Keywords: Congenital toxoplasmosis, humans, prevention, Toxoplasma gondii, toxoplasmosis, worldwide
Abstract
The morbidity due to congenital toxoplasmosis in humans is very high. Most of these infected children are likely to develop symptoms of clinical toxoplasmosis. Sequelae in fetus resulting from Toxoplasma gondii infections in women who become infected with this parasite during pregnancy can be devastating and enormous efforts are directed in some countries to prevent these consequences. Here, an update on congenital toxoplasmosis in humans, especially the rate of congenital infections in humans worldwide, is provided. Although several countries have surveillance programmes, most information on the rate of congenital transmission is from France and Brazil. Because of compulsory national screening programme in France to detect and treat women with recently acquired T. gondii infection with anti-toxoplasma therapy, the rate of congenital transmission and the severity of disease in children are declining. Infections by this parasite are widely prevalent in Brazil. The severity of clinical toxoplasmosis in Brazilian children is very high and may be associated with the genetic characteristics of T. gondii isolates prevailing in animals and humans in Brazil. Virtually little or no information is available on this topic from China, India and other countries in Asia.
Introduction
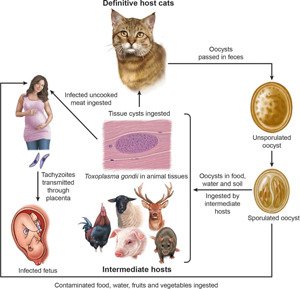
Toxoplasmosis, caused by the protozoan parasite, Toxoplasma gondii, is a worldwide zoonosis, and infections are widely prevalent in humans and animals (Dubey and Beattie, 1988; Dubey, 2010). Toxoplasmosis can cause serious illness in humans of all ages, and in particular immunosuppressed patients and neonates (Robert-Gangneux and Dardé, 2012; Torgerson and Mastroiacovo, 2013; Peyron et al., 2016; Dardé et al., 2020; McLeod et al., 2020). Although seroprevalence in Europe has declined in the past 2 decades, a very high prevalence is still prevalent in many countries (McLeod et al., 2020).
Sequelae in fetus resulting from T. gondii infections in women who become infected with this parasite during pregnancy can be devastating and enormous efforts are directed in some countries to prevent these consequences. Here an update on congenital toxoplasmosis in humans is provided, especially on the rate of congenital infections in humans worldwide.
Background of congenital toxoplasmosis
After the ingestion of food or water contaminated with T. gondii, there is parasitemia, and tachyzoites can invade the placenta if the woman is pregnant. Humans have haemochorial placenta. Nearly half of fetuses whose mothers become infected during pregnancy escape T. gondii infection. The global rate of transmission during pregnancy is 29% (Dunn et al., 1999). The stage of gestation at the time of mother's infection may determine the transmission of T. gondii to fetus. In general, the transmission of T. gondii is more efficient in the later half of gestation, mostly related to the anatomy and immune factors. For example, the thickness of the placenta varies with gestation; in early pregnancy, placental barrier of humans is 50–100 μm thick and progressively decreases to 2.5–5 μm at the end of pregnancy, allowing tachyzoites to more easily invade trophoblasts by the end of the gestational course (Blaszkowska and Góralska, 2014). Additionally, the internal cytotrophoblast layer is discontinuous, with its cells number decreasing during the gestational period. Infection early in gestation is clinically more severe, as reduced expression of Toll-like receptors in trophoblast cells during the first trimester of pregnancy may indicate a reduced ability of early placental cells to engage the immune response to intrauterine infection (Blaszkowska and Góralska, 2014).
Transplacental transmission of T. gondii infection generally occurs when a woman becomes infected during pregnancy. Rarely, congenital transmission occurs in women infected just before pregnancy or during chronic infection (Villena et al., 1998; Elbez-Rubinstein et al., 2009). In addition, in immunosuppressed women, reactivation/reinfection of an infection acquired before pregnancy can lead to congenital toxoplasmosis but is rare (Peyron et al., 2016).
Transplacental infection can lead to a wide variety of manifestations in the fetus and infant including spontaneous abortion, stillbirth; it can also cause severe disease in live infant, but most children are asymptomatic at birth. Although T. gondii may sometimes cause sporadic abortion, there is no evidence that it causes habitual abortion. Most severe cases of prenatally acquired toxoplasmosis were reported first with the predominant manifestation of encephalomyelitis. Historically, the first confirmed case of congenital toxoplasmosis was in an infant girl who was delivered full term by Caesarean section on 23 May 1938 at Babies Hospital, New York (Wolf et al., 1939). The girl developed convulsive seizures at 3 days of age and lesions were noted in the maculae of both eyes through an ophthalmoscope. She died of toxoplasmosis when 1-month-old and an autopsy was performed. At post-mortem, brain, spinal cord and right eye were removed for examination. Free and intracellular T. gondii were found in the lesions of encephalomyelitis and retinitis, and viable T. gondii was isolated in mice, rats and rabbits inoculated with tissues from the girl (Wolf et al., 1939).
In early 1950s, Dr Albert Sabin proposed a triad of signs: hydrocephalus or microcephalus, intracranial calcification and retinochoroiditis. This triad has been useful in drawing attention to prenatal toxoplasmosis. A better understanding came from the work of Eichenwald (1960), who found asymptomatic and clinical toxoplasmosis in many children in 1950s in Austria. Although the study by Eichenwald (1960) from selected cases before treatment (prenatal and postnatal) of infected children became a routine, it pointed that toxoplasmosis can cause serious illness in children including both generalized and neurological disease (Dubey and Beattie, 1988). As stated earlier, the most common manifestation of prenatal toxoplasmosis is ocular disease, sometimes presented as microphthalmy, cataracts, strabismus or nystagmus and even total blindness.
Hydrocephalus is the most dramatic sign of congenital toxoplasmosis, and occurs in approximately 4% of symptomatic children (Hutson et al., 2015). Initially, it was considered to be due to the blockage of aqueduct of Sylvius. Recently, 4 anatomical patterns of hydrocephalus were reported: (i) obstruction of aqueduct of Sylvius, occurring in 43% of cases, (ii) obstruction of foramina of Monroe occurring in 25% of cases, (iii) mixed aqueductal and foraminal obstruction, occurring in 11% of cases, and (iv) with no obstructive pathogenesis, and was seen in 21% of cases (Hutson et al., 2015). Ocular symptoms are the most common signs of congenital toxoplasmosis.
Most prenatal infections are sub-clinical at birth. Disease, if present in the neonatal period, is likely to be severe, invariably with neurological signs and often with signs of generalized infection. Such patients rarely recover without serious sequelae. Disease appearing in the first few months of life is usually less severe and is manifested by nystagmus, convulsions, bulging fontanelle and abnormal increase in skull circumference. Such patients sometimes develop normally.
It is likely that many cases of prenatal toxoplasmosis are missed because of difficulty in diagnosis. Couvreur et al. (1984), who diagnosed prenatal toxoplasmosis in 210 babies aged 0–10 months, found premature birth, intrauterine growth retardation or both in 17%, hyperbilirubinemia in 10%, hydrocephaly or microcephaly in 9%, intracranial calcification in 11% and retinochoroiditis in 22%. Infection was fatal in 2 of the 210, severe in 10%, mild without neurological signs in 34% and subclinical in 55%. It is noteworthy that over half of the babies born with T. gondii infection had no clinical manifestations. As noted by Eichenwald (1960) earlier, in congenitally infected children, virtually all organ systems may be affected (Peyron et al., 2016).
An important question concerns the subsequent fate of subclinically infected babies. Many of them develop retinochoroiditis, although it may not manifest until later in childhood, or even in adult life. In a follow-up of 11 congenitally infected children without symptoms at birth, 9 (82%) developed lesions within 20 years; 4 (36.6%) of them developed retinal scars that impaired vision, 5 (45.5%) developed scars without affecting vision (Koppe et al., 1986). In a 14-year follow-up of 327 congenitally infected children in Lyon, France, 95 (29%) had lesions despite treatment for toxoplasmosis. At the final examination, 60 (18%) had lesions only in the eyes, 35 (11%) had CNS lesions (intracerebral calcification in 31, hydrocephalus in 6 and microcephalus in 1) (Wallon et al., 2004). In a study in the USA, 11 of 120 congenitally infected children (many with obvious symptoms) recruited in a treatment programme died within 4 years despite treatment (McLeod et al., 2006a).
The morbidity of congenital toxoplasmosis in children is very high and true suffering may be underestimated (Havelaar et al., 2007; Bénard et al., 2008; Stillwaggon et al., 2011; Torgerson and Mastroiacovo, 2013; El Bissati et al., 2018; Binquet et al., 2019; Picone et al., 2020). One study estimated 1.2 million disability-adjusted life years and an estimated 190 100 cases globally (Torgerson and Mastroiacovo, 2013).
