Abstract
Background and Objectives
Optic neuritis is the most common optic neuropathy in young adults and a frequent manifestation of multiple sclerosis. Its clinical course is pertinent to the design of visual pathway neuroprotection trials.
Methods
This is a secondary analysis of longitudinal data from the TONE trial, which included 103 patients from 12 German academic tertiary centers with acute unilateral optic neuritis as a clinically isolated syndrome and baseline high-contrast visual acuity <0.5 decimal. Patients were randomized to 1,000 mg methylprednisolone i.v./d plus either erythropoietin (33,000 IU/d) or placebo (saline solution) for 3 days. They were followed up at standardized intervals with a battery of tests including high-contrast visual acuity, low-contrast letter acuity, contrast sensitivity, visual fields, visual evoked potentials, and retinal optical coherence tomography. At 6 months, participants answered a standardized questionnaire on vision-related quality of life (NEI-VFQ 25). We describe the disease course with mixed-effects piecewise linear models and calculate structure-function correlations using Pearson r. Because erythropoietin had no effect on the visual system, we use pooled (treatment-agnostic) data.
Results
Patients experienced initial rapid and then decelerating improvements of visual function with thinning of inner and thickening of outer retinal layers. At 6 months, visual parameters were positively correlated with inner and negatively correlated with outer retinal thickness changes. Peripapillary retinal nerve fiber layer thinning predominantly occurred in sectors without previous swelling. At 6 months, macular ganglion cell and inner plexiform layer thinning was weakly correlated with the P100 peak time (r = −0.11) and moderately correlated with the amplitude of visual evoked potentials (r = 0.35). Only functional outcomes were at least moderately correlated with vision-related quality of life.
Discussion
The longitudinal data from this large study cohort may serve as a reference for the clinical course of acute optic neuritis. The pattern of correlation between visual evoked potentials and inner retinal thinning may argue that the latter is mostly due to ganglion cell loss, rather than dysfunction. Visual pathway neuroprotection trials with functional outcomes are needed to confirm that candidate drugs will benefit patients' vision-related quality of life.
Trial Registration Information
ClinicalTrials.gov, NCT01962571.
Introduction
Optic neuritis is a frequent manifestation of multiple sclerosis (MS) and the most common optic neuropathy in young adults. Because its natural course is comparatively short, it has become a popular setting for clinical neuroprotection trials.1,2 With a mounting interest in short and cost-effective trials has come the need for high-quality data on the early course of glucocorticosteroid-treated optic neuritis, representing the likely course in a placebo group. The gold standard in this regard remains the Optic Neuritis Treatment Trial (ONTT), a multicentric study conducted in the 1980s that followed up over 400 participants with acute optic neuritis at standardized intervals with a battery of visual tests. Owing to the limitations of its time, the ONTT was not able to include 2 outcomes that have since proven to be of immense value: optical coherence tomography (OCT), which quantifies thicknesses of retinal neuronal layers on a micrometer scale, and low-contrast letter acuity, the psychophysical parameter which most closely mirrors disease-induced subjective changes.
More recent clinical cohorts which include these outcomes are typically limited by a much lower number of participants, irregular follow-up, monocentric recruitment, inclusion of patients with long-standing MS (who are apt to preexisting visual system changes), and a limited number of clinical outcomes.1,3-5 We aim to address these limitations and provide a new reference on the early course of glucocorticosteroid-treated acute optic neuritis, based on longitudinal data from 12 German tertiary academic centers acquired in the TONE study, described below.
Methods
In a randomized multicenter trial (TONE, ClinicalTrials.gov registration NCT01962571), our group investigated the safety and efficacy of high-dose erythropoietin (EPO) as an add-on to methylprednisolone pulse therapy in 103 patients with a first episode of acute unilateral optic neuritis as a clinically isolated syndrome and baseline high-contrast visual acuity <3/6 (0.5 decimal). Patients had to present within 10 days of disease onset and test negative for AQP4 antibodies to be included. They were recruited from 12 German academic tertiary referral centers between November 25, 2014, and October 9, 2017. All subjects received high-dose methylprednisolone (1,000 mg/d) for 3 days. They were randomized (1:1) to receive either 33,000 IU/d EPO or placebo (saline solution) as an adjunct, to be administered on the same days as methylprednisolone. We found that erythropoietin administration had no effect on the visual system.6,7 For this investigation, we thus used pooled (treatment-agnostic) data of all analyzed TONE participants. This amounts to a well-characterized disease cohort that received methylprednisolone in a standardized fashion as the only potentially visually effective drug.
