Abstract
Live attenuated simian immunodeficiency viruses (SIV), such as nef deletion mutants, are the most effective vaccines tested in the SIV-macaque model so far. To modulate the antiviral immune response induced by live attenuated SIV vaccines, we had previously infected rhesus monkeys with a nef deletion mutant of SIV expressing interleukin 2 (SIV-IL2) (B. R. Gundlach, H. Linhart, U. Dittmer, S. Sopper, S. Reiprich, D. Fuchs, B. Fleckenstein, G. Hunsmann, S. Stahl-Hennig, and K. Überla, J. Virol. 71:2225–2232, 1997). In the present study, SIV-IL2-infected macaques and macaques infected with the nef deletion mutant SIVΔNU were challenged with pathogenic SIV 9 to 11 months postvaccination. In contrast to the results with naive control monkeys, no challenge virus could be isolated from the SIV-IL2- and SIVΔNU-infected macaques. However, challenge virus sequences could be detected by nested PCR in some of the vaccinated macaques. To determine the role of immune responses directed against Env of SIV, four vaccinated macaques were rechallenged with an SIV-murine leukemia virus (MLV) hybrid in which the env gene of SIV had been functionally replaced by the env gene of amphotropic MLV. All vaccinated macaques were protected from productive infection with the SIV-MLV hybrid in the absence of measurable neutralizing antibodies, while two naive control monkeys were readily infected. Since the SIV-MLV hybrid uses the MLV Env receptor Pit2 and not CD4 and a coreceptor for virus entry, chemokine inhibition and receptor interference phenomena were not involved in protection. These results indicate that the protective responses induced by live attenuated SIV vaccines can be independent of host immune reactions directed against Env.
Despite extensive efforts, no safe and effective vaccine is yet available to protect against human immunodeficiency virus (HIV) type 1 (HIV-1) infection. Inoculation of rhesus monkeys with simian immunodeficiency viruses (SIV) is a useful model to study the efficacy of different vaccination strategies. The most effective vaccines in the SIV-macaque model are live attenuated SIV such as nef deletion mutants. Macaques previously infected with attenuated immunodeficiency viruses were protected from high-dose challenges with cell-free and cell-associated pathogenic virus strains (1, 9, 27, 41). The protective capacity increased with length of time of vaccination, although protection could be achieved as early as 8 and 10 weeks postinfection in some animals (27, 41). There was a direct correlation between the ability of the vaccine virus to replicate in the host and the degree of protection that was conferred (23, 41). This correlation between protection and the replicative capacity of the vaccine virus in the host (23, 41) may hamper attempts to further attenuate vaccine viruses without a loss of the capacity to induce protection. One way to circumvent this problem may be to enhance the immunogenicity of a more attenuated vaccine virus to afford the same degree of protection as that obtained with a less attenuated virus. Such a result might be achieved by local coexpression of viral antigen and immunostimulating cytokines. Therefore, we replaced the nef gene of SIVmac239 with the interleukin 2 (IL-2) coding region (15) and obtained SIV-IL2. The course of SIV-IL2 infection in rhesus monkeys was similar to the course of infection with the nef deletion mutant SIVΔNU, although mean capsid antigen levels and urinary neopterin levels were higher in the SIV-IL2-infected macaques than in the SIVΔNU-infected animals during the acute phase of infection (15). To determine the effect of IL-2 expression on vaccine protection, SIV-IL2- and SIVΔNU-infected macaques were challenged with pathogenic SIVmac239.
A number of effector mechanisms may mediate vaccine protection. Increased levels of neutralizing antibodies (6, 8, 23, 41), high cytotoxic T-lymphocyte (CTL) activity (25), and detectable T-helper-cell proliferation after challenge (30) were found to correlate with protection. Due to their inhibitory activity, chemokines (4, 7, 29) and other soluble factors released from CD8-positive cells (2, 22) may also be involved. In addition, nonimmunological mechanisms such as interference between vaccine virus and challenge virus may be responsible for protection (23, 27). If the latter were the case, live attenuated immunodeficiency viruses could hardly be used in humans, since long-term persistence of the vaccine virus at levels that can compete with the challenge virus likely would be required. Therefore, it is important to understand the mechanisms mediating protection prior to the use of live attenuated HIV-1 vaccines in humans. However, since no inbred monkey strains are available to allow cell transfer experiments, it is difficult to establish any causal relationship between a potential mechanism and protection.
With vaccine and challenge viruses containing env genes from heterologous immunodeficiency viruses, protection was observed in the absence of detectable neutralizing antibodies or antibodies cross-reacting with challenge virus Env at the time of challenge (5, 12, 25, 31, 33), suggesting that neutralizing antibodies were not required for protection by live attenuated immunodeficiency virus vaccines. To further evaluate the importance of Env-directed immune responses, SIV-IL2- and SIVΔNU-infected macaques were challenged with an SIV-murine leukemia virus (MLV) hybrid in which the env gene of SIV was functionally replaced by the env gene of amphotropic MLV. The lack of homology between SIV Env and MLV Env allowed the importance of the Env-specific humoral and cellular immune responses to be analyzed. Since cell entry by MLV Env is not inhibited by chemokines (10), it was also possible to investigate a potential contribution of chemokines to vaccine protection. The role of receptor interference could also be evaluated, since SIV and MLV use different receptors for entry into cells.
MATERIALS AND METHODS
Viruses and cell cultures.