The risk of congenital infection is lowest when mother becomes infected in the first trimester (10–15%) and highest when mother acquires infection during the third trimester (Table 1). If maternal infection occurs early in pregnancy, it results in fewer infected babies, but they are more severely affected than the greater number of infected babies born when infection is acquired later in pregnancy. The highest risk to the fetus is when infection is acquired at 10–24th week of gestation. As will be seen in Table 1, most of this information on the transmission of congenital toxoplasmosis is derived from studies in France. In their pioneering study, Desmonts and Couvreur (1974a, b) reported congenital toxoplasmosis in 210 children born at 1 hospital in Paris; approximately 26% were subclinically infected at birth. Only about 10% were clinically affected – 6% mildly and 4% severely, and up to 3% died in the neonatal period. In a subsequent study from the same hospital, Daffos et al. (1994) reported clinical outcome in 148 infected fetuses of 2030 mothers who seroconverted during pregnancy. Based on ultrasound examinations, they found that 48, 12 and 3% of fetuses had cerebral ventricular dilatations when mothers became infected in early (<16 weeks), middle (17–23 weeks) and late (after 24 weeks) gestation, respectively (Daffos et al., 1994). In a multicentre European study of 255 congenitally infected children, gestational age at the time of seroconversion in mothers was correlated with cerebral lesions but not retinochoroiditis (Gras et al., 2005). In this study, 51 of 255 infants had 1 or more lesions, and 9 had both intracranial and ocular lesions. Of these, 4 of 55 children died (at 13 months, 11 months, 3 months and 7 days of age). The mother of the baby that died at 1 week of age had seroconverted between 5 and 31 days of gestation and she had received spiramycin prophylaxis from 32nd week until delivery (Gras et al., 2005). The frequency and severity of clinical disease in congenitally infected children in France and Austria has decreased dramatically in the last decade, perhaps because of improved early detection and treatment. Ultrasound sonography can aid the determination of severity of lesions in the fetus (Codaccioni et al., 2020).
Table 1.
Rate of congenital Toxoplasma gondii transmission according to the gestational age of maternal seroconversion in Francea
| Reference | Desmonts and Couvreur (1984) | Hohlfeld et al. (1994) | Pratlong et al. (1994) | Dunn et al. (1999) | The SYROCOTT (2007) | Villena et al. (2010) | Wallon et al. (2013) |
|---|---|---|---|---|---|---|---|
| Gestational age (weeks) | n = 489b | n = 2081b | n = 187b | n = 603b | n = 1438b | n = 235b | n = 1624b |
| <16 | 9.1 | 3.7 | 3.9 | 6 | 15 | 7.2 | 10.0 |
| 16–28 | 28.2 | 16.5 | 17.1 | 40 | 44 | 35.3 | 20.0 |
| >28 | 59.3 | 28.9 | 53.1 | 72 | 71 | 57.4 | 55.8 |
Women who seroconverted during pregnancy were treated with anti-T. gondii drugs to prevent fetal transmission and damage to the fetus.
No. of women who seroconverted during pregnancy.
A recent study in France retrospectively evaluated 88 cases of congenital toxoplasmosis with ultrasound anomalies diagnosed by fetal medicine experts, 45 (51.1%) had one or more cerebral lesions, the most common lesion being intracranial hyperechogenic cerebral nodular foci (Codaccioni et al., 2020). In Table 2, initial data from screening studies are listed; at that time only a few follow-up studies on infected children were undertaken. Subsequently, many of these children were followed clinically for 4 or more years, and data included in the study were reported (The SYROCOT, 2007). Of 691 congenitally infected children from Europe, Brazil, Colombia and the USA, 24% had at least 1 clinical manifestation, 185 had ocular lesions and 13% had intracerebral calcification (The SYROCOT, 2007).
Table 2.
Congenital T. gondii infection in humans based on prenatal or postnatal screeninga
| Country | No. screened (years) | Infected children | Incidence rate | Symptomatic childrenb | Reference |
|---|---|---|---|---|---|
| Australia | 18 908 (1986–1989) | 3 | 1:6300 | 0 | Walpole et al. (1991) |
| Austria | 63 416 pregnant women (2000–2007) | 66 pregnant with primoinfection | Seroconversion rate in mothers 0.17%; no data on congenital infection | No data | Sagel et al. (2011) |
| Austria | 5545 | 4c | 1:1386 | No data | Prusa et al. (2013) |
| Austria | 1 387 680 (1992–2008) | 141 | 1: 10 000 | 7 died/terminated (4 spontaneous abortion, 2 hydrocephalus terminations, 1 porencephaly). Of the 17 live infants all had ICC, including microphthalmos in 2; 12 had neurological deficits within the first year of life | Prusa et al. (2015b) |
| Brazil | >364 130 (1995–2009) | >195 (newborn screening) | 5–23/10 000 | Data summarized in Dubey et al. (2012) | Neto et al. (2004); The SYROCOT (2007); Gilbert et al. (2008) |
| Brazil (Minas Gerais) | 146 307 (2006–2007) | 190 (newborn screening) | 13: 10 000 | >142 (142 RC, 39 ICC, 12 hydrocephalus, 10 microcephaly, 46 hearing loss, 4 died) | Vasconcelos-Santos et al. (2009); de Resende et al. (2010) |
| Brazil (Sergipe) | 15 204 (1999) newborns | 6 (newborn screening) | 4: 10 000 | 4 (3 RC, 1 ICC) | Inagaki et al. (2012) |
| Brazil (Goiás) | 246-newborns (2003–2011) | 162 (prenatal screening) | No data | 128 (50 RC, 51 ICC, 19 brain ventriculomegaly or hydrocephalus, 66 severe generalized disease, 13 blinds, 7 died) | Avelino et al. (2014) |
| Brazil (Paraná) | 31 (2000–2010) | 29 (prenatal or postnatal screening) | No data | 26 symptomatic at 1 month (16 RC, 13 ICC, 6 hydrocephalus, 2 microphthalmia, 1 microcephaly, 3 hearing loss) | Capobiango et al. (2014) |
| Brazil (Rio Grande do Sul) | 41 305 (2004–2014) | 24 (prenatal screening) | 6: 10 000 | 19 (13 RC, 9 ICC, 1 hydrocephalus, 5 microcephaly, 5 hearing loss, 1 cataract, 1 spastic). Of 5 children born asymptomatic, 3 remained IgG at 12 months, 1 became IgG negative at 15 months, and 1 lost to follow-up | Bischoff et al. (2016) |
| Brazil (Rondônia) | 102 963 (4 years) | 126 (newborn screening) | 12: 10 000 | 126 newborns with symptoms suggestive of congenital toxoplasmosis | Paraguassú-Chaves et al. (2019) |
| Brazil (Paraná) | 65 375 (2002–2016) | 39 (29 prenatal screening) | 6: 10 000 (15-year average) | 15 without clinical data, 12 of 24 symptomatic (12 RC, 3 ICC, 2 hydrocephalus) | Takahashi et al. (2019) |
| Brazil (Rio Grande do Sul) | 77 follow-up neonates, infants or children (1996–2017) | 77 infected children followed up to a median of 10 years (2–25) (65 prenatal or postnatal screening) | No data | 62 (55 RC, 44 ICC, 18 brain ventriculomegaly, 4 hydrocephalus, 9 microcephaly, 3 hearing loss) new RC lesions after the first year in 29 patients, with peaks at 4–5 and 9–14 years | Lago et al. (2021) |
| Colombia | 937 | 1 | No data | RC | Gomez-Marin et al. (1997) |
| Colombia | 2786 women + 522 newborns | 17d | 14:2786 | 4 RC, 4 ICC, 3 RC | Gómez (2005); Gallego-Marín et al. (2006); Gomez-Marin et al. (2007); The SYROCOT (2007) |
| Colombia | 15 333 (2003–2008) | 15 | No data | 7 symptomatic, 3 died in first month after birth. Hydrocephalus in 1, ICC in 3, RC in 3 | Gómez-Marin et al. (2011) |
| Denmark | 89 873 | 27 | 1:3328 | 5 (hydrocephalus and RC in 1, ICC and RC 1, ICC and retarded in 1, blind and retarded in 1) | Lebech et al. (1999); Gilbert and Peckham (2001) |
| Finland | 16 733 (1988–1989) | 4 | 1:4481 | 2 (ICC and RC in 1), no additional cases in 5-years follow-up | Lappalainen et al. (1995) |
| France | 30 768 | 36 | 1:854 | 7 (RC 7, CNS 1) | Philippe et al. (1988) |
| France | Children from 1206 infected mothers (1987–2000) | 366 | No data | 65 (ICC 24, RC 46) | The SYROCOT (2007) |
| France | 818 700 (2007) | 272e | 3.4: 10 000 | 11 prenatal (6 abortions, 5 fetal deaths), 28 symptomatic (ICC 21, 3 hydrocephalus, 4 macular chorioretinitis) | Villena et al. (2010) |
| France | Children from 1,624 infected mothers (1992–2008) | 207f | No data | 32 (RC), 22 (ICC), 5 hydrocephalus, 2 hepatosplenomegaly | Wallon et al. (2013) |
| Germany | 262 912 (1999–2002) | 55 | 1:4762 | 12 (ICC 5, RC 2, ICC and RC 4, hydrocephalus, ICC and RC 1) | Schmidt et al. (2006) |
| Greece | 63 suspected (2006–2009) | 21 | 4.5–5.1 per 100 000 births | 14 confirmed and 7 probable cases. 10 symptomatic at birth (RC in 5) | Aptouramani et al. (2012) |
| Hungary | 17 735 (1987–1994) | 0 | No data | No data | Szénási et al. (1997) |
| Italy | 28 247 (1996–2000) | 2 | 1: 14 123 | 0 at birth, new RC in 3 in 1-year follow-up | Valcavi et al. (1995) |
| Italy | Children from 43 infected mothers (1996–2000) | 15 | No data | 3 (ICC 3, RC 3) | The SYROCOT (2007) |
| Morocco | 48 890 (2015) | 21 | 4–8/10 000 births | No data | El-Bissati et al. (2018) |
| The Netherlands | 28 049 (1987–1988) | 12 | No data | 3 (ICC 1, RC 3) | Gilbert and Peckham (2001), The SYROCOT (2007) |
| The Netherlands | 10 008 (2006) | 18 | No data | No follow-up | Kortbeek et al. (2009) |
| Norway | 35 940 (1992–1993) | 11 | 1 (RC with loss of vision) | Jenum et al. (1998) | |
| Norway | Children from 33 infected mothers (1992–1994) | 17 | No data | 6 (ICC 4, RC 3) | The SYROCOT (2007) |
| Panama | 2,326 pregnant women screening and newborn babies testing (2017–2018) | 9 | 3.8/1000 live births | No data | Flores et al. (2021) |
| Poland | >27 516 (1998–2000) | 20 | No data | 5 (ICC 5, RC 1) | Paul et al. (2000, 2001a, b); The SYROCOT (2007) |
| Spain | 16 362 women surveyed (1999) | 5g | No data | 4 born asymptomatic | Muñoz Batet et al. (2004) |
| Sweden | 35 000 (1992–1993; 1997–1998) | 3 | No data | 1 (ICC, RC) | Evengård et al. (1999, 2001) |
| USA | >635 000 (1986–1992) | 50 + 5 | 1: 10 000 | 19 (14 CNS, 9 RC), 1–6-year follow-up in 39 children, new eye lesions in 4 | Guerina et al. (1994); Guerina (1994) |
Modified from Dubey (2010).
