Abstract
Objectives
Histiocytic disorders are pathologic expansions of myeloid cells in multiple organs, including the CNS. They share activation of the MAP kinase pathway due to either BRAFV600E variant or other variants in the RAS-RAF-MEK-ERK pathway. The rarity and heterogeneity of the disease only enable therapy through pathophysiologic considerations.
Methods
We present 2 histiocytosis cases without BRAF sequence variants that affect the CNS, one with Erdheim-Chester disease and the other with an unspecified histiocytosis, and their diagnostic and therapeutic challenges.
Results
In both cases, comprehensive analysis of the RAS-RAF-MEK-ERK signaling pathway secured the diagnosis. Treatment with the MEK inhibitor cobimetinib brought the disease to a complete halt. However, side effects such as thrombosis and serous macular edema made it necessary to reduce cobimetinib dosage. Low-dose cobimetinib maintenance medication was successful in preventing recurrence of histiocytic disease.
Discussion
CNS involvement of histiocytic disorders can lead to detrimental neurologic symptoms. MEK inhibitors are effective treatment options for some of these patients. Since side effects are common, according to our cases we propose a low-dose treatment of 20 mg per day to balance treatment effects with side effects.
Classification of Evidence
This case report provides Class IV evidence. This is a single observational study without controls.
Introduction
Histiocytoses encompass several rare conditions that can be best described as inflammatory myeloid-derived neoplastic disorders.1 They are characterized by a proliferation of myeloid cells, mainly tissue-resident macrophages or dendritic cells, presenting with an inflammatory infiltrate that is mostly responsible for the organ-specific lesions and symptoms. Of 5 distinguished groups of histiocytosis, the most prominent "L" group consists of Langerhans cell histiocytosis and xanthogranuloma syndrome, both mainly affecting children, and Erdheim-Chester (ECD) syndrome.2 ECD represents a non-Langerhans cell histiocytic disorder with multiorgan infiltration of CD68+CD1a– histiocytes. In up to 90%, sclerosis of the long tubular bones occurs, often without symptoms. CNS involvement occurs in 30%–50% of cases and is the main predictor of a poor prognosis.3 Patients with CNS-associated symptoms most often suffer from central diabetes insipidus, ataxia, nerve palsy, or cephalgia.1 Up to 25% of patients develop exophthalmos due to retrobulbar infiltrates. Altered RAS-RAF-MEK-ERK signaling because of somatic variant of the BRAF proto-oncogene at the codon V600 (BRAFV600E) is the most common genetic alteration associated with the disease.4 Detection of the variant is important as treatment options with BRAF inhibitors such as vemurafenib are available.5 For those 50% of patients with wild-type BRAF, clear treatment options are missing. Because histiocytic disorders have been proposed to be ERK-dependent, treating patients with BRAF wild-type histiocytosis with the MEK1/MEK2 inhibitor cobimetinib has been suggested6 and in few cases performed7 (eFigure 1). In this study, we present 2 patients with L-type histiocytosis with prominent CNS involvement and complete and long-term response to cobimetinib treatment.
Case Presentations
Case 1
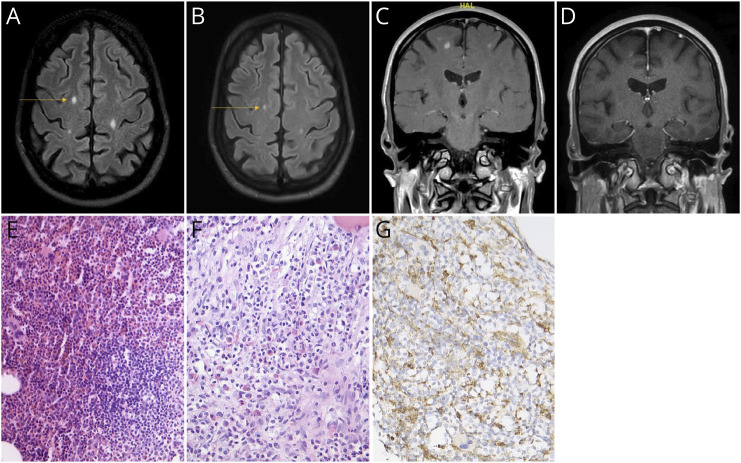
In 2016, a 51-year-old man suffered a traumatic knee injury. Right knee MRI showed symmetric sclerosis of the femur, consistent with asymptomatic ECD. Four years later, he experienced dysarthria and dizziness, followed by ataxia and fine motor dysfunction. An MRI scan in March 2020 showed subcortical T2 lesions in the right superior frontal gyrus, right peritrigonal region, left centrum semiovale, and left central region as well as cerebellar and subcortical contrast enhancement. MRI of the spinal cord showed no abnormalities. However, osteolytic lesions were found in the 10th thoracic vertebra. CSF analysis was without any pathologic findings. A vertebral biopsy confirmed ECD diagnosis, revealing foamy CD68-positive macrophage accumulation (Figure 1) and wild-type BRAF. Oral administration of cobimetinib 60 mg/d for 21 days followed by a 7-day break was started in May 2020. Because of diarrhea, treatment was reduced to 40 mg cobimetinib per day. Neurologic symptoms stabilized with medication. In early 2021, the patient experienced blurred vision in the left eye and was diagnosed with a central retinal vein thrombosis of the left retinal vein and cystic macular edema of both eyes. Assuming the symptoms were treatment-related, cobimetinib was stopped. In the following months, there was progressive gait instability and new MRI lesions. This led to a second treatment course in July 2022, which was subsequently discontinued because of persistent diarrhea. Cobimetinib was resumed in July 2023 at 20 mg/d with 7 days off per month; neither symptom, MRI progression, nor side effects occurred as of November 2023 (Figure 1).
