Keywords: Cervus nippon, genetic diversity, gGAPDH, Japan, Megatrypanum, phylogeny, rDNA, sika deer, taxonomy, Trypanosoma theileri
Abstract
The taxonomy of ruminant Trypanosoma theileri and its relatives (Kinetoplastida: Trypanosomatidae) is controversial, with recent phylogenetic studies segregating T. theileri in cattle and other ruminants worldwide into two major genetic lineages (the TthI and TthII clades) based on genetic markers. In the present study, T. theileri-like trypanosomes isolated from Honshu sika deer (Cervus nippon) in the western Japan (YMG isolate) were genetically characterized using a number of genetic markers. Sika deer trypanosomes of the YMG isolate were genetically different from the Trypanosoma sp. TSD1 isolate previously recorded from Hokkaido sika deer in northern Japan, with the former trypanosome isolate being genetically closer to European cervid trypanosomes and the bovine T. theileri TthII lineage. In contrast, the latter isolate exhibited greater relatedness to North American cervid trypanosomes and the bovine T. theileri TthI lineage, although a clear genetic distinction between these was apparent. Furthermore, trypanosomes in Honshu sika deer from the central part of Japan harboured additional genetic diversity and were closer to either TSD1 or YMG isolates, while distinct from known T. theileri-related genotypes. Importantly, cervids and wild ruminants worldwide might harbour divergent descendants of a T. theileri ancestor, which exhibit rigid host specificity to either bovines or cervid species.
Introduction
Trypanosoma theileri Laveran, 1902 (Euglenozoa: Kinetoplastida: Trypanosomatidae) is a cosmopolitan trypanosome of bovines, including domestic cattle, Zebu cattle, water buffaloes, European bison Bison bonasus (L.) and various African antelopes. It is transmitted via horse-flies, members of Tabanidae (Touré, 1968; Hoare, 1972; Schlafer, 1979; Kingston et al., 1986; Böse et al., 1987a, 1987b; D'Alessandro and Behr, 1991). Although appreciable numbers of T. theileri are occasionally found in the blood of cattle and other bovines, the infection is latent without any specific symptoms in healthy-looking ruminants (Hoare, 1972; D'Alessandro and Behr, 1991), detectable only via haemoculture (Touré, 1968; Schlafer, 1979) prior to the genetic era. Extremely low levels of parasitaemia persist, however, often for more than one year (Hoare, 1972). Classically, large-sized stercorarian trypanosomes such as T. theireli in mammals are classified in the subgenus Megatrypanum Hoare, 1964 (Section Stercoraria). The hosts of Megatrypanum trypanosomes include ruminants (Table 1), monotremes, marsupials, insectivores, bats, edentates and monkeys, among others (Hoare, 1972; Kingston and Morton, 1975; Kingston et al., 1992; Wita and Kingston, 1999; Hamilton et al., 2004, 2005; Thompson et al., 2014). Recent phylogenetic studies have demonstrated that this subgenus is solely based on morphological criteria and is clearly polyphyletic, lacking evolutionary as well as taxonomic relevance, thus considered nomen nudum in the latest classification (Stevens et al., 1999; Hamilton et al., 2005, 2007). In the present study, however, the term ‘Megatrypanum-type’ is used solely for convenience in order to specify trypanosomes of unique T. theileri-like morphology without taxonomical implications.
Table 1.
Nominal ruminant Trypanosoma spp. of Megatrypanum-type morphologya
| Species | Representative hosts recorded |
|---|---|
| T. theileri Laveran, 1902 | |
| Cattle, water buffalo, bison, various African antelopes, etc. | |
| T. ingens Bruce, Bruce, Hamerton, Bateman et Mackie, 1909 | |
| Cattle in Africa, African antelopes (Uganda, Congo, etc.), African and Asian chevrotains | |
| T. mazamarum Mazza, Romaña et Fiora, 1932 | |
| Brocket deer (Mazama spp.) in South America | |
| T. melophagium (Flu, 1908) Nöller, 1917 | |
| Sheep | |
| T. threodori Hoare, 1931 | |
| Goat | |
| T. cervi Kingston et Morton, 1975 | |
| North American cervids (elk, Alaskan moose, white-tailed deer, mule deer, reindeer) | |
| Red deer in Poland | |
| T. stefanskii Kingston, Bobek et Perzanowski, 1992 | |
| European cervids (roe deer, red deer, fallow deer) | |
| T. trinaperronei Teixeira, Camargo et García, 2020 | |
| White-tailed deer in Venezuela and North America | |
Since most of Megatrypanum-type species from non-bovines (see Table 1) were identified based on morphological characterization using trypanosomes occasionally found on blood smears, it was difficult to assume their precise taxonomic positions or relationships with T. theileri in the past. However, strict host specificity has been demonstrated via cross-infection experiments of T. theileri in sheep, T. melophagium in cattle (Hoare, 1972; D'Alessandro and Behr, 1991) and T. theileri in deer (Böse et al., 1987a; Fisher et al., 2013), suggesting their taxonomic relationships as independent species. A successful experimental infection of American bison Bison bison (L.) with T. theileri of cattle origin has been previously reported, indicating that cattle and bison share trypanosome species (Kingston et al., 1986). With regard to the Megatrypanum-type trypanosomes in wild ruminants, including deer, cross-infection experiments are a challenge, with the oral application of tabanids containing cervid trypanosomes being successful only in fallow deer and not in cattle calves (Böse et al., 1987a), indicative of the parasites’ rigid host specificity. Due to the taxonomic uncertainty with regard to deer trypanosomes, in the present study, ‘T. cf. cervi’ and ‘T. cf. stefanskii’ are used for convenience to describe Megatrypanum-type trypanosomes prevalent in North American and European cervids, respectively.
Modern molecular technology utilising various genetic markers has demonstrated a close evolutional relationship between T. theileri and Megatrypanum-type trypanosomes in wild ruminants (Rodrigues et al., 2003, 2006, 2010a, 2010b; Hamilton et al., 2005, 2009; Gibson et al., 2010; Garcia et al., 2011a, 2011b, 2020; Martinković et al., 2012; Fisher et al., 2013). In parallel, these studies have highlighted the genetic divergence of T. theileri and related Megatrypanum-type trypanosomes of different host and geographical origins based on multiple genetic markers such as the ribosomal RNA gene (rDNA), the glycosomal glyceraldehyde-3-phosphate dehydrogenase (gGAPDH) gene, the spliced leader RNA (SL) gene and cathepsin L-like cysteine protease (CatL-like) genes (Rodrigues et al., 2006, 2010a, 2010b; Garcia et al., 2011a, 2011b, 2020; Martinković et al., 2012; Yokoyama et al., 2015; Pacheco et al., 2018).
In the present study, trypanosomes isolated from Honshu sika deer Cervus nippon aplodontus (Heude, 1884) in Yamaguchi Prefecture, the westernmost part of Honshu Island, Japan, were genetically characterized based on the multiple genetic markers mentioned above. The Megatrypanum-type trypanosome isolate, referred to as YMG, was distinct from the TSD1 isolate from a Hokkaido sika deer C. nippon yesoensis (Heude, 1884) on Hokkaido Island, the northernmost part of Japan, recorded previously (Hatama et al., 2007). In other words, the present study demonstrated that two distinct genetic lineages of cervid Megatrypanum-type trypanosomes, fairly closer to ‘T. cf. stefanskii’ and ‘T. cf. cervi’, are distributed across Japan, having Honshu sika deer (at least C. n. aplodontus) and Hokkaido sika deer (C. n. yesoensis) as natural hosts, respectively. Furthermore, the analyses of additional cervid trypanosomes in the Kii Peninsula around the central part of Honshu suggest the more complicated genetic diversity of Megatrypanum-type sika deer trypanosomes.