CNS, central nervous system signs; ICC, intracerebral calcification; NS, not stated; RC, retinochoroiditis.
Of 3708 mothers, 7 seroconverted during pregnancy. One infected fetus with abnormal prenatal ultrasound identified by prenatal screening, and infections in 3 babies were found by cord blood screening.
Of 2786 screened, 19 mothers seroconverted during pregnancy and 14 children were born infected. Three children were found infected by screening of 532 newborns; 8 of these infected children were selected for The SYROCOT analysis because diagnosis of cerebral lesion was done with tomography; those studies based on ultrasound were discarded (personnel communication from Gomez-Marin to J.P.D-February 25, 2021).
In 2007, there were 818 700 live births in France through the national screening programme; 272 congenital T. gondii-infections were recognized; 160 infections were diagnosed postnatally (130 at the age of 2 months, 22 between 2 months and 1 year). Of 235 cases, infection was acquired in first trimester in 17 (7%), 83 (35%) in the second trimester and 135 (58%) in third trimester.
Of 1624 mothers who seroconverted during pregnancy. Lesions in utero in 5, 20 at birth, 21 after birth. RC lesions discovered at birth in 2, in the first year in 20 and third year in 5.
In a multicentre retrospective study of 16 362 women in Barcelona in 1999, seroprevalence was 28.6% with primary infection of 1.02 per 1000 susceptible women; 9 of 12 susceptible women seroconverted during pregnancy. Out of 5 infected children, 4 were asymptomatic at birth; outcome of fifth infant was not stated.
As stated earlier, 60–70% of babies born from infected mothers escape infection. The severity of toxoplasmosis in the fetus or the infant is not related to the degree of symptoms of T. gondii infection in the mother. In 1 study that retrospectively examined risk factors among women who gave birth to infected children, 52% could not recall being sick (Boyer et al., 2005). In another study from a hospital in Lyon, France, of 603 women who seroconverted during pregnancy, only 36 (5%) had clinical symptoms (Dunn et al., 1999). In 161 of these 603 women (504 were treated for toxoplasmosis), infection was transmitted to their fetuses; 5 of them aborted, 3 were stillborn. Most of the 153 live born children were followed for 54 months after birth; 41 (27%) developed clinical signs (33 had retinochoroiditis, 14 had intracerebral calcification, 8 had combinations of signs, 1 child died when 8 days old) (Dunn et al., 1999).
The severity of symptoms is primarily related to the trimester of pregnancy when the mother becomes infected with T. gondii (Table 1). However, T. gondii genotype might be another factor, and this topic was reviewed recently (Dardé et al., 2020; Mcleod et al., 2020). Toxoplasma gondii strains are grouped into clonal Type I, II, III and atypical, based on different systems of genotyping (Dardé et al., 2020). Type II and III T. gondii strains predominate in Europe and North America, and Type I is rare. A different situation prevails in South America. The T. gondii strains from Brazil are mostly atypical and clonal strains are rare. In France, most strains isolated from congenital infections are Type II and the severity of congenital toxoplasmosis is related to the trimester of pregnancy when mother becomes infected (Dardé et al., 2020). However, in Brazil, women who became infected during the third trimester of pregnancy during oocyst-associated outbreaks of T. gondii had congenitally infected children with severe toxoplasmosis (Conceição et al., 2021). The severity of congenital toxoplasmosis in Brazil in comparison with France is thought to be associated with atypical T. gondii genotypes (Dubey et al., 2012). However, compared with France, relatively few strains from congenitally infected children in Brazil have been isolated and fully genotyped; 14 genotypes were reported (Carneiro et al., 2013). There was no association of genotype with the severity of ocular lesions. However, in the USA, by using a serotyping assay, ocular toxoplasmosis and different anatomical patterns of hydrocephalus were associated with T. gondii Type II than in non-Type II (McLeod et al., 2012). It should be noted that serotyping has limited efficiency in distinguishing genotypes.
Estimates of congenital transmission
An estimate of the incidence of clinically manifest prenatal toxoplasmosis may be obtained in 3 ways: first, from reports of observed cases and, second, from calculations based on the infection rate during pregnancy, and third, from screening of babies at birth. Representative examples of estimates of congenital infections based primarily on screening of mothers during pregnancy are given in Table 2. Many countries have some surveillance programmes (van der Giessen et al., 2021). Congenital infections noted during acute outbreaks of toxoplasmosis summarized recently (Dubey, 2021) were excluded from Table 2. Data from the National Collaborative Chicago-based Toxoplasmosis Study in the USA (Boyer et al., 2005, 2011; McLeod et al., 2006a, b, 2009, 2020) are not included in Table 2.
More information is available from countries that have screening (prenatal or postnatal) programmes (Tables 2 and 3).
Table 3.
Congenital toxoplasmosis (CT) in children, based on cases reported at national level in the surveillance system (Toxosurv/National Reference Centre on Toxoplasmosis)a
| Year | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total CT cases (%) | 272 (0.031) | 268 (0.030) | 266 (0.032) | 244 (0.029) | 188 (0.022) | 204 (0.025) | 179 (0.022) | 216 (0.026) | 246 (0.030) | 195 (0.024) | 153 (0.019) | 151 (0.020) |
| Prevalence CT diagnosed at birth | 160 | 153 | 158 | 135 | 132 | 107 | 122 | 152 | 158 | 122 | 89 | 80 |
| Children born with CT | 234 | 233 | 228 | 207 | 173 | 176 | 165 | 205 | 225 | 172 | 128 | 120 |
| Prevalence CT symptomatic at birth | 26 | 27 | 25 | 18 | 26 | 15 | 17 | 15 | 20 | 15 | 12 | 12 |
| Prevalence severe forms CT at birth | 8 | 8 | 8 | 5 | 9 | 7 | 5 | 4 | 5 | 2 | 5 | 3 |
| Mortality | 12 | 17 | 25 | 12 | 5 | 13 | 9 | 7 | 5 | 5 | 4 | 9 |
| Number of pregnancies | 867 308 | 866 810 | 824 641 | 832 799 | 822 621 | 790 290 | 811 510 | 818 565 | 798 948 | 783 640 | 783 640 | 719 737 |
Courtesy of Toxosurv/National Reference Centre on Toxoplasmosis.