SIV-IL2 and SIVΔNU (SIVmac239) were described previously (15). An SIVmac239 nef-open (18) challenge virus stock was prepared on rhesus monkey peripheral blood mononuclear cells (PBMCs). The median tissue culture infective dose (TCID50) was 105.8/ml when the virus stock was tested on C8166 cells as described previously (17). The median monkey infective dose (MID50) was 106/ml when 10-fold dilutions (10−3 to 10−7) of the virus stock were injected intravenously into two monkeys each. Construction of MuSIV+, which only differs from MuSIV (32) by the presence of a full-length nef gene, will be described elsewhere (32a). An MuSIV+ stock (was prepared on rhesus monkey PBMCs (38), and the TCID50 of the virus stock (104.8/ml) was determined on C8166 cells (17). Positive cultures were identified by immunoperoxidase staining with serum from an SIV-infected macaque essentially as described previously (28). The only modification was the use of concanavalin A-coated microtiter plates instead of poly-l-lysine-coated plates to increase the adherence of the cells. The minimal number of PBMCs of infected macaques required for virus isolation in cocultures with C8166 or Raji cells was determined as a measure of cell-associated viral load (16). Positive cultures were identified by immunoperoxidase staining as described above.
Infection of rhesus monkeys.
Animals were housed at the German Primate Center in Göttingen, Germany. Care of the monkeys and collection of specimens were carried out in accordance with institutional guidelines as described previously (36). One hundred MID50s of SIVmac239 was injected intravenously into eight rhesus monkeys at 38 weeks (monkeys 7738-IL2, 7741-IL2, 7742-IL2, 7756ΔNU, 7761ΔNU, and 7763ΔNU; see legend to Fig. 1 for explanation of designations) or 46 weeks (monkeys 7744-IL2 and 7755ΔNU) after infection with SIV-IL2 or SIVΔNU (15). Two rhesus monkeys of Indian origin (seronegative for SIV, type D retroviruses, and simian T-cell leukemia virus type 1) were infected intravenously with SIVmac239 at the same time and at the same dose. Monkeys 7738-IL2, 7741-IL2, 7761ΔNU, and 7763ΔNU were rechallenged intravenously with 1,000 TCID50s of MuSIV+. Again, two naive rhesus monkeys were infected with MuSIV+ in parallel as a control.
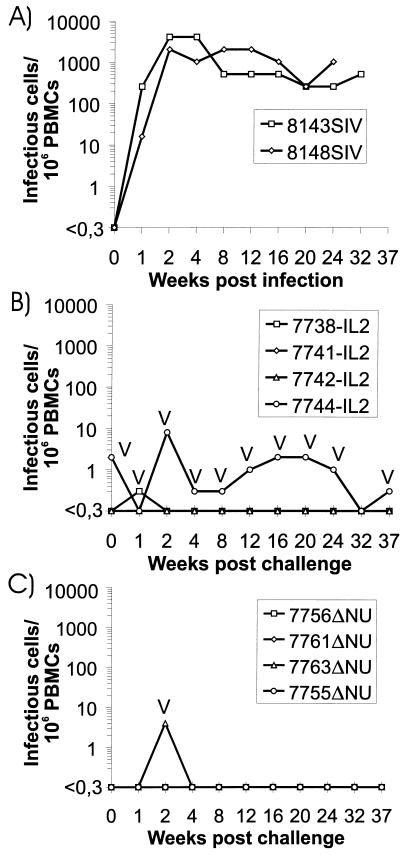
FIG. 1.
Cell-associated viral load after inoculation of naive rhesus monkeys (A), SIV-IL2 infected-rhesus monkeys (B), and SIVΔNU-infected rhesus monkeys (C) with SIVmac239. Infectious cells/106 PBMCs were calculated from the minimal number of PBMCs required for virus isolation in cocultures with C8166 cells. Isolates were characterized by PCR as described in Materials and Methods. V, vaccine virus. The four-digit numbers are monkey designations, and the letters following the numbers indicate the virus with which the monkeys were first infected: IL2, SIV-IL2; ΔNU, SIVΔNU; SIV, SIVmac239.
PCR.
To characterize isolates recovered at different times after infection, CEMx174 cells infected with the different isolates were lysed in buffer K (50 mM KCl, 15 mM Tris, 2.5 mM MgCl2, 0.5% Tween 20, 100 μg of proteinase K per ml) and subjected to PCR. With primers SL16 and SL17 (20), which flank the deletions in nef and the U3 region, fragments were amplified from the lysates under the following conditions: 94°C for 2 min and 39 cycles of 40 s at 94°C, 1 min at 61°C, and 1 min at 72°C. After each cycle, the extension time was prolonged by 1 s. PCR products were size separated by agarose gel electrophoresis side by side with PCR products derived from plasmids containing full-length nef, SIV-IL2, or SIVΔNU sequences. This procedure allowed us to discriminate between these viruses. To detect challenge virus sequences without prior cultivation, PBMCs or lymph node cells were lysed in buffer K. Genomic DNA was purified by phenol-chloroform extraction and concentrated by ethanol preipitation, if necessary. Genomic DNA (1 to 10 μg) was used in a 100-μl PCR for 25 cycles with primers S9261s (5′-TAC-TCC-AGA-GGC-TCT-CTG-CGA-3′) and S10241a (5′-GCG-ACT-GAA-TAC-AGA-GCG-AAA-TGC-AGT-3′) under the conditions described above. A 1-μl quantity of the PCR product was amplified in a second round for 39 cycles with primers SL16 and S10020a (5′-GGT-ATC-TAA-CAT-ATG-CCT-CAT-AAG-T-3′), which does not bind to SIV-IL2 or SIVΔNU, thereby allowing selective amplification of full-length nef sequences. With this nested PCR, a minimum of four copies of nef could be detected in the presence of 10 μg of genomic DNA.
