spearman test. Differences were considered statistically significant when P < 0.05.
Results
Of the 179 inhabitants of Siebra, 127 (70.95%) participated in the study; 55 (43.31%) were men and 72 (56.69%) were women, with an average age of 27.2 years. The prevalence for S. mansoni, according to the KK technique, was 48.82% (62/127).
Stratification of parasitic load by EPG showed 29.03% (18/62) with heavy intensity infections, 16.13% (10/62) with moderate intensity infections and 54.84% (34/62) with low intensity infections. The geometric mean EPG of the participants was 390.3 [95% confidence interval (CI) 230.5–530.1].
The POC-CCA results showed 57 positives (44.88%), 27 ‘trace’ (21.26%) and 43 negatives (33.86%). The positivity index for schistosomiasis by POC-CCA was 66.14% (84/127), considering (t+) and 44.88% (57/127), considering (t−) (Table 1).
Positive results are stratified in Table 2 by age group and diagnostic method used. Children aged 10–14 years presented the highest percentage of positivity. Of the positive individuals, identified by the KK technique, 29% were in the age groups under 15 years and approximately 34% in the age groups between 19 and 29 years. From 50 years onwards, the positivity rates reduced to approximately 10%, in both diagnostic methods used.
Table 2.
Prevalence in the different methods used, stratified by age group
| Age group (years) | Total | Kato-Katz | POC-CCA | |
|---|---|---|---|---|
| No. of positives (%) | POC-CCA (t+) (%) | POC-CCA (t−) (%) | ||
| 2–4 | 5 | 0 | 3 (3.57) | 2 (3.51) |
| 5–9 | 19 | 7 (11.29) | 12 (14.29) | 9 (15.79) |
| 10–14 | 13 | 11 (17.74) | 11 (13.10) | 9 (15.79) |
| 15–19 | 10 | 6 (9.68) | 9 (10.71) | 7 (12.28) |
| 20–24 | 12 | 9 (14.52) | 11 (13.10) | 7 (12.28) |
| 25–29 | 14 | 6 (9.68) | 9 (10.71) | 6 (10.53) |
| 30–39 | 25 | 10 (16.13) | 14 (16.67) | 8 (14.03) |
| 40–49 | 12 | 7 (11.29) | 7 (8.33) | 3 (5.26) |
| ⩾50 | 17 | 6 (9.68) | 8 (9.52) | 6 (10.53) |
A greater concordance was identified between the KK and POC-CCA results when trace was considered negative and the diagnostic failure was higher when trace was considered positive. Of the individuals who were egg positive by the KK technique, 25.8% were negative by POC-CCA (t−). Among the 43 individuals negative by POC-CCA (t+), 20.93% (9/43) were egg positive by the KK technique. Only 25.93% (7/27) of the individuals who had trace POC-CCA had detectable eggs in their stool (Table 3).
Table 3.
Analysis of agreement and diagnostic accuracy of POC-CCA, compared to KK results
| POC-CCA (t+) | Kato-Katz | POC-CCA (t−) | Kato-Katz | ||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Total | Positive | Negative | Total | ||
| Positive | 53 | 31 | 84 | Positive | 46 | 11 | 57 |
| Negative | 9 | 34 | 43 | Negative | 16 | 54 | 70 |
| Total | 62 | 65 | 127 | Total | 62 | 65 | 127 |
| Sensitivity | 85.48% (95% CI 74.22–93.14) | Sensitivity | 74.19% (95% CI 61.50–84.47) | ||||
| Specificity | 52.31% (95% CI 39.54–64.85) | Specificity | 83.08% (95% CI 71.73–91.24) | ||||
| PPV | 63.10% (95% CI 56.51–69.23) | PPV | 80.87% (95% CI 70.53–87.06) | ||||
| NPV | 79.07% (95% CI 66.42–87.83) | NPV | 77.14% (95% CI 68.57–83.92) | ||||
| Accuracy | 68.50% (95% CI 59.67–76.45) | Accuracy | 78.74% (95% CI 70.60–85.50) | ||||
| κ | 0.37 (95% CI 0.21–0.54) | κ | 0.57 (95% CI 0.43–0.72) | ||||
CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; k, Kappa coefficient.