Most of the estimates of congenital infections are from studies that are 10–40 years old, but they are listed in Table 2 to provide perspective. Only Austria and France have compulsory screening of pregnant women for T. gondii infection. Rates of congenital transmission are difficult to compare among countries because of the different methodology used. In some studies, only seroconversion during pregnancy was reported (Sagel et al., 2011). One could guess/estimate congenital infection based on the assumption of 50% rate of transmission from mother to fetus. Although there is no national screening for toxoplasmosis in Brazil, valuable information has been obtained from testing of children at few hospitals; these studies were reviewed in detail previously (Dubey et al., 2012) and summarized here in Table 2.
France
Much of the information on congenital toxoplasmosis is derived from studies in France (Tables 1–3). Mass screening of women during pregnancy was initiated by Georges Desmonts in Paris, France in the 1960s looking at seroconversion in women during pregnancy and the transmission of T. gondii to the fetus (Desmonts and Couvreur, 1974a, b); the screening programme became mandatory in France in 1992. France has a population around 65 million, with less than 900 000 pregnancies. All women are screened for T. gondii antibodies at their first prenatal visit and those with IgG antibodies are not tested further. Seroconversion data are sought through monthly screening and seroconverted women are followed clinically by ultrasound examinations and treated with anti-T. gondii therapy to prevent transmission to the fetus or fetal damage. In Lyon, in a cohort of 603 pregnant women with confirmed toxoplasmosis, the overall maternal–fetal transmission rate was 29% (95% CI 25–33), which marked a sharp increase in risk with the duration of gestation from 6% at 13 weeks to 72% at 36 weeks (Dunn et al., 1999).
Most complete data on the estimates of congenital toxoplasmosis were recently made available from France; 2 or 3 infants were infected per 10 000 live births (Table 3). Based on 12-year data, the number of congenitally infected children decreased from 272 (0.031%) in 2007 to 151 (0.018%) in 2018, including the severe congenital infections (Table 3).
Austria
Austria has a population of around 9 million and is one of the first countries to establish a screening programme for T. gondii infection during pregnancy (Thalhammer, 1973, 1978). During 1992–2008, 1 387 689 women/infants were tested for T. gondii infection by serology, molecular tests and cord blood screening (Prusa et al., 2013, 2015a, b, 2017). Annually 8.5% per 10 000 women acquired T. gondii infection during pregnancy with an estimated 1 congenitally infected infant per 10 000 pregnancies (Prusa et al., 2015b). Data regarding clinical outcome are summarized in Table 2. Rationale was provided by the Austrian Government concerning the cost savings because of prenatal screening (Prusa et al., 2017).
Other European countries
There are no recent data on congenital transmission. Data in the past 2 decades are summarized in Table 2. The national screening programme in Denmark was discontinued because of low rate of congenital transmission and the cost-effectiveness (Table 2).
Africa
Little information is available from Africa. A review paper stated 21 cases of congenital toxoplasmosis from 6 hospitals in 3 geographic areas of Morocco (El Bissati et al., 2018). Based on the number of live births annually in these 6 centres, 4–8 congenitally infected children were born per 10 000 births but details are lacking (El Bissati et al., 2018). There are many unconfirmed reports of congenital toxoplasmosis and spontaneous abortion associated with toxoplasmosis in Egypt; these were recently reviewed by Abbas et al. (2020). Most of these reports are based on serological results on single samples from pregnant women, and detection of DNA in the placenta.
USA
A neonatal screening was initiated in Massachusetts, USA in 1980s (Guerina et al., 1994). Prenatal screening is based on testing mothers for T. gondii seroconversion during pregnancy and postnatal screening is often sampling of cord blood or heel pricks of the newborn combined with phenylketonuria testing (Guthrie cards). However, most of these data were based on prevalence at birth without a follow-up. The 1994 study revealed a low rate of congenital transmission (Table 2).
As stated earlier, through the National Collaborative Chicago-based Toxoplasmosis Study, information has been gained concerning epidemiology, clinical presentation, treatment of congenitally infected children in the USA (Boyer et al., 2005, 2011; McLeod et al., 2006a, 2009, 2020).
Brazil
Brazil has a population of around 213 million. Although there is no national screening of T. gondii in pregnant women or children in Brazil, several centres are performing screening based on convenience and affordability. Data summarized by Dubey et al. (2012), up to 2011, indicated 5–23 congenital infections per 10 000 births. Often the sampling was based on who could pay for the tests, and under these circumstances, there will be lower representation of samples from low economic groups. There is also the possibility of false negativity based on IgM testing because many infants with congenital toxoplasmosis are negative for IgM antibodies at birth. There is no national reference laboratory for the confirmation of T. gondii serological testing in Brazil.
The most comprehensive study on congenital toxoplasmosis in children is from the State of Minas Gerais, Brazil (Vasconcelos-Santos et al., 2009; de Resende et al., 2010; Carneiro et al., 2013). In this study, blood samples were collected from 146 307 newborns at 1560 public health care centres in 853 cities in the state of Minas Gerais. All serological testing was performed in one laboratory initially using an IgM-ELISA capture test kit (Toxo IgMQ-Preven, Symbiosis, Leme, Brazil) and results were confirmed on further testing for IgA antibodies by ELISA, and IgG and IgM anti-T. gondii (enzyme-linked fluorometric assay, VIDAS, BioMérrieux SA, Lyon, France), using blood samples from infants and their mothers. Additionally, infected children were followed clinically months after delivery (Vasconcelos-Santos et al., 2009; de Resende et al., 2010). Congenital toxoplasmosis was suspected in 235 infants (1 in 622), and confirmed in 190 children (1 in 770 live births); this figure of 1 per 770 live births does not include in utero mortality due to toxoplasmosis nor infants negative for IgM antibodies at birth (Dubey et al., 2012).
Of the 106 infected children identified early in screening programme, 46 (43.4%) had hearing loss; 4 of these had sensorineural hearing loss and 13 had conductive hearing loss (de Resende et al., 2010). Most of these children had ophthalmic lesions (Vasconcelos-Santos et al., 2009). One hundred and forty-two (79.8%) out of 178 children that underwent ophthalmic examination at 2 months of age had ocular lesions. In 113 of these children, lesions were bilateral; 46.3% of them had macular lesions (de Resende et al., 2010). Viable T. gondii was isolated by mouse bioassay from peripheral blood in 27 (15.2%) out of 178 children when they were 4 months or older (Carneiro et al., 2013). To our knowledge, this is the highest rate of parasitemia demonstrated in congenitally infected children. Genetically, 14 of the 24 isolates tested by 10 PCR-RFLP markers revealed 14 genotypes, distinct from those in Europe (Dardé et al., 2020).
A recent study from a hospital in Porto Alegre, Brazil reported long-term follow-up of 77 congenitally infected children from a retrospective investigation of patients 1996–2017 (Lago et al., 2021). The children were followed for 2–25 years (Table 2). Most children had ocular lesions (55 children) and 44 had intracerebral calcification, a hallmark of congenital toxoplasmosis. Fewer ocular lesions were detected in children who were treated before they were 4 months old (35.2%) vs those treated after they were 12 months old (77.8%), clearly revealing the benefit of early treatment. Two peaks of retinochoroiditis were detected between 4–5 and 9–14 years (Lago et al., 2021). Other lesions in these children were hydrocephalus in 4, microcephalus in 9 and hearing loss in 3 (Lago et al., 2021).
Based on limited studies, both the rate of congenital infection and the severity of disease in congenitally infected children are higher in Brazil than in Europe. This topic was discussed by Dubey et al. (2012) and is repeated here. This conclusion was based on a comparison of ocular lesions in 30 children in Brazil with 281 children in Europe using a similar methodology. In these 30 Brazilian children, the ocular lesions were more extensive and more likely to involve the area of the retina affecting the central vision than in the European children, despite the fact that most of the Brazilian children had been treated for toxoplasmosis for 12 months (Gilbert et al., 2008). This study also concluded that the Brazilian children had a 5 times higher risk of severe toxoplasmosis than children in Europe. In another report, the risk of intracranial lesions detected by computed tomography scan was much higher in Brazilian children than in children in Europe (The SYROCOT, 2007). Some of these differences are thought to be related to the genetic makeup of the T. gondii strains in humans in Brazil. Indeed, the T. gondii strains from the Minas Gerais study had atypical genotype compared with most of the strains from congenitally infected children in France that were mostly Type II (Ajzenberg et al., 2002; Dardé et al., 2020). In addition to genotype, several other factors should not be ignored including the host genetics, the environment, cultural and economic factors.
Other South American and Central American countries
More data have been reported from Colombia, and the pattern of clinical manifestations and prevalence is like from Brazil (Table 2). In a selected survey, 15 congenital infections were identified among 15 333 women sampled (Gómez-Marin et al., 2011). No information is available from other countries in this region.
China, India and other Asian countries
There is little or no information on the rates of congenital transmission of T. gondii in these countries.