Immunological methods.
PBMCs were phenotypically characterized by three-color fluorescence analysis on a FACScan flow cytometer (Becton-Dickinson, Heidelberg, Germany). For determination of lymphocyte subsets, a gate was set on forward and side light scattering to include T cells, B cells, and NK cells with a minimum of contaminating macrophages. These cell populations were defined by monoclonal antibodies against CD3 (FN18; M. Jonkers, Biomedical Primate Research Center, Rijswijk, The Netherlands), CD20 (H299; Coulter, Krefeld, Germany), CD16 and CD56 (3G8 and B159, respectively; Immunotech, Hamburg, Germany), and CD14 (RM052; Immunotech). CD4+ T cells (OKT4; Ortho, Neckargemuend, Germany) were further differentiated into naive and memory T-helper cells on the basis of low-level or high-level (CD29+) expression of CD29 (4B4; Coulter).
The humoral immune response of infected monkeys against SIV antigens was determined by an enzyme-linked immunosorbent assay (ELISA) with pelleted, whole SIVmac251 as an antigen as described previously (37). Antibody titers against MLV Env were determined by immunofluorescence analysis. 293T cells were transfected with the MLV env expression plasmid pHIT456 as described previously (35). Two days after transfection, cells were air dried on glass slides and fixed in ice-cold acetone for 15 min. Serial dilutions of sera (starting with a 1:20 dilution) in phosphate-buffered saline containing 5% bovine serum albumin were incubated with transfected and mock-transfected 293T cells, washed, and stained with a 1:50 dilution of a fluorescein isothiocyanate (FITC)-labelled secondary rabbit anti-human immunoglobulin G (IgG) antibody (DAKO, Glostrup, Denmark). In a blinded fashion, the highest antibody dilution giving clear staining of transfected but not mock-transfected 293T cells was determined and taken as the anti-MLV Env antibody titer. Neutralizing antibody titers were determined on CEMx174-SEAP cells, which express the secreted alkaline phosphatase gene under the control of the SIV long terminal repeat, essentially as described previously (24). Sera (20 μl) were diluted with 180 μl of RPMI medium containing 10% fetal calf serum. This dilution was inactivated at 56°C for 30 min and further diluted in seven twofold steps. Serial dilutions (25 μl) were incubated with 25 μl of an MuSIV+ stock in triplicate for 2 h. CEMx174-SEAP cells (150 μl) (2 × 105 cells/ml) were added and incubated for 3 days. The SEAP activity of the culture supernatants was determined with a Phospha-Light-kit (Tropix, Bedford, Mass.).
RESULTS
To study the effect of IL-2 on SIV replication, pathogenesis, and immunogenicity, we had previously infected four rhesus monkeys with SIV-IL2 and four with SIVΔNU. The expression of IL-2 by the nef deletion mutant did not fundamentally alter the attenuated phenotype of this nef deletion mutant, although there was a slight increase in virus replication and immune stimulation during the acute phase of infection (15). The consequences of IL-2 expression by a live attenuated virus on vaccine protection were analyzed by intravenous inoculation of 100 MID50s of pathogenic SIVmac239 at 38 and 46 weeks after infection with SIV-IL2 or SIVΔNU. At the time of challenge, virus could be isolated only from one of the SIV-IL2-infected monkeys (7744-IL2) and not from the other, SIV-IL2- or SIVΔNU-infected monkeys (Fig. 1B and C). The virus recovered from monkey 7744-IL2 contained a deletion in the inserted IL-2 coding region similar to that in a virus recovered from this monkey 16 weeks after infection with SIV-IL2 (15). In contrast, virus containing a full-length IL-2 coding region could be isolated from monkey 7741-IL2 up to 28 weeks postinfection (data not shown). When genomic DNA from the PBMCs of SIV-IL2-infected monkeys was analyzed 32 weeks postinfection by nested PCR, deletions in the IL-2 coding region of SIV-IL2 were detected in all animals (data not shown). A full-length IL2 coding region was present at the same time in genomic DNA isolated from the PBMLs of three of the four SIV-IL2-infected monkeys (data not shown). No apparent differences were observed in the SIV antibody titers between SIV-IL2- and SIVΔNU-infected macaques prior to or at the time of challenge (Table 1). SIV-specific T-helper-cell proliferation was detectable in all SIV-IL2- and SIVΔNU-infected macaques 2 weeks prior to challenge and in all infected macaques but 7738-IL2 at the time of challenge (data not shown).
TABLE 1.
SIV antibody titers after challenge with SIVmac239
| Animal | Antibody titera (103) at the following wk postchallenge with SIVmac239:
|
|||
|---|---|---|---|---|
| −12 | 0 | 24 | 37 | |
| 7738-IL2 | 51 | 102 | 51 | 51 |
| 7741-IL2 | 102 | 205 | 102 | 205 |
| 7742-IL2 | 51 | 51 | 51 | 51 |
| 7744-IL2 | 102b | 102 | 102 | 205 |
| 7755ΔNU | 102b | 102 | 51 | 51 |
| 7756ΔNU | 26 | 26 | 26 | 51 |
| 7761ΔNU | 205 | 410 | 410 | 410 |
| 7763ΔNU | 205 | 205 | 205 | 205 |
| 8143SIV | ND | 0 | 819 | ND |
| 8149SIV | ND | 0 | 102 | ND |
Serum dilution giving absorbance values twice the background values. ND, not done.