The agreement, calculated using the kappa index, considering POC-CCA (t+) and KK, was 68% (k = 0.37), considered as a discrete agreement (standard error: 0.0859). Sensitivity was 85.48% and specificity was 52.31%, with a PPV of 63.10%, NPV of 79.07% and accuracy of 68.50% (Table 3). When we considered POC-CCA (t−), we observed that the agreement improved to 78% (k = 0.57), considered as a moderate agreement (standard error: 0.0728). Sensitivity dropped to 74.19%, but specificity increased to 83.08%. PPV, NPV and accuracy were 80.87, 77.14 and 78.74%, respectively (Table 3).
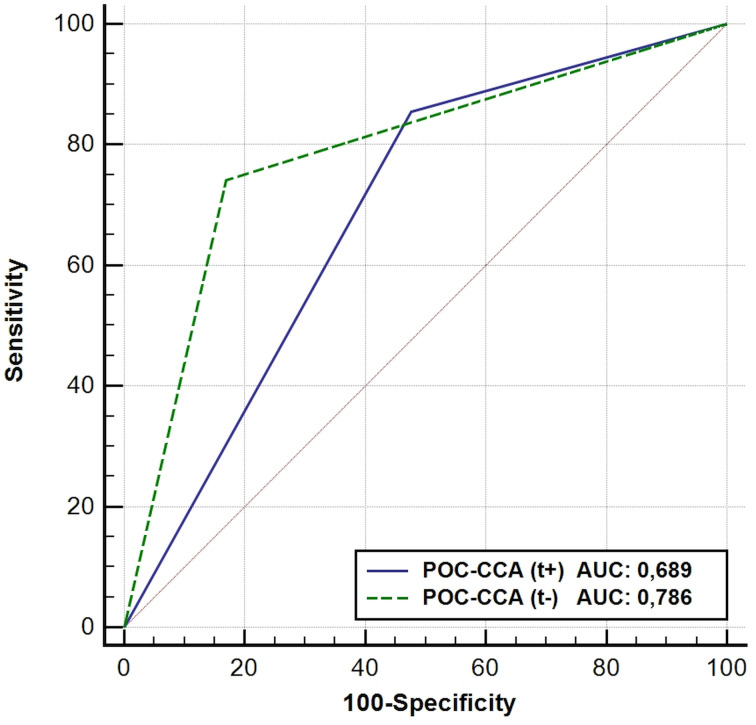
When ROC curve analyses were performed using the KK results as reference, POC-CCA (t+) had smaller sensitivity. The POC-CCA, when the trace was considered negative, had higher discriminatory power, with an area under the curve (AUC) of 0.786 (Fig. 1).
Fig. 1.
ROC curves and AUC of CCA results using KK as reference.
Table 4 presents the classification of POC-CCA results, stratified by G score and the semi-quantitative scale. The semi-quantitative scale classified 47.37% (27/57) of the positive individuals as 3+ (high reactivity), 21.05% (12/57) as 2+ (moderate reactivity) and 31.58% (18/57) as 1+ (low reactivity). G10 was the only class that showed 100% correlation with individuals with heavy intensity parasitic infection. Among the negative and POC-CCA (t+) individuals who had S. mansoni eggs in their feces, 87.50% had low intensity infections, with a maximum of six eggs in a total of six slides analysed. Another important finding is that POC-CCA (t−) failed to detect infection in 16 (22.86%) egg-positive individuals.
Table 4.
Results of POC-CCA, classified by G-score and semi-quantitative scale, ELISA, KK and parasitic load
| POC-CCA | ELISA | Kato-Katz | ||||||
|---|---|---|---|---|---|---|---|---|
| G score | Visual score* | Parasitic load (n and EPG mean) | ||||||
| Classes | n | R | NR | Negative | Low | Moderate | Heavy | |
| 1 | 43 | Neg. | 18 | 25 | 33 | 09 (12, 74) | 00 | 00 |
| 2 | 16 | Trace | 06 | 10 | 12 | 04 (22, 58) | 00 | 00 |
| 3 | 11 | Trace | 08 | 03 | 08 | 03 (17, 27) | 00 | 00 |
| 4 | 10 | 1+ | 04 | 06 | 06 | 04 (51, 78) | 00 | 00 |
| 5 | 08 | 1+ | 04 | 04 | 03 | 04 (34, 98) | 01 (180) | 00 |
| 6 | 06 | 2+ | 04 | 02 | 02 | 03 (22, 1) | 01 (243, 9) | 01 (531, 9) |
| 7 | 06 | 2+ | 05 | 01 | 01 | 02 (51, 6) | 01 (187, 9) | 02 (1.251) |
| 8 | 14 | 3+ | 13 | 01 | 00 | 04 (56, 95) | 05 (271, 78) | 05 (595, 92) |
| 9 | 06 | 3+ | 06 | 00 | 00 | 01 (39, 9) | 02 (289, 95) | 03 (1.086, 63) |
| 10 | 07 | 3+ | 07 | 00 | 00 | 00 | 00 | 07 (1.619, 04) |
*Visual score based on the semi-quantitative scale; R, reactive; NR, non-reactive.