Prophylactic treatment during pregnancy and prenatal screening
Prevention of infection of the fetus by prophylactic treatment of the mother depends on the delay between maternal infection and its transmission to the fetus. It is also hoped that if infection is already present in the fetus, treatment may limit its ill effects. Treatment is begun as soon as possible during the prenatal period. In Austria and France, it is by spiramycin before the 20th week of pregnancy and thereafter by pyrimethamine and sulfonamide (Picone et al., 2020).
A large European multicentre cohort study found no evidence that pre-natal treatment with either spiramycin or sulphonamide combined with pyrimethamine influenced maternal transmission (Gilbert et al., 2001). The relative risk of mother-to-child transmission of T. gondii compared to Lyon, France (women treated) was 1.24 in Austria (women treated), 0.59 in Denmark (no treatment) and 0.65 in the Netherlands (50% treated, 50% not treated) (Gilbert et al., 2001). A meta-analysis of 22 European cohorts found weak evidence that treatment started within 3 weeks of seroconversion reduced mother-to-child transmission compared with treatment started after 8 weeks or more (The SYROCOTT, 2007). On the other hand, the number of congenital infections, based on the mothers testing screening in Lyon, France, decreased after 1992 (46.6% after 1992 vs 59.4% from 1987–1991) when screening of susceptible women became mandatory and antenatal treatment was initiated as soon as the diagnosis was made (Wallon et al., 2013). In a recent randomized multicentre trial of sulfadiazine plus pyrimethamine (SzP), and spiramycin (Sp) in 143 mothers (73 SzP group, 70 Sp group) who seroconverted during pregnancy, the transmission rate of congenital toxoplasmosis was 2-fold lower in the SzP group (18.5%) than the Sp group (30%) (Mandelbrot et al., 2018). Cerebral lesions were noted in 0/73 in the SzP group vs 6 of 70 in the Sp group, indicating the effectiveness of therapy in reducing damage to fetal tissues.
One current practice is to start treatment with spiramycin if the woman becomes infected in the first or early second trimester of pregnancy, and then perform amniocentesis to detect fetal infection (Montoya, 2018). If fetal infection is detected, then therapy is switched to pyrimethamine and sulfadiazine. Pyrimethamine and sulfadiazine may be used initially in the late second and third trimesters when acute infection is detected.
French T. gondii programme is based on serological screening of mothers to give prophylactic measures to seronegative women to avoid maternal infection. When seroconversion occurs during pregnancy, in complement to prophylactic treatment, a prenatal diagnosis is recommended. This prenatal diagnosis is based on monthly ultrasonography examination and molecular testing for T. gondii DNA on amniotic fluid. Amniocentesis must be performed after 18 amenorrhea weeks and 4 weeks after maternal infection. In case of detection of DNA (PCR positive) in amniotic fluid, diagnosis of congenital toxoplasmosis is made. However, if the PCR is negative, this does not mean that the fetus is free of congenital infection. Children must be followed at birth to detect congenital infection. In France, since surveillance of congenital toxoplasmosis by the National Reference Centre for Toxoplasmosis (beginning in 2007), approximately 10% of false-negative diagnoses are identified annually (data from NRC for toxoplasmosis). One explanation for these observations is that T. gondii is arrested in the placenta and crosses the barrier a few days to weeks later.
Each country needs to evaluate the cost of screening pregnant women, treatment of congenitally infected children and human suffering based on resources and the prevalence of T. gondii in general population (Scallan et al., 2011; Jones et al., 2018; Suijkerbuijk et al., 2018; Binquet et al., 2019; Bobić et al., 2019). Stillwaggon et al. (2011) provided an extensive guideline for estimating the costs of preventive maternal screening for and the social costs resulting from toxoplasmosis based on studies in Europe and the USA. While estimating these costs, the value of all resources used or lost should be considered, including the cost of medical and non-medical services, wages lost, cost of in-home care, indirect costs of psychological impacts borne by the family for lifetime care of a substantially cognitively impaired child; cost of fetal death was estimated to be 5 million dollars (Stillwaggon et al., 2011). A study on the cost-effectiveness of screening from Austria estimated a lifetime cost of 103 Euros per birth under prenatal screening compared with 323 Euros without screening (Prusa et al., 2017). Although it is unethical to value human life in terms of dollars, each nation must balance public funding for all the needs of its people, including the prevention of crippling ailments.
Where it has been carried out with thoroughness, persistence and determination, as in France, education appears to have contributed to a reduction in the incidence of T. gondii infection during pregnancy. It should be included in the instructions given in antenatal clinics and by obstetricians and midwives dealing with individual patients. Individual instruction given in person is likely to be most effective but should be supplemented by booklets printed in the various languages of the patients and by videos in the waiting rooms of antenatal clinics.
Acknowledgements
This research was supported in part by an appointment of Camila K. Cerqueira-Cézar and Fernando H. A. Murata to the Agricultural Research Service (ARS) Research Participation administered by the Oak Ridge Institute for Science and Education (ORISE) through an inter-agency agreement between the US Department of Energy (DOE) and the US Department of Agriculture (USDA). ORISE was managed by ORAU under DOE contract number DE-SC 0014664. All opinions expressed in this paper were the authors' and did not necessarily reflect the policies and views of USDA, ARS, DOE or ORAU/ORISE.
Author contributions
J.P.D. and I.V. wrote the review; F.H.A.M., C.K.C. and O.C.H.K. helped with literature and evaluation of data.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Ethical standards
Not applicable.
Conflict of interest
None.
References
- Abbas IE, Villena I and Dubey JP (2020) A review on toxoplasmosis in humans and animals from Egypt. Parasitology 147, 135–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajzenberg D, Cogné N, Paris L, Bessières MH, Thulliez P, Filisetti D, Pelloux H, Marty P and Dardé ML (2002) Genotype of 86 Toxoplasma gondii isolates associated with human congenital toxoplasmosis, and correlation with clinical findings. Journal of Infectious Diseases 186, 684–689. [DOI] [PubMed] [Google Scholar]
- Aptouramani M, Theodoridou M, Syrogiannopoulos G, Mentis A, Papaevangelou V, Gaitana K, Daponte A, Hadjichristodoulou C and The Toxoplasmosis Study Group of the Greece-Cyprus Pediatric Surveillance Unit (2012) A dedicated surveillance network for congenital toxoplasmosis in Greece, 2006–2009: assessment of the results. BMC Public Health 12, 1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avelino MM, Amaral WN, Rodrigues IMX, Rassi AR, Gomes MBF, Costa TL and Castro AM (2014) Congenital toxoplasmosis and prenatal care state programs. BMC Infectious Diseases 14, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénard A, Petersen E, Salamon R, Chêne G, Gilbert R and Salmi LR and for the European Toxo Prevention Study Group (EUROTOXO) (2008) Survey of European programmes for the epidemiological surveillance of congenital toxoplasmosis. Eurosurveillance 13, 18834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binquet C, Lejeune C, Seror V, Peyron F, Bertaux AC, Scemama O, Quantin C, Béjean S, Stillwaggon E and Wallon M (2019) The cost-effectiveness of neonatal versus prenatal screening for congenital toxoplasmosis. PLoS ONE 14, e0221709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff AR, Friedrich L, Cattan JM and Uberti FAF (2016) Incidence of symptomatic congenital toxoplasmosis during ten years in a Brazilian hospital. Pediatric Infectious Disease Journal 35, 1313–1316. [DOI] [PubMed] [Google Scholar]