We report examination data that were obtained at baseline and at weeks 4, 16, and 26. These included affected and fellow eye measurements of high-contrast visual acuity (ETDRS chart score), low-contrast letter acuity (2.5% Sloan chart score), contrast sensitivity (log), visual fields, visual evoked potentials (VEPs) P100 amplitudes and peak times, as well as OCT of the peripapillary retinal nerve fiber layer and posterior pole. A detailed description of the procedures is available in the eMethods. Acquisition, handling, and reporting of OCT data are in accordance with the OSCAR-IB and APOSTEL recommendations.8,9 The report follows the STROBE guidelines.10
Statistical Analyses
The statistical analysis was performed using R (version 3.6.2) with the RStudio interface. The analysis set comprised data from the 103 patients in the TONE trial who received at least one dose of the study medication and for whom at least one follow-up OCT was available. In consensus with the American Statistical Association's statement on p values11 and recent addendum,12 we report outcomes as adjusted mean estimates with 95% confidence intervals. We modeled longitudinal (time course) data using mixed-effects piecewise linear models (lme4 and lspline packages, versions 1.1-27.1 and 1.0-0) with spline points at 4 and 16 weeks as well as random intercepts and random slopes per patient. The peripapillary retinal nerve fiber layer thickness change was calculated relative to the unaffected eye at baseline. Changes in macular retinal layer thicknesses were calculated relative to the same (affected) eye at baseline. They were modeled without random intercepts because the intercept (change at baseline) is zero. Models for visual evoked potentials P100 amplitudes and peak times were additionally adjusted for the study center (details and justification in eMethods). The reported mean estimates are population estimates (fixed effects). Correlation analyses were performed using Pearson r. We considered correlations with 0.1 ≤ |r| < 0.3 to be weak, 0.3 ≤ |r| < 0.5 to be moderate, and |r| ≥ 0.5 to be strong.13
Standard Protocol Approvals, Registrations, and Patient Consents
The TONE study (NCT01962571) was approved by the ethics committee of the University of Freiburg, Germany, and the institutional review boards of all participating sites. All participants provided written informed consent.
Data Availability
Individual participant data of the TONE trial, including data dictionaries, will be made available to researchers with a methodologically sound proposal.
Results
Study Population
The cohort included 71 female (69%) and 32 male (31%) participants with an overall median age of 30 years (IQR 25 to 36), of whom 51 (50%) had received placebo and 52 (50%) had received erythropoietin. Their detailed characteristics are listed in eTable 1.
Time Course of Structural Outcomes
Change in Peripapillary Retinal Nerve Fiber Layer Thickness (3.5-mm Circle)
Compared with the unaffected eye, there was a mean global peripapillary retinal nerve fiber layer (pRNFL) thickening (swelling) of 14.8 µm (95% CI 9.1–20.5) at baseline. This was more pronounced in the superior and inferior poles compared with the temporal sector. The global pRNFL thickness decreased by 2.1 µm/week in the first 4 weeks (−3.3 to −0.9) and then by 1.7 µm/week (−2.0 to −1.3) until week 16. A small amount of pRNFL thinning took place between weeks 16 and 26 (−0.2 µm/wk [−0.3 to −0.1], Figure 1A). The relative thinning (% change) was more pronounced in sectors with less thickening at baseline (eFigure 1). Change estimates for all sectors of the 3.5-mm peripapillary circle are listed in eTable 2. Estimates in the 4.1-mm and 4.7-mm peripapillary circles are tabulated in eTable 3 and eTable 4, respectively.
Figure 1. Time Course of Inner Retinal Layer Thickness Changes.

Triangles are individual data points. Thick black lines are estimated population means. Dashed lines are per-patient estimates. mGCIPL = macular ganglion cell and inner plexiform layer; mRNFL = macular retinal nerve fiber layer; pRNFL = peripapillary retinal nerve fiber layer. * = Compared with the unaffected eye at baseline.
Change in Macular Retinal Layer Thicknesses
Macular layers showed no evidence of thickening at baseline (data not shown). The disease course was characterized by initial rapid thinning of the inner retinal layers (Figure 1, B and C) and thickening of the outer retinal layers (Figure 2). Macular thickness changes generally concluded after 4 months. The exception was the outer nuclear layer, whose thickness gradually declined between week 16 and 26 (Figure 2B, eTable 5).