Determined at week −22.
After inoculation of SIVmac239 into two naive control monkeys, high cell-associated viral loads were observed 2 weeks postinfection and persisted for the entire observation period (Fig. 1A). In contrast, four macaques previously infected with SIV-IL2 (Fig. 1B) or SIVΔNU (Fig. 1C) had low or undetectable cell-associated viral loads. From monkey 7744-IL2, which was virus isolation positive at the time of challenge, virus could be isolated repeatedly after challenge. Characterization by PCR revealed the presence of the vaccine virus but not the challenge virus (Fig. 1B). Similarly, isolates recovered 1 and 2 weeks after challenge from monkeys 7738-IL2 and 7763ΔNU, respectively, contained the vaccine virus and not the challenge virus (Fig. 1B and C). However, with a sensitive nested PCR, full-length nef sequences were detected in PBMCs or lymph node cells isolated 6 or 37 weeks after challenge from all SIVΔNU-infected animals and two of the SIV-IL2-infected animals (Table 2). The load of the challenge virus must have been rather low, since only one or two of five independent PCRs were positive in each of the two PCR-positive SIV-IL2-infected macaques and only one of four independent PCRs was positive in each of the SIVΔNU-infected macaques (Table 2).
TABLE 2.
Detection of full-length nef sequences after challenge with SIVmac239 and MuSIV+a
| Animal | Detection of full-length nef sequences at the indicated wk postchallenge with:
|
||||
|---|---|---|---|---|---|
| SIVmac239
|
MuSIV+
|
||||
| 6 | 37 | 37 (LN) | 2 | 4 | |
| 7738-IL2 | −/− | − | −/− | − | +/− |
| 7741-IL2 | +/+ | − | −/− | + | −/− |
| 7742-IL2 | −/− | − | −/− | ND | ND |
| 7744-IL2 | −/− | − | +/− | ND | ND |
| 7755ΔNU | + | − | −/− | ND | ND |
| 7756ΔNU | + | − | −/− | ND | ND |
| 7761ΔNU | + | − | −/− | − | −/− |
| 7763ΔNU | − | + | −/− | + | −/− |
Nested PCR was performed on DNA from PBMCs or lymph node cells (LN). Results obtained from the same DNA sample in two independent PCRs are separated by a slash. +, sequences were detected; −, sequences were not detected; ND, not done.
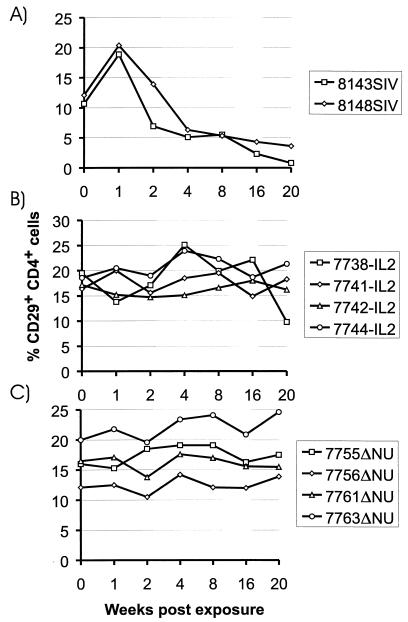
The PCR data suggest that the spread of the challenge virus was controlled efficiently in the absence of sterilizing immunity. Determination of SIV antibody titers did not reveal an increase in antibody titers after challenge (Table 1). Challenge virus replication must have been too low to induce an anamnestic humoral immune response. Although the viral load determinations did not give any evidence for progression to AIDS in the vaccinated macaques, the percentage of CD29+ CD4+ lymphocytes was determined, since a drop in this population is an early prognostic marker for a decline in immune function in humans (3, 11) and macaques (19, 26). A reduction in the percentage of CD29+ CD4+ cells was observed in the control monkeys (Fig. 2A) but not in the vaccinated monkeys (Fig. 2B and C). A reduction in the percentage of CD29+ CD4+ cells in macaque 7738-IL2 at week 20 was only transient, since normal values were obtained for a follow-up sample (data not shown). The CD4/CD8 ratio did not decrease in the vaccinated monkeys, but a two- to threefold reduction was seen in the naive control monkeys (data not shown).
FIG. 2.
Percentage of CD29+ CD4+ cells in peripheral blood lymphocytes of macaques infected with SIVmac239 (A), SIV-IL2 (B), or SIVΔNU (C). Numbers of CD29+ CD4+ cells are expressed as a percentage of total lymphocytes.
Concordant with the laboratory findings, both naive control animals succumbed to AIDS-like disease. Monkey 8148SIV died 33 weeks postinfection due to massive Pneumocystis carinii (PC) pneumonia. PC structures were detectable in the lung alveoli, and lung tissue was also PC positive when analyzed by immunohistochemistry. The other control monkey had generalized lymphadenopathy and severe splenomegaly. It had to be euthanatized 45 weeks postinfection due to partial paralysis of the lower extremities and urinary retention. Necropsy findings included multiple neoplasias in the thoracic and pelvic cavities; these neoplasias were histologically identified as nodal and extranodal malignant lymphoma. Since all vaccinated macaques were clinically asymptomatic, SIV-IL2 and SIVΔNU induced efficient control of challenge virus replication and solid protection against the pathogenic consequences of SIV infection in the absence of sterilizing immunity.