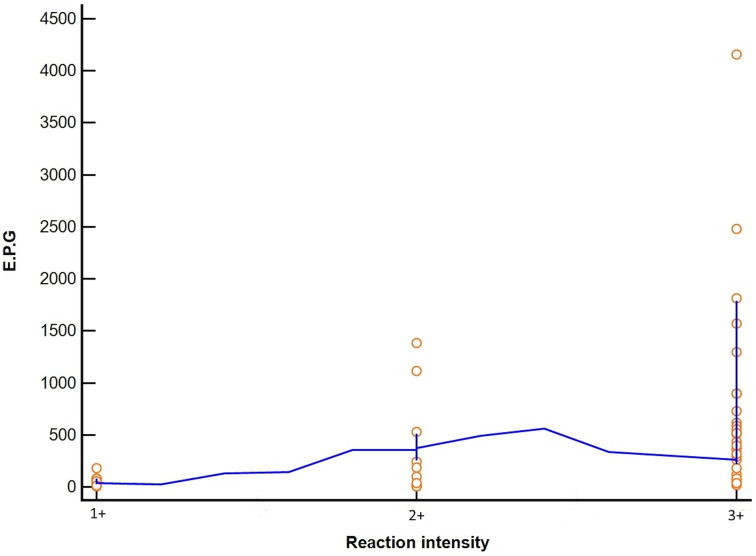
Evaluating the agreement only between the positive individuals in the POC-CCA (G4–G10), that is, considering trace as negative, we found a weak agreement between the parasitic load found by KK and the intensity of the immunochromatographic reaction defined by the semi-quantitative scale, with an agreement index of 54%, κ of 0.29 and standard error of 0.1128. The correlation between these tests by the Spearman calculation was moderate (r = 0.54; P < 0.001; 95% CI 0.305–0.723) (Fig. 2).
Fig. 2.
Correlation between intensity of POC-CCA test bands (G4–G10), classified by semi-quantitative scale, and comparison with parasitic loads according to the KK technique.
We reevaluated the nine POC-CCA ‘trace’ individuals who were KK positive using the POC-CCA filtration (POC-CCA-FLT) technique as well as eight POC-CCA negative individuals who were egg positive. All subjects with ‘trace’ results before urine filtration were positive after the antigen concentration step, five increased to 1+, three to 2+ and one to 3+. Among the eight individuals with eggs that were initially POC-CCA negative, six (75%) became positive, with low reaction intensity (four were 1+ and two were 2+) and two remained negative (25%).
When we evaluated POC-CCA and ELISA results in KK positive individuals, separated by intensity of infection, the ELISA was positive for 90.32% of the individuals with eggs in their stool and was 100% reactive in individuals with moderate and heavy intensity EPG (Table 5).
Table 5.
Results of ELISA and POC-CCA techniques stratified according to the parasite load determined by the KK method
| POC-CCA | Kato-Katz (EPG) | |||||||
|---|---|---|---|---|---|---|---|---|
| Negative | Low | Moderate | Heavy | |||||
| ELISA | ELISA | ELISA | ELISA | |||||
| R | NR | R | NR | R | NR | R | NR | |
| Positive | 02 (1.58%) | 10 (7.87%) | 14 (11.02%) | 04 (3.15%) | 10 (7.87%) | 0 | 18 (14.17%) | 0 |
| Trace | 07 (5.51%) | 13 (10.24%) | 07 (5.51%) | 0 | 0 | 0 | 0 | 0 |
| Negative | 10 (7.87%) | 23 (18.11%) | 07 (5.51%) | 02 (1.58%) | 0 | 0 | 0 | 0 |
EPG, Eggs per gram of feces; R, reactive; NR, non-reactive.