Figure 2. Time Course of Outer Retinal Layer Thickness Changes.
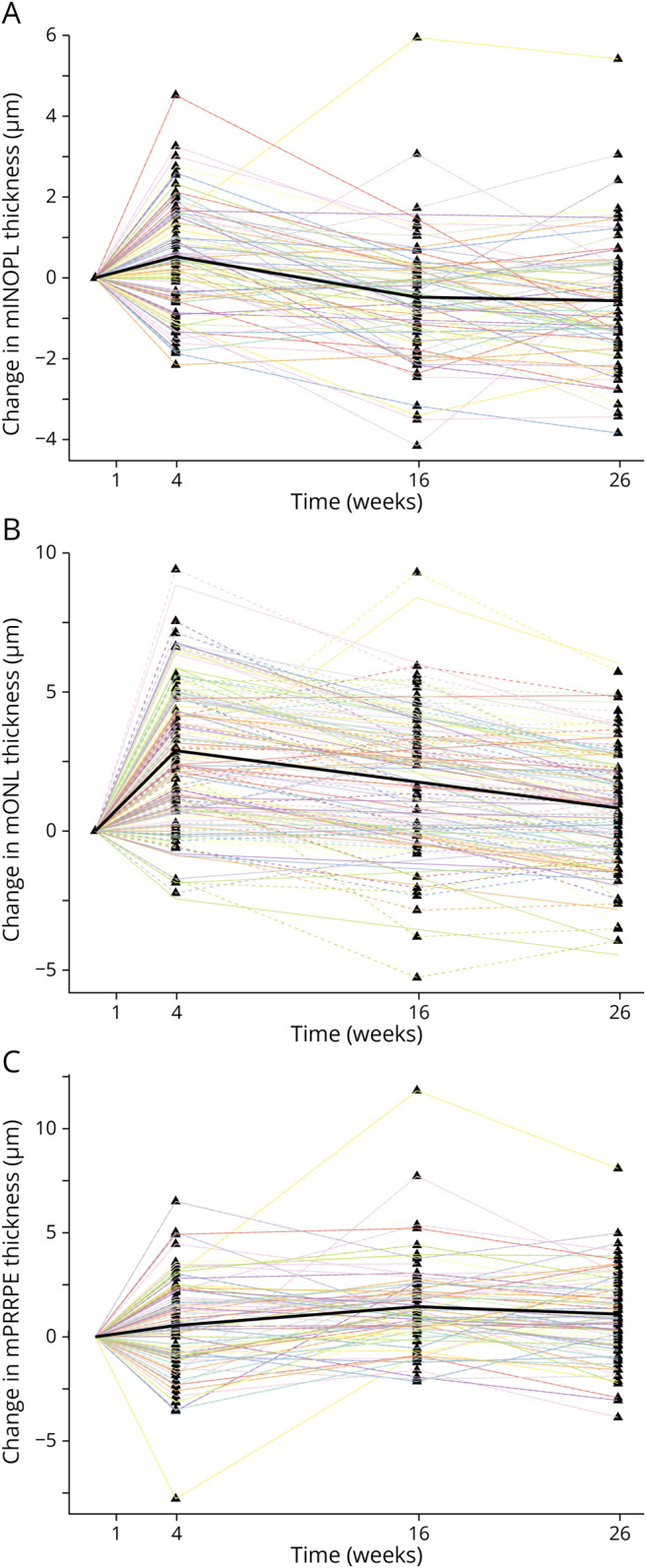
Triangles are individual data points. Thick black lines are estimated population means. Dashed lines are per-patient estimates. mINOPL = macular inner nuclear and outer plexiform layer; mONL = macular outer nuclear layer; mPRRPE = macular photoreceptor layer and retinal pigment epithelium.
The initial change was most pronounced in the macular ganglion cell and inner plexiform layer (mGCIPL), which lost 1.5 µm of thickness per week in the first 4 weeks (95% CI −1.7 to −1.3). It continued thinning at a slower pace until week 16 (−0.2 µm/wk [−0.3 to −0.2]) and then remained stable (0.0 µm/wk [95% CI −0.1 to 0.0]). The macular retinal nerve fiber layer (mRNFL) followed a similar trajectory (Figure 1, B and C,eTable 5). Per-sector analysis revealed that mGCIPL thinning was more prominent in the inner ETDRS sectors, whereas mRNFL thinning was more prominent in the outer sectors (eFigure 2).