- Blaszkowska J and Góralska K (2014) Parasites and fungi as a threat for prenatal and postnatal human development. Annals of Parasitology 60, 225–234. [PubMed] [Google Scholar]
- Bobić B, Villena I and Stillwaggon E (2019) Prevention and mitigation of congenital toxoplasmosis. Economic costs and benefits in diverse settings. Food and Waterborne Parasitology 16, e00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer KM, Holfels E, Roizen N, Swisher C, Mack D, Remington J, Withers S, Meier P, McLeod R and The Toxoplasmosis Study Group (2005) Risk factors for Toxoplasma gondii infection in mothers of infants with congenital toxoplasmosis: implications for prenatal management and screening. American Journal of Obstetrics and Gynecology 192, 564–571. [DOI] [PubMed] [Google Scholar]
- Boyer K, Hill D, Mui E, Wroblewski K, Karrison T, Dubey JP, Sautter M, Noble AG, Withers S, Swisher C, Heydemann P, Hosten T, Babiarz J, Lee D, Meier P, McLeod R and Toxoplasmosis Study Group (2011) Unrecognized ingestion of Toxoplasma gondii oocysts leads to congenital toxoplasmosis and causes epidemics in North America. Clinical Infectious Diseases 53, 1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capobiango JD, Breganó RM, Navarro IT, Rezende Neto CP, Casella AMB, Mori FMRL, Pagliari S, Inoue IT and Reiche EMV (2014) Congenital toxoplasmosis in a reference center of Paraná, Southern Brazil. Brazilian Journal of Infectious Diseases 18, 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro ACAV, Andrade GM, Costa JGL, Pinheiro BV, Vasconcelos-Santos DV, Ferreira AM, Su C, Januário JN and Vitor RWA (2013) Genetic characterization of Toxoplasma gondii revealed highly diverse genotypes for isolates from newborns with congenital toxoplasmosis in Southeastern Brazil. Journal of Clinical Microbiology 51, 901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codaccioni C, Picone O, Lambert V, Maurice P, Pomar L, Winer N, Guibaud L, Lavergne RA, Saliou AH, Quinio D, Benachi A, Noel C, Ville Y, Cuillier F, Pomares C, Ferret N, Filisetti D, Weingertner AS, Vequeau-Goua V, Cateau E, Benoist G, Wallon M, Dommergues M, Villena I and Mandelbrot L (2020) Ultrasound features of fetal toxoplasmosis: a contemporary multicenter survey in 88 fetuses. Prenatal Diagnosis 40, 1741–1752. [DOI] [PubMed] [Google Scholar]
- Conceição AR, Belucik DN, Missio L, Gustavo Brenner L, Henrique Monteiro M, Ribeiro KS, Costa DF, Valadão MCDS, Commodaro AG, de Oliveira Dias JR and Belfort R Jr (2021) Ocular findings in infants with congenital toxoplasmosis after a toxoplasmosis outbreak. Ophthalmology S0161–6420, 00195-0. [DOI] [PubMed] [Google Scholar]
- Couvreur J, Desmonts G, Tournier G and Szusterkac M (1984) Etude d'une série homogène de 210 cas de toxoplasmose congénitale chez des nourrissons âgés de 0 à 11 mois et dépistés de façon prospective. Annales de Pédiatrie 31, 815–819. [PubMed] [Google Scholar]
- Daffos F, Mirlesse V, Hohlfeld P, Jacquemard F, Thulliez P and Forestier F (1994) Toxoplasmosis in pregnancy. Lancet (London, England) 344, 541. [DOI] [PubMed] [Google Scholar]
- Dardé ML, Mercier A, Su C, Khan A and Grigg ME (2020) Molecular epidemiology and population structure of Toxoplasma gondii. In Weiss LM and Kim K (eds), Toxoplasma gondii: The Model Apicomplexan – Perspectives and Methods, 3rd Edn. London: Academic Press, pp. 63–116. [Google Scholar]
- de Resende LM, de Andrade GMQ, de Azevedo MF, Perissinoto J, Vieira ABC and CTBG-UFMG (2010) Congenital toxoplasmosis: auditory and language outcomes in early diagnosed and treated children. Scientia Medica 20, 13–19. [Google Scholar]
- Desmonts G and Couvreur J (1974a) Congenital toxoplasmosis. A prospective study of 378 pregnancies. New England Journal of Medicine 290, 1110–1116. [DOI] [PubMed] [Google Scholar]
- Desmonts G and Couvreur J (1974b) Toxoplasmosis in pregnancy and its transmission to the fetus. Bulletin of the New York Academy of Medicine 50, 146–159. [PMC free article] [PubMed] [Google Scholar]
- Desmonts G and Couvreur J (1984) Toxoplasmose congénitale. Etude prospective de l'issue de la grossesse chez 542 femmes atteintes de toxoplasmose acquise en cours de gestation. Annales de Pédiatrie 31, 805–809. [PubMed] [Google Scholar]
- Dubey JP (2010) Toxoplasmosis of Animals and Humans, 2nd Edn. Boca Raton, Florida, USA: CRC Press. [Google Scholar]
- Dubey JP (2021) Outbreaks of clinical toxoplasmosis in humans: five decades of personal experience, prospective, and lessons learned. Parasite and Vectors 14, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP and Beattie CP (1988) Toxoplasmosis of Animals and Man. Boca Raton, Florida, USA: CRC Press. [Google Scholar]
- Dubey JP, Lago EG, Gennari SM, Su C and Jones JL (2012) Toxoplasmosis in humans and animals in Brazil: high prevalence, high burden of disease, and epidemiology. Parasitology 139, 1375–1424. [DOI] [PubMed] [Google Scholar]
- Dunn D, Wallon M, Peyron F, Petersen E, Peckham C and Gilbert R (1999) Mother-to-child transmission of toxoplasmosis: risk estimates for clinical counselling. Lancet (London, England) 353, 1829–1833. [DOI] [PubMed] [Google Scholar]
- Eichenwald HF (1960) A study of congenital toxoplasmosis with particular emphasis on clinical manifestations, sequelae and therapy in human toxoplasmosis. In Siim JC (ed.), Human Toxoplasmosis, 2nd Edn. Copenhagen: Munksgaard, pp. 41–49. [Google Scholar]
- Elbez-Rubinstein A, Ajzenberg D, Dardé ML, Cohen R, Dumètre A, Yera H, Gondon E, Janaud JC and Thulliez P (2009) Congenital toxoplasmosis and reinfection during pregnancy: case report, strain characterization, experimental model of reinfection, and review. Journal of Infectious Diseases 199, 280–285. [DOI] [PubMed] [Google Scholar]
- El Bissati K, Levigne P, Lykins J, Adlaoui EB, Barkat A, Berraho A, Laboudi M, El Mansouri B, Ibrahimi A, Rhajaoui M, Quinn F, Murugesan M, Seghrouchni F, Gómez-Marín JE, Peyron F and McLeod R (2018) Global initiative for congenital toxoplasmosis: an observational and international comparative clinical analysis. Emerging Microbes & Infections 7, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evengård B, Lilja G, Capraru T, Malm G, Kussofsky E, Öman H and Forsgren M (1999) A retrospective study of seroconversion against Toxoplasma gondii during 3000 pregnancies in Stockholm. Scandinavian Journal of Infectious Diseases 31, 127–129. [DOI] [PubMed] [Google Scholar]
- Evengård B, Petersson K, Engman ML, Wiklund S, Ivarsson SA, Teär-Fahnehjelm K, Forsgren M, Gilbert R and Malm G (2001) Low incidence of Toxoplasma infection during pregnancy and in newborns in Sweden. Epidemiology and Infection 127, 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores C, Villalobos-Cerrud D, Borace J, Fábrega L, Norero X, Sáez-Llorens X, Moreno MT, Restrepo CM, Llanes A, Quijanda RM, De Guevara ML, Guzmán G, de la Guardia V, García A, Lucero MF, Wong D, McLeod R, Soberon M and Caballero EZ (2021) Epidemiological aspects of maternal and congenital toxoplasmosis in Panama. Pathogens 10, 764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego-Marín C, Henao AC and Gómez-Marín JE (2006) Clinical validation of a western blot assay for congenital toxoplasmosis and newborn screening in a hospital in Armenia (Quindio) Colombia. Journal of Tropical Pediatrics 52, 107–112. [DOI] [PubMed] [Google Scholar]
- Gilbert RE and Peckham CS (2001) Prenatal screening for Toxoplasma infection. In Joynson DHM and Wreghitt TG (eds), Toxoplasmosis. A Comprehensive Clinical Guide. Cambridge University Press, pp. 214–240. [Google Scholar]
- Gilbert RE, Gras L, Wallon M, Peyron F, Ades AE and Dunn DT (2001) Effect of prenatal treatment on mother to child transmission of Toxoplasma gondii: retrospective cohort study of 554 mother-child pairs in Lyon, France. International Journal of Epidemiology 30, 1303–1308. [DOI] [PubMed] [Google Scholar]