Table 5 shows the classification of the stratified results of KK positivity in relation to the POC-CCA and ELISA, of 28/62 (45.2%) individuals with high and moderate EPG, 100% were positive in the POC-CCA, and all were reactive in the ELISA technique. Of 34/62 (54.8%) who presented low EPG, the POC-CCA (+) detected 21/34 (61.8%), and the ELISA 28/34 (82.35%).
Discussion
The locality of Siebra (Maruim, SE, Brazil) is a highly endemic area, with schistosomiasis prevalence >45%. The average parasite load of the population was 380.3 EPG, where the majority (57.14%) presented low parasite load, but almost 30% had heavy intensity infections. These rates can be explained by the water contact activities of the community (92.74% of the population reports contact), and very poor access to drinking water (9.50%) and sanitary sewage (6.70%). These environmental characteristics are similar in most of the hyperendemic areas, where the poor sanitation conditions contribute to transmission (Saucha et al., 2015).
The high prevalence of schistosomiasis in this area is worrying because it is concentrated in children and economically active age groups, leading to persistence in disease transmission and increasing the risk development of severe pathology, along with an increase in the disability-adjusted life-year index, which directly affects the population's living conditions and perpetuates the cycle of poverty (Nascimento and de Oliveira, 2010). Data from the Schistosomiasis Control Program (PCE) show that of the 6248 parasitological tests (KK technique) performed in the municipality of Maruim between 2000 and 2011, 26.2% were positive. Of these, 48.9% had low parasitic load, 30.7% had moderate intensity infections and 20.4% had heavy intensity infections. Only patients who had S. mansoni eggs in their feces were treated and no other control action was developed by the PCE. As of 2012, the programme did not carry out activities in the municipality as a result of cuts in the federal, state and municipal government health budgets and the prioritization of financial resources towards combating arboviruses (Brasil, 2017).
The POC-CCA (t−) had similar prevalence results to the KK method; however, the number of false negatives (16/127) resulted in only a moderate agreement between these methods. Among the POC-CCA negative (20.93%) and trace (25.92%) participants, some were egg positive with a very low parasitic load (3.9–48 EPG). Other studies performed in Brazil had negative POC-CCA results in individuals with light intensity infections, with most discordant results occurring when trace results were considered positive (Bezerra et al., 2018; Oliveira et al., 2018; Sousa et al., 2019). We also found that some POC-CCA positive individuals were egg negative by KK, similar to other studies showing POC-CCA is more sensitive than stool microscopy (Ashton et al., 2011; Colley et al., 2013; Sousa-Figueiredo et al., 2013).
When trace was considered positive, prevalence was possibly overestimated, as there was an increase in sensitivity but a reduction in specificity and PPV. These data are similar to those of studies conducted in communities on the shores of Lake Victoria, Africa (Standley et al., 2010) and Januária in northern Minas Gerais, Brazil (Oliveira et al., 2018). These results in disparate regions suggest that POC-CCA has more accurate results when trace is considered negative.
The intensity of the POC-CCA reaction (semi-quantitative scale) showed moderate (r = 0.54) correlation with parasitic load based on EPG but agreement between tests was poor (κ = 0.29). The correlation may have been affected by the wide range of EPG values in persons with a class 3+ POC-CCA. Individuals with G8 and G9 POC-CCA scores had diverse parasitic loads (19.9–1812 EPG). Category G10, also class 3+, was the only one in which all individuals had a high parasite load (516–4156 EPG). Class 2+ (G6 and G7) also included individuals with very low egg counts. Our test agreement was similar to what Casacuberta et al. (2016) found in a highly endemic area in southeastern Uganda (r = 0.660) and what Silveira et al. (2016) observed in low and moderate intensity areas in southeastern Brazil (r = 0.625). However, the agreement rate (κ = 0.29) in our study was much lower than seen in southeastern Brazil (κ = 0.66). This suggests that EPG cannot be inferred based only on the intensity of the POC-CCA reaction.