Changes in the outer retina were most prominent in the outer nuclear layer, whose thickness increased by 0.7 µm/wk in the first 4 weeks (95% CI 0.6–0.9) and then gradually declined by 0.1 µm/wk (−0.1 to −0.1) until week 26. The inner nuclear and outer plexiform layer thickness followed a similar trajectory but reverted to stable baseline values by week 16. The thickening in the combined photoreceptor and retinal pigment epithelium stratum was more gradual, peaking at week 16 and remaining stable at a slightly elevated level (Figure 2, A–C, eTable 5).
Microcystic Macular Edema
At week 26, 2 affected eyes of 2 participants had microcystic macular edema. It did not appear on macular scans at baseline, week 4, and week 16 in any subject.
Time Course of Functional Visual Outcomes
There was rapid improvement in all subjective functional outcomes in the first 4 weeks, followed by a tapering in the pace of recovery (eTable 6). In contrast to retinal thinning, which concluded by week 16, functional improvements continued until week 26.
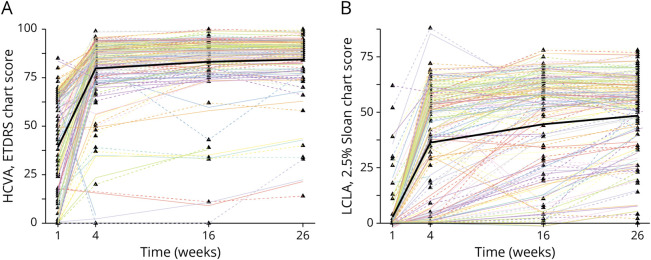
At baseline, high-contrast visual acuity (HCVA) was distributed bimodally with local maxima at zero and Snellen 3/6 (0.5 decimal, the upper limit allowed by the inclusion criteria). On further visits, its distribution was normal, with few outliers on the lower spectrum. It improved by a mean of 10.1 ETDRS letters/week (95% CI: 8.7 to 11.6) in the first 4 weeks, with only subtle changes thereafter (Figure 3A). The overall recovery was good: At week 26, 75 of 103 patients (73%) achieved a HCVA of 85 or better, corresponding to Snellen 20/20 or 1.0 decimal. However, there remained an impairment of 6.1 ETDRS letters compared with the unaffected eye (95% CI: 3.3 to 8.9), and 6 of 103 individuals (6%) had vision worse than Snellen 3/6 (0.5 decimal).
Figure 3. Time Course of Visual Acuity.
Triangles are individual data points. Thick black lines are estimated population means. Dashed lines are per-patient estimates. HCVA = high-contrast visual acuity; LCLA = low-contrast letter acuity.
At baseline, 90 of 103 patients (87%) had a low-contrast letter acuity (LCLA) score of less than 5, meaning that they were not able to complete the first line of the chart. On subsequent visits, LCLA scores were more widely distributed and there were more outliers in the lower spectrum compared with HCVA (Figure 3B). The initial improvement was less pronounced (mean change until week 4: 8.4 letters/week [95% CI 7.1–9.5]). Recovery continued until week 26 (0.7 letter/week [0.4–1.0] in weeks 4–16, then 0.4 letters/week [0.1–0.6]). At week 26, an impairment of 16.4 letters remained compared with the unaffected eye (95% CI 12.0–20.9).
The changes in contrast sensitivity (eFigure 3), the visual field mean defect (eFigure 4), and the VEP P100 amplitude (Figure 4A) followed similar general trajectories. The model for the VEP P100 peak time estimated an increase in the first 4 weeks and then a gradual decline until week 26 (Figure 4B). This is further discussed in the supplement. Change estimates for all models are listed in eTable 6.
Figure 4. Time Course of Electrophysiologic Outcomes.
Triangles are individual data points. Thick black lines are estimated population means. Dashed lines are per-patient estimates. VEP = visual evoked potential.
Structure-Function Correlation and Vision-Related Quality of Life
Vision-related quality of life was weakly correlated with the change in pRNFL thickness (Pearson r = 0.15). Its association with the mGCIPL thickness change fell below the threshold of a weak correlation (r = 0.08). The correlation of the pRNFL thickness change with functional outcomes was generally stronger than or similar to that of the mGCIPL thickness change (Table). The functional outcome with the strongest correlation to vision-related quality of life was high-contrast visual acuity (r = 0.60). Low-contrast letter acuity and contrast sensitivity were moderately correlated with vision-related quality of life (|r| within 0.38–0.42).