- Gilbert RE, Freeman K, Lago EG, Bahia-Oliveira LMG, Tan HK, Wallon M, Buffolano W, Stanford MR, Petersen E and for the European Multicentre Study on Congenital Toxoplasmosis (2008). Ocular sequelae of congenital toxoplasmosis in Brazil compared with Europe. PLoS Neglected Tropical Diseases 2, e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Marin JE, Montoya-de-Londono MT and Castano-Osorio JC (1997) A maternal screening program for congenital toxoplasmosis in Quindio, Colombia and application of mathematical models to estimate incidences using age-stratified data. American Journal of Tropical Medicine and Hygiene 57, 180–186. [DOI] [PubMed] [Google Scholar]
- Gomez-Marin JE, Gonzalez MM, Montoya MT, Giraldo A and Castaño JC (2007) A newborn screening programme for congenital toxoplasmosis in the setting of a country with less income. Archives of Disease in Childhood 92, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Marin JE, de-la-Torre A, Angel-Muller E, Rubio J, Arenas J, Osorio E, Nuñez L, Pinzon L, Mendez-Cordoba LC, Bustos A, de-la-Hoz I, Silva P, Beltran M, Chacon L, Marrugo M, Manjarres C, Baquero H, Lora F, Torres E, Zuluaga OE, Estrada M, Moscote L, Silva MT, Rivera R, Molina A, Najera S, Sanabria A, Ramirez ML, Alarcon C, Restrepo N, Falla A, Rodriguez T and Castaño G (2011) First Colombian multicentric newborn screening for congenital toxoplasmosis. PLoS Neglected Tropical Diseases 5, e1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez JE (2005) Evaluación del tratamiento de la toxoplasmosis gestacional en una cohorte colombiana. Infectio 9, 16–23. [Google Scholar]
- Gras L, Wallon M, Pollak A, Cortina-Borja M, Evengard B, Hayde M, Petersen E, Gilbert R and European Multicenter Study on Congenital Toxoplasmosis (2005) Association between prenatal treatment and clinical manifestations of congenital toxoplasmosis in infancy: a cohort study in 13 European centres. Acta Paediatrica 94, 1721–1731. [DOI] [PubMed] [Google Scholar]
- Guerina NG (1994) Congenital infection with Toxoplasma gondii. Pediatric Annals 23, 138–142. [DOI] [PubMed] [Google Scholar]
- Guerina NG, Hsu HW, Meissner HC, Maguire JH, Lynfield R, Stechenberg B, Abroms I, Pasternack MS, Hoff R, Eaton RB, Grady GF and the New England Regional Toxoplasma Working Group (1994) Neonatal serologic screening and early treatment for congenital Toxoplasma gondii infection. New England Journal of Medicine 330, 1858–1863. [DOI] [PubMed] [Google Scholar]
- Havelaar AH, Kemmeren JM and Kortbeek LM (2007) Disease burden of congenital toxoplasmosis. Clinical Infectious Diseases 44, 1467–1474. [DOI] [PubMed] [Google Scholar]
- Hohlfeld P, Daffos F, Costa JM, Thulliez P, Forestier F and Vidaud M (1994) Prenatal diagnosis of congenital toxoplasmosis with a polymerase-chain-reaction test on amniotic fluid. New England Journal of Medicine 331, 695–699. [DOI] [PubMed] [Google Scholar]
- Hutson SL, Wheeler KM, McLone D, Frim D, Penn R, Swisher CN, Heydemann PT, Boyer KM, Noble AG, Rabiah P, Withers S, Montoya JG, Wroblewski K, Karrison T, Grigg ME and McLeod R (2015) Patterns of hydrocephalus caused by congenital Toxoplasma gondii infection associate with parasite genetics. Clinical Infectious Diseases 61, 1831–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki ADM, Carvalheiro CG, Cipolotti R, Gurgel RQ, Rocha DA, Pinheiro KS, Araújo RM, Lima DRR, Winandy JL and Mussi-Pinhata MM (2012) Birth prevalence and characteristics of congenital toxoplasmosis in Sergipe, North-east Brazil. Tropical Medicine and International Health 17, 1349–1355. [DOI] [PubMed] [Google Scholar]
- Jenum PA, Stray-Pedersen B, Melby KK, Kapperud G, Whitelaw A, Eskild A and Eng J (1998) Incidence of Toxoplasma gondii infection in 35,940 pregnant women in Norway and pregnancy outcome for infected women. Journal of Clinical Microbiology 36, 2900–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Kruszon-Moran D, Elder S, Rivera HN, Press C, Montoya JG and McQuillan GM (2018) Toxoplasma gondii infection in the United States, 2011–2014. American Journal of Tropical Medicine and Hygiene 98, 551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppe JG, Loewer-Siege DH and Roever-Bonnet H (1986) Results of 20-year follow-up of congenital toxoplasmosis. Lancet (London, England) 327, 254–256. [DOI] [PubMed] [Google Scholar]
- Kortbeek LM, Hofhuis A, Nijhuis CDM and Havelaar AH (2009) Congenital toxoplasmosis and DALYs in the Netherlands. Memórias do Instituto Oswaldo Cruz 104, 370–373. [DOI] [PubMed] [Google Scholar]
- Lago EG, Endres MM, Scheeren MFC and Fiori HH (2021) Ocular outcome of Brazilian patients with congenital toxoplasmosis. Pediatric Infectious Disease Journal 40, e21–e27. [DOI] [PubMed] [Google Scholar]
- Lappalainen M, Koskiniemi M, Hiilesmaa V, Ämmälä P, Teramo K, Koskela P, Lebech M, Raivio KO, Hedman K and the Study Group (1995) Outcome of children after maternal primary Toxoplasma infection during pregnancy with emphasis on avidity of specific IgG. Pediatric Infectious Disease Journal 14, 354–361. [DOI] [PubMed] [Google Scholar]
- Lebech M, Andersen O, Christensen NC, Hertel J, Nielsen HE, Peitersen B, Rechnitzer C, Larsen SO, Nørgaard-Pedersen B, Petersen E and the Danish Congenital Toxoplasmosis Study Group (1999) Feasibility of neonatal screening for Toxoplasma infection in the absence of prenatal treatment. Lancet (London, England) 353, 1834–1837. [DOI] [PubMed] [Google Scholar]
- Mandelbrot L, Kieffer F, Sitta R, Laurichesse-Delmas H, Winer N, Mesnard L, Berrebi A, Le Bouar G, Bory JP, Cordier AG, Ville Y, Perrotin F, Jouannic JM, Biquard F, d'Ercole C, Houfflin-Debarge V, Villena I, Thiébaut R and the TOXOGEST Study Group (2018) Prenatal therapy with pyrimethamine + sulfadiazine vs spiramycin to reduce placental transmission of toxoplasmosis: a multicenter, randomized trial. American Journal of Obstetrics and Gynecology 219, 386.e1–386.e9. [DOI] [PubMed] [Google Scholar]
- McLeod R, Boyer K, Karrison T, Kasza K, Swisher C, Roizen N, Jalbrzikowski J, Remington J, Heydemann P, Noble AG, Mets M, Holfels E, Withers S, Latkany P, Meier P and Toxoplasmosis Study Group (2006a) Outcome of treatment for congenital toxoplasmosis, 1981–2004: the national collaborative Chicago-based, congenital toxoplasmosis study. Clinical Infectious Diseases 42, 1383–1394. [DOI] [PubMed] [Google Scholar]
- McLeod R, Khan AR, Noble GA, Latkany P, Jalbrzikowski J, Boyer K and for the Toxoplasmosis Study Group (2006b) Severe sulfadiazine hypersensitivity in a child with reactivated congenital toxoplasmic chorioretinitis. Pediatric Infectious Disease Journal 25, 270–272. [DOI] [PubMed] [Google Scholar]
- McLeod R, Kieffer F, Sautter M, Hosten T and Pelloux H (2009) Why prevent, diagnose and treat congenital toxoplasmosis? Memórias do Instituto Oswaldo Cruz 104, 320–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod R, Boyer KM, Lee D, Mui E, Wroblewski K, Karrison T, Noble AG, Withers S, Swisher CN, Heydemann PT, Sautter M, Babiarz J, Rabiah P, Meier P, Grigg ME and the Toxoplasmosis Study Group (2012) Prematurity and severity are associated with Toxoplasma gondii alleles (NCCCTS, 1981–2009). Clinical Infectious Diseases 54, 1595–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod R, Cohen W, Dovgin S, Finkelstein L and Boyer KM (2020) Human Toxoplasma infection. In Weiss LM and Kim K (eds), Toxoplasma gondii: The Model Apicomplexan – Perspectives and Methods, 3rd Edn. London: Academic Press, pp. 117–227. [Google Scholar]
- Montoya JG (2018) Systematic screening and treatment of toxoplasmosis during pregnancy: is the glass half full or half empty? American Journal of Obstetrics and Gynecology 219, 315–319. [DOI] [PubMed] [Google Scholar]
- Muñoz Batet C, Llobet CG, Morros TJ, Domenech LV, Soler MS, Sala IS, Mestres JB, Ponte ED, Lite JL, Andreu LM, Sánchez CJ and Romeu MB (2004) Toxoplasmosis y embarazo. Estudio multicéntrico realizado en 16.362 gestantes de Barcelona. Medicina Clínica 123, 12–16. [DOI] [PubMed] [Google Scholar]
- Neto EC, Rubin R, Schulte J and Giugliani R (2004) Newborn screening for congenital infectious diseases. Emerging Infectious Diseases 10, 1069–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraguassú-Chaves CA, Dantas LRM, de Almeida FM, Serruta AJ, Leite JMS, Neto LSL, Calderaro IFN and Xavier DFB (2019) Incidence of congenital toxoplasmosis in newborn infant in the western Amazon, Brazil. International Journal of Advanced Engineering Research and Science 6, 659–670. [Google Scholar]