Materials and methods
Sample collection and trypanosome detection
To control the population size of sika deer (C. n. aplodontus) and wild boars Sus scrofa leucomystax Temminck in rural areas of Shimonoseki City, Yamaguchi Prefecture, Japan, a maximum of 1500 animals for each species are shot throughout the year based on the annual plan introduced by the municipal office. Wild mammals shot as part of this program were analysed in the present study. Cardiac blood samples from 75 male and 40 female deer were collected monthly throughout a period between 9 November 2013 and 2 August 2014. In the same period, cardiac blood samples were also collected from 65 wild boars (21 males, 39 females and five animals of unknown sex). The blood was transferred from clean syringes to sterile vacuum blood collection tubes with EDTA-2Na (TERUMO Co., Shibuya-ku, Tokyo, Japan), kept at 4 °C, and transported to the laboratory of Yamaguchi University within 5 h after collection. Thin blood smears for each sample were taken for microscopic examination after Giemsa staining. For a survey of Borrelia infection in deer, 0.2 mL of blood from individual samples was added to 4.0 mL of modified Barbour-Stoenner-Kelly (BSK) medium, using MEM-alpha (BioWest, Essen, Germany) as a substrate for CMRL-1066, and incubated at 32 °C. Bacterial growth checks were performed on day 7 of culture and at arbitrary intervals thereafter. When free or aggregated trypanosomes were detected in the culture supernatant, these were collected in Eppendorf tubes, washed repeatedly with cold MEM medium and preserved at −20 °C until use. A few drops of the supernatant with trypanosomes were placed on a clean glass slide, air dried, fixed in 100% methanol and processed for Giemsa staining. Stained specimens were examined with a ×100 oil immersion objective lens, photographed at a magnification of ×1000 and edited with Adobe® Photoshop® ver. 11.0 (Adobe Systems, San Jose, California, USA). Photographs were then printed at a high magnification. Printed photographs were analysed as described previously (Sato et al., 2008).
Similarly, one and 10 cardiac blood samples were obtained from deer hunted in Tanabe City, Wakayama Prefecture, on 20 November 2008, and in Kushimoto Town, Wakayama Prefecture, on 14 October 2009, respectively. Hunting was conducted under the municipal annual plan to control the population size of sika deer. The blood samples were collected in sterile vacuum blood collection tubes with EDTA-2Na kept at 4 °C and were transported to the laboratory of Hyogo University of Health Sciences within 24 h after collection. Thin blood smears for each sample were taken for microscopic examination after Giemsa staining. These 11 deer blood samples were not used for culture, but for DNA extraction.
From slaughtered cattle at the Nanko Division of Osaka Municipal Central Market, 175 blood samples were collected on four separate days during the period between 10 and 31 August 2015. These cattle were bred in 19 prefectures throughout Japan and included 93 Japanese black breed, seven Holstein-Friesian breed, and 75 F1 between the Japanese black and Holstein-Friesian breeds. Blood samples were collected in sterile vacuum blood collection tubes with EDTA-2Na kept at 4 °C and were transported to the laboratory of Yamaguchi University within 32 h of collection. Thin blood smears for each sample were taken for microscopic examination after Giemsa staining. Simultaneously, approximately 0.2 mL of each blood sample were added to two culture wells of 24-well culture plates (MS-80240; Sumitomo Bakelite Co., Shinagawa-ku, Tokyo, Japan), containing 1.5 mL well−1 of RPMI1640 medium (Nissui Pharmaceutical Co., Sugamo, Tokyo, Japan) supplemented with 0.3% L-glutamine, 100 U mL−1 penicillin, 100 μg mL−1 streptomycin, 0.25 μg mL−1 amphotericin B and 10% heat-inactivated fetal bovine serum, in addition to feeder cells placed according to Sato et al. (2003). Feeder cells used in the present study were derived from the primary cell culture of kidney cells from a deer fetus. Culture was maintained under sterile conditions of 5% CO2 at 37 °C and checked weekly under an inverted microscope.
Blood and culture smear slides were deposited in the Meguro Parasitological Museum, Tokyo, Japan.
DNA extraction, polymerase chain reaction (PCR) and nucleotide sequencing
Parasite DNA was extracted from the primary trypanosome-positive haemocultures of deer collected in Yamaguchi Prefecture and cattle using an Illustra™ tissue and cells genomicPrep Mini Spin Kit (GE Healthcare UK, Buckinghamshire, UK) according to the instructions of the manufacturer. Blood DNA of Wakayama Prefecture deer was extracted from 0.2 mL of each blood sample using a nucleic acid purification kit, MagExtractor™-Genome (TOYOBO, Dojima Hama, Osaka, Japan), according to the instructions of the manufacturer. PCR amplification of partially overlapping rDNA fragments was performed in a 20 μL solution containing a DNA polymerase, Blend Taq-Plus (TOYOBO), and primers, as previously described (Sato et al., 2005). The PCR cycling protocol for rDNA fragments included 3 min at 94 °C, 40 cycles of 45 s at 94 °C, 1 min at 64, 62 or 60 °C (according to primer pairs), and 1 min at 72 °C, followed by a final extension step at 72 °C for 7 min. The hypervariable region (V7/V8) of the 18S rDNA was amplified via nested PCR using the TRY927F and TRY927R primer pair in the first round and SSU561F with SSU561R in the second round (see Sato et al., 2005). The gGAPDH gene was amplified via nested PCR with degenerate primers G3 and G5 in the first round, followed by G1 and G4a or G1 and G4b in the second round, as previously described (Hamilton et al., 2004; Sato et al., 2008). PCR amplification of the catalytic domain-encoding region of CatL-like genes was performed using a combination of primers DTO154 (5′-ACA GAA TTC CAG GGC CAA TGC GGC TCG TGC TGG-3′) and DTO155 (5′-TTA AAG CTT CCA CGA GTT CTT GAT GAT CCA GTA-3′) according to Cortez et al. (2009). The PCR cycling protocol for CatL-like genes was 3 min at 94 °C, 35 cycles of 1 min at 94 °C, 1 min at 56 °C and 1 min at 72 °C, followed by a final extension step at 72 °C for 10 min. The PCR amplification of SL genes was performed using a combination of primers LSL1 (5′-TTC TGT ACT TCA TGG TAT G-3′) and LSL2 (5′-CCA ATG AAG TAC AGA AAC TG-3′) as per Rodrigues et al. (2010a). The PCR cycling protocol for SL genes was 3 min at 94 °C, 35 cycles of 1 min at 94 °C, 2 min at 50 °C and 2 min at 72 °C, followed by a final extension at 72 °C for 10 min. When direct sequencing was not satisfactory, the purified PCR products were cloned into a plasmid vector, pTA2 (TArget Clone™; TOYOBO), and transformed into Escherichia coli JM109 (TOYOBO) according to the manufacturer's instructions. Following propagation, the plasmid DNA was extracted using a FastGene Plasmid Mini Kit (NIPPON Genetics Co.), and inserts from multiple independent clones, at least three, were sequenced using universal M13 forward and reverse primers.
Alignment and phylogenetic analysis
The rDNA, gGAPDH gene, CatL-like gene and SL gene sequences of trypanosome isolates obtained in the present study (DDBJ/EMBL/GenBank accession nos. LC618030–LC618052) and related sequences retrieved from the DDBJ/EMBL/GenBank databases were aligned using the CLUSTAL W multiple alignment program (Thompson et al., 1994) with subsequent manual adjustment. The Mfold web server (Zuker, 2003) was employed for predicting the secondary structure of partial 18S rDNA molecules (V7/V8 hypervariable region) using the energy minimization approach for each sequence. The accession numbers of the sequences analysed for each gene fragment are given in Supplementary Table 1 and figures showing phylogenetic trees. Regions judged to be poorly aligned and characters with a gap in any sequences were excluded from subsequent analyses: 623 characters, of which 198 were variable, remained for subsequent gGAPDH gene analysis; 450 characters, of which 90 were variable, remained for subsequent CatL-like gene analysis; and 658 characters, of which 119 were variable, remained for subsequent SL gene analysis. Additional CatL-like gene analysis including T. trinaperronei sequences (accession nos. MN747149–MN747155) was conducted using 256 characters, of which 60 were variable. Trypanosoma trinaperronei Teixeira, Camargo et García, 2020 is a recently proposed Megatrypanum-type trypanosome species from the white-tailed deer in Venezuela (García et al., 2020). Maximum likelihood (ML) analysis was performed with the program PhyML (Guindon and Gascuel, 2003; Dereeper et al., 2008), available on the ‘phylogeny.fr’ website (http://www.phylogeny.fr/). The probability of inferred branches was assessed by the approximate likelihood-ratio test, an alternative to the non-parametric bootstrap estimation of branch support (Anisimova and Gascuel, 2006). Relationships of different 18S rDNA V7/V8 genotypes were visualized using an automated haplotype network layout and visualization software ‘HapStar’, downloaded at http://fo.am/hapstar (Teacher and Griffiths, 2011). As variable lengths of the hypervariable 18S rDNA arising due to sites of nucleotide insertion/deletion (indel) were applied to this software in the present study, the position of a deleted nucleotide was treated as ‘X’ character, and the numbers of nucleotide differences between sequences were counted for the analysis (cf. Supplementary Table 2).