POC-CCA-FLT improved accuracy over conventional POC-CCA; however, we observed that some samples (25%) continue to show false negative results (KK positive but and negative in conventional POC-CCA and POC-CCA-FLT). By contrast, all trace individuals in the conventional POC-CCA who were KK positive had a positive POC-CCA-FLT result. A study conducted in two cities in southeastern Brazil, where the POC-CCA and POC-CCA-FLT tests were evaluated compared to the KK results, also showed that the POC-CCA FLT had superior results in the diagnosis of schistosomiasis compared to traditional POC-CCA (Grenfell et al., 2018). However, the addition of the filtration step makes the test cease to be point-of-care. It also increases costs because adding the 30 kDa filter (MRCF0R030, Merck Millipore, Darmstadt, Germany) raises the price by 102.46%.
Although POC-CCA (t−) detected fewer egg-positive individuals compared to POC-CCA (t+), it still had a higher diagnostic accuracy based on percent agreement, the kappa index, accuracy and AUC. However, for decision-making regarding individual or community-wide treatment, both missed a considerable number of infections (over 20%), which could lead to undertreatment and a perpetuation of schistosomiasis transmission. But if we consider that in an area of high endemicity, the main objective is to reduce prevalence of high intensity infections to <5% and prevent development of severe disease, whether trace is considered positive or negative becomes irrelevant, since the POC-CCA (45.75 and 67.72%, t− and t+ respectively) and KK (48.73%) result in the same mass treatment decision determined by the Brazilian Ministry of Health (Brasil, 2019).
Of the 127 individuals analysed by ELISA, 74 were reactive, suggesting that 59.05% of the residents of Siebra had contact with the parasite. The tests used showed 100% agreement in individuals with heavy and moderate intensity parasitic loads. Those classified with light intensity infections by KK showed some false negative by ELISA and POC-CCA. Although these losses were not significant, our data show that both the POC-CCA and the ELISA have differences diagnostic reactivities in individuals with low intensity infections.
Studies in both Brazil and Africa show that POC-CCA has greater sensitivity compared to KK (Shane et al., 2011; Chernet et al., 2017; Fuss et al., 2018), however it is worth mentioning that in our study we found a number of individuals who were POC-CCA negative with the presence of eggs, as reported in other studies as well (Siqueira et al., 2016; Lindholz et al., 2018).
Conclusion and future directions
Our data suggest that POC-CCA (t−) results are closer to those KK (individuals with heavy intensity infections) and show a better agreement between tests, leading to higher diagnostic accuracy. Nevertheless, there are still a high number of false negatives even in an area of high endemicity. A moderate positive correlation was observed between the reaction of POC-CCA intensity with parasitic load, but with large disagreement of individual results between tests, calling into question the use of the POC-CCA semi-quantitative scale to estimate infection intensity.
As observed by others in Brazil, POC-CCA-FLT improves the test accuracy and sensitivity compared to conventional POC-CCA. However, the addition of this one step makes the test no longer point-of-care and doubles the test costs.
Acknowledgements
We thank the Secretary of State of Health of Sergipe and the Secretary of Health of the municipality of Maruim for their technical support, the people of the Siebra community for their collaboration in implementing this project; Dr Govert van Dam of the University of Leiden in the Netherlands for giving us the parameters for reading the POC-CCA and for donating the Rapid Medical Diagnostics POC-CCA kits; Dr William Evan Secor from the Division of Parasitic Diseases and Malaria, Centers for Disease Control and Prevention, Atlanta, GA, USA, for the ELISA support. We also acknowledge The Federal University of Sergipe and CAPES for granting scholarships to the postgraduate students involved in this study.
Author contributions
Conceived of or designed study: FSMB, MCCP and LB; performed research: DFB, FSMB and MCCP; analysed data: FSMB, DFB and MCCP; contributed new methods or models: RTF, AGV and LB; wrote the paper: DFB and MCCP; wrote sections and revising it critically: DFB, MCCP, LB, AGV, RTF and FSMB. All the authors approved the final version of the manuscript.
Financial support
This work was supported by the CAPES – Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (grant for DFB).
Ethical standards
The authors assert that all procedures contributing to this study comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The study was approved by the Ethics Committee of the Federal University of Ceará – UFC (No. 2647566) and by the Ethics Committee of the Federal University of Sergipe UFS (No. 2806891).