Figure 1. Case 1: MRI and Histology of Erdheim-Chester Syndrome.
The patient presenting with subcortical edematous lesions in T2-weighted cranial MRI (A) before treatment in 06/2023 and (B) under therapy with cobimetinib 20 mg/d in 09/2023. (C) Contrast-enhanced T1-weighted MRI in coronar orientation of the area marked with an arrow in (A) in 06/2023. (D) T1-weighted cranial MRI in coronar orientation of the area marked in (B) in 09/2023 under therapy with 20 mg/d cobimetinib. Hematoxylin staining of vertebral tissue showing (E) reactive lymphocytes and some multinucleated giant cells as well as (F) kidney eosinophils. (G) Histiocytes were CD68-positive.
Case 2
In 2016, a 39-year-old woman experienced menstrual irregularities and weight gain. A brain MRI identified a tumor located at the infundibulum of the pituitary gland. CSF revealed lymphocytic pleocytosis (63 cells/μL) and positive oligoclonal bands selectively in the CSF (type 2) without other pathologic findings. In the biopsy of the infundibular mass, cellular morphology was suspicious for a histiocytic tumor presenting a strong CD68 positivity. Lesions contained CD3-positive T cells, CD20-positive B cells, as well as a strong gliosis with GFAP and S100 reactivity. Phosphorylation of STAT1 and STAT3 led to the differential diagnosis of neurosarcoidosis.8 A thoracical and abdominal CT scan as well as an FDG-PET remained without suspicious findings for metastases or lymphadenopathy. As cerebral lesions progressed to the left parahippocampal gyrus and temporal lobe, lacking tumor characteristics, but with signs of inflammation in the CSF, the patient underwent infliximab treatment from April 2017 to September 2017. Owing to progression, treatment was changed to rituximab for 2 cycles. However, neither rituximab nor steroids or tofacitinib were able to halt enlargement of the inflammatory mass. Infiltration of the optic tracts and chiasm caused bitemporal hemianopsia. In addition, hyperintensities on T2-weighted images with contrast enhancement in T1 appeared in the inferior third ventricle, medial part of the left temporal lobe, subcortical area of the right frontal lobe, and right cerebellum (Figure 2). Histology from the transsphenoidal tumor resection in 2019 was, again, suggestive of histiocytosis without further specification. Targeted NGS did not reveal oncogenic sequence variants; however, a strong phosphorylation of ERK was detected, indicating activation of the end-stage RAS/MEK pathway (Figure 2). This made histiocytosis, not otherwise specified, without the BRAFV600E variant the most likely diagnosis. Cobimetinib treatment in March 2021 led to the regression of MRI lesions and contrast enhancement within 3 months. However, in April 2021, the patient developed dyspnea attributed to pulmonary embolism, prompting a reduction in cobimetinib dosage from 60 mg to 40 mg per day. In follow-up examinations, macula edema and reduction of cardial functions were recorded. After remission of the tumor lesions, no new neurologic symptoms occurred. However, bilateral hemianopsia and reduced vision in the left eye remained, presumably because of irreversible damage during transsphenoidal surgery. Because the patient showed no signs of disease activity, the cobimetinib dose was further reduced to 20 mg/d in March 2023. Follow-up brain MRI, in June and finally in December 2023, revealed no contrast enhancement or enlargement of lesions (Figure 2). No side effects were recorded.