Results
Incidence of trypanosomes in deer and cattle
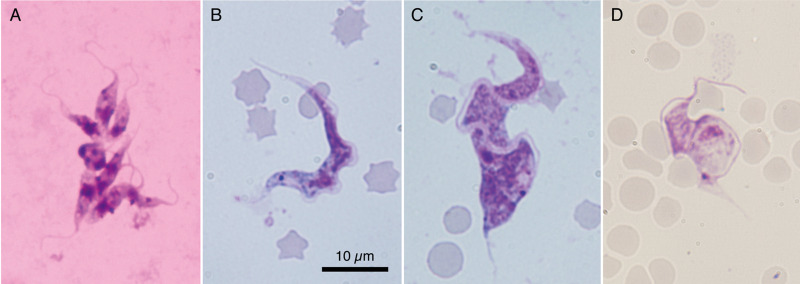
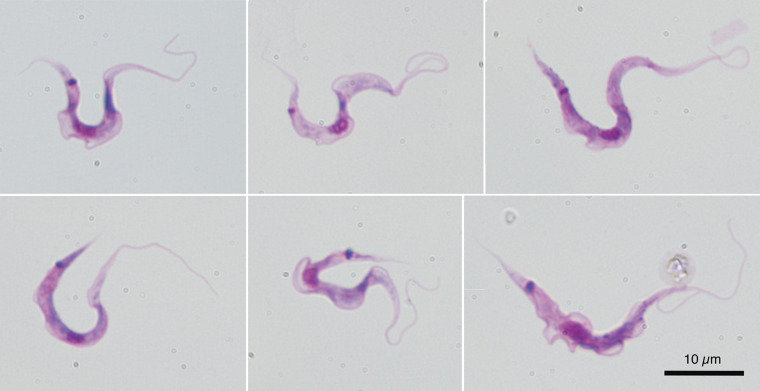
Blood samples were collected monthly from two to 28 deer in Yamaguchi Prefecture, and blood culture analysis detected trypanosome infection in eight (10.7%) male and five (12.5%) female deer (Fig. 1A). Microscopic detection of trypanosomes on the blood smear was thoroughly attempted but unsuccessful except for in one blood sample with a few trypomastigotes (Fig. 1B and C). Their measurements (n = 4) are summarized in Supplementary Table 3 for comparison with other ruminant Megatrypanum-type Trypanosoma spp. Wild boar blood culture detected no trypanosome infection. Six out of 11 (54.5%) deer from Wakayama Prefecture were positive for trypanosomes, as determined via nested PCR screening targeting the hypervariable region of 18S rDNA. Microscopic examination of blood smears revealed only one bloodstream form in a deer from Wakayama Prefecture (Fig. 1D). Haemoculture of 175 cattle blood samples identified six (3.43%) positive animals bred and reared in the western part of Japan (see Supplementary Table 1). Microscopic examination of blood smears revealed no trypanosomes in the peripheral blood of slaughtered cattle. Metacyclic trypomastigotes of T. theileri in haemoculture are shown in Fig. 2.
Fig. 1.
Trypanosoma theileri detected in the peripheral blood of sika deer in Japan. Cluster of epimastigotes in the culture supernatant (A); YMG isolate trypomastigotes in the blood smears (B and C); and a trypomastigote of the TNB isolate (D). The specimens have been stained with Giemsa. All photographs are shown at the same magnification with the scale bar in photograph B.
Fig. 2.
Metacyclic trypomastigotes of cultured Trypanosoma theileri from OSK isolates, originating from the peripheral blood of cattle in Japan. The specimens have been stained with Giemsa. All photographs are shown at the same magnification with the scale bar at the lower right.
Characterization of the rDNA of sika deer trypanosomes and cattle T. theileri in the western part of Japan
Three haemoculture samples of deer from Yamaguchi Prefecture (YMG-11, 14 and 15) exhibited no genetic variation in the rDNA sequence over 6777-bp length (DDBJ/EMBL/GenBank accession no. LC618030). Similarly, all six cultured trypanosome samples of cattle blood (OSK-16, 120, 148, 150, 155 and 172) showed no genetic variation in the rDNA sequence over 6132-bp length (DDBJ/EMBL/GenBank accession no. LC618031).
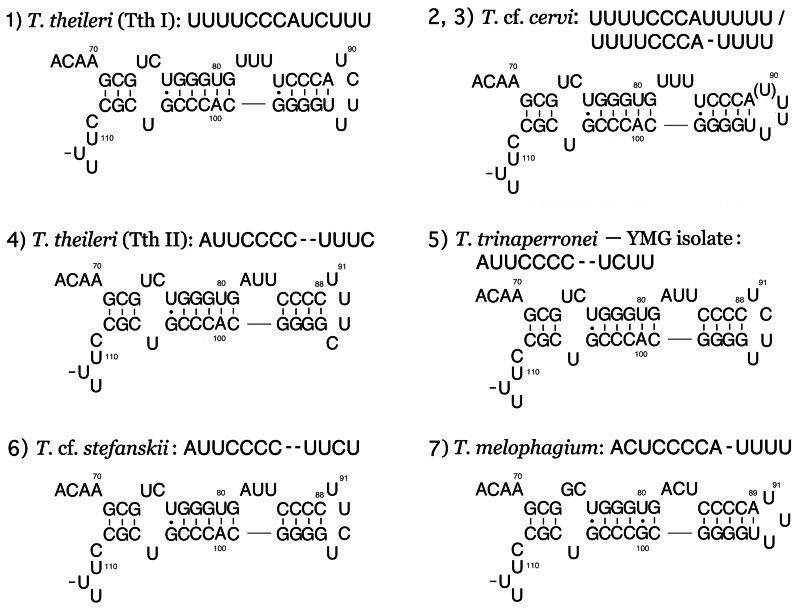
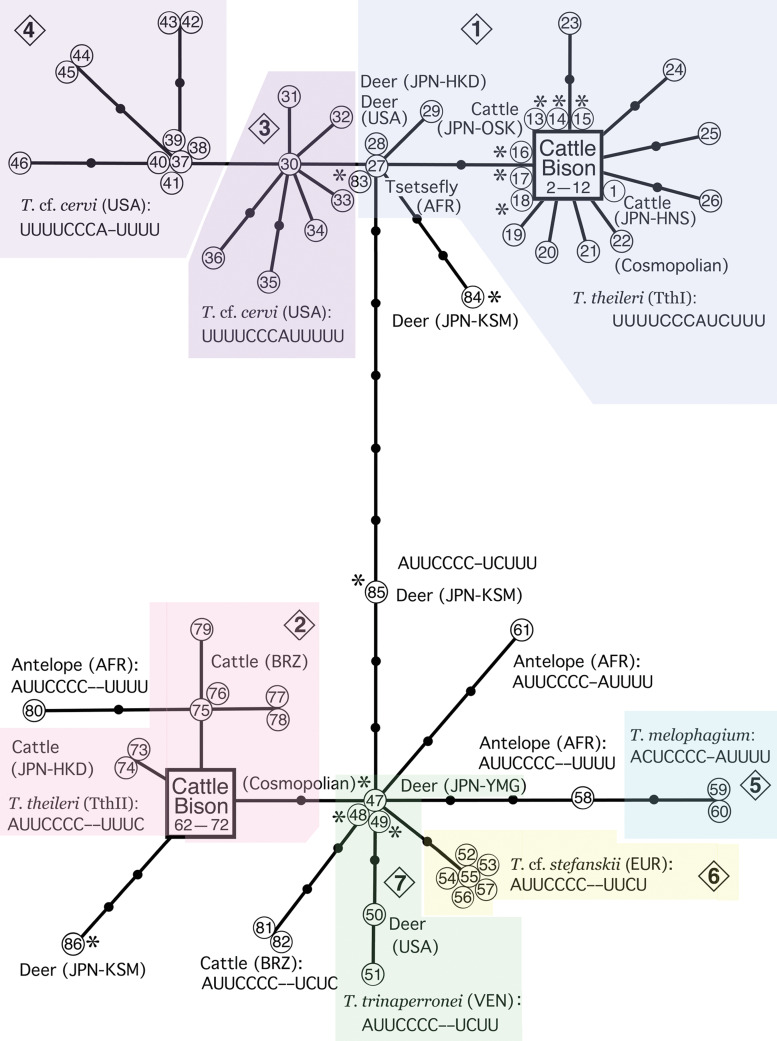
The hypervariable region of 18S rDNA is frequently utilized for detecting stercorarian trypanosomes in terrestrial vertebrates. The sequence was compared with those of T. theileri from cattle and bisons, T. melophagium from sheep, and other Megatrypanum-type trypanosomes from a variety of wild ruminants such as deer and antelopes (Supplementary Table 2). Compared sequences were divided into seven groups based on the hair-pin tip structure of the hypervariable region, corresponding to the 82nd to 92nd nucleotide of the amplicon as determined via nested PCR using the SSU561F and SSU561R primer pair for YMG isolate trypanosomes (LC618030), or to 82nd to 94th nucleotides of the T. theileri KM strain (TthI lineage) amplicon recorded in cattle from Japan (AB007814), as shown in Fig. 3. The relationships between ruminant Megatrypanum-type trypanosomes of various origins retrieved from the DDBJ/EMBL/GenBank databases are shown in Fig. 4. Genotype 1 (loop-tip sequence UUUUCCCAUCUUU) and Genotype 4 (AUUCCCC––UUUC) represented T. theileri from domestic ruminants, corresponding to the ‘TthI’ and ‘TthII’ genotypes of T. theileri described by Rodrigues et al. (2006, 2010a, 2010b) and Garcia et al. (2011a, 2011b).