Table.
Correlation of Outcome Measures at Week 26
| Change in retinal layer thickness | Patient-reported outcome | ||||||
| pRNFLa | mRNFL | mGCIPL | mINOPL | mONL | mPRRPE | NEI-VFQ | |
| pRNFL thickness change, µm | 1 | 0.63 | 0.76 | 0.04 | −0.28 | −0.25 | 0.15 |
| mGCIPL thickness change, µm | 0.76 | 0.73 | 1 | 0.23 | −0.23 | −0.18 | 0.08 |
| LCLA, 2.5% Sloan chart score | 0.57 | 0.37 | 0.53 | −0.04 | −0.12 | −0.28 | 0.39 |
| CS, log | 0.56 | 0.11 | 0.42 | −0.09 | −0.07 | −0.19 | 0.42 |
| HCVA, ETDRS chart score | 0.48 | 0.15 | 0.25 | −0.07 | −0.17 | −0.24 | 0.60 |
| Visual field MD, dB | −0.46 | −0.35 | −0.25 | −0.05 | 0.05 | 0.31 | −0.34 |
| VEP P100 amplitude, µV | 0.35 | 0.24 | 0.35 | 0.04 | −0.04 | −0.15 | 0.31 |
| VEP P100 peak time, ms | −0.31 | −0.09 | −0.11 | 0.13 | 0.34 | 0.18 | −0.07 |
Abbreviations: CS = contrast sensitivity; HCVA = high-contrast visual acuity; LCLA = low-contrast letter acuity; MD = mean defect; mGCIPL = macular ganglion cell and inner plexiform layer; mINOPL = macular inner nuclear and outer plexiform layer; mONL = macular outer nuclear layer; mPRRPE = macular photoreceptor layer and retinal pigment epithelium; mRNFL = macular retinal nerve fiber layer; pRNFL = peripapillary retinal nerve fiber layer; VEP = visual evoked potential.
Values are Pearson r. Thickness changes are relative to the affected eye at baseline except for the pRNLF thickness change, which is relative to the unaffected eye at baseline.
Compared with the unaffected eye at baseline.
Discussion
The data from the TONE study provide a unique opportunity to investigate the change dynamics after acute optic neuritis. This study resembles the Optic Neuritis Treatment Trial in its follow-up pattern but adds retinal optical coherence tomography and 2.5% low-contrast letter acuity to the test battery. We found a striking reduction in low-contrast letter acuity compared with high-contrast visual acuity at baseline, the majority of patients being unable to read past the first line on the 2.5% Sloan chart. Mild clinically apparent disc swelling in the affected eye was present in 23% of patients. On OCT, baseline pRNFL thickening was most pronounced in the superior and inferior poles while axons in the temporal sector and papillomacular bundle suffered the most subsequent percent thinning. This finding may argue against the notion that swelling of the superficial pRNFL is indicative of future atrophy—a pattern that would be distinct from other optic neuropathies such as NAION (non-arteritic anterior ischemic optic neuropathy). The predilection of relative thinning toward the temporal sectors might implicate metabolic stress as a component of axonal degeneration14 because the axons in the papillomacular bundle are particularly vulnerable. At present, we advise caution in interpreting this finding because previous reports have identified a more uniform pattern of per-sector pRNFL thinning.15
On follow-up, patients experienced improvements in all tested parameters of visual function, thinning of inner retinal layers, and a transient thickening of outer retinal layers. Changes were most dynamic in the first month. Inner retinal layer thickness plateaued after 4 months while other changes attenuated more gradually. Together with previous data from monocentric cohorts,1,3-5 this indicates that 4 months' follow-up is sufficient for clinical neuroprotection trials that measure the mGCIPL thickness. Because pRNFL thickness and more so low-contrast letter acuity still changed meaningfully between months 4 and month 6, we suggest that trials with these outcomes should follow up for at least 6 months. Because differences in mGCIPL thickness can also be detected with fewer participants compared with pRNFL thickness,1 trials with this outcome can be comparatively cost-efficient. Measuring pRNFL rather than mGCIPL thickness has the advantages of quantifying all optic nerve neurons, rather than only those whose cell bodies are located in the macula, and of being more closely associated with vision-related quality of life. However, detecting change that is truly meaningful to patients will require functional outcomes because only these were at least moderately correlated with vision-related quality of life, a finding that is consistent with previous literature.16-18 While additional instruments such as the 10-item Neuro-ophthalmic Supplement could have provided additional insights, there remains a general need to develop robust patient-reported outcome measures in optic neuritis.19
Inner retinal layer thinning in optic neuritis is a well-characterized1,4,5,20 and cogent finding as the mRNFL and mGCIPL correspond to the axons and cell bodies with underlying synapses of the inflamed retinal ganglion cell, respectively. The degree to which this thinning is due to structural changes in living cells or represents cell death has yet to be conclusively shown—a distinction with profound implications for the possibility of longer term neuroprotection or neurorestoration. In this cohort, mGCIPL thinning at 6 months was moderately correlated with the VEP amplitude (r = 0.35), but only weakly correlated with VEP P100 peak times (r = −0.11), a pattern that more likely represents retinal ganglion cell loss than dysfunction. A similar pattern of VEP changes was previously observed in relation to optic nerve thinning on MR studies21 and in a smaller cross-sectional OCT study of 46 patients with MS-associated ON.18