Figure 2. Case 2: MRI and Histology of Histiocytosis With Malignant Infiltration of the Optic Nerve and Chiasma.
T2 FLAIR sequence in transversal orientation (A) before treatment with cobimetinib in 04/2019 and (B) under therapy with cobimetinib in 06/2023. (C) Contrast-enhanced (CE) T1 sequence in transversal orientation in 04/2019. (D) Strong reduction of contrast enhancement in CE T1 sequence in transversal orientation in 06/2023. (E–G) Cerebral tissue of histiocytosis not otherwise specified obtained by brain biopsy. (E) Morphological evidence for histiocytes in hematoxylin-eosin (H&E) staining as well as immunohistochemical detection of (F) CD186 and (G) phosphorylated ERK (pERK) suggesting a strong induction of the end stage of the RAS-RAF-MEK-ERK signaling pathway.
Discussion
Both cases of histiocytic disorders without BRAF sequence variants that affect the CNS exhibit remarkable treatment responses to the MEK inhibitor cobimetinib, even at reduced dosage.
Histiocytosis with affection of the CNS is rare. To facilitate treatment based on the underlying pathophysiology, a comprehensive evaluation is necessary, encompassing clinical assessment, imaging, and histologic analysis.9 MRI lesions, particularly subcortical lesions in ECD, can be misleading and nonspecific. However, in the first case, CNS symptoms were preceded by bone abnormalities suggestive of ECD and confirmed by biopsy of the affected vertebra. In the second case, the findings were less clear, requiring the consideration of alternative diagnoses such as neurosarcoidosis and neoplasm. A brain biopsy revealed dysregulation of the RAS-RAF-MEK-ERK pathway. Because both patients lacked the BRAFV600E variant, the analysis of ERK phosphorylation was decisive for treatment decisions.
In both our cases, cobimetinib resulted in clinical stabilization and reduction in CNS infiltrates. This aligns with a prior study where patients with histiocytosis were effectively treated using a MEK 1/2 inhibitor, irrespective of their BRAFV600E variant status.6 However, in both instances, suspected side effects such as thrombosis and cystoid macular edema prompted a temporary halt and subsequent dose reduction, as previously described during cobimetinib treatment.10,11 MEK inhibitors have been approved for use in various cancers and tumor predisposition syndrome.12-14 Because of the rarity of histiocytic disorders, there are no trials aiming for dose dependency of BRAF or MEK inhibitors, but it is well know that most patients treated with BRAF inhibitors relapse when treatment is stopped.15,16
In conclusion, in these 2 cases of histiocytosis involving the CNS, even low-dose cobimetinib effectively mitigated the pathologic activation of the RAS-RAF-MEK-ERK pathway. This was recorded with clinical and imaging outcomes. Given the observed adverse effects, a general dose reduction in histiocytosis is warranted. Thus, we suggest a treatment approach of histiocytic disorders lacking the BRAFV600E variant with cobimetinib 60 mg/d until remission, followed by maintaining the response with a low dose of 20–40 mg/d.
Acknowledgment
The authors thank the patients for their consent to publish these case reports.
Appendix. Authors
| Name | Location | Contribution |
| Charlotte Schubert, MD | Institute of Neuroimmunology and Multiple Sclerosis (INIMS); Department of Neurology, University Medical Center Hamburg-Eppendorf, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Insa Schiffmann, MD | Institute of Neuroimmunology and Multiple Sclerosis (INIMS); Department of Neurology, University Medical Center Hamburg-Eppendorf, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Said C. Farschtschi, MD | Department of Neurology, University Medical Center Hamburg-Eppendorf, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Jean-François Emile, MD, PhD | Pathology Department, Paris-Saclay University, Versailles SQY University (UVSQ), EA4340-BECCOH, Assistance Publique-Hôpitaux de Paris (APHP), Ambroise-Paré Hospital, Boulogne-Billancourt, France | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Manuel A. Friese, MD | Institute of Neuroimmunology and Multiple Sclerosis (INIMS); Department of Neurology, University Medical Center Hamburg-Eppendorf, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Study Funding
The authors report no targeted funding.