Fig. 3.
Seven genotypes of ruminant Megatrypanum-type trypanosomes based on the putative secondary structure of the hair-pin loop of the 18S rDNA hypervariable region.
Fig. 4.
Relationships of ruminant Megatrypanum-type trypanosome isolates based on nucleotide sequence differences in the hypervariable region of 18S rDNA, illustrated via HapStar network analysis. Detailed data about the nucleotide sequences of analysed isolates are shown in Supplementary Table 1, and numbers in the circles or squares of this figure correspond to material numbers shown in Supplementary Table 2. Numbers 1–7 in the rhombus indicate genotypes shown in Fig. 3.
A majority of North American deer trypanosomes (‘T. cf. cervi’) represented Genotypes 2 (UUUUCCCAUUUUU) and 3 (UUUUCCCA–UUUU), having loop-tip nucleotide sequences closer to Genotype 1 (T. theileri TthI lineage). The TSD1 isolate of Megatrypanum-type trypanosomes, previously reported from a Hokkaido sika deer in the northernmost part of Japan, had a loop-tip nucleotide sequence identical to Genotype 1 and close loop-tip nucleotide sequences identical to Genotypes 2 and 3 (‘T. cf. cervi’). In contrast, a majority of European deer trypanosomes (‘T. cf. stefanskii’) were of Genotype 6 (AUUCCCC––UUCU), having a loop-tip nucleotide sequence closer to Genotype 4 (T. theileri TthII lineage; AUUCCCC––UUUC). YMG isolates of Honshu sika deer trypanosomes and T. trinaperronei from the white-tailed deer in Venezuela and North America shared the same loop-tip nucleotide sequence of Genotype 5 (AUUCCCC––UCUU), which was closer to Genotype 4 (T. theileri TthII lineage) and Genotype 6 (‘T. cf. stefanskii’).
Trypanosomes from the sika deer in Tanabe and Kushimoto, Wakayama Pref. (TNB872, KSM941, 944, 946, 948 and 949 isolates) had several diverse genotypes, distinct from the YMG isolate (Genotype 5), representing Genotype 1 and additional non-specified genotypes (AUUCCCC––AUUC, AUUCCCC––ACUC or AUUCCCC––GUUU) (DDBJ/EMBL/GenBank accession no. LC618032–LC618036). Multiple genotypes were often found in a single host deer (TNB872, KSM946, KSM944 and KSM948). Megatrypanum-type trypanosomes from various antelopes in Africa and other isolates exhibited further additional genotypes (Fig. 4).
Characterization of the ITS1 sequence of sika deer trypanosomes and cattle T. theileri in the western part of Japan
The ITS1 nucleotide sequence of the YMG isolate obtained in the present study was 282-bp long. Of T. theileri and ruminant Megatrypanum-type trypanosomes, 149 ITS1 sequences retrieved from the DDBJ/EMBL/GenBank databases (see Supplementary Table 1), 186–272-bp in length, were aligned and grouped into 10 major groups. Alignment revealed a great amount of indels, including some repeated units of a few nucleotides. Of T. theileri TthI, the lowest nucleotide identity recorded among 27 sequences was 90.8%, excluding indels. Similarly, of T. theileri TthII from cattle in Brazil, Croatia and Japan, the lowest nucleotide identity among 25 retrieved sequences was 80.6%. OSK isolates of T. theileri TthII (LC618031) had identical ITS1 sequences with the species isolated from cattle in Mongolia (LC440408), Italy (MK163554), Austria (KY412803), USA (JX178186), the Philippines (LC546921) and Brazil (HQ664817). Between 52 nucleotide sequences of TthI and TthII T. theileri lineages, the lowest identity was 45.1%.
Trypanosoma trinaperronei from white-tailed deer from Venezuela and Texas (MN752208, MN752209 and JX178172) had highly similar ITS1 sequences (99.57–100% with one indel over 232/233-bp of length), but exhibited lower similarity to the YMG isolate (<90.52% with a great number of indels), although their 18S rDNA was of Genotype 5 (Fig. 4). Venezuelan and North American isolates of T. trinaperronei and ‘T. cf. stefanskii’ D30 (HQ664845 or AY773714) had ITS sequences with a similarity of 88.79% and frequent indels. ITS1 sequences of the YMG isolate and ‘T. cf. stefanskii’ D30 exhibited 83.86% similarity with several indels. Between the YMG isolate of Genotype 5 and TSD isolate of Genotype 1 (AB569248), ITS regions showed 76.70% nucleotide identity with a great amount of indels.
Characterization of gGAPDH sequences of sika deer trypanosomes
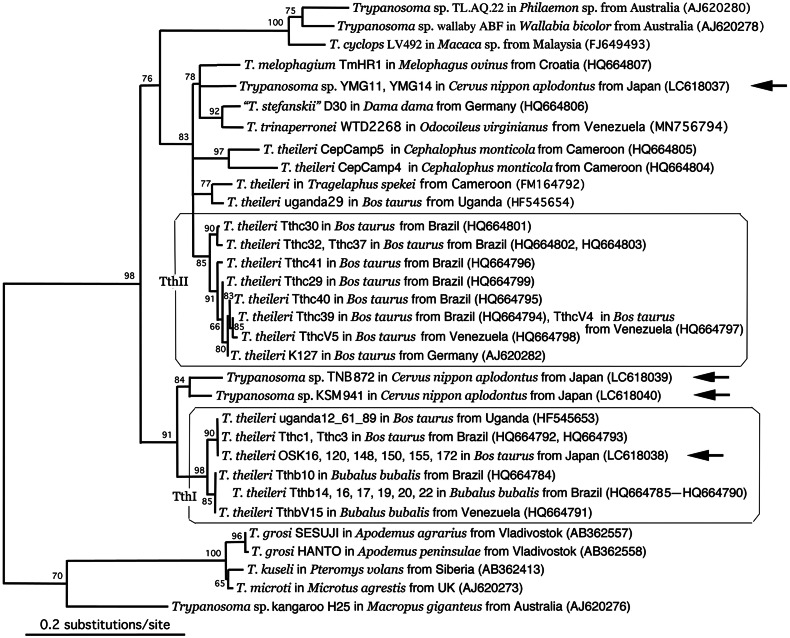
Two gGAPDH nucleotide sequences of sika deer trypanosomes YMG-11 and YMG-14 isolates were identical to each other (DDBJ/EMBL/GenBank accession no. LC618037). An ML phylogenetic tree constructed with sequences available at the DDBJ/EMBL/GenBank databases is shown in Fig. 5, and the phylogenetic relationships of different taxa were almost similar to those observed based on the 18S rDNA. Intra-lineage identities of T. theileri nucleotide sequences were fairly high [the lowest identities in the TthI and TthII lineages were 98.94% (838/847) and 98.11% (831/847), respectively], and inter-lineage identities of nucleotide sequences varied between 91.97% (779/847) and 93.27% (790/847).