- Paul M, Petersen E, Pawlowski ZS and Szczapa J (2000) Neonatal screening for congenital toxoplasmosis in the Poznań region of Poland by analysis of Toxoplasma gondii-specific IgM antibodies eluted from filter paper blood spots. Pediatric Infectious Disease Journal 19, 30–36. [DOI] [PubMed] [Google Scholar]
- Paul M, Jaworska A, Petersen E, Szczapa J and Twardosz-Pawlik H (2001a) Neonatal screening for congenital toxoplasmosis. International Journal for Parasitology 31, 116–121. [Google Scholar]
- Paul M, Petersen E and Szczapa J (2001b) Prevalence of congenital Toxoplasma gondii infection among newborns from the Poznań region of Poland: validation of a new combined enzyme immunoassay for Toxoplasma gondii-specific immunoglobulin A and immunoglobulin M antibodies. Journal of Clinical Microbiology 39, 1912–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron F, Wallon M, Kieffer F and Garweg J (2016) Toxoplasmosis. In Wilson CB, Nizet V, Maldonado YA, Remington JS and Klein JO (eds), Infectious Diseases of the Fetus and the Newborn Infant, 8th Edn. Philadelphia, PA, USA: Elsevier. pp. 949–1042. [Google Scholar]
- Philippe F, Lepetit D, Grancher MF, Morel A and Henocq A (1988) Pourquoi surveiller les enfants dont les mères ont eu une séroconversion de toxoplasmose pendant la grossesse? Réalité et risque de l'infection toxoplasmique congénitale infraclinique de l'enfant (Enquête portant sur 30768 naissances). Annales de Pédiatrie 35, 5–10. [PubMed] [Google Scholar]
- Picone O, Fuchs F, Benoist G, Binquet C, Kieffer F, Wallon M, Wehbe K, Mandelbrot L and Villena I (2020) Toxoplasmosis screening during pregnancy in France: opinion of an expert panel for the CNGOF. Journal of Gynecology Obstetrics and Human Reproduction 49, 101814. [DOI] [PubMed] [Google Scholar]
- Pratlong F, Boulot P, Issert E, Msika M, Dupont F, Bachelard B, Sarda P, Viala JL and Jarry D (1994) Fetal diagnosis of toxoplasmosis in 190 women infected during pregnancy. Prenatal Diagnosis 14, 191–198. [DOI] [PubMed] [Google Scholar]
- Prusa AR, Kasper DC, Olischar M, Husslein P, Pollak A and Hayde M (2013) Evaluation of serological prenatal screening to detect Toxoplasma gondii infections in Austria. Neonatology 103, 27–34. [DOI] [PubMed] [Google Scholar]
- Prusa AR, Kasper DC, Pollak A, Gleiss A, Waldhoer T and Hayde M (2015a) The Austrian toxoplasmosis register, 1992–2008. Clinical Infectious Diseases 60, e4–e10. [DOI] [PubMed] [Google Scholar]
- Prusa AR, Kasper DC, Pollak A, Olischar M, Gleiss A and Hayde M (2015b) Amniocentesis for the detection of congenital toxoplasmosis: results from the nationwide Austrian prenatal screening program. Clinical Microbiology and Infection 21, 191e1–191e8. [DOI] [PubMed] [Google Scholar]
- Prusa AR, Kasper DC, Sawers L, Walter E, Hayde M and Stillwaggon E (2017) Congenital toxoplasmosis in Austria: prenatal screening for prevention is cost-saving. PLoS Neglected Tropical Diseases 11, e0005648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Gangneux F and Dardé ML (2012) Epidemiology of and diagnostic strategies for toxoplasmosis. Clinical Microbiology Reviews 25, 264–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagel U, Krämer A and Mikolajczyk RT (2011) Incidence of maternal Toxoplasma infections in pregnancy in Upper Austria, 2000–2007. BMC Infectious Diseases 11, 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL and Griffin PM (2011) Foodborne illness acquired in the United States – major pathogens. Emerging Infectious Diseases 17, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt DR, Hogh B, Andersen O, Fuchs J, Fledelius H and Petersen E (2006) The national neonatal screening programme for congenital toxoplasmosis in Denmark: results from the initial four years, 1999–2002. Archives of Disease in Childhood 91, 661–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillwaggon E, Carrier CS, Sautter M and McLeod R (2011) Maternal serologic screening to prevent congenital toxoplasmosis: a decision-analytic economic model. PLoS Neglected Tropical Diseases 5, e1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suijkerbuijk AWM, van Gils PF, Bonačić Marinović AA, Feenstra TL, Kortbeek LM, Mangen MJJ, Opsteegh M, de Wit GA and van der Giessen JWB (2018) The design of a social cost-benefit analysis of preventive interventions for toxoplasmosis: an example of the One Health approach. Zoonoses and Public Health 65, 185–194. [DOI] [PubMed] [Google Scholar]
- Szénási Z, Ozsvár Z, Nagy E, Jeszenszky M, Szabó J, Gellén J, Végh M and Verhofstede C (1997) Prevention of congenital toxoplasmosis in Szeged, Hungary. International Journal of Epidemiology 26, 428–435. [DOI] [PubMed] [Google Scholar]
- Takahashi AFS, Bioni HO, Souza JM, Takizawa MGMH and Paiva JE (2019) Toxoplasmose congênita na cidade de Cascavel/PR no período de 2002–2016. Revista Thêma et Scientia 9, 260–267. [Google Scholar]
- Thalhammer O (1973) Prevention of congenital toxoplasmosis. Neuropädiatrie 4, 233–237. [DOI] [PubMed] [Google Scholar]
- Thalhammer O (1978) Prevention of congenital infections. In Thalhammer O, Baumgarten K and Pollak A (eds), Perinatal Medicine, 6th European Congress. Vienna: PSG Publishing Co. Massachusetts, pp. 44–51. [Google Scholar]
- The SYROCOT (Systematic Review on Congenital Toxoplasmosis) Study Group (2007) Effectiveness of prenatal treatment for congenital toxoplasmosis: a meta-analysis of individual patients’ data. Lancet (London, England) 369, 115–122. [DOI] [PubMed] [Google Scholar]
- Torgerson PR and Mastroiacovo P (2013) The global burden of congenital toxoplasmosis: a systematic review. Bulletin of the World Health Organization 91, 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcavi PP, Natali A, Soliani L, Montali S, Dettori G and Cheezi C (1995) Prevalence of anti-Toxoplasma gondii antibodies in the population of the area of Parma (Italy). European Journal of Epidemiology 11, 333–337. [DOI] [PubMed] [Google Scholar]
- van der Giessen J, Deksne G, Gómez-Morales MA, Troell K, Gomes J, Sotiraki S, Rozycki M, Kucsera I, Djurković-Djaković O and Robertson LJ (2021) Surveillance of foodborne parasitic diseases in Europe in a One Health approach. Parasite Epidemiology and Control 13, e00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos-Santos DV, Azevedo DOM, Campos WR, Oréfice F, Queiroz-Andrade GM, Carellos EVM, Romanelli RMC, Januário JN, Resende LM, Martins-Filho OA, Carneiro ACAV, Vitor RWA, Caiaffa WT and UFMG Congenital Toxoplasmosis Brazilian Group (2009) Congenital toxoplasmosis in southeastern Brazil: results of early ophthalmologic examination of a large cohort of neonates. Ophthalmology 116, 2199–2205. [DOI] [PubMed] [Google Scholar]
- Villena I, Chemla C, Quereux C, Dupouy D, Leroux B, Foudrinier F and Pinon JM (1998) Prenatal diagnosis of congenital toxoplasmosis transmitted by an immunocompetent woman infected before conception. Prenatal Diagnosis 18, 1079–1081. [PubMed] [Google Scholar]
- Villena I, Ancelle T, Delmas C, Garcia P, Brézin AP, Thulliez P, Wallon M, King L, Goulet V and Toxosurv network and National Reference Centre for Toxoplasmosis (2010) Congenital toxoplasmosis in France in 2007: first results from a national surveillance system. Euro Surveillance 15, 19600. [DOI] [PubMed] [Google Scholar]
- Wallon M, Kodjikian L, Binquet C, Garweg J, Fleury J, Quantin C and Peyron F (2004) Long-term ocular prognosis in 327 children with congenital toxoplasmosis. Pediatrics 113, 1567–1572. [DOI] [PubMed] [Google Scholar]
- Wallon M, Peyron F, Cornu C, Vinault S, Abrahamowicz M, Kopp CB and Binquet C (2013) Congenital Toxoplasma infection: monthly prenatal screening decreases transmission rate and improves clinical outcome at age 3 years. Clinical Infectious Diseases 56, 1223–1231. [DOI] [PubMed] [Google Scholar]
- Walpole IR, Hodgen N and Bower C (1991) Congenital toxoplasmosis: a large survey in Western Australia. Medical Journal of Australia 154, 720–724. [DOI] [PubMed] [Google Scholar]
- Wolf A, Cowen D and Paige B (1939) Human toxoplasmosis: occurrence in infants as an encephalomyelitis verification by transmission to animals. Science (New York, N.Y.) 89, 226–227. [DOI] [PubMed] [Google Scholar]