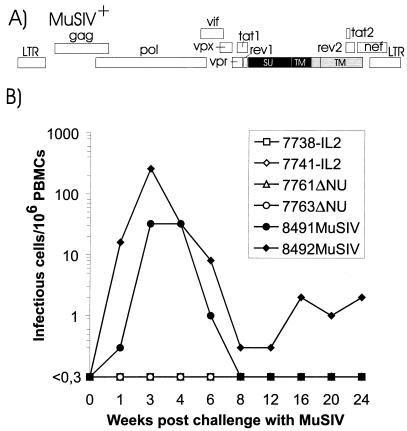
To further examine the mechanisms involved in protection, two of the SIV-IL2-infected macaques and two of the SIVΔNU-infected macaques which were protected from productive SIVmac239 infection were exposed intravenously to 1,000 TCID50s of an SIV-MLV hybrid virus (MuSIV+) 37 weeks after the first challenge. In MuSIV+ (Fig. 3A), the env gene of SIVmac239 is functionally replaced by the env gene of amphotropic MLV as previously described for MuSIV (32). MuSIV+ replicates in CD4− cell lines and is inhibited by an anti-MLV Env antibody (data not shown). Due to the lack of homology between MLV and SIV, SIV Env-directed immune responses should not cross-react with MLV Env. Indeed, no MLV Env binding antibodies and no MLV Env neutralizing antibodies could be detected at the time of challenge (Table 3).
FIG. 3.
Challenge of vaccinated macaques with MuSIV+. (A) Map of the SIV-MLV hybrid MuSIV+. MLV-derived sequences are marked by black boxes; inactivated reading frames are shaded. (B) Cell-associated viral load in macaques challenged with MuSIV+. Infectious cells/106 PBMCs were calculated from the minimal number of PBMCs required for virus isolation in cocultures with Raji cells. LTR, long terminal repeat; SU, surface protein; TM, transmembrane protein.
TABLE 3.
Antibody titers against MLV Env
| Animal | Titer of the following antibodies against MLV Env at the indicated wk postchallenge with MuSIV+:
|
|||
|---|---|---|---|---|
| Binding
|
Neutralizing
|
|||
| 0 | 16 | 0 | 16 | |
| 7738-IL2 | <1:20 | <1:20 | <1:20 | <1:20 |
| 7741-IL2 | <1:20 | <1:20 | <1:20 | <1:20 |
| 7761ΔNU | <1:20 | <1:20 | <1:20 | <1:20 |
| 7763ΔNU | <1:20 | <1:20 | <1:20 | <1:20 |
| 8491MuSIV | <1:20 | 1:320 | <1:20 | 1:320 |
| 8492MuSIV | <1:20 | 1:320 | <1:20 | 1:640 |
In two naive control monkeys infected with MuSIV+ in parallel, the cell-associated viral load determined on CD4− Raji cells revealed peak titers of up to 250 infectious units/106 PBMCs (Fig. 3B). In contrast, no virus could be isolated from any of the vaccinated macaques by use of cocultures with Raji cells (Fig. 3B). When CD4+ C8166 cells were used for coculturing, virus could be isolated only from monkey 7761ΔNU 8 weeks postchallenge and from monkey 7763ΔNU 20 and 24 weeks postchallenge. Since the isolates contained a deletion in the nef gene, the vaccine virus was isolated, but MuSIV+ or SIVmac239 was not. For three of the vaccinated macaques, full-length nef sequences could not be detected in PBMCs or lymph nodes at the time of the second challenge in three independent PCRs. For two of the macaques, full-length nef sequences were detectable shortly after the second challenge in one of three independent PCRs (Table 2). This finding could have been due to low-level infection with MuSIV+ or reactivation of the first challenge virus. If infection with MuSIV+ occurred, replication must have been rather weak, since neither of the vaccinated macaques developed antibodies against MLV Env (Table 3). In contrast, antibodies and neutralizing antibodies against MLV Env were detectable in the naive control monkeys 16 weeks after infection with MuSIV+ but not prior to infection (Table 3). This seroconversion confirms infection of the naive control animals but not of the vaccinated animals with MuSIV+. Analyses of SIV antibody titers in the vaccinated macaques after challenge with MuSIV+ did not reveal an anamnestic immune response (Table 4), which could have indicated infection with MuSIV+.
TABLE 4.
SIV antibody titers after challenge with MuSIV+
| Animal | Antibody titera (103) at the following wk postchallenge with MuSIV+:
|
|||
|---|---|---|---|---|
| 0 | 4 | 8 | 16 | |
| 7738-IL2 | 51 | 51 | 51 | 26 |
| 7741-IL2 | 205 | 205 | 205 | 205 |
| 7761ΔNU | 410 | 205 | 205 | 410 |
| 7763ΔNU | 205 | 205 | 410 | 205 |
| 8491MuSIV | 0 | 0.05 | 0.2 | 0.2 |
| 8492MuSIV | 0 | 0.2 | 0.1 | 0.2 |
Serum dilution giving absorbance values twice the background values.