Informed consent
Written informed consent was obtained from all individual participants included in the study. The parents/guardians and their children provided written consent for to participate in the research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Adriko M, Standley CJ, Tinkitina B, Tukahebwa EM, Fenwick A, Fleming FM, Sousa-Figueiredo JC, Stothard JR and Kabatereine NB (2014) Evaluation of circulating cathodic antigen (CCA) urine-cassette assay as a survey tool for Schistosoma mansoni in different transmission settings within Bugiri District, Uganda. Acta Tropica 136, 50–57. [DOI] [PubMed] [Google Scholar]
- Ashton RA, Stewart BT, Petty N, Lado M, Finn T, Brooker S and Kolaczinski JH (2011) Accuracy of circulating cathodic antigen tests for rapid mapping of Schistosoma mansoni and S. haematobium Infections in Southern Sudan. Tropical Medical International Health 16, 1099–1103. [DOI] [PubMed] [Google Scholar]
- Bärenbold O, Garba A, Colley DG, Fleming FM, Haggag AA, Ramzy R, Assaré RK, Tukahebwa EM, Mbonigaba JB, Bucumi V, Kebede B, Yibi MS, Meité A, Coulibaly JT, N'Goran EK, Tchuem Tchuenté LA, Mwinzi P, Utzinger J and Vounatsou P (2018) Translating preventive chemotherapy prevalence thresholds for Schistosoma mansoni from the Kato-Katz technique into the point-of-care circulating cathodic antigen diagnostic test. PLoS Neglected Tropical Disease 12, e0006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhe N, Medhin G, Erko B, Smith T, Gedamu S, Bereded D, Moore R, Habte E, Redda A, Gebre-Michael T and Gundersen SG (2004) Variations in helminth faecal egg counts in Kato–Katz thick smears and their implications in assessing infection status with Schistosoma mansoni. Acta Tropica 92, 205–212. [DOI] [PubMed] [Google Scholar]
- Bezerra FSM, Leal JKF, Sousa MS, Pinheiro MCC, Ramos AN, Silva-Moraes V and Katz N (2018) Evaluating a point-of-care circulating cathodic antigen test (POC-CCA) to detect Schistosoma mansoni infections in a low endemic area in north-eastern Brazil. Acta Tropica 182, 264–270. [DOI] [PubMed] [Google Scholar]
- Brasil, Ministério da Saúde (2017) Programa de Vigilância e Controle da Esquistossomose. TabNet Win32 3.0. – Alagoas. Secretaria de Vigilância em Saúde. Programa de Controle da Esquistossomose, website: http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sinan/pce/cnv/pcese.def (Accessed 27 April 2020).
- Brasil, Ministério da Saúde. Secretaria de Vigilância em Saúde (2019) Esquistossomose: causas, sintomas, tratamento, diagnóstico e prevenção. Editora MS. https://www.saude.gov.br/saude-de-a-z/esquistossomose. [Google Scholar]
- Casacuberta-Partal M, Kinunghi S, Vennervald BJ and Olsen A (2016) Evaluation and optimization of the circulating cathodic antigen (POC-CCA) cassette test for detecting Schistosoma mansoni infection by using image analysis in school children in Mwanza Region, Tanzania. Parasite Epidemiology and Control 1, 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casacuberta-Partal M, Hoekstra, PT, Kornelis D, van Lieshout L and van Dam GJ (2019). Standard Operating Procedure (SOP) for scoring POC-CCATM using G-scores – Version 2, website: https://ars.els-cdn.com/content/image/1-s2.0-S0001706X19307983-mmc2.pdf (Accessed 28 October 2019).