The second major structural change was a transient thickening in outer retinal layers, confirming findings from smaller monocentric cohorts.1,4,22,23 A possible mechanism suggested by Gabilondo et al.4 is an accumulation of microglia because of transitory edema mediated by Müller cell dysfunction. Although outer retinal thickening in our study was negatively associated with visual function, the magnitude of these retinal changes was very subtle. At the current time, they are best interpreted on a population level. Maturing technologies, such as adaptive optics OCT, might provide more accurate quantification that can also be interpreted in individual patients.24
While the TONE study found no treatment effect of EPO in the visual system, we cannot exclude subtleties that may have led to a higher variance in the pooled data. Some caution is, therefore, warranted because of our pooled analysis of EPO and placebo recipients. Finally, our data and analyses address the clinical course of optic neuritis under high-dose methylprednisolone therapy and do not pertain to untreated patients, for whom there remains a literature gap in longitudinal OCT data. The effect of time to treatment initiation is currently being investigated in a large-scale international prospective cohort by the Acute Optic Neuritis Network.25
The limitations of the TONE study design have been discussed.6 Most notably, a possible inadvertent inclusion of a small number of patients with anti-myelin oligodendrocyte glycoprotein (MOG)-associated optic neuritis may have led to an overestimation of clinical recovery. The overall prevalence of MOG optic neuritis in a population such as ours is expected to be low because subsequent serum analysis of ONTT patients26 revealed anti-MOG positivity in only 1.7%. Furthermore, MOG-associated optic neuritis typically presents with clinically severe optic disc swelling, an exclusion criterion in our study. We therefore believe that confounding by anti-MOG–positive patients, if present, is likely to be quantitively small. Previously clinically unapparent episodes of optic neuritis and retrograde transsynaptic atrophy from potential central inflammatory lesions are other possible but unmeasured confounders.
Our analysis confirms the feasibility of acute optic neuritis as a model for clinical neuroprotection and remyelination. This study builds upon previous works by including a larger number of patients who uniformly presented with acute optic neuritis as a clinically isolated syndrome, recruitment from multiple centers, standardized follow-up with a multimodal functional characterization of the visual system, and optical coherence tomography including macular outer retinal layer segmentation. We expect our findings to be applicable to most central European patients presenting with acute idiopathic or multiple sclerosis–associated optic neuritis and a baseline high-contrast visual acuity of less than 0.5 decimal. They should provide a reasonable expectation of the disease course in a methylprednisolone-treated placebo group in a clinical trial and may serve as a future reference on the disease course of optic neuritis.
Acknowledgment
The authors thank all participants who took part in the TONE trial. The authors also acknowledge support by the Open Access Publication Fund of the University of Freiburg.
Glossary
- EPO
erythropoietin
- HCVA
high-contrast visual acuity
- LCLA
low-contrast letter acuity
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- OCT
optical coherence tomography
- ONTT
Optic Neuritis Treatment Trial
- pRNFL
peripapillary retinal nerve fiber layer
- VEP
visual evoked potentials
Appendix. Authors
| Name | Location | Contribution |
| Sebastian Küchlin, MD | Eye Center, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Gabriele Ihorst, PhD | Clinical Trials Unit, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Sven P. Heinrich, PhD | Eye Center, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany` | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Pablo Márquez Neila, PhD | ARTORG Center for Biomedical Engineering Research, University of Bern, Switzerland | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Philipp Albrecht, MD | Department of Neurology, Maria Hilf Clinics Mönchengladbach; Department of Neurology, Medical Faculty, Heinrich Heine-Universität Düsseldorf, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design |
| Martin J. Hug, PhD | Pharmacy, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Ricarda Diem, MD | Department of Neurology and National Center for Tumor Diseases, Faculty of Medicine, University Hospital Heidelberg, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design |
| Wolf A. Lagrèze, MD | Eye Center, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design |
Study Funding
The authors report no targeted funding.