DISCUSSION
Macaques preinfected with an IL-2-expressing nef deletion mutant of SIV were protected from productive infection after challenge with a high dose of pathogenic SIVmac239 9 and 11 months after vaccination. Although no challenge virus could be isolated, nested PCR indicated the presence of challenge virus sequences in two of the SIV-IL2-infected macaques either 6 or 37 weeks postchallenge. Rather than that sterilizing immunity was induced, the spread of the challenge virus seemed to be controlled efficiently in these two monkeys. Since all animals vaccinated with SIVΔNU were protected to a similar degree following challenge with SIVmac239, there is no evidence that SIV-IL2 is superior to SIVΔNU with respect to vaccine efficiency. An earlier challenge might help to clarify whether protective responses are induced faster by SIV-IL2 than by SIVΔNU. However, since the viral load in SIV-IL2-infected macaques was higher than that in SIVΔNU-infected macaques during the acute phase of infection (15), SIV-IL2 does not seem to be a safer vaccine than SIVΔNU. Therefore, other cytokines, such as gamma interferon (14), might be better suited to further attenuate nef deletion mutants of SIV without affecting protective properties.
To analyze the importance of Env for vaccine protection, four macaques which were immunized with SIV-IL2 or SIVΔNU and which were previously protected from productive infection following SIVmac239 challenge were exposed to MuSIV+, an SIV-MLV hybrid in which the env gene of SIV is functionally replaced by the env gene of amphotropic MLV. All four macaques were protected from this challenge, as indicated by a lack of challenge virus isolation, the absence of seroconversion to MLV Env, and a missing anamnestic immune responses. However, since there is no established mechanism which could mediate sterilizing immunity against MuSIV+, low-level infection with MuSIV+ probably occurred, in agreement with our PCR data. Env-independent mechanisms must have controlled MuSIV+ replication in the vaccinated macaques. Therefore, a number of Env-dependent mechanisms that have been proposed to be involved in protection induced by live attenuated SIV vaccines did not seem to be required. Since SIV and MLV belong to different interference groups (34), protection did not depend on receptor interference. Although the kinetics of viral load and strength of protection argue against interference phenomena between vaccine and challenge viruses, Env-independent interference phenomena (39) still cannot be excluded.
Chemokines could also play an important role in the control of immunodeficiency virus infection. RANTES, MIP1α, MIP1β, and SDF inhibit immunodeficiency virus replication by preventing the interaction of Env with its coreceptors, members of the chemokine receptor family (4, 7, 10, 13, 29). This inhibition is highly specific for the coreceptor used by the respective Env and can be overcome by pseudotyping with an immunodeficiency virus Env targeting a different coreceptor. Since MLV Env-mediated cell entry is not inhibited by the chemokines analyzed (10) and since MLV Env uses an unrelated receptor (40), protection against MuSIV+ did not seem to depend on chemokine inhibition. In addition, protection occurred in the absence of neutralizing antibodies. Evidence for protection in the absence of neutralizing antibodies was previously obtained by use of a chimeric SIV in which the env gene of SIV was replaced by the env gene of HIV-1 (SHIV) as a challenge virus (5, 12, 33). Since no antibodies directed against Env of the challenge virus were detected at the time of challenge, protection was most likely mediated by cytotoxic T cells directed against the SIV genes present in the challenge virus. However, cellular immune responses recognizing Env of the vaccine virus and Env of the challenge virus could not be excluded. After challenge, cross-reacting, Env-specific T-helper cells might allow more rapid production of antibodies directed against Env of the challenge virus and thereby facilitate control of the challenge virus. When monkeys infected with attenuated SHIV were challenged with pathogenic SIV, they were protected in two studies (25, 31) but not in another one (21). The different outcomes after challenge with SHIV or SIV could have been due to the fact that the SHIV used as the vaccine virus was too attenuated to induce high levels of protection. Alternatively, since the SHIV used as the challenge virus in the earlier experiments seemed to be less pathogenic than SIV, induction of protection against SHIV might have been easier to achieve than that against fully pathogenic SIV. Like the SHIV used in the previous challenge studies, MuSIV+ is also attenuated.
With more virulent challenge viruses or homologous challenge viruses, Env-dependent mechanisms might facilitate protection. In addition, it seems likely that the best protection is achieved by vaccines eliciting a combination of antiviral immune responses. Nevertheless, this study shows that live attenuated SIV vaccines can protect against viruses carrying an unrelated env gene. Since inhibition by chemokines, neutralization by antibodies, and receptor interference did not seem to be required for protection, Env-independent effector mechanisms, such as cytotoxic T cells directed against other viral proteins, must have been responsible. Immunization with viral proteins other than Env should also be considered for vaccination against HIV.
ACKNOWLEDGMENTS
This work was supported by grants from the Bundesministerium für Bildung und Forschung (SIV Collaborative Research Project) and the Deutsche Forschungsgemeinschaft (SFB 466, Teilprojekt B4).
We thank M. Wirth and U. Sauer for excellent technical assistance, F. Kirchhoff for helpful discussions, and G. Hunsmann and B. Fleckenstein for continuous support.