- Chernet A, Kling K, Sydow V, Kuenzli E, Hatz C, Utzinger J, van Lieshout L, Marti H, Nickel B, Labhardt ND and Neumayr A (2017) Accuracy of diagnostic tests for Schistosoma mansoni infection in asymptomatic Eritrean refugees: serology and point-of-care circulating cathodic antigen against stool microscopy. Clinical Infectious Diseases 65, 568–574. [DOI] [PubMed] [Google Scholar]
- Colley DG, Binder S, Campbell C, King CH, Tchuenté LAT, N'Goran EK, Erko B, Karanja D, Kabatereine NB, van Lieshout L and Rathbun S (2013) A five-country evaluation of a point-of-care circulating cathodic antigen urine assay for the prevalence of Schistosoma mansoni. The American Journal of Tropical Medicine and Hygiene 88, 426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danso-Appiah A, Minton J, Boamah D, Otchere J, Asmah RH, Rodgers M, Bosompem KM, Eusebi P and De Vlas SJ (2016) Accuracy of point-of-care testing for circulatory cathodic antigen in the detection of schistosome infection: systematic review and meta-analysis. Bulletin of the World Health Organization 94, 522–33A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss A, Mazigo HD, Tappe D, Kasang C and Mueller A (2018) Comparison of sensitivity and specificity of three diagnostic tests to detect Schistosoma mansoni infections in school children in Mwanza region, Tanzania. PLoS ONE 13, e0202499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenfell RFQ, Taboada D, Coutinho LA, Pedrosa MLC, Assis JV, Oliveira MSP, Cruz RR, Almeida A, Silva-Moraes V, Katz N and Coelho PMZ (2018) Innovative methodology for point-of-care circulating cathodic antigen with rapid urine concentration for use in the field for detecting low Schistosoma mansoni infection and for control of cure with high accuracy. Transactions of the Royal Society of Tropical Medicine and Hygiene 112, 1–7. [DOI] [PubMed] [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística (2016) Área Territorial Oficial 2016. Rio de Janeiro, Brasil: IBGE, https://cidades.ibge.gov.br/brasil/se/maruim/panorama (Accessed 28 October 2019). [Google Scholar]
- Katz N (2018) Inquérito Nacional de Prevalência da Esquistossomose mansoni e Geo-helmintoses. Minas Gerais, BR: CPqRR. 76 p, website: http://tabnet.datasus.gov.br/cgi/sinan/inpeg/RelatorioINPEG.pdf (Accessed 19 October 2018). [Google Scholar]
- Katz N, Chaves A and Pellegrino J (1972) A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Revista do Instituto de Medicina Tropical de Sao Paulo 14, 397–400. [PubMed] [Google Scholar]
- Kittur N, Castleman JD, Campbell CH, King CH, Colley DG and Colley DG (2016) Comparison of Schistosoma mansoni prevalence and intensity of infection, as determined by the circulating cathodic antigen urine assay or by the Kato-Katz fecal assay: a systematic review. The American Journal of Tropical Medicine and Hygiene 94, 605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholz CG, Favero V, CdM V, Candido RRF, de Souza RP, dos Santos RR, Morassutti AL, Bittencourt HR, Jones MK, Pierre TGSt and Graeff-Yeixeira C (2018) Study of diagnostic accuracy of Helmintex, Kato-Katz, and POC-CCA methods for diagnosing intestinal schistosomiasis in Candeal, a low intensity transmission area in northeastern Brazil. PLoS Neglected Tropical Diseases 12, e0006274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento GL and de Oliveira MRF (2014) Severe forms of schistosomiasis mansoni: epidemiologic and economic impact in Brazil, 2010. Transactions of the Royal Society of Tropical Medicine and Hygiene 108, 29–36. [DOI] [PubMed] [Google Scholar]
- Okoyo C, Simiyu E, Njenga SM and Mwandawiro C (2018) Comparing the performance of circulating cathodic antigen and Kato-Katz techniques in evaluating Schistosoma mansoni infection in areas with low prevalence in selected counties of Kenya : a cross- sectional study. BMC Public Health 18, 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira WJ, Magalhães FDC, Elias AMS, de Castro VN, Favero V, Lindholz CG, Oliveira ÁA, Barbosa FS, Gil F, Gomes MA, Graeff-Teixeira C, Enk MJ, Coelho PMZ, Carneiro M, Negrão-Corrêa DA and Geiger SM (2018) Evaluation of diagnostic methods for the detection of intestinal schistosomiasis in endemic areas with low parasite loads: saline gradient, Helmintex, Kato-Katz and rapid urine test. PLoS Neglected Tropical Diseases 12, e0006232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubaba O, Chimbari MJ, Soko W, Manyangadze T and Mukaratirwa S (2018) Validation of a urine circulating cathodic antigen cassette test for detection of Schistosoma haematobiumin uMkhanyakude district of South Africa. Acta Tropica 182, 161–165. [DOI] [PubMed] [Google Scholar]