Fig. 5.
ML phylogenetic tree of ruminant Megatrypanum-type trypanosomes based on the 623 characters of gGAPDH gene nucleotide sequences. The species name of isolates is followed by the host species, country of collection and the DDBJ/EMBL/GenBank accession number in parentheses. Newly obtained sequences are indicated by arrows. Representative rodent trypanosomes and a Megatrypanum-type Trypanosoma sp. from a kangaroo were used as an outgroup due to their close phylogenetic relationships with the T. theileri clade (Hamilton et al., 2004).
The gGAPDH nucleotide sequence of the YMG isolate exhibited higher identity to those of T. theileri TthII lineage [95.28% (807/847)−95.75% (811/847)] than those of T. theileri TthI lineages [highest 92.21% (781/847)]. The same YMG isolate nucleotide sequence showed 95.97% (785/818) identity with that of the ‘T. cf. stefanskii’ D30 isolate from a fallow deer in Germany (HQ664806) and 96.58% (818/847) identity with that of T. trinaperronei WTD2268 in a white-tailed deer from Venezuela (MN756794). Between the T. trinaperronei WTD2268 (MN756794) and ‘T. cf. stefanskii’ D30 isolate, a gGAPDH nucleotide identity of 97.76% (828/847) was observed. Two gGAPDH nucleotide sequences of the KSM isolate harboured 96.08% (616/638) similarity between each other, as well as 91.36% (772/845) and 91.69% (585/638) similarity with the YMG isolate sequence.
Characterization of CatL-like and SL gene nucleotide sequences of sika deer trypanosomes
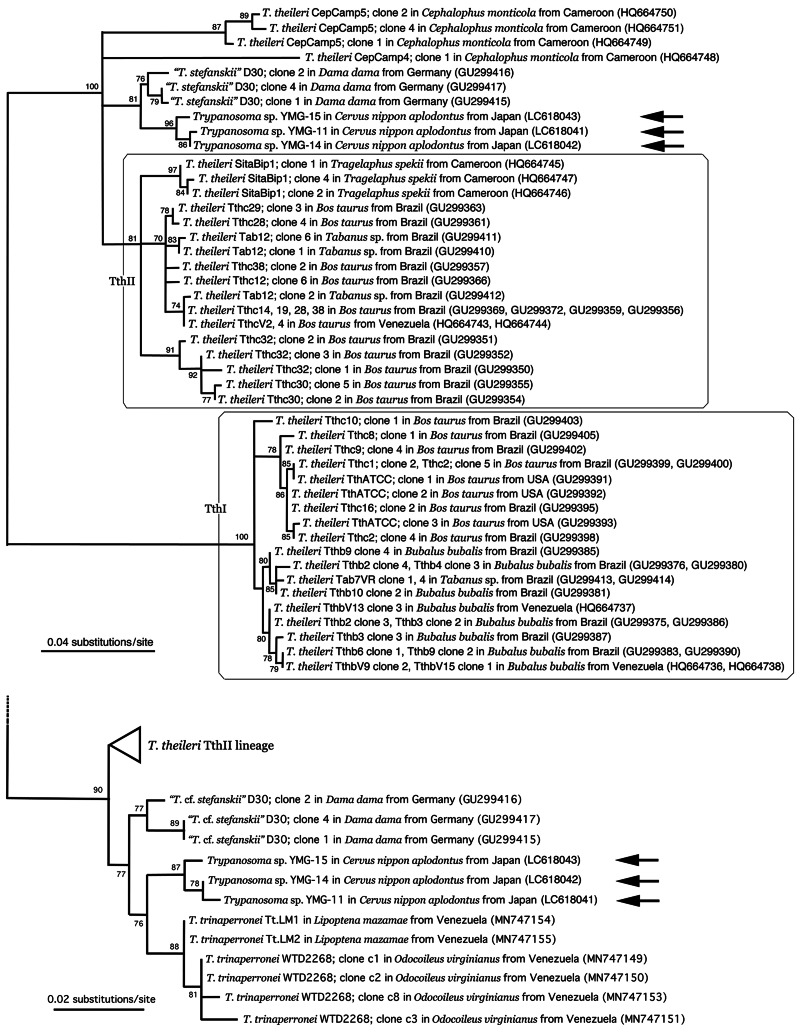
CatL-like gene sequences, 450-bp in length, were obtained with one each for the YMG-11 (LC 618041), YMG-14 (LC 618042) and YMG-15 (LC 618043) isolates with identities ranging between 96.00% (432/450) and 99.78% (449/450). In the ML phylogenetic tree, deer Megatrypanum-type trypanosomes formed an independent clade from the TthI and TthiII lineages of T. theileri and other African ruminant Megatrypanum-type trypanosomes (Fig. 6). Nucleotide identities between the D30 isolate from Cervus dama in Germany (‘T. cf. stefanskii’) and the YMG isolate ranged between 94.44% (425/450) and 97.78% (440/450). Nucleotide identities of T. trinaperronei and the YMG isolate ranged between 92.97% (238/256) and 98.05% (251/256), while those of T. trinaperronei and the ‘T. cf. stefanskii’ D30 ranged between 97.27% (249/256) and 98.44% (252/256).
Fig. 6.
Unrooted ML phylogenetic tree of ruminant Megatrypanum-type trypanosomes based on the 450 characters (upper) and 256 characters of CatL-like gene nucleotide sequences (lower). The species name of isolates is followed by the host species, country of collection and the DDBJ/EMBL/GenBank accession number in parentheses. Newly obtained sequences are indicated by arrows.
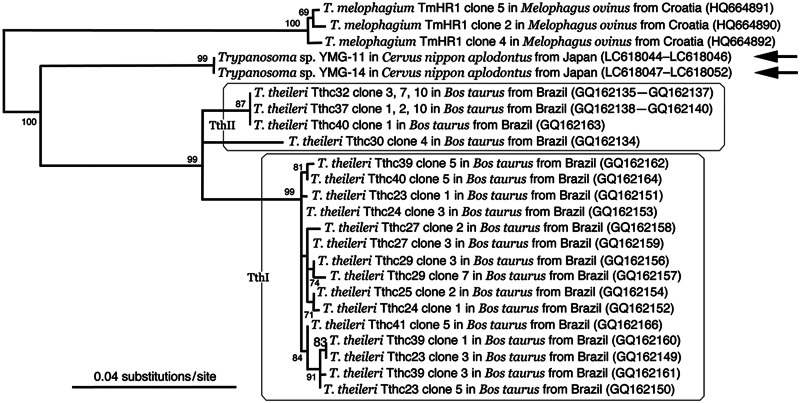
As for the SL gene of Honshu sika deer trypanosomes, three YMG-11 clones and six YMG-14 clones were sequenced, and all of them differed in length, ranging between 891 and 922-bp due to some repeated units of a few nucleotides (LC618044–LC618052). To construct an ML phylogenetic tree based on SL sequences, these short repeated units were omitted from the analysis. YMG-11 and YMG-14 sequences formed an independent branch from T. melophagium as well as T. theileri TthI and TthII lineages (Fig. 7).
Fig. 7.
Unrooted ML phylogenetic tree of ruminant Megatrypanum-type trypanosomes based on the 658 characters of SL gene nucleotide sequences. The species name of isolates is followed by the host species, country of collection and the DDBJ/EMBL/GenBank accession number in parentheses. Newly obtained sequences are indicated via arrows.