REFERENCES
- 1.Almond N, Kent K, Cranage M, Rud E, Clarke B, Stott E J. Protection by attenuated simian immunodeficiency virus in macaques against challenge with virus-infected cells. Lancet. 1995;345:1342–1344. doi: 10.1016/s0140-6736(95)92540-6. [DOI] [PubMed] [Google Scholar]
- 2.Baier M, Werner A, Bannert N, Metzner K, Kurth R. HIV suppression by interleukin-16. Nature. 1995;378:563. doi: 10.1038/378563a0. [DOI] [PubMed] [Google Scholar]
- 3.Blatt S P, McCarthy W F, Bucko Krasnicka B, Melcher G P, Boswell R N, Dolan J, Freeman T M, Rusnak J M, Hensley R E, Ward W W, Barnes D, Hendrix C W. Multivariate models for predicting progression to AIDS and survival in human immunodeficiency virus-infected persons. J Infect Dis. 1995;171:837–844. doi: 10.1093/infdis/171.4.837. [DOI] [PubMed] [Google Scholar]
- 4.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–832. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 5.Bogers W M, Niphuis H, ten Haaft P, Laman J D, Koornstra W, Heeney J L. Protection from HIV-1 envelope-bearing chimeric simian immunodeficiency virus (SHIV) in rhesus macaques infected with attenuated SIV: consequences of challenge. AIDS. 1995;9:13–18. [PubMed] [Google Scholar]
- 6.Clements J E, Montelaro R C, Zink M C, Amedee A M, Miller S, Trichel A M, Jagerski B, Hauer D, Martin L N, Bohm R P, Murphey-Corb M. Cross-protective immune responses induced in rhesus macaques by immunization with attenuated macrophage-tropic simian immunodeficiency virus. J Virol. 1995;69:2737–2744. doi: 10.1128/jvi.69.5.2737-2744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 8.Cole K S, Rowles J L, Jagerski B A, Murphey-Corb M, Unangst T, Clements J E, Robinson J, Wyand M S, Desrosiers R C, Montelaro R C. Evolution of envelope-specific antibody responses in monkeys experimentally infected or immunized with simian immunodeficiency virus and its association with the development of protective immunity. J Virol. 1997;71:5069–5079. doi: 10.1128/jvi.71.7.5069-5079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniel M D, Kirchhoff F, Czajak S C, Sehgal P K, Desrosiers R C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 10.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 11.Dolan M J, Clerici M, Blatt S P, Hendrix C W, Melcher G P, Boswell R N, Freeman T M, Ward W, Hensley R, Shearer G M. In vitro T cell function, delayed-type hypersensitivity skin testing, and CD4+ T cell subset phenotyping independently predict survival time in patients infected with human immunodeficiency virus. J Infect Dis. 1995;172:79–87. doi: 10.1093/infdis/172.1.79. [DOI] [PubMed] [Google Scholar]
- 12.Dunn C S, Hurtel B, Beyer C, Gloecker L, Ledger T N, Moog C, Kieny M P, Methali M, Schmitt D, Gut J P, Kirn A, Aubertin A M. Protection of SIVmac-infected macaque monkeys against superinfection by a simian immunodeficiency virus expressing envelope glycoproteins of HIV type 1. AIDS Res Hum Retroviruses. 1997;13:913–922. doi: 10.1089/aid.1997.13.913. [DOI] [PubMed] [Google Scholar]
- 13.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 14.Giavedoni L, Ahmad S, Jones L, Yilma T. Expression of gamma interferon by simian immunodeficiency virus increases attenuation and reduces postchallenge virus load in vaccinated rhesus macaques. J Virol. 1997;71:866–872. doi: 10.1128/jvi.71.2.866-872.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gundlach B R, Linhart H, Dittmer U, Sopper S, Reiprich S, Fuchs D, Fleckenstein B, Hunsmann G, Stahl-Hennig C, Überla K. Construction, replication, and immunogenic properties of a simian immunodeficiency virus expressing interleukin 2. J Virol. 1997;71:2225–2232. doi: 10.1128/jvi.71.3.2225-2232.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoch J, Lang S M, Weeger M, Stahl Hennig C, Coulibaly C, Dittmer U, Hunsmann G, Fuchs D, Müller J, Sopper S, Fleckenstein B, Überla K. vpr deletion mutant of simian immunodeficiency virus induces AIDS in rhesus monkeys. J Virol. 1995;69:4807–4813. doi: 10.1128/jvi.69.8.4807-4813.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson V, Byington R E. Quantitative assays for virus infectivity. In: Aldovini A, Walker B D, editors. Techniques in HIV research. New York, N.Y: Stockton Press; 1990. pp. 71–76. [Google Scholar]
- 18.Kestler H W, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 19.Kneitz C, Kerkau T, Müller J, Coulibaly C, Stahl-Hennig C, Hunsmann G, Hünig T, Schimpl A. Early phenotypic and functional alterations in lymphocytes from simian immunodeficiency virus infected macaques. Vet Immunol Immunopathol. 1993;36:239–255. doi: 10.1016/0165-2427(93)90022-v. [DOI] [PubMed] [Google Scholar]
- 20.Lang S M, Weeger M, Stahl Hennig C, Coulibaly C, Hunsmann G, Müller J, Müller-Hermelink H K, Fuchs D, Wachter H, Daniel M M, Desrosiers R C, Fleckenstein B. Importance of vpr for infection of rhesus monkeys with simian immunodeficiency virus. J Virol. 1993;67:902–912. doi: 10.1128/jvi.67.2.902-912.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Letvin N L, Li J, Halloran M, Cranage M P, Rud E W, Sodroski J. Prior infection with a nonpathogenic chimeric simian-human immunodeficiency virus does not efficiently protect macaques against challenge with simian immunodeficiency virus. J Virol. 1995;69:4569–4571. doi: 10.1128/jvi.69.7.4569-4571.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy J A, Mackewitz C E, Barker E. Controlling HIV pathogenesis: the role of the noncytotoxic anti-HIV response of CD8+ T cells. Immunol Today. 1996;17:217–223. doi: 10.1016/0167-5699(96)10011-6. [DOI] [PubMed] [Google Scholar]