- Sanneh B, Joof E, Sanyang AM, Renneker K, Camara Y, Sey AP, Jagne S, Baldeh I, Ceesay SJ, Sambou SM and Ogoussan K (2017) Field evaluation of a schistosome circulating cathodic antigen rapid test kit at point-of-care for mapping of schistosomiasis endemic districts in The Gambia. PLoS ONE 12, e0182003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucha CVV, da Silva JAM, Amorim LB, Saucha CVV, da Silva JAM and Amorim LB (2015) Condições de saneamento básico em áreas hiperendêmicas para esquistossomose no estado de Pernambuco em 2012. Epidemiologia e serviços de saúde: revista do Sistema Unico de Saúde do Brasil 24, 497–506. 10.5123/S1679-49742015000300015. [DOI] [Google Scholar]
- Shane HL, Verani JR, Abudho B, Montgomery SP, Blackstock AJ, Mwinzi PNM, Butler SE, Karanja DM and Secor WE (2011) Evaluation of urine CCA assays for detection of Schistosoma mansoni infection in Western Kenya. PLoS Neglected Tropical Diseases 5, e951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira AMS, Costa EGD, Ray D, Suzuki BM, Hsieh MH, de Fraga LAO and Caffrey CR (2016) Evaluation of the CCA immuno-chromatographic test to diagnose Schistosoma mansoni in Minas Gerais State, Brazil. PLoS Neglected Tropical Diseases 10, e0004357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira LMV, Couto FFB, Taboada D, Oliveira ÁA, Carneiro NFF, Oliveira E, Coelho PMZ and Katz N (2016) Performance of POC-CCA® in diagnosis of schistosomiasis mansoni in individuals with low parasite burden. Revista da Sociedade Brasileira de Medicina Tropical 49, 341–347. [DOI] [PubMed] [Google Scholar]
- Sousa-Figueiredo C, Betson M, Kabatereine NB and Stothard JR (2013) The urine circulating cathodic antigen (CCA) dipstick : a valid substitute for microscopy for mapping and point-of-care diagnosis of intestinal schistosomiasis. PLoS Neglected Tropical Diseases 7, e2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa MS, Van Dam GJ, Pinheiro MCC, de Dood CJ, Peralta JM, Peralta RHS, Daher EF, Corstjens P and Bezerra FSM (2019) Performance of an ultra-sensitive assay targeting the circulating anodic antigen (CAA) for detection of Schistosoma mansoni infection in a low endemic area in Brazil. Frontiers in Immunology 10, 682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standley CJ, Lwambo NJS, Lange CN, Kariuki HC, Adriko M and Stothard JR (2010) Performance of circulating cathodic antigen (CCA) urine-dipsticks for rapid detection of intestinal schistosomiasis in schoolchildren from shoreline communities of Lake Victoria. Parasites & Vectors 3, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchuem Tchuenté L-A, Kueté Fouodo CJ, Kamwa Ngassam RI, Sumo L, Dongmo Noumedem C, Kenfack CM, Gipwe NF, Nana ED, Stothard JR and Rollinson D (2012) Evaluation of circulating cathodic antigen (CCA) urine-tests for diagnosis of Schistosoma mansoni infection in Cameroon. PLoS Neglected Tropical Diseases 6, e1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utzinger J, Becker SL, van Lieshout L, van Dam GJ and Knopp S (2015) New diagnostic tools in schistosomiasis. Clinical Microbiology Infection 21, 529–542. [DOI] [PubMed] [Google Scholar]
- van Dam GJ, Wichers JH, Ferreira TMF, Ghati D, van Amerongen A and Deelder AM (2004) Diagnosis of schistosomiasis by reagent strip test for detection of circulating cathodic antigen. Journal of Clinical Microbiology 42, 5458–5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2011) Helminth Control in School-age Children, 2nd Edn. Geneva, Switzerland: World Health Organization, https://apps.who.int/iris/bitstream/handle/10665/44671/9789241548267_eng.pdf;jsessionid=6AB4B6B9C7371391A17F852524C1031A?sequence=1 (Accessed 12 February 2020). [Google Scholar]
- World Health Organization (2020a) Schistosomiasis. Situation and Trends. Geneva, Switzerland: World Health Organization, https://www.who.int/gho/neglected_diseases/schistosomiasis/en/ (Accessed 28 October 2020). [Google Scholar]
- World Health Organization (2020b) Schistosomiasis. Key Facts. Geneva, Switzerland: World Health Organization, https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (Accessed 11 February 2020). [Google Scholar]