Disclosure
S. Küchlin: doctoral fellowship with funding from the University of Freiburg, faculty of medicine and the Else Kröner Fresenius foundation; G. Ihorst: funding from Novartis, funding from Janssen Pharmaceutica; S.P. Heinrich: no relevant conflicts of interest; P. Márquez Neila: no relevant conflicts of interest; P. Albrecht: grants personal fees and non-financial support from Novartis, grants personal fees and non-financial support from Biogen, grants personal fees and non-financial support from Merz, grants personal fees and non-financial support from Roche, grants personal fees and non-financial support from Celgene, personal fees and non-financial support from Teva, personal fees and non-financial support from Ipsen, personal fees and non-financial support from Allergan, personal fees and non-financial support from Merck, grants and personal fees from Sanofi, personal fees from Janssen Cilag, personal fees from Sandoz, all outside of the submitted work; M.J. Hug: grants from Bristol-Myers Squibb, speaker's honoraria from Amgen, speaker's honoraria from Baxter Deutschland, speaker's honoraria from CSL Behring, speaker's honoraria from Biotest Pharma, speaker's honoraria from Fresenius Kabi, speaker's honoraria from Leo Pharma, speaker's honoraria from Merck Serono, speaker's honoraria from Novartis Pharma, speaker's honoraria from Pfizer, speaker's honoraria from Roche Pharma, speaker's honoraria from Sun Pharmaceuticals Industries; R. Diem: funding by the BMBF, funding by the DFG, funding by the Hertie Foundation; W.A. Lagrèze: grants from the German Federal Ministry for Education and Research (BMBF), grants from the German Research Foundation (DFG). Go to Neurology.org/NN for full disclosures.
References
- 1.Andorrà M, Alba-Arbalat S, Camos-Carreras A, et al. . Using acute optic neuritis trials to assess neuroprotective and remyelinating therapies in multiple sclerosis. JAMA Neurol. 2020;77(2):234-244. doi: 10.1001/jamaneurol.2019.3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toosy AT, Mason DF, Miller DH. Optic neuritis. Lancet Neurol. 2014;13(1):83-99. doi: 10.1016/S1474-4422(13)70259-X [DOI] [PubMed] [Google Scholar]
- 3.Henderson AP, Altmann DR, Trip AS, et al. . A serial study of retinal changes following optic neuritis with sample size estimates for acute neuroprotection trials. Brain. 2010;133(9):2592-2602. doi: 10.1093/brain/awq146 [DOI] [PubMed] [Google Scholar]
- 4.Gabilondo I, Martínez-Lapiscina EH, Fraga-Pumar E, et al. . Dynamics of retinal injury after acute optic neuritis: retinal Injury in ON. Ann Neurol. 2015;77(3):517-528. doi: 10.1002/ana.24351 [DOI] [PubMed] [Google Scholar]
- 5.Costello F, Pan YI, Yeh EA, Hodge W, Burton JM, Kardon R. The temporal evolution of structural and functional measures after acute optic neuritis. J Neurol Neurosurg Psychiatry. 2015;86(12):1369-1373. doi: 10.1136/jnnp-2014-309704 [DOI] [PubMed] [Google Scholar]
- 6.Lagrèze WA, Küchlin S, Ihorst G, et al. . Safety and efficacy of erythropoietin for the treatment of patients with optic neuritis (TONE): a randomised, double-blind, multicentre, placebo-controlled study. Lancet Neurol. 2021;20(12):991-1000. doi: 10.1016/S1474-4422(21)00322-7 [DOI] [PubMed] [Google Scholar]
- 7.Küchlin S, Ihorst G, Grotejohann B, et al. . Treatment with erythropoietin for patients with optic neuritis: long-term follow-up. Neurol Neuroimmunol Neuroinflamm. 2023;10(4):e200067. doi: 10.1212/NXI.0000000000200067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz-Herranz A, Balk LJ, Oberwahrenbrock T, et al. . The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2016;86(24):2303-2309. doi: 10.1212/WNL.0000000000002774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schippling S, Balk L, Costello F, et al. . Quality control for retinal OCT in multiple sclerosis: validation of the OSCAR-IB criteria. Mult Scler. 2015;21(2):163-170. doi: 10.1177/1352458514538110 [DOI] [PubMed] [Google Scholar]