Discussion
In the current work, molecular genetic analyses of cultured trypanosomes isolated from the blood of Honshu sika deer in Japan (YMG isolate) revealed a unique divergent of cervid trypanosomes, distinct from either the TthI or TthII lineages of T. theileri, T. melophagium in domestic ruminants or cervid Megatrypanum-type trypanosomes in Europe (‘T. cf. stefanskii’) as well as those in the Americas (‘T. cf. cervi’ and T. trinaperronei). Further, the YMG isolate was also distinct from a previously known Japanese isolate of sika deer trypanosomes (TSD1 isolate) collected from a Hokkaido sika deer in the northern part of Japan (Hatama et al., 2007), which was genetically closer to ‘T. cf. cervi’ or bovine T. theileri TthI (Fig. 4). It is likely that these two lineages of sika deer trypanosomes represent different phylogenetic populations of the parasite maintained by the southern and northern populations of sika deer in Japan, which were recently identified to harbour differences in their mitochondrial genes (Tamate et al., 1998; Nagata et al., 1999; Ohdachi et al., 2010). These two host groups arrived in the eastern part of the Eurasian continent prior to their dispersal to the Japanese Archipelago from (1) the Korean Peninsula to Kyushu and Honshu Islands (southern group) and (2) the northern part of the continent (through Sakhalin Island) to Hokkaido Island (northern group). Phylogenetic segregation of Japanese sika dear into two genetically distinct populations, i.e. southern and northern groups, divides the Honshu sika deer (a subspecies of sika deer C. nippon Temminck, 1838) based on morphological aspects. The Kii Peninsula (Wakayama Prefecture), where additional sika deer trypanosomes were collected (TNB/KSM isolate), is located near the border of distribution between the southern and northern Honshu sika deer groups. Accordingly, it was hypothesized that TNB/KSM isolates might resemble either YMG, TSD1 or a mix of both isolates.
TNB/KSM isolates exhibited multiple genotypes, closer to either YMG or TSD1 isolates from Japanese sika deer, but identical to neither. The nucleotide variation observed in TNB/KSM trypanosome isolates was exceptional when considering the homogeneous nucleotide sequences of T. theileri from all six OSK culture isolates. Similarly, the 18S rDNA of all three YMG cultured trypanosome isolates were identical. Other researchers have also reported unexpected variations of T. theileri sequences using field-collected blood samples without culture (Rodrigues et al., 2010b; Garcia et al., 2011a). It is uncertain whether this phenomenon can be ascribed to the source of PCR templates (trypanosomes in the original blood or grown in the culture), or endogenous features of the target genes analysed. Due to the use of field-collected blood, only two additional gGAPDH sequences were obtained (Fig. 5). The two gGAPDH sequences of TNB/KSM isolates showed a close relationship to the TthI lineage of T. theileri, and the same isolates exhibited an identical hair-pin loop-tip sequence (CCCAUCUUU) with the TthI lineage of bovine T. theileri (Fig. 4). Although sister phylogenetic relationships of Trypanosoma cyclops Weinman, 1972 as well as trypanosomes from Australian marsupials and terrestrial leeches to the T. theileri TthII clade are shown in Fig. 5 as in previous studies (Hamilton et al., 2005, 2007, 2009), the latest study by Ellis et al. (2021) has demonstrated that the T. cyclops clade containing newly collected leech trypanosomes forms a sister group to the T. theileri clade (including both TthI and TthII lineages, cervid T. theileri, and T. melophagium), indicating the monophyletic nature of ruminant Megatrypanum-type trypanosomes.
Using morphological criteria (e.g. pronounced undulating membrane, distinctly shorter free flagella and the greater body sizes of ‘T. cervi’ compared to ‘T. theileri’) and host specificity, ‘T. cervi’ in North American cervids and ‘T. stefanskii’ from European cervids were described as independent species (Kingston and Morton, 1975; Kingston et al., 1985, 1992). Morphological variations in the bloodstream form of deer trypanosomes were reported to hamper precise species identification (Kingston et al., 1985, 1992). Based on isoenzyme profile differences, Böse et al. (1993) suggested that there are probably at least two different species of cervid Megatrypanum-type trypanosomes in Europe, one parasitizing roe deer and the other infecting red deer and fallow deer. Using the same biochemical technique, Dirie et al. (1990) described the uniqueness of Megatrypanum-type trypanosomes in Swedish reindeer (Rangifer tarandus) compared to those found in American cervids and Swedish moose (Alces alces). The mosaic distribution of at least two Megatrypanum-type cervid trypanosomes has not yet been demonstrated via rDNA and gGAPDH sequencing, although Fisher et al. (2013) identified two genetic lineages of Megatrypanum-type trypanosomes from the white-tailed deer in Texas based on the rDNA sequences. Recently, Garcia et al. (2020) proposed a new species T. trinaperronei for one cervid trypanosome lineage found in the white-tailed deer in Venezuela, and they postulated its evolution from the ancestor originating from Eurasia after trans-continental movement through the Bering Strait via the Bering Land Bridge and the Panama Isthmus. According to this T. trinaperronei evolutionary scenario in Venezuela, the discovery of the YMG isolate in Japanese sika deer is essential for connecting the European cervid trypanosome D30 isolate from fallow deer (Germany) to the newly proposed species. The distribution of the TSD1 isolate, closely related to ‘T. cf. cervi’, might be explained by the reverse trans-continental movement of some deer from North America to Siberia through the Bering Land Bridge during the glacial period. At the same time, it can be postulated that limited research on cervid Megatrypanum-type trypanosomes and the use of haemoculture might constrain the field discovery of existing trypanosome lineages, as suggested in this study (TNB/KSM isolates; see Fig. 4). Garcia et al. (2011b) suggested that new genotypes (genetic lineages) are yet to be discovered in unexplored regions after they identified unknown local genotypes of T. theileri in cattle from Thailand.
Molecular research on T. theileri in cattle using the above-described genetic markers has suggested that phylogenetic relationships among trypanosome sequences are congruent with phylogeny based on other gene sequences (Cortez et al., 2009; Garcia et al., 2020), although the resolution degree of lineages or population diversity differs based on the gene markers assessed in relation to the inherent nucleotide conservation/divergence as well as sequencing span. Consistently, molecular studies on T. theileri indicated that the species of bovine ‘T. theileri’ include at least two major lineages (TthI and TthII) distributed throughout the world, probably via the anthropogenic dispersal of their hosts, i.e. domestic ruminants (Rodrigues et al., 2006, 2010a, 2010b), even though the distribution of other T. theileri genotypes has been suggested in cattle from Thailand, Philippines or Sri Lanka as well as antelopes in Africa (Garcia et al., 2011a; Auty et al., 2012; Yokoyama et al., 2015). Based on the nucleotide sequences of the rDNA hypervariable region, Megatrypanum-type trypanosomes in North American and European cervids are divided into at least two major lineages, both distinct yet closer to TthI and TthII lineages of T. theileri in cattle, which might correspond to species classically grouped as ‘T. cf. cervi’ and ‘T. cf. stefanskii’, respectively (Fig. 4). Prior to this study, the characterization of T. trinaperronei as T. theileri-like trypanosomes in white-tailed deer across the North and Central Americans (Garcia et al., 2020) seemed reasonable, since trypanosomes of the TthII-related lineage of cervid trypanosomes (‘T. cf. stefanskii’, typically observed in Europe) represent a special case in the North American continent where TthI-related lineage of cervid trypanosomes might be prevalent (‘T. cf. cervi’, typically observed in North America). The current study partially confirms their speculation, as the YMG isolate, which has closer phylogenetic relationships with ‘T. cf. stefanskii’ and T. trinaperronei, is distributed in Japan, which is located in the Far East, as well as near the continental border between the Old World and the New World. Currently, the new YMG isolate seems to occupy an intermediate phylogenetic position between the aforementioned two species, raising the question of where to draw the geographical boundary between species. Furthermore, another lineage of sika deer trypanosomes closer to either ‘T. cf. stefanskii’ or ‘T. cf. cervi’ (TNB/KSM isolates) were isolated in the Japanese mainland (Honshu island), in addition to the previously reported sika deer TSD1 trypanosomes isolate, which is phylogenetically closer to ‘T. cf. cervi’, but still genetically distinct from it. It is highly probable that multiple diverse lineages of Megatrypanum-type trypanosomes can be obtained from cervids in the Far East and other continents. Thus, in order to avoid taxonomic confusion, we propose that T. theileri and T. theileri-like trypanosomes in ruminants are classified solely as ‘T. theileri’, and are differentiated by lineage tags as has been recently done by various researchers (Rodrigues et al., 2006, 2010a, 2010b; Garcia et al., 2011a, 2011b, 2020; Ybañez et al., 2013; Weerasooriya et al., 2016). As far as retaining bovine ‘T. theileri’ for both TthI and TthII lineages since this nomenclature is widely accepted at present, we suggest that ‘T. melophagium’, ‘T. cervi’, ‘T. stefanskii’ and ‘T. trinaperronei’ should be synonymous to T. theileri regardless of host specificity. The taxonomic classification of three other species, i.e. T. ingens, T. mazamarum and T. threodori, remains uncertain due to a lack of genetic information. When considering that multiple lineages of cervid T. theileri (e.g. Japanese sika deer trypanosome isolates, genetically closer to either bovine T. theileri TthI or TthII, but identical to neither) could be isolated from an individual host or a local population, the use of different species names for each lineage is not feasible. Furthermore, as observed for the YMG isolate, it is difficult to define a clear border between the YMG isolate and T. trinaperronei or ‘T. cf. stefanskii’, even if they use different vectors, as emphasized by Votýpka et al. (2015) with regard to species classification. Further collection of samples and genetic data of T. theileri and T. theileri-like trypanosomes (i.e. Megatrypanum-type) from wild ruminants in different regions and continents is of great relevance for understanding their biodiversity and biogeography or, in other words, the clonal structure of T. theileri.