Disclosure
C. Schubert has received speaker honoraria from Alexion, Desitin and TAD and has received compensation for advice for Alexion and Argenx. None of these are related to this study. S.C. Farschtschi has received speaker honoraria from AstraZeneca and Alexion and compensation for advice or lecturing from Alexion not related to this study. M.A. Friese has received speaker honoraria from Merck and Roche has received compensation for advice or lecturing for Alexion, Merck, Lundbeck, Roche, and Sudo Biosciences; none of these are related to this study. The other authors report no conflicts of interest. Go to Neurology.org/NN for full disclosures.
References
- 1.McClain KL, Bigenwald C, Collin M, et al. Histiocytic disorders. Nat Rev Dis Prim. 2021;7(1):73. doi: 10.1038/S41572-021-00307-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127(22):2672-2681. doi: 10.1182/BLOOD-2016-01-690636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnaud L, Hervier B, Néel A, et al. CNS involvement and treatment with interferon-α are independent prognostic factors in Erdheim-Chester disease: a multicenter survival analysis of 53 patients. Blood. 2011;117(10):2778-2782. doi: 10.1182/BLOOD-2010-06-294108 [DOI] [PubMed] [Google Scholar]
- 4.Jouenne F, Chevret S, Bugnet E, et al. Genetic landscape of adult Langerhans cell histiocytosis with lung involvement. Eur Respir J. 2020;55(2):1901190. doi: 10.1183/13993003.01190-2019 [DOI] [PubMed] [Google Scholar]
- 5.Haroche J, Cohen-Aubart F, Emile JF, et al. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood. 2013;121(9):1495-1500. doi: 10.1182/BLOOD-2012-07-446286 [DOI] [PubMed] [Google Scholar]
- 6.Diamond EL, Durham BH, Ulaner GA, et al. Efficacy of MEK inhibition in patients with histiocytic neoplasms. Nature. 2019;567(7749):521-524. doi: 10.1038/S41586-019-1012-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen Aubart F, Emile JF, Maksud P, et al. Efficacy of the MEK inhibitor cobimetinib for wild-type BRAF Erdheim-Chester disease. Br J Haematol. 2018;180(1):150-153. doi: 10.1111/BJH.14284 [DOI] [PubMed] [Google Scholar]
- 8.Damsky W, Thakral D, Emeagwali N, Galan A, King B. Tofacitinib treatment and molecular analysis of cutaneous sarcoidosis. N Engl J Med. 2018;379(26):2540-2546. doi: 10.1056/NEJMOA1805958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goyal G, Heaney ML, Collin M, et al. Erdheim-Chester disease: consensus recommendations for evaluation, diagnosis, and treatment in the molecular era. Blood. 2020;135(22):1929-1945. doi: 10.1182/BLOOD.2019003507 [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal A, Taychert M, Hasanin L, Doll D, Basuino MG, Hasanein H. Erdheim-Chester disease: a case report of BRAF V600E-negative, MAP2K1-positive ECD diagnosed by blood next-generation sequencing assay and a brief literature review. Oncology (Williston Park). 2023;37(7):298-302. doi: 10.46883/2023.25921001 [DOI] [PubMed] [Google Scholar]
- 11.Heinzerling L, Eigentler TK, Fluck M, et al. Tolerability of BRAF/MEK inhibitor combinations: adverse event evaluation and management. ESMO Open. 2019;4(3):e000491. doi: 10.1136/ESMOOPEN-2019-000491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross AM, Wolters PL, Dombi E, et al. Selumetinib in children with inoperable plexiform neurofibromas. N Engl J Med 2020;382(15):1430-1442. doi: 10.1056/NEJMOA1912735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF -mutated melanoma. N Engl J Med. 2014;371(20):1867-1876. doi: 10.1056/NEJMOA1408868 [DOI] [PubMed] [Google Scholar]
- 14.Eng C, Kim TW, Bendell J, et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019;20(6):849-861. doi: 10.1016/S1470-2045(19)30027-0 [DOI] [PubMed] [Google Scholar]
- 15.Cohen Aubart F, Emile JF, Carrat F, et al. Targeted therapies in 54 patients with Erdheim-Chester disease, including follow-up after interruption (the LOVE study). Blood. 2017;130(11):1377-1380. doi: 10.1182/BLOOD-2017-03-771873 [DOI] [PubMed] [Google Scholar]
- 16.Donadieu J, Larabi IA, Tardieu M, et al. Vemurafenib for refractory multisystem langerhans cell histiocytosis in children: an international observational study. J Clin Oncol. 2019;37(31):2857-2865. doi: 10.1200/JCO.19.00456 [DOI] [PMC free article] [PubMed] [Google Scholar]