- 10.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 11.Wasserstein RL, Lazar NA. The ASA statement on p-values: context, process, and purpose. Am Statistician. 2016;70(2):129-133. doi: 10.1080/00031305.2016.1154108 [DOI] [Google Scholar]
- 12.Wasserstein RL, Schirm AL, Lazar NA. Moving to a world beyond “p < 0.05.”. Am Statistician. 2019;73(sup1):1-19. doi: 10.1080/00031305.2019.1583913 [DOI] [Google Scholar]
- 13.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Routledge; 2013. [Google Scholar]
- 14.Adiele RC, Adiele CA. Metabolic defects in multiple sclerosis. Mitochondrion. 2019;44:7-14. doi: 10.1016/j.mito.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 15.Costello F, Hodge W, Pan Y, Eggenberger E, Coupland S, Kardon R. Tracking retinal nerve fiber layer loss after optic neuritis: a prospective study using optical coherence tomography. Mult Scler. 2008;14(7):893-905. doi: 10.1177/1352458508091367 [DOI] [PubMed] [Google Scholar]
- 16.Mowry EM, Loguidice MJ, Daniels AB, et al. . Vision related quality of life in multiple sclerosis: correlation with new measures of low and high contrast letter acuity. J Neurol Neurosurg Psychiatry. 2009;80(7):767-772. doi: 10.1136/jnnp.2008.165449 [DOI] [PubMed] [Google Scholar]
- 17.Balk LJ, Coric D, Nij Bijvank JA, Killestein J, Uitdehaag BM, Petzold A. Retinal atrophy in relation to visual functioning and vision-related quality of life in patients with multiple sclerosis. Mult Scler. 2018;24(6):767-776. doi: 10.1177/1352458517708463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longbrake EE, Lancia S, Tutlam N, Trinkaus K, Naismith RT. Quantitative visual tests after poorly recovered optic neuritis due to multiple sclerosis. Mult Scler Relat Disord. 2016;10:198-203. doi: 10.1016/j.msard.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panthagani J, O'Donovan C, Aiyegbusi OL, et al. . Evaluating patient-reported outcome measures (PROMs) for future clinical trials in adult patients with optic neuritis. Eye. 2023;37(15):3097-3107. doi: 10.1038/s41433-023-02478-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petzold A, Balcer LJ, Calabresi PA, et al. . Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2017;16(10):797-812. doi: 10.1016/S1474-4422(17)30278-8 [DOI] [PubMed] [Google Scholar]
- 21.Burton EV, Greenberg BM, Frohman EM. Optic neuritis: a mechanistic view. Pathophysiology. 2011;18(1):81-92. doi: 10.1016/j.pathophys.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 22.Kaufhold F, Zimmermann H, Schneider E, et al. . Optic neuritis is associated with inner nuclear layer thickening and microcystic macular edema independently of multiple sclerosis. PLoS ONE. 2013;8(8):e71145. doi: 10.1371/journal.pone.0071145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Louzi OA, Bhargava P, Newsome SD, et al. . Outer retinal changes following acute optic neuritis. Mult Scler. 2016;22(3):362-372. doi: 10.1177/1352458515590646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller DT, Kurokawa K. Cellular-scale imaging of transparent retinal structures and processes using adaptive optics optical coherence tomography. Annu Rev Vis Sci. 2020;6(1):115-148. doi: 10.1146/annurev-vision-030320-041255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asseyer S, Asgari N, Bennett J, et al. . The acute optic neuritis network (ACON): study protocol of a non-interventional prospective multicenter study on diagnosis and treatment of acute optic neuritis. Front Neurol. 2023;14:1102353. doi: 10.3389/fneur.2023.1102353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen JJ, Tobin WO, Majed M, et al. . Prevalence of myelin oligodendrocyte glycoprotein and aquaporin-4–IgG in patients in the optic neuritis treatment trial. JAMA Ophthalmol. 2018;136(4):419-422. doi: 10.1001/jamaophthalmol.2017.6757 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual participant data of the TONE trial, including data dictionaries, will be made available to researchers with a methodologically sound proposal.