Acknowledgements
We would like to express our sincere thanks to the local hunters and municipal officers engaged in the protection of wildlife in Shimonoseki City, Yamaguchi Prefecture, as well as Kushimoto Town and Tanabe City, Wakayama Prefecture, Japan, who made this study possible.
Author contributions
A.T., K.M., A.S.-I. and H.S. conceived and designed the study. I.R., A.S., M.E., A.T., A.S.-I., K.S. and H.S. conducted data gathering. I.R., A.S. and M.E. wrote the article, and A.T., K.M. and H.S. edited it.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Ethical standards
Not applicable.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182021001360.
click here to view supplementary material
Data
Representative blood films collected in the present work were deposited in the Meguro Parasitological Museum, Tokyo, Japan. The nucleotide sequences obtained in the present study are available from the DDBJ/EMBL/GenBank databases under the accession nos. LC618030–LC618052.
Conflict of interest
None.
References
- Anisimova M and Gascuel O (2006) Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Systematic Biology 55, 539–552. [DOI] [PubMed] [Google Scholar]
- Auty H, Anderson NE, Picozzi K, Lembo T, Mubanga J, Hoare R, Fyumagwa RD, Mable B, Hamill L, Cleaveland S and Welburn SC (2012) Trypanosome diversity in wildlife species from the Serengeti and Luangwa Valley ecosystems. PLoS Neglected Tropical Diseases 6, e1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böse R, Friedhoff KT and Olbrich S (1987a) Transmission of Megatrypanum trypanosomes to Cervus dama by Tabanidae. Journal of Protozoology 34, 110–113. [DOI] [PubMed] [Google Scholar]
- Böse R, Friedhoff KT, Olbrich S, Büscher G and Domeyer I (1987b) Transmission of Trypanosoma theileri to cattle by Tabanidae. Parasitology Research 73, 421–424. [DOI] [PubMed] [Google Scholar]
- Böse R, Petersen K, Pospichal H, Buchanan N and Tait A (1993) Characterization of Megatrypanum trypanosomes from European Cervidae. Parasitology 107, 55–61. [DOI] [PubMed] [Google Scholar]
- Cortez AP, Rodrigues AC, Garcia HA, Neves L, Batista JS, Bengaly Z, Paiva F and Teixeira MMG (2009) Cathepsin L-like genes of Trypanosoma vivax from Africa and South America – characterization, relationships and diagnostic implications. Molecular and Cellular Probes 23, 44–51. [DOI] [PubMed] [Google Scholar]
- D'Alessandro PA and Behr MA (1991) Trypanosoma lewisi and its relatives. In Kreier JP and Baker JR (eds), Parasitic Protozoa 2nd Edn. vol 1. San Diego, CA, USA: Academic Press, pp. 225–263. [Google Scholar]
- Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie J-M and Gascuel O (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Research 36, 465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirie MF, Bornstein S, Wallbanks KR, Molyneux DH and Steen M (1990) Comparative studies on Megatrypanum trypanosomes from cervids. Tropical Medicine and Parasitology 41, 198–202. [PubMed] [Google Scholar]
- Ellis J, Barratt J, Kaufer A, Pearn L, Armstrong B, Johnson M, Park Y, Downey L, Cao M, Neill L, Lee R, Ellis B, Tyler K, Lun Z-R and Stark D (2021) A new subspecies of Trypanosoma cyclops found in the Australian terrestrial leech Chtonobdella bilineata. Parasitology 148, 1125–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AC, Schuster G, Cobb WJ, James AM, Cooper SM, Peréz de León AA and Holman PJ (2013) Molecular characterization of Trypanosoma (Megatrypanum) spp. infecting cattle (Bos taurus), white-tailed deer (Odocoileus virginianus), and elk (Cervus elaphus canadensis) in the United States. Veterinary Parasitology 197, 29–42. [DOI] [PubMed] [Google Scholar]
- Garcia HA, Kamyingkird K, Rodrigues AC, Jittapalapong S, Teixeira MMG and Desquesnes M (2011a) High genetic diversity in field isolates of Trypanosoma theileri assessed by analysis of cathepsin L-like sequences disclosed multiple and new genotypes infecting cattle in Thailand. Veterinary Parasitology 180, 363–367. [DOI] [PubMed] [Google Scholar]
- Garcia HA, Rodrigues AC, Martinkovic F, Minervino AHH, Campaner M, Nunes VLB, Paiva F, Hamilton PB and Teixeira MMG (2011b) Multilocus phylogeographical analysis of Trypanosoma (Megatrypanum) genotypes from sympatric cattle and water buffalo populations supports evolutionary host constraint and close phylogenetic relationships with genotypes found in other ruminants. International Journal for Parasitology 41, 1385–1396. [DOI] [PubMed] [Google Scholar]
- Garcia HA, Blanco PA, Rodrigues AC, Rodrigues CMF, Carmen SA, Takata CSA, Campaner M, Camargo EP and Teixeira MMG (2020) Pan-American Trypanosoma (Megatrypanum) trinaperronei n. sp. in the white tailed deer Odocoileus virginianus Zimmermann and its deer ked Lipoptena mazamae Rondani, 1878: morphological, developmental and phylogeographical characterization. Parasites & Vectors 13, 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W, Pilkington JG and Pemberton JM (2010) Trypanosoma melophagium from the sheep ked Melophagus ovinus on the island of St Kilda. Parasitology 137, 1799–1804. [DOI] [PubMed] [Google Scholar]
- Guindon S and Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52, 696–704. [DOI] [PubMed] [Google Scholar]
- Hamilton PB, Stevens JR, Gaunt MW, Gidley J and Gibson WC (2004) Trypanosomes are monophyletic: evidence from genes for glyceraldehyde phosphate dehydrogenase and small subunit ribosomal RNA. International Journal for Parasitology 34, 1393–1404. [DOI] [PubMed] [Google Scholar]
- Hamilton PB, Stevens JR, Gidley J, Holz P and Gibson WC (2005) A new lineage of trypanosomes from Australian vertebrates and terrestrial bloodsucking leeches (Haemadipsidae). International Journal for Parasitology 35, 431–443. [DOI] [PubMed] [Google Scholar]
- Hamilton PB, Stevens JR and Gibson WC (2007) Patterns of co-evolution between trypanosomes and their hosts deduced from ribosomal RNA and protein-coding gene phylogenies. Molecular Phylogenetics and Evolution 44, 15–25. [DOI] [PubMed] [Google Scholar]
- Hamilton PB, Adams ER, Njiolou F, Gibson WC, Cuny G and Herder S (2009) Phylogenetic analysis reveals the presence of the Trypanosoma cruzi clade in African terrestrial mammals. Infection, Genetics and Evolution 9, 81–86. [DOI] [PubMed] [Google Scholar]
- Hatama S, Shibahara T, Muzuki M, Kadota K, Uchida I and Kanno T (2007) Isolation of a Megatrypanum trypanosome from sika deer (Cervus nippon yesoensis) in Japan. Veterinary Parasitology 149, 56–64. [DOI] [PubMed] [Google Scholar]
- Hoare CA (1972) The Trypanosomes of Mammals: A Zoological Monograph. Oxford, UK: Blackwell Scientific Publications. [Google Scholar]
- Kingston N and Morton JK (1975) Trypanosoma cervi sp. n. from elk (Cervus canadensis) in Wyoming. Journal of Parasitology 61, 17–23. [PubMed] [Google Scholar]
- Kingston N, Franzmann A and Maki L (1985) Redescription of Trypanosoma cervi (Protozoa) in moose, Alces alces, from Alaska and Wyoming. Proceedings of the Helminthological Society of Washington 52, 54–59. [Google Scholar]