- 23.Lohman B L, McChesney M B, Miller C J, McGowan E, Joye S M, Van Rompay K K, Reay E, Antipa L, Pedersen N C, Marthas M L. A partially attenuated simian immunodeficiency virus induces host immunity that correlates with resistance to pathogenic virus challenge. J Virol. 1994;68:7021–7029. doi: 10.1128/jvi.68.11.7021-7029.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Means R E, Greenough T, Desrosiers R C. Neutralization sensitivity of cell culture-passaged simian immunodeficiency virus. J Virol. 1997;71:7895–7902. doi: 10.1128/jvi.71.10.7895-7902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller C J, McChesney M B, Lu X, Dailey P J, Chutkowski C, Lu D, Brosio P, Roberts B, Lu Y. Rhesus macaques previously infected with simian-human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239. J Virol. 1997;71:1911–1921. doi: 10.1128/jvi.71.3.1911-1921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morphey-Corb M, Martin L N, Davison-Fairburn B, Montelaro R C, Miller M, West M, Ohkawa S, Baskin G, Zhang J, Putney S D, Allison A C, Eppstein D A. A formalin-inactivated whole SIV vaccine confers protection in macaques. Science. 1989;246:1293–1297. doi: 10.1126/science.2555923. [DOI] [PubMed] [Google Scholar]
- 27.Norley S, Beer B, Binninger-Schinzel D, Cosma C, Kurth R. Protection from pathogenic SIVmac challenge following short-term infection with a Nef-deficient attenuated virus. Virology. 1996;219:195–205. doi: 10.1006/viro.1996.0237. [DOI] [PubMed] [Google Scholar]
- 28.Norley S G, Lower J, Kurth R. Insufficient inactivation of HIV-1 in human cryo poor plasma by beta-propiolactone: results from a highly accurate virus detection method. Biologicals. 1993;21:251–258. doi: 10.1006/biol.1993.1082. [DOI] [PubMed] [Google Scholar]
- 29.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J, Arenzana-Seisdedos F, Schwartz O, Heard J, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 30.Petry H, Dittmer U, Stahl Hennig C, Coulibaly C, Makoschey B, Fuchs D, Wachter H, Tolle T, Morys Wortmann C, Kaup F J, Jurkiewitz E, Lüke W, Hunsmann G. Reactivation of human immunodeficiency virus type 2 in macaques after simian immunodeficiency virus SIVmac superinfection. J Virol. 1995;69:1564–1574. doi: 10.1128/jvi.69.3.1564-1574.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quesada-Rolander M, Mäkitalo B, Thorstensson R, Zhang Y-J, Castanos-Velez E, Biberfeld G, Putkonen P. Protection against mucosal SIVsm challenge in macaques infected with a chimeric SIV that expresses HIV type 1 envelope. AIDS Res Hum Retroviruses. 1996;12:993–999. doi: 10.1089/aid.1996.12.993. [DOI] [PubMed] [Google Scholar]
- 32.Reiprich S, Gundlach B R, Fleckenstein B, Überla K. Replication-competent chimeric lenti-oncovirus with expanded host cell tropism. J Virol. 1997;71:3328–3331. doi: 10.1128/jvi.71.4.3328-3331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Reiprich, S., et al. Unpublished data.
- 33.Shibata R, Siemon C, Czajak S C, Desrosiers R C, Martin M A. Live, attenuated simian immunodeficiency virus vaccines elicit potent resistance against a challenge with a human immunodeficiency virus type 1 chimeric virus. J Virol. 1997;71:8141–8148. doi: 10.1128/jvi.71.11.8141-8148.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sommerfelt M A, Weiss R A. Receptor interference groups of 20 retroviruses plating on human cells. Virology. 1990;176:58–69. doi: 10.1016/0042-6822(90)90230-o. [DOI] [PubMed] [Google Scholar]
- 35.Soneoka Y, Cannon P M, Ramsdale E E, Griffiths J C, Romano G, Kingsman S M, Kingsman A J. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 1995;23:628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stahl-Hennig C, Herchenröder O, Nick S, Evers M, Stille-Siegener M, Jentsch K D, Kirchhoff F, Tolle T, Gatesman T J, Lüke W, Hunsmann G. Experimental infection of macaques with HIV-2ben, a novel HIV-2 isolate. AIDS. 1990;4:611–617. doi: 10.1097/00002030-199007000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Stahl-Hennig C, Voss G, Nick S, Petry H, Fuchs D, Wachter H, Coulibaly C, Lüke W, Hunsmann G. Immunization with Tween-ether-treated SIV adsorbed onto aluminum hydroxide protects monkeys against experimental SIV infection. Virology. 1992;186:588–596. doi: 10.1016/0042-6822(92)90025-k. [DOI] [PubMed] [Google Scholar]
- 38.Überla K, Stahl Hennig C, Böttiger D, Mätz-Rensing K, Kaup F J, Li J, Haseltine W A, Fleckenstein B, Hunsmann G, Öberg B, Sodroski J. Animal model for the therapy of acquired immunodeficiency syndrome with reverse transcriptase inhibitors. Proc Natl Acad Sci USA. 1995;92:8210–8214. doi: 10.1073/pnas.92.18.8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volsky D J, Simm M, Shahabuddin M, Li G, Chao W, Potash M J. Interference to human immunodeficiency virus type 1 infection in the absence of downmodulation of the principal virus receptor, CD4. J Virol. 1996;70:3823–3833. doi: 10.1128/jvi.70.6.3823-3833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss R A, Tailor C S. Retrovirus receptors. Cell. 1995;82:531–533. doi: 10.1016/0092-8674(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 41.Wyand M S, Manson K H, Garcia-Moll M, Montefiori D, Desrosiers R C. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]