- Kingston N, Thomas G, McHolland L, Williams ES, Trueblood MS and Maki L (1986) Experimental transmission of Trypanosoma theileri to bison. Proceedings of the Helminthological Society of Washington 53, 198–203. [Google Scholar]
- Kingston N, Bobek B and Perzanowski K (1992) Description of Trypanosoma (Megatrypanum) stefanskii sp. n. from roe deer (Capreolus capreolus) in Poland. Journal of the Helminthological Society of Washington 59, 89–95. [Google Scholar]
- Martinković F, Matanović K, Rodrigues AC, Garcia HA and Teixeira MMG (2012) Trypanosoma (Megatrypanum) melophagium in the sheep ked Melophagus ovinus from organic farms in Croatia: phylogenetic inferences support restriction to sheep and sheep keds and close relationship with trypanosomes from other ruminant species. Journal of Eukaryotic Microbiology 59, 134–144. [DOI] [PubMed] [Google Scholar]
- Nagata J, Masuda R, Tamate HB, Hamasaki S, Ochiai K, Asada M, Tatsuzawa S, Suda K, Tado H and Yoshida MC (1999) Two genetically distinct lineages of the sika deer, Cervus nippon, in Japanese Islands: comparison of mitochondrial D-loop region sequences. Molecular Phylogenetics and Evolution 13, 511–519. [DOI] [PubMed] [Google Scholar]
- Ohdachi SD, Ishibashi Y, Iwasa MA and Saitoh T (2010) The Wild Mammals of Japan. Kyoto, Japan: Shoukadoh Book Sellers. [Google Scholar]
- Pacheco TDA, Marcili A, da Costa AP, Witter R, Melo ALT, Boas RV, Chitarra CS, Dutra V, Nakazato L and Pacheco RC (2018) Genetic diversity and molecular survey of Trypanosoma (Megatrypanum) theileri in cattle in Brazil's western Amazon region. Revista Brasileira de Parasitologia Veterinária 27, 579–583. [DOI] [PubMed] [Google Scholar]
- Rodrigues AC, Campaner M, Takata CS, Dell'Porto A, Milder RV, Takeda GF and Teixeira MMG (2003). Brazilian isolates of Trypanosoma (Megatrypanum) theileri: diagnosis and differentiation of isolates from cattle and water buffalo based on biological characteristics and randomly amplified DNA sequences. Veterinary Parasitology 116, 185–207. [DOI] [PubMed] [Google Scholar]
- Rodrigues AC, Paiva F, Campaner M, Stevens JR, Noyes HA and Teixeira MMG (2006) Phylogeny of Trypanosoma (Megatrypanum) theileri and related trypanosomes reveals lineages of isolates associated with artiodactyl hosts diverging on SSU and ITS ribosomal sequences. Parasitology 132, 215–224. [DOI] [PubMed] [Google Scholar]
- Rodrigues AC, Garcia HA, Batista JS, Minervino AHH, Góes-Cavalcante GG, Silva FMD, Ferreira RC, Campaner M, Paiva F and Teixeira MMG (2010a) Characterization of spliced leader genes of Trypanosoma (Megatrypanum) theileri: phylogeographical analysis of Brazilian isolates from cattle supports spatial clustering of genotypes and parity with ribosomal markers. Parasitology 137, 111–122. [DOI] [PubMed] [Google Scholar]
- Rodrigues AC, Garcia HA, Ortiz PA, Cortez AP, Martinkovic F, Paiva F, Batista JS, Minervino AH, Campaner M, Pral EM, Alfieri SC and Teixeira MMG (2010b) Cysteine proteases of Trypanosoma (Megatrypanum) theileri: cathepsin L-like gene sequences as targets for phylogenetic analysis, genotyping diagnosis. Parasitology International 59, 318–325. [DOI] [PubMed] [Google Scholar]
- Sato H, Ishita K, Matsuo K, Inaba T, Kamiya H and Ito M (2003) Persistent infection of Mongolian jirds with a non-pathogenic trypanosome, Trypanosoma (Herpetosoma) grosi. Parasitology 127, 357–363. [DOI] [PubMed] [Google Scholar]
- Sato H, Osanai A, Kamiya H, Obara Y, Jiang W, Zhen W, Chai J, Une Y and Ito M (2005) Characterization of SSU and LSU rRNA genes of three Trypanosoma (Herpetosoma) grosi isolates maintained in Mongolian jirds. Parasitology 130, 157–167. [DOI] [PubMed] [Google Scholar]
- Sato H, Leo N, Katakai Y, Takano J, Akari H, Nakamura S and Une Y (2008) Prevalence and molecular phylogenetic characterization of Trypanosoma (Megatrypanum) minasense in the peripheral blood of small Neotropical primates after a quarantine period. Journal of Parasitology 94, 1128–1138. [DOI] [PubMed] [Google Scholar]
- Schlafer DH (1979) Trypanosoma theileri: a literature review and report of incidence in New York cattle. Cornell Veterinarian 69, 411–425. [PubMed] [Google Scholar]
- Stevens J, Teixeira MMG, Bingle LEH and Gibson WC (1999) The taxonomic position and evolutionary relationships of Trypanosoma rangeli. International Journal for Parasitology 29, 749–757. [DOI] [PubMed] [Google Scholar]
- Tamate HB, Tatsuzawa S, Suda K, Izawa M, Doi T, Sunagawa K, Miyahira F and Tado H (1998) Mitochondrial DNA variations in local populations of the Japanese sika deer, Cervus nippon. Journal of Mammalogy 79, 1396–1403. [Google Scholar]
- Teacher AGF and Griffiths DJ (2011) HapStar: automated haplotype network layout and visualization. Molecular Ecology Resources 11, 151–153. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG and Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22, 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Godfrey SS and Thompson RCA (2014) Trypanosomes of Australian mammals: a review. International Journal for Parasitology: Parasites and Wildlife 3, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touré S (1968) Description complémentaire de Trypanosoma theileri Laveran, 1902. Mention particulière des formes observées en Casamance (Sénégal). Revue d'Elevage et de Médecine Vétérinaire des Pays Tropicaux 21, 365–373. [PubMed] [Google Scholar]
- Votýpka J, d'Avila-Levy CM, Grellier P, Maslov DA, Lukeš J and Yurchenko V (2015) New approaches to systematics of Trypanosomatidae: criteria for taxonomic (re)description. Trends in Parasitology 31, 460–469. [DOI] [PubMed] [Google Scholar]
- Weerasooriya G, Sivakumar T, Lan DTB, Long PT, Takemae H, Igarashi I, Inoue N and Yokoyama N (2016) Epidemiology of bovine hemoprotozoa parasites in cattle and water buffalo in Vietnam. Journal of Veterinary Medical Science 78, 1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wita I and Kingston N (1999) Trypanosoma cervi in red deer, Cervus elaphus, in Poland. Acta Parasitologica 44, 93–98. [Google Scholar]
- Ybañez AP, Sivakumar T, Ybañez RH, Vincoy MR, Tingson JA, Perez ZO, Gabotero SR, Buchorno LP, Inoue N, Matsumoto K, Inokuma H and Yokoyama N (2013) Molecular survey of bovine vector-borne pathogens in Cebu, Philippines. Veterinary Parasitology 196, 13–20. [DOI] [PubMed] [Google Scholar]
- Yokoyama N, Sivakumar T, Fukushi S, Tattiyapong M, Tuvshintulga B, Kothalawala H, Silva SS, Igarashi I and Inoue N (2015) Genetic diversity in Trypanosoma theileri from Sri Lankan cattle and water buffaloes. Veterinary Parasitology 207, 335–341. [DOI] [PubMed] [Google Scholar]
- Zuker M (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Research 31, 3406–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182021001360.
click here to view supplementary material
Data Availability Statement
Representative blood films collected in the present work were deposited in the Meguro Parasitological Museum, Tokyo, Japan. The nucleotide sequences obtained in the present study are available from the DDBJ/EMBL/GenBank databases under the accession nos. LC618030–LC618052.